Introduction
Steroid-induced osteonecrosis of the femoral head
(ONFH), a serious orthopedic disease caused by long-term or
excessive use of glucocorticoids, is a bone disorder primarily
presenting femoral head collapse, resulting in hip joint
dysfunction and eventually total hip arthroplasty (1,2). The
prevalence rate of ONFH in Japan is ~0.0182%, while in South Korea
it is ~0.0289% (3). Considering
that, there is a pressing need to understand the specific molecular
mechanism behind ONFH and ascertain potential therapeutic
biomarkers.
The tripartite motif (TRIM) family of proteins
belonging to the subfamily of E3 ubiquitin ligases has been shown
to be implicated in diversified human diseases, such as tumors,
inflammatory, infectious, neuropsychiatric disorders, chromosomal
abnormalities as well as developmental diseases (4). As a member of the TRIM family,
tripartite motif-containing protein 21 (TRIM21) can function as an
E3 ligase dependent on its RING domain (5). Initially, TRIM21 was identified to
act as a regulator of immune responses and participate in
autoimmune diseases (5-7).
A recent study revealed that TRIM21 has low expression during the
osteogenic process of mesenchymal stem cells and TRIM21 negatively
regulates the osteogenic capacity of mesenchymal stem cells both
in vitro and in vivo (8). Nevertheless, the effects of TRIM21 on
the process of ONFH remain to be elucidated.
Nuclear factor erythroid 2-related factor 2 (Nrf2)
which is modulated by Kelch-like Ech-associated protein-1 (Keap1)
is regarded as a principal component of the cellular defense system
responding to various types of endogenous and exogenous insults
(9). Numerous studies showed that
the Keap1/Nrf2 pathway participates in a multitude of biological
events including metabolism, cell proliferation and cell death
(10,11). Notably, dysregulation of the
Nrf2/Keap1 pathway has been shown to be involved in the process of
ONFH (12). Concurrently, TRIM21
was reported to block the Keap1/Nrf2 pathway in
hepatocarcinogenesis (13).
The present study sought to unravel the regulatory
role of TRIM21 in the development of steroid-induced ONFH and to
identify whether its action mechanism was associated with the
Keap1/Nrf2 pathway.
Materials and methods
Cell culture and treatment
Minimal essential medium α (MEMα; Corning, Inc.)
supplemented with 10% fetal bovine serum (FBS; Wuhan Saios
Biotechnology Co., Ltd.) was adopted for the incubation of murine
preosteoblast MC3T3-E1 (Wuhan Saios Biotechnology Co., Ltd.) cells
at 37˚C with 5% CO2. Dexamethasone (Dex; Shanghai
Macklin Biochemical Co., Ltd.) at the concentration of 1 µM was
used to treat MC3T3-E1 cells for 24 h at 37˚C (14). Additionally, cells were treated
with 10 µM Nrf2 inhibitor ML385 (Shanghai Macklin Biochemical Co.,
Ltd.) for 24 h at 37˚C (15)
before treatment with Dex. To stimulate osteogenic differentiation,
the osteogenesis-inducing medium containing 10% FBS, 5 mM
L-glycerophosphate, 100 nM Dex and 50 mg/ml ascorbic acid was
applied to the culture of MC3T3-E1 cells at 80% confluence and
incubated for 7 days at 37˚C.
Transfection protocol
MC3T3-E1 cells at the logarithmic phase were seeded
in a 6-well plate (1x105 cells/well) and were incubated
at 37˚C until they reached 80% confluence. Using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.), MC3T3-E1 cells were established with a stable
knockdown of TRIM21 using 50 nmol/l short hairpin RNAs (shRNAs) for
TRIM21 (sh-TRIM21#1, sense 5'-GAACCTGGACACGTTAGATAT-3', antisense
5'-ATATCTAACGTGTCCAGGTTC-3'; sh-TRIM21#2, sense
5'-TTGTCTCCTTCTACAACATAA-3', antisense 5'-TTATGTTGTAGAAGGAGACAA-3')
or 50 nmol/l control shRNA (sh-NC, sense 5'-TACGGAGGACTCGATCTAG-3',
antisense 5'-CTAGATCGAGTCCTCCGTA-3') ordered from Shanghai
GenePharma Co., Ltd., according to the manufacturer's protocol at
37˚C for 48 h. Following 48 h of culture at 37˚C, cells were
harvested for the subsequent experiments.
Cell Counting Kit-8 (CCK-8) assay
MC3T3-E1 cells subjected to transfection and
indicated treatment were plated into 96-well plates (3,000
cells/well). After being incubated for 2 h with 10 µl CCK-8
solution (Beyotime Institute of Technology), the absorbance was
recorded at 450 nm by using a microplate reader (BMG-Labtech,
Ltd.).
Lactate dehydrogenase (LDH) release
assay
The cytotoxicity in the supernatants of MC3T3-E1
cells subjected to centrifugation at 8,000 x g for 10 min at 4˚C
was examined with an LDH assay kit (Nanjing Jiancheng
Bioengineering Institute). Absorbance was reco.
Evaluation of reactive oxygen species
(ROS) production
A 2 µM 2,7-dichloro-dihydrofluorescein diacetate
(DCFH-DA; Shanghai Aladdin Biochemical Technology Co., Ltd.)
solution was added to the MC3T3-E1 cells, previously subject to
transfection and indicated treatment, for 30 min of incubation at
37˚C in the dark. Following washing with PBS, the fluorescence
intensity was observed under a fluorescence microscope (Zeiss
GmbH).
Detection of oxidative stress
indexes
Following centrifugation at 8,000 x g for 10 min at
4˚C, superoxide dismutase (SOD; cat. no. S930985), glutathione
peroxidase (GSH-Px; cat. no. G930918) and malonaldehyde (MDA; cat.
no. M930417) contents were evaluated using specific assay kits from
Shanghai Macklin Biochemical Co., Ltd. The absorbance values were
recorded using a microplate reader.
Flow cytometry analysis
An Annexin V-fluorescein isothiocyanate (FITC)
apoptosis detection kit (Nanjing KeyGen Biotech Co., Ltd.) was used
to assess the apoptosis of MC3T3-E1 cells subjected to transfection
and indicated treatment. Cells were plated into 6-well plates and
incubated at 37˚C. MC3T3-E1 cells were suspended in the binding
buffer containing Annexin-V-FITC and PI. The apoptosis rate was
detected using flow cytometry (BD FACSCalibur; BD Biosciences).
Data were analyzed using FlowJo software v7.6.1 (Tree Star, Inc.).
The apoptotic rate was calculated as the percentage of early + late
apoptotic cells.
Measurement of caspase 3 activity
Caspase 3 activity was examined with a caspase-3
activity assay kit (cat. no. ab252897; Abcam) according to the
manufacturer's instructions. Cell lysates were incubated with
caspase-3 substrate DEVD-AFC for 2 h at 37˚C in the dark. The
fluorescence was observed using a fluorescence plate reader
(excitation at 400 nm and emission at 505 nm).
Alkaline phosphatase (ALP) staining
and Alizarin red S (ARS) staining
Following osteogenic differentiation, MC3T3-E1 cells
previously subjected to transfection and indicated treatment were
fixed in 4% paraformaldehyde for 15 min at 37˚C, before being
incubated with ALP staining solution (MK BioScience Co., Inc.) for
4 h or ARS solution (Shanghai Macklin Biochemical Co., Ltd.) for 30
min at 37˚C. The images were captured under an inverted light
microscope (Zeiss GmbH).
Reverse transcription-quantitative
(RT-q) PCR
Extraction of total RNA from 1x104
MC3T3-E1 cells was conducted using TRIzol® reagent
(Thermo Fisher Scientific, Inc.). The cDNA was synthesized using
the PrimeScript RT reagent kit (Takara Bio, Inc.) according to the
instructions provided by the manufacturer. qPCR was performed on
the ABI 7500 Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with cDNA as templates using SYBR Green
PCR Master Mix Reagents (Takara Bio, Inc.) according to the
manufacturer's instructions. The following thermocycling conditions
were used for qPCR: Initial denaturation for 2 min at 94˚C;
followed by 35 cycles for 30 sec at 94˚C and 45 sec at 55˚C. The
calculation of relative mRNA levels was performed based on the
2-∆∆Cq method (16).
GAPDH was employed as the internal reference. Specific primer
sequences were: TRIM21 forward, 5'-CCTGGTTAGATTCCACGGCA-3' and
reverse, 5'-TGAACTGCCCCCATTCTTCC-3'; and GAPDH forward,
5'-GCCTCCTCCAATTCAACCCT-3' and reverse, 5'-CTCGTGGTTCACACCCATCA-3'.
All experiments were replicated three times.
Western blotting
After the preparation of cellular lysates using RIPA
buffer (Beyotime Institute of Biotechnology), the quantification of
protein concentration was performed using the BCA method (Beyotime
Institute of Biotechnology). Total protein (40 µg protein per lane)
was separated on 10% gels using SDS-PAGE and then transferred onto
the polyvinylidene difluoride membranes. After blocking with 5% BSA
(Beyotime Institute of Biotechnology) for 1.5 h at room
temperature, the membranes were then immunoblotted with primary
antibodies including TRIM21 (cat. no. ab207728; 1:1,000; Abcam),
B-cell lymphoma 2 (Bcl-2; cat. no. ab196495; 1:2,000; Abcam), Bcl-2
associated X (Bax; cat. no. ab3191; 1:1,000; Abcam), osterix (Osx;
cat. no. ab209484; 1:1,000; Abcam), runt-related transcription
factor 2 (RUNX2; cat. no. ab236639; 1:1,000; Abcam), bone
morphogenetic protein 2 (BMP2; cat. no. ab284387; 1:1,000; Abcam),
Nrf2 (cat. no. 12721; 1:1,000; Cell Signaling Technology, Inc.),
Keap1 (cat. no. ab119403; 1:1,000; Abcam), heme oxygenase-1 (HO-1;
cat. no. ab189491; 1:2,000; Abcam), NAD(P)H:quinone oxidoreductase
1 (NQO1; cat. no. ab80588; 1:10,000; Abcam) and GAPDH (cat. no.
ab181603; 1:10,000; Abcam) at 4˚C overnight, before being probed
with HRP-conjugated secondary antibody (cat. no. ab6721; 1:2,000;
Abcam) for 1 h at room temperature. Protein signals were visualized
using chemiluminescence reagents (MilliporeSigma). The western
blotting images of proteins were processed using ImageJ software
(version 1.8.0; National Institutes of Health) with GAPDH as the
loading control.
Statistical analysis
Data are presented as mean ± standard deviation of
three independent experiments and analyzed using GraphPad Prism 8
software (GraphPad Software, Inc.; Dotmatics). Statistical
comparison between two groups was made using an unpaired student's
t-test. One-way analysis of variance followed by Tukey's post hoc
test was used to compare the effects of more than two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
TRIM21 knockdown increases viability
and reduces cytotoxicity in Dex-treated MC3T3-E1 cells
To investigate the role of TRIM21 in ONFH, Dex was
initially used for the establishment of a cell model of ONFH in
MC3T3-E1 cells. Subsequently, TRIM21 mRNA levels and protein
expression were evaluated using RT-qPCR and western blotting,
respectively, and TRIM21 expression was noticeably increased in
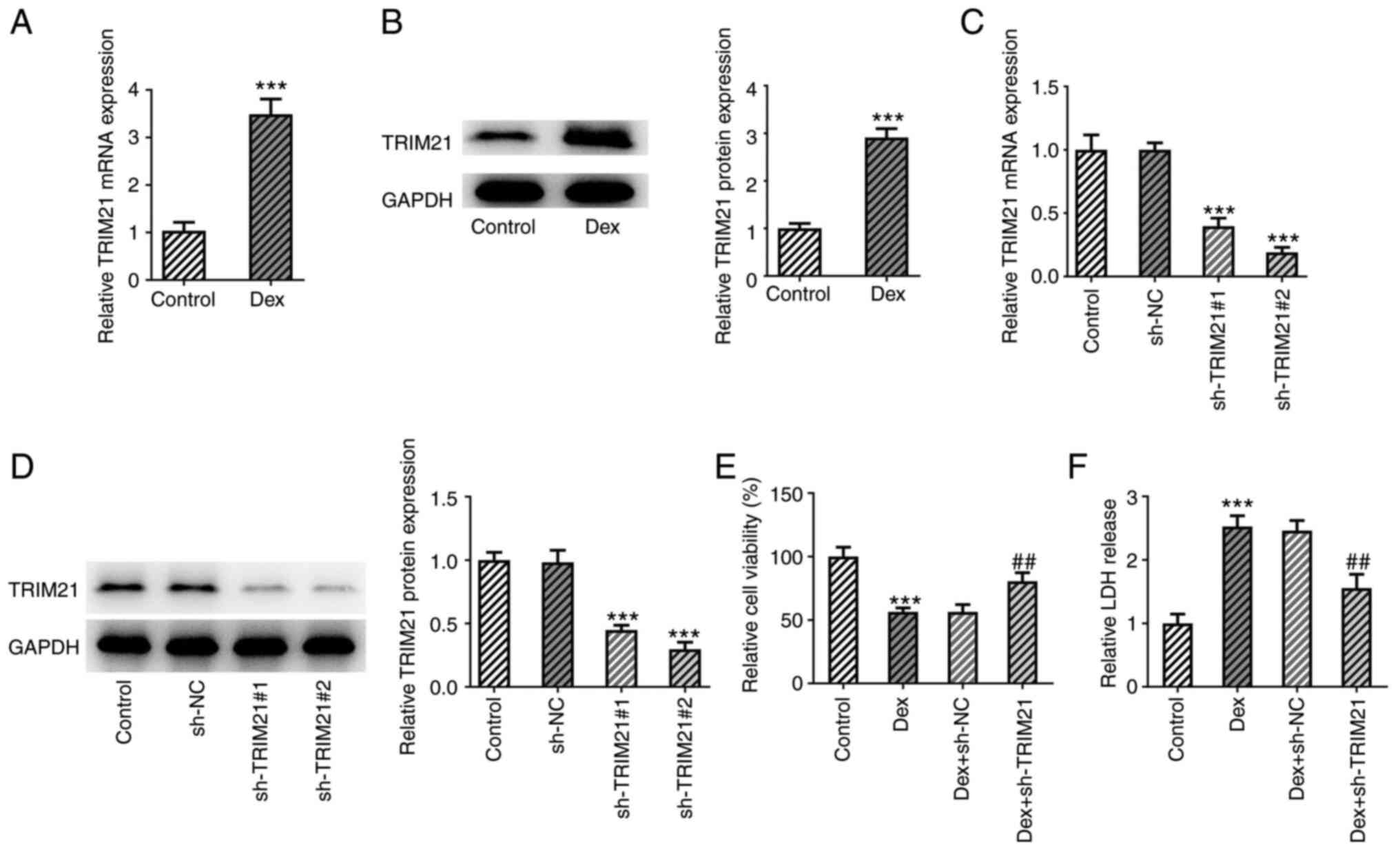
MC3T3-E1 cells administered with Dex (Fig. 1A and B). Moreover, TRIM21 expression was
distinctly decreased after transfection of sh-TRIM21#1/2 (Fig. 1C and D). Therefore, sh-TRIM21#2 was chosen for
the follow-up assays as TRIM21 exhibited lower expression in the
sh-TRIM21#2 group. Based on the data from the CCK-8 assay, the
viability was diminished in MC3T3-E1 cells exposed to Dex and the
viability of Dex-treated MC3T3-E1 cells was improved through the
knockdown of TRIM21 (Fig. 1E). In
addition, the results from the LDH assay showed that the stimulated
LDH release in Dex-challenged MC3T3-E1 cells was decreased when
TRIM21 was silenced (Fig. 1F). In
conclusion, TRIM21 knockdown suppressed Dex-stimulated viability
loss and LDH release in MC3T3-E1 cells.
TRIM21 knockdown mitigates oxidative
stress and apoptosis of Dex-challenged MC3T3-E1 cells
At the same time, as elucidated using DCFH-DA
staining, TRIM21 silencing decreased Dex-induced ROS production in
MC3T3-E1 cells (Fig. 2A). Dex
exposure diminished SOD and GSH-Px activities whilst upregulating
MDA content in MC3T3-E1 cells, which were all partially reversed by
silencing of TRIM21 (Fig. 2B-D).
The apoptosis of MC3T3-E1 cells was evaluated upon exposure to Dex.
As shown in Fig. 2E and F, Dex treatment markedly increased the
apoptotic rate of MC3T3-E1 cells, which was then decreased
following TRIM21 knockdown. The increased caspase 3 activity that
was found in MC3T3-E1 cells challenged with Dex was decreased when
TRIM21 was knocked down (Fig. 2G).
Western blotting also implied that Dex treatment resulted in the
upregulation of Bax expression and the downregulation of Bcl-2
expression in MC3T3-E1 cells (Fig.
2H). However, in MC3T3-E1 cells treated with Dex, transfection
of sh-TRIM21#2 clearly reduced Bax expression although is
strengthened Bcl-2 expression. Overall, TRIM21 knockdown impeded
Dex-stimulated oxidative stress and apoptosis in MC3T3-E1
cells.
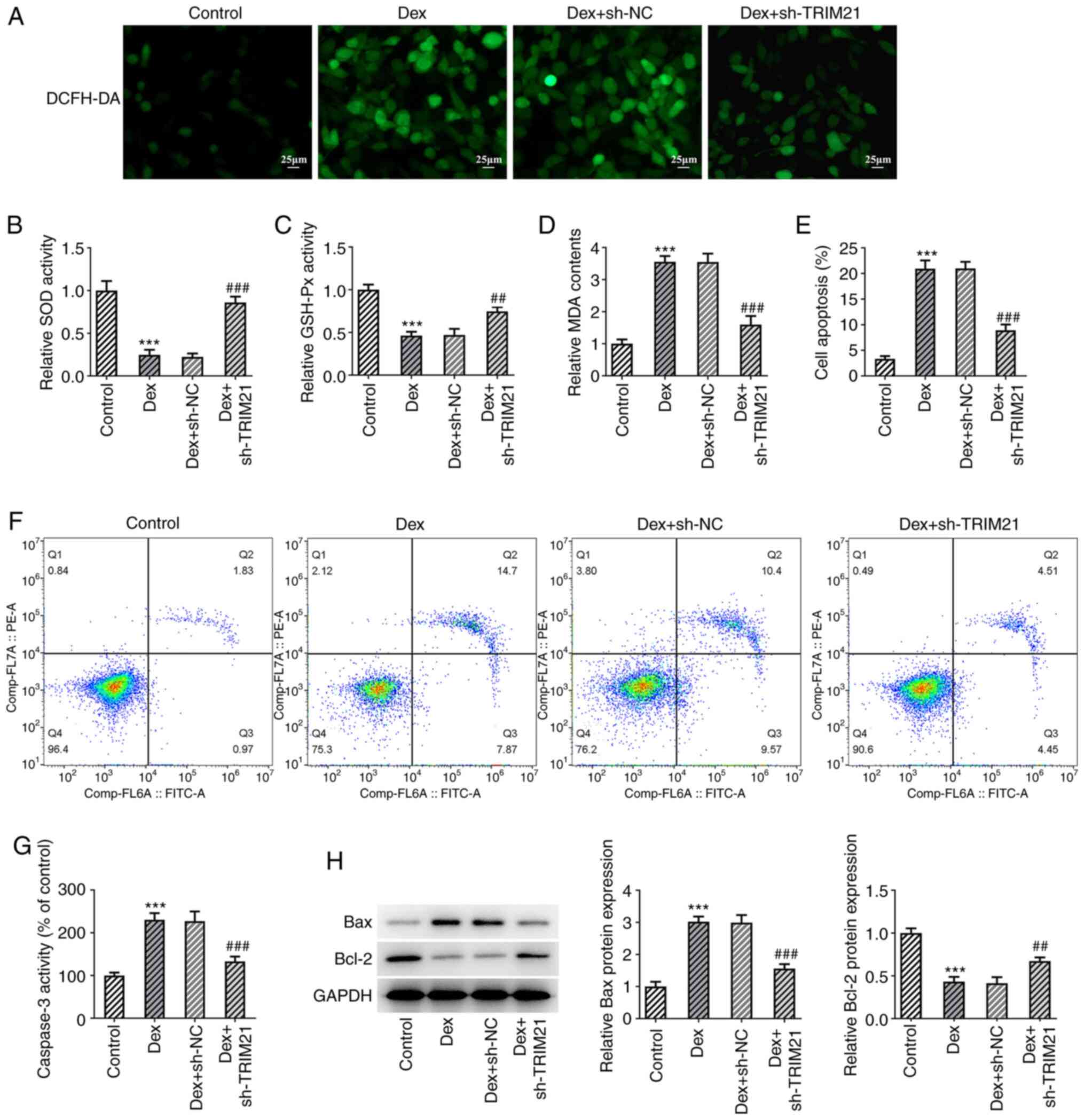 | Figure 2Interference with TRIM21 mitigates
oxidative stress and apoptosis of Dex-challenged MC3T3-E1 cells.
(A) DCFH-DA staining evaluated ROS generation. The levels of (B)
SOD, (C) GSH-Px and (D) MDA were detected using the corresponding
kits. (E and F) Flow cytometry analysis of cell apoptosis. (G)
Caspase 3 activity as measured by a kit. (H) Western blotting
tested the expression of apoptosis-associated proteins.
***P<0.001 vs. control group; ##P<0.01,
###P<0.001 vs. Dex + sh-NC group. TRIM21, tripartite
motif-containing protein 21; Dex, dexamethasone; DCFH-DA,
2,7-dichloro-dihydrofluorescein diacetate; ROS, reactive oxygen
species; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase;
MDA, malonaldehyde; sh-, short hairpin RNA; sh-NC, sh-RNA negative
control. |
Interference with TRIM21 contributes
to the osteogenic differentiation of MC3T3-E1 cells exposed to
Dex
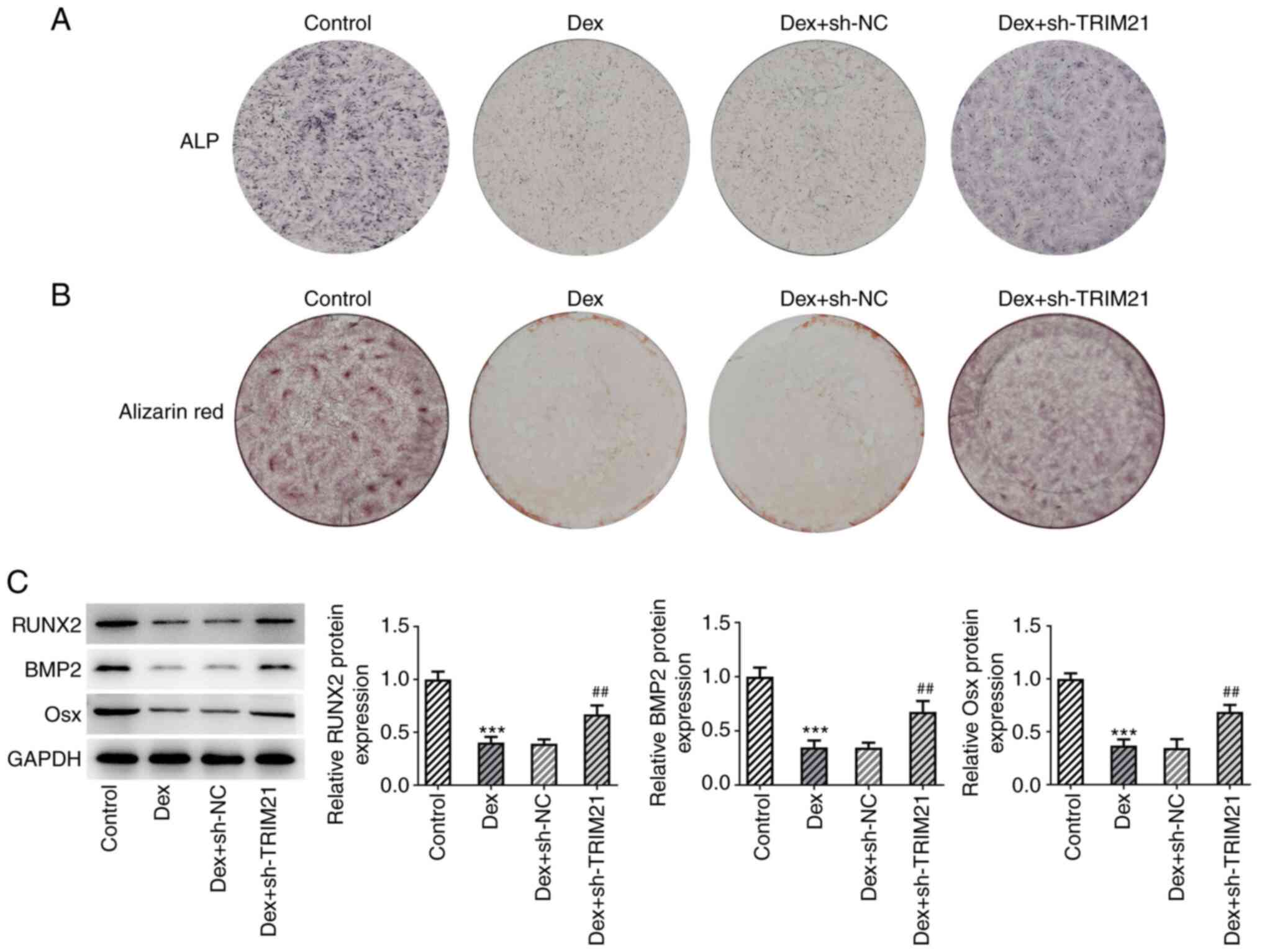
Using ALP staining, it was observed that the
decreased ALP activity in MC3T3-E1 cells treated with Dex increased
again following TRIM21 knockdown (Fig.
3A). Furthermore, the data from the ARS staining showed that
the attenuated calcium accumulation in MC3T3-E1 cells caused by Dex
treatment was aggravated by the TRIM21 knockdown (Fig. 3B). Western blotting analysis of
osteogenic differentiation-related markers also indicated that Dex
exposure decreased Osx, RUNX2 and BMP2 protein expressions in
MC3T3-E1 cells, which were partly restored following knockdown of
TRIM21 (Fig. 3C). In summary,
TRIM21 knockdown exacerbated the differentiation of Dex-treated
MC3T3-E1 cells into osteoblasts.
TRIM21 knockdown activates the
anti-oxidant Keap1/Nrf2 pathway
The expression of Keap1/Nrf2 pathway-related
proteins was investigated by the present study because the
Keap1/Nrf2 pathway was shown to play an inhibitory role in
oxidative stress (17). The
decreased Nrf2, HO-1 and NQO1 expression and the increased Keap1
expression in Dex-challenged MC3T3-E1 cells were all restored after
TRIM21 was knocked down (Fig. 4),
suggesting that TRIM21 inhibition might activate Keap1/Nrf2
signaling in Dex-induced MC3T3-E1 cells.
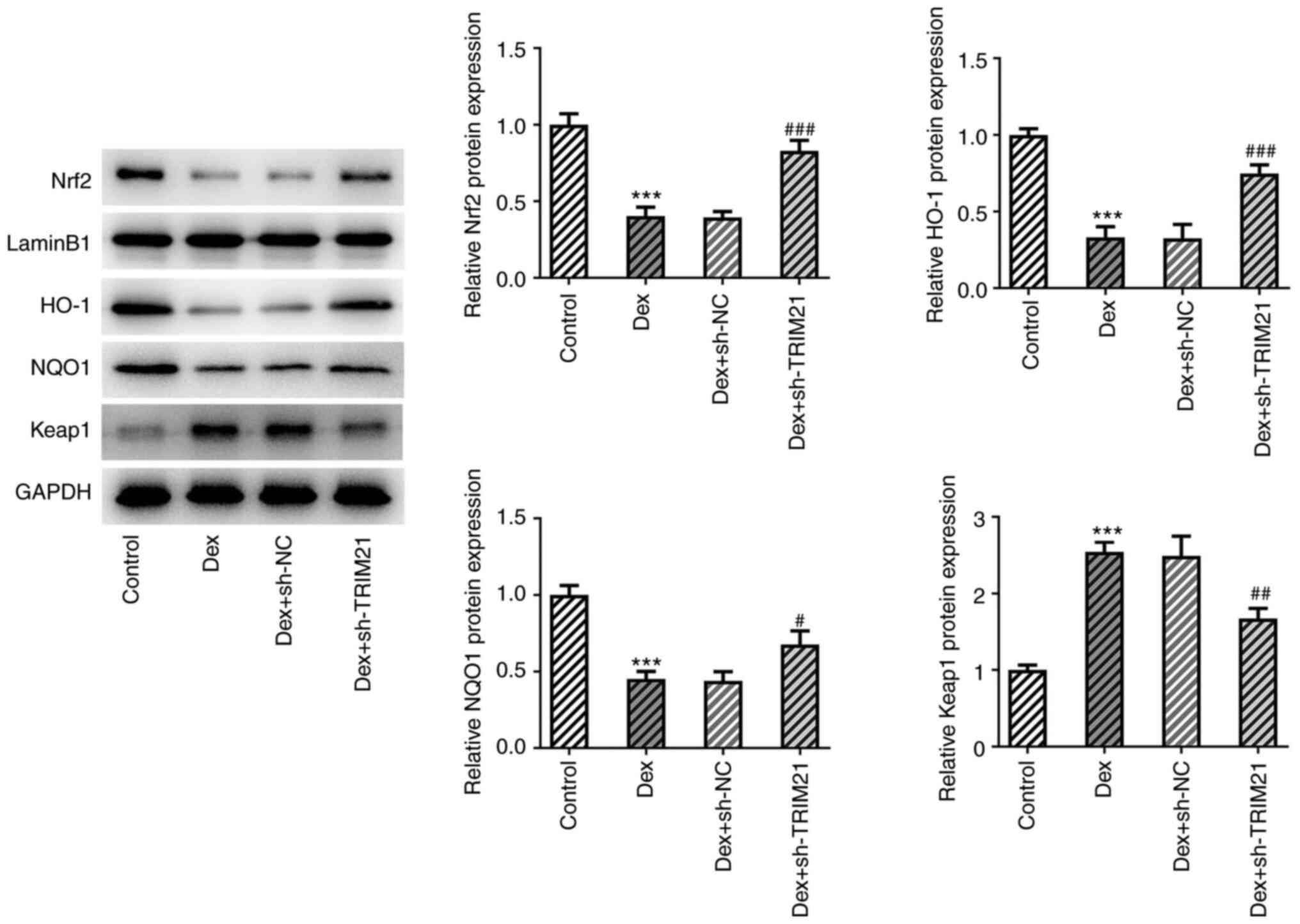 | Figure 4TRIM21 deficiency activates the
anti-oxidant Keap1/Nrf2 pathway. Western blotting was used to
examine the expression of proteins involved in the Keap1/Nrf2
pathway. ***P<0.001 vs. control group;
#P<0.05, ##P<0.01,
###P<0.001 vs. Dex + sh-NC group. TRIM21, tripartite
motif-containing protein 21; Keap1, Kelch-like ECH-associated
protein 1; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1,
heme oxygenase-1; NQO1, NAD(P)H:quinone oxidoreductase 1; sh-,
short hairpin RNA; sh-NC, sh-RNA negative control; Dex,
dexamethasone. |
TRIM21 knockdown activates Keap1/Nrf2
signaling to hamper the oxidative stress and apoptosis and drives
the osteogenic differentiation in Dex-treated MC3T3-E1 cells
The present study employed the Nrf2 inhibitor ML385
to test the hypothesis that TRIM21 might participate in the
biological events in the Dex-induced cellular model of ONFH by
mediating Keap1/Nrf2 signaling. Compared with the Dex group, ROS
and MDA levels were decreased whilst SOD and GSH-Px activities were
increased in Dex + sh-TRIM21 group (Fig. 5A-D). However, ROS (detected by
DCFH-DA staining) and MDA levels were upregulated while SOD and
GSH-Px activities were downregulated in the Dex + sh-TRIM21 + ML385
group compared with the Dex + sh-TRIM21 group. In addition, the
weakened apoptotic rate, accompanied by the declined caspase 3
activity, Bax expression and elevated Bcl-2 expression in
Dex-exposed MC3T3-E1 cells transfected with TRIM21 shRNA were all
partially counteracted by pretreatment with ML385 (Fig. 5E-H). Additionally, TRIM21 deletion
improved ALP activity and calcium accumulation in MC3T3-E1 cells
challenged with Dex, which were then partially abrogated by ML385,
implying that the stimulatory role of TRIM21 knockdown in the
osteogenic differentiation of Dex-exposed MC3T3-E1 cells was
abolished by inactivation of Nrf2 (Fig. 6A and B). This finding was also evidenced by the
decreased Osx, RUNX2 and BMP2 expressions in Dex + sh-TRIM21 +
ML385 group compared with the Dex + sh-TRIM21 group (Fig. 6C). In conclusion, inhibition of
Nrf2 partly counteracted the effects of TRIM21 knockdown on the
oxidative stress, apoptosis and osteogenic differentiation in
Dex-treated MC3T3-E1 cells.
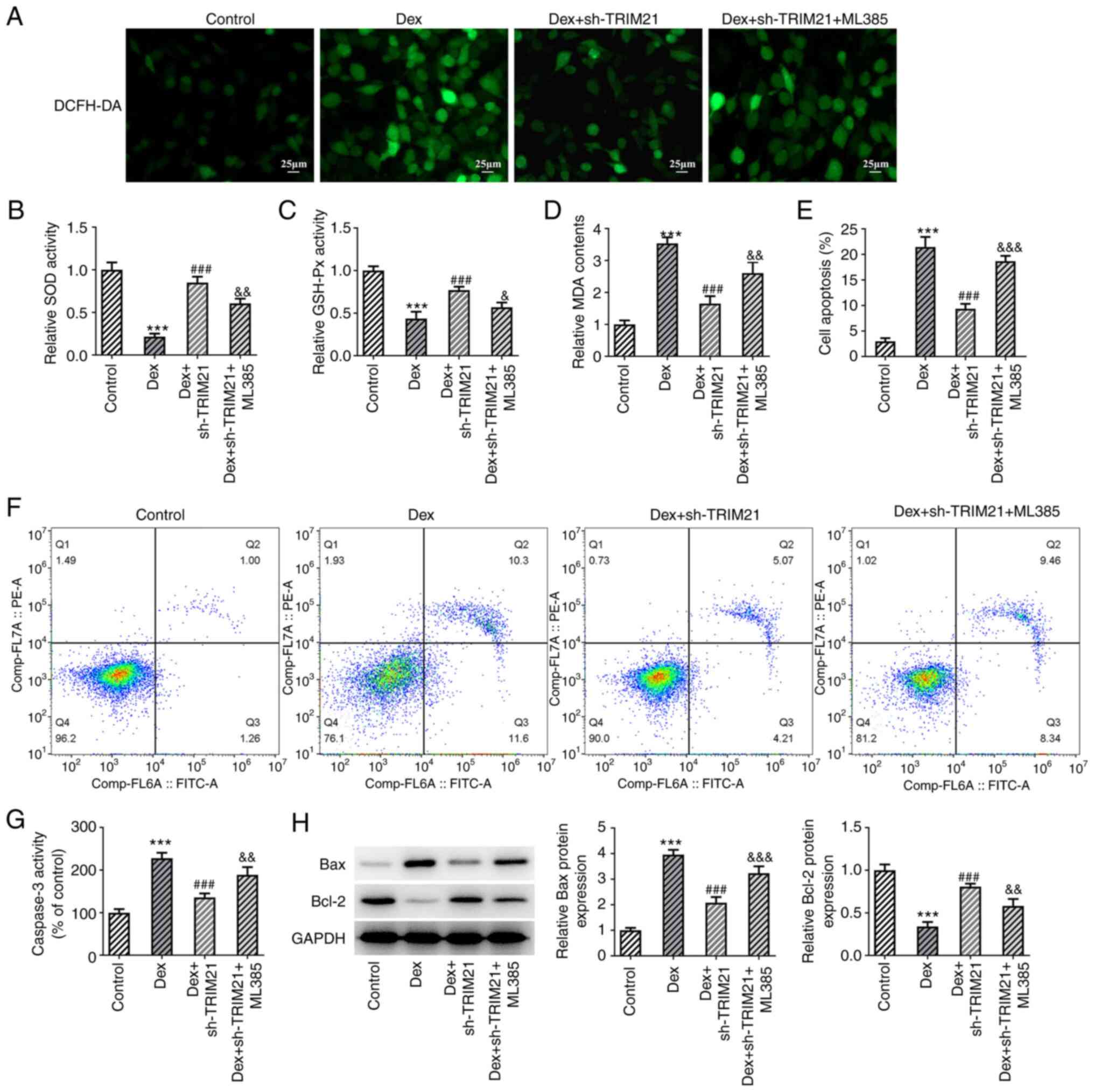 | Figure 5TRIM21 deletion activates Kelch-like
ECH-associated protein 1/nuclear factor erythroid 2-related factor
2 signaling to hamper oxidative stress and apoptosis in Dex-treated
MC3T3-E1 cells. (A) DCFH-DA staining evaluated reactive oxygen
species generation. The levels of (B) SOD, (C) GSH-Px and (D) MDA
were detected by the corresponding kits. (E and F) Flow cytometry
analysis of cell apoptosis. (G) Caspase 3 activity as measured by a
kit. (H) Western blotting of apoptosis-associated proteins.
***P<0.001 vs. control group;
###P<0.001 vs. Dex group; &P<0.05,
&&P<0.01,
&&&P<0.001 vs. Dex + sh-TRIM21 group.
TRIM21, tripartite motif-containing protein 21; Dex, dexamethasone;
DCFH-DA, 2,7-dichloro-dihydrofluorescein diacetate; SOD, superoxide
dismutase; GSH-Px, glutathione peroxidase; MDA, malonaldehyde; sh-,
short hairpin RNA; sh-TRIM21, sh-RNA targeting TRIM21. |
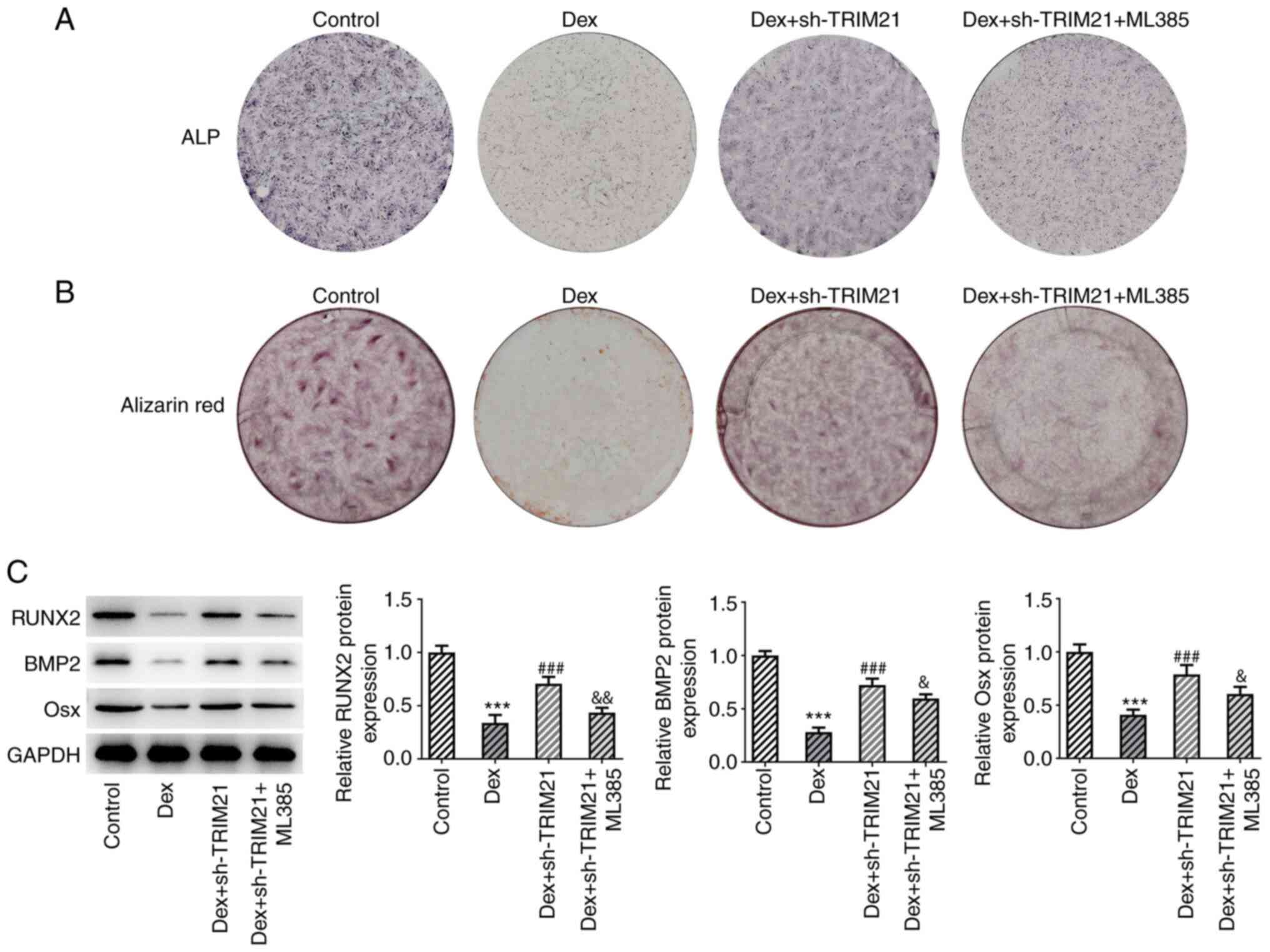 | Figure 6TRIM21 depletion activates Keap1/Nrf2
signaling to drive the osteogenic differentiation in Dex-treated
MC3T3-E1 cells. (A) ALP staining measured ALP activity. (B)
Alizarin red S staining estimated calcium salt deposition. (C)
Western blotting tested the expression of osteogenic
differentiation-associated proteins. ***P<0.001 vs.
control group; ###P<0.001 vs. Dex group;
&P<0.05, &&P<0.01 vs. Dex +
sh-TRIM21 group. TRIM21, tripartite motif-containing protein 21;
Keap1, Kelch-like ECH-associated protein 1; Nrf2, nuclear factor
erythroid 2-related factor 2; Dex, dexamethasone; ALP, alkaline
phosphatase; sh-, short hairpin RNA; sh-TRIM21, sh-RNA targeting
TRIM21; RUNX2, runt-related transcription factor 2; BMP2, bone
morphogenetic protein 2; Osx, osterix. |
Discussion
Osteoblasts, which are the sole bone-forming cells,
are responsible for bone formation and maintenance of bone mass.
Osteoblast dysfunction is one of the pivotal mechanisms leading to
steroid-induced ONFH (18) and
autologous osteoblast cell implantation may be regarded as an
effective therapy for ONFH (19).
Administration with glucocorticoids, extensively applied for the
treatment of autoimmune and inflammatory diseases as
immunosuppressive and anti-inflammatory drugs, is the most common
non-traumatic cause of ONFH (20).
As an artificially synthetic glucocorticoid, Dex has also been
implicated in the process of ONFH. In particular, Dex has been
proposed to suppress the differentiation and stimulate apoptosis,
autophagy, ferroptosis and oxidative stress in osteoblasts
(21-23).
Osteoblast cells caused by stimulation with 1 µM Dex for 24 h have
been widely used as a cell model to explore the mechanisms
involving in steroid-related ONFH (14,24,25).
Thus, the mechanism underlying Dex-induced ONFH was investigated in
the present study after murine MC3T3-E1 preosteoblast cells were
exposed to Dex.
A growing number of studies have underlined the
pivotal roles of TRIM21 in tumorigenesis (26), immune response (5) and autophagy (27). TRIM21 is downregulated during the
osteogenic process of mesenchymal stem cells (8). In addition, Liu et al
(28) demonstrated that TRIM21
expression is elevated in bone specimens from osteoporosis patients
and ovariectomy-induced osteoporotic mice. Their findings also
supported the hypothesis that TRIM21 depletion promotes bone
formation by enhancing the osteogenic differentiation of bone
marrow mesenchymal stem cells and elevating the activity of
osteoblast. In the present study, TRIM21 expression was raised in
MC3T3-E1 cells challenged with Dex and the viability loss and LDH
release in Dex-treated MC3T3-E1 cells were both reversed by
silencing of TRIM21. Additionally, enhanced osteoblast apoptosis
has been documented to play a crucial role in the development and
maintenance of bones and to further the progression of
glucocorticoids-induced ONFH (29,30).
By contrast, in Dex-exposed MC3T3-E1 cells, knockdown of TRIM21
attenuated the apoptotic ability of MC3T3-E1 cells, reduced caspase
3 activity and pro-apoptotic Bax expression and enhanced
anti-apoptotic Bcl-2 expression. Comparing the results of cell
activity and apoptosis of Dex-treated MC3T3-E1, it was found that
the cell viability of Dex and Dex + shNC groups was ~50%, but the
cell apoptosis rate in that two groups was only ~20%. The reason
for this result may be that there are other forms of cell death
involved in this process, such as necrosis and ferroptosis
(31,32). Oxidative stress, resulting in ROS
accumulation, is the leading cause of osteoblast injury and death
(33). Altered ROS generation can
facilitate osteoblast apoptosis and deplete the expression of
antioxidant enzymes leading to ONFH (34). Meanwhile, TRIM21 reduction has been
reported to inactivate oxidative stress in intervertebral disc
degeneration (35) and atrial
remodeling (36). Consistent with
this, the present study showed that TRIM21 knockdown decreased
Dex-induced ROS production, decreased SOD and GSH-Px activities and
increased MDA activity in MC3T3-E1 cells.
Osteogenic differentiation is deemed a pivotal
determinant in bone regeneration (37). Abnormal bone remodeling and
osteoblastic bone formation have been shown to participate in the
occurrence and progression of ONFH (38). Belonging to the BMP family, BMP2,
which plays a major role in bone development and remodeling, is a
well-established promoter of osteoblastic differentiation and bone
formation (39). Runx2 is the main
downstream regulator of the BMP signaling pathway that regulates
the expression of several osteogenic genes (40). Osx is indispensable for osteogenic
differentiation and its overexpression is related to the
enhancement of osteogenic differentiation (41). Notably, the absence of TRIM21 may
potentiate osteogenic differentiation and decrease RUNX2 expression
in mesenchymal stem cells (8). In
the current study, after TRIM21 was knocked down in MC3T3-E1 cells
challenged with Dex, the ALP activity was increased, calcium salt
deposition was strengthened and Osx, RUNX2 and BMP2 protein
expressions were all increased.
Furthermore, an emerging study has underlined that
TRIM21 may serve an inhibitor role in Keap1/Nrf2 signaling in
hepatocarcinogenesis (13). Nrf2,
a member of the Cap-n-Collar family of basic leucine zipper
proteins, is regarded as a major anti-oxidative modulator by
contributing to the transcription of antioxidant genes (42). As a main repressor protein of Nrf2,
Keap1 facilitates the polyubiquitination of the Nrf2 protein and
drives proteasome-dependent Nrf2 degradation (42). Under unstressed conditions, Keap1
binds to Nrf2 and promotes its degradation. Once the cells are
damaged, Nrf2 will dissociate from Keap1, translocate into the
nucleus and subsequently activate various genes, including
HO-1(42). Abundant evidence has
demonstrated that activation of Keap1/Nrf2 signaling can mitigate
oxidative stress in the process of steroid-induced ONFH (18). Li et al (43) showed that the Nrf2 knockdown
exacerbates apoptosis, oxidative stress and inflammation while
inhibiting the osteogenic differentiation of high
glucose-stimulated MC3T3-E1 cells. The present data also
demonstrated that Nrf2 inhibitor ML385 partially counteracted the
effects of TRIM21 deletion on apoptosis, oxidative stress as well
as osteogenic differentiation in Dex-treated MC3T3-E1 cells.
Collectively, the present study showed that TRIM21
interference activated the Keap1/Nrf2 pathway to antagonize
Dex-triggered apoptosis, oxidative stress and osteogenic
differentiation decrease in MC3T3-E1 cells, thereby relieving the
progression of steroid-induced ONFH. All these outcomes emphasize
that TRIM21 may be valued as a potential target for steroid-induced
ONFH. Nevertheless, this study only explored the effect of TRIM21
on mouse preosteoblast cells. Further experiments associated with
osteoclast will be analyzed in the future. In addition, the other
signaling pathways downstream of TRIM21, such as the YAP1/β-catenin
signaling pathway (28), the
intervention timing and possible screening models for high-risk
patients will also be investigated in the next studies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JS and XM conceived and designed the study. JS, LC,
XW and XM conducted the experiments. LC and XW performed the
literature search and data extraction. JS drafted the manuscript
and XM revised it. All authors read and approved the final version
of the manuscript. JS and XM confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pan J, Lu L, Wang X, Liu D, Tian J, Liu H,
Zhang M, Xu F and An F: AIM2 regulates vascular smooth muscle cell
migration in atherosclerosis. Biochem Biophys Res Commun.
497:401–409. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hines JT, Jo WL, Cui Q, Mont MA, Koo KH,
Cheng EY, Goodman SB, Ha YC, Hernigou P, Jones LC, et al:
Osteonecrosis of the femoral head: An updated review of ARCO on
pathogenesis, staging and treatment. J Korean Med Sci.
36(e177)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ando W, Sakai T, Fukushima W, Kaneuji A,
Ueshima K, Yamasaki T, Yamamoto T and Nishii T: Working group for
ONFH guidelines and Sugano N. Japanese orthopaedic association 2019
guidelines for osteonecrosis of the femoral head. J Orthop Sci.
26:46–68. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Watanabe M and Hatakeyama S: TRIM proteins
and diseases. J Biochem. 161:135–144. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jones EL, Laidlaw SM and Dustin LB:
TRIM21/Ro52-roles in innate immunity and autoimmune disease. Front
Immunol. 12(738473)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ben-Chetrit E, Chan EK, Sullivan KF and
Tan EM: A 52-kD protein is a novel component of the SS-A/Ro
antigenic particle. J Exp Med. 167:1560–1571. 1988.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ben-Chetrit E, Fox RI and Tan EM:
Dissociation of immune responses to the SS-A (Ro) 52 and 60-kd
polypeptides in systemic lupus erythematosus and Sjögren's
syndrome. Arthritis Rheum. 33:349–355. 1990.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xian J, Liang D, Zhao C, Chen Y and Zhu Q:
TRIM21 inhibits the osteogenic differentiation of mesenchymal stem
cells by facilitating K48 ubiquitination-mediated degradation of
Akt. Exp Cell Res. 412(113034)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ulasov AV, Rosenkranz AA, Georgiev GP and
Sobolev AS: Nrf2/Keap1/ARE signaling: Towards specific regulation.
Life Sci. 291(120111)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fan Z, Wirth AK, Chen D, Wruck CJ, Rauh M,
Buchfelder M and Savaskan N: Nrf2-Keap1 pathway promotes cell
proliferation and diminishes ferroptosis. Oncogenesis.
6(e371)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Song MY, Lee DY, Chun KS and Kim EH: The
role of NRF2/KEAP1 signaling pathway in cancer metabolism. Int J
Mol Sci. 22(4376)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang N, Sun H, Xue Y, Zhang W, Wang H, Tao
H, Liang X, Li M, Xu Y, Chen L, et al: Inhibition of MAGL activates
the Keap1/Nrf2 pathway to attenuate glucocorticoid-induced
osteonecrosis of the femoral head. Clin Transl Med.
11(e447)2021.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Wang F, Zhang Y, Shen J, Yang B, Dai W,
Yan J, Maimouni S, Daguplo HQ, Coppola S, Gao Y, et al: The
ubiquitin E3 ligase TRIM21 promotes hepatocarcinogenesis by
suppressing the p62-Keap1-Nrf2 antioxidant pathway. Cell Mol
Gastroenterol Hepatol. 11:1369–1385. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Han D, Gu X, Gao J, Wang Z, Liu G, Barkema
HW and Han B: Chlorogenic acid promotes the Nrf2/HO-1
anti-oxidative pathway by activating
p21Waf1/Cip1 to resist
dexamethasone-induced apoptosis in osteoblastic cells. Free Radic
Biol Med. 137:1–12. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hu Q, Zuo T, Deng L, Chen S, Yu W, Liu S,
Liu J, Wang X, Fan X and Dong Z: β-Caryophyllene suppresses
ferroptosis induced by cerebral ischemia reperfusion via activation
of the NRF2/HO-1 signaling pathway in MCAO/R rats. Phytomedicine.
102(154112)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Suzuki T, Takahashi J and Yamamoto M:
Molecular basis of the KEAP1-NRF2 signaling pathway. Mol Cells.
46:133–141. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lu Z and Han K: SMAD4 transcriptionally
activates GCN5 to inhibit apoptosis and promote osteogenic
differentiation in dexamethasone-induced human bone marrow
mesenchymal stem cells. Steroids. 179(108969)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Palekar G: Hip preservation with
autologous osteoblast cell-based treatment in osteonecrosis of the
femoral head. Orthopedics. 44:e183–e189. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kerachian MA, Séguin C and Harvey EJ:
Glucocorticoids in osteonecrosis of the femoral head: A new
understanding of the mechanisms of action. J Steroid Biochem Mol
Biol. 114:121–128. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fang L, Zhang G, Wu Y, Li Z, Gao S and
Zhou L: SIRT6 prevents glucocorticoid-induced osteonecrosis of the
femoral head in rats. Oxid Med Cell Longev.
2022(6360133)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun F, Zhou JL, Wei SX, Jiang ZW and Peng
H: Glucocorticoids induce osteonecrosis of the femoral head in rats
via PI3K/AKT/FOXO1 signaling pathway. PeerJ.
10(e13319)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Peng P, Nie Z, Sun F and Peng H:
Glucocorticoids induce femoral head necrosis in rats through the
ROS/JNK/c-Jun pathway. FEBS Open Bio. 11:312–321. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Deng S, Dai G, Chen S, Nie Z, Zhou J, Fang
H and Peng H: Dexamethasone induces osteoblast apoptosis through
ROS-PI3K/AKT/GSK3β signaling pathway. Biomed Pharmacother.
110:602–608. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhou M, Liu L, Xu Y, Jiang J, Liu G and
Zhai C: Effects of osteoblast autophagy on glucocorticoid-induced
femoral head necrosis. Jt Dis Relat Surg. 31:411–418.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Alomari M: TRIM21-a potential novel
therapeutic target in cancer. Pharmacol Res.
165(105443)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kimura T, Jain A, Choi SW, Mandell MA,
Schroder K, Johansen T and Deretic V: TRIM-mediated precision
autophagy targets cytoplasmic regulators of innate immunity. J Cell
Biol. 210:973–989. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu RX, Gu RH, Li ZP, Hao ZQ, Hu QX, Li
ZY, Wang XG, Tang W, Wang XH, Zeng YK, et al: Trim21 depletion
alleviates bone loss in osteoporosis via activation of
YAP1/β-catenin signaling. Bone Res. 11(56)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nie Z, Chen S and Peng H: Glucocorticoid
induces osteonecrosis of the femoral head in rats through
GSK3β-mediated osteoblast apoptosis. Biochem. Biophys Res Commun.
511:693–699. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mutijima E, De Maertelaer V, Deprez M,
Malaise M and Hauzeur JP: The apoptosis of osteoblasts and
osteocytes in femoral head osteonecrosis: Its specificity and its
distribution. Clin Rheumatol. 33:1791–1795. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Guo S, Mao L, Ji F, Wang S, Xie Y, Fei H
and Wang XD: Activating AMP-activated protein kinase by an α1
selective activator compound 13 attenuates dexamethasone-induced
osteoblast cell death. Biochem Biophys Res Commun. 471:545–552.
2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sun F, Zhou JL, Liu ZL, Jiang ZW and Peng
H: Dexamethasone induces ferroptosis via P53/SLC7A11/GPX4 pathway
in glucocorticoid-induced osteonecrosis of the femoral head.
Biochem Biophys Res Commun. 602:149–155. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang YH, Li B, Zheng XF, Chen JW, Chen K,
Jiang SD and Jiang LS: Oxidative damage to osteoblasts can be
alleviated by early autophagy through the endoplasmic reticulum
stress pathway-implications for the treatment of osteoporosis. Free
Radic Biol Med. 77:10–20. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Deng S, Zhou JL, Fang HS, Nie ZG, Chen S
and Peng H: Sesamin protects the femoral head from osteonecrosis by
inhibiting ROS-induced osteoblast apoptosis in rat model. Front
Physiol. 9(1787)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zheng J, Chang L, Bao X, Zhang X, Li C and
Deng L: TRIM21 drives intervertebral disc degeneration induced by
oxidative stress via mediating HIF-1α degradation. Biochem Biophys
Res Commun. 555:46–53. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu X, Zhang W, Luo J, Shi W, Zhang X, Li
Z, Qin X, Liu B and Wei Y: TRIM21 deficiency protects against
atrial inflammation and remodeling post myocardial infarction by
attenuating oxidative stress. Redox Biol. 62(102679)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bharadwaz A and Jayasuriya AC: Osteogenic
differentiation cues of the bone morphogenetic protein-9 (BMP-9)
and its recent advances in bone tissue regeneration. Mater Sci Eng
C Mater Biol Appl. 120(111748)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tingart M, Beckmann J, Opolka A, Matsuura
M, Wiech O, Grifka J and Grässel S: Influence of factors regulating
bone formation and remodeling on bone quality in osteonecrosis of
the femoral head. Calcif Tissue Int. 82:300–308. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Halloran D, Durbano HW and Nohe A: Bone
morphogenetic protein-2 in development and bone homeostasis. J Dev
Biol. 8(19)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu DD, Zhang CY, Liu Y, Li J, Wang YX and
Zheng SG: RUNX2 regulates osteoblast differentiation via the BMP4
signaling pathway. J Dent Res. 101:1227–1237. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Han Y, Kim YM, Kim HS and Lee KY:
Melatonin promotes osteoblast differentiation by regulating osterix
protein stability and expression. Sci Rep. 7(5716)2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Baird L and Yamamoto M: The molecular
mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol.
40:e00099–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li X, Yu X, He S and Li J: Dipeptidyl
peptidase 3 is essential for maintaining osteoblastic
differentiation under a high-glucose environment by inhibiting
apoptosis, oxidative stress and inflammation through the modulation
of the Keap1-Nrf2 pathway. Int Immunopharmacol.
120(110404)2023.PubMed/NCBI View Article : Google Scholar
|