Introduction
One of the most severe complications of tuberculosis
(TB) is intracranial tuberculomas. While tuberculoma is observed in
1.8% of patients with TB, ~25% of these cases present as isolated
central nervous system (CNS) tuberculoma (1,2).
Tuberculomas can be easily confused with diseases such as lymphoma,
glioblastoma, metastatic masses, fungal infections, toxoplasmosis
and neurocysticercosis (3,4). Contrast-enhanced computed tomography
and magnetic resonance imaging (MRI) are instrumental in the
diagnosis of tuberculoma. However, the optimal diagnostic imaging
tool is MRI (2,5).
Patients receiving anti-TB drugs should be monitored
for side effects, including nausea, vomiting, abdominal pain and
hepatotoxicity (6,7). Isoniazid (INH), rifampin (RIF) and
pyrazinamide (PZA) are all hepatotoxic. RIF can discolor urine and
cause thrombocytopenia. Ethambutol (E) can produce dose-dependent
optic neuritis and peripheral neuropathy. PZA can cause various
cutaneous reactions, asymptomatic hyperuricemia and gouty
arthritis. Streptomycin can cause ototoxicity and vertigo (8,9). INH
can also cause peripheral neuropathy, but this reaction can be
prevented by pyridoxine supplementation (10,11).
Another important point is that paradoxical deterioration in the
clinical status of patients with CNS tuberculoma may be observed
after an increased inflammatory response during the return of the
immune system in patients whose steroid treatment is reduced or
discontinued. Immune reconstitution inflammatory syndrome (IRIS) is
a life-threatening condition with a poor prognosis (6,12).
This case report presents a rare case of CNS
tuberculoma in a pediatric patient who developed hepatotoxicity
twice, followed by IRIS, and whose disease was controlled with
different regimens of anti-TB drugs and prolonged steroid
treatment.
Case report
A 7-year-old female patient who had no underlying
disease was started on oral antibiotic treatment with a preliminary
diagnosis of sinusitis at another medical center where the patient
had presented 3 weeks previously with complaints of headache, loss
of appetite, nausea and vomiting. During follow-up, the patient
complained of difficulty stepping on the right foot and walking
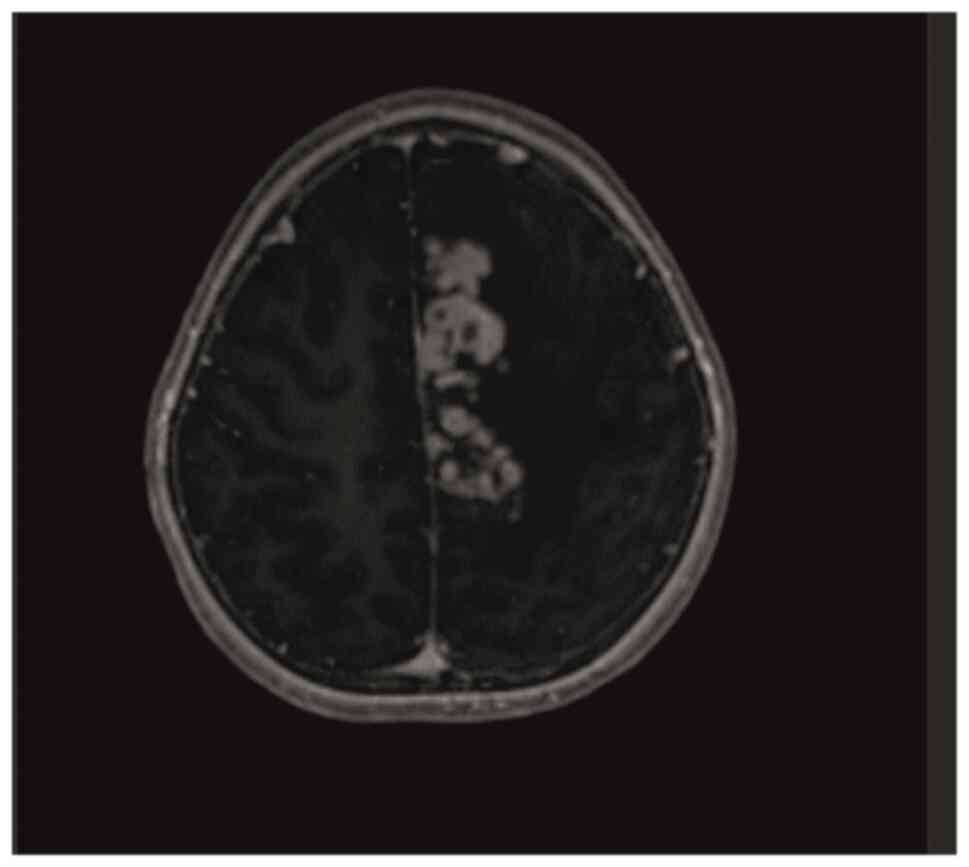
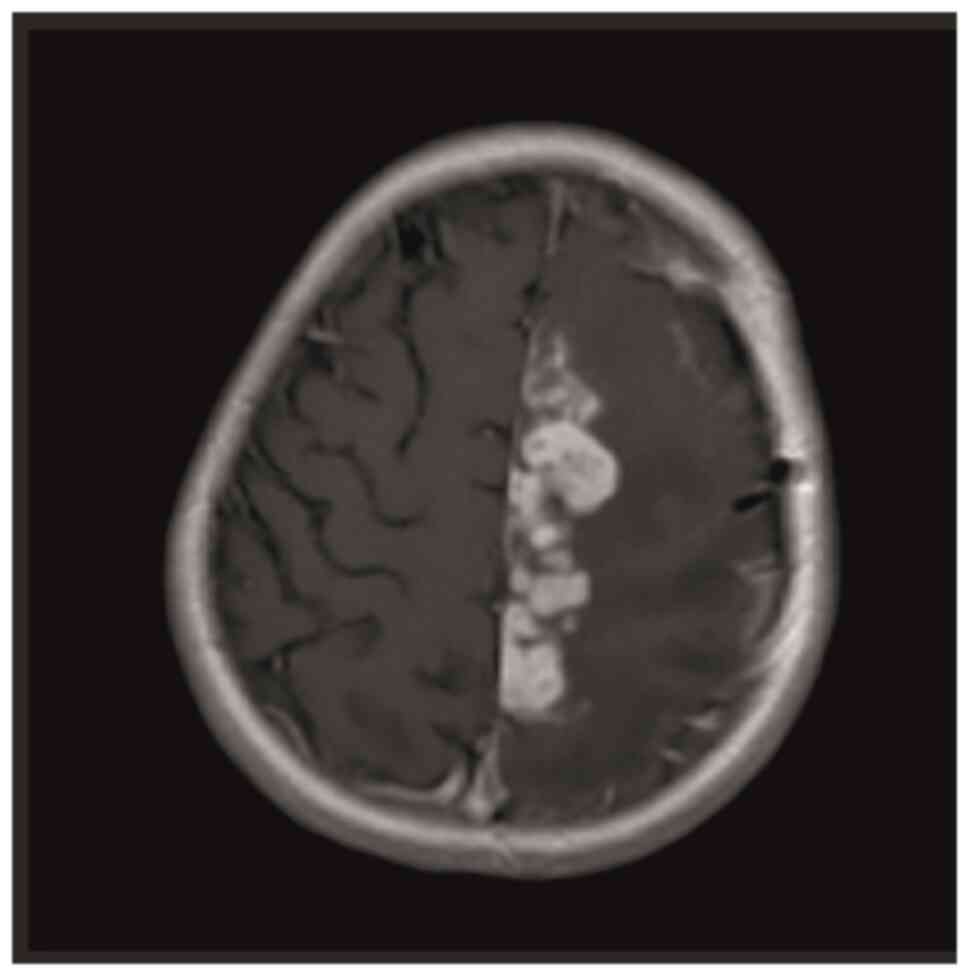
with a dragging right foot. Contrast-enhanced brain MRI performed
for these symptoms revealed multiple lesions in the left
frontoparietal region, the largest of which was 13x7 mm, covering
an area of ~7x5 cm, with massive vasogenic edema extending to the
corpus callosum. Most lesions demonstrated prominent peripheral
ring-like contrast enhancement, with certain lesions displaying a
nodular, closely juxtaposed appearance. In addition, a 7-mm shift
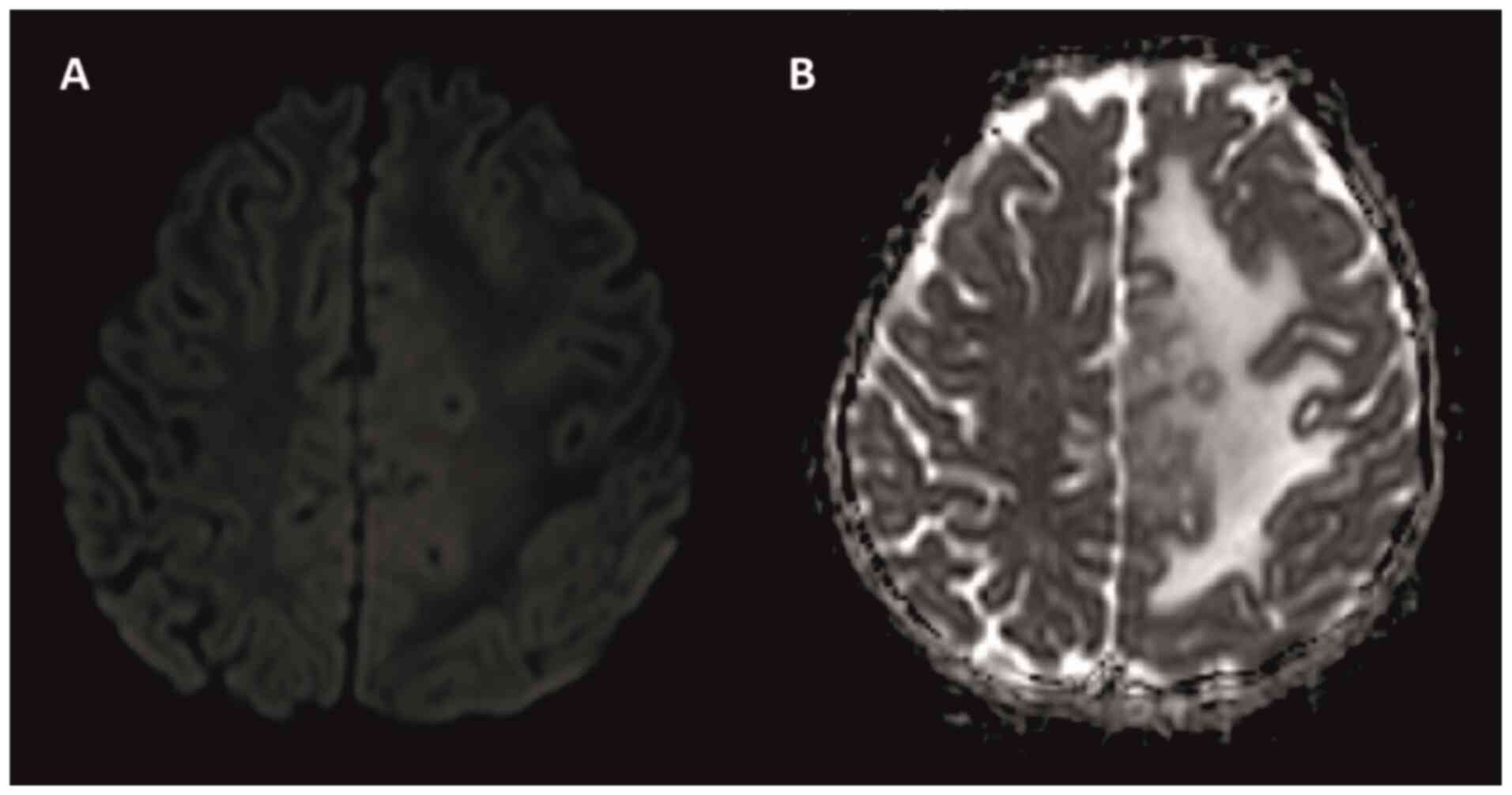
to the right in the midline was observed (Fig. 1). No contrast enhancement was
observed on diffusion MRI (Fig. 2A
and B). In April 2022, the patient
was admitted to the Department of Pediatric Infection of Balcalı
Hospital (Sarıçam, Turkey) with a preliminary diagnosis of brain
tuberculosis and a mass. On initial examination, the patient's
general condition was moderate and consciousness was clear. In the
neurological examination of the patient based on the data of the
Turkish Neurological Association, the Babinski sign was positive on
the left, gaze limitation was positive on the left and muscle
strength in the lower extremities was 4/5(13). No other pathological findings were
noted. Empiric INH, RIF, PZA, E and steroid at 2 mg/kg were started
with a prediagnosis of tuberculoma.
The white blood cell count was 13.300/µl (normal
range, 4.500-15.500/µl), hemoglobin was 13 g/dl (normal range,
11.5-15.5 g/dl), platelets were 494.000/µl (normal range,
150.000-450.000/µl), liver and renal function tests were normal,
the erythrocyte sedimentation rate was 43/h (normal range, 0-20/h)
and C-reactive protein was 1.82 mg/l (normal range, 0-0.8 mg/l).
According to standard laboratory tests, anti-human immunodeficiency
virus, as well as toxoplasma IgM and IgG were negative. An
ophthalmic examination revealed papillary congestion. A
contrast-enhanced MRI of the spine, chest and abdomen was normal.
There was no known history of TB in the family. Interferon-γ
release assay (IGRA) was positive in the study conducted with the
QuantiFERON®-TB Gold Plus ELISA kit (cat no. 0590-0301)
according to the manufacturer's instructions (14). A tuberculin skin test performed
with PPD Tuberculin 5 TU/0.1 ml solution revealed a result of 0x0
mm (15). A Bacille
Calmette-Guérin (BCG) scar was present (16). For a definitive diagnosis, the
patient's intracranial mass was removed as much as possible.
According to standard laboratory tests, cerebrospinal fluid (CSF)
obtained during surgery showed no growth in TB culture. In this CSF
sampling, no microorganisms were seen in the Acid-fast stain and
the cell count showed 80/mm3 erythrocytes. However, CSF
biochemistry could not be performed due to insufficient sample
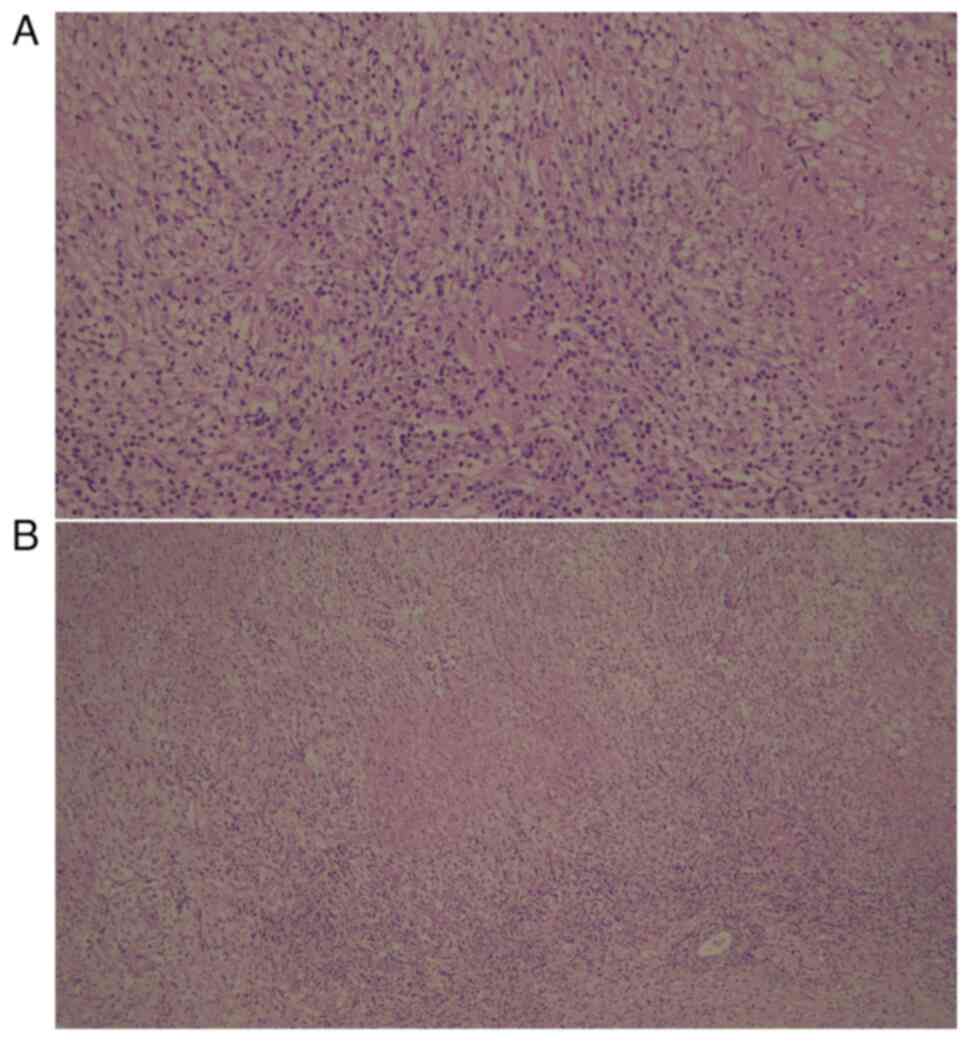
size. While necrotizing granulomatous inflammation was detected in
the patient's intracranial pathology samples (Fig. 3A and B), IgG4-related disease,
Aspergillus, Bartonella, Tularemia, Epstein-Barr
virus, Cytomegalovirus, Brucella and Toxoplasma
gondii tests performed in the Public Health Laboratory and
private external laboratories found negative results. The results
of the analyses indicated that the samples were negative for the
presence of parasites and viruses. The evaluations were made as
standard hospital microbiology tests and by the Public Health
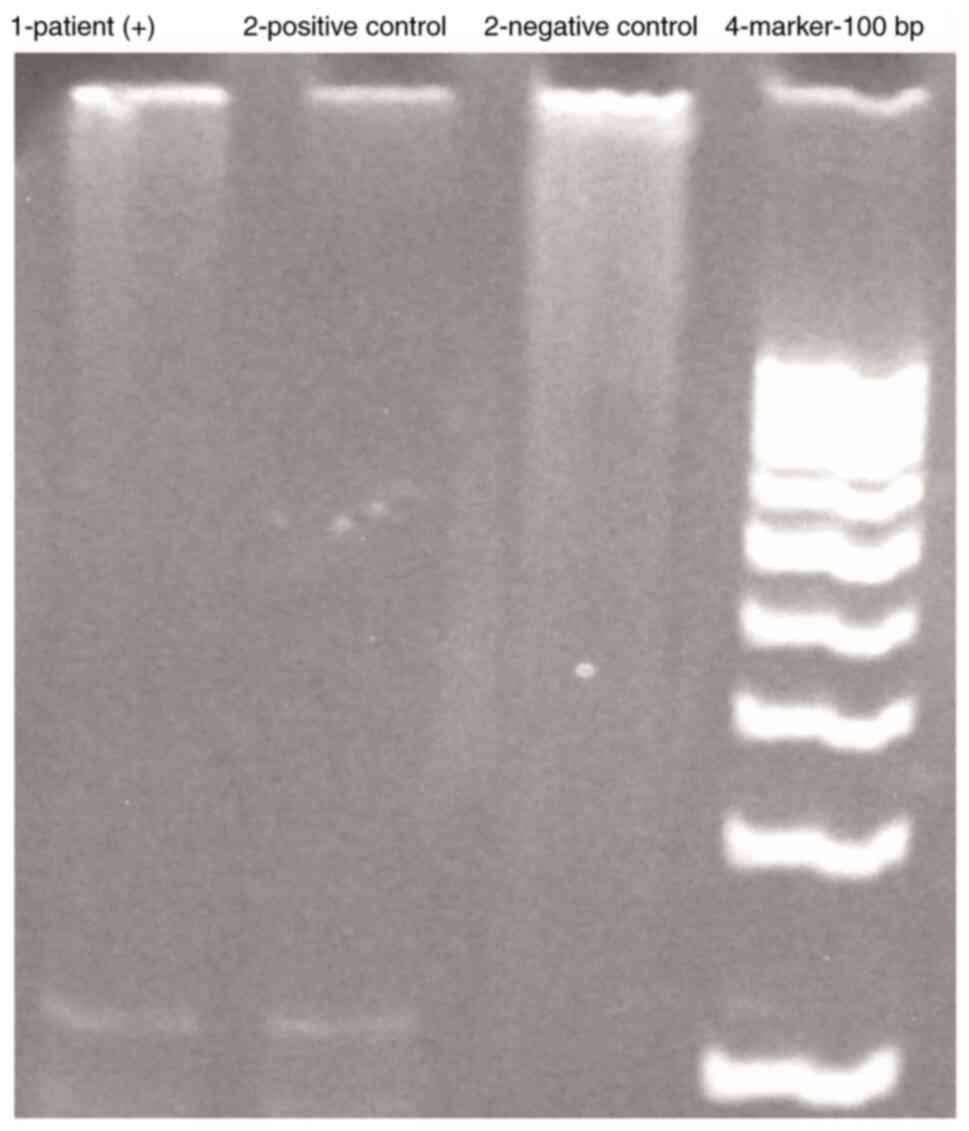
Laboratory. In the pathology sample, the TB PCR test gave a
positive result (Fig. 4; the
materials were run on the same gel strip). The patient result was
compared to positive and negative control DNA samples maintained at
the laboratory and the 123 bp alignment was detected as positive.
For the PCR method, mycobacterial DNA was isolated first solation
of Mycobacterium DNA Using Chelex-100 Solution (10%)/DNA
Extraction from BACTEC 12 B Bottle, and purified. The PCR product
of the 65-kDa heat shock protein region specific to all
mycobacteria was cut with restriction enzymes and mycobacterial
species were identified. Mycobacterial DNA was detected using
Mycobacterium tuberculosis Complex Specific IS6110 PCR. The
primers TB4 (sequence, 5'-CCT-GCG-AGC-GTA-GGC-GTC-GG-3') and TB5
(sequence, 5'-CTC-GTC-CAG-CGC-CGC-TTC-GG-3') were used for the
detection of Mycobacterium tuberculosis by PCR. Samples were
run on a 7.5% polyacrylamide gel containing 2% ethidium bromide for
electrophoresis and then examined by UV analysis. The observation
of an amplification compound of 123 bp was considered to indicate a
positive result (17). The
patient's standard immunodeficiency and rheumatological test
results performed in the standard serology and biochemistry
laboratory were found to be normal. The diagnosis of malignancy was
excluded.
Since the biopsy was positive for necrotizing
granulomatous inflammation and IGRA and the TB PCR test was
positive, other causes were ruled out and the patient was diagnosed
with isolated CNS tuberculoma. The patient's family was questioned
again for TB and it was revealed that a relative of the uncle had
TB. There was no close contact with this distant relative. The
entire family was screened for TB (tuberculin skin test and lung
X-ray). At the first examination, the classical 4-drug regimen
(INH, RIF, PZA and E) antituberculosis treatment was started and
continued for 2 months. Thereafter, maintenance treatment was
initiated. Steroid treatment was continued at a dose of 2 mg/kg for
~2 months and was tapered and discontinued at the follow-up visit.
During the fifth month of treatment, the patient developed vomiting
and abdominal pain. The aspartate aminotransferase (AST) level was
914 U/l (normal range, 15-42 U/l) and the alanine transaminase
(ALT) level was 900 U/l (normal range, 8-38 U/l), and the treatment
was discontinued. This condition was considered drug-induced
hepatotoxicity. After a 15-day interruption, anti-TB treatment was
resumed because AST/ALT levels had returned to normal. In the
seventh month of treatment, the patient again had nausea, vomiting
and abdominal pain, and the AST/ALT level was 912/128 U/l, so the
anti-TB treatment was interrupted for the second time. Treatment
failure and IRIS were considered due to edema and lesion
progression found on the MRI taken at the 7th month of anti-TB
treatment (Fig. 5). The patient
was started on a non-hepatotoxic regimen and 3% saline for cerebral
edema. Hypertonic saline was given as a 20-min infusion at 2-5
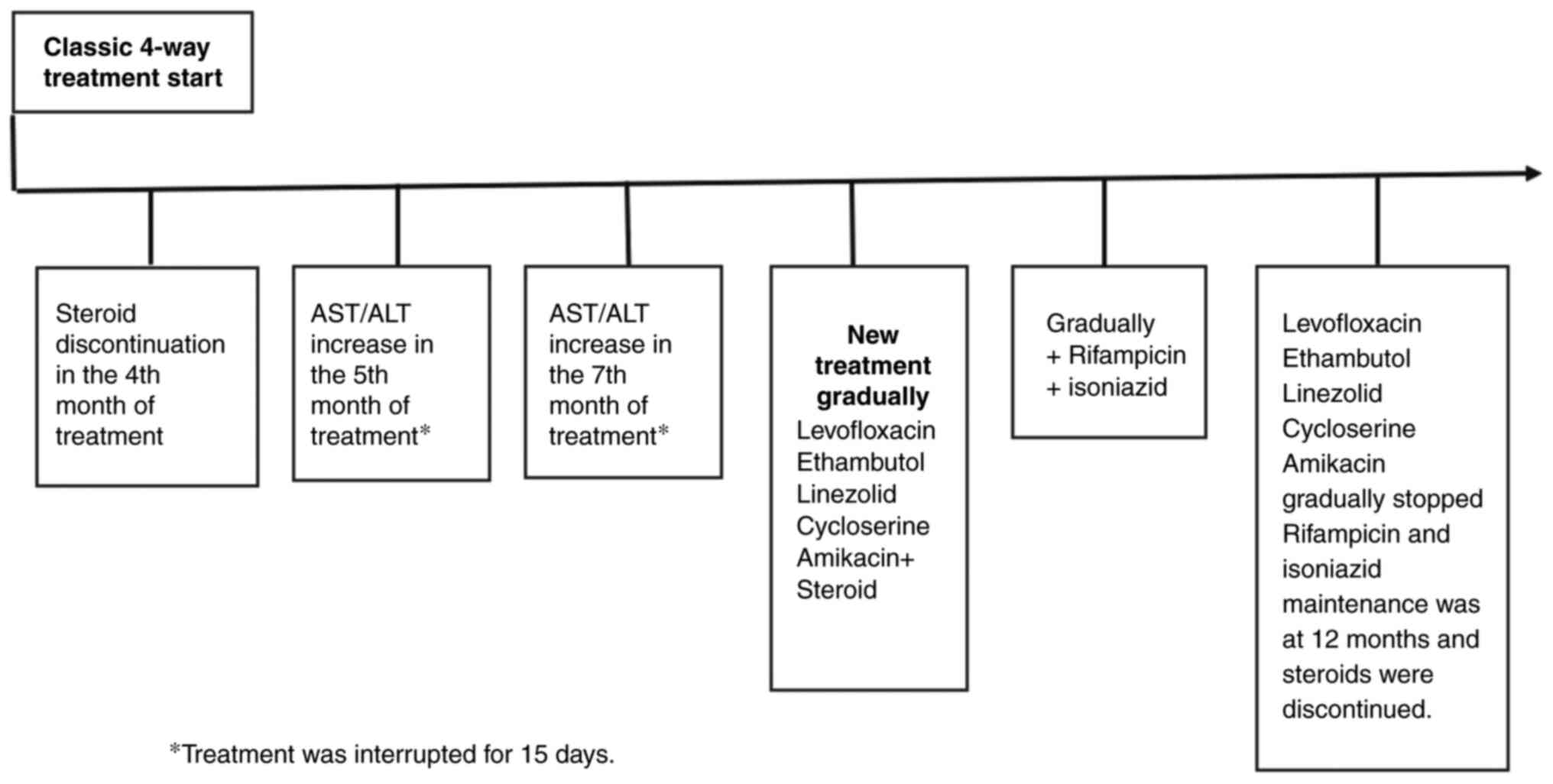
ml/kg. Oral levofloxacin, ethambutol, linezolid, cycloserine and
intramuscular amikacin were started. Steroid was restarted at 2
mg/kg. When the AST/ALT levels had reached normal levels, RIF and
then INH were gradually added to the anti-TB regimen. The treatment
regimen is presented in Fig. 6.
During the hospital stay, the patient received treatment in an
isolated room. During the follow-up, the patient could not go to
school and received education at home. Crowded places were also
avoided.
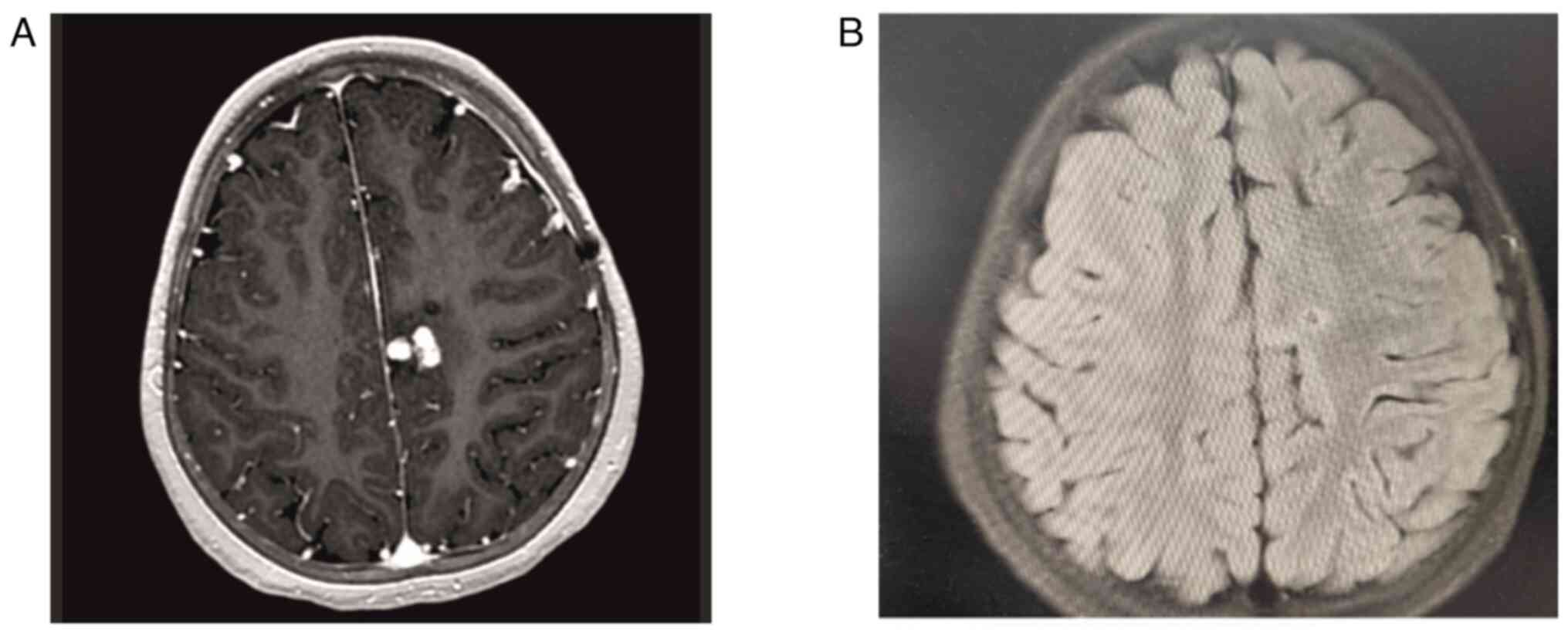
Brain MRI (Fig. 7A
and B) showed regression of the
lesions in the fifth and 12th months of non-classical anti-TB
regimen. Currently, the patient is in the 12th month of INH and RIF
maintenance therapy, and steroid treatment has been tapered and
discontinued. During the follow-up, the patient's neurological
deficit regressed and radiological examinations showed improvement.
The patient is being monitored and the planned duration of
treatment is at least 18 months based on the clinical and MRI
findings. There is currently no clear consensus on the usual
treatment for this condition.
Discussion
TB continues to be one of the most common infectious
causes of death (18). BCG
vaccine, which has a historical importance in the prevention of TB,
is known to have been administered to >4 billion individuals
within its 100-year history (19).
The prevention and reduction of pulmonary and systemic TB also
reduces the incidence of CNS TB (20). CNS tuberculomas represent a
distinct form of TB. Despite their rarity, they are of significant
clinical importance due to their propensity to result in
significant neurological complications in affected individuals
(21).
Tuberculomas present as space-occupying lesions that
vary in size and location, and clinical symptoms depend on these
factors. Symptoms may include headache (50%), fever (14%), loss of
consciousness (27%) and seizures (12-18%) (22). The diagnosis of cerebral
tuberculoma is challenging due to the absence of distinctive
clinical manifestations (23).
Although histopathological analysis provides great advantages for
definitive diagnosis, it is a difficult procedure (24). Early initiation of treatment in
cerebral tuberculoma is associated with better treatment outcomes,
and early diagnosis and complete treatment can reduce morbidity,
mortality and the emergence of drug-resistant disease (20,25).
Although there was no evidence of TB at the initial presentation of
the case of the current study, anti-TB treatment was immediately
initiated due to suspicion on brain imaging. The diagnosis was
subsequently proven by both histopathological and molecular
methods.
Studies are ongoing for the rapid and accurate
diagnosis of TB, and Immuno-(I)-PCR, which is based on the
detection of potential Mycobacterium tuberculosis antigens,
has been shown to be useful (26,27).
I-PCR, an ultrasensitive method, has been shown to provide a
several-fold increase in sensitivity compared to ELISA by combining
it with the amplification capacity of PCR with ELISA.
Antigens/antibodies were detected in TB patients by I-PCR (28,29).
Determination of moderately high choline/creatine ratios by
magnetic resonance spectroscopy has been shown to be a useful
method in the diagnosis of CNS tuberculoma (30). The aim is to provide early and
correct diagnosis and treatment, following which the patient's
response to treatment is good and sequelae can be reduced. It is
also a protective factor against the development of drug
resistance. Therefore, these studies are of high importance.
During treatment, it is necessary to be careful
regarding drug side effects and conditions such as IRIS. The most
common side effect of anti-TB drugs is hepatotoxicity.
Hepatotoxicity continues to be an important problem because anti-TB
treatment must be stopped or changed. If transaminase levels exceed
3 to 5 times the upper limit of normal or serum bilirubin exceeds
2.5 mg/l, it is recommended that hepatotoxic drugs should be
changed to 2nd- or 3rd-line options (10,11).
In the study conducted by Yurdakul et al (31), ALT enzyme elevation was found in
23.7% of patients receiving anti-TB treatment and complications
requiring drug discontinuation were found in 11.3%.
Increased immune response with steroid tapering or
discontinuation is noteworthy with respect to IRIS. In general, the
condition of IRIS is characterized by an improvement or reversal of
the immune system, as well as a worsening of the pre-existing
infection or the emergence of an acute symptomatic infection
(10,32). In the case of the present study,
the side effect of elevated liver enzymes developed in the fifth
and seventh months of anti-TB treatment, and steroid treatment was
discontinued in the fourth month of anti-TB treatment. The
appearance of new tuberculomas in the MRI performed as a result of
the second treatment interruption created a challenging situation.
Treatment was resumed with both steroid and non-hepatotoxic
regimens. To avoid confusion in these patients, follow-up brain
imaging should not be delayed in cases where anti-TB drugs may need
to be stopped. If necessary, non-classical anti-TB drug regimens
should be considered earlier.
It is difficult to predict whether IRIS will occur
during TB treatment and studies are ongoing on this subject.
Detection of TB-lipoarabinomannan (LAM), a TB cell wall
glycoprotein, in urine samples is helpful in risk stratification
because LAM antigen positivity is associated with disseminated TB
and low CD4+ T-cell counts. A positive association between LAM
positivity and TB-IRIS has been reported (33). A recent study suggests that CD4+
T-cell activation markers may predict TB-IRIS and that a
combination of CD4+ and CD8+ T-cell markers may be helpful in
diagnosing TB-IRIS (34).
The duration of treatment in intracranial
tuberculoma is uncertain. The prevailing opinion is that the
typical course of treatment should last between 12 and 18 months
(35,36). In the study conducted by Li et
al (36), patients with
tuberculoma were given treatment for 18 months. Furthermore, in a
study examining isolated brainstem tuberculomas, treatment was
typically administered for a minimum of 18 months or until the
tuberculoma regressed (37). Based
on the clinical and brain MRI findings, the treatment duration for
the present case was planned to be at least 18 months.
In conclusion, the prognosis of the disease is
directly related to early diagnosis and the timing of initiation of
anti-TB treatment. It should always be kept in mind that IRIS may
occur in cases with tuberculoma and that anti-TB drug side effects
may develop. In the present case of CNS tuberculoma, it was
uncertain whether the progression of the patient's lesions was the
result of IRIS or the interruption of anti-TB treatment due to drug
side effects. Steroid treatment was started for IRIS and a
different regimen was started for CNS tuberculoma. It is important
to follow-up such patients clinically and at certain intervals with
cranial MRI. We believe that there is no standardization in control
MRI and that control imaging should be performed every 6 months for
the slightest new neurological complaint, transition to maintenance
therapy or to evaluate the response to treatment. The current case
is notable due to its rare and non-classical treatment regimen.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
FTC, UC, FK, OK, OOG and DA conceived the idea of
the study. FTC, UC, FK, OK, NT, EB, AU and AD were involved in the
study design. FTC, UC, FK, OK, NT, EB, AU, OOG, DA and AD collected
and/or processed the data. FTC, UC, FK, OK, NT, EB, AU, OOG, DA and
AD analysed and/or commented on the data. FTC, UC, FK, OK, NT, EB,
AU, OOG, DA and AD performed the literature review. FTC, UC, FK and
OK wrote the manuscript. FTC, UC, FK, OK, NT, EB, AU, OOG and DA
critically reviewed the manuscript. All of the authors participated
in the design and analysis of the study, and they have read and
approved the final version. FTC, UC, FK, OK, NT, EB, AU, OOG, AD
and DA confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Verbal consent was obtained from the patient and
written consent was obtained from the patient's mother for the
publication of the present case report and accompanying associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Matsumoto Y, Aikawa H, Narita S, Tsutsumi
M, Yoshida H, Etou H, Sakamoto K, Inoue R, Nii K and Kazekawa K:
Intracranial tuberculoma in non-immunosuppressive state. Neurol Med
Chir (Tokyo). 53:259–262. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Perez-Malagon CD, Barrera-Rodriguez R,
Lopez-Gonzalez MA and Alva-Lopez LF: Diagnostic and neurological
overview of brain tuberculomas: A review of literature. Cureus.
13(e20133)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Verma R and Gupta R: Multiple
ring-enhancing lesions: Diagnostic dilemma between
neurocysticercosis and tuberculoma. BMJ Case Rep.
2014(bcr2013202528)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yang M, Zhang JT, Yao Y, Tan QC, Gao T,
Tian CL, Huang X and Yu SY: A clinical study of miliary brain
tuberculomas in China. Jpn J Infect Dis. 69:231–235.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li CR, Li YZ, Li YM and Zheng YS: Dynamic
and contrast enhanced CT imaging of lung carcinoma, pulmonary
tuberculoma, and inflammatory pseudotumor. Eur Rev Med Pharmacol
Sci. 21:1588–1592. 2017.PubMed/NCBI
|
|
6
|
Sindgikar SP, Narayanaswamy B, Alexander
LM and Kanavu R: Paradoxical immune reconstitution inflammatory
syndrome in neurotuberculosis. BMJ Case Rep.
14(e243739)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Salman N, Somer A and Yalçın I (eds):
Paediatric Infectious Diseases. Akademi Kitabevi, İstanbul, 2015
(In Türkiye).
|
|
8
|
Saukkonen JJ, Cohn DL, Jasmer RM, Schenker
S, Jereb JA, Nolan CM, Peloquin CA, Gordin FM, Nunes D, Strader DB,
et al: An official ATS statement: Hepatotoxicity of
antituberculosis therapy. Am J Respir Crit Care Med. 174:935–952.
2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Alsayed SSR and Gunosewoyo H:
Tuberculosis: Pathogenesis, current treatment regimens and new drug
targets. Int J Mol Sci. 24(5202)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dhiman RK, Saraswat VA, Rajekar H, Reddy C
and Chawla YK: A guide to the management of tuberculosis in
patients with chronic liver disease. J Clin Exp Hepatol. 2:260–270.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Abbara A, Chitty S, Roe JK, Ghani R,
Collin SM, Ritchie A, Kon OM, Dzvova J, Davidson H, Edwards TE, et
al: Drug-induced liver injury from antituberculous treatment: A
retrospective study from a large TB centre in the UK. BMC Infect
Dis. 17(231)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Turhan A, Tosun Tasar P, Timur Ö,
Karaşahin Ö, Gökgül A and Binici DN: Immune reconstitution
inflammatory syndrome during anti-tuberculous therapy: A case
report. Tepecik Eğit ve Araşt Hast Dergisi. 29:103–106. 2019.(In
Turkish).
|
|
13
|
Erdoğan FF and Demir S (Ed): (2018).
Neurological Evaluation in Infants and Children. Nörolojik
Değerlendirme, Türk Nöroloji Derneği, Galenos Yayınevi, İstanbul
(In Turkish).
|
|
14
|
QuantiFERON-TB Gold Plus ELISA
Instructions for Use, March. 2023, QIAGEN GmbH QIAGEN Strasse 1,
40724 Hilden, Almanya.
|
|
15
|
Menentoğlu B, Şimşek C and Hatipoğlu N:
Administration and interpretation of PPD test. J Pediatr Inf.
15:57–62. 2021.(In Turkish).
|
|
16
|
Rümke HC: BCG: An almost 100-year-old
vaccine. Ned Tijdschr Geneeskd. 164(D5146)2020.PubMed/NCBI(In Dutch).
|
|
17
|
Saniç A, Eroğlu C and Kizirgil A:
Identification of mycobacterium species by PCR and RFLP methods.
21. Yüzyılda Tüberküloz Sempozyumu ve II. Tüberküloz Laboratuvar
Tanı Yöntemleri Kursu, Samsun, 2003 (In Turkish).
|
|
18
|
Gemnani R, Saboo K, Wanjari A, Kumar S and
Acharya S: Tuberculoma masquerading as a sixth cranial nerve palsy
in a young patient: A case report. Cureus.
16(e59469)2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Aslan G and Alkaya D: One hundred of
tuberculosis vaccine: History of bacille calmette-guérin-could BCG
vaccination induce trained immunity? Turk J Immunol. 10:12–21.
2022.
|
|
20
|
Cherian A, Ajitha KC, Iype T and Divya KP:
Neurotuberculosis: An update. Acta Neurol Belg. 121:11–21.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ech-cherif El kettani N, Jerguigue H,
Karouache A, El quessar A, El hassani MR, Chakir N, Boukhrissi N
and Jiddane M: No 5 Imaging of encephalic tuberculomas. J Radiol.
85(1517)2004.
|
|
22
|
Dian S, Ganiem AR, Te Brake LH and van
Laarhoven A: Current insights into diagnosing and treating
neurotuberculosis in adults. CNS Drugs. 37:957–972. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gupta RK and Kumar S: Central nervous
system tuberculosis. Neuroimaging Clin N Am. 21:795–814, vii-viii.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
DeLance AR, Safaee M, Oh MC, Clark AJ,
Kaur G, Sun MZ, Bollen AW, Phillips JJ and Parsa AT: Tuberculoma of
the central nervous system. J Clin Neurosci. 20:1333–1341.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Maheswari EU, Bhoopathy RM, Bhanu K and
Anandan H: Clinical spectrum of central nervous system tuberculosis
and the efficacy of revised national tuberculosis control program
in its management. J Neurosci Rural Pract. 10:71–77.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mehta PK, Singh N, Dharra R, Dahiya B,
Sharma S, Sheoran A, Gupta KB, Chaudhary D, Mehta N and Varma-Basil
M: Diagnosis of tuberculosis based on the detection of a cocktail
of mycobacterial antigen 85B, ESAT-6 and cord factor by immuno-PCR.
J Microbiol Methods. 127:24–27. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Singh N, Sreenivas V, Gupta KB, Chaudhary
A, Mittal A, Varma-Basil M, Prasad R, Gakhar SK, Khuller GK and
Mehta PK: Diagnosis of pulmonary and extrapulmonary tuberculosis
based on detection of mycobacterial antigen 85B by immuno-PCR.
Diagn Microbiol Infect Dis. 83:359–364. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mehta PK, Raj A, Singh NP and Khuller GK:
Detection of potential microbial antigens by immuno-PCR
(PCR-amplified immunoassay). J Med Microbiol. 63(Pt 5):627–641.
2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mehta PK, Dahiya B, Sharma S, Singh N,
Dharra R, Thakur Z, Mehta N, Gupta KB, Gupta MC and Chaudhary D:
Immuno-PCR, a new technique for the serodiagnosis of tuberculosis.
J Microbiol Methods. 139:218–229. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ahlawat S, Dabla S, Kumar V, Singh M, Bala
K and Mehta PK: Role of immuno-polymerase chain reaction (I-PCR) in
resolving diagnostic dilemma between tuberculoma and
neurocysticercosis: A case report. Am J Case Rep. 19:599–603.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yurdakul A, Çalışır H, Taci N, Çelik N and
Öğretensoy M: Hepatotoxicity occured during anti-tuberculosis
treatment. Toraks Dergisi. 4:16–20. 2003.(In Turkish).
|
|
32
|
Kılınc F, Çay Ü, Çetin FT, Tapac N, Ozgur
Gundeslioglu O and Alabaz D: Intracranial tuberculoma developing
during the treatment of a case with tuberculous meningitis caused
by the zoonotic mycobacterium caprae. Klin Padiatr: Feb 6, 2024
(Epub ahead of print).
|
|
33
|
Bulterys MA, Wagner B, Redard-Jacot M,
Suresh A, Pollock NR, Moreau E, Denkinger CM, Drain PK and Broger
T: Point-of-care urine LAM tests for tuberculosis diagnosis: A
status update. J Clin Med. 9(111)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tibúrcio R, Barreto-Duarte B, Naredren G,
Queiroz ATL, Anbalagan S, Nayak K, Ravichandran N, Subramani R,
Antonelli LRV, Satagopan K, et al: Dynamics of T-lymphocyte
activation related to paradoxical tuberculosis-associated immune
reconstitution inflammatory syndrome in persons with advanced HIV.
Front Immunol. 12(757843)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Marais S, Van Toorn R, Chow FC, Manesh A,
Siddiqi OK, Figaji A, Schoeman JF and Meintjes G: Tuberculous
Meningitis International Research Consortium. Management of
intracranial tuberculous mass lesions: how long should we treat
for? Wellcome Open Res. 4(158)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li H, Liu W and You C: Central nervous
system tuberculoma. J Clin Neurosci. 19:691–695. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sadashiva N, Tiwari S, Shukla D, Bhat D,
Saini J, Somanna S and Devi BI: Isolated brainstem tuberculomas.
Acta Neurochir (Wien). 159:889–897. 2017.PubMed/NCBI View Article : Google Scholar
|