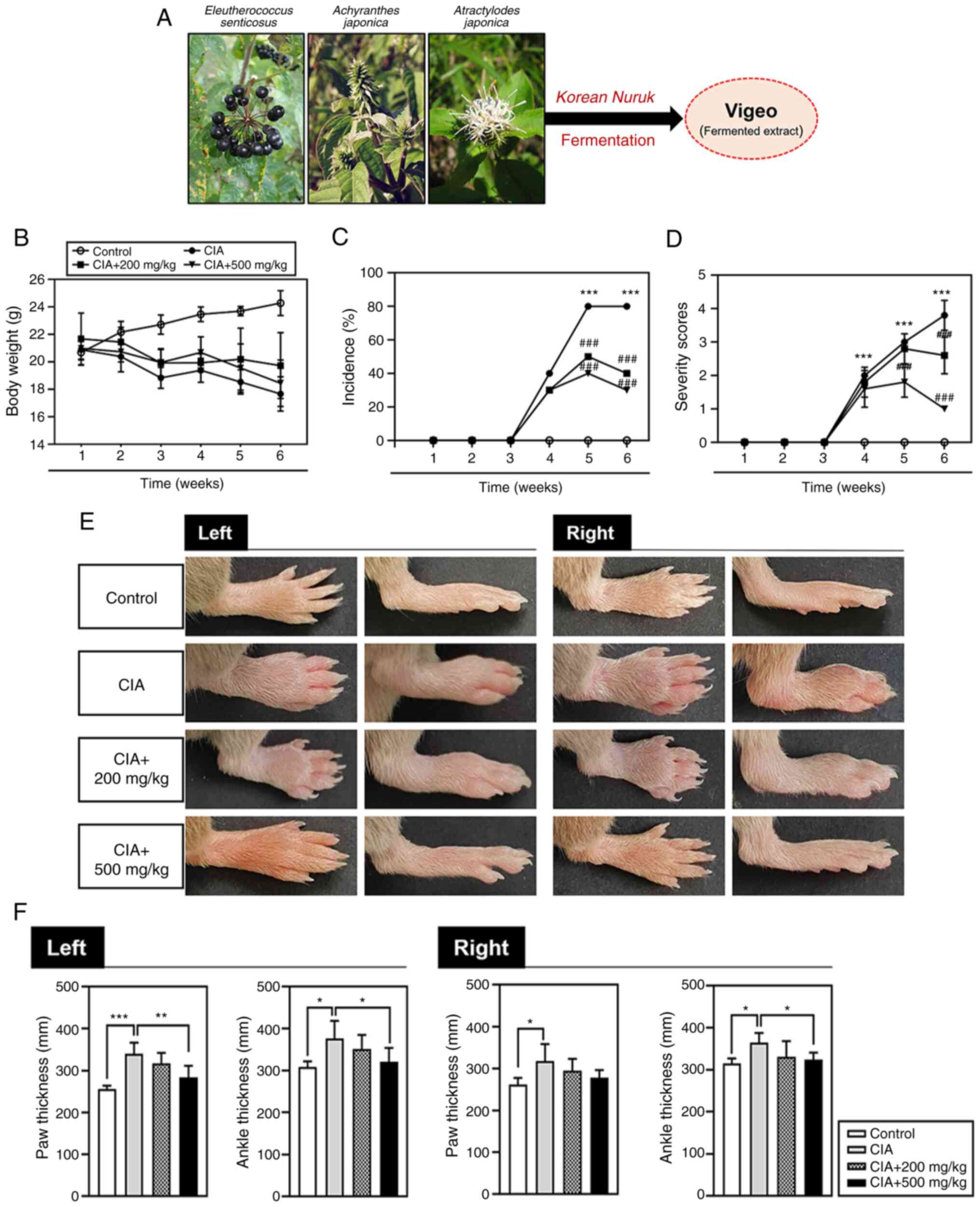
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory
disorder characterized by joint inflammation, excessive
proliferation of synovial tissue and destruction of bone and
cartilage, resulting in pain and loss of function (1). Under normal physiological conditions,
bone remodeling by osteoblasts and osteoclasts occurs continuously,
maintaining a balance between bone synthesis and degradation.
However, in pathological conditions such as RA, this homeostasis is
disrupted, resulting in uncontrolled formation of osteoclasts,
which are highly specialized cells crucial for bone resorption
(2).
Over the last 20 years, significant progress has
been made in understanding the pathogenesis of RA. Autologous IgG
antibodies, diagnostic markers of RA and anti-cyclic citrullinated
peptide antibodies lead to hypertrophy and proliferation of the
synovial membrane (3).
Additionally, the involvement of fibroblasts, T cells, macrophages,
mast cells, dendritic cells and B cells has been demonstrated
(4). Macrophages activated by T
cells secrete pro-inflammatory cytokines, including TNF-α, IL-6 and
IL-1β. These cytokines promote tissue destruction through the
activation of synovial fibroblasts and chondrocytes in the joint
(5) and by inducing osteoclast
differentiation. TNF-α is additionally produced by activated T
cells and is involved in inflammation-induced bone resorption,
which aids bone destruction by upregulating the expression of
receptor activator of the nuclear factor κB (RANK) and its ligand
(RANKL) and the secretion of macrophage colony-stimulating factor
in osteoblasts and stromal cells (6). IL-6 production is frequently
dysregulated in RA patients and is essential for synovial
inflammation. A novel immunotherapeutic agent that is an IL-6
receptor antagonist reduces bone remodeling and supports bone
maintenance in patients with RA (7). IL-6 is also involved in other
diseases associated with accelerated bone turnover, such as Paget's
disease and multiple myeloma (8).
IL-1β is a key pro-inflammatory cytokine that is highly expressed
in patients with RA. IL-1 induces RANKL expression, which promotes
osteoclast differentiation through the production of prostaglandin
E in the bone tissue. It also affects bone resorption via an
alternative pathway (9-11).
Therefore, natural drugs that downregulate the production of
inflammatory cytokines may be useful for the treatment of RA.
Vigeo is a natural product with anti-osteoporotic
effects that contains a mixture of three substances fermented using
Korean nuruk: Eleutherococcus senticosus,
Achyranthes japonica and Atractylodes japonica. Vigeo has
previously been shown to effectively inhibit RANKL-induced
osteoclast differentiation by downregulating the phosphorylation
activities of the Akt, IκB and MAPK signaling pathways. It also
significantly suppresses bone loss in a lipopolysaccharide
(LPS)-induced mouse model of osteoporosis. Furthermore, Vigeo
suppresses the levels of the bone resorption marker C-terminal
telopeptide-1 in the serum of these mice (12).
The present study aimed to determine the effect of
Vigeo administration on bone resorption and the immune response in
a mouse model of RA. It confirmed the anti-inflammatory effects of
Vigeo in RA by evaluating bone loss in affected joints.
Materials and methods
Animals
A total of 20 healthy 8-week-old male DBA/1J mice
weighing 19-23 g were purchased from Central Lab Animal Inc. The
mice were randomly divided into four groups, each consisting of
five mice: i) Control, ii) collagen-induced arthritis (CIA) group,
iii) CIA + 200 mg/kg Vigeo (low dose) and iv) CIA + 500 mg/kg Vigeo
(high dose). All animals were housed at a constant temperature
(22±2˚C) and 50-55% humidity with a 12 h light/dark cycle. Animal
experimental protocols were approved (approval no. WKU21-67) by the
Institutional Animal Care and Use Committee (IACUC) of Wonkwang
University (Iksan, Republic of Korea) and all experiments were
conducted in accordance with the guidelines of the IACUC of
Wonkwang University.
Reagents
Vigeo was purchased from Panax Bio Co. Ltd. and
prepared and fermented as previously described (12) (Fig.
1A). Briefly, dried Eleutherococcus senticosus (135 g),
Achyranthes japonica (78 g) and Atractylodes japonica
(78 g) were boiled in hot water for 3 h in an extractor
(Cosmos-660; Kyungseo E&P Co., Ltd.). Korean traditional
fermentation starter, nuruk (1 kg), and fresh yeast (4 g)
were mixed and fermented in 1.5 l distilled water (DW) for 96 h.
The hot water extract was mixed with popped rice (3 kg) and
fermented at 26˚C for 15 days. The resulting mixture of
nuruk-fermented extract (Vigeo) was carefully freeze-dried
and diluted using ultrapure water.
CIA-RA in vivo mouse model and
Immunization of DBA/1J mice with type II collagen (CII)
A solution to induce a primary immune response in
DBA/1J mice was prepared by dissolving CII lyophilized powder (cat.
no. 20022; lot no. 200297; Chondrex, Inc.) in acetic acid to a
concentration of 2 mg/ml. Next, the collagen mixture was mixed with
complete Freund's adjuvant (CFA; cat. no. 7001; lot. no. 200200;
Chondrex, Inc.) in a 1:1 ratio and the final mixture was emulsified
on ice using a homogenizer. The working solution (100 µl) was
gently and slowly injected into the tail veins of the mice on day
0. The second booster injection consisted of CII mixed with an
incomplete Freund's adjuvant (IFA; cat. no. 7002; lot no. 200341;
Chondrex, Inc.) was injected as described above on day 21. Mice
received either 200 or 500 mg/kg Vigeo or DW orally daily from days
22-41. All the mice were sacrificed on day 42.
Micro computed tomography (micro-CT)
based quantification of paw-joint architecture
Micro-CT 3D images were obtained using a SkyScan
1173 high-energy micro-CT scanner (Bruker Corporation) on both the
left and right hind paws. The scans were performed using source
currents and voltages of 60 mA and 130 µV, respectively and the
data acquired from the scanned paws were analyzed with Bruker CTAn
software version 1.6 (build 22) by OBEN. Measured bone parameters
included total volume (TV; mm3), bone volume (BV;
mm3), bone volume/total volume (BV/TV; %) and total
porosity (%). Parameter quantifications and assessments were
performed as described previously by Perilli et al (13). Diameters of the volumes of interest
(VOIs) were determined in three segments of the paw region: Region
A, VOIs in the total paw volume (PV phalange-carpal joint); Region
B, VOIs in the radiocarpal (RC) to metacarpal (MCP) joint (RC-MCP
radiocarpal-metacarpal); and Region C, VOIs in the radiocarpal (RC)
joint.
Histological analysis
Joint tissues were fixed in 10% neutral-buffered
formalin for 24 h, washed in PBS, decalcified for 21 days in a 12%
EDTA solution to remove the moisture remaining in the tissue, the
alcohol concentration was adjusted step by step and the dehydration
process was performed at 24-26˚C for 12 h each [70, 80, 90 and 100%
EtOH (I), 100% EtOH (II) and 100% EtOH (III)]. Dehydrated tissues
were immersed in xylene/EtOH (1:1) solution, followed by 100%
xylene I, II, III solution for 3 h each. Subsequent to this
clearing, the ankle tissue was infiltrated with 55˚C paraffin wax
solution three times, each lasting 3 h, to facilitate paraffin
permeation. To detect the cartilage in the paraffin-embedded tissue
sections, hematoxylin (cat. no. 3540MIRA01; lot. no. 90104; BBC
Biochemical) and eosin (cat. no. MA0101015MIRA01; lot. no. 89546;
BBC Biochemical) (H&E), safranin O (cat. no. S8884; lot. no.
MKBT3301V; MilliporeSigma), fast green (cat. no. F7258; lot. no.
MKBR3297V; MilliporeSigma) and toluidine blue (cat. no. T3260; lot.
no. MKBQ1663V; MilliporeSigma) staining methods were used. The
sections were incubated with different staining reagents for
varying durations at 24-26˚C. The reaction times for each staining
reagent are as follows: Hematoxylin-2 min; eosin-1 min; safranin
O-5 min; fast green-5 min; toluidine blue-3 min.
Cytokine analysis
Blood samples were collected from mice by drawing
300-500 µl of blood from the orbital sinus after inhalation
anesthesia with isoflurane and centrifuged at 1,000 x g for 15 min
at 4˚C. The inhalation of 4% isoflurane for 2 min in the anesthesia
box induced sufficient anesthesia and was then maintained at
1.0-1.5% isoflurane, from which mice quickly recovered within
~50-80 sec after their removal from the anesthesia box under
control conditions. Enzyme-linked immunosorbent assay (ELISA) kits
(R&D Systems, Inc.) were used to measure the serum levels of
TNF-α (cat. no. MTA00B; lot. no. P362560), IL-6 (cat. no. M6000B;
lot. no. P364710) and IL-1β (cat. no. MLB00C; lot. no.
P361801).
Statistical analysis
Each experiment was performed at least three times
and data were analyzed and expressed as the mean ± standard
deviation. Statistical significance was determined using a one-way
analysis of variance followed by Tukey's multiple-comparisons test
using SPSS 14.0 (SPSS, Inc.). P<0.05 was considered to indicate
a statistically significant difference.
Results
Vigeo attenuates arthritic severity in
CIA-DBA/1J mice
To determine the therapeutic effect of Vigeo
treatment in mice with arthritis a CIA mouse model was selected, an
in vivo experimental model of RA that is commonly used. CIA
was induced in DBA/1J mice by primary immunization with
CFA-emulsified CII (day 0) and secondary immunization with
IFA-emulsified CII (day 21). The therapeutic effect of Vigeo in CIA
mice was confirmed by oral administration of DW, or 200 or 500
mg/kg Vigeo daily, beginning on the day after the second
immunization (day 22). The total duration of the experiment was 42
days, after which the mice were sacrificed. Visible symptoms of
arthritis in the CIA mice gradually appeared after the second
intravenous injection. Loss of appetite and body weight was
observed on day 14. However, weight loss in the mice receiving
Vigeo tended to be reduced when compared with that in the CIA group
(Fig. 1B). In the CIA group,
common arthritic symptoms such as joint edema began to appear 5
days after the second immunization on day 26 and tended to worsen
from days 32 to 42. By contrast, the paw and ankle edema was
significantly improved in the Vigeo oral administration group when
compared with that in the CIA group (Fig. 1C and D). Moreover, the increase in paw and
ankle thickness also improved with Vigeo treatment (Fig. 1E and F).
Vigeo alleviates bone and joint
destruction in CIA-DBA/1J mice
The most severe effect of RA is bone erosion in the
joints. Articular destruction and bone damage were confirmed in
hind paws using micro computed tomography (micro-CT; Fig. 2A). Micro-CT images enabled
quantification of bone changes in CIA mice (Fig. 2B and C). Furthermore, the analysis showed that
due to edema and increased total porosity resulting from bone
erosion, the TV was increased in CIA mice when compared with that
in the control group. By contrast, the CIA group BV and BV/TV
parameters were significantly lower than those in the control
group. Following Vigeo treatment, the 200 and 500 mg/kg Vigeo group
Larsen scores showed diminished TV and total porosity when compared
with those in the CIA group. The high-dose Vigeo-treatment group
also showed noticeably reduced TV and total porosity in the
radiocarpal-metacarpophalangeal joint region (Fig. 2B and C).
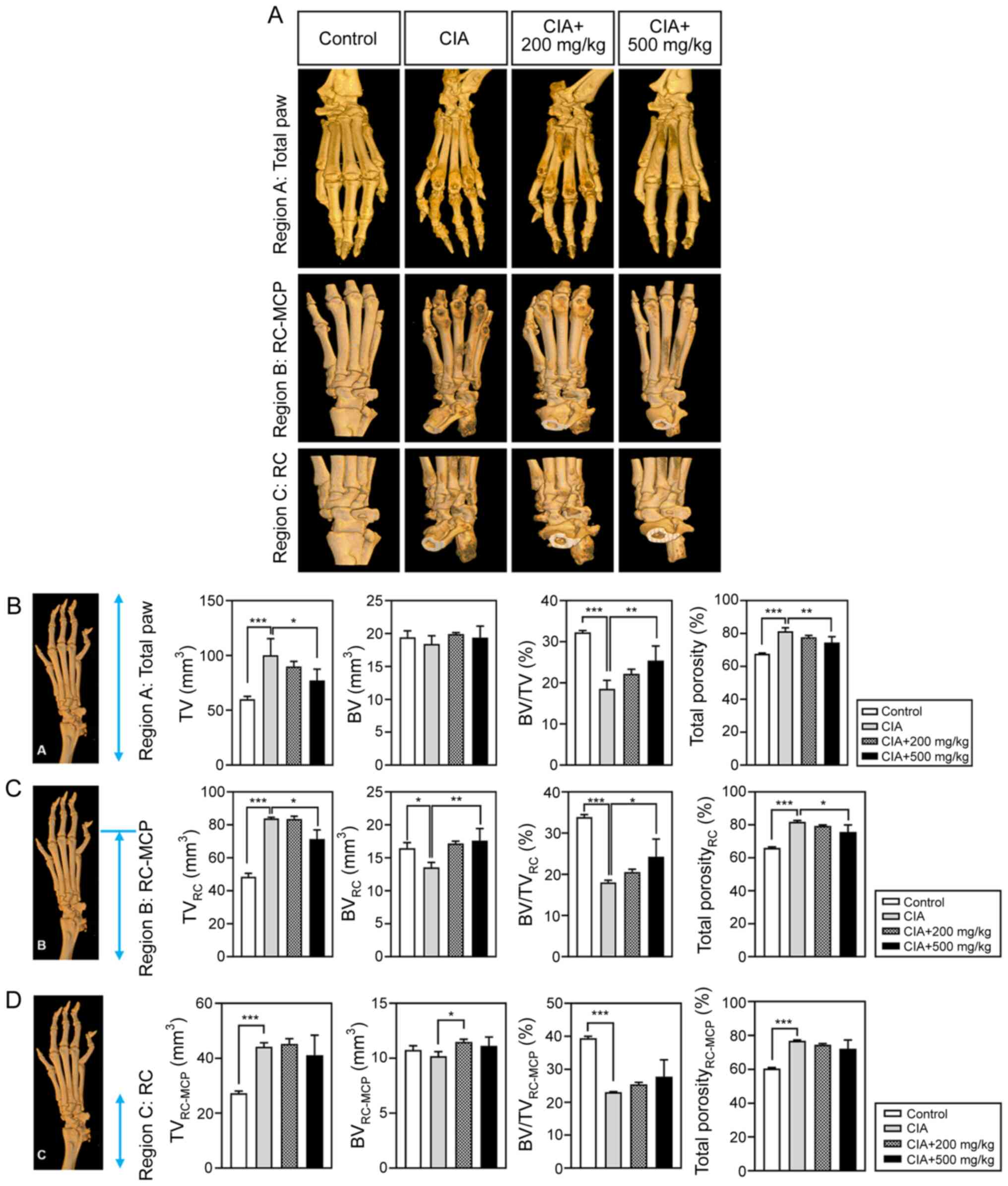 | Figure 2Evaluation of the anti-inflammatory
effects of Vigeo in CIA-RA mice. Arthritis-induced joints were
evaluated according to specific regions. The 3D images of each paw
were captured and the paw VOIs were analyzed with a micro-CT
analyzer in Regions (A-C). The images shown in B, C and D are
presented to show the analysis regions in the same image. TV
(mm3), BV (mm3), BV/TV (%) and total porosity
(%) were determined in Region A from the phalanges-carpal to the RC
joint, Region B from RC-MCP joint and Region C in the RC joint. (D)
Data are represented as the mean ± standard deviation (n=5).
*P<0.05, **P<0.01,
***P<0.001 vs. the control group or the CIA control
group. CIA, collagen-induced arthritis; RA, rheumatoid arthritis;
VOI, volume of interest; CT, computed tomography; TV, tissue
volume; BV, bone volume; RC, radiocarpal; MCP, metacarpal. |
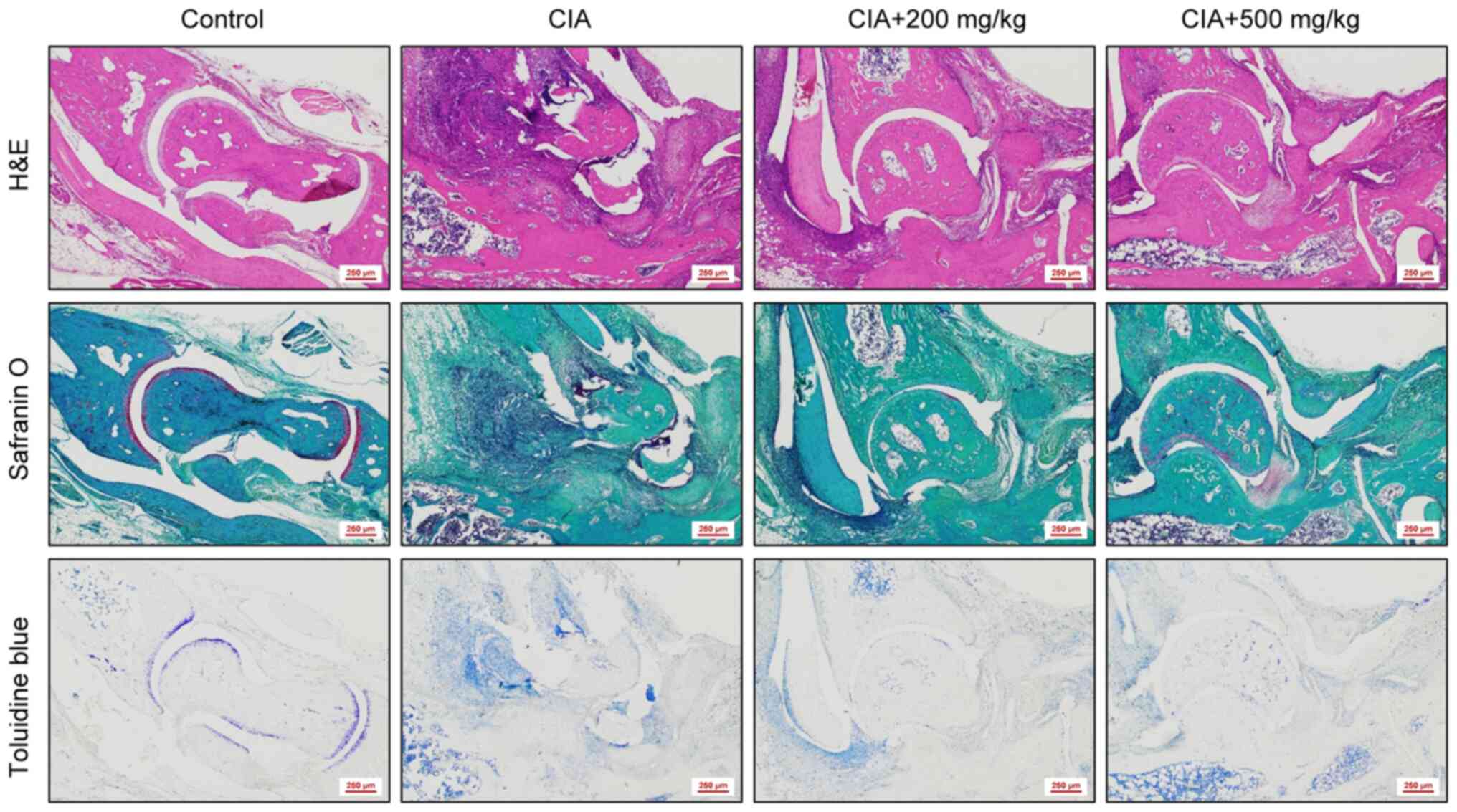
Vigeo suppresses inflammation and
joint destruction in CIA-DBA/1J mice
The present study conducted histological analyses of
the mouse ankle joints to determine the degree of joint destruction
and inflammation. H&E staining confirmed that the ankle joint
region containing cartilage and bone was preserved in mice from the
control group compared with that in the CIA group, which showed
severe cartilage erosion. Joint destruction in mice from both the
low- and high-dose Vigeo treatment groups was improved when
compared with the CIA group. Safranin O/fast green staining was
used as a visual indicator of the extent of cartilage damage.
Joints from control group mice showed well-maintained red cartilage
tissue by safranin O staining, but joints from CIA mice had
atypical red cartilage tissue in a disorganized shape due to
significant cartilage destruction. By contrast, the preservation of
joint cartilage in Vigeo-treated mice was superior compared with
that in mice from the CIA group. Finally, toluidine blue staining
was performed to evaluate the cartilage matrix. This test revealed
that Vigeo treatment somewhat improved violet-stained cartilage
regions by inhibiting the cartilage matrix destruction observed in
the joints of CIA mice (Fig.
3).
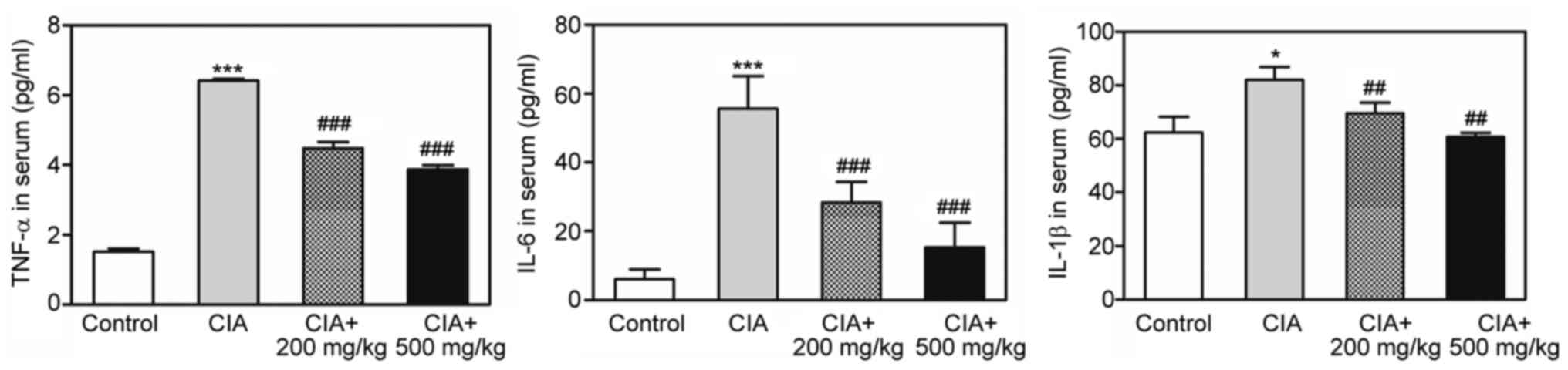
Vigeo inhibits the production of
pro-inflammatory cytokines in CIA-DBA/1J mice
RA is mainly driven by continuously high levels of
pro-inflammatory factors, including TNF-α, IL-6 and IL-1β (5). To test whether Vigeo treatment
affected the production of proinflammatory cytokines in CIA mice,
the present study determined the serum levels of these cytokines
using ELISA. Serum levels of TNF-α, IL-6 and IL-1β levels were all
increased in mice from the CIA group when compared with the control
group. However, the increase of cytokine secretion in CIA mice was
significantly inhibited by both low and high concentrations of
Vigeo when compared with the untreated CIA group (Fig. 4).
Discussion
For thousands of years, natural products have been
regarded as therapeutic tools worldwide and have been developed
alongside human culture. The production of substances with
beneficial bioactivity has mainly been achieved through various
traditional herbal separation and purification methods that were
developed based on environmental, climatic and geographical factors
(14,15). Fermentation is one of the main
methods used by the food industry to manipulate and grow various
types of bacteria, yeasts and fungi (16). However, in recent years,
fermentation has been applied in various fields such as the mass
production of highly complex substances including insulin,
alternative proteins and antibiotics (17). In addition, the chemical and
biological diversity of the natural products produced by microbial
fermentation can improve quality and promote the absorption rate of
active substances in the body. In particular, screening suitable
disease targets by utilizing the secondary metabolites of
microorganisms as new drugs has increasingly been attempted
(18).
RA is a severe systemic and chronic autoimmune
disease that is typically accompanied by the deformation and
destruction of joint tissue and bone, with symptoms such as pain,
stiffness and inflammation gradually appearing over several weeks
(19,20). Despite successes in RA treatment
with currently available therapeutics, these drugs are not always
effective and concerns regarding adverse effects, including
hepatotoxicity, primary gastrointestinal symptoms and respiratory
symptoms, persist (21).
Therefore, the present study chose a different approach through the
application of safe and natural therapeutic agents (22). Previously, we reported a novel
effect of Vigeo, a fermented extract of Eleutherococcus
senticosus, Achyranthes japonica and Atractylodes
japonica based on Korean nuruk, on osteoclast
differentiation and function in LPS-induced inflammatory bone
diseases, including osteoporosis. Vigeo strongly inhibited
RANKL-induced Akt, IκB and MAPK signaling pathways and
significantly suppressed mRNA and protein expression of c-Fos and
nuclear factor of activated T cells 1 (downstream transcription
factors in osteoclasts) and the expression of osteoclast
differentiation marker genes (12). Previous studies (23-25)
have demonstrated the efficacy of the three components of Vigeo in
bone diseases, such as osteoporosis and RA. Eleutherococcus
senticosus extracts have been shown to prevent ovariectomy
(OVX)-induced osteoporosis in a rat model and to decrease the serum
levels of factors that indicate high bone turnover, such as
alkaline phosphatase, type I collagen and osteocalcin (23). Achyranthes japonica improves
the serum levels of insulin-like growth factor (IGF) and
IGF-binding protein 3, which are essential stimulators of bone
formation in response to osteoporosis induced by OVX in rats
(24). Extracts of Atractylodes
japonica in 70% ethanol reduced osteoclast differentiation by
decreasing NF-κB activation via RANKL in bone marrow-derived
macrophages in vitro and protected against osteoporotic
symptoms in a RANKL-injection mouse model of bone loss in
vivo (25). The ability of
each of the three plant extracts to inhibit osteoporosis in animal
models suggests the possibility of preventing or treating
osteoporosis using the newly produced Vigeo fermented with Korean
nuruk.
Based on the anti-osteoporotic effects of the three
plant extracts, the present study was designed to determine the
synergistic effects of these different components in a CIA mouse
model in vivo. It was hypothesized that Vigeo would have
excellent therapeutic effects in reducing bone loss in a CIA mouse
model of RA. RA is a systemic inflammatory disease mainly
characterized by chronic inflammation in the joint synovium and
infiltration of inflammatory factors that causes joint destruction
and disability (1). The present
study found that Vigeo significantly inhibited arthritic symptoms
such as paw edema in CIA-RA mice. As shown in Fig. 2, micro-CT analysis revealed
inhibition of joint damage and Fig.
3 shows that joint synovial hyperplasia and cartilage and bone
erosion were attenuated by Vigeo administration at a dose of 500
mg/kg, as determined by histology. T cells, macrophages and
synovial cells have crucial roles in RA pathogenesis (4). Various inflammatory cytokines,
including TNF-α, IL-6 and IL-1β, are produced by these cells and
high levels of these cytokines are observed in patients with RA
(5). The regulation of cytokine
levels is a potential therapeutic strategy for RA treatment
(4,5). TNF-α is expressed primarily by
macrophages and synovial cells as well as activated T cells within
RA inflamed joints (26) and the
involvement of TNF-α in arthritic bone destruction has been
demonstrated in several studies (27-29).
hTNF-tg mice develop chronic inflammatory arthritis with
hyperplasia of the synovium, inflammatory infiltrates of the joint
space, pannus formation and cartilage and bone destruction.
Application of TNF-specific neutralizing antibodies in CIA mice
reduces disease activity and bone damage (27). IL-1 is produced by activated
macrophages and synovial fibroblasts in RA joints (30). In models of arthritis,
overexpression of IL-1α or IL-1β, or deficiency of soluble IL-1Ra
result in the development of a disease associated with bone and
cartilage destruction (31). IL-6
is produced by various cell types in the inflamed RA bone
microenvironment, including macrophages, fibroblast-like synovial
cells and chondrocytes (32).
Reports show that IL-6-deficient mice are protected from
inflammation and bone destruction in an antigen-induced arthritis
model (33-35).
The present study found that Vigeo significantly
diminished the production of pro-inflammatory cytokines in CIA-RA
mice. Therefore, the inhibitory effects of Vigeo on bone and
cartilage loss in this mouse model may be attributed to the
reduction in cytokine levels observed following treatment with
Vigeo.
However, the present study had some limitations. As
a study on signal transduction pathways related to synovial
proliferation and angiogenesis during RA onset, the absence of
experiments confirming changes in the VEGF and VEGFR/PI3K/AKT
pathways in joint tissue and the results on the regulation of
pro-inflammatory cytokines in patients remains a limitation. Also,
the key targets focusing on the metabolism and potential compounds
of Vigeo to treat RA still require further studies and verification
and a detailed study of the appropriate dosing regimen of Vigeo is
needed for clinical RA application.
In summary, the present study suggested the
potential use of Vigeo in the treatment of inflammatory arthritis
accompanied by pain, severe bone loss and joint deformity (Fig. S1). Vigeo significantly abrogated
synovial inflammation and joint, cartilage and bone loss in a
CIA-RA mouse model. The therapeutic properties of Vigeo may be
attributed to the suppression of pro-inflammatory cytokine
production. It is suggested that Vigeo may be an effective
therapeutic agent for the treatment of RA.
Supplementary Material
Schematic of the therapeutic efficacy
of Vigeo in a collagen-induced arthritis mouse model
(Eleutherococcus senticosus-https://commons.wikimedia.org/wiki/File:Eleutherococcus_senticosus_kz05.jpg,
Achyranthes japonica-https://en.wikipedia.org/wiki/Achyranthes_japonica,
Atractylodes japonica-https://www.wikidata.org/wiki/Q10893998).
*P<0.05, ***P<0.001 vs. the control
group and ##P<0.01, ###P<0.001 vs. the
CIA control group. CIA, collagen-induced arthritis; RA, rheumatoid
arthritis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a grant from the
Basic Science Research Program through the National Research
Foundation of Korea (NRF), funded by the Ministry of Education
(grant no. NRF-2022R1C1C2010740).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JYK and MSL designed the study and revised the
manuscript. YHC, CHL, SYE and GDP performed the experiments. YHC,
CHL and CHC analyzed the data. YHC and CHL drafted the manuscript.
JYK and MSL confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
WKU21-67) and followed the guidelines of the Ethics Committee for
Experimental Animals of Wonkwang University (Iksan, Republic of
Korea). Among the medicinal ingredients of Vigeo used in the
experimental study, Eleutherococcus senticosus belongs to
Endangered wildlife level Ⅱ and was not collected from the wild but
purchased from Hwacheon Gasiogalpi Farm in Gandong-myeon, Hwacheon,
Gangwon-do (Gangwon, Republic of Korea). It does not collect or
damage internationally endangered species designated and announced
by the Minister of Environment in accordance with the Convention on
International Trade in Endangered Species of Wild Plants (CITES)
(http://www.cites.org) and the United Nations. It
complies with International Union for Conservation of Nature (IUCN)
standards for natural conservation (https://s3.amazonaws.com/iucnredlist-newcms/staging/public/attachments/3154/reg_guidelines_en.pdf)
and adheres to permitting procedures for protected species.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aletaha D and Smolen JS: Diagnosis and
management of rheumatoid arthritis: A review. JAMA. 320:1360–1372.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Firestein GS: Evolving concepts of
rheumatoid arthritis. Nature. 423:356–361. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wu CY, Yang HY and Lai JH:
Anti-citrullinated protein antibodies in patients with rheumatoid
arthritis: Biological effects and mechanisms of immunopathogenesis.
Int J Mol Sci. 21(4015)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Petrelli F, Mariani FM, Alunno A and
Pexeddu I: Pathogenesis of rheumatoid arthritis: One year in review
2022. Clin Exp Rheumatol. 40:475–482. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kondo N, Kuroda T and Kobayashi D:
Cytokine networks in the pathofenesis of rheumatoid arthritis. Int
J Mol Sci. 22(10922)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Romas E, Gillespie MT and Martin TJ:
Involvement of receptor activator of NFkappaB ligand and tumor
necrosis factor-alpha in bone destruction in rheumatoid arthritis.
Bone. 30:340–346. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rossi JF, Lu ZY, Jourdan M and Klein B:
Interleukin-6 as a therapeutic target. Clin Cancer Res.
21:1248–1257. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pandolfi F, Franza L, Carsi V, Altamura S,
Andriollo G and Nucera E: Interleukin-6 in rheumatoid arthritis.
Int J Mol Sci. 21(5238)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li B, Wang P, Jiao J, Wei H, Xu W and Zhou
P: Roles of the RANKL-RANK axis in immunity-implications for
pathogenesis and treatment of bone metastasis. Front Immunol.
13(824117)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Levescot A, Chang MH, Schnell J,
Nelson-Maney N, Yan J, Martínez-Bonet M, Grieshaber-Bouyer R, Lee
PY, Wei K, Blaustein RB, et al: IL-1β-driven osteoclastogenic Tregs
accelerate bone erosion in arthritis. J Clin Invest.
131(e141008)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Allard-Chamard H, Carrier N, Dufort P,
Durand M, de Brum-Fernandes AJ, Boire G, Komarova SV, Dixon SJ,
Harrison RE, Manolson MF and Roux S: Osteoclasts and their
circulating precursors in rheumatoid arthritis: Relationships with
disease activity and bone erosions. Bone Rep.
12(100282)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Eun SY, Cheon YH, Park GD, Chung CH, Lee
CH, Kim JY and Lee MS: Anti-osteoporosis effects of the
Eleutherococcus senticosus, Achyranthes japonica, and
Atractylodes japonica mixed extract fermented with
nuruk. Nutrients. 13(3904)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Perilli E, Cantley M, Marino V, Crotti TN,
Smith MD, Haynes DR and Dharmapatni AASSK: Quantifying not only
bone loss, but also soft tissue swelling, in a murine inflammatory
arthritis model using micro-computed tomography. Scand J Immunol.
81:142–150. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Salminen S, Collado MC, Endo A, Hill C,
Lebeer S, Quigley EMM, Sanders ME, Shamir R, Swann JR, Szajewska H
and Vinderola G: The international scientific association of
probiotics and prebiotics (ISAPP) consensus statement on the
definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol.
18:649–667. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shen B: A new golden age of natural
products drug discovery. Cell. 163:1297–1300. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen GQ and Liu X: On the future
fermentation. Microb Biotechnol. 14:18–21. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shah AA, Qian C, Liu Z, Wu J, Sultana N,
Mobashar M, Wanapat M and Zhong X: Evaluation of biological and
chemical additives on microbial community, fermentation
characteristics, aerobic stability, and in vitro gas production of
SuMu No. 2 elephant grass. J Sci Food Agric. 101:5429–5436.
2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ramírez-Rendon D, Passari AK,
Ruiz-Villafán B, Rodríquez-Sanoja R, Sánchez S and Demain AL:
Impact of novel microbial secondary metabolites on the pharma
industry. Appl Microbiol Biotechnol. 106:1855–1878. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Orange DE, Blachere NE, DiCarlo EF, Mirza
S, Pannellini T, Jiang CS, Frank MO, Parveen S, Figgie MP,
Gravallese EM, et al: Rheumatoid arthritis morning stiffness is
associated with synovial fibrin and neutrophils. Arthritis
Rheumatol. 72:557–564. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang A and Lee YC: Mechanisms for joint
pain in rheumatoid arthritis (RA): From cytokines to central
sensitization. Curr Osteoporos Rep. 16:603–610. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cai W, Gu Y, Cui H, Cao Y, Wang X, Yao Y
and Wang M: The efficacy and safety of mainstream medications for
patients with cDMARD-Naïve rheumatoid arthritis: A network
meta-analysis. Front Pharmacol. 9(183)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Simon LS and Yocum D: New and future drug
therapies for rheumatoid arthritis. Rheumatology (Oxford). 39
(Suppl 1):S36–S42. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lim DW, Kim JG, Lee YS, Cha SH and Kim YT:
Preventive effects of Eleutherococcus senticosus bark
extract in OVX-induced osteoporosis in rats. Molecules.
18:7998–8008. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kim JS, Lee SW, Kim SK, Na SW and Kim YO:
Osteoprotective effect of extract from Achyranthes japonica
in ovariectomized rats. J Exerc Rehabil. 10:372–377.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ha H, An H, Shim KS, Kim T, Hwang YH and
Ma JY: Ethanol extract of Atractylodes macrocephala protects
bone loss by inhibiting osteoclast differentiation. Molecules.
18:7376–7388. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Choy EH and Panayi GS: Cytokine pathways
and joint inflammation in rheumatoid arthritis. N Engl J Med.
344:907–916. 2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Keffer J, Probert L, Cazlaris H,
Georgopoulos S, Kaslaris E, Kioussis D and Kollias G: Transgenic
mice expressing human tumour necrosis factor: A predictive genetic
model of arthritis. EMBO J. 10:4025–4031. 1991.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Redlich K, Hayer S, Ricci R, David JP,
Tohidast-Akrad M, Kollias G, Steiner G, Smolen J, Wagner EF and
Schett G: Osteoclasts are essential for TNF-alpha-mediated joint
destruction. J Clin Invest. 110:1419–1427. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Shealy DJ, Wooley PH, Emmell E, Volk A,
Rosenberg A, Treacy G, Wagner CL, Mayton L, Griswold DE and Song
SYR: Anti-TNF-alpha antibody allows healing of joint damage in
polyarthritic transgenic mice. Arthritis Res. 4(R7)2002.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Niki Y, Yamada H, Seki S, Kikuchi T,
Takaishi H, Toyama Y, Fujikawa K and Tada N: Macrophage- and
neutrophil-dominant arthritis in human IL-1 alpha transgenic mice.
J Clin Invest. 107:1127–1135. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Thomson BM, Saklatvala J and Chambers TJ:
Osteoblasts mediate interleukin 1 stimulation of bone resorption by
rat osteoclasts. J Exp Med. 164:104–112. 1986.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Okamoto H, Yamamura M, Morita Y, Harada S,
Makino H and Ota Z: The synovial expression and serum levels of
interleukin-6, interleukin-11, leukemia inhibitory factor, and
oncostatin M in rheumatoid arthritis. Arthritis Rheum.
40:1096–1105. 1997.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kobayashi H, Ohshima S, Nishioka K,
Yamaguchi N, Umeshita-Sasai M, Ishii T, Mima T, Kishimoto T, Kawase
I and Saeki Y: Antigen induced arthritis (AIA) can be transferred
by bone marrow transplantation: evidence that interleukin 6 is
essential for induction of AIA. J Rheumatol. 29:1176–1182.
2002.PubMed/NCBI
|
|
34
|
Ohshima S, Saeki Y, Mima T, Sasai M,
Nishioka K, Nomura S, Kopf M, Katada Y, Tanaka T, Suemura M and
Kishimoto T: Interleukin 6 plays a key role in the development of
antigen-induced arthritis. Proc Natl Acad Sci USA. 95:8222–8226.
1998.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Boe A, Baiocchi M, Carbonatto M, Papoian R
and Serlupi-Crescenzi O: Interleukin 6 knock-out mice are resistant
to antigen-induced experimental arthritis. Cytokine. 11:1057–1064.
1999.PubMed/NCBI View Article : Google Scholar
|