Introduction
The prevalence of diabetes is increasing worldwide,
posing a significant threat to various body systems, including
large blood vessels, microvessels and nerves (1,2),
thereby resulting in a spectrum of diabetes-related complications,
such as diabetic nephropathy, retinopathy and cardiomyopathy
(3). However, the insidious and
multifactorial nature of diabetic hearing loss often results in it
being a clinically underdiagnosed complication of diabetes, as
highlighted by the American Diabetes Association (4). A significant prevalence of hearing
impairment among individuals with type II diabetes has been
identified, ranging from 34.4 to 60.2% (5). Hearing loss and diabetes share some
common risk factors since damage to the insulin system can lead to
problems anywhere in the body. Insulin receptors, glucose
transporters and components of insulin signaling are present in the
sensory receptors and supporting cells of the cochlea, suggesting
that hearing is susceptible to impairment by glucose utilization
(6). Notably, comprehensive
meta-analyses have confirmed the persistent correlation between
diabetes and hearing impairment, irrespective of diabetes type,
underscoring the need to identify the intricate mechanisms linking
diabetes to auditory dysfunction (7). Oxidative stress induced by elevated
glucose levels is recognized as a pivotal factor precipitating
cellular apoptosis in diabetes (8). Central to cellular aerobic
respiration, reactive oxygen species (ROS) regulation and various
signaling pathways (9),
mitochondria have emerged as a crucial focal point for
understanding the disease mechanism and formulating innovative
therapeutic interventions.
Apelins are primarily found in endocardial and
vascular endothelial cells, suggesting that they may originate from
these tissues. Central sources of apelin, such as from
magnocellular neurons, also contribute to circulating Apelins.
Apelins act in an autocrine/paracrine manner (10). In the context of regulating glucose
levels, the role of Apelin-13, which is a critical regulator of
lipid metabolism, has assumed significance. Recent research has
highlighted the diverse protective roles of Apelin-13 in mitigating
diabetic complications, such as promoting nitric oxide production
and ameliorating renal tissue fibrosis in diabetic nephropathy
(11). Moreover, Apelin-13 has
been demonstrated to possess the potential to improve glucose and
lipid metabolism in mice with gestational diabetes, and to reduce
oxidative stress and inflammation via activation of the PI3K/AKT
pathway. In addition, it confers a protective effect on pancreatic
islets, thereby improving pregnancy outcomes (12). Diabetes is an independent risk
factor for vascular calcification (13), and Apelin-13 may reduce high
glucose (HG)-induced calcification of mouse aortic vascular smooth
muscle cells by inhibiting ROS-mediated DNA damage (14). Notably, its potential in preventing
noise-induced cochlear damage and hearing loss (15), as well as in enhancing the survival
of hair cell-like cells against oxidative stress damage (16), is gaining attention. However, the
precise role of Apelin-13 in diabetes-induced hearing loss remains
to be elucidated, necessitating further exploration.
Given that cochlear hair cells are highly sensitive
to damage (17), they were adopted
as an in vitro research model in the present study, and the
hypothesis that Apelin-13 could play a protective role against HG
in cochlear hair cells by maintaining mitochondrial function was
proposed. The present study set the experimental foundation for the
application of Apelin-13 as a potential treatment strategy for
diabetic hearing loss.
Materials and methods
Cell culture and treatment
The mouse cochlear hair cell line HEI-OC1 (cat. no.
M8-0401; OriCell, Guangzhou Cyagen Biotech Co. Ltd.) was cultured
in DMEM supplemented with 5% FBS and 1% penicillin-streptomycin
(all from Gibco; Thermo Fisher Scientific, Inc.) in an atmosphere
containing 5% CO2 at 37˚C. HEI-OC1 cells were starved
without serum for 12 h to synchronize cell proliferation, after
which, they were pretreated with Apelin-13 (0.01, 0.05 and 0.1 nM;
Selleck Chemicals) for 24 h (18)
and then cultured under HG (25 mM glucose) conditions. For
mechanistic studies, HEI-OC1 cells were pretreated with the
endoplasmic reticulum (ER) stress agonist tunicamycin (TM; 5 µg/ml;
Selleck Chemicals) for 6 h.
Cell Counting Kit-8 (CCK-8)
HEI-OC1 cells were seeded in a 96-well plate
(5x103/well), pretreated with Apelin-13 (0.01, 0.05 and
0.1 nM) for 24 h and cultured with or without HG for 48 h. CCK-8
reagent (10 µl; Dalian Meilun Biology Technology Co., Ltd.) was
then added to the wells and, after 2 h of co-culture at 37˚C, the
absorbance at 450 nm was recorded using a microplate reader (Tecan
Group, Ltd.). Cell viability was calculated based on a standard
curve.
Oxidative stress indicators
Oxidative stress indicators, including
malondialdehyde (MDA; cat. no. S0131; Beyotime Institute of
Biotechnology), ROS (cat. no. HL10124.3; Haling), superoxide
dismutase (SOD; cat. no. S0101) and glutathione peroxidase (GSH-Px;
cat. no. S0056; both from Beyotime Institute of Biotechnology),
were determined in HEI-OC1 cells using commercial assay kits
according to the manufacturers' instructions. The cells were lysed
in an ice bath, centrifuged at 10,000 x g for 10 min at 4˚C, and
the supernatants were collected for subsequent determination. The
samples were incubated with specific working solutions and the
absorbance was recorded.
Flow cytometry
Apoptosis of HEI-OC1 cells was determined using flow
cytometry (FACSCanto; BD Biosciences) with an Annexin V-FITC/PI
assay kit (cat. no. KGA108; Nanjing KeyGen Biotech Co., Ltd.). The
experiment was performed according to the kit manufacturer's
instructions. HEI-OC1 cells were gathered and centrifuged at 300 x
g for 5 min at 4˚C, the supernatant was discarded and the cells
were washed once with PBS. After further centrifugation, the cells
were suspended in 500 µl 1X Annexin V binding buffer, and were then
treated with 5 µl Annexin V-FITC and 5 µl PI. HEI-OC1 cells were
incubated in the dark for 15 min and apoptosis was analyzed using
FlowJo 10.6.2 software (BD Biosciences).
Caspase3 activity
Routine cell lysis and supernatant collection
following centrifugation were performed as aforementioned, followed
by protein quantification. Subsequently, 45 µl Reaction buffer, 50
µl samples to be tested and 5 µl Ac-DEVD-pNA (cat. no. E-CK-A383;
Elabscience Biotechnology, Inc.) were sequentially mixed. The
absorbance was recorded at 405 nm after incubation at 37˚C for 1-2
h.
Mitochondrial membrane potential (MMP)
and mitochondrial ROS (mtROS)
JC-1 probe (HY-K0601) and MitoSOX Red probe (cat.
no. HY-D1055; MedChemExpress) were separately used to identify MMP
and mtROS. The probes were dissolved in DMSO and diluted to a 2 µM
working solution with serum-free DMEM. HEI-OC1 cells were incubated
with working solution for 20 min in the dark, and then washed twice
with PBS. Images were then acquired under a fluorescence microscope
(Olympus Corporation).
ATP level detection
ATP levels in HEI-OC1 cells were measured using an
ATP detection kit (cat. no. S0026; Beyotime Institute of
Biotechnology). The supernatant of the cell lysate was obtained as
aforementioned. ATP levels were measured according to the kit's
instructions, and the absorbance was recorded at 405 nm.
Western blotting
Proteins were extracted from HEI-OC1 cells after
lysis using RIPA buffer (cat. no. BL504A; Biosharp; Labgic
Technology Co., Ltd.) on ice, and the protein concentration was
determined using the bicinchoninic acid method and normalized.
Subsequently, SDS-polyacrylamide gel electrophoresis on 6 or 8%
gels was performed to separate proteins (20 µg/lane). Protein
samples were then transferred to PVDF membranes (MilliporeSigma)
and the membranes were blocked with 5% BSA (Sigma-Aldrich; Merck
KGaA) at room temperature for 1 h. Diluted primary antibodies
against CHOP (cat. no. 15204-1-AP; 1:1,000; Proteintech Group,
Inc.), GRP78 (cat. no. 11587-1-AP; 1:5,000; Proteintech Group,
Inc.), XBP1 (cat. no. 24168-1-AP; 1:1,000; Proteintech Group,
Inc.), phosphorylated (p)-PERK (cat. no. 3179; 1:1,000; Cell
Signaling Technology, Inc.), PERK (3192; 1:1,000; Cell Signaling
Technology, Inc.) and β-actin (cat. no. 81115-1-RR; 1:10,000;
Proteintech Group, Inc.) were used to incubate the membranes at 4˚C
overnight, followed by incubation with a HRP-conjugated secondary
antibody (cat. no. SA00001-2; 1:5,000; Proteintech Group, Inc.) at
37˚C for 1 h. The ECL solution (Vazyme Biotech Co., Ltd.) was then
added to evenly cover the membranes for 1 min, and densitometric
analysis was performed using the Image-Pro Plus 7.0 software (Media
Cybernetics, Inc.).
Statistical analysis
Data are presented as the mean ± SD of three
independent experiments, and data analysis was performed using SPSS
25.0 software (IBM Corp.). Data were analyzed using one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Apelin-13 inhibits oxidative stress
and apoptosis in HG-treated HEI-OC1 cells
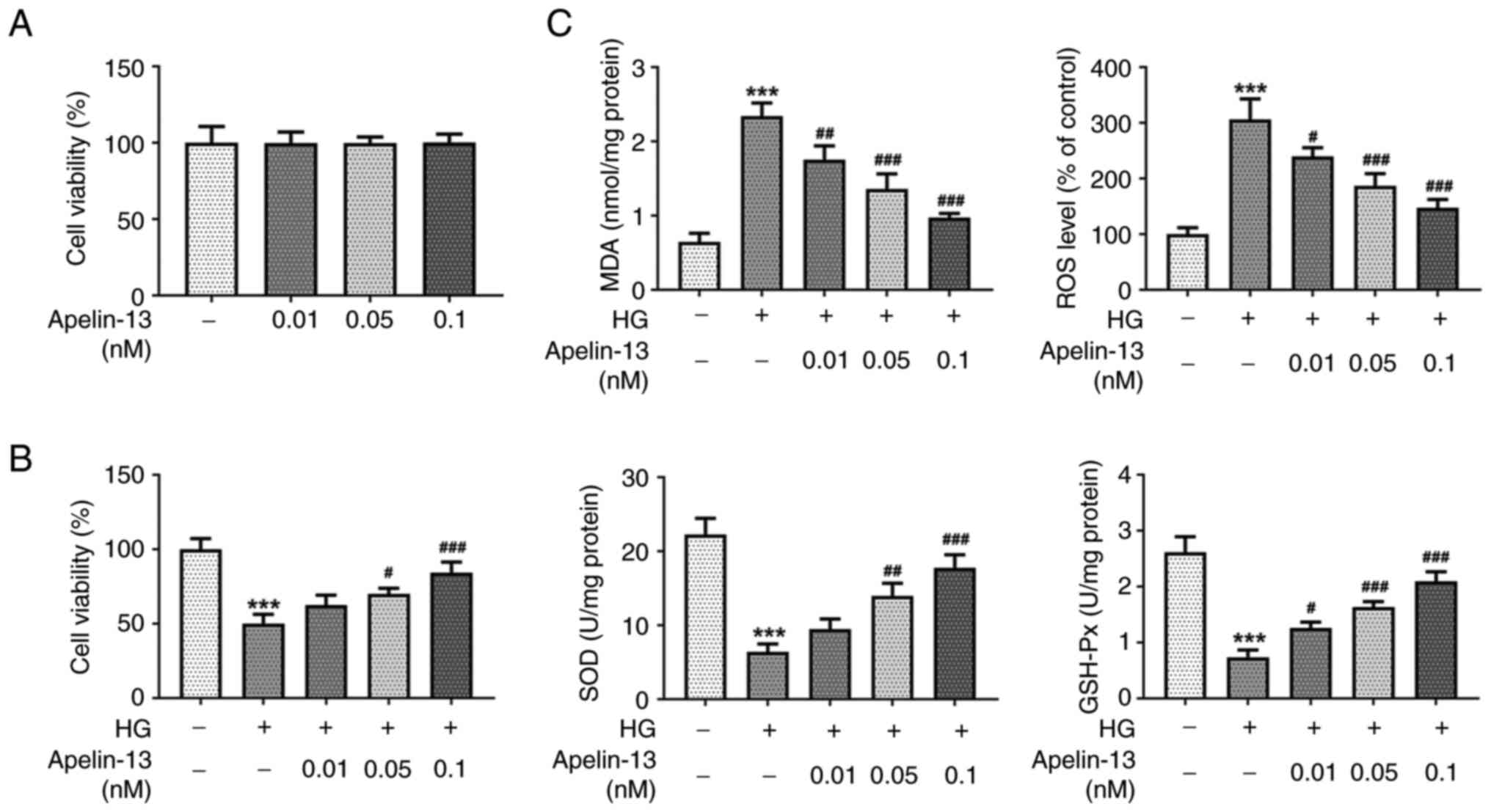
The results of the CCK-8 assay exhibited that
Apelin-13 at concentrations of 0.01, 0.05 and 0.1 nM had no
significant effect on HEI-OC1 cell viability (Fig. 1A). However, in HG-treated cells, HG
caused a decrease in cell viability. As the concentration of
Apelin-13 treatment increased, the cell viability increased in a
concentration-dependent manner (Fig.
1B). The levels of oxidative stress in HEI-OC1 cells were
assessed by determining changes in indicators. Compared with those
in the control group, the MDA and ROS levels in the HG group were
significantly increased, while the activities of SOD and GSH-Px
were significantly decreased. Apelin-13 treatment could reduce MDA
and ROS levels, and increase SOD and GSH-Px activities in
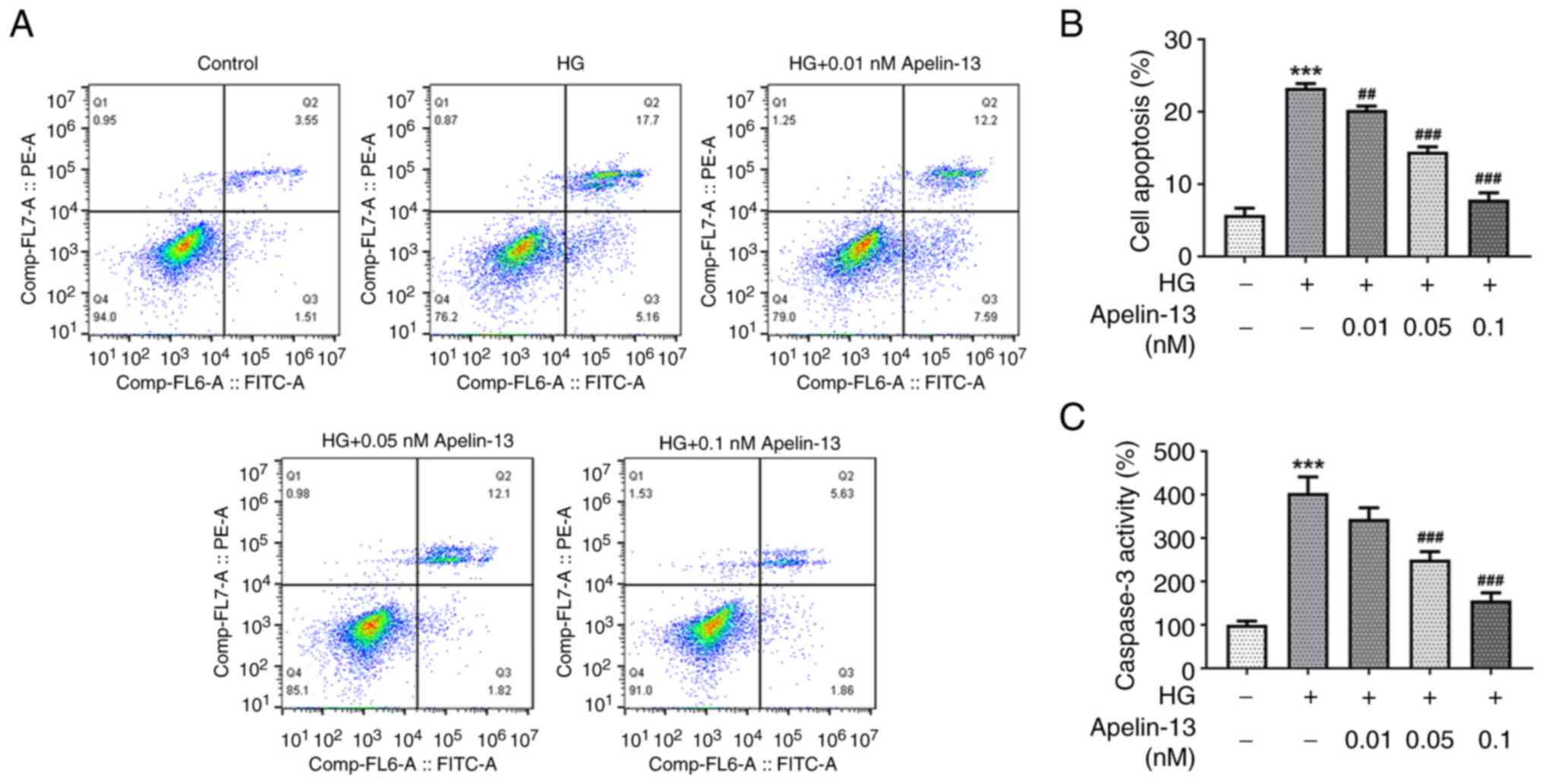
HG-treated cells in a concentration-dependent manner (Fig. 1C). Flow cytometric analysis
revealed that the proportion of apoptotic cells increased in the HG
group, and Apelin-13 treatment could significantly reduce cell
apoptosis (Fig. 2A and B). Meanwhile, caspase3 activity in
HEI-OC1 cells displayed an upward trend under the influence of HG,
and Apelin-13 reduced the increase in caspase3 activity in a
concentration-dependent manner (Fig.
2C).
Apelin-13 reduces mitochondrial
dysfunction in HG-treated HEI-OC1 cells
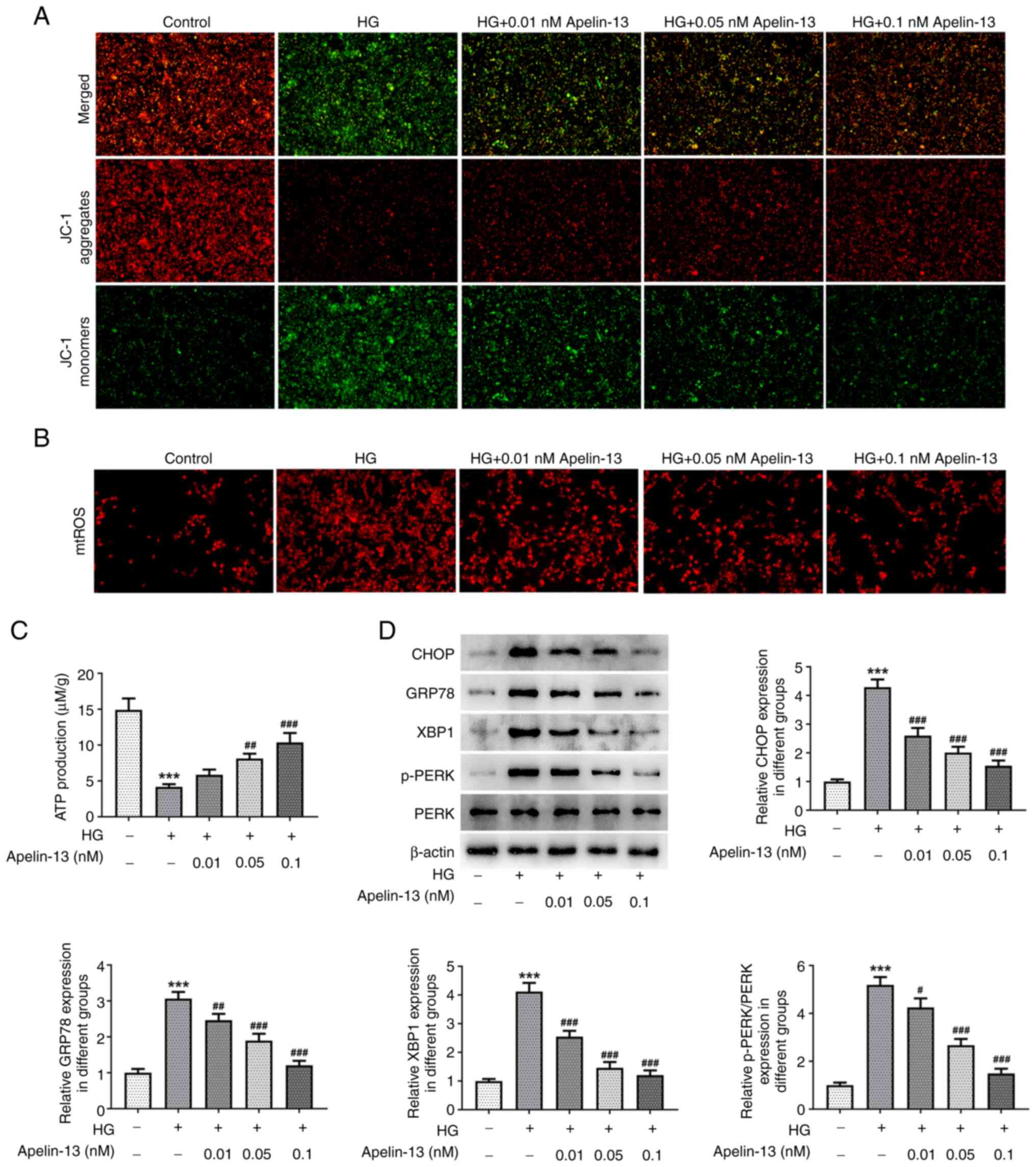
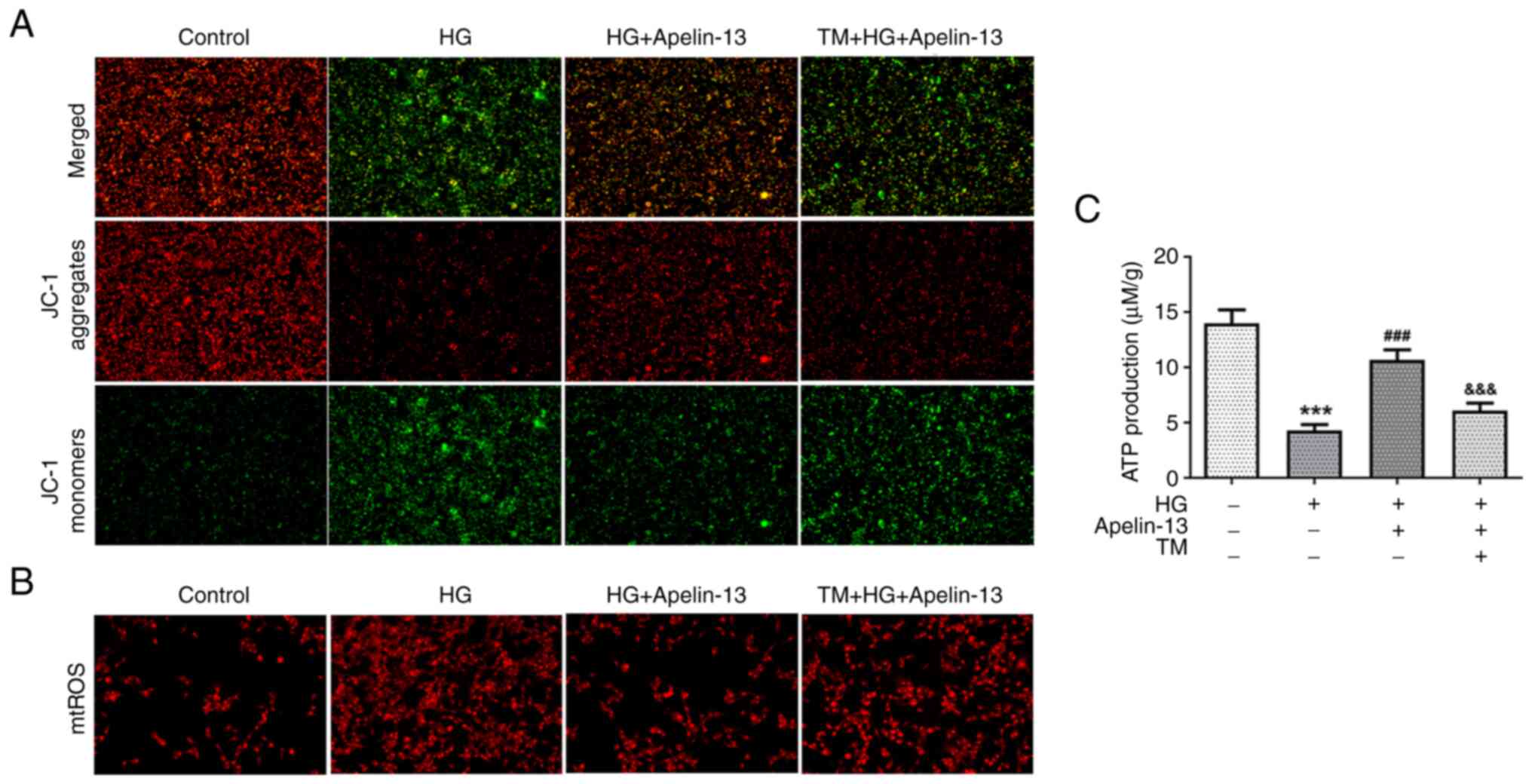
The JC-1 probe detected a decrease in aggregates in
HEI-OC1 cells in the HG group, accompanied by an increase in
monomers, indicating a decrease in MMP. Apelin-13 treatment
maintained MMP, and the group treated with a concentration of 0.1
nM had the best effect (Fig. 3A).
In addition, HG induced an increase in mtROS production and
Apelin-13 decreased the amount of mtROS in a
concentration-dependent manner (Fig.
3B). Notably, ATP production of HEI-OC1 cells in the HG group
was impaired and lower ATP levels were detected than those in the
control group. As the concentration of Apelin-13 treatment
increased, ATP production levels were restored in a
concentration-dependent manner (Fig.
3C). Western blotting revealed that HG induced ER stress, and
the expression levels of related proteins CHOP, GRP78, XBP1 and
p-PERK/PERK in HEI-OC1 cells were significantly increased. However,
their levels were also affected by Apelin-13 treatment and
demonstrated a downward trend compared with those in the HG group
(Fig. 3D).
ER stress agonist reverses the effects
of Apelin-13
To further explore the potential role of ER stress
in the regulatory effects of Apelin-13, HEI-OC1 cells were
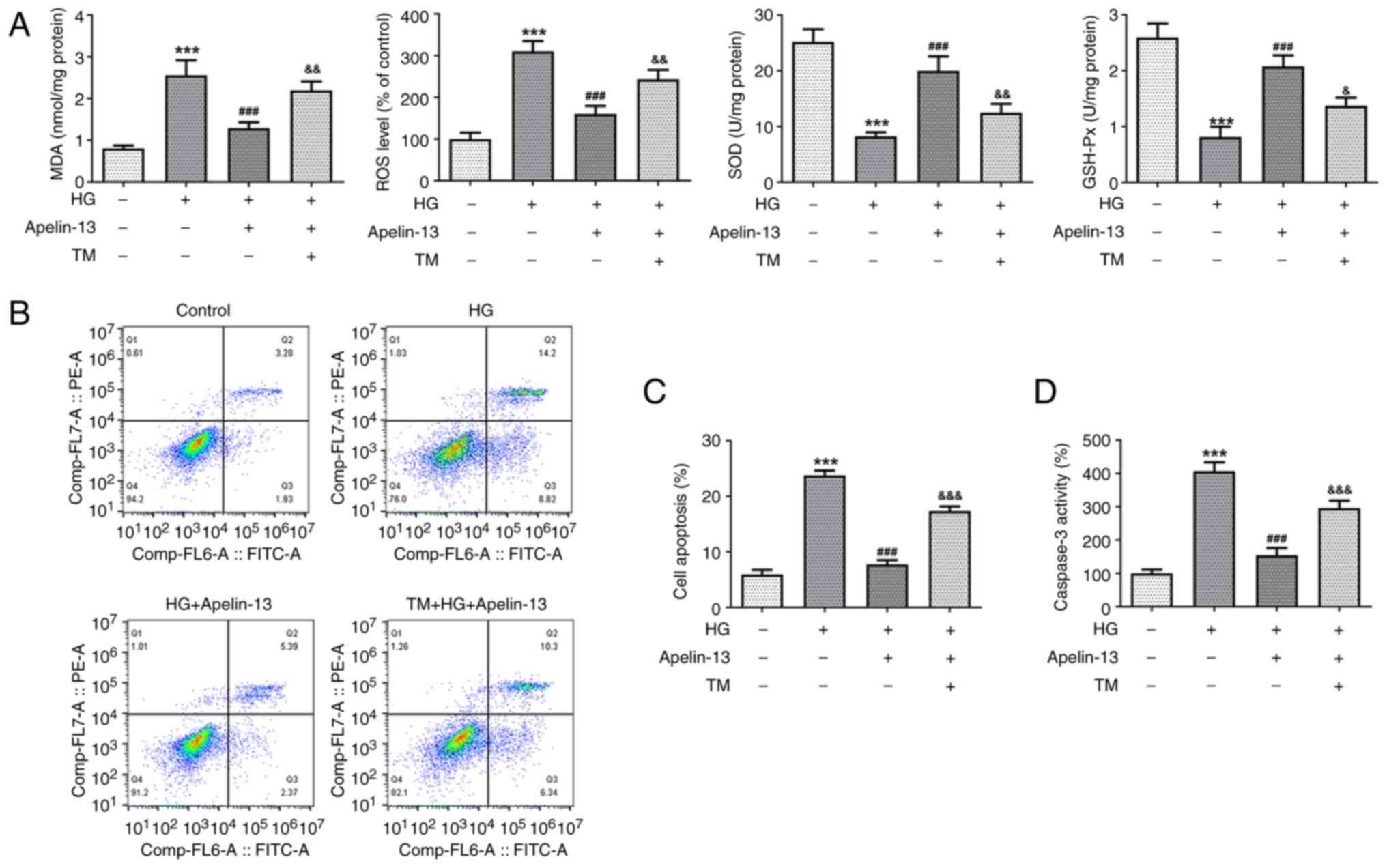
pretreated with TM to stimulate ER stress. Compared with in the HG
+ Apelin-13 group, additional TM treatment significantly increased
MDA and ROS levels, while SOD and GSH-Px activities were decreased
(Fig. 4A). The results of flow
cytometry (Fig. 4B and C) and the caspase3 kit (Fig. 4D) revealed that TM elevated the
proportion of apoptotic cells and intracellular caspase3 activity.
These results indicated that TM destroyed the inhibitory effect of
Apelin-13 on cellular oxidative stress and apoptosis. Subsequently,
MMP was re-evaluated to confirm the effect of TM on mitochondrial
function. Additional TM treatment lowered the MMP (Fig. 5A), with a concomitant increase in
mtROS, as reflected using the MitoSOX Red probe (Fig. 5B). Similarly, the ATP levels
increased by Apelin-13 were affected by TM, resulting in a
significant decrease in cellular ATP production (Fig. 5C).
Discussion
The gradual onset and complex manifestation of
diabetic hearing loss contribute to the lack of research on it,
compounded by the intricate structure and relative inaccessibility
of the inner ear (19). Consistent
with hearing loss caused by ototoxicity, noise overstimulation, or
aging, hair cells in the cochlea are preferentially affected
(20). Hearing is not just a
passive response to stimuli, the human auditory system is enhanced
by active processes in the cochlear hair cells that amplify sound
signals hundreds of times (21).
Cochlear hair cell damage is the major cause of hearing loss and
cells cannot regenerate spontaneously after damage (22). Therefore, the present study focused
on cochlear hair cells. Elucidating the pathogenesis of diabetic
hearing loss based on hair cells is necessary to offer insights
crucial for early diagnosis, prevention and effective management
strategies. Existing research has highlighted the detrimental
impact of heightened glucose or fat levels on diabetes, resulting
in increased mitochondrial load and the accumulation of damaged
mitochondria within cells (23-25).
The present study, employing JC-1 and MitoSOX Red probes combined
with ATP detection, successfully demonstrated the adverse effects
of HG intervention, including diminished MMP, reduced ATP
production and an increase in mtROS within HEI-OC1 cells. These
results indicated that mitochondrial damage increases upon HG
treatment, which is consistent with the conclusions of previous
studies. Notably, the therapeutic administration of Apelin-13
alleviated these aberrations. Additionally, the decrease in MMP can
promote the further activation of procaspase3 by apoptotic bodies,
thereby activating the caspase apoptotic pathway. In the present
study, it was found that caspase3 activity was decreased after
Apelin-13 treatment, and flow cytometry results revealed that cell
apoptosis was also decreased. These findings indicated that
Apelin-13 had potential in preserving mitochondrial function and
impeding the progression of apoptotic signaling pathways within the
cochlear hair cells. A recent study has also revealed that diabetic
hearing loss is accompanied by mitochondrial structural or
functional damage and activation of endogenous apoptotic pathways
(26), which further supports that
the inhibition of mitochondrial damage and apoptosis by Apelin-13
found in this research is beneficial to alleviating hearing
loss.
Furthermore, the current findings elucidated the
regulatory impact of Apelin-13 on ER stress-related proteins,
reinforcing its role in mediating ER stress. Apelin-13 exhibits
therapeutic potential in diabetic cardiovascular complication by
reducing the unfolded protein response and endothelial dysfunction
induced by the glycolytic metabolite methylglyoxal (27). It can be concluded that this
finding is similarly consistent with the conclusion of the present
study because the disease background is also a complication of
diabetes and can affect ER-related functions. Notably, the observed
parallels between the protective effects of Apelin-13 on the
survival of dopaminergic neurons in Parkinson's disease mouse
models via ER stress (28)
underscore the multifaceted nature of its regulatory mechanisms.
Furthermore, variations in serum Apelin-12 levels observed between
patients with diabetes and controls accentuate the significance of
Apelin as clinical markers (29).
However, to date, there are a lack of studies elucidating the
specific role of the isoform Apelin-13 in clinical diabetes. Hence,
further research is warranted to reveal the clinical significance
of Apelin-13.
In addition, earlier research has pointed to the
complexities in understanding the interplay between hyperglycemia
and hearing impairment, with some evidence indicating that blood
glucose control, rather than diabetes per se, may be the
primary determinant of hearing loss (6). It was considered by the authors that
no conclusion can be made at present. Notably, the administration
of exogenous Apelin-13 has demonstrated the potential to enhance
glucose metabolism (30). In
normal and diabetic mice, glucose utilization is improved in
response to Apelin-13, which results in increased hypothalamic
nitric oxide release. Data suggest the identification of oral
Apelin-13 administration as a new potential target for the
treatment of metabolic disorders (31). Therefore, whether stemming from
diabetes or elevated blood glucose levels, Apelin-13 appears to
possess the capacity to address both factors. It should not be
ignored that Apelin is found to have a short half-life in the human
body, and rapid receptor desensitization can be achieved by
coupling β-arrestins (32). Apelin
analogues with enhanced biological activity and resistance to
degradation have now been developed, compared with the endogenous
peptide. For example, the apelin-13 analog MM07 increases forearm
blood flow in human volunteers and is considered an excellent
vasodilator (33). The development
of analogs can avoid peptide degradation and circumvent the side
effects of β-arrestin signaling pathway. Additionally, small
molecule apelin agonists are also under development (34), and these studies suggest different
apelin-based therapeutic strategies. Currently, Apelin-13 or its
analogs are not used to treat various forms of hearing loss because
clinical research on Apelin is in its infancy. Nevertheless,
according to the results in the present study, it is considered by
the authors it has great potential in hearing loss. Diabetes often
involves insulin resistance or impaired insulin signaling, which
can lead to mitochondrial dysfunction. The current results may
indicate that Apelin-13 acts independently of the insulin signaling
pathway. Additionally, elevated glucose levels lead to increased
oxidative stress, damaging cellular components including
mitochondria. The ability of Apelin-13 to reduce mitochondrial
dysfunction suggests its potential role in reducing oxidative
stress within cochlear hair cells, which may contribute to maintain
their function and vitality under diabetic conditions.
In conclusion, the present study elucidated the
protective role of Apelin-13 in ameliorating HG-induced
mitochondrial functional impairment in cochlear hair cells by
inhibiting ER stress. This research paves the way for harnessing
the Apelin-13 signaling system as a promising therapeutic strategy
for combating diabetic hearing loss. While existing studies have
underscored the physiological role of Apelin-13 in glucose
metabolism, further research is warranted to unravel the complex
nuances of the Apelin system (35), encompassing novel regulatory
ligands and other Apelin isoforms. These are the limitations to the
present study, which also include the use of single mouse-derived
cells, and the exploration of the role of Apelin-13 and its
analogues on glucose metabolism and diabetic hearing loss in the
in vivo environment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZH and JG contributed to study design, implementing
methods and drafting the manuscript. JG and TH contributed to
methods and data analysis, and confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Knapp M, Tu X and Wu R: Vascular
endothelial dysfunction, a major mediator in diabetic
cardiomyopathy. Acta Pharmacol Sin. 40:1–8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Avogaro A and Fadini GP: Microvascular
complications in diabetes: A growing concern for cardiologists. Int
J Cardiol. 291:29–35. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Demir S, Nawroth PP, Herzig S and Ekim
Üstünel B: Emerging targets in type 2 diabetes and diabetic
complications. Adv Sci (Weinh). 8(e2100275)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Glessner M: American diabetes
association-75th scientific sessions (June 5-9, 2015-Boston,
Massachusetts, USA). Drugs Today (Barc). 51:383–386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gupta S, Eavey RD, Wang M, Curhan SG and
Curhan GC: Type 2 diabetes and the risk of incident hearing loss.
Diabetologia. 62:281–285. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Samocha-Bonet D, Wu B and Ryugo DK:
Diabetes mellitus and hearing loss: A review. Ageing Res Rev.
71(101423)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Horikawa C, Kodama S, Tanaka S, Fujihara
K, Hirasawa R, Yachi Y, Shimano H, Yamada N, Saito K and Sone H:
Diabetes and risk of hearing impairment in adults: A meta-analysis.
J Clin Endocrinol Metab. 98:51–58. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Darenskaya MA, Kolesnikova LI and
Kolesnikov SI: Oxidative stress: Pathogenetic role in diabetes
mellitus and its complications and therapeutic approaches to
correction. Bull Exp Biol Med. 171:179–189. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rizwan H, Pal S, Sabnam S and Pal A: High
glucose augments ROS generation regulates mitochondrial dysfunction
and apoptosis via stress signalling cascades in keratinocytes. Life
Sci. 241(117148)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chapman FA, Maguire JJ, Newby DE,
Davenport AP and Dhaun N: Targeting the apelin system for the
treatment of cardiovascular diseases. Cardiovasc Res.
119:2683–2696. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gao Z, Zhong X, Tan YX and Liu D:
Apelin-13 alleviates diabetic nephropathy by enhancing nitric oxide
production and suppressing kidney tissue fibrosis. Int J Mol Med.
48(175)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zheng XD, Huang Y and Li H: Regulatory
role of Apelin-13-mediated PI3K/AKT signaling pathway in the
glucose and lipid metabolism of mouse with gestational diabetes
mellitus. Immunobiology. 226(152135)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee SJ, Lee IK and Jeon JH: Vascular
calcification-new insights into its mechanism. Int J Mol Sci.
21(2685)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang P, Wang AP, Yang HP, Ai L, Zhang HJ,
Wang YM, Bi YL, Fan HH, Gao J, Zhang HY and Liu JZ: Apelin-13
attenuates high glucose-induced calcification of MOVAS cells by
regulating MAPKs and PI3K/AKT pathways and ROS-mediated signals.
Biomed Pharmacother. 128(110271)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Khoshsirat S, Abbaszadeh HA, Peyvandi AA,
Heidari F, Peyvandi M, Simani L and Niknazar S: Apelin-13 prevents
apoptosis in the cochlear tissue of noise-exposed rat via Sirt-1
regulation. J Chem Neuroanat. 114(101956)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Niknazar S, Abbaszadeh HA, Peyvandi H,
Rezaei O, Forooghirad H, Khoshsirat S and Peyvandi AA: Protective
effect of [Pyr1]-apelin-13 on oxidative stress-induced apoptosis in
hair cell-like cells derived from bone marrow mesenchymal stem
cells. Eur J Pharmacol. 853:25–32. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xing Y, Ji Q, Li X, Ming J, Zhang N, Zha D
and Lin Y: Asiaticoside protects cochlear hair cells from high
glucose-induced oxidative stress via suppressing AGEs/RAGE/NF-κB
pathway. Biomed Pharmacother. 86:531–536. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen G, Liang X, Han Q, Mai C, Shi L, Shao
Z, Hong Y, Lin F, Li M, Hu B, et al: Apelin-13 pretreatment
promotes the cardioprotective effect of mesenchymal stem cells
against myocardial infarction by improving their survival. Stem
Cells Int. 2022(3742678)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Alizadeh Y, Jalali MM and Sehati A:
Association of different severity of diabetic retinopathy and
hearing loss in type 2 diabetes mellitus. Am J Otolaryngol.
43(103383)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fettiplace R and Nam JH: Tonotopy in
calcium homeostasis and vulnerability of cochlear hair cells. Hear
Res. 376:11–21. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hudspeth AJ: Integrating the active
process of hair cells with cochlear function. Nat Rev Neurosci.
15:600–614. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Yeom J, Park J and Park JY: Fluid dynamic
simulation for cellular damage due to lymphatic flow within the
anatomical arrangement of the outer hair cells in the cochlea.
Comput Biol Med. 161(106986)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen Y, Xin Y, Cheng Y and Liu X:
Mitochondria-endoplasmic reticulum contacts: The promising
regulators in diabetic cardiomyopathy. Oxid Med Cell Longev.
2022(2531458)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mao H, Chen W, Chen L and Li L: Potential
role of mitochondria-associated endoplasmic reticulum membrane
proteins in diseases. Biochem Pharmacol. 199(115011)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yao L, Liang X, Qiao Y, Chen B, Wang P and
Liu Z: Mitochondrial dysfunction in diabetic tubulopathy.
Metabolism. 131(155195)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lyu AR, Kim TH, Shin SA, Kim EH, Yu Y,
Gajbhiye A, Kwon HC, Je AR, Huh YH, Park MJ and Park YH: Hearing
impairment in a mouse model of diabetes is associated with
mitochondrial dysfunction, synaptopathy, and activation of the
intrinsic apoptosis pathway. Int J Mol Sci. 22(8807)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim S, Kim S, Hwang AR, Choi HC, Lee JY
and Woo CH: Apelin-13 inhibits methylglyoxal-induced unfolded
protein responses and endothelial dysfunction via regulating AMPK
pathway. Int J Mol Sci. 21(4069)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhu J, Dou S, Jiang Y, Chen J, Wang C and
Cheng B: Apelin-13 protects dopaminergic neurons in MPTP-induced
Parkinson's disease model mice through inhibiting endoplasmic
reticulum stress and promoting autophagy. Brain Res. 1715:203–212.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Castan-Laurell I, El Boustany R, Pereira
O, Potier L, Marre M, Fumeron F, Valet P, Gourdy P, Velho G and
Roussel R: Plasma apelin and risk of type 2 diabetes in a cohort
from the community. Diabetes Care. 43:e15–e16. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dray C, Debard C, Jager J, Disse E,
Daviaud D, Martin P, Attané C, Wanecq E, Guigné C, Bost F, et al:
Apelin and APJ regulation in adipose tissue and skeletal muscle of
type 2 diabetic mice and humans. Am J Physiol Endocrinol Metab.
298:E1161–E1169. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fournel A, Drougard A, Duparc T, Marlin A,
Brierley SM, Castro J, Le-Gonidec S, Masri B, Colom A, Lucas A, et
al: Apelin targets gut contraction to control glucose metabolism
via the brain. Gut. 66:258–269. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pope GR, Tilve S, McArdle CA, Lolait SJ
and O'Carroll AM: Agonist-induced internalization and
desensitization of the apelin receptor. Mol Cell Endocrinol.
437:108–119. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chapman FA, Nyimanu D, Maguire JJ,
Davenport AP, Newby DE and Dhaun N: The therapeutic potential of
apelin in kidney disease. Nat Rev Nephrol. 17:840–853.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Read C, Nyimanu D, Williams TL, Huggins
DJ, Sulentic P, Macrae RGC, Yang P, Glen RC, Maguire JJ and
Davenport AP: International union of basic and clinical
pharmacology. CVII. Structure and Pharmacology of the apelin
receptor with a recommendation that elabela/toddler is a second
endogenous peptide ligand. Pharmacol Rev. 71:467–502.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li C, Cheng H, Adhikari BK, Wang S, Yang
N, Liu W, Sun J and Wang Y: The role of apelin-APJ system in
diabetes and obesity. Front Endocrinol (Lausanne).
13(820002)2022.PubMed/NCBI View Article : Google Scholar
|