Introduction
Preterm delivery (PTD) is commonly defined as live
birth occurring before the completion of 37 weeks of pregnancy.
Worldwide, ~15 million newborns are delivered preterm every year
with a global PTD rate of 11% (1).
Most of the cases occur spontaneously, while ~30% are performed for
medical causes such as pre-eclampsia or eclampsia and fetal growth
restriction (2). While the
underlying cause of PTD is not fully clear, it has been suggested
that infection, inflammation and vascular diseases may have a role
in its pathophysiology (3).
PTD constitutes a major cause of perinatal and
childhood mortality causing 18% of deaths in children and 35%
deaths in neonates (1).
Additionally, PTD is a well-recognized risk factor for several
maternal complications later in life. Research has shown that PTD
increases the risk of cardiovascular disorders at 3 years
post-partum by alteration of systolic blood pressure and lipid
levels (4). A different study has
found an increase in systolic and diastolic blood pressure up to 20
years post-partum in women with PTD vs. those delivering at term
(5). Amongst other diseases, the
risk of type-2 diabetes mellitus and hypercholesterolemia is also
increased in the first 10 years after PTD (6).
Diabetes and hypertension in turn are important risk
factors for chronic kidney disease (CKD) in adults. Indeed, CKD is
a major global health concern and is the 16th most common cause of
mortality around the world (7). Up
to 16% of the global population is affected by CKD with mortality
rates as high as 60% in end-stage renal disease (ESRD) (8). Owing to the high morbidity and
mortality of CKD and ESRD, there have been efforts to identify and
control risk factors that lead to the development of the
disease.
Previously, there have been several studies linking
PTD to the long-term risk of maternal CKD (9-11).
Since chronic hypertension and hypertensive disorders of pregnancy
have both been linked with the risk of CKD (7,12),
and there is a close association between PTD and hypertensive
disorders (5), it is plausible
that PTD could be a risk factor for CKD later in life. In this
context, a detailed literature search was conducted to
systematically analyze and a quantitative analysis was also
conducted to examine the association between PTD and the risk of
maternal CKD.
Materials and methods
Literature source and search
strategy
The protocol of the review was registered and then
published on PROSPERO (registration no. CRD42023422085; https://www.crd.york.ac.uk/prospero/).
PRISMA guidelines were followed (13). Studies for the present review were
searched in the electronic databases of PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase
(https://www.embase.com/landing?status=grey), CENTRAL
(https://www.cochranelibrary.com/central) and Scopus
(https://www.scopus.com/home.uri). Gray
literature was searched on Google Scholar (https://scholar.google.com/). Two reviewers performed
the search separately which was completed on 15th May 2023. The
databases were examined with key words consisting of ‘chronic
kidney disease’, ‘renal disease’, ‘dialysis’, ‘pregnancy’,
‘maternal’, ‘complications’, ‘preterm birth’ and ‘preterm delivery’
in different combinations. Details of the search are further
provided in Table SI. All search
results were congregated and deduplicated electronically. The
titles and abstracts of all articles were screened to identify
relevant studies. Non-relevant articles were excluded and the
remaining underwent full-text analysis. The reviewers carefully
screened these studies based on the following criteria for further
inclusion. Any disagreements were solved by consensus. The
reference lists of the included studies were also examined for any
other missed articles.
Inclusion criteria
Studies conducted on a population of pregnant women
were included. The exposure was PTD. The comparative group was
pregnant women without PTD. The outcome variable was CKD in the
mother. Studies were to report adjusted effect size with 95%
confidence intervals (CI) of the association between PTD and risk
of maternal CKD. Both case-control and cohort studies were eligible
provided they were published in peer-reviewed journals.
Studies reporting outcomes for the offspring,
studies not reporting adjusted data and studies using the same
database with the same study period were excluded. Additionally,
review articles, case reports and non-English language studies were
not considered.
Extracted data and outcomes
The studies underwent data extraction using a
pre-formatted table. Two reviewers independently retrieved data on
the author's name, year of publication, the database for the study,
location, study type, sample size, inclusion criteria, the
definition of PTD, type of renal disorder and its method of
identification, percentage of women with PTD and renal disorder in
the study, confounders adjusted and follow-up period. Study details
were then cross-matched and any discrepancies were resolved in
discussion with the third author.
Risk of bias analysis
Two reviewers judged the quality of the study based
on Newcastle Ottawa Scale (NOS) (14). The NOS has three domains:
Representativeness of the study cohort, comparability and
measurement of outcomes. Points are given based on the preformatted
questions. The final score of a study can range from 0-9.
Statistical analysis
Review Manager (RevMan; v.5.3; The Cochrane
Collaboration) was employed for combining data from included
studies. The pooled association between PTD and the risk of
maternal CKD was presented as a hazard ratio (HR) with 95% CIs in
the form of a forest plot. The meta-analysis was conducted in a
random-effects model. Since the studies presented data on CKD or
ESRD in the maternal population, subgroup analyses were conducted
for all CKD and ESRD.
Funnel plots were generated using RevMan to judge
publication bias. The I2 statistic was the tool to
determine inter-study heterogeneity. I2<50% indicated
low and >50% indicated substantial heterogeneity. A sensitivity
analysis was performed to check for outliers in the analysis. One
study at a time was removed from the meta-analysis and the results
were regenerated. The results of the sensitivity analysis were
presented in tabular form.
Results
Search results
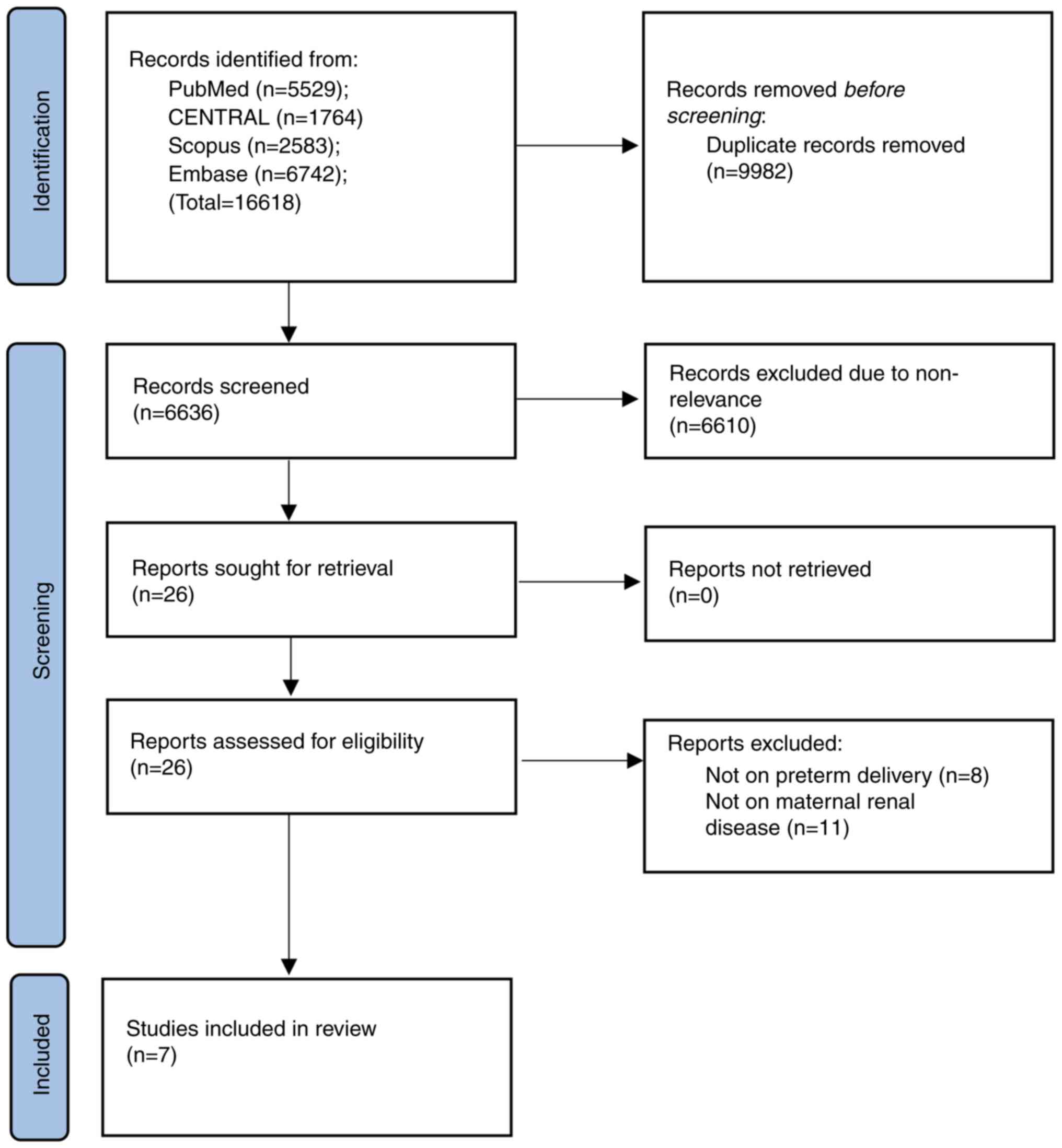
The outcomes of every step of the search strategy
are presented in a flowchart form (Fig. 1). The initial search yielded 16,618
articles. Amongst these, 9,982 duplicate studies were eliminated.
The remaining 6,636 records were screened for primary eligibility.
The reviewers selected 26 for full-text review. Of these, a total
of 7 reviews (9-11,15-18)
made it to the final review while the rest were not eligible.
Details of included studies
Data from the studies are revealed in Table I. While the publication years of
the studies were from 2010 to 2021, the cohorts included in them
dated back up to 1967. Three of the studies were from Scandinavia,
while the remaining were from Israel, Canada, Germany and Iran. All
were cohort studies retrospectively examining their respective
databases. The two Norwegian and one Iranian study consisted of a
limited sample size with <3,500 participants. The remaining
studies were with large sample sizes ranging from 99,338 to
1,943,716 participants. PTD was defined as birth after <37 of
gestation across most studies. In addition, women with baseline CKD
were excluded from all studies. In total, 3 studies examined the
risk of ESRD, 3 different studies were on CKD while 1 study
reported the association between PTD and both CKD and ESRD. Renal
disorders were identified by clinical evaluation of estimated
glomerular filtration rate (eGFR) in only 1 study. The other
studies identified renal disease by using international
classification of disease (ICD) codes, hospitalization episodes for
renal disease, or by the treatment modality (dialysis/renal
transplantation). The confounders adjusted for the association
varied across the studies. The follow-up duration ranged from 5.4
years to up to 37 years. As per the author's judgment, the studies
were of moderate quality receiving a NOS score of 7 or 8.
 | Table IDetails of included studies. |
Table I
Details of included studies.
| First author,
year | Location | Database | Study type | Sample size | Inclusion
criteria | Definition of preterm
birth | Preterm birth
(%) | Identification of
renal disorder | Renal disorder
(%) | Confounders
adjusted | Follow-up | NOS score | (Refs.) |
|---|
| Sandvik et al,
2010 | Norway | Medical Birth
Registry & Norwegian Renal Registry (1967-1994) | RC | 1,481 | Women with single
pregnancies with preexisting diabetes | <37 weeks | 25.1 | ESRD; identified as
undergoing dialysis or renal transplantation | 3.2 | Year of birth, age,
marital status, stillbirth, congenital malformations of offspring,
caesarean section in first pregnancy | Up to 37 years | 7 | (18) |
| Vikse et al,
2010 | Norway | Medical Birth
Registry & Norwegian kidney biopsy registry (1988-2005) | RC | 582 | Not specified | <37 weeks | 9.5 | ESRD; identified as
undergoing dialysis or renal transplantation | 13 | Maternal age, eGFR,
proteinuria, diastolic blood pressure, duration of renal disease,
interstitial fibrosis and inflammation | Up to 16 years | 8 | (10) |
| Pariente et
al, 2017 | Israel | Soroka University
Medical Center (1988-2012) | RC | 99,338 | All women with
pregnancies | <37 weeks | 16.4 | CKD; identified by
hospitalization episode | 0.13 | Preeclampsia diabetes
mellitus and indicated preterm delivery | Mean 11.2 years | 7 | (9) |
| Dai et al,
2018 | Canada | Canadian Institute
for Health Information (1993-2002) | RC | 1,598,043 | All women with
pregnancies | NR | 6.3 | ESRD; identified by
hospitalization episode | 0.03 | Maternal age,
region, time period, obesity, preterm delivery, intrauterine death,
fetal distress, placental disorders/abruption, oligohydramnios,
prolonged pregnancy, postpartum haemorrhage, deep vein thrombosis,
cardiac disease, blood transfusion, caesarean delivery | Median 15
years | 8 | (11) |
| Barrett et
al, 2020 | Sweden | Swedish Medical
Birth Register & Swedish Renal Register (1973-2012) | RC | 1,943,716 | All women with
singleton pregnancies | <37 weeks | 8.4 | CKD & ESRD;
identified by ICD codes | CKD: 0.92 ESRD:
0.06 | Maternal age, year
of delivery, country of origin, education level, body mass index,
smoking during pregnancy, gestational diabetes, preeclampsia,
parity, and inter-pregnancy interval | Median 20.6
years | 8 | (17) |
| Goetz et al,
2021 | Germany | AOK
Baden-Wuerttemberg insurance database (2010-2017) | RC | 193,152 | All women with
singleton pregnancies | <37 weeks | 6.6 | CKD; identified by
ICD codes | 0.71 | Maternal age,
diabetes, or gestational diabetes as well as obesity and
dyslipidemia | Mean 5.4 years | 8 | (16) |
| Naz et al,
2021 | Iran | Tehran Lipid and
Glucose Study | RC | 3,035 | All women with at
least one pregnancy | <37 weeks | 6.9 | CKD; identified by
eGFR | 36.5 | Smoking, parity,
age at first delivery, body mass index, educational level,
preeclampsia, and gestational diabetes mellitus | Median 16
years | 7 | (15) |
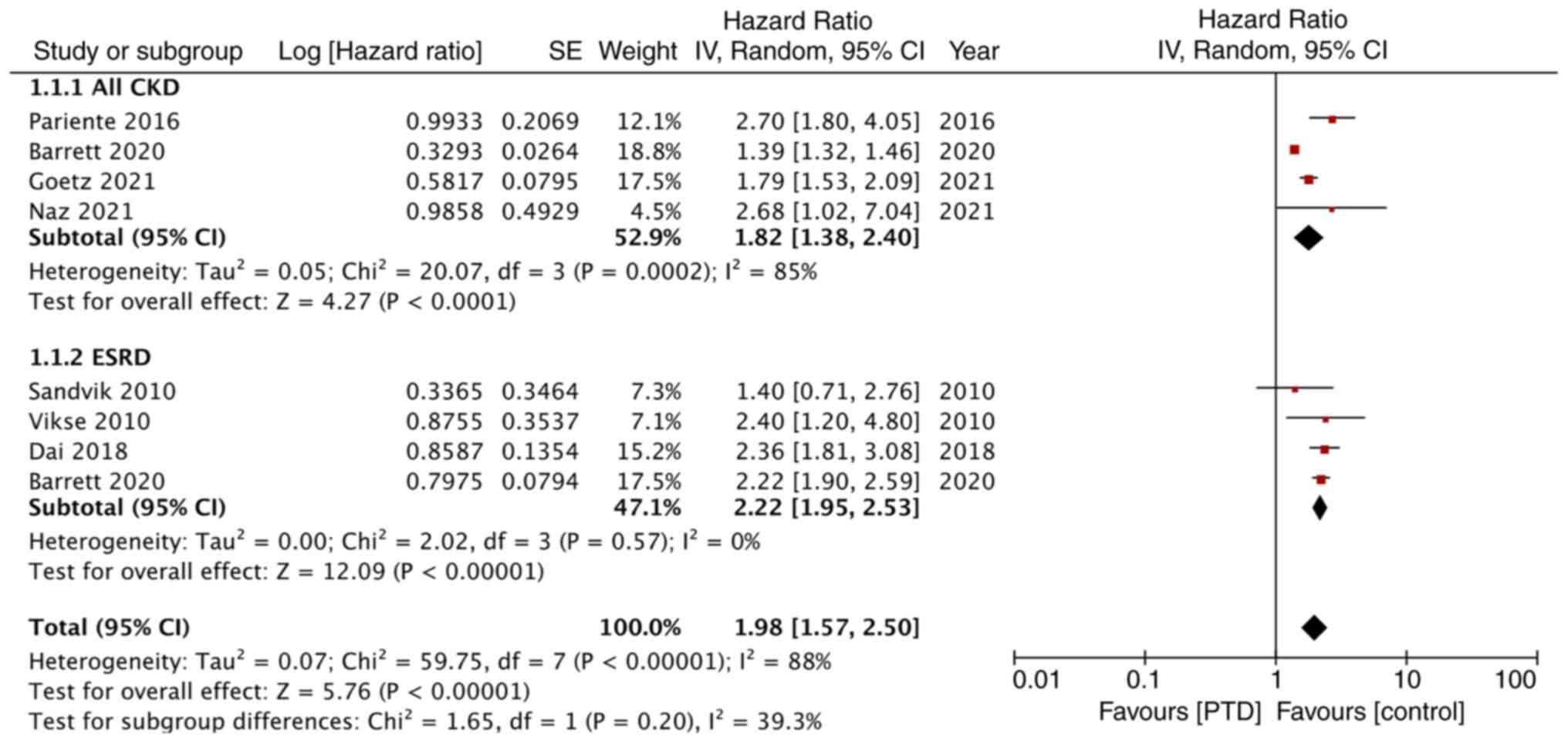
Meta-analysis
The meta-analysis of the association between PTD and
maternal renal disease is shown in Fig. 2. Pooled analysis of 4 studies found
that women with PTD had a statistically significant increased risk
of CKD in the long term (HR: 1.82; 95% CI: 1.38, 2.40;
I2=85%). Similarly, the meta-analysis also revealed a
statistically significant increased risk of ESRD amongst women with
PTD as compared with those without PTD (HR: 2.22; 95% CI: 1.95,
2.53; I2=0%). Overall, the pooled analysis demonstrated
a significantly higher incidence of renal disorders with PTD (HR:
1.98; 95% CI: 1.57, 2.50; I2=88%). There was no gross
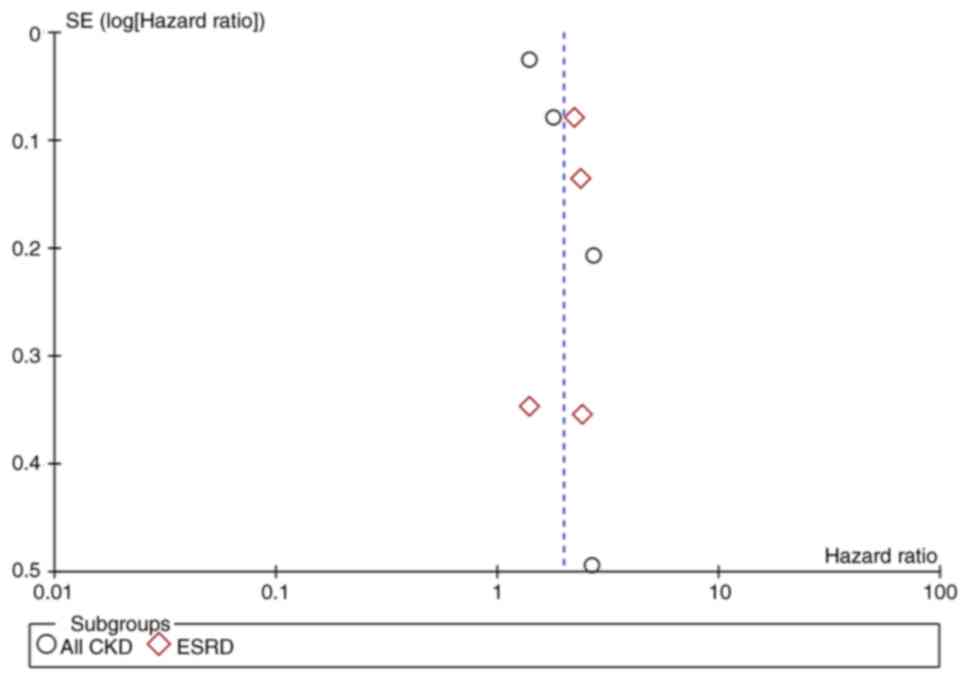
asymmetry on the funnel plot indicating no publication bias
(Fig. 3).
To examine if any study had a major effect on the
outcomes, a sensitivity analysis was performed (Table II). On the exclusion of studies
from the meta-analysis of CKD or ESRD, there was no change in the
significance of the results.
 | Table IISensitivity analysis. |
Table II
Sensitivity analysis.
| A, CKD |
|---|
| Excluded study,
author(s), year | HR (95% CI) | (Refs.) |
|---|
| Pariente et
al, 2017 | 1.61
(1.26-2.06) | (9) |
| Barrett et
al, 2020 | 2.13
(1.54-2.94) | (17) |
| Naz et al,
2021 | 1.77
(1.33-2.35) | (15) |
| Goetz et al,
2021 | 2.01
(1.15-3.51) | (16) |
| B, ESRD |
| Excluded study,
author(s), year | HR (95% CI) | (Refs.) |
| Sandvik et
al, 2010 | 2.26
(1.98-2.58) | (18) |
| Vikse et al,
2010 | 1.96
(1.53-2.49) | (10) |
| Dai et al,
2018 | 1.92
(1.50-2.45) | (11) |
| Barrett et
al, 2020 | 1.93
(1.51-2.45) | (17) |
Discussion
Pregnancy has been often considered as a metabolic
stress test for females which uncovers any intrinsic vascular
disease and endothelial anomalies (19). Indeed, several pregnancy-related
complications including gestational diabetes, hypertensive
disorders of pregnancy and intra-uterine growth restriction have
been linked with adverse long-term maternal cardiovascular
disorders (20). Nevertheless,
little is known about the relationship between adverse pregnancy
outcomes and the future risk of CKD in females. Barrett et
al (21) in a systematic
review and meta-analysis showed that gestational diabetes and
pre-eclampsia are both associated with increased risk of CKD and
ESRD. The same review also examined the relationship between PTD
and the risk of ESRD but could include just 3 studies in the
meta-analysis. In the present study, the authors undertook an
updated literature search and included several new studies to
assess whether PTD is an independent risk factor for maternal CKD
and ESRD.
The results of the current review revealed that
women with PTD have an increased risk of renal disorders later in
life as compared with those delivering at term. It was noted that
PTD increases the risk of CKD by 82% and the risk of ESRD is
increased by an alarming 122%. The results were more or less
consistent across all included studies with only minor differences
in the risk estimates in their respective populations. On the
singular exclusion of one study at a time, the results still
demonstrated a consistently increased risk of CKD and ESRD in the
mother. While the results concurred with the prior review of Barett
et al (21), it has
important differences. The previous studies were solely on ESRD and
included only 3 studies. In the present study, the authors included
4 additional studies and conducted separate analyses for both CKD
and ESRD thereby providing the most recent and comprehensive
evidence on the research question.
Despite several studies showing a positive
association between PTD and CKD/ESRD, the underlying pathological
mechanism is not clearly understood. As both PTD and CKD have
multiple risk factors, the higher risk of CKD with PTD may be
mediated by several shared mechanisms. Catov et al (22) identified that PTD increases the
risk of metabolic syndrome later in life independent of other
pregnancy complications and pre-pregnancy metabolic status.
Metabolic syndrome is a known independent risk factor for CKD which
is primarily mediated via insulin resistance and excess free-fatty
acid production (23). Secondly, a
systemic proinflammatory profile has been associated with an
increased risk of PTD (24).
C-reactive protein, a pro-inflammatory marker is found in increased
quantities in mothers with PTD and the same marker is an
independent predictor of CKD (25,26).
Metabolic diseases such as diabetes, hypertension and obesity; all
of which are associated with a pro-inflammatory phase are known to
increase the risk of PTD (27). In
addition, placental dysfunction is an important component of these
metabolic diseases which increases the risk of PTD (16). It leads to increased release of
proinflammatory and antiangiogenic cytokines which hasten
endothelial dysfunction and systemic atherosclerosis; all of which
result in end-organ dysfunction. Taking into account such common
pathophysiological mechanisms it is tenable that PTD is a result of
the baseline subclinical predisposition to future CKD in women with
a shared risk profile (17).
Moreover, the role of reactive oxygen species, which are
overproduced at the end of the pregnancy and during labor, is
important (28). Oxidative stress
can cause endothelial dysfunction and microvascular damage, leading
to kidney injury, interstitial fibrosis and proteinuria (29).
One of the most important confounders in the
association between PTD and CKD or ESRD can be pre-eclampsia which
is a strong risk factor for the latter (12). PTD is frequently performed as an
iatrogenic procedure for pre-eclampsia and intrauterine growth
restriction and these factors could lead single-handedly to CKD.
Therefore, the next valid question is if the risk of CKD increases
in both spontaneous and iatrogenic PTD. Nevertheless, limited
literature exists to answer this. Barrett et al (17) have revealed that the risk of CKD or
ESRD is increased in both spontaneous and iatrogenic PTD with the
risk being stronger with the latter. In addition, the risk was
still increased based on different gestational ages and even with
very/extreme PTD. Similar results were demonstrated by Pariente
et al (9) who reported
significantly increased risk in both spontaneous and induced
PTD.
There are limitations to the present meta-analysis.
Foremost, the retrospective and database-derived nature of the data
is prone to selection bias and data-entry errors. Secondly, the
high heterogeneity of the meta-analysis of CKD is a cause of
concern, and hence results may be cautiously interpreted. The
scarce number of studies in the meta-analysis also prevented any
detailed subgroup analysis and meta-regression. Thirdly, only one
study estimated the outcome by actual measurements of eGFR. The
remaining studies used ICD codes or identified patients with renal
hospitalization episodes. The latter may be an important source of
bias affecting the credibility of results. Furthermore, there was
no assessment of the stage of CKD in the included studies and a
separate analysis was possible only for stage 5 CKD (ESRD). The
distribution of CKD severity and its progression in PTD females
remains unclear. Furthermore, the adjusted confounders were not the
same across studies. Some studies included important factors like
pre-eclampsia and gestational diabetes while others did not.
Several other unknown variables could have been missed and hence
the current measure should not be considered fool-proof. Lastly,
the data were derived from a very limited geographical location and
hence its generalizability is questionable.
Despite these limitations, the present study
provided the best possible evidence on the link between PTD, CKD
and ESRD in females. A detailed and comprehensive search was
undertaken of multiple databases involving two independent
reviewers. Only adjusted measures were used to avoid baseline
confounding. A separate analysis was conducted for CKD and ESRD
with sensitivity analysis to check for outliners.
The current findings have important clinical
implications. The prevalence of CKD is significantly increasing
with stage 3 and higher disease being prevalent in 12% of the
global female population (30).
Given the results of the review, the women's obstetric history
should be an important component during the assessment of CKD.
Female patients with PTD should be counseled and closely monitored
for future risk of CKD to prevent deterioration to ESRD.
Additionally, further robust studies which take into account the
limitations of the current literature are needed to enhance the
quality of evidence and to identify risk-reducing
interventions.
Women with PTD could be at increased risk of future
CKD and ESRD. The limited number of studies and retrospective
nature of data are important limitations. Further studies are
needed to supplement the available evidence.
Supplementary Material
Search strategy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WW conceived and designed the study. YC, XZ, QZ and
QS collected the data and performed the literature search. QZ and
QS conducted the meta-analysis. WW was involved in the writing of
the manuscript. All authors have read and approved the final
manuscript. YC and XZ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Walani SR: Global burden of preterm birth.
Int J Gynaecol Obstet. 150:31–33. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Goldenberg RL, Culhane JF, Iams JD and
Romero R: Epidemiology and causes of preterm birth. Lancet.
371:75–84. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Romero R, Espinoza J, Kusanovic JP, Gotsch
F, Hassan S, Erez O, Chaiworapongsa T and Mazor M: The preterm
parturition syndrome. BJOG. 113 (Suppl 3):S17–S42. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Perng W, Stuart J, Rifas-Shiman SL,
Rich-Edwards JW, Stuebe A and Oken E: Preterm birth and Long-Term
maternal cardiovascular health. Ann Epidemiol. 25:40–45.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Catov JM, Lewis CE, Lee M, Wellons MF and
Gunderson EP: Preterm birth and future maternal blood pressure,
inflammation, and intimal-medial thickness: The CARDIA study.
Hypertension. 61:641–646. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tanz LJ, Stuart JJ, Williams PL, Missmer
SA, Rimm EB, James-Todd TM and Rich-Edwards JW: Preterm delivery
and maternal cardiovascular disease risk factors: The nurses'
Health Study II. J Womens Health (Larchmt). 28:677–685.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen TK, Knicely DH and Grams ME: Chronic
kidney disease diagnosis and management: A review. JAMA.
322:1294–1304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
O'connor NR and Corcoran AM: End-stage
renal disease: Symptom management and advance care planning. Am Fam
Physician. 85:705–710. 2012.PubMed/NCBI
|
|
9
|
Pariente G, Kessous R, Sergienko R and
Sheiner E: Is preterm delivery an independent risk factor for
long-term maternal kidney disease? J Matern Fetal Neonatal Med.
30:1102–1107. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vikse BE, Hallan S, Bostad L, Leivestad T
and Iversen BM: Previous preeclampsia and risk for progression of
biopsy-verified kidney disease to end-stage renal disease. Nephrol
Dial Transplant. 25:3289–3296. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dai L, Chen Y, Sun W and Liu S:
Association between hypertensive disorders during pregnancy and the
subsequent risk of end-stage renal disease: A Population-Based
Follow-Up study. J Obstet Gynaecol Can. 40:1129–1138.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Oishi M, Iino K, Tanaka K, Ishihara K,
Yokoyama Y, Takahashi I and Mizunuma H: Hypertensive disorders of
pregnancy increase the risk for chronic kidney disease: A
population-based retrospective study. Clin Exp Hypertens.
39:361–365. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. Int J Surg. 88(105906)2021.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Wells G, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses. Sci Educa: Jan, 2000.
|
|
15
|
Saei Ghare Naz M, Rahmati M, Azizi F and
Ramezani Tehrani F: Risk of chronic kidney disease in women with a
history of preterm delivery: Tehran lipid and glucose study. J
Nephrol. 34:1621–1629. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Goetz M, Müller M, Gutsfeld R, Dijkstra T,
Hassdenteufel K, Brucker SY, Bauer A, Joos S, Colombo MG,
Hawighorst-Knapstein S, et al: An observational claims data
analysis on the risk of maternal chronic kidney disease after
preterm delivery and preeclampsia. Sci Rep.
11(12596)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Barrett PM, McCarthy FP, Evans M,
Kublickas M, Perry IJ, Stenvinkel P, Kublickiene K and Khashan AS:
Risk of Long-Term renal disease in women with a history of preterm
delivery: A population-based cohort study. BMC Med.
18(66)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sandvik MK, Iversen BM, Irgens LM,
Skjaerven R, Leivestad T, Søfteland E and Vikse BE: Are adverse
pregnancy outcomes risk factors for development of end-stage renal
disease in women with diabetes? Nephrol Dial Transplant.
25:3600–3607. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rangaswami J, Naranjo M and Mccullough PA:
Preeclampsia as a form of type 5 cardiorenal syndrome: An
Underrecognized Entity in Women's Cardiovascular Health.
Cardiorenal Med. 8:160–172. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
O'Kelly AC, Michos ED, Shufelt CL, Vermunt
JV, Minissian MB, Quesada O, Smith GN, Rich-Edwards JW, Garovic VD,
El Khoudary SR and Honigberg MC: Pregnancy and reproductive risk
factors for cardiovascular disease in women. Circ Res. 130:652–672.
2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Barrett PM, McCarthy FP, Kublickiene K,
Cormican S, Judge C, Evans M, Kublickas M, Perry IJ, Stenvinkel P
and Khashan AS: Adverse pregnancy outcomes and Long-Term maternal
kidney disease: A systematic review and Meta-analysis. JAMA Netw
Open. 3(e1920964)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Catov JM, Althouse AD, Lewis CE, Harville
EW and Gunderson EP: Preterm delivery and metabolic syndrome in
women followed from prepregnancy through 25 years later. Obstet
Gynecol. 127:1127–1134. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Laguardia HA, Hamm LL and Chen J: The
metabolic syndrome and risk of chronic kidney disease:
Pathophysiology and intervention strategies. J Nutr Metab.
2012(652608)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rodie VA, Freeman DJ, Sattar N and Greer
IA: Pre-eclampsia and cardiovascular disease: Metabolic syndrome of
pregnancy? Atherosclerosis. 175:189–202. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Banaem LM, Mohamadi B, Jaafarabadi MA and
Moghadam NA: Maternal serum C-reactive protein in early pregnancy
and occurrence of preterm premature rupture of membranes and
preterm birth. J Obstet Gynaecol Res. 38:780–786. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kugler E, Cohen E, Goldberg E, Nardi Y,
Levi A and Krause I, Garty M and Krause I: C reactive protein and
long-term risk for chronic kidney disease: A historical prospective
study. J Nephrol. 28:321–327. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Berger H, Melamed N, Davis BM, Hasan H,
Mawjee K, Barrett J, McDonald SD, Geary M and Ray JG: Impact of
diabetes, obesity and hypertension on preterm birth:
Population-based study. PLoS One. 15(e0228743)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Menon R: Oxidative stress damage as a
detrimental factor in preterm birth pathology. Front Immunol.
5(567)2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Duni A, Liakopoulos V, Roumeliotis S,
Peschos D and Dounousi E: Oxidative stress in the pathogenesis and
evolution of chronic kidney disease: Untangling Ariadne's Thread.
Int J Mol Sci. 20(3711)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hill NR, Fatoba ST, Oke JL, Hirst JA,
O'Callaghan CA, Lasserson DS and Hobbs FD: Global prevalence of
chronic kidney Disease-A systematic review and meta-analysis. PLoS
One. 11(e0158765)2016.PubMed/NCBI View Article : Google Scholar
|