Introduction
An unapproved prescription describes the provision
of a medicine without marketing authorization for human use,
whereas an off-label prescription describes the use of an
authorized drug outside of the recommended age group, indication,
dosage, route of administration or frequency of use (1). Pharmaceutical trials of medicines
that are not tested in children may result in off-label or
unapproved drug prescriptions provided to children (2). Despite legislative enforcement on
authorized drugs, approved pediatric formulations are ineffective
for the pharmaceutical industry (3). A function of the Mutual Recognition
Agreements signed by The European Union (EU) and third-country
authorities is to assess drug safety and efficacy of medicines
prior to public release (4).
However, testing of drug safety and efficacy in children raises
ethical concerns, and therefore, the number of pediatric clinical
trials is limited (5,6). This may lead to clinicians
prescribing drugs to children using data from adult clinical
trials, which could lead to increased numbers of unapproved and
off-label prescriptions provided to children (7). Off-label and unauthorized
prescriptions present significant risks to children (8). However, a previous study reported
that, within a 2-month period of observation, the prevalence of
off-label and unlicensed drug use was increased in both pediatric
intensive care units (PICUs) and neonatal intensive care units
(NICUs), compared with a study at the same location 16 years prior,
despite the anticipated impact of current regulatory efforts
(9).
As of early 1937, 353 patients were exposed to
Elixir sulfanilamide. Nearly one-third of them, 34 children and 71
adults, soon died of acute kidney failure (10). A total of 25 years later, use of
the over-the-counter sleeping pill thalidomide was reported to
induce teratogenesis in consumers in America, Australia and Europe
(11). Furthermore, in 1971,
diethylstilbestrol (a non-steroidal estrogen medication), 30 years
after its approval by the Food and Drug Administration, was
discontinued due to its association with breast cancer (12). These occurrences necessitated
stringent drug regulations to ensure efficacy of approved drugs and
safeguard the health of their users. The prescription of off-label
and unlicensed drugs to children may potentially be associated with
toxicity and adverse drug effects (13). A specific condition that may be
associated with the use of off-label and unlicensed drugs in
children is bipolar depression in depressive episodes. Currently
available antidepressants in children are unsatisfactory; for
example, a number of systematic reviews have indicated that
antidepressant methylphenidate and memantine have high non-response
rates and non-tolerance (14).
High non-response rates and non-tolerance in children are also
associated with second-generation antipsychotic drugs such as
amisulpride, clozapine, olanzapine and risperidone (15).
Differences in use of off-label and unauthorized
prescriptions between countries may be attributed to varying
stringency of drug regulatory authorities and pharmaceutical
spending budgets, which are associated with gross domestic product
(GDP) (16). The lack of
pediatric-specific medications may contribute to off-label and
unlicensed prescriptions. Multi-collaborative efforts involving
researchers, medical staff, industry regulators and policy makers
may be required to establish pediatric formulations in the future.
To the best of our knowledge, this is the first study to estimate
the global pooled prevalence of off-label and unlicensed
prescriptions given to hospitalized children in NICUs, PICUs or
standard pediatric wards, using meta-analysis.
Materials and methods
Literature search strategy
The present systematic review adhered to the
preferred reporting items for systematic reviews and meta-analyses
(PRISMA) guidelines (17). A
comprehensive literature search was conducted on January 12, 2023
using the PubMed (https://pubmed.ncbi.nlm.nih.gov/), Scopus (https://www.elsevier.com/zh-cn/products/scopus/search),
Web of Science (https://www.webofscience.com/wos) and Google Scholar
databases (https://scholar.google.com/) for articles published
between 1990 and 2023. Medical subject headings and key word
queries were used across all databases. The PubMed database was
searched using the key words, ‘off label use’ and ‘prevalence’, in
combination with ‘children’. The other databases used queries such
as ‘prevalence’, ‘off label drug use’ or ‘unlicensed drug use’,
alongside ‘children’, with the language preference set to English
and document type set to articles. Only observational studies that
reported prevalence of off-label or unlicensed prescribing in
hospitalized pediatric patients and published in English were
included. Reviews, duplicates and non-full text articles were
excluded.
Data extraction
Data search and extraction was conducted by two
independent researchers using EndNote (version X9; Clarivate Plc.).
The researchers independently selected the most relevant articles
using the titles and abstracts of articles. Full-text articles were
selected and assessed for eligibility. An independent third party
arbitrated discrepancies. Data were extracted from each article
using a standardized form with the following fields: Author, year,
country, mean age, prevalence of off-label and unlicensed drug use,
study design, sample size, study setting, total prescriptions,
prescription drugs, and off-label and unlicensed prescriptions.
Risk of bias and quality
assessment
The Joanna Briggs Institute critical assessment
checklist (18) was used to assess
the quality of included studies, which included the likelihood of
bias in design, implementation and analysis (Table SI). The checklist has nine
questions, answers to which are either yes, no, unclear or not
applicable. High quality studies achieve a minimum of 5 affirmative
responses.
Statistical analysis
The primary outcome of the present study was
prevalence of off-label and unlicensed prescriptions to children.
The following analyses were conducted: Pooled prevalence of
off-label prescriptions among i) ICU and ii) general pediatric ward
patients, and pooled prevalence of unlicensed prescriptions among
iii) ICU and iv) general pediatric ward patients. The prevalence of
off-label or unlicensed prescriptions was calculated by dividing
the number of off-label or unlicensed prescriptions by total number
of prescriptions. STATA (version 16; StataCorp LP) software was
used to calculate SE and CI values, and for forest map generation.
The random-effects model was used regardless of the outcome of the
heterogeneity analysis. I2>50% was considered to
indicate high heterogeneity. Subgroup analysis was conducted using
data from the International Monetary Fund on global GDP
distribution (16). Sensitivity
analysis was performed by the exclusion of individual studies one
at a time and those of insufficient quality were excluded. Egger's
test and funnel plots were used to evaluate publication bias. The
χ2 test was used for categorical data. P<0.05 was
considered to indicate a statistically significant difference.
Results
Literature search and screen
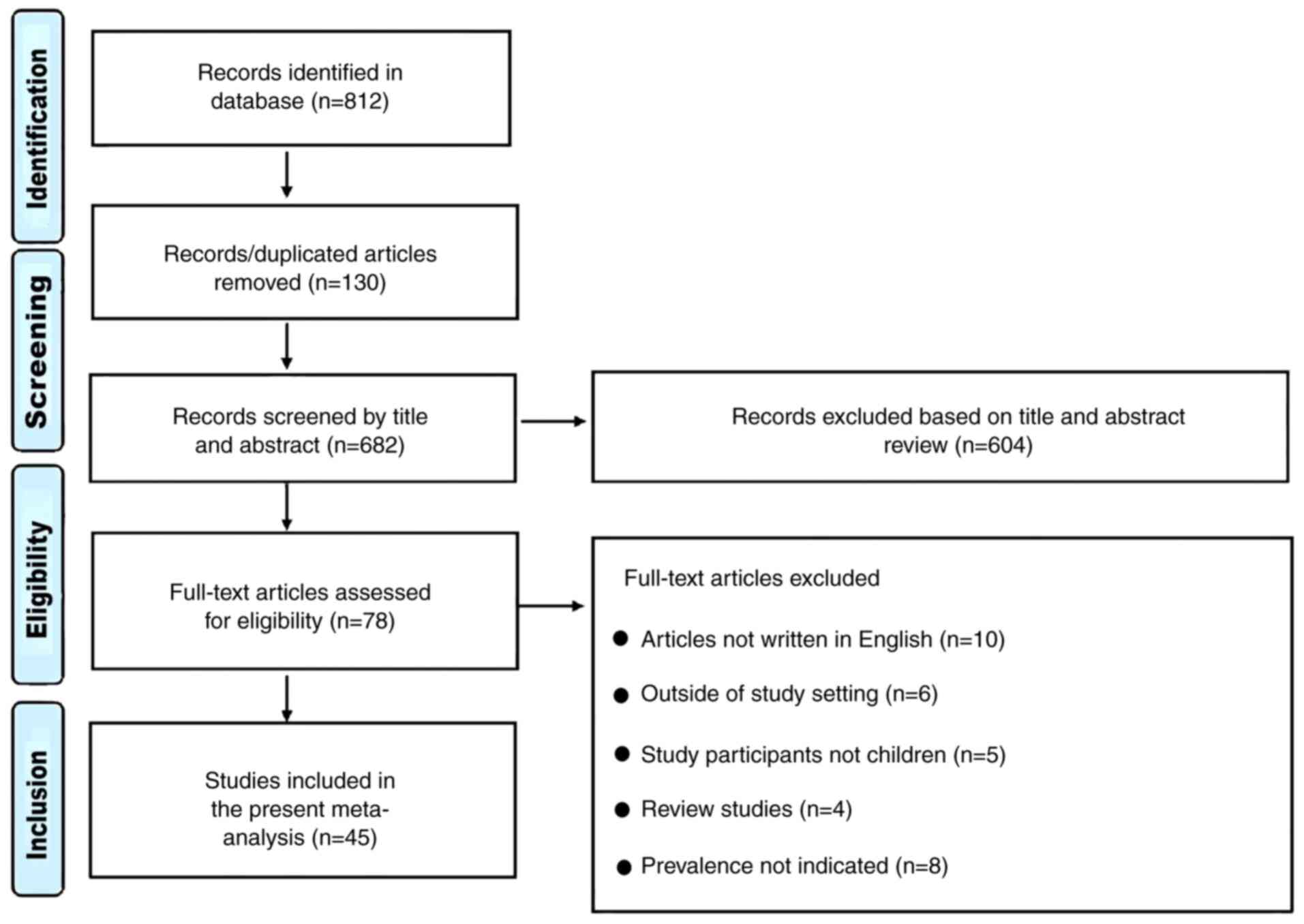
A total of 812 articles were examined, of which 130
duplicates were excluded (Fig. 1).
Subsequently, 604 articles were excluded following examination of
the title, full text and abstract, which left 78 studies. Further
exclusions included 10 studies that were not written in English, 8
that did not report on prevalence, 6 studies that were conducted
outside the NICU, PICU or general pediatric ward, 4 reviews and 5
studies that involved mixed participants (adults and children). A
total of 45 studies were included in the present study (9,10,19-61).
Study characteristics
The 45 articles included in the meta-analysis
included 26 prospective, 7 retrospective and 12 cross-sectional
studies (Table I). Patient sample
size varied from 40 to 13,426, with a range of 240-8,891
prescriptions issued. Of the 45 studies, 22 originated from Europe,
13 from Asia, three each from South America and Africa and 2 each
from North America and Australia. Off-label prevalence was reported
in all articles (10.0-87.0%), while 34 studies reported the
prevalence of unlicensed prescriptions (1.3-79.0%). In total, 34
(75.6%) studies reported prevalence of both off-label and
unlicensed medication prescriptions among participants. All studies
recruited participants from either the NICU, PICU or general ward,
with 23 studies conducted in general pediatric wards. The highest
prevalence of off-label drug use was reported in Estonia (87%),
followed by Ethiopia (75%). The lowest off-label prevalence was in
Italy (9%), followed by Zimbabwe (10%). The highest unlicensed drug
prevalence was reported in Indonesia (79.0%).
 | Table ICharacteristics of included
studies. |
Table I
Characteristics of included
studies.
| | Prevalence, % | | Number | |
|---|
| First author,
year | Country of
residence | Patient age | Off-label | Unlicensed | Study design | No. of
patients | Study setting | Total
prescriptions | Off-label and
unlicensed | (Refs.) |
|---|
| Turner et
al, 1999 | UK | 1.0 ya | 35.3 | - | Prospective | 936 | PICU | 4,455 | 1,574 | (19) |
| Conroy et
al, 1999 | UK | 26.0-36.0 w | 54.7 | 9.9 | Prospective | 70 | NICU | 455 | 294 | (20) |
| Pandofini et
al, 2002 | Italy | 3.7 yb | 60.0 | - | Prospective | 1,461 | General ward | 4,255 | 2,547 | (21) |
| Barr et al,
2002 | Israel | <28.0 d | 59.0 | 16.0 | Prospective | 105 | NICU | 525 | 397 | (22) |
| Jong et al,
2002 | Netherlands | 16.7 ya | 43.0 | 28.0 | Prospective | 293 | General ward | 1,017 | 728 | (23) |
| Conroy et
al, 2003 | UK | - | 54.6 | - | Prospective | 51 | PICU | 1,574 | 859 | (24) |
| Jong et al,
2004 | UK | 8.7 yb | 20.3 | 16.8 | Retrospective | 13,426 | General ward | 5,253 | 1,947 | (25) |
| Neubert et
al, 2004 | Germany | <18.0 y | 26.4 | 1.3 | Prospective | 178 | General ward | 740 | 198 | (26) |
| Bajcetic et
al, 2005 | Belgrade | 4.0 h -18.0 y | 48.0 | 11.0 | Prospective | 544 | General ward | 2,037 | 1,202 | (27) |
| Di Paolo et
al, 2006 | Sweden | 3.0-14.0 y | 25.0 | 24.0 | Prospective | 60 | General ward | 483 | 236 | (28) |
| Kaisi et al,
2007 | Zimbabwe | 1.0-5.0 y | 10.0 | 31.0 | Prospective | 300 | General ward | 300 | 123 | (29) |
| Santos et
al, 2008 | Brazil | 2.0 yb | 39.6 | 55.0 | Prospective | 272 | General ward | 1,450 | 623 | (30) |
| Bavdekar et
al, 2009 | India | 3.6±3.7
yb | 70.6 | - | Prospective | 300 | PICU | 2,237 | 1,579 | (31) |
| Lass et al,
2011 | Estonia | <28.0 d | 87.0 | - | Prospective | 490 | NICU | 1,981 | 1,723 | (32) |
| Palčevski et
al, 2012 | Croatia | 6.8 mb | 13.3 | 11.9 |
Cross-sectional | 691 | General ward | 1,643 | 412 | (33) |
| Oguz et al,
2012 | Turkey | 32.5±4.7
wb | 33.5 | 28.8 | Prospective | 464 | NICU | 1,315 | 819 | (34) |
| Ballard et
al, 2013 | Australia | 2.6 yb | 36.0 | - | Retrospective | 300 | General ward | 887 | 283 | (35) |
| Kieran et
al, 2014 | Ireland | 35.0 wb | 39.0 | 19.0 | Prospective | 110 | NICU | 900 | 522 | (36) |
| Silva et al,
2015 | Portugal | 36.1±4.0
wb | 27.9 | 4.4 |
Cross-sectional | 218 | NICU | 1,011 | 326 | (37) |
| Lee et al,
2013 | Malaysia | 2.0 yb | 34.1 | 27.3 | Prospective | 168 | PICU | 1,295 | 795 | (38) |
| Ribeiro et
al, 2013 | Portugal | 6.2±4.9
yb | 32.2 | - | Retrospective | 700 | General ward | 724 | 233 | (39) |
| Lindell-Osuagwu
et al, 2014 | Finland | 1.0-12.0 y | 51.0 | - | Prospective | 123 | NICU | 1,054 | 538 | (40) |
| Laforgia et
al, 2014 | Italy | 37.0 wb | 37.4 | 11.4 |
Cross-sectional | 126 | NICU | 483 | 236 | (41) |
| Langerová et
al, 2014 | Italy | 1.0-15.0
yb | 9.0 | 1.3 | Retrospective | 4,282 | General ward | 8,559 | 879 | (42) |
| Luedtke et
al, 2014 | USA | 3.0 w-15.0 y | 57 | - | Prospective | 40 | General ward | 240 | 136 | (43) |
| Riou et al,
2015 | France | 34.0 wb | 59.5 | 5.2 | Prospective | 910 | NICU | 8,891 | 5,752 | (44) |
| Joret-Descout et
al, 2015 | France | 5.1 yb | 36.5 | 3.2 |
Cross-sectional | 120 | General ward | 315 | 125 | (45) |
| Jobanputra et
al, 2015 | India | 6.3±1.7
wb | 41.3 | 21.0 | Prospective | 482 | PICU | 1,789 | 1,114 | (46) |
| Berdkan et
al, 2016 | Lebanon | 3.5 yb | 30.2 | 11.1 | Retrospective | 500 | PICU | 2,054 | 848 | (47) |
| Cuzzolin et
al, 2016 | Italy | 3.3 wb | 59.0 | 14.5 |
Cross-sectional | 220 | NICU | 720 | 529 | (48) |
| Corny et al,
2016 | Canada | 10.9 yb | 38.2 | 8.3 |
Cross-sectional | 308 | General ward | 2,145 | 997 | (49) |
| Ramadaniati et
al, 2017 | Indonesia | 2.0 yb | 50.8 | 15.1 | Retrospective | 67 | General ward | 1,553 | 1,023 | (50) |
| Tefera et
al, 2017 | Ethiopia | 4.5±4.3
yb | 75.8 | - | Prospective | 243 | General ward | 800 | 607 | (51) |
| Teigen et
al, 2017 | Norway | 0.0-17.0 y | 44.0 | 26.0 |
Cross-sectional | 179 | General ward | 930 | 650 | (52) |
| Nir-Neuman et
al, 2018 | Israel | 33.0-38.0 w | 64.8 | 5.9 | Prospective | 134 | NICU | 1,069 | 756 | (9) |
| Costa et al,
2018 | Brazil | 2.4±4.4
wb | 49.3 | 24.6 | Prospective | 220 | NICU | 17,421 | 12,869 | (53) |
| Mazhar et
al, 2018 | Saudi Arabia | 12.0 db | 29.7 | 12.9 | Prospective | 138 | NICU | 583 | 248 | (54) |
| Aamir et al,
2018 | Pakistan | 8.0±18.5
db | 52.1 | 33.4 | Prospective | 1,300 | General ward | 3,448 | 2,948 | (55) |
| Landwehr et
al, 2019 | Australia | 6.0±4.7
yb | 54.0 | 1.6 |
Cross-sectional | 190 | General ward | 1,160 | 644 | (10) |
| Dornelles et
al, 2019 | Brazil | 18.0 mb | 44.6 | 27.0 | Prospective | 157 | PICU | 1,328 | 951 | (56) |
| Kouti et al,
2019 | Iran | 34.0±4.4
wb | 38.1 | 1.9 |
Cross-sectional | 193 | NICU | 1,049 | 420 | (57) |
| Tukayo et
al, 2020 | Indonesia | 1.8 yb | 71.5 | 79.0 |
Cross-sectional | 200 | General ward | 1,961 | 1,557 | (58) |
| Gidey et al,
2020 | Ethiopia | 0.0-28.0 d | 67.6 | 23.6 |
Cross-sectional | 122 | NICU | 366 | 334 | (59) |
| García-López et
al, 2020 | Italy | 9.0 mb | 39.6 | 12.9 |
Cross-sectional | 85 | General ward | 1,198 | 630 | (60) |
| AlAzmi et
al, 2021 | Saudi Arabia | 4.0 yb | 39.4 | - | Retrospective | 326 | General ward | 865 | 341 | (61) |
Overall pooled prevalence of off-label
and unlicensed drug use
A total of 45 articles were included in the
meta-analysis, which involved 60,997 off-label and unlicensed drugs
prescribed to patients in the NICU, PICU or general wards. Due to
the number of study designs used, a random-effect meta-analysis
model was used to assess total number of prescriptions, and
off-label and unlicensed prescriptions issue. The global prevalence
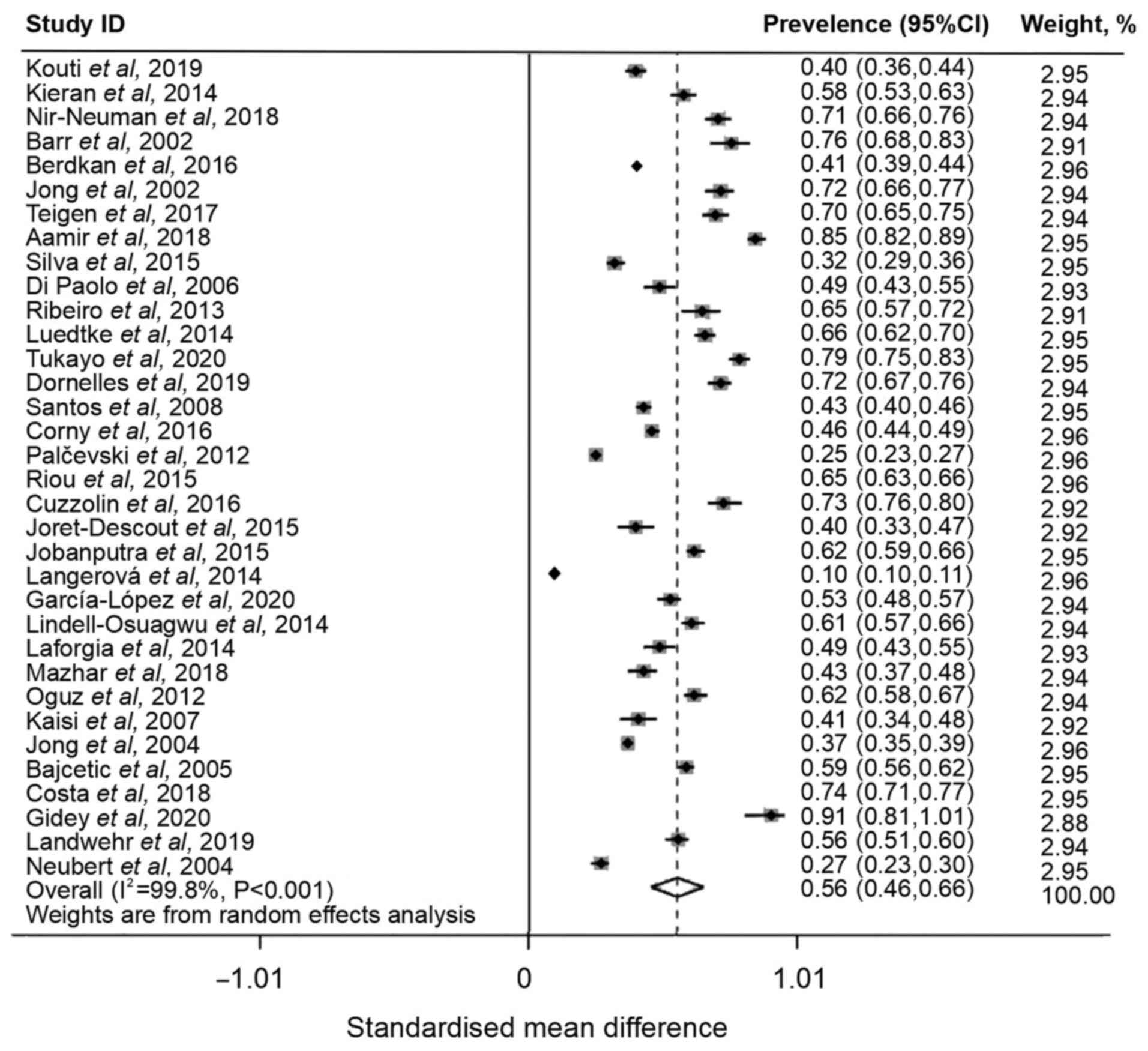
of off-label and unlicensed prescriptions among pediatric or
neonatal patients was 56% (95% CI, 0.46-0.66), with high
heterogeneity (I2=99.8%; Fig. 2). As significant heterogeneity was
observed, sensitivity analysis was performed. Sensitivity analysis
indicated that the pooled prevalence remained notably stable upon
exclusion of each study (Fig.
S1). Sensitivity analysis was performed to assess the impact of
excluding studies that had a small sample size of prescriptions.
Following the exclusion of 12 studies that had <1,000
prescriptions, the overall combined prevalence decreased to 55% and
demonstrated high heterogeneity (I2=99.4%; Figs. S2 and S3). The exclusion of articles with a
small sample size did not significantly alter the global prevalence
of off-label and unlicensed drug prescriptions to children. The
high heterogeneity of included studies may be due to methodological
differences of study designs. The exclusion of studies with a high
risk of bias did not significantly alter the prevalence rates of
off-label and unlicensed drug prescriptions (Fig. S1, Fig. S2 and Fig. S3) to children, as calculated from
the sensitivity analysis .
Overall pooled prevalence of off-label
or unlicensed drug use among pediatric patients
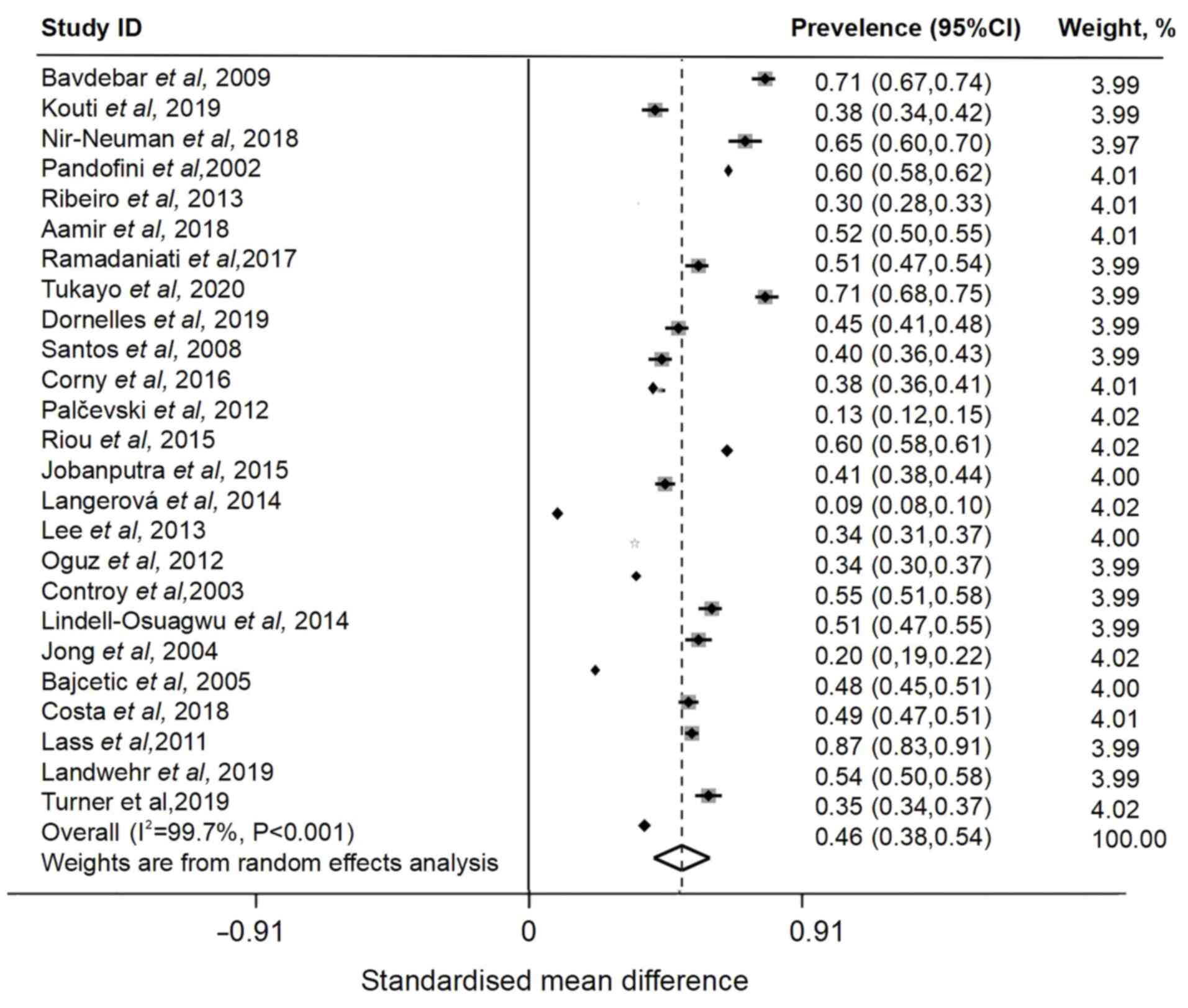
The prevalence of off-label prescriptions given to
admitted pediatric patients was 46% (95% CI, 0.38-0.54), which was
decreased compared with overall prevalence and had high
heterogeneity (I2=99.7%) (Fig. 3). Of 45 included articles, 34
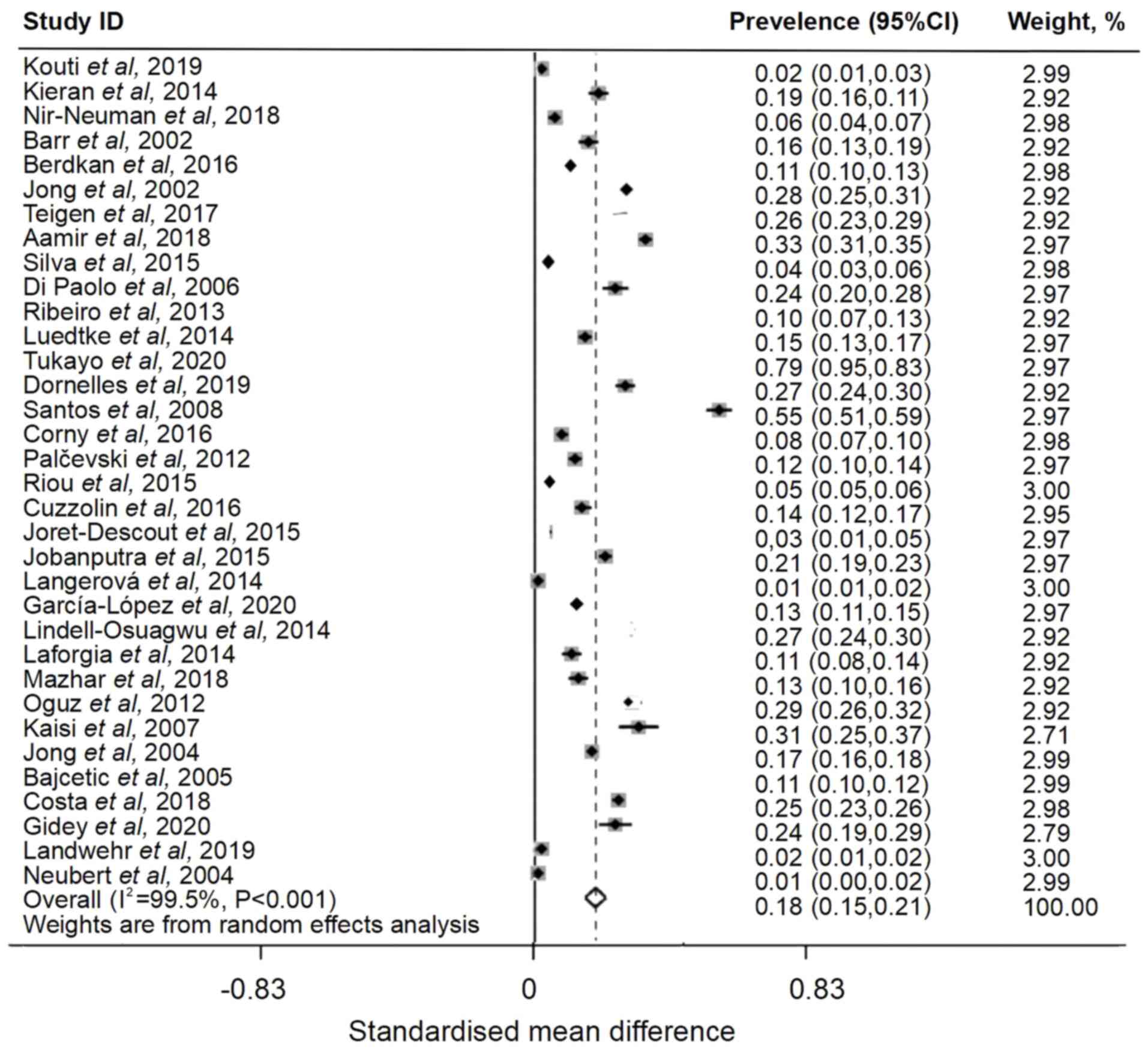
reported unlicensed drug usage in hospitalized children. A total of
10,126 prescriptions were unlicensed in the included articles. The
global pooled prevalence rate was 18% (I2=99.5%; 95% CI,
0.15-0.21; Fig. 4).
Subgroup analysis of prevalence of
off-label and unlicensed drug use according to the continent of
study
The subgroup analysis determined the combined
prevalence of off-label and unlicensed drugs used on each
continent. Africa had the highest prevalence rate at 66% (95% CI,
0.17-1.15), followed by Asia at 65% (95% CI, 0.52-0.78), South
America at 63% (95% CI, 0.42-0.84), North America at 56% (95% CI,
0.36-0.76), Australia at 56% (95% CI, 0.51-0.60) and Europe at 49%
(95% CI, 0.36-0.62) (Table
SII).
Subgroup analysis showed that Australia had the
highest prevalence of off-label drug use among pediatric patients
at 54% (95% CI, 0.50-0.58), followed by Asia at 52% (95% CI,
0.42-0.63), South America at 45% (95% CI, 0.39-0.50), Europe at 42%
(95% CI, 0.29-0.55) and North America at 38% (95% CI, 0.36-0.41)
(Table SII). The prevalence of
unlicensed medication prescriptions among pediatric patients in
South America was the highest at 36% (95% CI, 0.20-0.51), followed
by Africa at 27% (95% CI, 0.20-0.34), Asia at 25% (95% CI,
0.16-0.34), Europe at 12% (95% CI, 0.09-0.15), North America at 8%
(95% CI, 0.06-0.09) and Australia at 2% (95% CI, 0.02-0.03)
(Table SII).
Publication bias
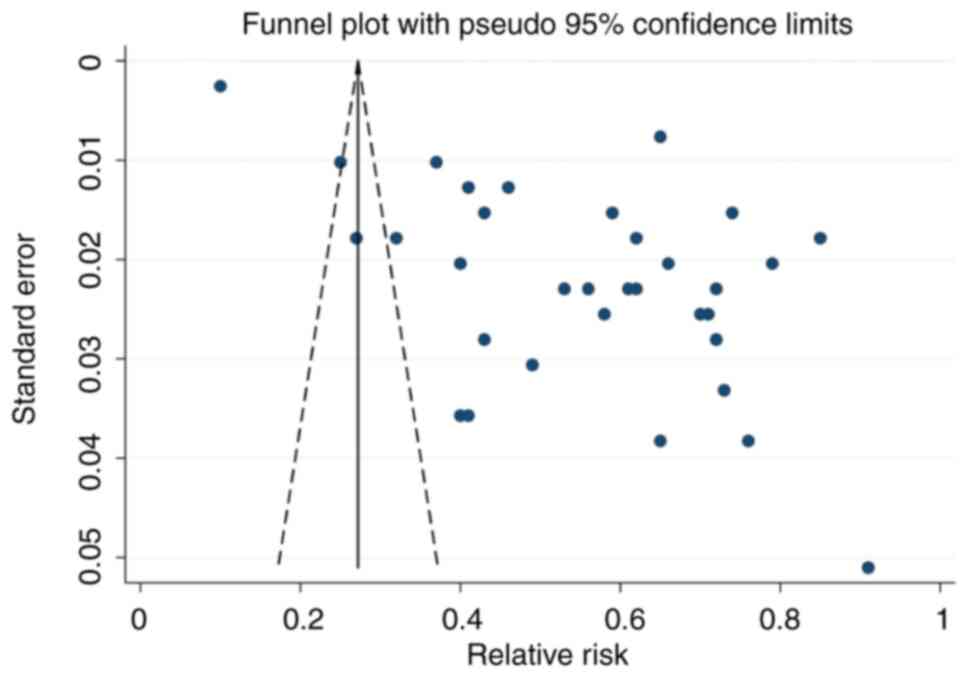
Funnel plot analysis indicated notable publication
bias based on the asymmetric shape of the funnel (Fig. 5). The range of effect sizes for
most studies was between 0.40 and 0.80, with a low standard error.
The regression-based Egger's test was significant (P=0.017), which
indicated heterogeneity and publication bias (Fig. S4). Non-parametric trim-and-fill
analysis identified and corrected funnel plot asymmetries in
studies reporting off-label and unlicensed prescribing. Despite a
degree of publication bias, the results of the present study could
still be considered robust.
Discussion
As available standardized pediatric prescription
data are limited, off-label and unlicensed medications are
frequently prescribed to pediatric patients. The prescription of
off-label and unlicensed drugs has been escalating among children
recently, and current regulations for the use of prescription
medications are considered ineffective in the pharmaceutical
industry (62). The present study
aimed to assess the global prevalence of off-label and unlicensed
drug prescriptions given to hospitalized children through
meta-analysis. All prescriptions in the included studies originated
from data obtained from patients that were admitted to the NICU,
PICU or general ward. Of 45 studies, 34 reported the prevalence of
prescriptions of both off-label and unlicensed medication given to
children. The overall global prevalence of off-label and unlicensed
medications among patients in the PICU or NICU was 56%. The highest
prevalence was in Asia (62%), followed by South America (53%) and
Europe (29.9%).
Following the exclusion of studies following
sensitivity analysis, the combined prevalence of off-label and
unlicensed medication use was 55% compared with an overall global
prevalence of 56% in all included studies. A previous meta-analysis
of 6 studies reported that off-label prescriptions constituted
93.5% of all medications prescribed (63); however, the incidence of unlicensed
medications was only 3.9%. A large proportion of medications were
prescribed to children <2 years of age in the aforementioned
meta-analysis (63). The present
study demonstrated that the prevalence of off-label prescriptions
given to admitted pediatric patients was 46% and that of unlicensed
prescriptions was 18%. A previous study demonstrated that the
frequency of off-label medication use to children was 14.5-35%,
although no meta-analysis or statistical analysis was performed
(64). Differences between the
aforementioned study and the present study may be attributed to
different sample sizes and examination of antipsychotic drugs
specifically in the previous study, whereas the present study
examined all types of prescribed drug. Differences could also be
attributed to differences in drug regulatory standards and
pharmaceutical budgets between different countries (13,14).
Subgroup analysis according to continent of study indicated the
overall pooled prevalence of off-label and unlicensed drugs used
Africa had the highest prevalence rate at 66%, followed by Asia at
65%, South America at 63%, North America at 56%, Australia at 56%
and Europe at 49%. As high heterogeneity was demonstrated across
the studies in the present meta-analysis, further studies on the
prevalence of off-label and unlicensed prescriptions to
hospitalized children are necessary.
A standardized definition of off-label and
unlicensed medications may be required to accurately assess their
frequency of use, risk factors and impact. Further prospective
clinical studies should evaluate the efficacy and safety of these
medications in the pediatric population. The prescription of
off-label and unlicensed drugs in pediatric primary healthcare
necessitates further investigation of their use by regulatory
agencies and the pharmaceutical industry. The elimination of the
prescription of off-label and unlicensed drugs may potentially
increase drug safety in children. In the future, clinicians could
report suspected adverse drug reactions, such as unipolar
depression, to relevant authorities to increase drug safety in
children.
The prescription of off-label and unlicensed drugs
may expose children to drug toxicity and adverse drug effects, due
to the pressure on clinicians to treat critically ill neonatal
patients. In addition to empirical treatment methods (the doctor's
treatment experience in the course of use), implementation of
enhanced safe drug usage is imperative. Collaborative engagement
between the pharmaceutical industry, clinical academics, healthcare
professionals, parents and national regulatory bodies is key to
prevent children from becoming 'therapeutic orphans'. To decrease
the risk of drug misuse in the pediatric population, pharmacists
should review medication charts. This, as well as drug labeling
(including patient and physician instructions), is an effective
method to avoid drug misuse (65).
Strict supervision of drug administration can also avoid adverse
drug reactions. To develop pediatric formulations,
multi-collaborative efforts of researchers, medical staff, industry
regulators and policymakers are required. Further evidence to
support the safety and efficacy of off-label drug prescriptions to
children and communication with legal guardians about proposed
treatment is essential in the future.
The present meta-analysis analyzed studies that
reported on the prevalence of off-label and unlicensed
prescriptions among pediatric patients, and had varying study
sample sizes, durations and designs. Different countries may
require distinct licensing information and drug package-leaflet
data. Additionally, only 2 studies each originated from North
America and Australia, which limited the scope of the present
study; therefore further research on this topic in North America
and Australia is warranted. These limitations potentially
contributed to the heterogeneity observed in the present analysis.
To address this high heterogeneity, the present study investigated
the source of heterogeneity, conducted sensitivity analysis and
used the random-effects model. In addition, PRISMA was used to
minimize reporting bias. To the best of our knowledge, the present
study is the first to report the pooled prevalence of off-label and
unlicensed prescriptions in hospitalized pediatric patients.
In conclusion, the present meta-analysis
demonstrated that the prevalence of off-label and unlicensed
prescriptions among pediatric patients was substantial and varied
across geographical regions. The differences between studies may be
attributed to methodological discrepancies and differences in
licensing and drug leaflet information across countries; therefore,
it could be recommended that drug authorities standardize pediatric
prescription practices in future.
Supplementary Material
Sensitivity analysis indicating that
the pooled prevalence remains notably stable upon exclusion of each
study.
Sensitivity analysis to assess the
impact of excluding studies that had a small sample size of
prescriptions.
Forest plot of prevalence of off-label
and unlicensed prescriptions in a pediatric population after
excluding studies that had a small sample size of
prescriptions.
Regression-based Egger's test
examining off-label and unlicensed prescriptions.
Quality assessment of included
articles using the Joanna Briggs Institute critical assessment
checklist.
Subgroup analysis by study design and
continent.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Research Project
on Traditional Chinese Medicine in Heilongjiang Province (grant no.
ZHY2022-080).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XY, JG and LY curated and analyzed the data. YT and
OB conceived the study and revised the manuscript. OB wrote, edited
and reviewed the manuscript. YT and OB confirm the authenticity of
all the raw data All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moulis F, Durrieu G and Lapeyre-Mestre M:
Off-label and unlicensed drug use in children population. Therapie.
73:135–149. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
van Riet-Nales DA, Schobben AF, Egberts TC
and Rademaker CM: Effects of the pharmaceutical technologic aspects
of oral pediatric drugs on patient-related outcomes: A systematic
literature review. Clin Ther. 32:924–938. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen J, Luo X, Qiu H, Mackey V, Sun L and
Ouyang X: Drug discovery and drug marketing with the critical roles
of modern administration. Am J Transl Res. 10:4302–4312.
2018.PubMed/NCBI
|
|
4
|
Thompson G, Barker CI, Folgori L, Bielicki
JA, Bradley JS, Lutsar I and Sharland M: Global shortage of
neonatal and paediatric antibiotic trials: Rapid review. BMJ Open.
7(e016293)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Joseph PD, Craig JC and Caldwell PHY:
Clinical trials in children. Br J Clin Pharmacol. 79:357–369.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gore R, Chugh PK, Tripathi CD, Lhamo Y and
Gautam S: Pediatric off-label and unlicensed drug use and its
implications. Curr Clin Pharmacol. 12:18–25. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Paine MF: Therapeutic disasters that
hastened safety testing of new drugs. Clin Pharmacol Ther.
101:430–434. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Leandro JA: ‘Risk-free rest and sleep:’
Jornal do Médico (Portugal) and the thalidomide disaster,
1960-1962. Hist Cienc Saude Manguinhos. 27:15–32. 2020.PubMed/NCBI View Article : Google Scholar : (In Portuguese,
English).
|
|
9
|
Nir-Neuman H, Abu-Kishk I, Toledano M,
Heyman E, Ziv-Baran T and Berkovitch M: Unlicensed and off-label
medication use in pediatric and neonatal intensive care units: No
change over a decade. Adv Ther. 35:1122–1132. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Landwehr C, Richardson J, Bint L, Parsons
R, Sunderland B and Czarniak P: Cross-sectional survey of off-label
and unlicensed prescribing for inpatients at a paediatric teaching
hospital in Western Australia. PLoS One.
14(e0210237)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kleeblatt J, Betzler F, Kilarski LL,
Bschor T and Köhler S: Efficacy of off-label augmentation in
unipolar depression: A systematic review of the evidence. Eur
Neuropsychopharmacol. 27:423–441. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shaikh M and Gandjour A: Pharmaceutical
expenditure and gross domestic product: Evidence of simultaneous
effects using a two-step instrumental variables strategy. Health
Econ. 28:101–122. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Mori AT, Meena E and Kaale EA: Economic
cost of substandard and falsified human medicines and cosmetics
with banned ingredients in Tanzania from 2005 to 2015: A
retrospective review of data from the regulatory authority. BMJ
Open. 8(e021825)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Moreira M and Sarraguça M: How can oral
paediatric formulations be improved? A challenge for the XXI
century. Int J Pharm. 590(119905)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Leucht S, Corves C, Arbter D, Engel RR, Li
C and Davis JM: Second-generation versus first-generation
antipsychotic drugs for schizophrenia: A meta-analysis. Lancet.
373:31–41. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
International Monetary Fund: Real GDP
growth. [Internet]. International Monetary Fund, Geneva, 2020.
https://www.imf.org/external/datamapper/NGDP_RPCH@WEO/OEMDC/ADVEC/WEOWORLD.
Accessed August 7, 2020.
|
|
17
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Barker TH, Stone JC, Sears K, Klugar M,
Tufanaru C, Leonardi-Bee J, Aromataris E and Munn Z: The revised
JBI critical appraisal tool for the assessment of risk of bias for
randomized controlled trials. JBI Evid Synth. 21:494–506.
2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Turner S, Nunn AJ, Fielding K and Choonara
I: Adverse drug reactions to unlicensed and off-label drugs on
paediatric wards: A prospective study. Acta Paediatr. 88:965–968.
1999.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Conroy S, McIntyre J and Choonara I:
Unlicensed and off label drug use in neonates. Arch Dis Child Fetal
Neonatal Ed. 80:F142–F144. 1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pandofini C, Impicciatore P, Provasi D,
Rocchi F, Campi R and Bonati M: Italian Paediatric Off-label
Collaborative Group. Off-label use of drugs in Italy: A
prospective, observational and multicentre study. Acta Paediatr.
91:339–347. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Barr J, Brenner-Zada G, Heiman E, Pareth
G, Bulkowstein M, Greenberg R and Berkovitch M: Unlicensed and
off-label medication use in a neonatal intensive care unit: A
prospective study. Am J Perinatol. 19:67–72. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jong GW, van der Linden PD, Bakker EM, van
der Lely N, Eland IA, Stricker BH and van den Anker JN: Unlicensed
and off-label drug use in a paediatric ward of a general hospital
in the Netherlands. Eur J Clin Pharmacol. 58:293–297.
2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Conroy S, Newman C and Gudka S: Unlicensed
and off label drug use in acute lymphoblastic leukaemia and other
malignancies in children. Ann Oncol. 14:42–47. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jong GW, Eland IA, Sturkenboom MC, van den
Anker JN and Strickerf BH: Unlicensed and off-label prescription of
respiratory drugs to children. Eur Respir J. 23:310–313.
2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Neubert A, Dormann H, Weiss J, Egger T,
Criegee-Rieck M, Rascher W, Brune K and Hinz B: The impact of
unlicensed and off-label drug use on adverse drug reactions in
paediatric patients. Drug Saf. 27:1059–1067. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bajcetic M, Jelisavcic M, Mitrovic J,
Divac N, Simeunovic S, Samardzic R and Gorodischer R: Off label and
unlicensed drugs use in paediatric cardiology. Eur J Clin
Pharmacol. 61:775–779. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Di Paolo ER, Stoetter H, Cotting J, Frey
P, Gehri M, Beck-Popovic M, Tolsa JF, Fanconi S and Pannatier A:
Unlicensed and off-label drug use in a Swiss paediatric university
hospital. Swiss Med Wkly. 136:218–222. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kaisi T, Maponga CC, Gavaza P and
Pazvakavambwa IE: An assessment of the extent of use of off-license
and unlicensed drugs on children at Parirenyatwa Hospital in
Harare, Zimbabwe. East Cent Afr J Pharm Sci. 9:3–7. 2007.
|
|
30
|
Santos DB, Clavenna A, Bonati M and Coelho
HL: Off-label and unlicensed drug utilization in hospitalized
children in Fortaleza, Brazil. Eur J Clin Pharmacol. 64:1111–1118.
2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bavdekar SB, Sadawarte PA, Gogtay NJ, Jain
SS and Jadhav S: Off-label drug use in a pediatric intensive care
unit. Indian J Pediatr. 76:1113–1118. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lass J, Käär R, Jõgi K, Varendi H,
Metsvaht T and Lutsar I: Drug utilisation pattern and off-label use
of medicines in Estonian neonatal units. Eur J Clin Pharmacol.
67:1263–1271. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Palčevski G, Skočibušić N and
Vlahović-Palčevski V: Unlicensed and off-label drug use in
hospitalized children in Croatia: A cross-sectional survey. Eur J
Clin Pharmacol. 68:1073–1077. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Oguz SS, Kanmaz HG and Dilmen U: Off-label
and unlicensed drug use in neonatal intensive care units in Turkey:
The old-inn study. Int J Clin Pharm. 34:136–141. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ballard CD, Peterson GM, Thompson AJ and
Beggs SA: Off-label use of medicines in paediatric inpatients at an
Australian teaching hospital. J Paediatr Child Health. 49:38–42.
2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kieran EA, O'Callaghan N and O'Donnell CP:
Unlicensed and off-label drug use in an Irish neonatal intensive
care unit: A prospective cohort study. Acta Paediatr.
103:e139–e142. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Silva J, Flor-de-Lima F, Soares H and
Guimarães H: Off-label and unlicensed drug use in neonatology:
Reality in a Portuguese University Hospital. Acta Med Port.
28:297–306. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lee JL, Redzuan AM and Shah NM: Unlicensed
and off-label use of medicines in children admitted to the
intensive care units of a hospital in Malaysia. Int J Clin Pharm.
35:1025–1029. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ribeiro M, Jorge A and Macedo AF:
Off-label drug prescribing in a Portuguese paediatric emergency
unit. Int J Clin Pharm. 35:30–36. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lindell-Osuagwu L, Hakkarainen M, Sepponen
K, Vainio K, Naaranlahti T and Kokki H: Prescribing for off-label
use and unauthorized medicines in three paediatric wards in
Finland, the status before and after the European Union Paediatric
regulation. J Clin Pharm Ther. 39:144–153. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Laforgia N, Nuccio MM, Schettini F,
Dell'Aera M, Gasbarro AR, Dell'Erba A and Solarino B: Off-label and
unlicensed drug use among neonatal intensive care units in Southern
Italy. Pediatr Int. 56:57–59. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Langerová P, Vrtal J and Urbánek K:
Incidence of unlicensed and off-label prescription in children.
Ital J Pediatr. 40(12)2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Luedtke KE and Buck ML: Evaluation of
off-label prescribing at a children's rehabilitation center. J
Pediatr Pharmacol Ther. 19:296–301. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Riou S, Plaisant F, Boulch DM, Kassai B,
Claris O and Nguyen KA: Unlicensed and off-label drug use: A
prospective study in French NICU. Acta Paediatr. 104:e228–e231.
2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Joret-Descout P, Prot-Labarthe S, Brion F,
Bataille J, Hartmann JF and Bourdon O: Off-label and unlicensed
utilisation of medicines in a French paediatric hospital. Int J
Clin Pharm. 37:1222–1227. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Jobanputra N, Save SU and Bavdekar SB:
Off-label and unlicensed drug use in children admitted to pediatric
intensive care units (PICU). Int J Risk Saf Med. 27:113–121.
2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Berdkan S, Rabbaa L, Hajj A, Eid B,
Jabbour H, El Osta NE, Karam L and Khabbaz LR: Comparative
assessment of off-label and unlicensed drug prescriptions in
children: FDA versus ANSM guidelines. Clin Ther. 38:1833–1844.
2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Cuzzolin L and Agostino R: Off-label and
unlicensed drug treatments in neonatal intensive care units: An
Italian multicentre study. Eur J Clin Pharmacol. 72:117–123.
2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Corny J, Bailey B, Lebel D and Bussières
JF: Unlicensed and off-label drug use in paediatrics in a
mother-child tertiary care hospital. Paediatr Child Health.
21:83–87. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ramadaniati HU, Tambunan T, Khairani S and
Adisty HS: Off-label and unlicensed prescribing in pediatric
inpatients with nephrotic syndrome in a major teaching hospital: An
Indonesian context. Asian J Pharm Clin Res. 10:355–359. 2017.
|
|
51
|
Tefera YG, Gebresillassie BM, Mekuria AB,
Abebe TB, Erku DA, Seid N and Beshir HB: Off-label drug use in
hospitalized children: A prospective observational study at Gondar
University Referral Hospital, Northwestern Ethiopia. Pharmacol Res
Perspect. 5(e00304)2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Teigen A, Wang S, Truong BT and Bjerknes
K: Off-label and unlicensed medicines to hospitalised children in
Norway. J Pharm Pharmacol. 69:432–438. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Costa HTML, Costa TX, Martins RR and
Oliveira AG: Use of off-label and unlicensed medicines in neonatal
intensive care. PLoS One. 13(e0204427)2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Mazhar F, Akram S, Haider N, Hadi MA and
Sultana J: Off-label and unlicensed drug use in hospitalized
newborns in a Saudi tertiary care hospital: A cohort study. Int J
Clin Pharm. 40:700–703. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Aamir M, Khan JA, Shakeel F, Shareef R and
Shah N: Drug utilization in neonatal setting of Pakistan: Focus on
unlicensed and off label drug prescribing. BMC Pediatr.
18(242)2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Dornelles AD, Calegari LH, de Souza L,
Ebone P, Tonelli TS and Carvalho CG: The unlicensed and off-label
prescription of medications in general paediatric ward: An
observational study. Curr Pediatr Rev. 15:62–66. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Kouti L, Aletayeb M, Aletayeb SMH, Hardani
AK and Eslami K: Pattern and extent of off-label and unlicensed
drug use in neonatal intensive care units in Iran. BMC Pediatr.
19(3)2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Tukayo BLA, Sunderland B, Parsons R and
Czarniak P: High prevalence of off-label and unlicensed paediatric
prescribing in a hospital in Indonesia during the period Aug.-Oct.
2014. PLoS One. 15(e0227687)2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Gidey MT, Gebretsadkan YG, Tsadik AG,
Welie AG and Assefa BT: Off-label and unlicensed drug use in Ayder
comprehensive specialized hospital neonatal intensive care unit.
Ital J Pediatr. 46(41)2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
García-López I, Vendrell MCM, Romero IM,
de Noriega I, González JB and Martino-Alba R: Off-label and
unlicensed drugs in pediatric palliative care: A prospective
observational study. J Pain Symptom Manage. 60:923–932.
2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
AlAzmi A, Alasmari Z, Yousef C, Alenazi A,
AlOtaibi M, AlSaedi H, AlShaikh A, AlObathani A, Ahmed O,
Goronfolah L and Alahmari M: Off-Label drug use in pediatric
out-patient care: A multi-center observational study. Hosp Pharm.
56:690–696. 2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Tsukamoto K, Carroll KA, Onishi T,
Matsumaru N, Brasseur D and Nakamura H: Improvement of pediatric
drug development: Regulatory and practical frameworks. Clin Ther.
38:574–581. 2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Shakeel S, Iffat W, Nesar S, Zaidi H and
Jamshed S: Exploratory findings of prescribing unlicensed and
off-label medicines among children and neonates. Integr Pharm Res
Pract. 9:33–39. 2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Andrade SRA, Santos PANM, Andrade PHS and
da Silva WB: Unlicensed and off-label prescription of drugs to
children in primary health care: A systematic review. J Evid Based
Med. 13:292–300. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Turner S, Gill A, Nunn T, Hewitt B and
Choonara I: Use of ‘off-label’ and unlicensed drugs in paediatric
intensive care unit. Lancet. 347:549–550. 1996.PubMed/NCBI
|