Introduction
Idiopathic Pulmonary Fibrosis (IPF) is a distinct
type of interstitial lung disease (ILD) with an unknown etiology,
characterized by low survival rates, predominantly affecting older
individuals (1,2). Despite IPF primarily affecting a
single organ, there are numerous comorbidities that can affect the
prognosis and alter the natural course of the disease (3,4). In
fact, sleep-related disorders, including Obstructive Sleep Apnea
(OSA), have been acknowledged as a significant comorbidity with a
high prevalence in individuals diagnosed with IPF (5-8).
OSA, a disorder with high prevalence, is frequently
overlooked and has significant associations with detrimental
consequences, notably cardiovascular disease and sudden death
(9-11).
Irrespective of the severity of IPF, the presence of OSA has been
found to be correlated with a poor outcome (12,13).
Furthermore, severe OSA in IPF patients has been strongly
associated with the presence of cardiovascular diseases and with
increased systemic oxidative stress and blood biomarkers associated
with lung fibrosis (14,15).
Established airway or blood biomarkers in lung
fibrosis are still missing, despite recent advances in the
pathobiology of the disease development (16,17).
However, elevated levels of Krebs von den Lungen-6 (KL-6),
Endothelin-1 (ET-1), and S100 calcium-binding protein A9 (S100A9)
have been observed in patients with IPF, indicating that they could
serve as biomarkers for diagnosis and prognosis (18,19).
Importantly, a comprehensive assessment of these biomarkers in
patients with OSA and IPF has not yet been conducted. Therefore,
the aim of this study was to investigate (1) potential differences in serum and
bronchoalveolar lavage (BAL) KL-6 levels, and serum ET-1 and S100A9
levels in IPF-OSA patients, and (2) test the hypothesis that assessment of
these biomarkers provides a characteristic OSA signature,
potentially valuable for diagnostic screening and treatment
monitoring.
Materials and methods
Study design
We performed a cross-sectional study on newly
diagnosed patients with IPF, who were admitted to the Sleep
Disorders Center at the Medical School of the University of Crete,
Greece, for the evaluation of OSA between December 2013 and
December 2017. In order to be included in the study, patients had
to have histologically confirmed IPF (usual interstitial pneumonia)
through surgical lung biopsy. Alternatively, if a surgical biopsy
was not performed, they were eligible if they met the diagnostic
criteria for IPF outlined by the American Thoracic Society,
European Respiratory Society, and American College of Chest
Physicians (2). Patients were
deemed eligible for inclusion in the study if they exhibited
clinical stability for a period of no less than 4 weeks prior to
enrollment and possessed an educational background surpassing
elementary school. The exclusion criteria were: refusal to
participate, previous OSA diagnosis, history of thoracic surgery or
surgery in the upper respiratory tract, Central Sleep Apnea
Syndromes, Congestive Heart Failure (NYHA III-IV), a history of
life-threatening arrhythmias, severe cardiomyopathy, significant
chronic kidney disease, untreated hypothyroidism, family or
personal history of mental illness, drug or alcohol abuse, sedative
use, severe cognitive impairment (MMSE score ≤9), concurrent
oncological diseases, history of narcolepsy or restless legs
syndrome.
All subjects provided written informed consent and
ethical approval was provided by the University Hospital Ethics
Committee of the University Hospital of Heraklion (IRB number: 1045
and 17030).
Initial visit-data collection
A detailed evaluation was conducted on all patients,
encompassing various aspects such as age, body mass index (BMI)
measurement, comprehensive medical history with a focus on
sleep-related symptoms, associated conditions, comorbidities,
smoking history, and alcohol intake. In addition, we performed
pulmonary function tests (PFTs), overnight attended polysomnography
(PSG) and measurement of arterial blood gases (ABGs).
Pulmonary function tests
All patients underwent PFTs and recording of
O2 saturation (SpO2) by noninvasive pulse oximetry. We
followed standardized procedures to conduct spirometry and assess
the carbon monoxide diffusing capacity of the lung (DLco) (20,21).
Spirometry was performed with the patient in the upright and supine
position. Furthermore, we utilized the gender-age-physiology (GAP)
index, a comprehensive prognostic staging system, to summarize the
clinical-functional severity in patients with IPF. This index
incorporates various clinical and physiological variables such as
gender, age, forced vital capacity (FVC), and DLco (22). The patients were categorized into
three stages based on the GAP index: stage 1 included 24 patients
with a GAP index ranging from 0 to 3, stage 2 consisted of 15
patients with a GAP index of 4 to 5, and stage 3 comprised 6
patients with a GAP index greater than 5.
Questionnaires
All patients filled out the Epworth Sleepiness Scale
(ESS), Beck Depression scale (BDS) and quality of life
questionnaire (Short-Form-36, SF-36).
Epworth sleepiness scale (ESS)
Currently, the ESS is the most commonly utilized
subjective test for assessing daytime sleepiness in clinical
settings. This self-administered questionnaire is straightforward
and consists of eight items. It measures the risk of falling asleep
in specific everyday situations. A score of 10 or below is
considered to be within the normal range. As the score increases
(ranging from 10 to 24), the level of reported daytime sleepiness
also increases (23).
Beck depression inventory (BDI). The 21-item
questionnaire is a widely recognized and extensively validated
self-report measure of depressive symptoms. The BDI assesses the
intensity of depressive symptoms experienced in the week prior. The
respondent rates each item by selecting one or more options,
ranging from 0 (no symptoms) to 3 (most severe level). Scores can
range from 0 to 63, representing the total of the highest level
endorsed on each item. Any score below 10 is considered to be
within the normal range (24).
Short-Form 36 Health Survey: This
questionnaire, consisting of 36 items, is a reliable and validated
tool for assessing the general health and quality of life. The
SF-36 health survey consists of eight domains, each scored on a
scale from 0 (worst) to 100 (best). The SF-36 scales are classified
into two dimensions, namely physical health and mental health. The
scale for the score is 0 to 100, with 100 representing the highest
quality of life and 0 representing the lowest (25).
Polysomnography (PSG)
Each patient underwent a single-night full
diagnostic Polysomnography (PSG) study (Alice 5, Diagnostics
System, Respironics, USA) following standard procedures, with
monitoring of the electroencephalogram (EEG) (using three EEG
derivations, frontal, central, and occipital), electro-oculogram,
electromyogram, flow (by oronasal thermistor and nasal air pressure
transducer), thoracic and abdominal respiratory effort (by
respiratory inductance plethysmography), pulse oximetry (SpO2), and
body position monitoring. Snoring was recorded by a microphone
placed on the anterior neck. The definition of apnea and hypopnea
followed the American Academy of Sleep Medicine (AASM) standard
criteria (26). The apnea-hypopnea
index (AHI), calculated as the number of apnea and hypopnea events
per hour of sleep, was used to diagnose OSA and assess its
severity. OSA was considered mild if the AHI was ≥5 per h but
<15 per h, as moderate if AHI was ≥15 per h but <30 per h,
and as severe if AHI was ≥30 per h.
Biomarker measurements
Once overnight polysomnography was completed, blood
samples were collected in the morning after fasting overnight.
Bronchoalveolar lavage (BAL) was obtained from patients with a
flexible bronchoscope wedged into a subsegmental bronchus of a
predetermined region of interest based on radiographical findings.
A total of 120 ml of normal saline were instilled in 60-ml
aliquots, retrieved by low suction. The BAL fractions were pooled
and split equally into two samples. Bronchoalveolar lavage fluid
(BALF) was passed through a Millipore filter to isolate cells in
suspension from debris and mucus. To pellet cells, samples were
centrifuged at 1,500 rpm for five minutes at room temperature (RT)
and BAL supernatant was stored in aliquots at -80˚C. KL-6 levels
were measured in the supernatant. Serum and BAL KL-6 levels were
measured using Nanopia KL-6 assay (Sekusui Diagnostics GMBH) with
OLYMPUS AU640, and were expressed as Units/ml according to the
manufacturer's instructions. The KL-6 level in healthy individuals
has a reference range of 105.3-401.2 U/ml according to
manufacturer's instructions. ET-1 serum concentration was measured
with Endothelin-1 Quantikine ELISA (R&D Systems, DET100) and
expressed as pg/ml, according to manufacturer's instructions. The
mean ± standard deviation (SD) ET-1 levels in healthy volunteers
are 1.24±0.35 pg/ml with a range of 0.47-2 pg/ml according to the
manufacturer. S100A9 serum concentration was measured with human
S100A9 DuoSet ELISA (R&D Systems, DY5578) and were expressed as
µg/ml according to manufacturer's instructions.
Statistical analysis
Results are presented as mean ± SD for continuous
variables if normally distributed, and as median (25-75th
percentile) if not. Absolute numbers (or percentages) are used to
represent qualitative variables. To compare groups, we used a
two-tailed unpaired t-test for independent samples (when data was
normally distributed) or a Mann-Whitney U test (when data was not
normally distributed) for continuous variables. For categorical
variables, Fisher's exact test or the χ2 test was used.
To determine the relationship between different parameters and
inflammatory biomarker levels, we used the Spearman's correlation
test (non-normally distributed data) to calculate correlation
coefficients for all the independent predictors. We included
various clinically relevant factors as independent variables, such
as age, gender, BMI, smoking history, comorbidities, AHI, oxygen
desaturation index (ODI), average and minimum SpO2 levels during
sleep, duration of time with SpO2 below 90%, arterial blood gas
(ABG) measurements, and spirometry data. In addition, we employed
multivariate linear regression analysis to investigate the
potential relationship between biomarker levels and indices of
respiratory function and severity of OSA. Potential explanatory
variables, such as age, gender, BMI, GAP index, PFTs, indices of
OSA severity, smoking status, and co-morbidities, were taken into
account when adjusting all models. A P-value less than 0.05 was
deemed to be statistically significant. Data were analyzed using
PAWP 17.0 software (SPSS Inc, Chicago, IL).
Results
Patient characteristics and
polysomnographic findings
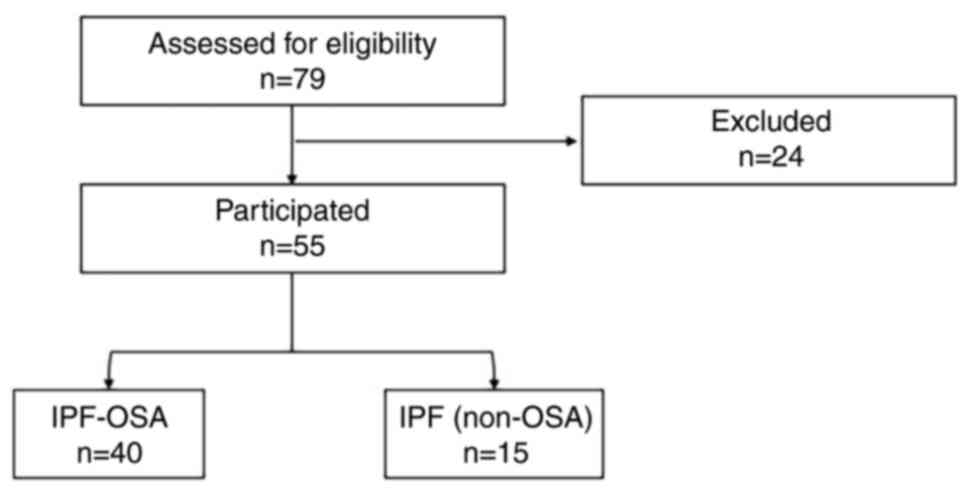
Fifty-five subjects (46 males, 9 females) were
included in the study (Fig. 1). At
the time of the study, all patients were treatment naïve and did
not receive corticosteroids or require oxygen supplementation.
Using AHI ≥15 for OSA diagnosis resulted in: 15 patients with IPF
(27%), and 40 IPF-OSA (73%). Table
I summarizes the clinical variables collected for the two
groups. Age, gender, BMI, comorbidities, smoking status and PFTs
were not different between the two groups (P>0.05). As expected,
AHI, ODI, mean SaO2, min SaO2, and Total
Sleep Time with oxygen saturation below 90% (TST 90) were worse in
the IPF-OSA group (Table II).
Concerning sleepiness, depressive symptoms, and quality of life,
patients with IPF-OSA showed more severe functional impairments,
which reached statistical significance only for Physical Component
Summary (PCS) of the SF-36 (56 vs. 67, P=0.03).
 | Table IBaseline demographics, spirometric
measurements and ABG analysis results of the included patients. |
Table I
Baseline demographics, spirometric
measurements and ABG analysis results of the included patients.
|
Characteristics | All patients
(n=55) | IPF (n=15) | IPF-OSA (n=40) | P-value |
|---|
| Age, years | 73.4±5.7 | 73.9±5.6 | 73.2±5.9 | 0.69 |
| Male, n (%) | 46(84) | 13(87) | 33(83) | 0.71a |
| BMI,
kg/m2 | 30.4±4.1 | 29.6±3.6 | 30.8±4.3 | 0.36 |
| Neck circumference,
cm | 41.2±2.9 | 41.6±2.4 | 41.1±3.0 | 0.62 |
| Waist
circumference, cm | 110.5±10.2 | 106.9±9.4 | 110.7±10.4 | 0.13 |
| Hip circumference,
cm | 106.9±8.6 | 105.6±6.9 | 107.4±9.1 | 0.51 |
| Smoking status, n
(%) | | | | 0.33b |
|
Current
smokers | 3(6) | 1(7) | 2(5) | |
|
Ex-smokers | 37(67) | 8(53) | 29(73) | |
| Comorbidities, n
(%) | | | | |
|
Diabetes
mellitus | 19(35) | 5(33) | 14(35) | 0.99b |
|
Hypertension | 30(55) | 8(53) | 22(55) | 0.91a |
|
Dyslipidaemia | 20(36) | 6(40) | 14(35) | 0.76b |
|
Ischemic
heart disease | 10(18) | 2(13) | 8(20) | 0.71b |
|
Atrial
fibrillation | 4(7) | 0 (0) | 4(10) | 0.57b |
|
Compensated
heart failure | 2(4) | 0 (0) | 2(5) | 0.99b |
|
Hypothyroidism | 7(13) | 3(20) | 4(10) | 0.38b |
|
COPD | 11(20) | 3(20) | 8(20) | 0.99b |
| GAP index, n
(%) | | | | 0.37b |
|
Stage I | 16(29) | 3(20) | 13(32) | |
|
Stage
II | 32(58) | 10(67) | 22(55) | |
|
Stage
III | 7(13) | 2(13) | 5(13) | |
| PFTs | | | | |
|
FVC, %
predicted | 75.4±17.0 | 74.9±18.1 | 75.6±16.9 | 0.89 |
|
FEV1/FVC | 82.0±6.0 | 83.4±6.1 | 81.5±6.0 | 0.31 |
|
TLC, %
predicted | 69.8±15.1 | 64.6±16.9 | 71.8±14.1 | 0.12 |
|
DLCO, %
predicted | 50.7±16.7 | 50.7±16.6 | 50.7±17.0 | 0.99 |
|
KCO, %
predicted | 87.0±22.9 | 88.8±22.0 | 86.4±23.5 | 0.73 |
| ABGs | | | | |
|
pH | 7.41±0.02 | 7.42±0.02 | 7.41±0.02 | 0.13 |
|
pPCO2,
mmHg | 39.4±3.7 | 38.5±3.1 | 39.7±3.9 | 0.32 |
|
pPO2,
mmHg | 71.2±8.2 | 70.2±8.7 | 71.6±8.2 | 0.59 |
|
HCO3-,
mmol/l | 25.1±2.0 | 24.9±1.5 | 25.2±2.2 | 0.73 |
 | Table IIBaseline PSG data and questionnaires
scores of the final sample. |
Table II
Baseline PSG data and questionnaires
scores of the final sample.
| Variables | All patients
(n=55) | IPF (n=15) | IPF-OSA (n=40) | P-value |
|---|
| Diagnostic PSG | | | | |
|
TRT,
min | 435±40 | 425±46 | 438±37 | 0.28 |
|
TST,
min | 235±57 | 216±67 | 242±52 | 0.13 |
|
SE, % | 54±12 | 51±14 | 55±11 | 0.22 |
|
WASO,
min | 153±52 | 155±64 | 153±48 | 0.92 |
|
NREM,
%TST | 91±3 | 91±3 | 91±3 | 0.99 |
|
SWS,
%TST | 7 (6-10) | 7 (7-10) | 7 (6-9) | 0.49 |
|
REM,
%TST | 9±3 | 9±3 | 9±3 | 0.99 |
|
AHI,
events/h | 22 (13-40) | 8 (4-12) | 26 (5-12) | <0.01 |
|
REM AHI,
events/h | 31 (13-48) | 6.5 (1.0-9.25) | 39 (30-58) | <0.01 |
|
AI, /h | 45±13 | 37±12 | 49±11 | <0.01 |
|
ODI,
events/h | 26 (18-40) | 9 (4-14) | 30 (23-54) | <0.01 |
|
Mean
SaO2, % | 92 (89-93) | 94 (92-94) | 91 (86, 92) | <0.01 |
|
Minimum
SaO2, % | 81 (78-84) | 86 (85-89) | 80 (74-82) | <0.01 |
|
TST90,
min | 40 (18-141) | 5 (0-10) | 95 (33-195) | <0.01 |
| Questionnaire
scores | | | | |
|
ESS | 7±5 | 7±5 | 8±5 | 0.53 |
|
ESS ≥10, n
(%) | 20(36) | 5(33) | 15(38) | 0.54a |
|
BDS | 11±5 | 10±5 | 11±6 | 0.51 |
|
BDS ≥10, n
(%) | 31(56) | 6(40) | 25(63) | 0.13b |
|
SF-36 | | | | |
|
PF | 62±22 | 70±16 | 39±23 | 0.07 |
|
RP | 56±29 | 67±29 | 52±28 | 0.08 |
|
BP | 76±22 | 77±18 | 75±23 | 0.78 |
|
GH | 54±17 | 60±16 | 51±17 | 0.11 |
|
PCS | 59±17 | 67±16 | 56±17 | 0.03 |
|
VT | 55±16 | 57±16 | 54±18 | 0.56 |
|
SF | 78±19 | 80±20 | 77±20 | 0.68 |
|
RE | 70±26 | 74±28 | 68±26 | 0.45 |
|
MH | 64±15 | 67±9 | 63±17 | 0.46 |
|
MCS | 64±15 | 70±16 | 61±15 | 0.08 |
Evaluation of KL-6, ET-1 and S100A9
levels
Table III shows
the results of the measurement of the three studied molecules;
KL-6, ET-1 and S100A9, in the whole IPF population and per group.
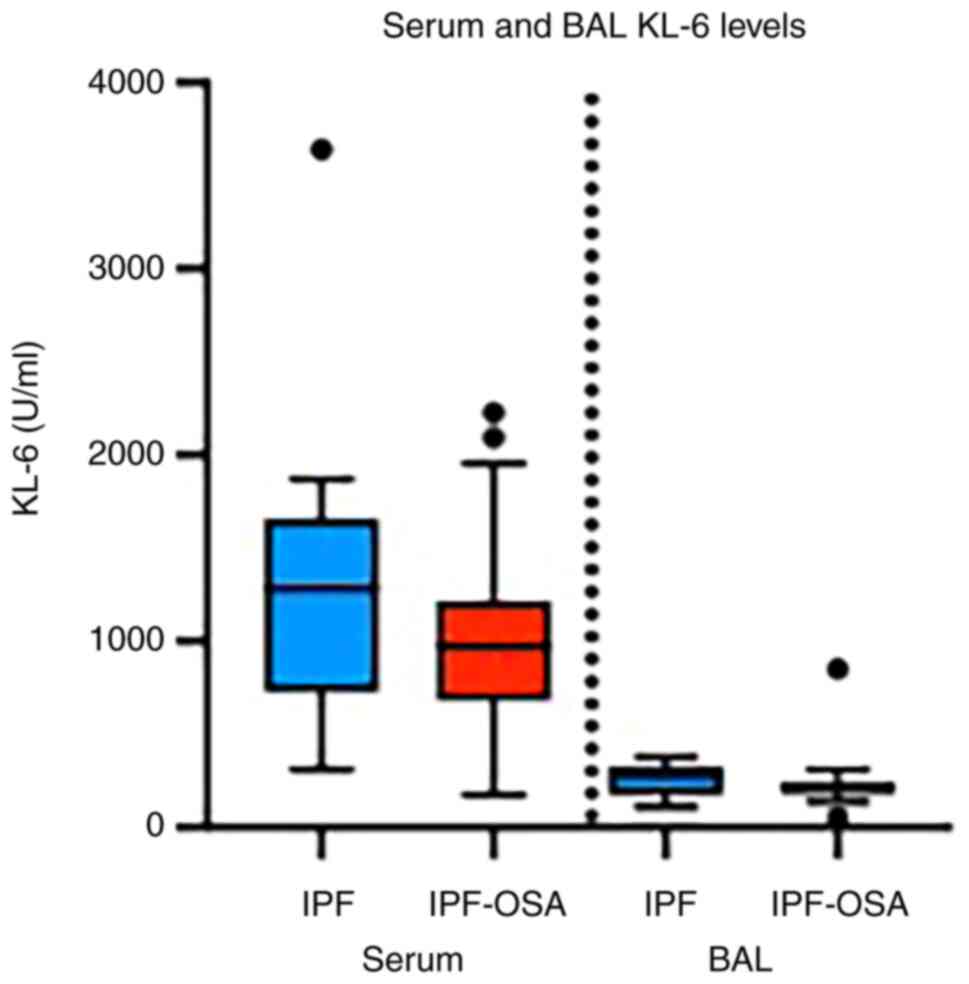
Serum KL-6 levels were found increased in comparison with BAL KL-6
levels in all IPF patients, while no difference was observed
between patients with IPF and patients with IPF and OSA
(P>0.05), either in serum or in BAL (Table III; Fig. 2).
 | Table IIILevels of serum KL-6, BAL KL-6, ET-1
and S100A9 in the entire cohort and in the two groups. |
Table III
Levels of serum KL-6, BAL KL-6, ET-1
and S100A9 in the entire cohort and in the two groups.
| Variables | All patients
(n=55) | IPF (n=15) | IPF-OSA (n=40) | P-value |
|---|
| Serum KL-6
(U/ml) | 1087
(726-1407) | 1280
(729-1659) | 975 (682-1217) | 0.12 |
| BAL KL-6
(U/ml) | 205 (183-294) | 271 (175-330) | 205 (183-245) | 0.59 |
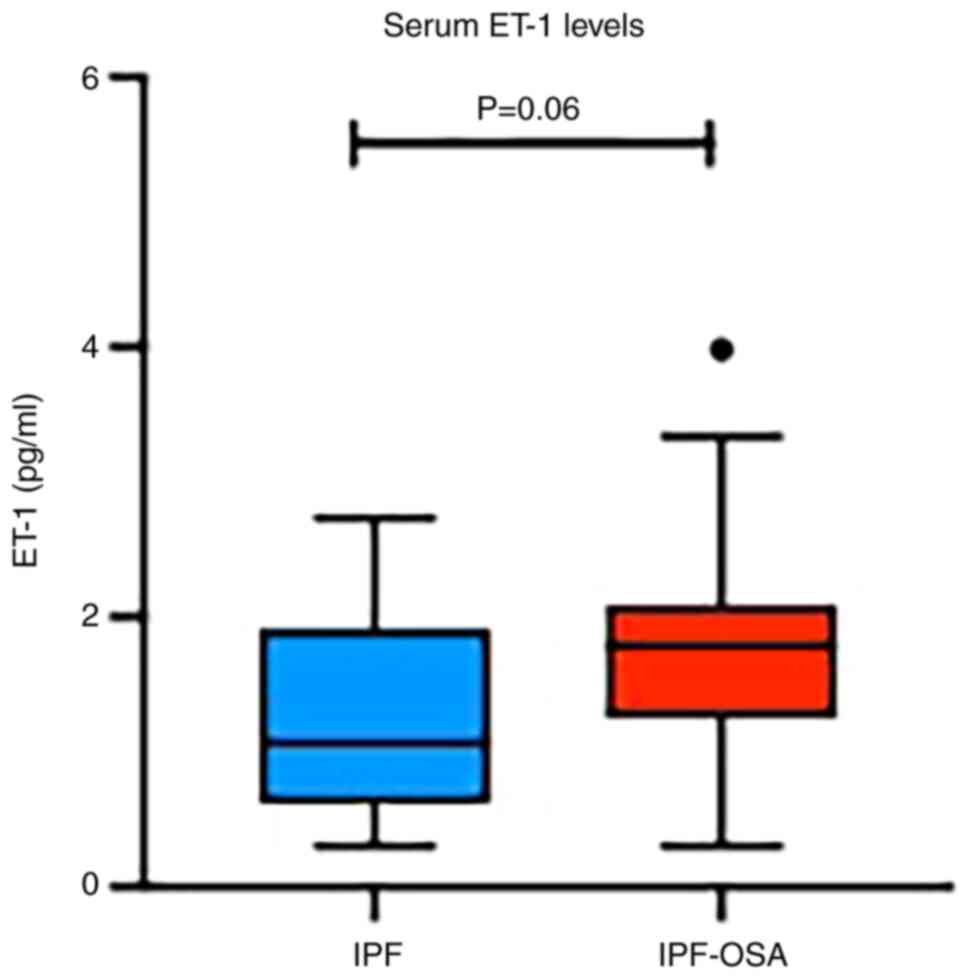
| ΕΤ-1 (pg/ml) | 1.74
(0.99-2.03) | 1.07
(0.63-1.90) | 1.78
(1.00-1.98) | 0.06 |
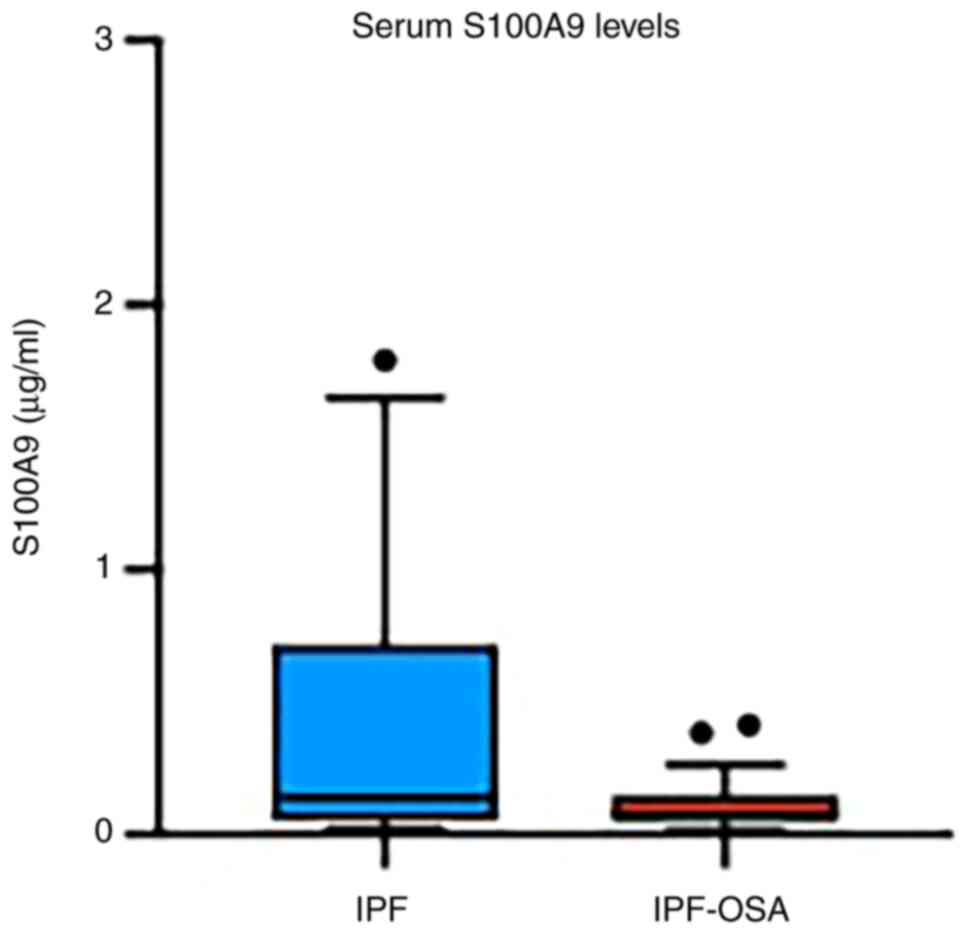
| S100Α9 (µg/ml) | 0.89
(0.05-0.17) | 0.14
(0.05-0.72) | 0.08
(0.05-0.15) | 0.23 |
Regarding ET-1, increased serum levels were detected
in the IPF-OSA group, compared with IPF patients without OSA,
although this result was marginally statistically significant
(P=0.06) (Table III; Fig. 3). Interestingly, subgroup analysis
based on OSA severity, revealed further increase of ET-1 in
patients with severe OSA (AHI ≥30) compared to IPF-non-OSA group
(1.74 vs. 1.07, P=0.07).
S100A9 serum levels were also evaluated, but no
significant difference was found between the two studied groups of
patients (Table III; Fig. 4).
Correlation analysis
In the whole studied population, significant
correlations were found between serum KL-6 levels and the severity
of IPF, assessed by GAP index (r=0.507, P=0.001), TLC (%) (r=-0.43,
P=0.008), DLCO (%) (r=-0.46, P=0.005), and KCO (%) (r=-0.38,
P=0.02) (Table SI). Serum levels
of KL-6 were still correlated with GAP index independently of
obesity, smoking, or indices of OSA severity (β=321.7, P=0.001).
Furthermore, BAL KL-6 levels were correlated with nocturnal mean
SaO2 (r=-0.49, P=0.028), even after adjustment for
obesity, smoking and GAP index (β=-25.273, P=0.04) and S100A9
levels (r=0.509, P=0.019). Correlations were also found between
ET-1 and GAP index (r=0.365, P=0.006), DLCO (%) (r=-0.53,
P<0.001), KCO (%) (r=-0.54, P<0.001) and TST90 (r=0.32,
P=0.021) (Table SII). However,
these correlations disappeared after adjustment for obesity and
smoking.
Furthermore, we analyzed the correlation between the
indices of OSA severity, arterial blood gases, pulmonary function,
and the levels of these markers separately in the two groups, in
order to evaluate a possible association of these molecules in
IPF-OSA patients. Interestingly, serum KL-6 was correlated with
nocturnal mean SaO2 only in the IPF group (r=0.73,
P=0.011), and BAL KL-6 correlated with AHI (r=0.55, P=0.04) and
with nocturnal mean SaO2 (r=-0.66, P=0.01) only in the
IPF-OSA group. All these correlations persisted, although with a
marginally statistical significance, after adjustments for obesity,
smoking and GAP index (β=221.718, P=0.05, β=3.566, P=0.04 and
β=-29.969, P=0.08 respectively).
ET-1 was correlated with TST90 (Table SII) in IPF-OSA group (r=0.32,
P=0.04), but this correlation disappeared after adjustments for
obesity, smoking and GAP index. In the IPF-OSA group S100A9 levels
were also correlated with indices of OSA severity (Table SIII), including TST90 (r=0.34,
P=0.03) and with a borderline significance after adjustments for
obesity, smoking and GAP index (β=0.015, P=0.06), ODI (r=0.36,
P=0.023) which persisted after adjustments for obesity, smoking and
GAP index (β=0.013, P=0.02), and nocturnal mean SaO2
(r=-0.32, P=0.04), but this correlation disappeared after
adjustments for obesity, smoking and GAP index.
Discussion
The prevalence of OSA exhibited a persistent upward
trend, in patients with IPF, with reported prevalence ranging from
10 to 88% in several cohorts (6-8,13,27-29).
The underlying pathophysiologic mechanisms of this relationship are
still not fully understood. However, there is data implicating
intermittent hypoxia and aging-related mechanisms in OSA, such as
oxidative stress and short telomere length, in the pathogenesis or
disease progression of pulmonary fibrosis (15,27).
On the other hand, it could be expected that restrictive lung
diseases, including IPF, are characterized by reduced lung volumes
which could induce upper airway instability and promote OSA
(30).
Our study investigated the levels of three molecules
that have been associated with lung fibrosis in order to evaluate
potential differences between IPF patients with and without OSA,
Particularly, we assessed the levels of ET-1 in serum, as well as
the levels of KL-6 in serum and BAL, and S100A9 in serum, in a
group of consecutive IPF patients who were suspected to have OSA.
Only newly diagnosed and treatment-naïve IPF patients were included
in the current study. Therefore, we evaluated patients upon IPF
diagnosis and investigated the presence of comorbid OSA. We
compared patients with both IPF and OSA vs. IPF without OSA,
although it remains indeterminable whether OSA or IPF developed
first in these patients. Our findings showed an increase in serum
ET-1 in IPF-OSA group, when compared with IPF patients without OSA,
while it was correlated statistically significant with OSA severity
parameters. In the whole studied population, ET-1 levels were
significantly correlated with GAP index and pulmonary function
tests. Moreover, independent associations of serum KL-6 levels and
IPF severity index were detected. Significant associations were
also noted in BAL KL-6 and serum S100A9 levels with specific OSA
severity parameters in IPF-OSA subgroup.
ET-1 is a peptide hormone primarily synthesized by
the vascular endothelium, initially described for its role in
vasoconstriction, although it has been also involved in pulmonary
fibrosis pathogenesis, as a mediator of transforming growth factor
(TGF)-b pathway and enhancer of fibroblasts differentiation and
collagen production (19,31,32).
Current literature has described an association between serum ET-1
levels and IPF severity, suggesting a role as a potential disease
biomarker (33,34). In our study, ET-1 levels were
increased in the IPF-OSA compared to IPF group (1.78 vs. 1.07), and
this difference seemed to be driven by the OSA severity.
Furthermore, ET-1 levels were associated with PFTs and indices of
OSA severity, although these correlations disappeared after
adjustment for obesity and smoking. As ET-1 levels in OSA patients
are associated with increased cardiovascular risk (35), larger and prospective studies are
needed to clarify the role of ET-1 in IPF patients with OSA.
KL-6, a mucin-like integral membrane glycoprotein,
localized to type II alveolar epithelial cells and bronchial
epithelial cells, has been found elevated in the blood of patients
with lung injury, while its levels have been also associated with
severity and prognosis in patients with pulmonary fibrosis
(36-38).
In our study, serum KL-6 levels were found increased in comparison
with BAL levels, and they were correlated with IPF severity, in
accordance with current literature (38). Importantly, our analysis in IPF-OSA
patients revealed that BAL KL-6 levels were correlated with indexes
of OSA severity, independently of obesity or smoking, implying a
possible further epithelial injury in IPF-OSA patients. While the
exact role of KL-6 as a biomarker for lung injury in OSA remains
unclear, a previous study with a small sample size indicated that
some patients with OSA had elevated circulating levels of KL-6,
proposing the possibility of subclinical lung injury in OSA
(39). Despite the potential
influence of comorbid obesity and smoking exposure on KL-6 levels
and lung diffusion capacity, our study demonstrated that even after
accounting for these factors, the correlation between serum KL-6
and pulmonary function impairment, as well as the relationship
between BAL KL-6 and indices of OSA severity, remained
statistically significant (30,31).
S100A9, additionally known as myeloid-related
protein 14 or calgranulin B, belongs to the family of
calcium-binding proteins S100. It is mostly expressed in
neutrophils, but also in endothelial cells and macrophages,
exerting immunomodulatory and profibrotic properties (40). Previous studies have suggested a
role of S100A9 for diagnosis and prognosis in interstitial lung
diseases (40-42).
In our study, serum S100A9 levels were correlated significantly
with indices of OSA severity in patients with IPF and OSA, even
after adjustments for obesity, smoking and GAP index. As far as we
are concerned, our study is the first one evaluating the levels of
S100A9 in serum of IPF patients with OSA, implying a potent
association with OSA severity, which should be further investigated
in larger cohorts.
The results of our study suggested that the levels
of ET-1, KL-6 and S100A9, previously described as potential
biomarkers for lung fibrosis, could also be associated with the
presence of OSA, as well as OSA severity, in IPF patients. Our
findings may provide a warning that lung injury is more prominent
in patients with IPF and OSA.
Given that the clinical characteristics,
progression, and mortality of IPF can be influenced by the presence
of multiple comorbidities like OSA, it is imperative to prioritize
the early identification and treatment of OSA alongside the
treatment of IPF (4,29). Given the fact that patients
commonly underestimate their symptoms and delay OSA diagnosis, it
is of great importance for the clinician to seek for biomarkers
that could imply a potential OSA signature in IPF patients, so
further evaluation for OSA co-existence can be suggested. Moreover,
a comprehensive understanding of the underlying pathogenetic
mechanisms that drive the development of IPF and OSA will shed
light to the identification of valuable biomarkers, which may be
used as additional screening OSA tools, as well as therapeutic
targets. Nonetheless, further studies are needed to uncover whether
detection and intervention on specific biomarkers are promising new
treatments for IPF patients with comorbid OSA.
Our study was limited by the relatively small number
of participants in our cohort, which could explain the failure to
detect statistically significant effects of many of the parameters
measured. Therefore, further extensive research is required to
validate those findings. Another limitation to be noted was that
levels of the studied molecules were not assessed after treatment
of OSA; thus, we could not provide data about their potent use as
evaluators of treatment effect. Finally, the limitations of the
cross-sectional design prevented us from drawing causal conclusions
or determining the direction of the effects we observed. However,
given that IPF is a relatively rare disease, the strength of our
study was that we collected serum as well as BAL from
well-characterized, treatment naïve IPF patients.
In conclusion, our findings showed increased serum
ET-1 levels in IPF-OSA patients in comparison with IPF patients
without OSA, and significant associations in serum ET-1, S100A9 and
BAL KL-6 levels with specific OSA severity parameters in IPF-OSA
group of patients. The data presented here may indicate that these
molecules might be used as biomarkers for IPF-OSA, probably
reflecting the additional lung injury of OSA in pulmonary fibrosis.
Larger studies should confirm our results maybe suggesting a
characteristic OSA signature with diagnostic screening value and
utility in treatment monitoring.
Supplementary Material
Correlations of serum Krebs von den
Lungen-6 measurements.
Correlations of serum endothelin-1
measurements.
Correlations of serum S100
calcium-binding protein A9 measurements.
Acknowledgements
This abstract was presented at the 24th Congress of
the European Sleep Research Society (September 25-28, 2018; Basel,
Switzerland), and was published as Abstract no. 679.
Funding
Funding: The present study was supported by a H.F.R.I. Research
Project for the Support of Faculty Members (grant no. 2143;
University of Crete).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
IB and SM contributed to the acquisition of human
samples, and confirm the authenticity of the raw data. SM, EV and
NT performed the bronchoscopies. ET and CK performed the
experiments. IB, SM and EV reviewed the literature. IB, SM, EV and
NT analyzed and interpreted the data. IB, EV, NT, SS and KMA were
involved in the drafting of the manuscript. SS and KMA supervised
the study and contributed to the conception and design of the
study. All authors were involved in the writing of the manuscript,
and all authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients who participated in the study. The study was approved by
the Ethics Committees of the University Hospital of Heraklion
(institutional review board no. 1045 and 17030; Crete, Greece).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Raghu G, Remy-Jardin M, Myers JL, Richeldi
L, Ryerson CJ, Lederer DJ, Behr J, Cottin V, Danoff SK, Morell F,
et al: Diagnosis of idiopathic pulmonary fibrosis. An official
ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care
Med. 198:e44–e68. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Raghu G, Remy-Jardin M, Richeldi L,
Thomson CC, Inoue Y, Johkoh T, Kreuter M, Lynch DA, Maher TM,
Martinez FJ, et al: Idiopathic pulmonary fibrosis (an Update) and
progressive pulmonary fibrosis in adults: An official
ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care
Med. 205:e18–e47. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cano-Jiménez E, Hernández González F and
Peloche GB: Comorbidities and complications in idiopathic pulmonary
fibrosis. Med Sci (Basel). 6(71)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Margaritopoulos GA, Antoniou KM and Wells
AU: Comorbidities in interstitial lung diseases. Eur Respir Rev.
26(160027)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mermigkis C, Bouloukaki I, Antoniou K,
Papadogiannis G, Giannarakis I, Varouchakis G, Siafakas N and
Schiza SE: Obstructive sleep apnea should be treated in patients
with idiopathic pulmonary fibrosis. Sleep Breath. 19:385–391.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mermigkis C, Stagaki E, Tryfon S, Schiza
S, Amfilochiou A, Polychronopoulos V, Panagou P, Galanis N,
Kallianos A, Mermigkis D, et al: How common is sleep-disordered
breathing in patients with idiopathic pulmonary fibrosis? Sleep
Breath. 14:387–390. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lancaster LH, Mason WR, Parnell JA, Rice
TW, Loyd JE, Milstone AP, Collard HR and Malow BA: Obstructive
sleep apnea is common in idiopathic pulmonary fibrosis. Chest.
136:772–778. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Karuga FF, Kaczmarski P, Szmyd B,
Białasiewicz P, Sochal M and Gabryelska A: The association between
idiopathic pulmonary fibrosis and obstructive sleep apnea: A
systematic review and meta-analysis. J Clin Med.
11(5008)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xie W, Zheng F and Song X: Obstructive
sleep apnea and serious adverse outcomes in patients with
cardiovascular or cerebrovascular disease: A PRISMA-compliant
systematic review and meta-analysis. Medicine (Baltimore).
93(e336)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shahar E, Whitney CW, Redline S, Lee ET,
Newman AB, Nieto FJ, O'Connor GT, Boland LL, Schwartz JE and Samet
JM: Sleep-disordered breathing and cardiovascular disease:
Cross-sectional results of the sleep heart health study. Am J
Respir Crit Care Med. 163:19–25. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gami AS, Howard DE, Olson EJ and Somers
VK: Day-night pattern of sudden death in obstructive sleep apnea. N
Engl J Med. 352:1206–1214. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bosi M, Milioli G, Fanfulla F, Tomassetti
S, Ryu JH, Parrino L, Riccardi S, Melpignano A, Vaudano AE,
Ravaglia C, et al: OSA and prolonged oxygen desaturation during
sleep are strong predictors of poor outcome in IPF. Lung.
195:643–651. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee JH, Jang JH, Park JH, Lee S, Kim JY,
Ko J, Jung SY, Kim DW, Hong S and Jang HJ: Prevalence and clinical
impacts of obstructive sleep apnea in patients with idiopathic
pulmonary fibrosis: A single-center, retrospective study. PLoS One.
18(e0291195)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gille T, Didier M, Boubaya M, Moya L,
Sutton A, Carton Z, Baran-Marszak F, Sadoun-Danino D, Israël-Biet
D, Cottin V, et al: Obstructive sleep apnoea and related
comorbidities in incident idiopathic pulmonary fibrosis. Eur
Respir. 49(1601934)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Melo NCV, Amorim FF and Santana ANC:
Connecting the dots: Hypoxia, pulmonary fibrosis, obstructive sleep
apnea, and aging. Am J Respir Crit Care Med.
191(966)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sindhu A, Jadhav U, Ghewade B, Wagh P and
Yadav P: Unveiling the diagnostic potential: A comprehensive review
of bronchoalveolar lavage in interstitial lung disease. Cureus.
16(e52793)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jee AS, Sahhar J, Youssef P, Bleasel J,
Adelstein S, Nguyen M and Corte TJ: Review: Serum biomarkers in
idiopathic pulmonary fibrosis and systemic sclerosis associated
interstitial lung disease-frontiers and horizons. Pharmacol Ther.
202:40–52. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lin L, Zhao Y, Li Z, Li Y, Wang W, Kang J
and Wang Q: Expression of S100A9 and KL-6 in common interstitial
lung diseases. Medicine (Baltimore). 101(e29198)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ross B, D'Orléans-Juste P and Giaid A:
Potential role of endothelin-1 in pulmonary fibrosis: From the
bench to the clinic. Am J Respir Cell Mol Biol. 42:16–20.
2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Graham BL, Steenbruggen I, Miller MR,
Barjaktarevic IZ, Cooper BG, Hall GL, Hallstrand TS, Kaminsky DA,
McCarthy K, McCormack MC, et al: Standardization of spirometry 2019
update. An official American thoracic society and European
respiratory society technical statement. Am J Respir Crit Care Med.
200:e70–e88. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Macintyre N, Crapo RO, Viegi G, Johnson
DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A,
Enright P, et al: Standardisation of the single-breath
determination of carbon monoxide uptake in the lung. Eur Respir J.
26:720–735. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ley B, Ryerson CJ, Vittinghoff E, Ryu JH,
Tomassetti S, Lee JS, Poletti V, Buccioli M, Elicker BM, Jones KD,
et al: A multidimensional index and staging system for idiopathic
pulmonary fibrosis. Ann Intern Med. 156:684–691. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Johns MW: A new method for measuring
daytime sleepiness: The Epworth sleepiness scale. Sleep.
14:540–545. 1991.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Beck AT, Steer RA and Carbin MG:
Psychometric properties of the beck depression inventory:
Twenty-five years of evaluation. Clin Psychol Rev. 8:77–100.
1988.
|
|
25
|
Martinez TY, Pereira CA, Dos Santos ML,
Ciconelli RM, Guimarães SM and Martinez JAB: Evaluation of the
short-form 36-item questionnaire to measure health-related quality
of life in patients with idiopathic pulmonary fibrosis. Chest.
117:1627–1632. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Berry RB, Brooks R, Gamaldo C, Harding SM,
Lloyd RM, Quan SF, Troester MT and Vaughn BV: AASM scoring manual
updates for 2017 (version 2.4). J Clin Sleep Med. 13:665–666.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mermigkis C, Chapman J, Golish J,
Mermigkis D, Budur K, Kopanakis A, Polychronopoulos V, Burgess R
and Foldvary-Schaefer N: Sleep-related breathing disorders in
patients with idiopathic pulmonary fibrosis. Lung. 185:173–178.
2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Schiza S, Mermigkis C, Margaritopoulos GA,
Daniil Z, Harari S, Poletti V, Renzoni EA, Torre O, Visca D,
Bouloukaki I, et al: Idiopathic pulmonary fibrosis and sleep
disorders: No longer strangers in the night. Eur Respir Rev.
24:327–339. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mermigkis C, Bouloukaki I and Schiza SE:
Sleep as a new target for improving outcomes in idiopathic
pulmonary fibrosis. Chest. 152:1327–1338. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Schiza SE, Bouloukaki I, Bolaki M and
Antoniou KM: Obstructive sleep apnea in pulmonary fibrosis. Curr
Opin Pulm Med. 26:443–448. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lagares D, Busnadiego O, García-Fernández
RA, Lamas S and Rodríguez-Pascual F: Adenoviral gene transfer of
endothelin-1 in the lung induces pulmonary fibrosis through the
activation of focal adhesion kinase. Am J Respir Cell Mol Biol.
47:834–842. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cantor J: Maximizing the therapeutic
effect of endothelin receptor antagonists in pulmonary fibrosis: A
paradigm for treating the disease. Int J Mol Sci.
25(4184)2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pulito-Cueto V, Genre F, López-Mejías R,
Mora-Cuesta VM, Iturbe-Fernández D, Portilla V, Sebastián Mora-Gil
M, Ocejo-Vinyals JG, Gualillo O, Blanco R, et al: Endothelin-1 as a
biomarker of idiopathic pulmonary fibrosis and interstitial lung
disease associated with autoimmune diseases. Int J Mol Sci.
24(1275)2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bellaye PS, Yanagihara T, Granton E, Sato
S, Shimbori C, Upagupta C, Imani J, Hambly N, Ask K, Gauldie J, et
al: Macitentan reduces progression of TGF-β1-induced pulmonary
fibrosis and pulmonary hypertension. Eur Respir J.
52(1701857)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Janssen C, Pathak A, Grassi G and Van De
Borne P: Endothelin contributes to the blood pressure rise
triggered by hypoxia in severe obstructive sleep apnea. J
Hypertens. 35:118–124. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang H, Chen L, Wu L, Huang J, Li H, Wang
X and Weng H: Diagnostic and prognostic predictive values of
circulating KL-6 for interstitial lung disease: A PRISMA-compliant
systematic review and meta-analysis. Medicine (Baltimore).
99(e19493)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang T, Shen P, Duan C and Gao L: KL-6 as
an immunological biomarker predicts the severity, progression,
acute exacerbation, and poor outcomes of interstitial lung disease:
A systematic review and meta-analysis. Front Immunol.
12(745233)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Soccio P, Moriondo G, d'Alessandro M,
Scioscia G, Bergantini L, Gangi S, Tondo P, Foschino Barbaro MP,
Cameli P, Bargagli E and Lacedonia D: Role of BAL and serum krebs
von den Lungen-6 (KL-6) in patients with pulmonary fibrosis.
Biomedicines. 12(269)2024.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lederer DJ, Jelic S, Basner RC, Ishizaka A
and Bhattacharya J: Circulating KL-6, a biomarker of lung injury,
in obstructive sleep apnoea. Eur Respir J. 33:793–796.
2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lee JU, Kim MK, Kim MS, Lee SJ, Park S
lee, Chang HS, Park JS and Park CS: S100 calcium-binding protein
A9, a potential novel diagnostic biomarker for idiopathic pulmonary
fibrosis. J Korean Med Sci. 39(e13)2024.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Araki K, Kinoshita R, Tomonobu N, Gohara
Y, Tomida S, Takahashi Y, Senoo S, Taniguchi A, Itano J, Yamamoto
KI, et al: The heterodimer S100A8/A9 is a potent therapeutic target
for idiopathic pulmonary fibrosis. J Mol Med (Berl). 99:131–145.
2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bennett D, Salvini M, Fui A, Cillis G,
Cameli P, Mazzei MA, Fossi A, Refini RM and Rottoli P: Calgranulin
B and KL-6 in bronchoalveolar lavage of patients with IPF and NSIP.
Inflammation. 42:463–470. 2019.PubMed/NCBI View Article : Google Scholar
|