Introduction
The coronavirus disease 2019 (COVID-19) is a highly
contagious viral illness, which has spread globally, affecting
millions of individuals worldwide (1). According to the World Health
Organization, as of October 2023, there have been >771 million
confirmed cases and >6 million deaths worldwide since the onset
of the COVID-19 pandemic. To be specific, Saudi Arabia reported
841,469 confirmed cases in 2023, while the United States reported
103,436,829 cases by October 2023. These figures underscore the
widespread impact of this disease (1).
Cancer patients, with their immunosuppressed status
due to their condition or its treatment, are at an increased risk
of infection in comparison to the general population. This
immunosuppression can lead to serious complications, potentially
resulting in delays of treatment and unnecessary hospitalizations,
which may adversely affect the disease prognosis (2). The immunocompromised state of cancer
patients may be attributed to antineoplastic therapies, supportive
medications such as steroids or the immunosuppressive nature of
cancer itself. In addition, immunomodulatory drugs, including
programmed cell death 1 and programmed cell death ligand 1
inhibitors, can alter the immune responses to infections (3,4).
Cancer patients, who are often at an advanced age (≥60 years) and
have one or more significant comorbidities, are at an increased
risk for COVID-19-related morbidity and mortality. These patients'
frequent interactions with the healthcare system through anticancer
therapies, monitoring and supportive care further elevate this risk
(4). Treatment for cancer within
14 days of a COVID-19 diagnosis has been identified as a risk
factor for developing severe complications, including acute
respiratory distress syndrome (28.6%), septic shock (3.6%) and
acute myocardial infarction (3.6%) (2). Among cancer patients diagnosed with
COVID-19, a study showed that 21% succumbed to the disease as
compared to 7.8% in non-cancer populations (5). Furthermore, research indicates that
cancer patients diagnosed with COVID-19 are more likely to require
hospitalization, intensive care unit (ICU) admission and mechanical
ventilation, irrespective of the cancer type or treatment. These
findings emphasize the importance of stringent infection control
measures and the necessity of treating cancer patients in
outpatient settings whenever feasible in order to decrease the risk
(2). Given the global prevalence
of cancer and the high transmissibility of severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2), understanding the disease
course and the factors affecting clinical outcomes in cancer
patients with COVID-19 is essential (4). However, most studies performed
examining cancer patients with COVID-19 have been single-center
investigations, with significant variability in both inclusion
criteria and outcomes. A common limitation is that many of these
research endeavours and studies are case series, making it
challenging to generalize findings to broader populations (5). Cancer patients represent a diverse
group and there is a need for a better understanding regarding
which patients, and which tumor- or treatment-related factors, are
associated with an increased risk of infection and related adverse
outcomes. This knowledge is crucial in determining whether an
elevated COVID-19 risk should influence cancer treatment approaches
(5).
The present study aims to evaluate cancer patients
in terms of clinical outcomes related to COVID-19 infection, with a
focus on the type of malignancy, mortality rates and other clinical
outcomes. The findings of this research could be instrumental in
protecting at-risk populations from COVID-19 or similar viral
infections, reducing disease progression, lowering mortality and
morbidity rates and ensuring optimal outcomes for cancer
patients.
Materials and methods
Literature search
A search was performed in the relevant databases,
including PubMed (https://pubmed.ncbi.nlm.nih.gov), Cochrane (https://pubmed.ncbi.nlm.nih.gov) and Embase
(https://www.elsevier.com/products/embase), starting
from August 2023 in a systematic manner. The search terms and key
words were ‘cancer’, ‘COVID-19’, ‘mortality’, ‘oncology’ and
‘impact’.
In accordance with the guidelines of Preferred
Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)
(6), the inclusion and exclusion
criteria and main outcomes of the present study were clarified in a
protocol, which was registered in International Prospective
Register of Systematic Reviews (PROSPERO; https://www.crd.york.ac.uk/prospero/; no.
CRD42023445522).
The collected studies were retrieved and downloaded
from their databases, followed by arrangement on a Google Drive
platform. The studies were arranged by folders denoting their year
of publication for subsequent screening and data analysis. The
focus was on studies relevant to the COVID-19 pandemic, so the
years searched were from 2020 to 2023. The search and screening
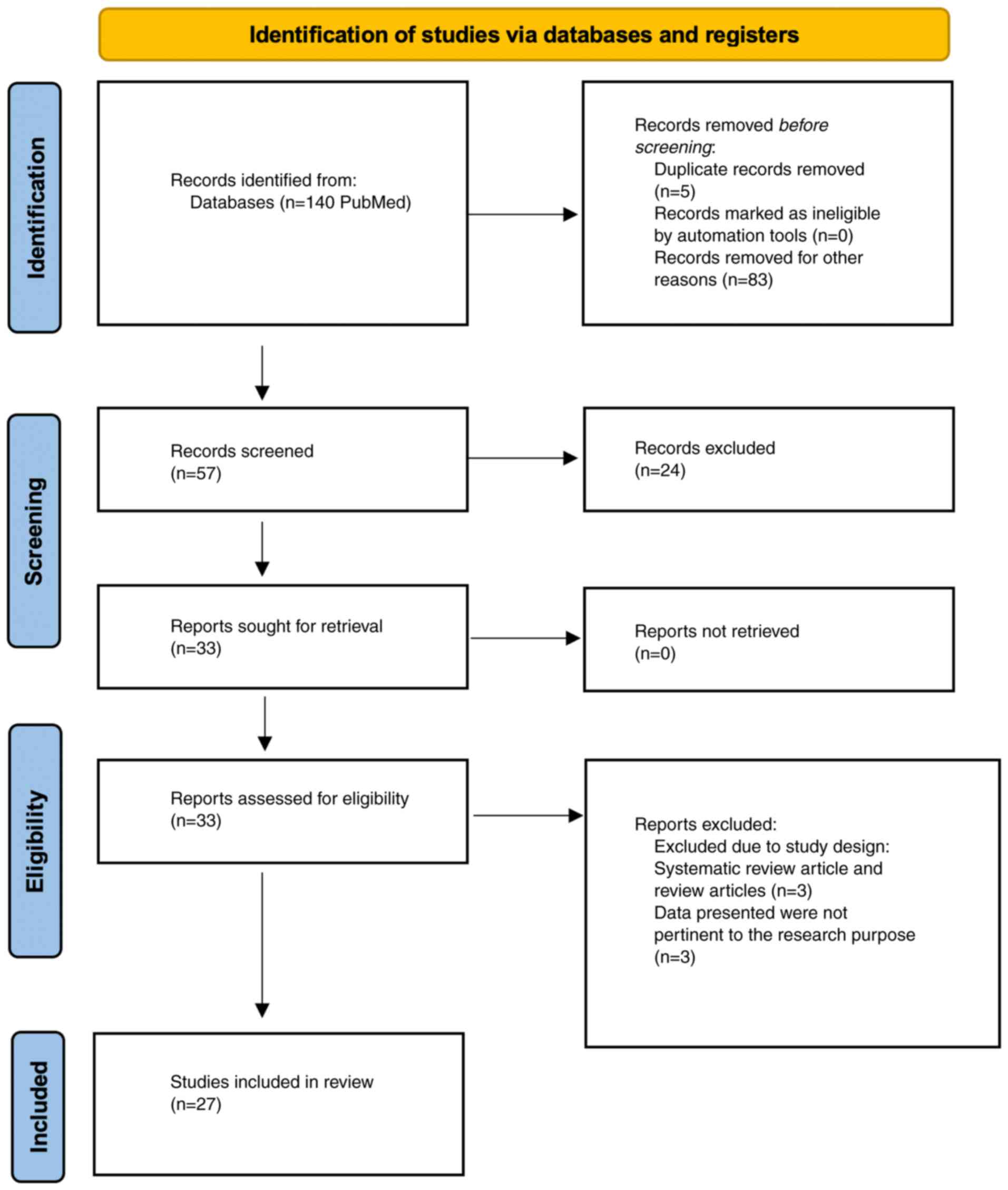
process of the studies is demonstrated in the flow chart (Fig. 1).
Eligibility criteria
The following criteria were required to be met for
the studies to be included in the present review: i) Patients
studied were diagnosed with any type of malignancy by any medically
recognized diagnostic criteria before developing COVID-19; ii)
patients were confirmed to have COVID-19 infection through any of
clinical or laboratory method; iii) any aspect of the patient's
malignancy was affected by COVID-19 infection, including their
treatment, management, screening and vaccination outcomes; iv) the
language of all included studies was confined to English.
The exclusion criteria were as follows: i) Patients
who were diagnosed with any type of malignancy after a confirmed
COVID-19 infection; ii) articles or studies categorized as case
reports or review articles; iii) studies with insufficient or
incomplete data to match any aspect of the inclusion criteria to
obtain a complete data analysis. All of the studies eligible for
the present review were evaluated by four authors (AhAA, TA, MeAA
and MoAA) and any disagreements were resolved through consulting an
author who was not part of the study's screening team (RA).
Data extraction and risk of bias
assessment
A team of five authors (AhAA, NA, TA, MeAA and MoAA)
performed the task of data extraction. The extracted content was
organized into the following categories: i) Study characteristics:
First author, publication year, type of study, sample size, number
of COVID-19 patients; ii) population characteristics: Average age
and gender; iii) outcomes for cancer patients: Mortality in cancer
patients without COVID-19, mortality in cancer patients with
COVID-19, delay in treatment and complications. The data were
cross-checked by the screening team consisting of four authors. At
any point through the process, any disagreement between two authors
was resolved or consulted by a third author (RA).
In further detail, 57 articles were transferred from
Mendeley (https://www.mendeley.com/search/) to Rayyan
(https://www.rayyan.ai) to undergo screening and
duplicate identification. Subsequently, four authors (AhAA, TA,
MeAA and MoAA) independently evaluated the articles based on their
titles. The team identified and resolved five instances of
duplication and addressed six disagreements through team
discussions. Furthermore, three independent authors (AhAA, TA and
MeAA) conducted full-text screening of the articles. Following the
screening of articles, data extraction was performed within an
Excel spreadsheet (Office 365; Version 16; Microsoft Corp.). Each
author extracted several articles, focusing on authors' names, year
of publication, country, sample size, number of COVID-19 patients,
type of cancer, average age, sex, primary outcomes (e.g.,
mortality), secondary outcomes (e.g., complications and treatment
obstacles) and concluding remarks. This process was thoroughly
reviewed by an author (RA) to ensure accuracy and completeness.
Statistical analysis
Meta-analysis was conducted on the studies, which
were extracted according to the guidelines from the PRISMA group
(6). In the statistical analysis
of events of mortality, the proportion (prevalence) of the total
participants was used as the summary statistic. The proportion
(prevalence) of mortality events among participants was used as the
summary statistic in order to indicate how common the condition was
in the study population. A random-effects model was used for
meta-analysis and inter-study heterogeneity was assessed using
χ2 and I2 statistics. The Q-test was used for
heterogeneity. Higher values of I2 and the χ2
statistic signified increased levels of inconsistency inter-studies
and P<0.001 was considered to provide evidence of significant
heterogeneity. Sensitivity analysis was conducted by sequentially
omitting one study at a time from the analysis to evaluate its
impact on the overall results and statistical significance. This
approach helped identify whether any single study
disproportionately influences the findings and allows for detection
of potential sources of heterogeneity across the included studies.
The meta-regression model was also used to determine whether gender
predominance was a source of heterogeneity. Statistical analysis
was performed using the ‘Meta’ package of R-Studio.
Risk of bias/quality assessment
The methodological quality of the observational
studies was assessed using the Newcastle-Ottawa scale (https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp)
by three independent reviewers (AhAA, TA and MeAA), with conflict
resolution achieved through mutual consensus or, if necessary,
involvement of a third party (RA). The assessment comprised three
sections, totaling nine components, examining study population
selection, comparability of factors and exposure ascertainment.
Each section featured 2 to 4 questions with ratings as high, low or
unclear risk of bias. Discrepancies in ratings underwent resolution
through discussion among reviewers (RA, AhAA and TA), with external
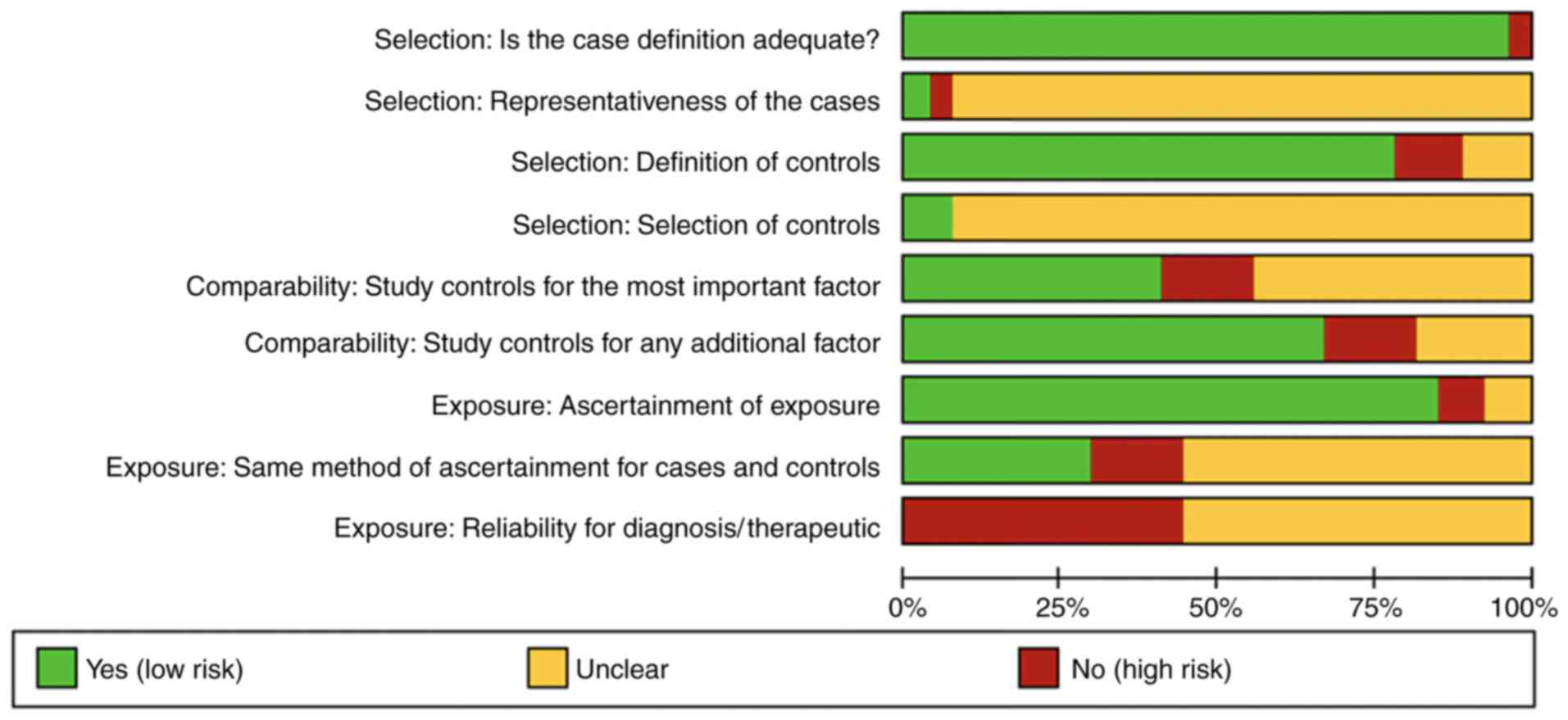
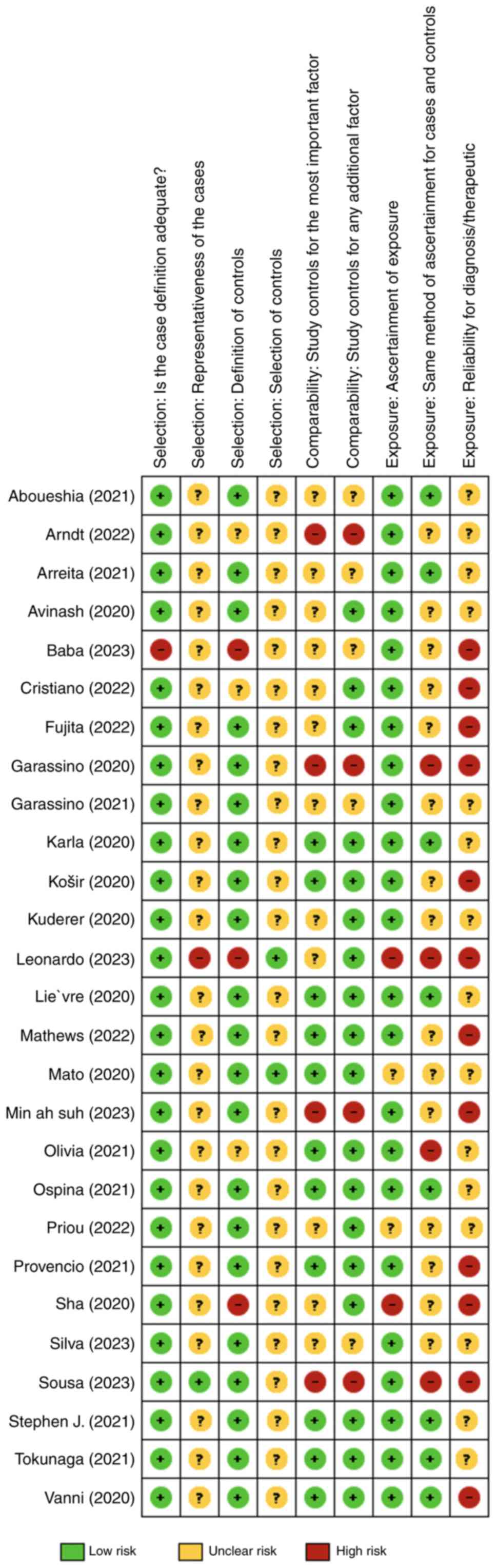
mediation available if disagreements persisted. Figs. 2 and 3 provide a comprehensive risk of bias
graph and summary, revealing generally low bias risk in study
selection domains, such as adequate cases and control definition.
However, other aspects demonstrated a higher average of risk of
bias, such as the way complications or exposures to risk factors
that were identified, measured or reported in the studies, as well
as the reliability of diagnostic criteria used, underscoring the
need for critical assessment in observational research.
Results
Sociodemographic characteristics. This review
examines the findings of 27 studies (4,7-32),
offering a detailed exploration of the interplay between COVID-19
and oncology. Initially, 140 studies were identified in accordance
the objective for the review with 5 studies removed due to
duplication, and 83 studies removed for additional reasons such as
different language and non-eligible articles like case reports and
brief reviews. Following the screening of 57 studies, 24 records
were further excluded, as they did not meet the inclusion criteria.
A total of 33 studies were further assessed for eligibility with 6
removed for reasons including the data not matching the study's
purpose. Finally, 27 studies were included in the review, as they
all met the eligibility criteria. The studies show diversity in
sample sizes, with the study by Lee et al (7), a General Community Survey, presenting
an extensive pool of 1,807,559 individuals, while a more focused
cross-sectional survey by Košir et al (8) involved 177 participants. Examining
the gender distribution within the COVID-19 patient cohorts
revealed noteworthy patterns. In the randomized clinical trial
(9), the BNT162b2 vaccine
recipients showed a notable 63.9% female majority. Conversely, a
retrospective cohort study by Solaini et al (10) displayed a balanced distribution
among COVID-19 patients. With a focus on the impact of COVID-19 on
cancer patients, Mathews et al (11) provided a detailed breakdown of 66
positive cases, demonstrating a nearly equal gender distribution
among these vulnerable individuals. Meanwhile, Lee et al
(7) reported 155 positive cases
among 23,266 individuals with cancer, emphasizing the real-world
implications of the virus in this specific population (Table I).
 | Table IStudy characteristics. |
Table I
Study characteristics.
| Author(s),
year | Type of study | Sample size | Number of COVID-19
patients | Average age,
years | Sex | (Refs.) |
|---|
| Solaini et
al, 2023 | Retrospective
cohort study | 632 | 205 | 71 | Male before
COVID-19, 254 (59.5%); Male after COVID-19, 124 (60.5%); Female
before COVID-19, 173 (40.5%); Female after COVID-19, 81
(39.5%) | (10) |
| Thomas et
al, 2022 | Randomized,
placebo-controlled, observer-blinded global phase 3 clinical
trial | 46,429 | 0 | Participants
received BNT162b2 vaccine: 64.0 (16-86) | Female received
BNT162b2 vaccine, 1,215 (63.9%); Female received placebo, 1,198
(62.7%); Total, 21,627 (49.1%) | (9) |
| Lee et al,
2020 | General community
survey | 1,807,559 | I) Total
Individuals: With cancer, 23,266; Without cancer, 1,784,293. II)
Positive COVID-19 rest reports: Among those with cancer, 155
reports; Among those without cancer, 10,249 reports | Not provided | Male without
cancer, 42.7%; Male with cancer, 55.2%; Male not on chemotherapy or
immunotherapy, 42.9%; Male on chemotherapy or immunotherapy,
45.8% | (7) |
| Košir et al,
2020 | Cross-sectional
survey | 177 | 0 | 29.33 | Female, 154 (87%);
Male, 20 (11%) | (8) |
| Dinmohamed et
al, 2020 | Nationwide
Netherlands cancer registry-based study | Not Mentioned | Not Mentioned | Not mentioned | Not mentioned | (12) |
| Mathews et
al, 2022 | Observational
study | 631 | PCR confirmed, 628;
Clinical diagnosis, 3 | Patients with
cancer who tested positive for COVID-19, 66; Patients who tested
positive for COVID-19, 62 | Male patients with
cancer who tested positive for COVID-19, 248; Female patients with
cancer who tested positive for COVID-19, 261; Male patients who
tested positive for COVID-19, 298; Female patients who tested
positive for COVID-19, 333 | (11) |
| Mendonça et
al, 2023 | Retrospective study
cohort | 29,796 | Not mentioned | The largest number
of cases occurred in males aged 55-74 years and females aged 50-69
years | Male, 11,255;
Female, 13,636 | (13) |
| Baba et al,
2023 | Retrospective | Two groups: 120 +
384=504 | 504 | Pre-covid, 53;
Pandemic, 54 | All female
(504) | (14) |
| Sha et al,
2020 | Retrospective | 161 | 161 | 57 | Male, 94; Female,
67 | (15) |
| Aboueshia et
al, 2021 | Retrospective | 260 | 57 | 58.6 | Female, 52.3 % | (16) |
| Rucinska and
Nawrocki, 2022 | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | (17) |
| Resende et
al, 2022 | Retrospective | 11,753 | 11,753 | Not mentioned | Most patients were
females | (18) |
| de Sousa et
al, 2023 | Observational
cross-sectional | 2019: 561,039 2020:
502,766 2021: 538,993 | Not mentioned | Not mentioned | Not mentioned | (19) |
| Arndt et al,
2023 | Prospective panel
survey | Mentioned each
month in 2020-2023 | Not mentioned | Not mentioned | Not mentioned | (20) |
| Kuderer et
al, 2020 | Cohort study | 928 | 928 | 66 | Female, 459 (49%);
Male, 468 (50%) | (4) |
| Vanni et al,
2020 | Retrospective
analysis | Not mentioned | Not mentioned | Not mentioned | Not mentioned | (21) |
| Garassino et
al, 2020 | Cross-sectional
component and a longitudinal cohort component | Not mentioned | 200 | 68 | Male, 141; Female,
59 | (22) |
| Tokunaga et
al, 2022 | Observational study
based on survey | The total number of
questionnaires sent was 744, but only 74% (551 out of 744) were
answered and analyzed | Not mentioned | Not mentioned | Not mentioned | (23) |
| Priou et al,
2022 | Retrospective
multicenter cohort study | 6,240 | Not mentioned | 68 | Female, 38% | (24) |
| Fujita et
al, 2022 | Retrospective
cohort study | 725 | Not mentioned | 73 | Male before
COVID-19, 298 (71.5%); Male after COVID-19, 209 (67.9%); Female
before COVID-19, 119 (28.5%); Female after COVID-19, 99
(32.1%) | (25) |
| Suh et al,
2023 | Retrospective
nationwide population-based study | It was mentioned
indirectly by the number of esophagogastroduodenoscopies on monthly
bases in 2019, 2020 and 2021 | Not Mentioned | Not mentioned | Not mentioned | (26) |
| Lara et al,
2021 |
Multi-institutional, retrospective,
observational cohort study | 193 | 193 | 65 | Not mentioned | (27) |
| Arrieta et
al, 2021 | Prospective cohort
study | 548 | 66 | Mean:
61.5±12.9 | Female, 312; Male,
236 | (28) |
| Provencio et
al, 2021 | Prospective
observational study | 447 | 447 | Mean: 67.1±9 | Male, 332; Female,
115 | (29) |
| Mato et al,
2020 | International
cohort study, multicentric | 198 | 198 | Mean: 70.5
(38-98) | Male, 63%; Female,
37% | (30) |
| Ospina et
al, 2021 | Analytical cohort
study | 742 | 720 | Not mentioned | Female, 403; Male,
339 | (31) |
| Lièvre et
al, 2020 | Retro-prospective
cohort study | 1,289 | 1,289 | Mean: 67
(19-100) | Female, 494; Male,
795 | (32) |
Mortality and complications among
cancer patients
This review study also encompassed various cancer
types and their outcomes during the COVID-19 pandemic demonstrated
in Table II, shedding light on
mortality rates, treatment delays and complications. In gastric
adenocarcinoma, Solaini et al (10) found a higher mortality rate in
COVID-19 patients (5.9%) compared to pre-COVID cases (2.6%), with
potential delays in diagnosis and treatment. Thomas et al
(9) observed no mortality in
patients with a history of malignancy, reporting a 94.4% vaccine
efficacy but highlighting higher adverse events in vaccine
recipients. Lee et al (7)
identified a 60% increased risk of COVID-19 in cancer patients,
with a twofold risk during chemotherapy/immunotherapy. Košir et
al (8) reported a 45% impact
on cancer treatment or care in adolescent and young adult patients.
Decreases in cancer diagnoses and barriers to care were noted by
Dinmohamed et al (12),
while Mathews et al (11)
reported a substantial increase in mortality for various cancers
during COVID-19. Breast cancer outcomes varied, with Baba et
al (14) finding no
significant difference in critical events, while Resende et
al (18) observed a lower
prevalence of early-stage breast cancer and a higher prevalence of
advanced-stage cases. In lung cancer, Sha et al (15) highlighted increased physical
discomfort and psychological distress, and Aboueshia et al
(16) reported higher mortality,
longer hospital stays and more unplanned reintubations in COVID-19
patients. The study by Kuderer et al (4) on invasive or hematological
malignancies indicated a mortality rate of 13%, with severe illness
in 26% and ICU admissions of 14% of cancer patients with COVID-19.
Vanni et al (21) warned of
potential increases in invasive surgeries due to screening program
suspensions. Patients with thoracic cancer, as per Garassino et
al (22), faced high mortality
and complications, while Tokunaga et al (23) noted a decrease in gastrectomies for
gastric cancer due to restricted surgical spots in hospitals
because of the pandemic. Lung cancer patients in the study by Priou
et al (24) saw no
significant impact of treatment delay on mortality (Study 2).
 | Table IIOutcomes for cancer patients. |
Table II
Outcomes for cancer patients.
| Study author and
year | Type of cancer | Mortality in cancer
patients (without COVID-19) | Mortality in COVID
cancer patients | Delay in
treatment | Complications and
conclusion | (Refs.) |
|---|
| Solaini et
al, 2023 | Gastric
adenocarcinoma | Pre-COVID-19
pandemic: Mortality occurred in 10 cases (2.6%) | Mortality occurred
in 9 cases (5.9%) | Potential delays in
diagnosis and treatment | Longer median times
from diagnosis to diagnostic work-up, chemotherapy and operation;
Higher rate of conversion to open surgery | (10) |
| Thomas et
al, 2022 | History of past or
active malignancy, including malignant tumors, benign tumors and
other non-specific neoplasms. | 0 | 0 | Not mentioned | 94.4% vaccine
efficacy in participants with neoplasm history; 3 COVID-19 cases in
participants with malignancy; Higher vaccine-related adverse events
in BNT162b2 recipients | (9) |
| Lee et al,
2021 | Not specific | Not mentioned | Not mentioned | Higher risk in
older participants and males; Symptom-based prediction models
indicating higher likelihood of predicted COVID-19 | 60% increased risk
of testing positive for COVID-19 in cancer patients; Twofold
increased risk with chemotherapy/immunotherapy; Higher risk of
hospitalization | (7) |
| Košir et al,
2020 | Not specific | Not mentioned | Not mentioned | Postponed/canceled
follow-up appointments, virtual appointments, postponed cancer
treatment/surgery, changes in treatment protocols | 45% of adolescent
and young adult patients reported an impact on cancer treatment or
care | (8) |
| Dinmohamed et
al, 2020 | Statistics of head
and neck cancers, gastrointestinal cancers, lung cancer, breast
cancer, gynecologic cancers, urological cancers, hematological
cancer and skin cancers | Not mentioned | Not mentioned | Not mentioned | Decrease in cancer
diagnoses; Barriers to consultation, transition to telehealth,
resource reallocation; Temporary halt of national screening
programs | (12) |
| Mathews et
al, 2022 | Breast,
gastrointestinal, lung, lymphoma, genitourinary, gynecologic,
leukemia, CNS and other (including thyroid cancer, skin cancer,
liposarcoma, head and neck cancer, carcinoma of unknown primary
origin). | 17 | 49 | Not mentioned | Not mentioned | (11) |
| Mendonça et
al, 2023 | Non-melanoma skin,
breast, thyroid, prostate, melanoma skin, colorectal, lung, cervix
uteri, kidney, stomach, oral cavity, oropharynx, larynx | Not mentioned | Not mentioned | Rapid screening and
prevention measures necessary | Newly diagnosed
cases declined; Changes in the stage of newly diagnosed
cancers | (13) |
| Baba et al,
2023 | Breast cancer | 2 | 7 | Not mentioned | No significant
difference in the incidence of critical events | (14) |
| Sha et al,
\2020 | Lung cancer | Not mentioned | Not mentioned | Not mentioned | Cancer patients
experienced increased physical discomfort and psychological
distress due to the pandemic's impact on their treatment and
overall well-being | (15) |
| Aboueshia et
al, 2021 | Not specific | Not mentioned | 7 | Higher frequency of
unplanned reintubation and longer hospital stays in cancer patients
with COVID-19 | Obesity and active
smoking associated with increased risk of mortality | (16) |
| Rucinska and
Nawrocki, 2022 | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | (17) |
| Resende et
al, 2022 | Breast cancer | Not mentioned | Not mentioned | Potential delay in
diagnosis and treatment initiation | The study found a
lower pre-valence of early-stage breast cancer (stage I-II) and a
higher prevalence of advanced-stage breast cancer (stage IV) during
the COVID-19 pandemic compared to the pre-pandemic period | (18) |
| de Sousa et
al, 2023 | Not specific | Not mentioned | Not mentioned | Mortality may be
more related to SARS-CoV-2 infection itself than to treatment
delays | There was a
significant stage shift towards more advanced stages (III and IV)
in 2020 and 2021 | (19) |
| Arndt et al,
2023 | Not mentioned | Not mentioned | Not mentioned | Delay in diagnostic
work-up | The provision of
care was reduced by 21% in the area of aftercare, by 12% in
psycho-oncological care and by 9% with respect to tumor surgery
compared to the time before COVID-19 | (20) |
| Kuderer et
al, 2020 | Invasive or
hematological malignancy | 0 | 121 (13%) patients
had died, all within 30 days of COVID-19 diagnosis | 116 (12%) required
mechanical ventilation | 242 (26%) patients
met the composite severe illness endpoint and 132 (14%) patients
were admitted to the ICU | (4) |
| Vanni et al,
2020 | Breast cancer | Not mentioned | Not mentioned | Not mentioned | Due to suspension
of screening programs, an increase in size and stage of breast
cancer presentation was observed, which may have led to an increase
in more invasive surgeries | (21) |
| Garassino et
al, 2020 | Any thoracic cancer
(NSCLC, SCLC, mesothelioma, thymic epithelial tumors and other
pulmonary neuro-endocrine neoplasms) | Not mentioned | 66 (33%) | 31 (53%) of 58
hospitalized patients with data on complete length of stay had a
prolonged hospitalization, defined as longer than 8 days | High mortality and
low admission to intensive care in patients with thoracic cancer.
13 (10%) of these patients were admitted to the ICU. Complications:
Pneumonia or pneumonitis, 125/157 (80%); acute respiratory distress
syndrome, 42/157 (27%); multi-organ failure, sepsis, coagulopathy,
bacterial, infection, arrhythmia, heart failure | (22) |
| Tokunaga et
al, 2022 | Gastric cancer | Not mentioned | Not mentioned | The number of
gastrectomies during the study period was <80% that of the
previous year | The number of
gastrectomies was lower than that in the previous year | (23) |
| Priou et al,
2022 | Lung cancer | Overall mortality
during 2018-2019, 125 (2%) | Not mentioned | The mortality may
have been more related to SARS-CoV-2 infection itself than to any
treatment delays | The rates of
non-metastatic lung cancer patients under going tumor resection,
non-surgical multimodal treatment and best supportive care before
vs. after the outbreak reached 42 vs. 42%, 49 vs. 50% and 9 vs. 8%,
respectively. | (24) |
| Fujita et
al, 2022 | Gastric cancer | Not mentioned | Not mentioned | The median time to
treatment was significantly shorter in patients during the COVID-19
pandemic (P<0.001) | In Japan, delays in
diagnosing patients with gastric cancer, probably due to refraining
from consultation, may have resulted in an increase in the
diagnosis of advanced-stage cancer. Furthermore, an increasing
proportion of patients required more invasive gastrectomy. | (25) |
| Suh et al,
2023 | Gastric cancer | Not mentioned | Not mentioned | Not mentioned | The oncologic
outcomes of gastric cancer during the COVID-19 pandemic may become
worse, as many cases of esophagogastroduodenoscopy and gastric
cancer management were suspended or delayed | (26) |
| Lara et al,
2022 | Gynecologic cancer
(including endometrial, ovarian, cervical and vulvar cancer) | Not mentioned | 34 (17.6%) | Not mentioned | The most common
complications secondary to COVID-19 infection were pulmonary,
cardio vascular and renal | (27) |
| Arrieta et
al, 2021 | Lung cancer,
mesothelioma or thymomas | Not mentioned | 61 | The impact of
COVID-19 on cancer care is evident in the observed delays and
challenges | Patients with
treatment adjustments during the studied period experienced a
median PFS of 10.9 months, while those without modifications had an
unreached median PFS | (28) |
| Provencio et
al, 2021 | NSCLC, SCLC, other
lung cancers | Not mentioned | 146 (32.7%) | 350 (78.3%)
patients were hospitalized, with a length of stay of 13.4±11.4
days | Nine of the 447
(2.0%) patients were admitted to the ICU | (29) |
| Mato et al,
2020 | Chronic lymphocytic
leukemia | Not mentioned | 66 deaths were
observed (33% case fatality rate) for this population identified
with symptomatic COVID-19 | Not mentioned | COVID-19-directed
therapies were administered as part of a clinical trial or
compassionate use protocol in 16 and 19% of patients, respectively.
Antiviral ritonavir (17%) and remdesivir (7%) | (30) |
| Ospina et
al, 2021 | Types of
malignancy: Breast, colorectal, prostate, head and neck, gastric,
lung, cervix, sarcoma, renal, ovary, melanoma, nonmelanoma skin,
neuroendocrine, anal vesicle, esophagus, osteosarcoma, thymus,
gastrointestinal, cholangiocarcinoma, penis, appendix, small
intestine, mesothelioma, adrenal gland, giant cells, CNS,
hepatocarcinoma, thyroid, bladder, uterus, germ, pancreas, and
unknown primary | Not mentioned | 96 (26.3%) | The frequency of
mechanical ventilation was higher as the decade of age increased
from 50 years, with a slight decrease after 70 years; a high
frequency of invasive ventilatory support was found in the group
aged 31-40 years | Secondary outcomes
included the requirement for noninvasive mechanical ventilation and
the requirement for invasive mechanical ventilation. A higher
frequency of invasive ventilation was evidenced in men. | (31) |
| Lièvre et
al, 2020 | Solid malignant
tumor | Not mentioned | 370 (29%) | 412 (42%) patients
required oxygen and 49 (5%) mechanical ventilation | Mortality and
COVID-19 severity in cancer patients are high and are associated
with general characteristics of patients | (32) |
Meta-analysis revealing overall
mortality
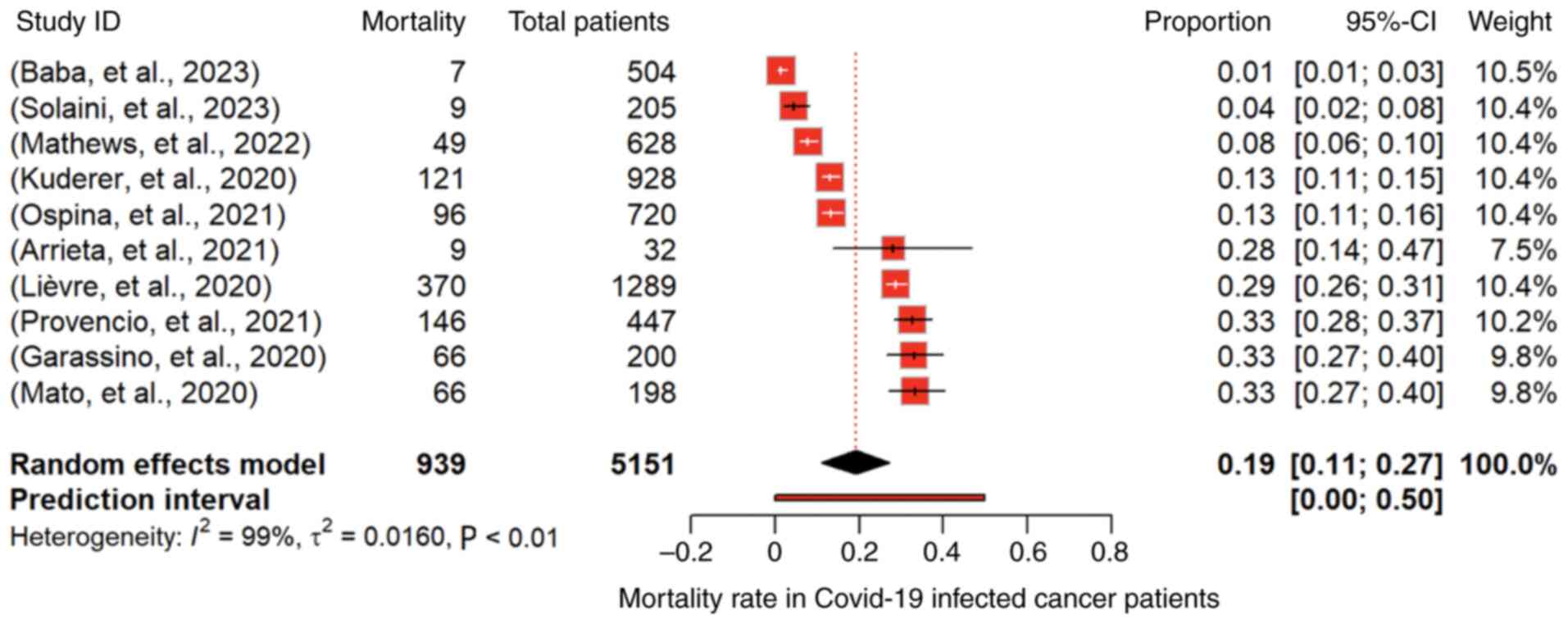
The prevalence of mortality in COVID-19-infected
individuals was assessed by 10 studies comprising 5,151 cancer
patients (4,10,11,14,22,28-32).
The pooled proportion, under a random-effects model, was 0.1913
(95% CI: 0.1109 to 0.2718; P<0.01), indicating a significant
overall mortality rate of 19.1% among cancer patients infected with
COVID-19 (Fig. 4). However,
substantial heterogeneity was evident (I2=98.7%),
highlighting diverse outcomes across studies. The Q-test for
heterogeneity was highly significant (P<0.0001). For
non-COVID-19 cancer patients, reported in 5 studies including
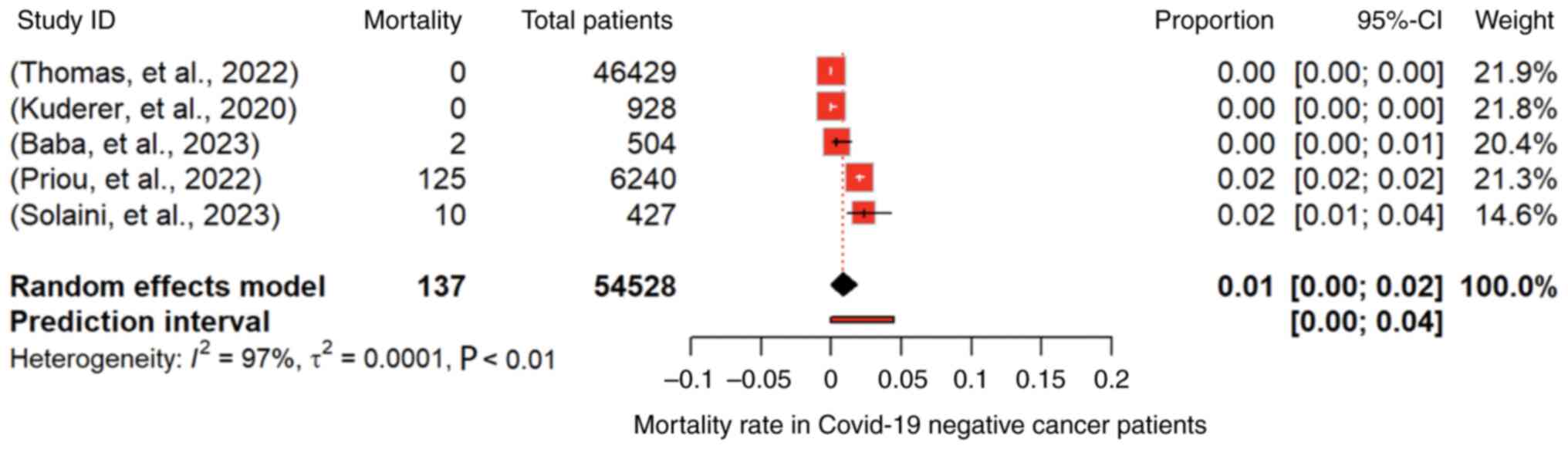
54,528 cancer patients (4,9,10,14,25),
the overall mortality rate was as low as 1% (95% CI: 0.00 to 0.02;
P<0.01) under a random-effects model (Fig. 5). However, substantial
heterogeneity was observed (I2=97.1%, P<0.01).
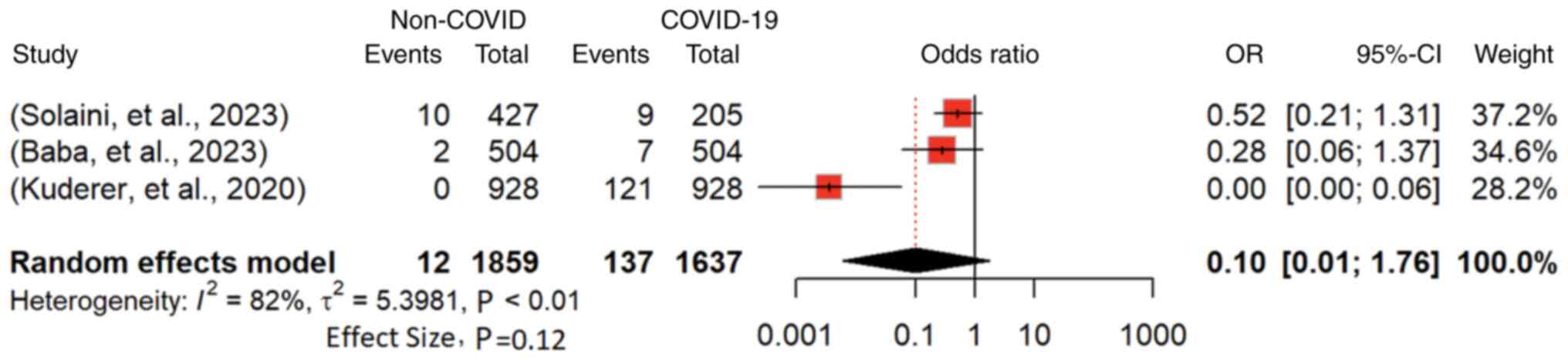
Regarding the risk of mortality in non-COVID-19 vs.
COVID-19 cancer patients, reported by 3 studies involving 3,496
cancer patients (4,10,14),
the odds ratio (OR) for mortality was 0.1036 (95% CI: 0.0061 to
1.7614; P<0.01) under a random-effects model (Fig. 6). The overall estimate suggests a
potentially decreased mortality risk for non-COVID-19 patients.
However, substantial heterogeneity (I2=82.1%; P<0.01)
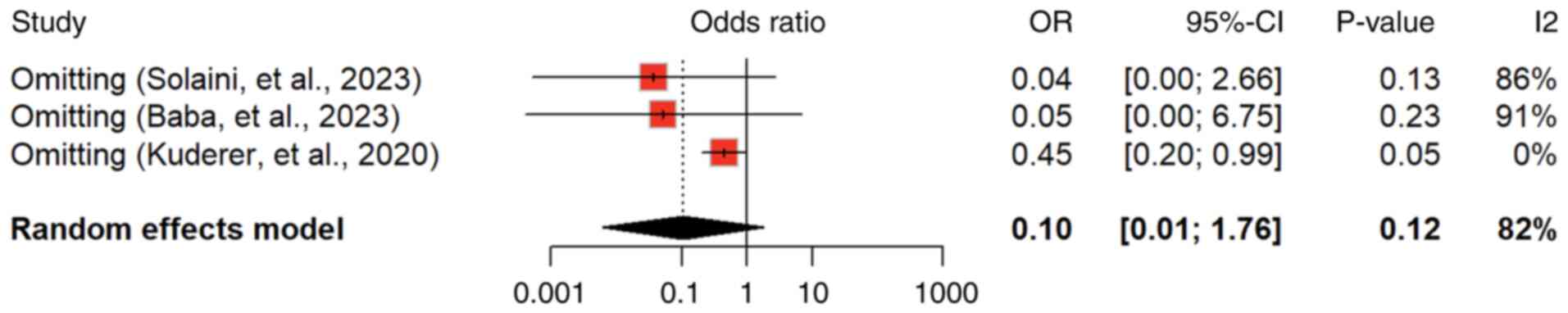
was observed, indicating variability among studies. Influential
analysis (sensitivity analysis) was identified by Kuderer et
al (4) as a potential source
of heterogeneity, and its omission led to a lower pooled estimate
(0.45, 95% CI: 0.20 to 0.99; P<0.01), implying a subgroup with
lower mortality risk (Fig. 7).
Discussion
In this comprehensive review of the intersection of
cancer and COVID-19, the findings revealed the complex dynamics
influencing outcomes among cancer patients during the pandemic. The
variation in sample sizes across studies, exemplified by the
general community survey conducted with an extensive pool of
1,807,559 individuals and the more focused cross-sectional survey
by Košir et al (8)
involving 177 participants, underscores the diverse methodologies
of different geographical samples and various health care systems
employed in understanding this intersection. The randomized
clinical trial reported by Thomas et al (9) revealed a significant 63.9% female
majority among BNT162b2 vaccine recipients, while the retrospective
cohort study conducted by Solaini et al (10) showcases a balanced distribution
among COVID-19 patients. Shifting the focus to the impact of
COVID-19 on cancer patients, Mathews et al (11) break down 66 positive cases,
revealing a nearly equal gender distribution within this vulnerable
group. Simultaneously, Lee et al's (7) report on 155 positive cases among
individuals with cancer accentuates the tangible real-world
implications of the virus within this specific population. These
findings collectively contribute to our understanding of the
interplay between COVID-19 and oncology.
This study thoroughly investigated the variability
in outcomes among different cancer types, particularly focusing on
why certain cancers, such as gastric adenocarcinoma and thoracic
cancers, may exhibit higher mortality rates in COVID-19 patients.
It provided an analysis of the biological and clinical factors that
could contribute to these disparities. For instance, the aggressive
nature of these cancers, combined with the immunosuppressive
effects of both the disease and its treatments, could exacerbate
the severity of COVID-19. The manuscript explores how these
patients' pre-existing conditions and the potential delay in
diagnosis due to the pandemic may have contributed to their
heightened vulnerability.
The present study also discusses the impact of
COVID-19 on cancer management and treatment decisions. It shows how
the pandemic has forced alterations in standard treatment
protocols, including delays in surgery, modifications in
chemotherapy regimens and the adoption of telemedicine for
consultations. It also sheds light on the ethical dilemmas faced by
oncologists in prioritizing treatment for patients with a higher
chance of survival during resource-scarce periods. Furthermore,
insights into how COVID-19 has affected surgical trends and the
implementation of chemotherapy protocols are well-documented,
emphasizing the need for adaptive strategies in oncological care
during global health crises.
In gastric adenocarcinoma, Fox et al (2022)
revealed a higher mortality rate in COVID-19 patients compared to
the pre-COVID era, underscoring the challenges posed by potential
delays in diagnosis and treatment (33). This aligns with earlier studies
emphasizing the importance of timely intervention in gastric
cancers to improve survival rates (34,35).
The observation of Thomas et al (9) of no mortality in individuals with a
history of malignancy, coupled with high vaccine efficacy,
corroborates with previous research on the potential protective
effects of vaccinations in cancer patients (36).
The increased risk of COVID-19 in cancer patients,
as reported by Lee et al (7), echoes concerns raised in earlier
studies about the vulnerability of cancer patients to infectious
diseases (37,38). Košir et al's (8) identification of a substantial impact
on adolescent and young adult cancer patients aligns with broader
discussions on the unique challenges faced by this demographic
group during the pandemic (39,40).
The decrease in cancer diagnoses and barriers to care highlighted
by Dinmohamed et al (12)
resonates with concerns raised in the early stages of the pandemic
regarding disruptions to routine healthcare services and the
downstream effects on cancer outcomes (41,42).
Breast cancer outcomes, as reported by Baba et
al (14) and Resende et
al (18), showcase the
variability in responses to the pandemic. While the former found no
significant difference in critical events, the latter identified a
stage shift towards more advanced cases. These findings contribute
to the ongoing discourse on the multifaceted impacts of COVID-19 on
breast cancer patients, necessitating tailored approaches to care
(43,44).
In lung cancer, the increased physical discomfort
and psychological distress reported by Sha et al (15) highlight the broader mental health
implications of the pandemic on cancer patients, an aspect that has
gained prominence in recent literature (45). Aboueshia et al (16) findings of higher mortality, longer
hospital stays and increased unplanned reintubations in COVID-19
patients with lung cancer emphasize the need for targeted
interventions in this vulnerable population, aligning with prior
research on the intersection of respiratory diseases and COVID-19
outcomes (46,47).
The study by Kuderer et al (4) on invasive or hematological
malignancies signifies the severity of COVID-19 in this patient
group. The observed mortality, severe illness and ICU admissions
are consistent with earlier reports on the heightened risks faced
by individuals with hematological malignancies during the pandemic
(48). Vanni et al
(21) caution about potential
increases in invasive surgeries due to screening program
suspensions, which echoes broader concerns about the collateral
damage on cancer care caused by pandemic-related disruptions
(49).
The association between hemogram parameters and
COVID-19 infection has been examined in various studies (50), and parameters including the
platelet-to-lymphocyte ratio (51)
were found to be related to the infection. Furthermore, the red
cell distribution width, a marker of anisocytosis in the hemogram,
has been associated with recurrent hospitalizations of patients
with COVID-19(52). Other
inflammatory markers were introduced as predictors of frailty in
diabetics during COVID-19(53). In
addition, the role of inflammation in cancer has been reported in
various studies (54,55). Furthermore, mortality is increased
when markers of inflammation are elevated (56).
The high mortality and complications faced by
patients with thoracic cancer, as highlighted by Passaro et
al (57), underscore the
critical need for specialized care in this population. Previous
studies reinforce the consistent challenges faced by patients with
thoracic cancer (3), emphasizing
the importance of maintaining continuity in care during pandemics
(58). Tokunaga et al's
(23) finding of a decrease in
gastrectomies for gastric cancer aligns with concerns about reduced
access to surgical interventions during the pandemic, potentially
impacting long-term outcomes (59,60).
Mullangi et al's (61) study on patients with lung cancer
presents a unique perspective, suggesting that mortality may be
more related to SARS-CoV-2 infection itself rather than to
treatment delays. This observation prompts further investigation
into the specific factors contributing to mortality in patients
with lung cancer during the pandemic, providing a basis for
tailored interventions (62).
The present meta-analysis accounts for various
potential confounding factors, including age, comorbidities and
cancer stage, when comparing mortality rates between
COVID-19-infected cancer patients and their non-COVID counterparts.
The study used multivariate analysis to determine the impact of
COVID-19 on cancer outcomes, ensuring that the differences observed
are not merely due to these confounders. This methodological
approach enhances the reliability of the findings, providing a
clearer understanding of how COVID-19 specifically affects cancer
mortality rates.
The pooled analysis of 10 studies involving 5,151
cancer patients infected with COVID-19 reveals a significant
overall mortality rate of 19.1%. This finding is consistent with
emerging evidence highlighting the high vulnerability of cancer
patients to severe outcomes of COVID-19(63). However, the substantial
heterogeneity (I2=98.7%) suggests diverse outcomes
across these studies, emphasizing the need for nuanced
interpretations. The observed variability may be attributed to
differences in patient populations, cancer types, treatment
modalities and healthcare infrastructure among the included
studies. The low P-value for the Q-test for heterogeneity further
underscores the significance of this observed heterogeneity
(P<0.0001). This variability underscores the complexity of the
interaction between COVID-19 and cancer, necessitating tailored
approaches to patient care (64).
By contrast, the overall mortality rate among
non-COVID cancer patients, as reported by 5 studies comprising
54,528 individuals (4,9,10,14,24),
was considerably lower at 0.01 (1%). This finding aligns with prior
research suggesting that cancer patients not infected with COVID-19
experience relatively lower mortality rates (65). However, similar to the
COVID-19-infected group, substantial heterogeneity is observed
(I2=97.1%, P<0.0001). The wide range of mortality
rates among non-COVID cancer patients could be attributed to
variations in cancer types, stages and treatment responses.
Regarding the risk of mortality, the OR for
non-COVID vs. COVID cancer patients was 0.1036 (95%CI: 0.0061 to
1.7614) based on 3 studies involving 3,496 cancer patients. The
overall estimate suggests a potential decrease in mortality risk
for non-COVID patients, indicating that cancer patients not
infected with COVID-19 may have a comparatively lower risk of
mortality (66). However, the
substantial heterogeneity (I2=82.1%) signals variability
among studies. Sensitivity analysis identified the study by Kuderer
et al (4) as a potential
source of heterogeneity. Its omission led to a lower pooled
estimate (0.4473, 95% CI: 0.2026 to 0.9878), implying a subgroup
with a lower mortality risk among non-COVID cancer patients. This
underscores the importance of considering the characteristics of
individual studies and potential sources of heterogeneity in
meta-analyses to derive more accurate and clinically relevant
conclusions. The identification of a subgroup with a lower
mortality risk could guide further research into factors
influencing outcomes in cancer patients not infected with
COVID-19.
This study clarifies that while COVID-19 may worsen
the prognosis for cancer patients, the mechanisms by which it does
so differ significantly from other chronic diseases. For instance,
the immune dysregulation caused by cancer and its treatment can
create a unique vulnerability to COVID-19 that is not present in
other conditions. It integrates these distinctions into its broader
analysis, providing an understanding of the intersection between
cancer and COVID-19.
This study carries significant implications for both
clinical practice and public health. The observed high
vulnerability of cancer patients to severe outcomes underscores the
need for tailored interventions and prioritized care. Clinicians
should be mindful of potential delays in diagnosis and treatment,
particularly in gastric adenocarcinoma, and consider personalized
strategies for diverse patient cohorts, as exemplified by the
variability in breast cancer responses. Furthermore, the study
highlights the broader mental health implications of the pandemic
on lung cancer patients, emphasizing the importance of holistic
care approaches. These implications necessitate ongoing efforts to
integrate pandemic-specific considerations into cancer care
protocols and public health strategies. The manuscript suggests
that guidelines are updated to reflect the challenges posed by
COVID-19, such as ensuring timely treatment while minimizing
infection risks. Recommendations for improving patient outcomes may
include vaccination strategies tailored to cancer patients
(10,14,18,21,23,25,26).
Future research should explore specific factors
influencing mortality in patients with lung cancer during the
pandemic, building on the unique perspective presented by Priou
et al (24). Additionally,
there is a critical need for comprehensive studies investigating
the long-term mental health impacts on lung cancer patients,
informed by Sha et al's (15) findings. Exploring the collateral
damage on cancer care, as raised by Vanni et al (21), requires in-depth investigations
into the consequences of disruptions in cancer screening programs.
Not all of the studies included in the present analysis adequately
controlled for key confounding factors, which could have led to the
introduction of bias into the pooled estimates. This variability in
controlling for confounders, such as patient demographics, disease
severity, cancer stage, comorbidities and treatment history, may
impact the comparability of the study's outcomes and the overall
robustness of the study's findings. In order to improve the
reliability and accuracy of future research, the usage of more
rigorous and multivariate models may be recommended, which can
better adjust for these critical confounders, as it will ensure
that the observed associations more accurately reflect true causal
relationships. In addition, further research should focus on
understanding the characteristics of the subgroup with a lower
mortality risk among non-COVID cancer patients, providing insights
for targeted interventions. Long-term outcomes in patients with
thoracic cancer, as emphasized by Garassino et al (22), warrant dedicated research efforts
to ensure continuous and specialized care during pandemics and
other healthcare disruptions.
Despite the comprehensive nature of the systematic
review and meta-analysis, several limitations need to be
acknowledged. The inherent heterogeneity across the included
studies highlights the diverse patient populations, cancer types
and treatment modalities considered. This heterogeneity underscores
the challenge of synthesizing data from studies with varying
methodologies and emphasizes the need for cautious interpretation.
The reliance on published literature may introduce publication
bias, as studies with positive or statistically significant results
are more likely to be published. This potential bias may affect the
generalizability of findings and should be considered when
extrapolating conclusions to the broader population. The dynamic
nature of the COVID-19 pandemic may introduce temporal biases, with
outcomes influenced by evolving healthcare practices, treatments
and vaccination strategies. Furthermore, the limitations of the
individual studies, such as varying sample sizes and methodologies,
could impact the overall robustness of the meta-analysis. In
addition, the COVID-19 pandemic has had significant effects on the
various aspects of oncological care, which include chemotherapy
protocols and surgical trends. For instance, surgical delays or
changes and modifications in chemotherapy administration schedules
have been widely reported as adaptations in order to mitigate the
risk of infection and to manage healthcare resource limitations.
However, due to the constraints of the included studies in the
current study, which often lacked detailed information on these
particular treatment adjustments, the present analysis was unable
to comprehensively evaluate the extent of these pandemic-related
impacts. Despite these limitations, the present study provides
valuable insights into the intersection of COVID-19 and oncology,
offering a foundation for future research and clinical
considerations.
In conclusion, the present review signifies the high
vulnerability of cancer patients to severe outcomes from COVID-19,
emphasizing the need for tailored interventions and prioritized
care. The variability in outcomes across different cancer types and
patient cohorts highlights the nuanced nature of this intersection.
Noteworthy patterns emerge, such as the differential mortality
rates in gastric adenocarcinoma patients during the pandemic and
the varied outcomes for vaccine recipients with a history of
malignancy. The increased risk of COVID-19 among cancer patients,
particularly during chemotherapy/immunotherapy, highlights the
vulnerability of this population. This study not only informs
immediate clinical considerations but also sets the stage for
future research, aimed at refining the current understanding of the
interaction between COVID-19 and oncology, ultimately improving
outcomes for this vulnerable population.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RAA: Protocol preparation and submission, manuscript
writing, proofreading, reviewing, editing, finalization of the
study. AhAA: Screening, data extraction, reviewing collected data,
manuscript writing. NIA: Data extraction, reviewing collected data,
manuscript writing. TAA, MeAA and MoAA: Screening, data extraction,
reviewing collected data, manuscript writing. MMMA, AbAA and LA:
Data extraction, reviewing collected data, manuscript writing. NAA:
Proofreading the manuscript, reviewing data, finalization of the
study. All authors have read and approved the final version of the
study. RAA and AhAA confirm the authenticity of the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cascella M, Rajnik M, Aleem A, Dulebohn SC
and Di Napoli R: Features, evaluation, and treatment of coronavirus
(COVID-19). In: StatPearls Publishing, Treasure Island (FL),
2020.
|
|
2
|
Al-Quteimat OM and Amer AM: The impact of
the COVID-19 pandemic on cancer patients. Am J Clin Oncol.
43:452–455. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gupta K, Gandhi S, Mebane A III, Singh A,
Vishnuvardhan N and Patel E: Cancer patients and COVID-19:
Mortality, serious complications, biomarkers, and ways forward.
Cancer Treat Res Commun. 26(100285)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kuderer NM, Choueiri TK, Shah DP, Shyr Y,
Rubinstein SM, Rivera DR, Shete S, Hsu CY, Desai A, de Lima Lopes G
Jr, et al: Clinical impact of COVID-19 on patients with cancer
(CCC19): A cohort study. Lancet. 395:1907–1918. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Johannesen TB, Smeland S, Aaserud S,
Buanes EA, Skog A, Ursin G and Helland Å: COVID-19 in cancer
patients, risk factors for disease and adverse outcome, a
population-based study from norway. Front Oncol.
11(652535)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lee KA, Ma W, Sikavi DR, Drew DA, Nguyen
LH, Bowyer RCE, Cardoso MJ, Fall T, Freidin MB, Gomez M, et al:
Cancer and risk of COVID-19 through a general community survey.
Oncologist. 26:e182–e185. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Košir U, Loades M, Wild J, Wiedemann M,
Krajnc A, Roškar S and Bowes L: The impact of COVID-19 on the
cancer care of adolescents and young adults and their well-being:
Results from an online survey conducted in the early stages of the
pandemic. Cancer. 126:4414–4422. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Thomas SJ, Perez JL, Lockhart SP,
Hariharan S, Kitchin N, Bailey R, Liau K, Lagkadinou E, Türeci Ö,
Şahin U, et al: Efficacy and safety of the BNT162b2 mRNA COVID-19
vaccine in participants with a history of cancer: Subgroup analysis
of a global phase 3 randomized clinical trial. Vaccine.
40:1483–1492. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Solaini L, Bencivenga M, Rosa F, D'ignazio
A, Marino E, Ministrini S, Sofia S, Sacco M, Mura G, Rausa E, et
al: Consequences of the COVID-19 pandemic on the diagnosis and
treatment of gastric cancer in referral centers in Italy. Tumori.
109:121–128. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mathews AS, Paul A, Yu IS, McGahan C,
Bhang E, Villa D, Gelmon K, Avina-Zubieta A, Gerrie AS, Lee U, et
al: The clinical impact of COVID-19 on patients with cancer in
British Columbia: An observational study. Heliyon.
12(e12140)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dinmohamed AG, Visser O, Verhoeven RHA,
Louwman MWJ, van Nederveen FH, Willems SM, Merkx MAW, Lemmens VEPP,
Nagtegaal ID and Siesling S: Fewer cancer diagnoses during the
COVID-19 epidemic in the Netherlands. Lancet Oncol. 21:750–751.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mendonça E Silva DR, Fernandes GA, França
E Silva ILA and Curado MP: Cancer stage and time from cancer
diagnosis to first treatment during the COVID-19 pandemic. Semin
Oncol. 50:60–65. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Baba K, Kawamoto M, Mamishin K, Uematsu M,
Kiyohara H, Hirota A, Takahashi N, Fukuda M, Kusuhara S, Nakajima
H, et al: The impact of the COVID-19 pandemic on perioperative
chemotherapy for breast cancer. Cancer Med. 12:12095–12105.
2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sha Z, Chang K, Mi J, Liang Z, Hu L, Long
F, Shi H, Lin Z, Wang X and Pei X: The impact of the COVID-19
pandemic on lung cancer patients. Ann Palliat Med. 9:3373–3378.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Aboueshia M, Hussein MH, Attia AS,
Swinford A, Miller P, Omar M, Toraih EA, Saba N, Safah H, Duchesne
J and Kandil E: Cancer and COVID-19: Analysis of patient outcomes.
Future Oncol. 17:3499–3510. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rucinska M and Nawrocki S: COVID-19
pandemic: Impact on cancer patients. Int J Environ Res Public
Health. 19(12470)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Resende CAA, Fernandes Cruz HM, Costa E
Silva M, Paes RD, Dienstmann R, Barrios CHE, Goncalves AC, Cascelli
FGA, Souto AKBA, Oliveira LC, et al: Impact of the COVID-19
pandemic on cancer staging: An analysis of patients with breast
cancer from a community practice in Brazil. JCO Glob Oncol.
8(e2200289)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
de Sousa CFPM, de Castro Junior G,
Starling MTM, Restini FCF, Rodrigues AN, de Castro Ribeiro HS,
Arruda GV, Hanna SA, de Moraes FY and Marta GN: Impact of the
COVID-19 outbreak on cancer staging in Brazil. Clin Oncol (R Coll
Radiol). 35:e404–e406. 2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Arndt V, Doege D, Fröhling S, Albers P,
Algül H, Bargou R, Bokemeyer C, Bornhäuser M, Brandts CH, Brossart
P, et al: Cancer care in German centers of excellence during the
first 2 years of the COVID-19 pandemic. J Cancer Res Clin Oncol.
149:913–919. 2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vanni G, Pellicciaro M, Materazzo M, Bruno
V, Oldani C, Pistolese CA, Buonomo C, Caspi J, Gualtieri P,
Chiaravalloti A, et al: Lockdown of breast cancer screening for
COVID-19: Possible scenario. In Vivo. 34:3047–3053. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Garassino MC, Whisenant JG, Huang LC,
Trama A, Torri V, Agustoni F, Baena J, Banna G, Berardi R, Bettini
AC, et al: COVID-19 in patients with thoracic malignancies
(TERAVOLT): First results of an international, registry-based,
cohort study. Lancet Oncol. 21:914–922. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tokunaga M, Yoshikawa T, Boku N, Nishida
Y, Tanahashi T, Yamada T, Haruta S, Etoh T, Hirahara N, Kawachi Y,
et al: Impact of COVID-19 on gastric cancer treatment in Japanese
high-volume centers: A JCOG stomach cancer study group survey. Surg
Today. 52:231–238. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Priou S, Lamé G, Zalcman G, Wislez M, Bey
R, Chatellier G, Cadranel J, Tannier X, Zelek L, Daniel C, et al:
Influence of the SARS-CoV-2 outbreak on management and prognosis of
new lung cancer cases, a retrospective multicentre real-life cohort
study. Eur J Cancer. 173:33–40. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fujita S, Sakuramoto S, Miyawaki Y,
Morimoto Y, Ebara G, Nishibeppu K, Oya S, Fujihata S, Lee S, Sugita
H, et al: Impact of the first era of the coronavirus disease 2019
pandemic on gastric cancer patients: A single-institutional
analysis in Japan. Int J Clin Oncol. 27:930–939. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Suh MA, Park SB, Kwak MS, Yoon JY and Cha
JM: Impact of the COVID-19 pandemic on esophagogastroduodenoscopy
and gastric cancer claims in South Korea: A nationwide,
population-based study. Yonsei Med J. 64:549–557. 2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lara OD, Smith M, Wang Y, O'Cearbhaill RE,
Blank SV, Kolev V, Carr C, Knisely A, McEachron J, Gabor L, et al:
COVID-19 outcomes of patients with gynecologic cancer in New York
City: An updated analysis from the initial surge of the pandemic.
Gynecol Oncol. 164:304–310. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Arrieta O, Lara-Mejía L, Bautista-GonzÁlez
E, Heredia D, Turcott JG, BarrÓn F, Ramos-Ramírez M,
Cabrera-Miranda L, Salinas Padilla MÁ, Aguerrebere M, et al:
Clinical impact of the COVID-19 pandemic in Mexican patients with
thoracic malignancies. Oncologist. 26:1035–1043. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Provencio M, Mazarico Gallego JM, Calles
A, Antoñanzas M, Pangua C, Mielgo Rubio X, Nadal E, Castro RL,
López-Martín A, Del Barco E, et al: Lung cancer patients with
COVID-19 in Spain: GRAVID study. Lung Cancer. 157:109–115.
2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mato AR, Roeker LE, Lamanna N, Allan JN,
Leslie L, Pagel JM, Patel K, Osterborg A, Wojenski D, Kamdar M, et
al: Outcomes of COVID-19 in patients with CLL: A multicenter
international experience. Blood. 136:1134–1143. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ospina AV, Bruges R, Mantilla W, Triana I,
Ramos P, Aruachan S, Quiroga A, Munevar I, Ortiz J, Llinás N, et
al: Impact of COVID-19 infection on patients with cancer:
Experience in a Latin American Country: The ACHOCC-19 study.
Oncologist. 26:e1761–e1773. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lièvre A, Turpin A, Ray-Coquard I, Le
Malicot K, Thariat J, Ahle G, Neuzillet C, Paoletti X, Bouché O,
Aldabbagh K, et al: Risk factors for coronavirus disease 2019
(COVID-19) severity and mortality among solid cancer patients and
impact of the disease on anticancer treatm: A French nationwide
cohort study (GCO-002 CACOVID-19). Eur J Cancer. 141:62–81.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fox L, Monroy-Iglesias MJ, Aggarwal A,
Haire K, Purushotham A, Spicer J, Papa S, Rigg A, Dolly S, Sullivan
R and Van Hemelrijck M: Association between COVID-19 burden and
delays to diagnosis and treatment of cancer patients in England. J
Cancer Policy. 31(100316)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Necula L, Matei L, Dragu D, Neagu AI,
Mambet C, Nedeianu S, Bleotu C, Diaconu CC and Chivu-Economescu M:
Recent advances in gastric cancer early diagnosis. World J
Gastroenterol. 25:2029–2044. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tan YK and Fielding JW: Early diagnosis of
early gastric cancer. Eur J Gastroenterol Hepatol. 18:821–829.
2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wu SY, Tung HJ, Huang KH, Lee CB, Tsai TH
and Chang YC: The protective effects of influenza vaccination in
elderly patients with breast cancer in Taiwan: A real-world
evidence-based study. Vaccines (Basel). 10(1144)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Coleman CN, Mansoura MK, Marinissen MJ,
Grover S, Dosanjh M, Brereton HD, Roth L, Wendling E, Pistenmaa DA
and O'Brien DM: Achieving flexible competence: Bridging the
investment dichotomy between infectious diseases and cancer. BMJ
Glob Health. 5(e003252)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lashley FR: Emerging infectious diseases:
Vulnerabilities, contributing factors and approaches. Expert Rev
Anti Infect Ther. 2:299–316. 2004.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tsamakis K, Gavriatopoulou M, Schizas D,
Stravodimou A, Mougkou A, Tsiptsios D, Sioulas V, Spartalis E,
Sioulas AD, Tsamakis C, et al: Oncology during the COVID-19
pandemic: Challenges, dilemmas and the psychosocial impact on
cancer patients. Oncol Lett. 20:441–447. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Glidden C, Howden K, Romanescu RG, Hatala
A, Scott I, Deleemans JM, Chalifour K, Eaton G, Gupta AA, Bolton
JM, et al: Psychological distress and experiences of adolescents
and young adults with cancer during the COVID-19 pandemic: A
cross-sectional survey. Psychooncology. 31:631–640. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Edge R, Meyers J, Tiernan G, Li Z,
Schiavuzzi A, Chan P, Vassallo A, Morrow A, Mazariego C, Wakefield
CE, et al: Cancer care disruption and reorganisation during the
COVID-19 pandemic in Australia: A patient, carer and healthcare
worker perspective. PLoS One. 16(e0257420)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cancino RS, Su Z, Mesa R, Tomlinson GE and
Wang J: The impact of COVID-19 on cancer screening: Challenges and
opportunities. JMIR Cancer. 6(e21697)2020.PubMed/NCBI View
Article : Google Scholar
|
|
43
|
Parmar HS, Nayak A, Gavel PK, Jha HC,
Bhagwat S and Sharma R: Cross talk between COVID-19 and breast
cancer. Curr Cancer Drug Targets. 21:575–600. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Brown JM, Wasson MD and Marcato P:
Triple-negative breast cancer and the COVID-19 pandemic: Clinical
management perspectives and potential consequences of infection.
Cancers (Basel). 13(296)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Blevins TR, Lo SB, Coker CA, Arrato NA,
Reisinger SA, Shields PG and Andersen BL: COVID-19 or cancer
stress? Anxiety and depressive symptoms in patients with advanced
lung cancer. Int J Behav Med. 31:325–330. 2024.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Beltramo G, Cottenet J, Mariet AS, Georges
M, Piroth L, Tubert-Bitter P, Bonniaud P and Quantin C: Chronic
respiratory diseases are predictors of severe outcome in COVID-19
hospitalised patients: A nationwide study. Eur Respir J.
58(2004474)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
He ZF, Zhong NS and Guan WJ: Impact of
chronic respiratory diseases on the outcomes of COVID-19. Arch
Bronconeumol. 58:5–7. 2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sahu KK and Cerny J: Managing patients
with hematological malignancies during COVID-19 pandemic. Expert
Rev Hematol. 13:787–793. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sud A, Jones ME, Broggio J, Loveday C,
Torr B, Garrett A, Nicol DL, Jhanji S, Boyce SA, Gronthoud F, et
al: Collateral damage: The impact on outcomes from cancer surgery
of the COVID-19 pandemic. Ann Oncol. 31:1065–1074. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Aktas G: Hematological predictors of novel
coronavirus infection. Rev Assoc Med Bras (1992). 67 (Suppl
1):S1–S2. 2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Khalid A, Ali Jaffar M, Khan T, Abbas Lail
R, Ali S, Aktas G, Waris A, Javaid A, Ijaz N and Muhammad N:
Hematological and biochemical parameters as diagnostic and
prognostic markers in SARS-COV-2 infected patients of Pakistan: A
retrospective comparative analysis. Hematology. 26:529–542.
2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Atak BM, Kahveci G, Bilgin S, Kurtkulagi
O, Duman TT, Demirkol ME and Aktas G: Haemoglobin and red cell
distribution width levels in internal medicine patients indicate
recurrent hospital admission during COVID-19. Fam Med Prim Care
Rev. 24:32–36. 2022.
|
|
53
|
Atak BM, Bilgin S, Kurtkulagi O, Kahveci
G, Duman TT, Sagdic T and Aktas G: Frailty in diabetic subjects
during COVID-19 and its association with HbA1c, mean platelet
volume and monocyte/lymphocyte ratio. Clin Diabetol. 11:119–126.
2022.
|
|
54
|
Catal O, Ozer B, Sit M, Aktas G and Erkol
H: The role of monocyte to lymphocyt ratio in predicting metastasis
in rectal cancer. Ann Med Res. 28(527)2021.
|
|
55
|
Sit M, Aktas G, Ozer B, Kocak MZ, Erkus E,
Erkol H, Yaman S and Savli H: Mean platelet volume: An overlooked
herald of malignant thyroid nodules. Acta Clin Croat. 58:417–420.
2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Karagoz I, Aktas G, Yoldas H, Yildiz I,
Ogun MN, Bilgi M and Demirhan A: Association between hemogram
parameters and survival of critically Ill patients. J Intensive
Care Med. 34:511–513. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Passaro A, Bestvina C, Velez Velez M,
Garassino MC, Garon E and Peters S: Severity of COVID-19 in
patients with lung cancer: Evidence and challenges. J Immunother
Cancer. 9(e002266)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Matenge S, Sturgiss E, Desborough J, Hall
Dykgraaf S, Dut G and Kidd M: Ensuring the continuation of routine
primary care during the COVID-19 pandemic: A review of the
international literature. Fam Pract. 39:747–761. 2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Lin JA, Braun HJ, Schwab ME, Pierce L,
Sosa JA and Wick EC: Pandemic recovery: Persistent disparities in
access to elective surgical procedures. Ann Surg. 277:57–65.
2023.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Mitura K: The impact of COVID-19 pandemic
on critical care and surgical services availability. Crit Care
Innov. 3:43–50. 2020.
|
|
61
|
Mullangi S, Aviki EM, Chen Y, Robson M and
Hershman DL: Factors associated with cancer treatment delay among
patients diagnosed with COVID-19. JAMA Netw Open.
5(e2224296)2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Addabbo F, Giotta M, Mincuzzi A, Minerba
AS, Prato R, Fortunato F, Bartolomeo N and Trerotoli P: No excess
of mortality from lung cancer during the COVID-19 pandemic in an
area at environmental risk: Results of an explorative analysis. Int
J Environ Res Public Health. 20(5522)2023.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z,
Zhang Z, You H, Wu M, Zheng Q, et al: Patients with cancer appear
more vulnerable to SARS-CoV-2: A multicenter study during the
COVID-19 outbreak. Cancer Discov. 10:783–791. 2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Poortmans PM, Guarneri V and Cardoso MJ:
Cancer and COVID-19: What do we really know? Lancet. 395:1884–1885.
2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Yang F, Shi S, Zhu J, Shi J, Dai K and
Chen X: Clinical characteristics and outcomes of cancer patients
with COVID-19. J Med Virol. 92:2067–2073. 2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Desai A, Gupta R, Advani S, Ouellette L,
Kuderer NM, Lyman GH and Li A: Mortality in hospitalized patients
with cancer and coronavirus disease 2019: A systematic review and
meta-analysis of cohort studies. Cancer. 127:1459–1468.
2021.PubMed/NCBI View Article : Google Scholar
|