Introduction
Recently, immune checkpoint inhibitors (ICIs) have
been used in the treatment of many cancers, including lung cancer,
and the management of immune-related adverse events (irAEs) caused
by ICIs is important. Although the time of irAE onset depends on
the type of ICI and whether they are used in combination, the
median onset time for most irAEs is 2 to 4 months (1,2).
However, it is generally difficult to predict the time of onset of
irAEs, since they can occur even in patients long after the start
of ICI treatment (2).
The optimal duration of ICI use in advanced
non-small cell lung cancer (NSCLC) has not been fully evaluated. In
clinical practice, many patients with advanced NSCLC continue
therapy beyond 2 years; however, in the pivotal trials that
established the use of ICIs in patients with NSCLC, patients were
treated with ICI therapy for up to 2 years (3).
The case of a lung cancer patient who responded to
anti-programmed death-1 (PD-1) antibody treatment, namely nivolumab
monotherapy, and continued for more than 4 years, and then
developed diabetes mellitus (DM) 3 years and 3 months and
polymyalgia rheumatica (PMR) 4 years and 5 months after starting
nivolumab treatment is reported.
Case report
In June 2014, a 54-year-old man was referred to
Kainan hospital complaining of pain in the right hip joint region
for the past two months. He was an ex-smoker (60 pack-years), was
on no regular medications, and had no medical history except for
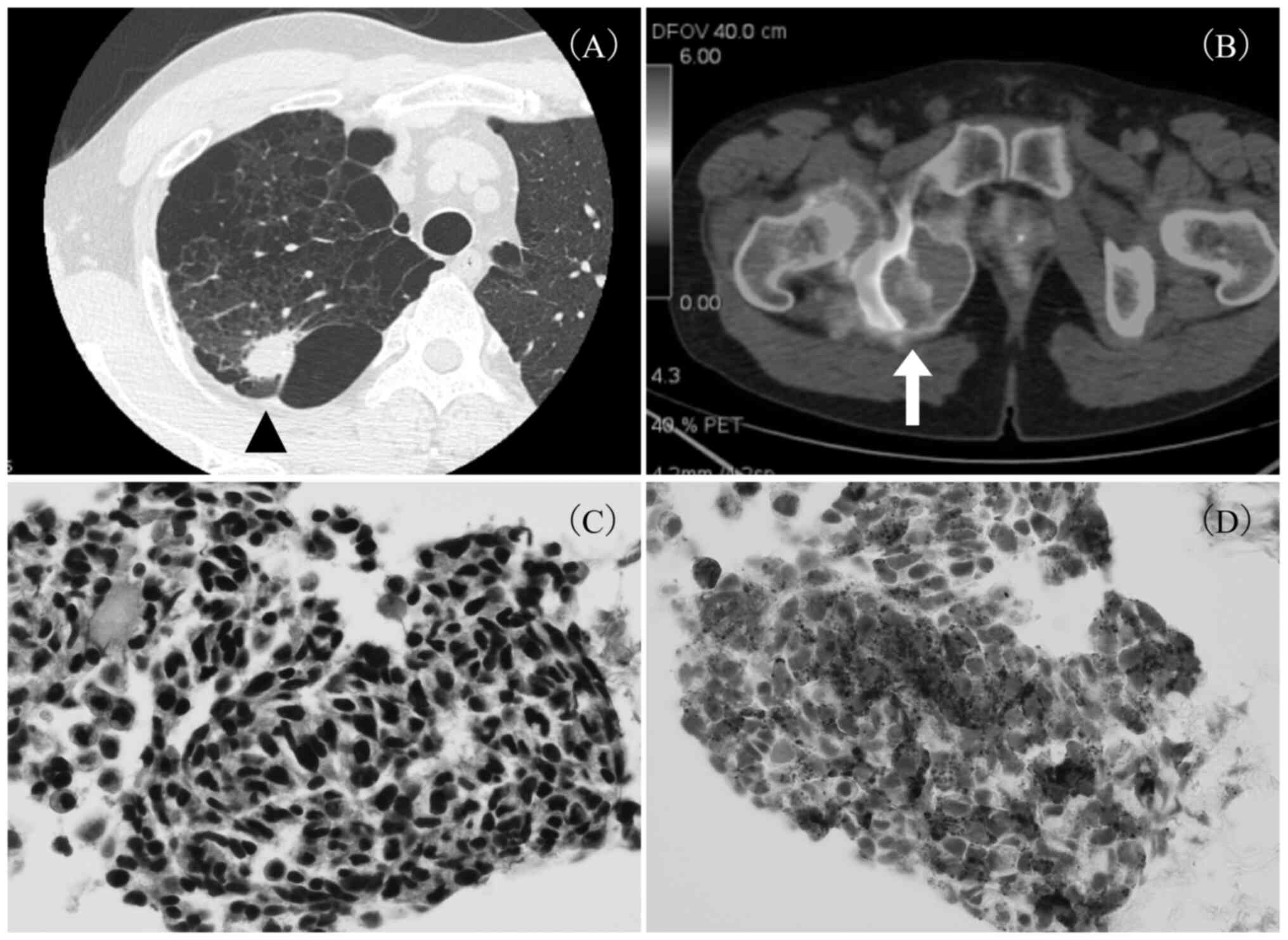
colitis. Computed tomography (CT) showed an ~25-mm nodule in the
right upper lobe of the lung (Fig.
1A) and a 50-mm mass in the right pelvic region. On
18F-fluorodeoxyglucose positron emission tomography-CT,
intense accumulation was seen in the right S2 nodule of the lung
[standardized uptake value (SUV) max: 15.2], right adrenal gland,
and a pelvic mass from the right sciatic/pubic bone to the right
internal obturator muscle (SUV max: 10.81) (Fig. 1B). On histopathological examination
of the transbronchial biopsy (TBB) specimen from the nodule in the
right lung, carcinoma with neuroendocrine features was identified;
hematoxylin and eosin staining showed small tumor cells with a
relatively high nucleus-to-cytoplasm ratio and hyperchromatic
nuclei (Fig. 1C), and
immunohistochemical analysis was positive for synaptophysin
(Fig. 1D), napsin A, Ki-67 (30%
positive), and thyroid transcription factor-1, and negative for p63
and chromogranin A. According to the 7th edition of TNM staging,
the patient was classified as having stage IV lung cancer
(T1bN0M1b). Mutation status was negative for epidermal growth
factor receptor and anaplastic lymphoma kinase gene, and the
programmed death-ligand 1 (PD-L1) tumor proportion score using the
22C3 antibody was less than 1%. In light of the pathologically
atypical presentation for small cell carcinoma and his good
performance status (PS), the decision was made to treat him
according to standard treatment guidelines for stage IV NSCLC.
From July 2014, he was treated with radiation
therapy for the pelvic lesion (45 Gy/15 Fr), as well as
chemotherapy (carboplatin + paclitaxel + bevacizumab for four
courses), which was followed by bevacizumab maintenance therapy.
After 15 cycles of maintenance therapy, the right adrenal
metastasis was larger, and a new left adrenal metastasis was
observed. Pemetrexed monotherapy was started as second-line therapy
(total of two courses), followed by docetaxel monotherapy as
third-line therapy (total of six courses). Although he had a period
of remission, in January 2018, re-enlargement of the right adrenal
metastasis was detected, and he was treated with radiation therapy
for the right adrenal lesion (50 Gy/25 Fr), as well as chemotherapy
(two courses of docetaxel monotherapy).
However, in February 2019, enlargement of the left
adrenal metastasis was detected, and he was administered nivolumab
monotherapy as fifth-line therapy. Prior to the initiation of
nivolumab treatment, the patient exhibited PS 0, with preserved
organ function and no suggestion of DM or autoimmune disease.
Nivolumab treatment was very effective, and a partial response was
achieved two months after the start of treatment. He continued
nivolumab treatment and achieved an almost complete response.
Therefore, discontinuation of nivolumab was considered after two
years of treatment, but nivolumab was continued as the patient
wanted to continue treatment due to concerns about relapse. At a
regular medical visit in May 2022, he complained of thirst and
polydipsia that had started a few days earlier, and his blood
glucose level, which had previously been within reference limits,
was increased to 468 mg/dl. Urinary ketones were negative,
pancreatic amylase was not elevated, and autoantibodies, including
anti-glutamic acid decarboxylase (GAD) antibodies, were negative.
He was diagnosed with DM and started on insulin therapy, after
which a decrease in the serum C-peptide level was observed.
Nivolumab treatment was resumed with continued
glycemic control, but one year after resumption of treatment,
muscle pain in the femoral area and neck appeared, which did not
improve with nonsteroidal anti-inflammatory drugs. Although
nivolumab treatment was discontinued, he developed worsening
arthralgia, muscle pain in both shoulders and knees, muscle
weakness, and weight loss. CT and magnetic resonance imaging showed
no obvious cancer progression, and rheumatoid factor,
anti-citrullinated protein antibody, antinuclear antibody,
anti-neutrophil cytoplasmic antibody, and anti-aminoacyl transfer
ribonucleic acid synthetase antibodies were negative, and
C-reactive protein was high (11.81 mg/dl), whereas the serum
creatine phosphokinase level was not elevated. Four years and 5
months after the start of nivolumab treatment, he was diagnosed
with PMR and treated with prednisolone (PSL) (10 mg daily).
Subsequent symptom control of his PMR was good, and PSL was tapered
and discontinued in ~7 months. Almost 20 months have passed since
the discontinuation of nivolumab treatment, but recurrence of the
lung cancer has not been observed.
Discussion
This was a rare case of a lung cancer patient
receiving nivolumab monotherapy for more than 4 years who developed
DM and PMR as irAEs. Although the median onset time for most irAEs
is 2 to 4 months (1,2), it is considered that patients should
always be carefully monitored for the development of irAEs during
ICI administration. Specifically, imaging studies, including chest
X-rays, and blood tests, including endocrine function tests and
cardiac markers, should be performed regularly, and non-specific
symptoms such as fatigue and appetite loss, which are common in
cancer patients, should also be considered as possibly related to
irAEs. Aberrant T cell activity is thought to be a prime factor in
the development of irAEs (4);
however, the type and timing of their development remain
challenging to predict due to the complexity of the underlying
mechanisms, which have not yet been fully elucidated.
In a Japanese study, 0.14-0.33% of patients
developed type 1 DM after anti-PD-1 antibody monotherapy, and the
median time from the date of the first anti-PD-1 antibody injection
to the onset of type 1 DM was 155 days (range: 13-504 days)
(5). Compared to Western patients,
Japanese patients are less likely to be positive for autoantibodies
such as anti-GAD antibodies, and ketosis and ketoacidosis are often
observed (5). In the present case,
DM developed more than 1,000 days after the start of nivolumab
treatment, which was a relatively long time to the onset of DM. In
addition, in the present case, anti-GAD antibodies and other
autoantibodies were negative, and there was no evidence of
ketosis.
With respect to PMR, 0.2-2.1% of patients developed
PMR after anti-PD-1 or anti-PD-L1 antibody administration, and the
time from the date of the first ICI injection to the onset of PMR
ranged from <1 to 53 months (6-8).
ICI-mediated PMR may have a milder course with less intense
inflammation than primary PMR, and ICI-mediated PMR can be managed
with a relatively low glucocorticoid dose (9). In addition, in a prospective
observational study including all cancer patients treated with
ICIs, the disease control rates in patients with rheumatic irAEs,
non-rheumatic irAEs, and those without irAEs were 85.7, 75.1, and
35.3%, respectively (7,8). In the present case, PMR developed 53
months after the start of nivolumab treatment, which was a
relatively long time to the onset of PMR. The patient was treated
with PSL 10 mg/day, the subsequent symptom control of PMR was good,
and the lung cancer was also well controlled.
Patients with advanced NSCLC were treated with ICI
therapy for up to 2 years in the pivotal trials that informed the
use of ICIs; in clinical practice, however, many patients continue
therapy beyond 2 years (3). In
addition, the CheckMate 153 trial suggested that continuing
nivolumab beyond 1-year improved outcomes (10). Although the optimal duration of ICI
use had not been adequately evaluated, a recent retrospective
cohort study showed that for patients with advanced NSCLC whose
disease is still responding to ICI therapy at 2 years, stopping
therapy and monitoring rather than continuing immunotherapy
indefinitely is a reasonable strategy with sustained clinical
benefit (3). In other research,
when comparing NSCLC patients treated with anti-PD-1 antibodies for
2 years with those who discontinued treatment and those who
continued treatment, in the continued treatment group, 19.5% of
patients had grade 1, 9.7% had grade 2, and 3.2% had grade 3-4
irAEs, whereas only 6.6% of patients had grade 1 irAEs in the
discontinued treatment group (11). Furthermore, there have been reports
of delayed irAEs such as adrenal insufficiency, hypothyroidism,
pneumonia, and alopecia after the end of treatment (3 to 28
months), even after short-term administration (12). In that report on delayed irAEs
(12), endocrine disorders were
the most common, followed by neurologic, cutaneous, and pulmonary
disorders, and corticosteroids were often used as an interim
treatment. In the present case, discontinuing nivolumab was
considered 2 years after the start of nivolumab treatment, but
nivolumab treatment was continued at the patient's request. At the
time this patient was treated, there was insufficient evidence to
support stopping immunotherapy in patients with NSCLC who were
responding well to treatment at 2 years, however, it might have
been necessary to make a decision not to continue nivolumab
treatment because of the risk of irAEs. It may also have been
necessary to more fully explain the risks of irAEs to the
patient.
In conclusion, a case of a lung cancer patient
successfully treated with nivolumab monotherapy who developed DM 3
years and 3 months after starting nivolumab and PMR 4 years and 5
months later was described. The underlying cause of delayed irAEs
and how long to follow up on irAEs remains to be elucidated and
warrants further investigation. This report provides oncologists
and pulmonologists with further insight into the importance of
long-term management of irAEs and the appropriate treatment
duration of immunotherapy. In addition, since irAEs have been
reported even after the completion of ICI administration, it should
be noted that long-term management of irAEs is necessary for
patients receiving and who have received ICIs.
Acknowledgements
The authors would like to thank Dr. Hidefumi Sato
(Department of Health Management, Kainan Hospital), Dr. Yoshiharu
Ozawa (Diabetes and Endocrinology, Kainan Hospital), Dr. Takuji
Tsuyuki (Department of Pathology, Kainan Hospital) and Dr.
Kaneshige Sasaki (Department of Rheumatology, Kainan Hospital) for
their assistance and stimulating discussions.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MakN analyzed and interpreted the patient data and
made a major contribution to writing the manuscript. HM contributed
to data collection and assisted in the preparation of the
manuscript. AH, MasN, SH, MK and NT advised on patient treatment
and assisted in patient data interpretation. In addition, AH, MasN,
SH, MK and NT confirmed the authenticity of all raw data, and
critically revised and edited the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written, informed consent for
the publication of the data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kanjanapan Y, Day D, Butler MO, Wang L,
Joshua AM, Hogg D, Leighl NB, Razak ARA, Hansen AR, Boujos S, et
al: Delayed immune-related adverse events in assessment for
dose-limiting toxicity in early phase immunotherapy trials. Eur J
Cancer. 107:1–7. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tang SQ, Tang LL, Mao YP, Li WF, Chen L,
Zhang Y, Guo Y, Liu Q, Sun Y, Xu C and Ma J: The pattern of time to
onset and resolution of immune-related adverse events caused by
immune checkpoint inhibitors in cancer: A pooled analysis of 23
clinical trials and 8,436 patients. Cancer Res Treat. 53:339–354.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sun L, Bleiberg B, Hwang WT, Marmarelis
ME, Langer CJ, Singh A, Cohen RB, Mamtani R and Aggarwal C:
Association between duration of immunotherapy and overall survival
in advanced non-small cell lung cancer. JAMA Oncol. 9:1075–1082.
2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Morad G, Helmink BA, Sharma P and Wargo
JA: Hallmarks of response, resistance, and toxicity to immune
checkpoint blockade. Cell. 184:5309–5337. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Baden MY, Imagawa A, Abiru N, Awata T,
Ikegami H, Uchigata Y, Oikawa Y, Osawa H, Kajio H, Kawasaki E, et
al: Characteristics and clinical course of type 1 diabetes mellitus
related to anti-programmed cell death-1 therapy. Diabetol Int.
10:58–66. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Le Burel S, Champiat S, Mateus C,
Marabelle A, Michot JM, Robert C, Belkhir R, Soria JC, Laghouati S,
Voisin AL, et al: Prevalence of immune-related systemic adverse
events in patients treated with anti-Programmed cell Death
1/anti-Programmed cell Death-Ligand 1 agents: A single-centre
pharmacovigilance database analysis. Eur J Cancer. 82:34–44.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kostine M, Rouxel L, Barnetche T, Veillon
R, Martin F, Dutriaux C, Dousset L, Pham-Ledard A, Prey S,
Beylot-Barry M, et al: Rheumatic disorders associated with immune
checkpoint inhibitors in patients with cancer-clinical aspects and
relationship with tumour response: A single-centre prospective
cohort study. Ann Rheum Dis. 77:393–398. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kita T, Araya T, Sakai T, Uchida Y,
Matsuoka H and Kasahara K: Nivolumab-Induced polymyalgia rheumatica
in a patient with lung adenocarcinoma. Am J Med Sci. 362:321–323.
2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vermeulen OCB, Brouwer E, Slart RHJA,
Sandovici M, Rutgers A, Hilterman TJ, Hiddinga B, Oosting SF,
Jalving M, de Heij AH, et al: Immune checkpoint inhibitor-mediated
polymyalgia rheumatica versus primary polymyalgia rheumatica:
Comparison of disease characteristics and treatment requirement.
Rheumatology (Oxford). 64:771–779. 2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Waterhouse DM, Garon EB, Chandler J,
McCleod M, Hussein M, Jotte R, Horn L, Daniel DB, Keogh G, Creelan
B, et al: Continuous versus 1-year fixed-duration nivolumab in
previously treated advanced non-small-cell lung cancer: CheckMate
153. J Clin Oncol. 38:3863–3873. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ardin C, Humez S, Leroy V, Ampere A,
Bordier S, Escande F, Turlotte A, Stoven L, Nunes D, Cortot A and
Gauvain C: Pursuit or discontinuation of anti-PD1 after 2 years of
treatment in long-term responder patients with non-small cell lung
cancer. Ther Adv Med Oncol. 15(17588359231195600)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Couey MA, Bell RB, Patel AA, Romba MC,
Crittenden MR, Curti BD, Urba WJ and Leidner RS: Delayed
immune-related events (DIRE) after discontinuation of
immunotherapy: Diagnostic hazard of autoimmunity at a distance. J
Immunother Cancer. 7(165)2019.PubMed/NCBI View Article : Google Scholar
|