Introduction
Large clinical trials have shown that sodium-glucose
co-transporter (SGLT-2) inhibitors provide good metabolic control
and cardiovascular (CV) benefits, such as reductions in overall CV
risk, hospitalization rates for heart failure and CV, and all-cause
mortality rates, with renoprotective effects as well. Large
investigational trials have demonstrated that representative drugs
from this class, such as empagliflozin or dapagliflozin, exhibited
beneficial primary and secondary effects regarding CV and
cerebrovascular (CVB) protection. These drugs act by blocking
glucose and sodium reabsorption in the kidneys, resulting in its
excretion through urine, and therefore, lowering blood glucose
levels and arterial hypertension (1). This medication has other effects,
such as reducing inflammation and oxidative stress, and assuming
these are the basis for the onset of CV or CVB events, it is
hypothesized that the use of SGLT-2 inhibitors may have additional
CV and CVB protective effects (1).
Cerebral artery aneurysm (CAA) is a condition that
can cause severe neurological deficits and potentially death. CAA
is characterized by abnormal dilatation of a portion of an artery
in the brain; due to this enlargement in volume, there is a
consequent high risk of rupture and subarachnoid hemorrhage
(2).
The symptoms of a ruptured CAA include double
vision, nausea, vomiting, stiff neck, sensitivity to light,
seizures, loss of consciousness and even cardiac arrest (3). Although not all patients who develop
a CAA require treatment, the ruptured aneurysms require prompt
intervention to save the patient's life. Interventions include
surgery (microvascular clipping), endovascular treatment (platinum
coil embolization) or implantation of flow diversion devices. Other
treatments may include anti-seizure drugs, calcium channel-blocking
drugs, the insertion of a shunt or rehabilitative therapy (3). The long-term management of CAA
focuses on reducing the risk factors and they emphasize the
necessity of lowering high blood pressure to a normal value such as
by reducing the use of alcohol and giving up smoking (3).
Causes and risk factors for CAA include a genetic
predisposition, smoking, arterial hypertension, diabetes and
atherosclerosis. Previously and at present, the primary method of
treatment for CAA is surgery, with the intention of preventing the
rupture. However, more recently, novel treatments have shown CV
benefits and are increasingly being used (4). Several genes have been described in
the literature as being implicated in the development of CAA, with
effects on vascular wall structure, extracellular matrix
homeostasis or inflammatory response. One such gene is COL3A1,
located on chromosome 2q32.2, which encodes type III collagen.
Mutations in this gene can disrupt collagen production, leading to
vascular wall weakening and predisposition to the formation of an
aneurysm (5).
Another gene associated with aneurysm development is
ELN, located on chromosome 7q11.23, which encodes elastin.
Mutations affecting elastin production result in increased vascular
rigidity, contributing to aneurysm susceptibility (6).
A different mechanism involves the degradation of
the extracellular matrix, which weakens arterial wall resistance.
This process can be exacerbated by increased expression of matrix
metalloproteinase (MMP)9 in smooth muscle cells and endothelium.
MMP9, located on chromosome 20q13.12, encodes an enzyme involved in
the degradation of collagen and elastin (7).
Additionally, genes such as RNF213 (chromosome
17q25.3) play a role in the development of cerebral arteries.
Mutations in RNF213 have been linked to an increased risk of CAA
due to fragile cerebral vessels prone to rupture (8).
In the present case study, the potential beneficial
effects of using SGLT-2 inhibitors in a patient with prior CAA and
at high risk for a CV event are described.
Case report
In March 2022, at the Diabetes Department of Marius
Nasta Institute of Pneumology (Bucharest, Romania), a 50-year-old
white/Caucasian male from an urban environment, with no history of
smoking, alcohol consumption or recreational drug use, was
admitted. The patient stated that he works in agriculture and runs
a grain farm. The patient's family history included a sister with
type 1 diabetes, but no known CV diseases were present in the
family. The patient was diagnosed with type 2 diabetes in 2013, at
the age of 38, with an initial glycated hemoglobin A1c (HbA1c) of
7.2% (normal range, 0-5.6%) without any signs and typical symptoms
of diabetes onset. For the treatment of diabetes, the patient
initially received medical nutritional therapy (diet and exercise)
and oral antidiabetics: First line of treatment with Metformin
according to the guidelines (9)
with an initial dose of 2,000 mg per day, a dose at which the
patient experienced gastrointestinal symptoms. Although lower doses
of metformin were tried, the patient could not tolerate this
treatment. After several attempts with the lowest possible dose and
several commercial preparations, Metformin treatment was ceased due
to the intolerance. The patient continued his treatment with diet
and exercise only, and over the years glycemic control was good,
with an HbA1c ≤6.0%.
The patient's medical history included type 2
diabetes, arterial hypertension (since 2013), dyslipidemia (since
2013), a surgically repaired aneurysm of the anterior communicating
artery (in 1998), a ruptured basilar artery tip aneurysm that was
subsequently embolized with a stent (in 2013), obesity [from the
age of 35 in 2010; a body mass index (BMI) of 31.72 kg/m²],
bronchiectasis (since 2020) diagnosed by a CT scan, non-alcoholic
fatty liver disease (NAFLD; since 2018) diagnosed by abdominal
ultrasound and diabetic neuropathy (since 2023) diagnosed by
peripheral sensitivity tests.
The onset of both CAAs was sudden and the primary
clinical symptoms for both were a severe headache along with
dizziness and nausea. Since this condition posed a threat to the
patient's life, an urgent surgical intervention was performed both
in 1998 and in 2013; in 1998, the CAA was surgically repaired and
in 2013 a stent was used for embolization.
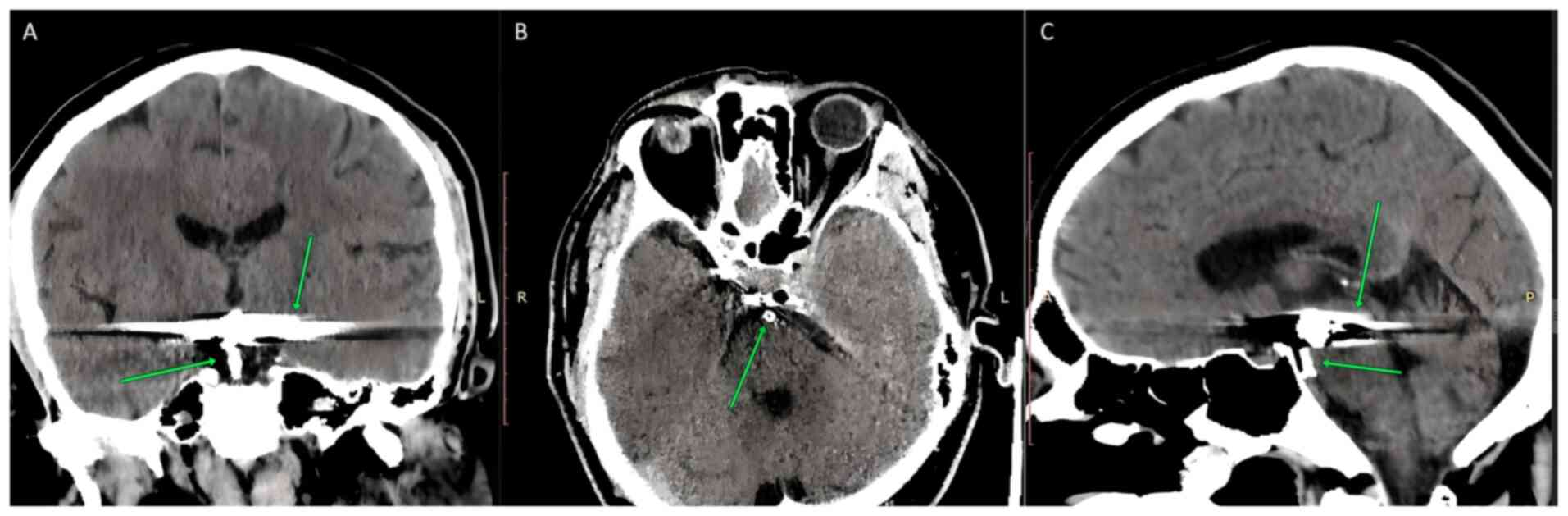
Fig. 1 shows the
stent that was used to repair the basilary artery tip aneurysm
(left image, arrow pointing at the lower side of the picture,
middle and right images, arrow pointing at the lower side of the
picture) and the metallic artifacts from pieces that were used for
the surgical repair of the CAA of the anterior communicating artery
(left and right side: arrows pointing in the upper side of the
picture). A computed tomography scan was used to acquire these
images.
The patient was treated with a long-acting
angiotensin-converting enzyme inhibitor-Perindopril, 5 mg once a
day (qd) (since February 2016), this being the first line of
treatment for arterial hypertension in diabetic patients based on
international guidelines (10);
β-blockers-Nebivololum 5 mg qd (since February 2016); antiplatelet
medication-acetylsalicylic acid, 75 mg qd since (since February
2020); and statins-Atorvastatin, 20 mg qd since (since February
2020) (Table I).
 | Table IMedical history of the patient. |
Table I
Medical history of the patient.
| Condition | Baseline
treatment | Start/end dates | Switch to other
treatments | Start/end dates |
|---|
| Type 2 diabetes | Metformin 2000
mg/day | December
2013-February 2014 | Empagliflozin 10
mg/day | March
2022-ongoing |
| Arterial
hypertension | Perindopril 5
mg/day | February
2016-ongoing | - | - |
| Arterial
hypertension | Nebivololum 5
mg/day | February
2016-ongoing | - | - |
|
Dyslipidemia/secondary CV prevention | Rosuvastatin 20
mg/day February 2020 | November 2013-20
mg/day | Atorvastatin | February
2020-ongoing |
| Secondary CV
prevention | Clopidogrel 75 mg/day
2020 | November
2013-February | Acetylsalicylic acid
75 mg/day | February
2020-ongoing |
In March 2022, the patient came to the hospital with
symptoms of shortness of breath, bilateral lower limb edema,
fatigue and a noticeable decline in quality of life; these symptoms
are consistent with the clinical symptomatology of heart failure.
The symptoms started ~2 weeks prior and got progressively worse up
to the point that the patient sought medical support. Given the
worsening of the symptoms, the patient's daily activities were
perturbed; he was no longer able to perform his daily tasks as he
did previously. At that time the patient was undergoing chronic
treatments for his pathologies, as aforementioned (detailed in
Table I).
Physical examination revealed a high blood pressure
of 144/82 mmHg, Grade I obesity, a BMI of 31.72 kg/m2
and abdominal obesity with a waist circumference of 112 cm and
bilateral lower limb edema.
The only available clinical examination for this
patient in 2022 was an electrocardiogram that did not reveal any
abnormal findings; the patient had a normal regular sinus rhythm
and a heart rate of 82 bpm. Other clinical examinations were not
available as, at that time, accessibility of the patient's medical
information was hampered by the COVID-19 pandemic.
Initial laboratory results for this patient, in
2022, are shown in Table II. The
patient had well-controlled type 2 diabetes; HbA1c was 5.2% (normal
range, 0-5.6%), but was outside the range regarding secondary
prevention of CV events, his total cholesterol, low-density
lipoprotein (LDL) cholesterol and triglycerides were outside
treatment targets for him; as a consequence, his overall CV risk
was very high (a score of SCORE 2 RISK=14.2%). SCORE 2 RISK is an
online risk assessment model that estimates the 10-year risk of CV
disease used in Europe; it is an algorithm that uses simple data
such as age, sex, tobacco use, systolic blood pressure, total
cholesterol and high-density lipoprotein to produce a numeric value
that translates into low risk, medium risk or high risk (<2%,
low risk; 2-4.9%, moderate risk; 5-9.9%, high risk; and ≥10%, very
high risk) (11).
 | Table IIBlood test results from 2022. |
Table II
Blood test results from 2022.
| Parameter | Value | Normal range |
|---|
| HbA1c, % | 5.2 | 0-5.6 |
| Cholesterol,
mg/dl | 229 | 120-200 |
| High-density
lipoprotein cholesterol, mg/dl | 50 | >40 in male
subjects |
| Low-density
lipoprotein cholesterol | 138 | <100 in a diabetic
patient and <55 in a patient with prior CV events (5) |
| Triglycerides,
mg/dl | 207 | 40-149 |
| Alanine
aminotransferase, UI/l | 17 | <37 |
| Aspartate
transaminase, UI/l | 29 | <40 |
| Creatinine,
mg/dl | 0.94 | 0.6-1.1 |
| eGFR, ml/min/1.73
m2 | 91 | >90 ml/min |
Due to the uncontrolled LDL levels, a change in
statin treatment was suggested to the patient; in February 2020,
rosuvastatin 20 mg qd was switched to atorvastatin 20 mg qd and the
patient did not experience any side effects of atorvastatin;
however, the patient refused to increase the dose of atorvastatin
due to the side effects he suffered with rosuvastatin [myalgias and
elevated creatine kinase levels when taking high doses of
rosuvastatin (20 mg)]. The patient did not experience any side
effects from atorvastatin.
In addition, the patient's blood pressure was not
adequately controlled, since the guidelines for a diabetic patient
suggests that blood pressure values should be <130/70 mmHg
(12). At this point, the patient
was referred to a cardiologist for a check-up and adjustment of his
antihypertensive treatment, but could not get an appointment since
the urgency of his condition was still low in the context of the
overall pandemic.
After reviewing the patient's case, it was decided
that the best course of action was the use of medication with
proven CV protective effects. Out of the available classes of drugs
for the treatment of diabetes, SGLT-2 inhibitors have shown CV
protective effects, and in clinical studies, SGLT-2 inhibitors have
shown that they can prolong the life of patients when given early
in the course of the disease (1).
In March 2022, SGLT-2 inhibitor (Empagliflozin, 10
mg qd) treatment was launched. This treatment was started despite
good glycemic control with a HbA1c of 5.2%, due to the CV benefits
of this class of drugs (1). The
patient's symptoms on presentation were more indicative of a heart
failure. However, it was not possible to run specific tests for
confirmation of heart failure (echocardiography and N-terminal pro
b-type natriuretic peptide levels) due to the low accessibility to
medical services during the pandemic. Based on clinical judgment, a
diagnosis of heart failure was assumed and it was decided to
administer treatment as a means of performing a therapeutic
test.
The patient returned for a follow-up evaluation in
March 2023, after 1 year of treatment, when he declared better
tolerance for exerting effort, lack of lower limb edema (also
revealed at the physical exam), a better quality of life and no
more shortness of breath.
At the follow-up physical examination, the patient
had a normal blood pressure of 128/70 mmHg, a heart rate of 75 bpm
and a lack of peripheral edema; however, the patient was still
classed as Grade I obesity (BMI, 30.25 kg/m) and the abdominal
obesity persisted (waist circumference, 109 cm). The patient
managed to lower his blood pressure and also managed to lose 3 kg
of body weight over the year from the initiation of SGLT2 inhibitor
treatment.
The patient was evaluated from a biological
perspective and the lab values showed a significant improvement in
the lipid profile with a slight decline in renal function, a known
temporary effect of initiation of SGLT-2 inhibitors. The results of
the lipid panel analysis improved with the normalization of
cholesterol and triglycerides values, but the patient was still
outside the normal range for LDL target for a diabetic patient with
a prior CV event; the target for him being <55 mg/dl (Table III).
 | Table IIIBlood test results from March
2023. |
Table III
Blood test results from March
2023.
| Parameter | Value | Normal range |
|---|
| HbA1c, % | 4.9 | 0-5.6 |
| Cholesterol,
mg/dl | 164 | 120-200 |
| High-density
lipoprotein cholesterol, mg/dl | 49 | >40 in male
subjects |
| Low-density
lipoprotein cholesterol, mg/dl | 91 | <100 in a diabetic
patient and <55 in a patient with prior CV events |
| Triglycerides,
mg/dl | 119 | 40-149 |
| Alanine
aminotransferase, UI/l | 22 | <37 |
| Aspartate
transaminase, UI/l | 34 | <40 |
| Creatinine,
mg/dl | 0.98 | 0.6-1.1 |
| eGFR, ml/min/1.73
m2 | 86 | >90 |
The patient was evaluated by echocardiography in
March 2023. The left ventricular ejection fraction was 55%,
diastolic function was normal, left ventricular hypertrophy was
present and systolic function was normal; the patient was diagnosed
with heart failure with preserved ventricular ejection fraction
(data not available).
Additionally, in March 2023, the patient underwent a
CT scan of the brain that showed no new onsets of aneurysmal
dilations, no new metal clip artifacts, no new aneurysms
post-embolization and no acute changes. The SCORE 2 RISK was 11%,
which was a reduction of 3.2% from the initial score registered in
2022; the patient continued with the treatment as part of his
further management since this treatment demonstrated improvement of
his clinical variables.
Discussion
The mechanism underlying the development of CAA
involves endothelial dysfunction, smooth muscle cell apoptosis and
vascular remodeling, and it typically occurs at the bifurcations of
cerebral arteries where, due to the high blood flow, a weakening of
the vessel wall can occur; this can also be exacerbated due to
endothelial injury (13). The
patient of the present study had a history of arterial
hypertension, an aneurysm of the anterior communicating artery that
had been operated on and a ruptured basilar artery aneurysm that
was embolized with a stent.
Another potential cause of an emerging aneurysm is
an imbalance in the actions of proteolytic enzymes, which can cause
a defect in the arterial wall matrix and exacerbate the brittleness
of a vessel (14). CAA may be
induced by inflammation and oxidative stress; inflammatory
cytokines, such as tumor necrosis factor-α (TNF-α) and
interleukin-6 (IL-6), have a well-established role in vascular
remodeling. The dysregulated action of inflammatory cytokines is
one of the core reasons for the onset of atherosclerosis, and
atherosclerosis along with the damage during remodeling of the
arterial wall are considered to be the main causes of the onset of
CAA (15). Oxidative stress can
also increase endothelial dysfunction, and along with inflammatory
cytokines, the damage to the arterial wall is exacerbated (16).
Obesity results in the presence of a
pro-inflammatory state, characterized by high levels of
inflammatory cytokines such as TNF-α and IL-6. The patient was
classed as obese since 2010, when at the age of 35 years, he was
classed as Grade 1 obese. Obesity is an additional risk factor for
the patient's history of CVB events, along with the arterial
hypertension mentioned previously (17).
SLGT-2 inhibitors have shown CV benefits in diabetic
and non-diabetic patients, with a significant reduction in
hospitalizations due to heart failure and also a significant
reduction in the onset of stroke in these patients (18). This underlined the choice to use
empagliflozin in the present case, and since the patient was a very
high-risk CV patient, it would be beneficial to suggest a treatment
for improved protection, particularly secondary prevention against
another CAA event.
SGLT2 inhibitors have been shown to reduce arterial
hypertension, and systolic and diastolic blood pressure, in
addition to the reduction in blood glucose levels; and these are
hypothesized to be key elements underlying the CV protective
effects of these drugs (4,19). Thus, it was hypothesized that
empagliflozin may potentially prevent another CAA event, since
lowering the blood pressure is known to help with the stabilization
of a pre-existing aneurysm and/or the onset of a new one,
considering that in the present case, the patient had hypertension
since 2013.
Another reason an SGLT-2 inhibitor was used in this
patient was the anti-inflammatory effect that this class has shown;
in large studies, SGLT-2 inhibitors lowered TNF-α and IL-6 levels
(1). This can reduce the
likelihood of CAA formation and prevent progression, as
inflammation is one of the primary causes of the onset of CAA
(5). Thus, empagliflozin was used
to reduce inflammation and reduce the vascular remodeling process
that leads to the formation of CAA.
Oxidative stress is also involved and plays a
crucial role in the development of endothelial dysfunction that can
lead to CAA through vascular disorders. SGLT-1 inhibitors have been
shown to inhibit NADPH oxidase and reduce overall oxidative stress
(20), and this was also
considered in the choice of using this treatment.
Another pleiotropic effect of SGLT-2 inhibitors is
reduced vascular stiffness and improved endothelial function
(21). These effects could help
prevent the onset of a CAA event and reduce the risk of rupture of
an existing aneurysm.
The renal benefits of SGLT-2 inhibitors are
well-established and are mediated by increased sodium excretion by
reducing fluid retention, and along with the reduction of arterial
hypertension, can lead to better CV hemodynamics (22). The use of SGLT2 inhibitors in a
diabetic patient, at a high risk of developing secondary renal
impairment from diabetes and hypertension, is a preventative
strategy (1), and in the present
case, considering the preexisting CAA, it was also a protective
measure due to the improvement in CV hemodynamics.
Patients with metabolic syndrome and type 2 diabetes
are at higher risk of liver dysfunction compared to the normal
population (23). These drugs,
SGLT-2 inhibitors, have a significant impact on blood glucose
levels as well as insulin sensitivity in peripheral tissues.
Furthermore, they are now recognized as effective agents in
diabetic patients with CV and renal complications owing to their
several pleiotropic effects. Recent evidence suggests that SGLT2
inhibitors interact with hepatic functions and SGLT2 inhibition
modulates liver function in pathways that are not yet well
defined.
NAFLD is a disorder that covers a spectrum of liver
lesions [steatosis, non-alcoholic steatohepatitis (NASH), fibrosis,
cirrhosis] that appears in the lack of or minimal alcohol
consumption, and in the absence of other causes of liver disease,
and is associated with metabolic impairments, including type 2
diabetes (24).
SGLT2 inhibitors have been shown to provide
therapeutic benefits for NAFLD, as they can reduce the mass of
stored fat in the liver and normalize its composition. Studies have
shown that SGLT2 inhibition using empagliflozin attenuates
inflammatory responses.
A clinical study has shown that long-term therapy
with SGLT2 inhibitors provides histological benefits for the livers
of patients with T2DM and NAFLD by significantly reducing steatosis
and normalizing liver tissue composition. Empagliflozin was found
to improve lobular inflammation, steatosis, bloating and fibrosis
(25).
SGLT2 inhibitors may serve as promising therapeutic
agents for patients with NASH. Current evidence strongly suggests
that SGLT2 inhibitors may improve liver failure in patients with
NAFLD either by promoting fat burning or by attenuating
inflammatory processes (25).
In the present case, the patient was also diagnosed
with NASH; therefore, based on the above, the use of SGLT-2
inhibitors may have improved hepatic function.
Studies have shown that the use of SGLT-2 inhibitors
reduced cerebral ischemia and also improved cardiovascular outcomes
following the modelling of a stroke in animals (26). This, together with the other
beneficial effects of reducing CV risk, CV events, reducing
arterial hypertension, reducing oxidative stress and improving
endothelial function in diabetic patients (1), underlined the rationale for the use
of empagliflozin in the patient of the present study.
In a study conducted by Jin et al (27), the effects of empagliflozin were
assessed in patients with abdominal aortic aneurysms (AAA), and it
was found that it could potentially benefit such patients. They
described that SGLT-2 inhibitors were present in the vascular wall
and played a key role in reducing endothelial dysfunction and also
in the remodeling of the arterial wall. Thus, empagliflozin can
protect patients with AAA by lowering the blood pressure, in-turn
reducing vascular wall stiffness, and therefore lower the risk of
an AAA rupture. The same mechanism may also underlie the beneficial
effects of empagliflozin in CAA, making it a drug that can benefit
several types of aneurysms. Thus, this drug has promising results
as a novel preventative and conservative treatment for AAA.
A study by Liu et al (28) showed that empagliflozin reduced
lipid levels and reduced systemic inflammation in non-diabetic
mouse models. By reducing oxidative stress in the vascular wall
alongside the effect of reducing blood pressure, this medication
may prevent damage to the vascular wall and therefore prevent the
onset of an aneurysm or the rupture of a pre-existing aneurysm.
However, the last two studies mentioned were
performed on non-diabetic populations, so it is unknown whether the
effects that this drug has on patients without diabetes are
replicated in diabetic patients; to the best of our knowledge,
there are no studies on the effect of empagliflozin on lipid levels
in diabetic patients.
The present study is limited by the fact that the
findings pertain to only one patient and are therefore not
universally applicable. In addition, the patient was a
white/Caucasian male from Eastern Europe, and thus, the findings
may not apply to individuals from other races, as genetic factors
may be at play, which were not assessed here. Another limitation
was the lack of cardiac ultrasound images given the limitations in
clinical availability due to the pandemic at the time.
In conclusion, SGLT-2 inhibitors may be a suitable
option for the prevention of CAA. To date, only a few cases have
been reported; thus, there is a need for further study and
potentially a clinical trial; however, the results do seem
promising. The effects, which include reducing blood pressure,
inflammation and oxidative stress, and improving endothelial
function, are all beneficial for patients with CV impairment, so
there is hope that these drugs will show benefits in patients with
CAA.
It is not possible to say with certainty whether the
use of SGLT-2 inhibitors in this patient definitively had a
protective effect from a further potential instance of a CAA.
However, the patient's quality of life was notably improved and CV
risk was reduced by 3.2%.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated in the present study may be
requested from the corresponding author.
Authors' contributions
OAP conceived the study, wrote and revised the
manuscript, and was in charge of Software and conducting the
clinical investigation. DR was in charge of Software and collection
of clinical data. MAB analyzed the data and was in charge of the
methodology of this research. GG collected the data, analyzed the
data, participated in the clinical investigation and was in charge
of project administration. RMN and GG supervised the study and the
corrections of the preliminary versions of the manuscript, also
were involved in the conceptualization of the research and
validated the manuscript. OAP, GG and MAB confirm the authenticity
of all the raw data. All authors have read and confirmed the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of his data and images.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
ChatGPT was used to correct the spelling and grammar
of the manuscript. During the preparation of this work, AI tools
were used to improve the readability and language of the
manuscript. The authors revised and edited the content produced by
the AI tools as necessary, and take full responsibility for the
final content presented in the manuscript.
References
|
1
|
Heerspink HJ, Perkins BA and Fitchett D:
Sodium-glucose cotransporter 2 inhibitors in the treatment of
diabetes mellitus: Cardiovascular outcomes and kidney disease.
Lancet. 396:1239–1253. 2020.
|
|
2
|
Mocco J, Ransom ER, Komotar RJ, Schmidt
JM, Sciacca RR, Mayer SA and Connolly ES Jr: Preoperative
prediction of long-term outcome in poor-grade aneurysmal
subarachnoid hemorrhage. Neurosurgery. 59:529–538. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
https://www.ninds.nih.gov/health-information/disorders/cerebral-aneurysms.
|
|
4
|
Wiebers DO, Whisnant JP, Huston J III,
Meissner I, Brown RD Jr, Piepgras DG, Forbes GS, Thielen K, Nichols
D, O'Fallon WM, et al: Unruptured intracranial aneurysms: Natural
history, clinical outcome, and risks of surgical and endovascular
treatment. Lancet. 362:103–110. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pepin M, Schwarze U, Superti-Furga A and
Byers PH: Clinical and genetic features of Ehlers-Danlos syndrome
type IV, the vascular type. N Engl J Med. 342:673–680.
2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kielty CM, Sherratt MJ and Shuttleworth
CA: Elastic fibres. J Cell Sci. 115:2817–2828. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang B, Henney A, Eriksson P, Hamsten A,
Watkins H and Ye S: Genetic variation at the matrix
metalloproteinase-9 locus on chromosome 20q12.2-13.1. Hum Genet.
105:418–423. 1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu W, Morito D, Takashima S, Mineharu Y,
Kobayashi H, Hitomi T, Hashikata H, Matsuura N, Yamazaki S, Toyoda
A, et al: Identification of RNF213 as a susceptibility gene for
moyamoya disease and its possible role in vascular development.
PLoS One. 6(e22542)2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
American Diabetes Association Professional
Practice Committee. 6. Glycemic targets: Standards of medical care
in diabetes-2022. Diabetes Care. 45 (Suppl 1):S83–S96.
2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ganesh J and Viswanathan V: Management of
diabetic hypertensives. Indian J Endocrinol Metab. 15 (Suppl
4):S374–S379. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
SCORE2 risk prediction algorithms. new
models to estimate 10-year risk of cardiovascular disease in
Europe. Eur Heart J. 42:2439–2454. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
American Diabetes Association Professional
Practice Committee. 10. Cardiovascular disease and risk management:
Standards of care in diabetes-2024. Diabetes Care. 47 (Suppl
1):S179–S218. 2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chalouhi N, Jabbour P and Tjoumakaris SI:
Cerebral aneurysms: Pathogenesis, diagnosis, and treatment. J
Neurosurg. 118:845–856. 2013.
|
|
14
|
Karsdal MA, Krarup H, Sand JMB,
Christensen PB, Gerstoft J, Leeming DJ, Weis N, Schaffalitzky de
Muckadell OB and Krag A: Review article: the efficacy of biomarkers
in chronic fibroproliferative diseases-early diagnosis and
prognosis, with liver fibrosis as an exemplar. Aliment Pharmacol
Ther. 40:233–249. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li J, Liu S, Li W, Hu S, Xiong J, Shu X,
Hu Q, Zheng Q and Song Z: Vascular smooth muscle cell apoptosis
promotes transplant arteriosclerosis through inducing the
production of SDF-1α. Am J Transplant. 12:2029–2043.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen B, Lu Y, Chen Y and Cheng J: The role
of Nrf2 in oxidative stress-induced endothelial injuries. J
Endocrinol. 225:R83–R99. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sanchez Corredera C, Tadepalli PS, Scaccia
J, Sibia AS and Mayrovitz HN: Implications of long sleep duration
on cardiovascular health: A systematic review. Cureus.
17(e77738)2025.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Neumiller JJ, Alicic RZ and Tuttle KR:
Therapeutic considerations for antihyperglycemic agents in diabetic
kidney disease. J Am Soc Nephrol. 28:2263–2274. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vasilenko LA and Zotov VV: Digitalization
of public administration in Russia: Risks, casuses, problems. Digit
Sociol. 3:4–16. 2020.(In Russian).
|
|
20
|
Liu Z, Ren Z, Zhang J, Chuang CC,
Kandaswamy E, Zhou T and Zuo L: Role of ROS and nutritional
antioxidants in human diseases. Front Physiol.
9(477)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dunn SR and St. John L: The impact of
SGLT2 inhibitors on endothelial function and vascular health: A
review. Vasc Pharmacol. 117:29–37. 2019.
|
|
22
|
Cherney DZ, Prestidge RL and Qiu W:
Effects of sodium-glucose cotransporter 2 inhibitors on kidney
function and proteinuria: A review of the mechanisms and clinical
effects. Can J Diabetes. 42:278–283. 2018.
|
|
23
|
Amzolini AM, Forțofoiu MC, Alhija AB,
Vladu IM, Clenciu D, Mitrea A, Forțofoiu M, Matei D, Diaconu M,
Tudor MS and Micu ES: Triglyceride and glucose index as a screening
tool for nonalcoholic liver disease in patients with metabolic
syndrome. J Clin Med. 11(3043)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Amzolini AM, Forţofoiu MC, Barău
Abu-Alhija A, Vladu IM, Clenciu D, Mitrea A, Forţofoiu M, Matei D,
Enăchescu V, Predescu OI and Micu ES: Triglyceride and glucose
index: A useful tool for non-alcoholic liver disease assessed by
liver biopsy in patients with metabolic syndrome? Rom J Morphol
Embryol. 62:475–480. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yaribeygi H, Maleki M, Jamialahmadi T,
Moallem SA and Sahebkar A: Hepatic benefits of sodium-glucose
cotransporter 2 inhibitors in liver disorders. EXCLI J. 22:403–414.
2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pawlos A, Broncel M, Woźniak E and
Gorzelak-Pabiś P: Neuroprotective effect of SGLT2 inhibitors.
Molecules. 26(7213)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jin Z, Deng H, Xiong S and Gao L:
Perspective of SGLT2i in the treatment of abdominal aortic
aneurysms. J Cardiovasc Pharmacol. 81:241–247. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu Y, Wu M, Xu B and Kang L:
Empagliflozin alleviates atherosclerosis progression by inhibiting
inflammation and sympathetic activity in a normoglycemic mouse
model. J Inflamm Res. 14:2277–2287. 2021.PubMed/NCBI View Article : Google Scholar
|