Introduction
Erectile dysfunction (ED) has been defined as the
inability to achieve and maintain a penile erection sufficiently to
allow satisfactory sexual performance (1). According to previous studies, ~52% of
men aged 40-70 years experience ED (2,3),
with projections estimating a global prevalence of 322 million
cases by 2025(4). ED adversely
affects psychosocial health and the quality of life (2). Penile erection is a complex
physiological process that requires arterial dilatation, relaxation
of the trabecular smooth muscle and activation of the corporeal
veno-occlusive mechanism, necessitating an adequately functioning
neural, vascular and endocrine environment (5). ED etiologically can be classified
into the following three classes: Organic (such as neurogenic,
hormonal, arterial, cavernosal, or drug-induced); psychogenic; and
mixed-type ED (2,4). However, this classification should be
used with caution, since the majority of patients with ED have a
mixed etiology (2). ED is mostly
of a mixed psychogenic and organic nature (4,6). An
important cause of psychogenic ED is performance anxiety, that is,
fear of inadequacy during sexual intercourse (7). Whilst developmental, cognitive,
emotional and interpersonal factors that predispose men to sexual
dysfunction have been previously identified in the etiology of
psychogenic ED, the etiology is now considered to be primarily
associated with a group of predisposing, triggering and maintaining
factors (4,8). Specifically, psychogenic ED has been
associated with performance anxiety and sexual confidence (8). However, depressive nature, loss of
self-esteem, relationship concerns and psychosocial stresses may
also be causes of psychogenic ED (9). By contrast, a number of factors have
been reported to be responsible for the etiology of organic ED.
There may be neurogenic causes namely multiple sclerosis, temporal
lobe epilepsy, Parkinson's disease, stroke, Alzheimer's disease,
spinal cord injury and cavernous nerve injury (radical pelvic
surgeries, such as radical prostatectomy) (4,6).
However, there may also be endocrinological causes, such as
testosterone deficiency or hypogonadism and hyperprolactinemia
(4,10). In addition, there may be
vasculogenic causes, including atherosclerosis, hypertension (HT),
hyperlipidemia, smoking, diabetes mellitus (DM) and pelvic
irradiation (4,6). Although neurogenic, endocrinological
and vasculogenic factors have been implicated in the development of
ED, the complete etiological spectrum remains to be fully
elucidated (4). Previous studies
have proposed a potential link between NUCB2/nesfatin-1, a satiety
regulator and ED (11,12).
NUCB2/nesfatin-1 is an adipocytokine that primarily
regulates food intake. Increased levels of nesfatin-1 in the
cerebroventricular system reduce food intake and the presence of an
antibody that neutralizes nesfatin-1 in this system stimulates
appetite (13). It has also been
shown to function (e.g. modulator, activator) in numerous systems,
such as the vascular, neural and hormonal systems (11,14,15),
and is expressed in testis (16).
In a study investigating the effect of NUCB2/nesfatin-1 on the
vascular system, it was previously found that NUCB2/nesfatin-1
modulated peripheral arterial contractility and suppressed the
vasodilator effect of nitric oxide (NO) (14), which brings to mind that the serum
NUCB2/nesfatin-1 level may be high in patients with ED. By
contrast, another previous study reported that NUCB2/nesfatin-1
could induce arterial vasodilation through significant changes in
NO/cyclic guanosine monophosphate (cGMP) activity (17).
Given the physiological pathways shared by
NUCB2/nesfatin-1 and penile erection, coupled with conflicting
results in previous studies, the present study aimed to explore the
relationship between NUCB2/nesfatin-1 and the presence and severity
of ED.
Materials and methods
Patients
The present prospective cross-sectional study was
conducted at the Department of Urology of the Health Sciences
University Bursa Medical Faculty of Medicine (Bursa, Turkey). The
protocol for the present study was approved by the Clinical
Research Committee of Bursa Yuksek Ihtisas Training and Research
Hospital (approval no. 2011-KAEK-25 2021/08-15), adhering to the
provisions of the Declaration of Helsinki (18). Written informed consent was
obtained from all participants.
Patient recruitment for the present study commenced
in August 2021 and ended in April 2023. The present study was
conducted at Bursa Yuksek Ihtisas Training and Research
Hospital.
Following a comprehensive physical examination and
medical history assessment, serum NUCB2/nesfatin-1, total
testosterone (TT), fasting blood glucose (FBG), hemoglobin A1c
(HbA1c), total cholesterol (TC), low-density
lipoprotein-cholesterol (LDL), high-density lipoprotein-cholesterol
(HDL), very low-density lipoprotein-cholesterol (VLDL), and
triglyceride (TG) levels were measured in all cases. Participants
aged >45 years underwent additional serum total
prostate-specific antigen (tPSA) testing.
Venous blood samples were collected (~10 ml per
patient) after 12 h overnight fasting (between 8 and 10 a.m. before
any significant physical activity), placed in vacutainer plastic
tubes (BD Biosciences) and processed according to clinical
laboratory protocols within 30 min after venipuncture. FBG (cat.
no. 3L82-22; Abbott Laboratories), HbA1c (cat. no. 030201002;
Lifotronic Technology Co., Ltd), TC (cat. no. 04S9230; Abbott
Laboratories), LDL (cat. no. 02R05-21; Archem Diognostic Ind. Ltd),
HDL (cat. no. 02R06-21; Archem Diognostic Ind. Ltd), VLDL, TG (cat.
no. 06T85-35; Archem Diognostic Ind. Ltd) and tPSA (cat. no. 7K70;
Abbott Laboratories) levels were then measured using commercially
available assay kits. Basal TT levels were determined using a
commercially available ELISA kit (cat. no. 2P13-28; Abbott
Laboratories) according to the manufacturer's protocols. Serum
samples for NUCB2/nesfatin-1 were frozen and stored at -70˚C and
measured using a standard Human Nesfatin-1 ELISA kit (cat. no.
E3063Hu; Bioassay Technology Laboratory) based on the sandwich
ELISA principle. The manufacturer's protocol was followed and no
changes were made to the standard protocol.
Groups
The present study included 43 men with ED (ED group)
and 40 healthy individuals (non-ED group). The participants were
evaluated using the Turkish version of the five-item International
Index of Erectile Function (IIEF-5) questionnaire (19). According to the IIEF-5 scores, ED
severity was categorized as follows: i) 5-7, severe ED; ii) 8-11,
moderate ED; iii) 12-16, mild-moderate ED; iv) 17-21, mild ED; and
v) 22-25, normal sexual function.
All participants underwent a comprehensive clinical
evaluation, including detailed medical and sexual history
histories, to identify underlying medical conditions that may cause
non-psychogenic ED, such as diabetes, cardiovascular disease or
hormonal imbalances associated with organic diseases. Certain
patients with ED had a medical condition that could cause
non-psychological ED (the number of patients with HT, DM and
coronary artery disease was 10, 11 and 5, respectively) (Table I), whilst others did not.
Individuals with infectious diseases, liver problems, renal
failure, a history of substance abuse or dependence, neoplasms or
autoimmune disorders and psychological disorders were excluded. ED
typically involves mixed (psychogenic and organic) etiologic
factors (2,4). Therefore, it was not possible to
separate patients with ED into purely organic or purely psychogenic
subtypes of ED. Therefore, in the present study, the patient group
was considered to consist mostly of the mixed type.
 | Table IComparison of the baseline
characteristics between the groups. |
Table I
Comparison of the baseline
characteristics between the groups.
|
Characteristics | ED group
(n=43) | Non-ED group
(n=40) | P-value |
|---|
| Age,
yearsa | 47.47±11.19 | 46.03±8.29 | 0.510 |
| Smoking status | | | 0.003 |
|
Smoker | 21 (48.8) | 7 (17.5) | |
|
Non-smoker | 22 (51.2) | 33 (82.5) | |
| Hypertension | | | 0.048 |
|
Present | 10 (23.25) | 3 (7.5) | |
|
Absent | 33 (76.75) | 37 (92.5) | |
| Diabetes
mellitus | | | 0.028 |
|
Present | 11 (25.58) | 3 (7.5) | |
|
Absent | 32 (74.42) | 37 (92.5) | |
| Coronary artery
disease | | | 0.435 |
|
Present | 5 (11.6) | 2(5) | |
|
Absent | 38 (88.4) | 38(95) | |
| Nesfatin-1,
ng/mlb | 0.64
(0.54-1.23) | 1.41
(0.63-4.1) | 0.019 |
| Fasting blood
glucose, mg/dlb | 96 (86-109) | 86.5
(83.25-101) | 0.111 |
| Hemoglobin A1c,
%b | 5.69
(5.09-6.36) | 5.39
(5.04-5.89) | 0.121 |
| Total testosterone,
ng/dlb | 435 (354-521) | 413 (368-571) | 0.668 |
| Total cholesterol,
mg/dla | 187.91±28.03 | 203.68±38.93 | 0.036 |
| Very low-density
lipoprotein-cholesterol, mg/dlb | 27 (18.8-37.6) | 29 (18.7-37.6) | 0.888 |
| Low-density
lipoprotein-cholesterol, mg/dla | 106.68±31.43 | 126.27±38.55 | 0.013 |
| Triglyceride,
mg/dlb | 107 (94-207) | 146 (141-188) | 0.685 |
| High-density
lipoprotein-cholesterol, mg/dla | 43.67±7.57 | 47.67±8.43 | 0.026 |
| Total
prostate-specific antigen, mg/dlb | 0.79
(0.56-1.24) | 0.81
(0.48-1.18) | 0.757 |
Statistical analysis
Data were processed and analyzed using the SPSS
v.21.0 software (IBM Corp.). To ensure accuracy, the Kolmogorov
Smirnov test was conducted for each group of continuous variables.
Normally distributed measurement data were presented as the mean ±
standard deviation and compared using the unpaired t-test.
Non-normally distributed data were expressed as median (P25, P75)
values and compared using the non-parametric Mann-Whitney U-test.
Categorical variables were presented as numbers and percentages and
compared using the χ2 and Fisher's exact tests. The
correlation between the serum NUCB2/nesfatin-1 level and ED
severity was assessed using Spearman's correlation coefficient.
Logistic regression analysis was used to identify risk factors and
predictors of ED. The diagnostic accuracy of serum NUCB2/nesfatin-1
for ED was evaluated using the area under the curve (AUC) values
obtained from receiver operating characteristic (ROC) analysis.
P<0.05 was considered to indicate a statistically significant
difference. The minimum required sample size was calculated to be
40 participants for each group with an effect size of 0.82, a
margin of error of 0.05 and a power of 0.95, using the two-tailed
independent-samples t-test. This calculation was based on a
previous study conducted by Ragab et al (12). Sample size estimation was
undertaken using G*Power v. 3.1.9.4(20).
Results
Baseline characteristics
The mean age of the participants was 46.77±9.87
(range, 25-67) years, with the ED and non-ED groups having mean
ages of 47.47±11.19 and 46.03±8.30 years, respectively. Age and
serum TT, FBG, HbA1c, VLDL, TG and tPSA values were found to be
statistically similar between the two groups (Table I). However, serum TC, LDL and HDL
values were found to be significantly higher in the non-ED group
compared with those in in the ED group (P<0.05). The mean serum
NUCB2/nesfatin-1 level in the ED group was found to be
significantly lower compared with that in the non-ED group
(P<0.05; Table I). The mean
IIEF-5 scores of the ED and non-ED groups were calculated to be
13.14±5.03 and 23.65±1.17, respectively. IIEF-5 scores were found
to be significantly higher in the non-ED group compared with those
in the ED group (P<0.001). The incidence of DM, HT and smoking
were more prevalent in the ED group compared with those in the
non-ED group (P<0.05; Table
I).
Association between NUCB2/nesfatin-1
and ED
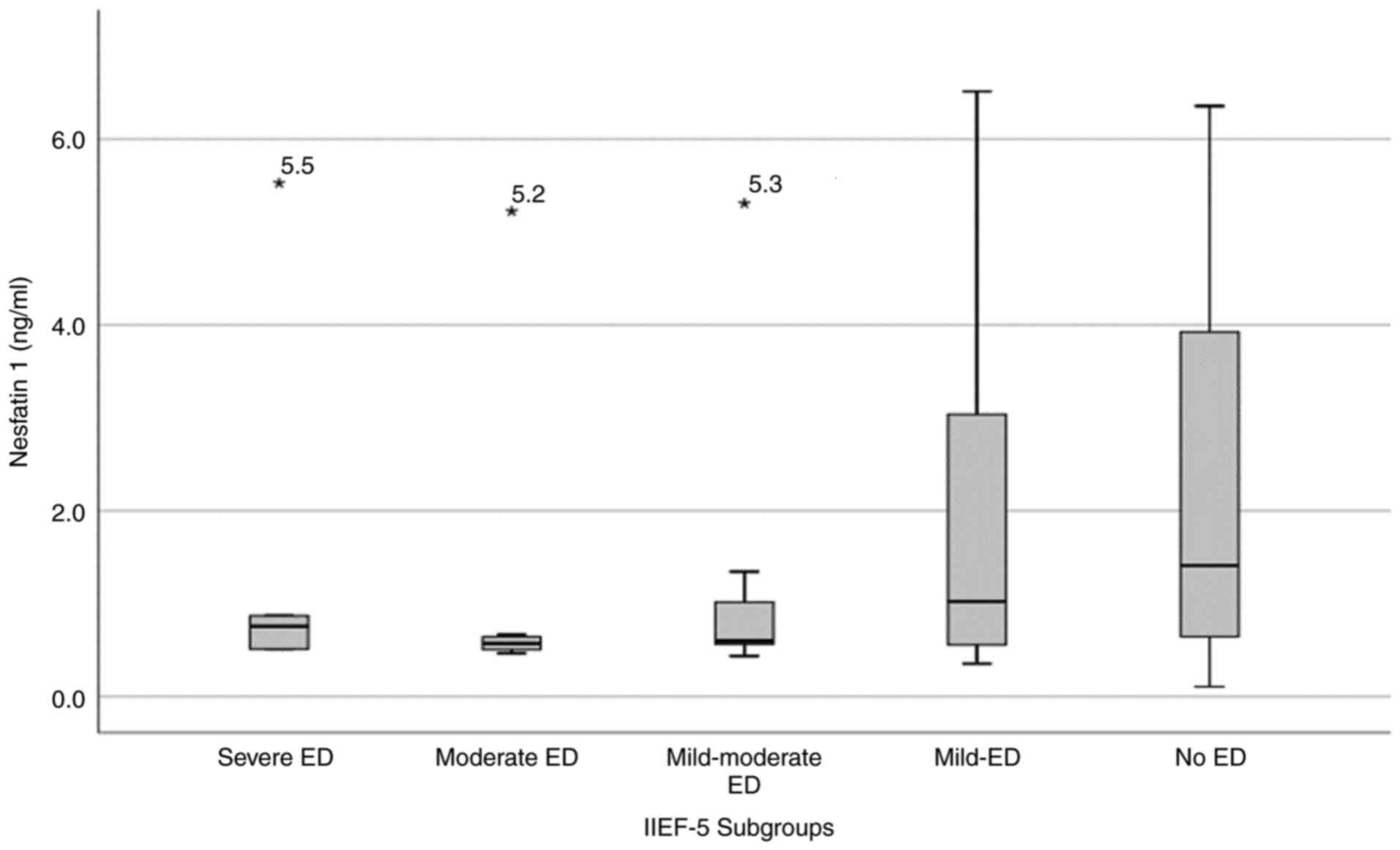
A weak negative correlation between serum
NUCB2/nesfatin-1 level and ED severity according to the IIEF-5
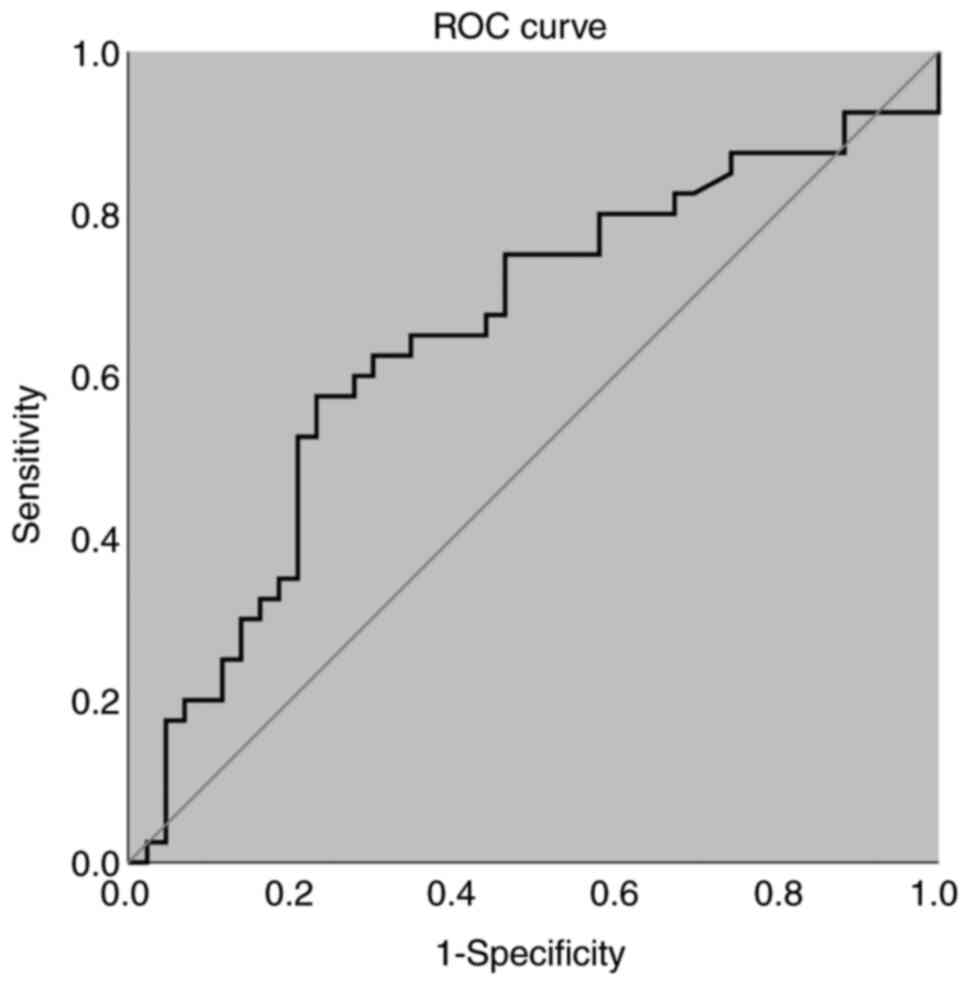
scores was found (r=-0.306; P=0.005; Fig. 1). Subsequent ROC curve analysis of
the serum NUCB2/nesfatin-1 revealed a cut-off value of 1.25 ng/ml
for distinguishing between the ED and non-ED groups (P<0.05),
with an AUC value of 0.650 (95% CI, 0.53-0.77; Fig. 2). However, multivariate logistic
regression analysis did not identify serum NUCB2/nesfatin-1 as a
predictor for ED, whereas smoking and DM increased the probability
of ED by 6.4 and 5.5 times, respectively (Table II).
 | Table IILogistic regression analysis of the
data for evaluation of possible risk factors and predictors of
erectile dysfunction in the present cohort. |
Table II
Logistic regression analysis of the
data for evaluation of possible risk factors and predictors of
erectile dysfunction in the present cohort.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variable | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Smoking habit | 4.5
(1.637-12.371) | 0.004 | 6.4
(2.138-19.184) | 0.001 |
| Hypertension | 3.7
(0.947-14.751) | 0.060 | | |
| Diabetes
mellitus | 4.2
(1.087-16.542) | 0.038 | 5.5
(1.272-24.275) | 0.023 |
| Nucleobindin
2/nesfatin-1 | 1.02
(0.999-1.047) | 0.062 | | |
| Total
cholesterol | 1.01
(1.001-1.028) | 0.041 | | |
| Low-density
lipoprotein-cholesterol | 1.01
(1.003-1.031) | 0.018 | | |
| High-density
lipoprotein-cholesterol | 1.06
(1.006-1.128) | 0.030 | | |
In the subgroup analysis, when the without HT
subgroups were compared, nesfatin-1 values were found to be
significantly lower in the ED group (P<0.05). Similarly, when
non-smokers were compared, nesfatin-1 values were found to be
significantly lower in the ED group (P<0.05). However, no
significance could be found in other subgroup analyses (Table III).
 | Table IIIComparison of the nesfatin-1 levels
between the subgroups based on with/without comorbidity. |
Table III
Comparison of the nesfatin-1 levels
between the subgroups based on with/without comorbidity.
| Patients | N | ED group | N | Non-ED group | P-value |
|---|
| Without DM | 32 | 6.24
(5.16-24.15) | 37 | 14.26
(6.45-39.22) | 0.660 |
| With DM | 11 | 7.42
(5.53-8.17) | 3 | 13.93
(4.87-13.93) | 0.312 |
| Without HT | 33 | 6.21
(5.31-14.66) | 37 | 14.26
(6.45-42.87) | 0.033 |
| With HT | 10 | 7.07
(5.41-12.58) | 3 | 12.71
(4.87-12.71) | 0.612 |
| Without
smoking | 22 | 6.48
(5.49-11.00) | 33 | 14.26
(6.45-48.06) | 0.034 |
| With smoking | 21 | 6.44
(5.19-15.02) | 7 | 12.71
(5.45-30.44) | 0.507 |
Discussion
The present study revealed that the serum
NUCB2/nesfatin-1 level was reduced in patients with ED whilst being
negatively correlated with ED severity. A cut-off value of 1.25
ng/ml for serum NUCB2/nesfatin-1 was established to differentiate
ED cases. The presence of DM and smoking emerged as risk factors
for ED, whilst serum NUCB2/nesfatin-1 was not a predictor of ED
according to logistic regression analysis.
Nesfatin-1, initially identified by Oh et al
(13) as a satiety molecule in the
hypothalamus, is derived from NUCB2(13). It is an 82-amino acid polypeptide
derived from the post-translational processing of hypothalamic
NUCB2, which consists of 396 amino acids (21,22).
This peptide is mainly produced in the hypothalamus, particularly
in the paraventricular nucleus, arcuate nucleus and nucleus of the
solitary tract. However, it is also synthesized in peripheral
tissues, such as the stomach, pancreas, adipose tissue and testes
(23). Nesfatin-1 has a molecular
weight of ~9.8 kDa and a half-life of 23.5 min (24,25).
Whilst the exact degradation mechanism of nesfatin-1 remains to be
fully understood, it is likely to be broken down by peptidases and
proteases (25). Although it shows
diurnal variations in its endogenous rhythm, there is no definitive
evidence to support the idea that it follows a circadian pattern
(26).
NUCB2/nesfatin-1 levels can be influenced by various
factors, including nutrient status, hormonal regulation and energy
homeostasis. Food intake significantly impacts its expression, with
levels decreasing during fasting and increasing after feeding
(27). A number of hormones, such
as insulin, glucagon and gonadotropins, can also regulate its
levels (28). Nesfatin-1 is
involved in glucose and lipid metabolism, enhancing insulin
secretion and action via stimulating insulin mRNA expression and/or
by promoting Ca2+ influx through L-type channels
(29). Its levels have been
demonstrated to be elevated in obesity but reduced in other
conditions, such as type 2 DM (30,31).
Nesfatin-1 can also been shown to modulate multiple signaling
pathways (e.g. AKT kinase/AMP-dependent protein kinase/mammalian
target of rapamycin pathway), including the mTOR/STAT3 signaling
pathway, which is instrumental in glucose homeostasis and hepatic
insulin sensitivity, although no specific receptor has been
identified (32). In addition,
this peptide serves various roles in growth (e.g. intrauterine and
postnatal), reproductive function, stress response and cancer
progression (28,33-36).
The question of whether diabetes, HT and smoking,
which are among the main etiological factors of ED, can mediate
effects on serum nesfatin-1 levels remain unknown. As previously
found by Zhai et al (37)
in a review and meta-analysis, studies evaluating the relationship
between circulating nesfatin-1 and diabetes have yielded
conflicting results (37). Whilst
a number of studies have demonstrated high levels of nesfatin-1 in
patients with type 2 diabetes (38,39),
others have reported lower levels of nesfatin-1 in such patients
(40-43).
In a previous study involving experimental HT models, no
significant difference was found between nesfatin-1 levels in
serum, urine and renal tissue samples of control and HT models
(Angiotensin II-induced model) (44). However, in another previous study,
where patients with essential HT were compared with the control
group, serum nesfatin-1 levels in the patient group were found to
be significantly higher (45). To
the best of our knowledge, in the current literature, no study on
the relationship between smoking and serum nesfatin-1 levels could
be found. Therefore, it was not possible to draw a conclusion on
the effect of diabetes, HT and smoking on serum nesfatin-1 levels
according to the present study.
Numerous studies have previously explored the
potential role of nesfatin-1 in female physiology, which included
animal models and clinical studies. Human studies have mostly
focused on polycystic ovary syndrome and gestational DM.
Controversial findings have been made regarding nesfatin-1 levels
in women with polycystic ovary syndrome, with studies suggesting
higher levels (46,47) and others suggesting lower levels
(48,49). In a previous meta-analysis,
nesfatin-1 concentrations in patients with gestational diabetes
were also conflicting. In total, three studies reported lower
circulating levels of nesfatin-1 compared with those of healthy
controls, whilst four studies observed higher levels of nesfatin-1
compared with those in healthy controls (50). In another study on premature
telarche and serum nesfatin-1 levels in young female individuals
(aged 4-8 years), serum nesfatin-1 levels were found to be
significantly higher in patients with premature telarche compared
with those in the healthy control group (51). In a previous animal study,
inhibition of the hypothalamic expression of nesfatin-1 was found
to delay puberty in female rats. Intracerebroventricular injection
of nesfatin-1 in adolescent female rats, especially during fasting,
was also observed to increase serum luteinizing hormone (LH) and
follicle stimulating hormone (FSH) levels (52). In another animal study, nesfatin-1
levels fluctuated during pregnancy in normal pregnant mice
(nesfatin-1 serum levels increased significantly on day 14.5 and
then decreased rapidly on day 19.5). Evidence has also been
provided that activation of Th17 cells, which may be an important
regulator of pregnancy maintenance, may be regulated by
nesfatin-1/NUCB2. Therefore, it was determined that nesfatin-1 may
be an important molecule for pregnancy and fertility (53). In a study by Kim et al
(54), NUCB2 mRNA and nesfatin-1
protein expression were detected in the ovaries and uterus of mice.
NUCB2/nesfatin-1 expression in both organs responded to various
hormonal manipulations (such as pregnant mare serum gonadotropin
administration, ovariectomy, 17β-estradiol injection) in the
direction of increase (administration of pregnant mare serum
gonadotropin, 17β-estradiol injection) or decrease (ovariectomy).
It was thereby concluded that NUCB2/nesfatin-1 expression in the
ovary and uterus of mice can be regulated through the
hypothalamus-pituitary-ovarian axis, where NUCB2/nesfatin-1 is a
local regulator of ovarian steroidogenesis and uterine function
(54). Although research on the
levels and physiologic roles of NUCB2/nesfatin-1 in women is
ongoing, available evidence suggests that it may influence various
aspects of female physiology, including reproductive functions and
stress responses (55).
However, whether nesfatin-1 serves a role in the
physiology of erection and/or ejaculation remains controversial. A
recent study by Chen et al (56) provided information on erection
physiology and ED pathophysiology. Using mouse models, a type 2
DM-like model was created and found that nesfatin-1 treatment had
an ameliorating effect on ED, a complication of DM. This previous
study also found nesfatin-1 can improve both glucose metabolism
disorders and diabetic ED in ED mice with type 2 DM, which may be
mediated by nesfatin-1 promoting the conversion of corpus
cavernosus smooth muscle cells to a contractile phenotype, through
the PI3K/AKT/mTOR signaling pathway. Intracavernosal pressures of
the diabetic ED group was found to be improved with nesfatin-1
treatment. The smooth muscle/collagen fiber ratio in the cavernosal
tissue, which had decreased with the development of diabetes,
increased by nesfatin-1 treatment and the phenotype structures of
muscle cells in this tissue, which were impaired with diabetes,
were improved by nesfatin-1 treatment (56). Although not directly associated
with erection and ejaculation physiology, there have been studies
investigating the role of nesfatin-1 in various reproductive
functions that may potentially affect erection and ejaculation
physiology. Gao et al (16)
found that nesfatin-1 is involved in the regulation of the
hypothalamo-pituitary-gonadal axis. Specifically, it was shown that
nesfatin-1 can affect the expression of the reproductive hormones
gonadotropin-releasing hormone, LH, FSH and testosterone, which are
critical for the maintenance of reproductive functions and erectile
function (16). In another study,
Ranjan et al (57) reported
that nesfatin-1 is expressed in various reproductive tissues,
including the testis, where it serves a role in spermatogenesis and
steroidogenesis. It was specifically found in Leydig cells and has
been shown to facilitate testosterone production and maturation of
testicular functions (57).
Nesfatin-1 has also been found to inhibit acrosome reaction in
sperm within the epididymis, which is a crucial step for
fertilization. This suggests that nesfatin-1 may have a regulatory
role in sperm maturation and function before ejaculation (58).
In the context of erection physiology, the NO/cGMP
signaling pathway serves a critical role in the regulation of
erectile function (59). NO is
synthesized from L-arginine by NO synthase (NOS) in endothelial
cells. NO is then released from the endothelium and the cavernous
nerve with sexual stimulation, subsequently activating the
guanylate cyclase, which converts GTP to cGMP. This conversion
reduces intracellular calcium levels, leading to the relaxation of
penile smooth muscles and increased blood flow, thereby
facilitating erection (4,60). Disruptions in any of these
processes can potentially result in ED (61).
Yamawaki et al (14) previously examined the possible
effect of nesfatin-1 on increasing blood pressure through the
modulation of endothelial function in rats. It was demonstrated
that the administration of nesfatin-1 to isolated mesenteric
arteries inhibited relaxation induced by sodium nitroprusside (a NO
donor) through the impairment of cGMP production. Furthermore,
intravenous administration of nestin-1 significantly increased
blood pressure and resisted SNP-induced blood pressure decreases
(14). Based on these
aforementioned results, it was therefore hypothesized to find a
higher serum NUCB2/nesfatin-1 level in patients with ED compared
with that in patients without ED. By contrast, serum nesfatin-1 of
patients in the ED group was found to be significantly lower
compared with that in patients in the non-ED group, corroborating
the findings reported by Ragab et al (12) and Sun et al (11).
In a previous study investigating the effects of
nesfatin-1 on the rat thoracic aorta, Barutcigil and Tasatargil
(17) reported that
NUCB2/nesfatin-1 did not affect the aortic tonus but induced
vasodilation in phenylephrine-constricted rat thoracic aorta. This
vasodilator effect was significantly abolished by the mechanical
removal of the endothelium and completely inhibited by both
molecules by the addition of N-nitro-L-arginine methyl ester (an
inhibitor of NOS) and H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one
(a soluble guanylate cyclase inhibitor) to tissue cultures.
Therefore, it was concluded that nesfatin-1 could induce arterial
vasodilation through endothelium-dependent mechanisms, which is
closely associated with the presence of endothelial cells and the
production of NO and cGMP in the rat thoracic aorta (17). In addition, previous human studies
have reported normal blood nesfatin-1 concentrations of <10
ng/ml (39,42). The discrepancies between the
results of the aforementioned studies may be due to the different
concentrations of nesfatin-1 administered. Yamawaki et al
(14) used a nesfatin-1
concentration of 100 ng/ml, higher compared with the 10 ng/ml
administered by Barutcigil and Tasatargil (17). The use of a higher concentration of
nesfatin-1 may have affected Ca2+ hemodynamics or the
NO/cGMP pathway. The concentration of nesfatin-1 administered in
the study by Yamawaki et al (14) exceeded that of normal human blood
levels, underscoring the need for further studies.
Mori et al (62) previously found that nesfatin-1 can
increase NO production in a dose-dependent manner in human
umbilical vein endothelial cells (62). Another study observed an increase
in cGMP levels in rat heart cells exposed to nesfatin-1(63). However, whether decreased serum
nesfatin-1 levels can lead to reduced NO and/or cGMP levels in
endothelial and/or smooth muscle cells remain unknown. This
necessitates further cellular and animal or human studies. To
confirm the present findings and elucidate the role of nesfatin-1
in erectile dysfunction, culturing human endothelial and smooth
muscle cells from penile tissues should be considered to
investigate the effects of varying nesfatin-1 levels on NO and cGMP
production. By manipulating nesfatin-1 concentrations in the
culture media, changes in NO and cGMP levels using appropriate
biochemical assays can be directly measured. Various techniques,
such as reverse transcription-quantitative PCR and Western
blotting, can be used to assess the expression levels of genes and
proteins involved in NO synthesis and cGMP signaling pathways in
response to altered nesfatin-1 levels. Developing animal models,
such as nesfatin-1 knockout mice or rats, to study the impact of
nesfatin-1 deficiency on erectile function should also be
considered. Erectile responses in such models can be evaluated
through intracavernosal pressure measurements following electrical
stimulation of the cavernous nerve. In addition, future studies can
administer nesfatin-1 to animal models with induced erectile
dysfunction to observe potential therapeutic effects. This may be
used to determine if nesfatin-1 supplementation can restore normal
erectile function and normalize NO/cGMP levels. If future studies
can show that low serum nesfatin-1 levels can cause a decrease in
the amount or activity of molecules important for erection
physiology, such as NO and cGMP, especially in penile tissue, it
will facilitate the understanding into the low serum nesfatin-1
levels observed in patients with ED, as reported in the present
study and previous studies by Ragab et al (12) and Sun et al (11). Although definitive conclusions
regarding the physiological effects of nesfatin-1 cannot be drawn
from the current literature, nesfatin-1 appears to be an important
molecule in the physiology of erection and the pathophysiology of
ED.
To determine whether nesfatin-1 can inhibit the
vasodilator effect of NO in the intracavernosal region during
sexual stimulation in patients with ED, a more challenging but
effective method would involve measuring and comparing
intracavernosal serum nesfatin-1 levels in patients with ED and
healthy men during sexual stimulation. This hypothesis warrants
further investigation.
Recent studies have explored the relationship
between nesfatin-1 and testosterone. Seon et al (64) demonstrated that nesfatin-1 was
regulated by testosterone, revealing a reduction in NUCB2 mRNA
expression in the pituitary glands of mice after castration, which
was reversed with testosterone replacement (64). Another previous study examined the
cellular distribution and regulatory patterns of NUCB2/nesfatin-1
in mammalian testes and revealed the specific expression of
NUCB2/nesfatin-1 in Leydig cells (28). Sun et al (11) previously reported a significantly
lower serum testosterone level in the ED group compared with that
in the control group, consistent with the lower nesfatin-1 levels
of the former (11). However, the
present study did not reveal a significant difference between the
serum testosterone levels of the two groups.
To establish the association of a factor with
disease, it would be ideal if the characteristics of the study
groups were as similar as possible, except for the presence of the
factor and the disease. Unfortunately, in the real world, achieving
study groups with identical characteristics is nearly impossible
for several reasons. Participants have inherent individual
differences in genetics, lifestyle and environmental exposures that
are difficult to control completely. In addition, ethical and
logistical constraints limit the ability to manipulate or control
certain factors in human studies. Random variations and unforeseen
confounding factors can also introduce differences that are not
accounted for at the outset. These aforementioned challenges render
it difficult to create perfectly matched study groups solely
differing by the factor and the disease in question.
A number of factors, such as age, diabetes and fat
mass, can also influence NUCB2/nesfatin-1 levels. It may be
beneficial to stratify subjects by age and then categorize them
according to their physical condition, such as by comparing
patients aged 40-50 years with diabetes and EDs with those without
EDs, which may provide more precise findings. With 43 subjects with
ED and 40 without ED included in the present study, the sample size
may not be sufficient. This is a limitation of the present study
and may be an aim of future studies.
As one of the results of this study, a weak negative
correlation was detected between serum NUCB2/nesfatin-1 level and
ED severity. This slight correlation suggests that it is uncertain.
This can be considered as a potential limitation of the present
study and may serve the purpose of future studies.
The present study has certain other limitations. The
sample size was relatively small. In addition, the quantity of
components in the NO/cGMP pathway was not evaluated or quantified.
Serum NUCB2/nesfatin-1 levels were also not measured during sexual
activity or upon stimulation.
The idea of investigating the development of ED and
the change in nesfatin-1 serum levels over time appears prudent. It
would undoubtedly be valuable to determine a correlation between
the serum nesfatin-1 levels of a group studied over time and ED
development and/or ED severity in the same investigated group to
show the ED/nesfatin-1 relationship.
In conclusion, the present study showed that serum
NUCB2/nesfatin-1 levels of patients with ED were significantly
lower compared with those of healthy individuals. There was also a
weak negative correlation between the serum NUCB2/nesfatin-1 level
and ED severity. Although this finding does not definitively
establish low levels of nesfatin-1 as a factor involved in the
etiology of ED, it suggests a potential association. Further
studies are needed to resolve the conflicting results regarding the
effect of NUCB2/nesfatin-1 on vascular physiology and to elucidate
the effects of this molecule on erectile physiology and/or
pathophysiology.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AK took part in protocol/project development, data
collection and management as well as manuscript writing/editing. AG
and AE took part in data analysis and manuscript writing/editing.
MG, ART and SC participated in data collection or management. RFK
and YU took part in the biochemical analysis of serum samples and
data collection. AK and AG confirm the authenticity of all the raw
data. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
1964 Declaration of Helsinki and its later amendments or comparable
ethical standards. The present study was approved by the Clinical
Research Committee of the Health Sciences University Bursa High
Specialty Training and Research Hospital (approval no. 2011-KAEK-25
2021/08-15). Written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
NIH Consensus Conference Impotence. NIH
consensus development panel on impotence. JAMA. 270:83–90.
1993.PubMed/NCBI
|
|
2
|
Sexual and Reproductive Health-Management
of erectile dysfunction-Uroweb.
|
|
3
|
Cho JW and Duffy JF: Sleep, sleep
disorders, and sexual dysfunction. World J Mens Health. 37:261–275.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shamloul R and Ghanem H: Erectile
dysfunction. Lancet. 381:153–65. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gratzke C, Angulo J, Chitaley K, Dai YT,
Kim NN, Paick JS, Simonsen U, Uckert S, Wespes E, Andersson KE, et
al: Anatomy, physiology, and pathophysiology of erectile
dysfunction. J Sex Med. 7:445–475. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Salonia A, Bettocchi C, Boeri L,
Capogrosso P, Carvalho J, Cilesiz NC, Cocci A, Corona G,
Dimitropoulos K, Gül M, et al: European association of urology
guidelines on sexual and reproductive health-2021 update: Male
sexual dysfunction. Eur Urol. 80:333–357. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Carson CC and Dean JD: Management of
Erectile Dysfunction in Clinical Practice. Springer Medical
Publishing, New York, NY, 2006.
|
|
8
|
Rosen RC: Psychogenic erectile
dysfunction. Classification and management. Urol Clin North Am.
28:269–278. 2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pastuszak AW: Current diagnosis and
management of erectile dysfunction. Curr Sex Health Rep. 6:164–176.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Janmohamed S and Bouloux PG: Endocrinology
of male sexual dysfunction. In: Male Sexual Dysfunction. Wiley,
Hoboken, NJ, pp30-47, 2017.
|
|
11
|
Sun W, Bi LK, Xie DD and Yu DX: Serum
nesfatin-1 is associated with testosterone and the severity of
erectile dysfunction. Andrologia. 52(e13634)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ragab A, Ahmed MH, Reda Sayed A,
EldinAbdelbary DAK and GamalEl Din SF: Serum nesfatin-1 level in
men with diabetes and erectile dysfunction correlates with
generalized anxiety disorder-7: A prospective comparative study.
Andrology. 11:307–315. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Oh IS, Shimizu H, Satoh T, Okada S, Adachi
S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, et al:
Identification of nesfatin-1 as a satiety molecule in the
hypothalamus. Nature. 443:709–712. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yamawaki H, Takahashi M, Mukohda M, Morita
T, Okada M and Hara Y: A novel adipocytokine, nesfatin-1 modulates
peripheral arterial contractility and blood pressure in rats.
Biochem Biophys Res Commun. 418:676–681. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ozcan M, Gok ZB, Kacar E, Serhatlioglu I
and Kelestimur H: Nesfatin-1 increases intracellular calcium
concentration by protein kinase C activation in cultured rat dorsal
root ganglion neurons. Neurosci Lett. 619:177–181. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gao X, Zhang K, Song M, Li X, Luo L, Tian
Y, Zhang Y, Li Y, Zhang X, Ling Y, et al: Role of Nesfatin-1 in the
Reproductive Axis of Male Rat. Sci Rep. 6(32877)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Barutcigil A and Tasatargil A: Effects of
nesfatin-1 on atrial contractility and thoracic aorta reactivity in
male rats. Clin Exp Hypertens. 40:414–420. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
World Medical Association. World medical
association declaration of helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Turunc T, Deveci S, Güvel S and
Peşkircioğlu L: The assessment of turkish validation with 5
question version of international index of erectile function
(IIEF-5). Turk J Urol. 33:45–49. 2007.
|
|
20
|
Faul F, Erdfelder E, Lang AG and Buchner
A: G*Power 3: A flexible statistical power analysis program for the
social, behavioral, and biomedical sciences. Behav Res Methods.
39:175–191. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Stengel A and Tache Y: Minireview:
Nesfatin-1-an emerging new player in the brain-gut, endocrine, and
metabolic axis. Endocrinology. 152:4033–4038. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Stengel A: Nesfatin-1-More than a food
intake regulatory peptide. Peptides. 72:175–183. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rupp SK, Wölk E and Stengel A: Nesfatin-1
Receptor: Distribution, signaling and increasing evidence for a G
protein-coupled receptor-A systematic review. Front Endocrinol
(Lausanne). 12(740174)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gonzalez R, Perry RL, Gao X, Gaidhu MP,
Tsushima RG, Ceddia RB and Unniappan S: Nutrient Responsive
Nesfatin-1 regulates energy balance and induces glucose-stimulated
insulin secretion in rats. Endocrinology. 152:3628–3637.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Aydin S: Role of NUCB2/nesfatin-1 as a
Possible Biomarker. Curr Pharm Des. 19:6986–6992. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Şahin Z: Could the change of anorexigenic
function of nesfatin-1 during the day be associated with circadian
rhythm? Troia Med J. 2022.
|
|
27
|
Hatef A, Shajan S and Unniappan S:
Nutrient status modulates the expression of nesfatin-1 encoding
nucleobindin 2A and 2B mRNAs in zebrafish gut, liver and brain. Gen
Comp Endocrinol. 215:51–60. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
García-Galiano D, Pineda R, Ilhan T,
Castellano JM, Ruiz-Pino F, Sánchez-Garrido MA, Vazquez MJ,
Sangiao-Alvarellos S, Romero-Ruiz A, Pinilla L, et al: Cellular
distribution, regulated expression, and functional role of the
anorexigenic peptide, NUCB2/Nesfatin-1, in the Testis.
Endocrinology. 153:1959–1971. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Riva M, Nitert MD, Voss U, Sathanoori R,
Lindqvist A, Ling C and Wierup N: Nesfatin-1 stimulates glucagon
and insulin secretion and beta cell NUCB2 is reduced in human type
2 diabetic subjects. Cell Tissue Res. 346:393–405. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Foo KS, Brauner H, Östenson CG and
Broberger C: Nucleobindin-2/nesfatin in the endocrine pancreas:
Distribution and relationship to glycaemic state. J Endocrinol.
204:255–263. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kadim BM and Hassan EA: Nesfatin-1-as a
diagnosis regulatory peptide in type 2 diabetes mellitus. J
Diabetes Metab Disord. 21:1369–1375. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wu D, Yang M, Chen Y, Jia Y, Ma ZA, Boden
G, Li L and Yang G: Hypothalamic nesfatin-1/NUCB2 knockdown
augments hepatic gluconeogenesis that is correlated with inhibition
of mTOR-STAT3 signaling pathway in rats. Diabetes. 63:1234–1247.
2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hofmann T, Elbelt U, Ahnis A, Rose M,
Klapp BF and Stengel A: Sex-specific regulation of
NUCB2/nesfatin-1: Differential implication in anxiety in obese men
and women. Psychoneuroendocrinology. 60:130–137. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu GM, Xu ZQ and Ma HS:
Nesfatin-1/nucleobindin-2 is a potent prognostic marker and
enhances cell proliferation, migration, and invasion in bladder
cancer. Dis Markers. 2018(4272064)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kim J, Chung Y, Kim H, Im E, Lee H and
Yang H: The tissue distribution of Nesfatin-1/NUCB2 in mouse. Dev
Reprod. 18:301–309. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cheng YY, Zhao XM, Cai BP, Ma LN, Yin JY
and Song GY: Nesfatin-1 in newborns: Relationship with endocrine
and metabolic and anthropometric measures. J Pediatr Endocrinol
Metab. 25:727–732. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhai T, Li SZ, Fan XT, Tian Z, Lu XQ and
Dong J: Circulating Nesfatin-1 levels and type 2 diabetes: A
systematic review and meta-analysis. J Diabetes Res.
2017(7687098)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Guo Y, Liao Y, Fang G, Dong J and Li Z:
Increased nucleobindin-2 (NUCB2) transcriptional activity links the
regulation of insulin sensitivity in Type 2 diabetes mellitus. J
Endocrinol Invest. 36:883–888. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
39
|
Zhang Z, Li L, Yang M, Liu H, Boden G and
Yang G: Increased plasma levels of nesfatin-1 in patients with
newly diagnosed type 2 diabetes mellitus. Exp Clin Endocrinol
Diabetes. 120:91–95. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Algul S, Ozkan Y and Ozcelik O: Serum
nesfatin-1 levels in patients with different glucose tolerance
levels. Physiol Res. 65:979–985. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Dai R, Deng G, Sun Z, Liu Z, Qian Y and
Han Y: Relation of serum and vitreous nesfatin-1 concentrations
with diabetic retinopathy. J Clin Lab Anal.
31(e22105)2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li QC, Wang HY, Chen X, Guan HZ and Jiang
ZY: Fasting plasma levels of nesfatin-1 in patients with type 1 and
type 2 diabetes mellitus and the nutrient-related fluctuation of
nesfatin-1 level in normal humans. Regul Pept. 159:72–77.
2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu F, Yang Q, Gao N, Liu F and Chen S:
Decreased plasma nesfatin-1 level is related to the thyroid
dysfunction in patients with type 2 diabetes mellitus. J Diabetes
Res. 2014(128014)2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Aydin MA, Aydogdu N, Tastekin E, Firat N
and Yalcinkaya Yavuz O: Investigation of the Relationship of
Nesfatin-1, Adropin Levels and Claudin-2, renalase immunoreactivity
with kidney function in an experimental hypertension model. P R
Health Sci J. 43:39–45. 2024.PubMed/NCBI
|
|
45
|
Kovalyova O, Ashcheulova T, Demydenko A,
Vizir M and Kochubiei O: Nesfatin-1 activity in patients with
essential hypertension and prediabetes, type 2 diabetes. Georgian
Med News. 44–49. 2017.PubMed/NCBI(In Russian).
|
|
46
|
Xu Y, Zhang H, Li Q, Lao K and Wang Y: The
role of nesfatin-1 expression in letrozole-induced polycystic
ovaries in the rat. Gynecol Endocrinol. 33:438–441. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sahin FK, Sahin SB, Ural UM, Cure MC,
Senturk S, Tekin YB, Balik G, Cure E, Yuce S and Kirbas A:
Nesfatin-1 and Vitamin D levels may be associated with systolic and
diastolic blood pressure values and hearth rate in polycystic ovary
syndrome. Bosn J Basic Med Sci. 15:57–63. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Varlı B, Şükür YE, Özmen B, Ergüder Bİ,
Sönmezer M, Berker B, Atabekoğlu C and Aytaç R: Anorexigenic
peptide (leptin, obestatin, nesfatin-1) levels and their impact on
assisted reproductive technology treatment outcomes in patients
with polycystic ovary syndrome. Clin Exp Reprod Med. 48:368–373.
2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Alp E, Görmüş U, Güdücü N and Bozkurt S:
Nesfatin-1 levels and metabolic markers in polycystic ovary
syndrome. Gynecol Endocrinol. 31:543–547. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sun J, Zhang D, Xu J, Chen C, Deng D, Pan
F, Dong L, Li S and Ye S: Circulating FABP4, nesfatin-1, and
osteocalcin concentrations in women with gestational diabetes
mellitus: A meta-analysis. Lipids Health Dis.
19(199)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Almasi N, Zengin HY, Koç N, Uçakturk SA,
İskender Mazman D, Heidarzadeh Rad N and Fisunoglu M: Leptin,
ghrelin, nesfatin-1, and orexin-A plasma levels in girls with
premature thelarche. J Endocrinol Invest. 45:2097–2103.
2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
García-Galiano D, Navarro VM, Roa J,
Ruiz-Pino F, Sánchez-Garrido MA, Pineda R, Castellano JM, Romero M,
Aguilar E, Gaytán F, et al: The anorexigenic neuropeptide,
nesfatin-1, is indispensable for normal puberty onset in the female
rat. J Neurosci. 30:7783–7792. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chung Y, Kim H, Im E, Kim P and Yang H: Th
17 Cells and Nesfatin-1 are associated with Spontaneous Abortion in
the CBA/j x DBA/2 Mouse Model. Dev Reprod. 19:243–252.
2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kim J, Sun S, Lee D, Youk H and Yang H:
Gonadotropin regulates NUCB2/nesfatin-1 expression in the mouse
ovary and uterus. Biochem Biophys Res Commun. 513:602–607.
2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hofmann T, Stengel A, Ahnis A, Buße P,
Elbelt U and Klapp BF: NUCB2/nesfatin-1 is associated with elevated
scores of anxiety in female obese patients.
Psychoneuroendocrinology. 38:2502–2510. 2013.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Chen K, Huang B, Feng J, Fan S, Hu Z, Ren
S, Tian H, Abdulkarem AL, Wang X, Tuo Y, et al: Nesfatin-1
regulates the phenotype transition of cavernous smooth muscle cells
by activating PI3K/AKT/mTOR signaling pathway to improve diabetic
erectile dysfunction. Heliyon. 10(e32524)2024.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ranjan A, Choubey M, Yada T and Krishna A:
Direct effects of neuropeptide nesfatin-1 on testicular
spermatogenesis and steroidogenesis of the adult mice. Gen Comp
Endocrinol. 271:49–60. 2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Kim S, Sun S, Kim M, Ha J, Seok E and Yang
H: NUCB2/nesfatin-1 suppresses the acrosome reaction in sperm
within the mouse epididymis. Anim Cells Syst (Seoul). 27:120–128.
2023.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Fazio L and Brock G: Erectile dysfunction:
Management update. Can Med Assoc J. 170:1429–1437. 2004.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Burnett AL: Nitric oxide in the penis:
Physiology and pathology. J Urol. 157:320–324. 1997.PubMed/NCBI
|
|
61
|
Priviero FB, Leite R, Webb RC and Teixeira
CE: Neurophysiological basis of penile erection. Acta Pharmacol
Sin. 28:751–755. 2007.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Mori Y, Shimizu H, Kushima H, Saito T,
Hiromura M, Terasaki M, Koshibu M, Ohtaki H and Hirano T:
Nesfatin-1 suppresses peripheral arterial remodeling without
elevating blood pressure in mice. Endocr Connect. 8:536–546.
2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Angelone T, Filice E, Pasqua T, Amodio N,
Galluccio M, Montesanti G, Quintieri AM and Cerra MC: Nesfatin-1 as
a novel cardiac peptide: Identification, functional
characterization, and protection against ischemia/reperfusion
injury. Cell Mol Life Sci. 70:495–509. 2013.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Seon S, Jeon D, Kim H, Chung Y, Choi N and
Yang H: Testosterone Regulates NUCB2 mRNA expression in male mouse
hypothalamus and pituitary gland. Dev Reprod. 21:71–78.
2017.PubMed/NCBI View Article : Google Scholar
|