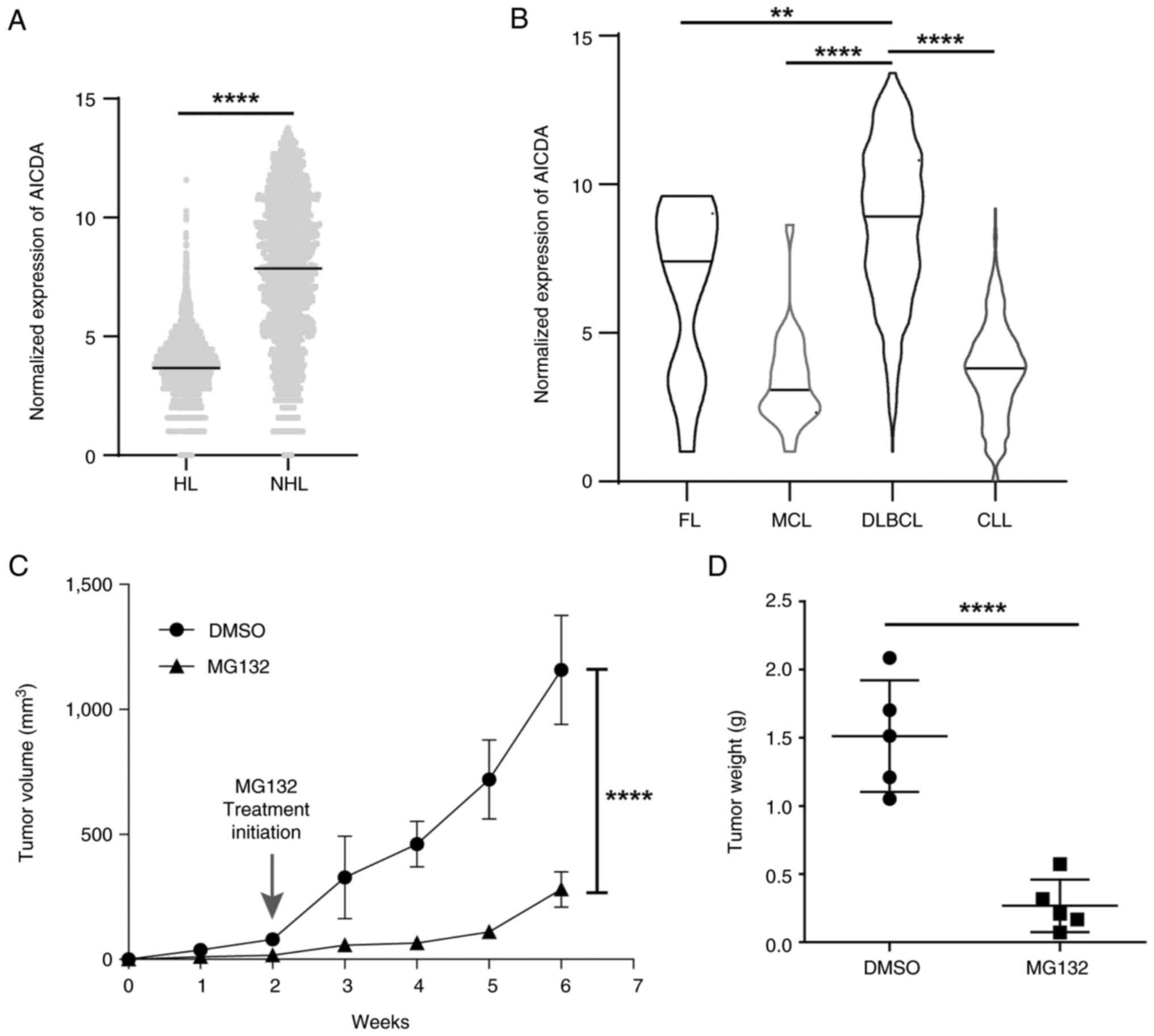
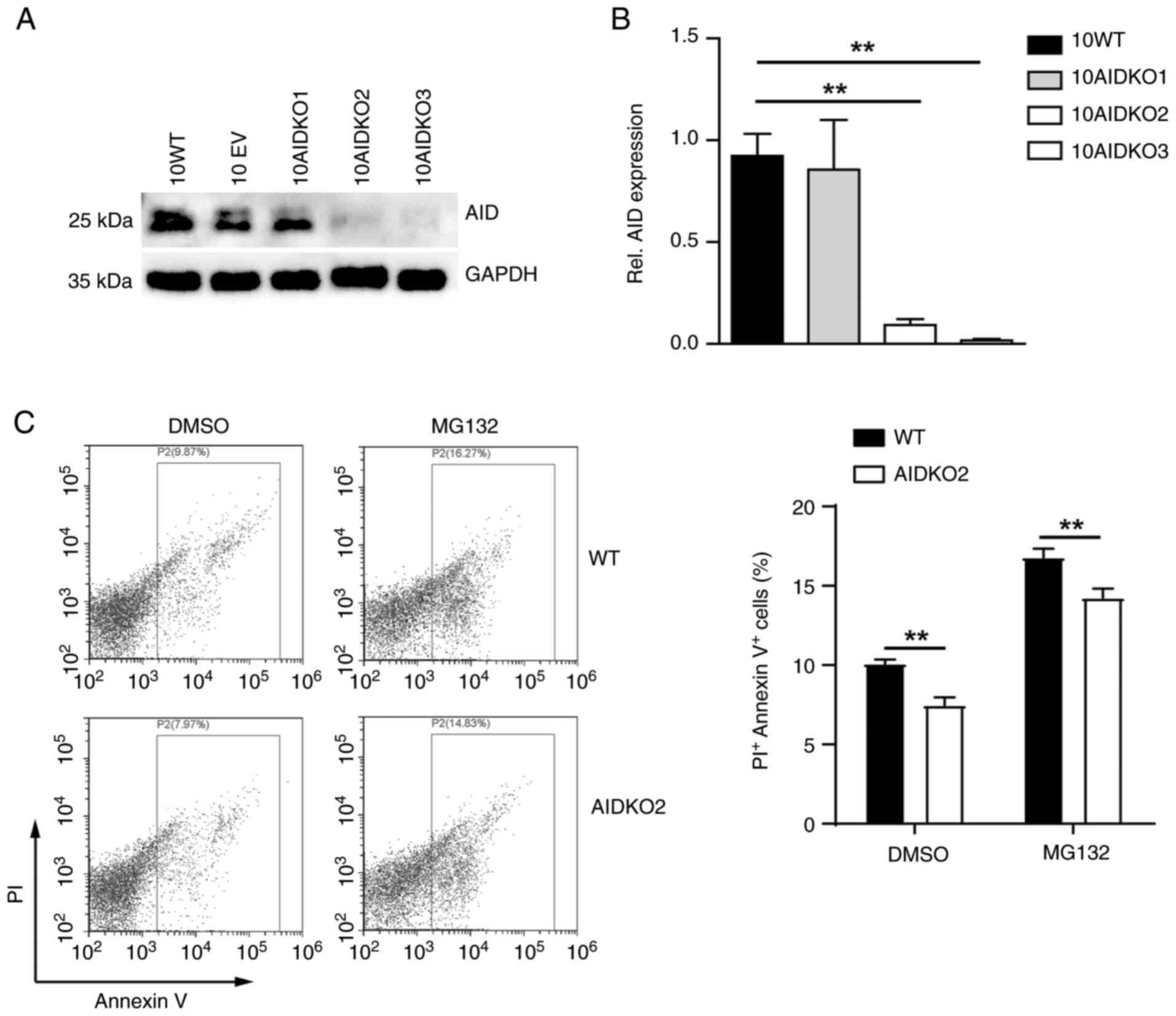
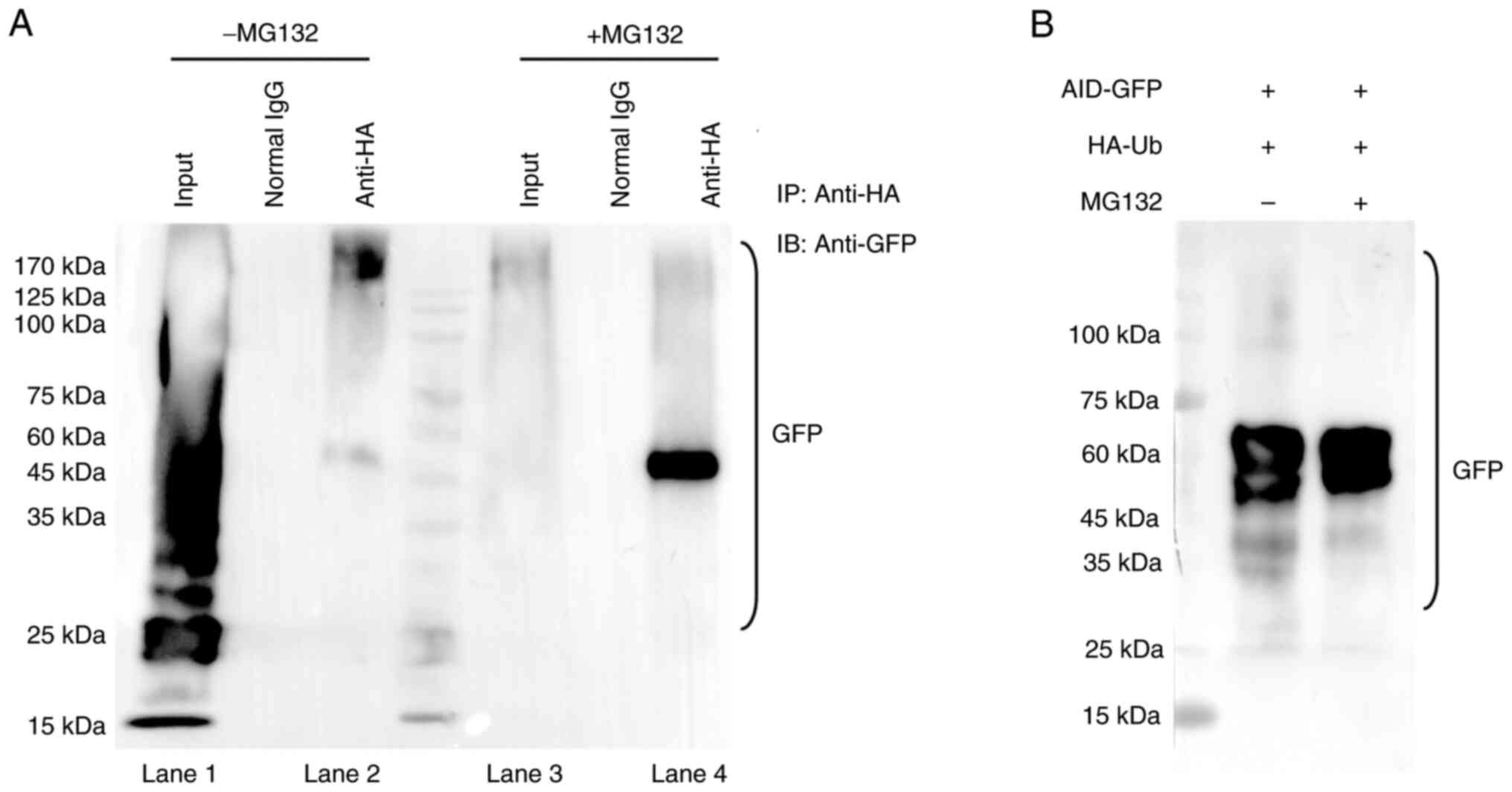
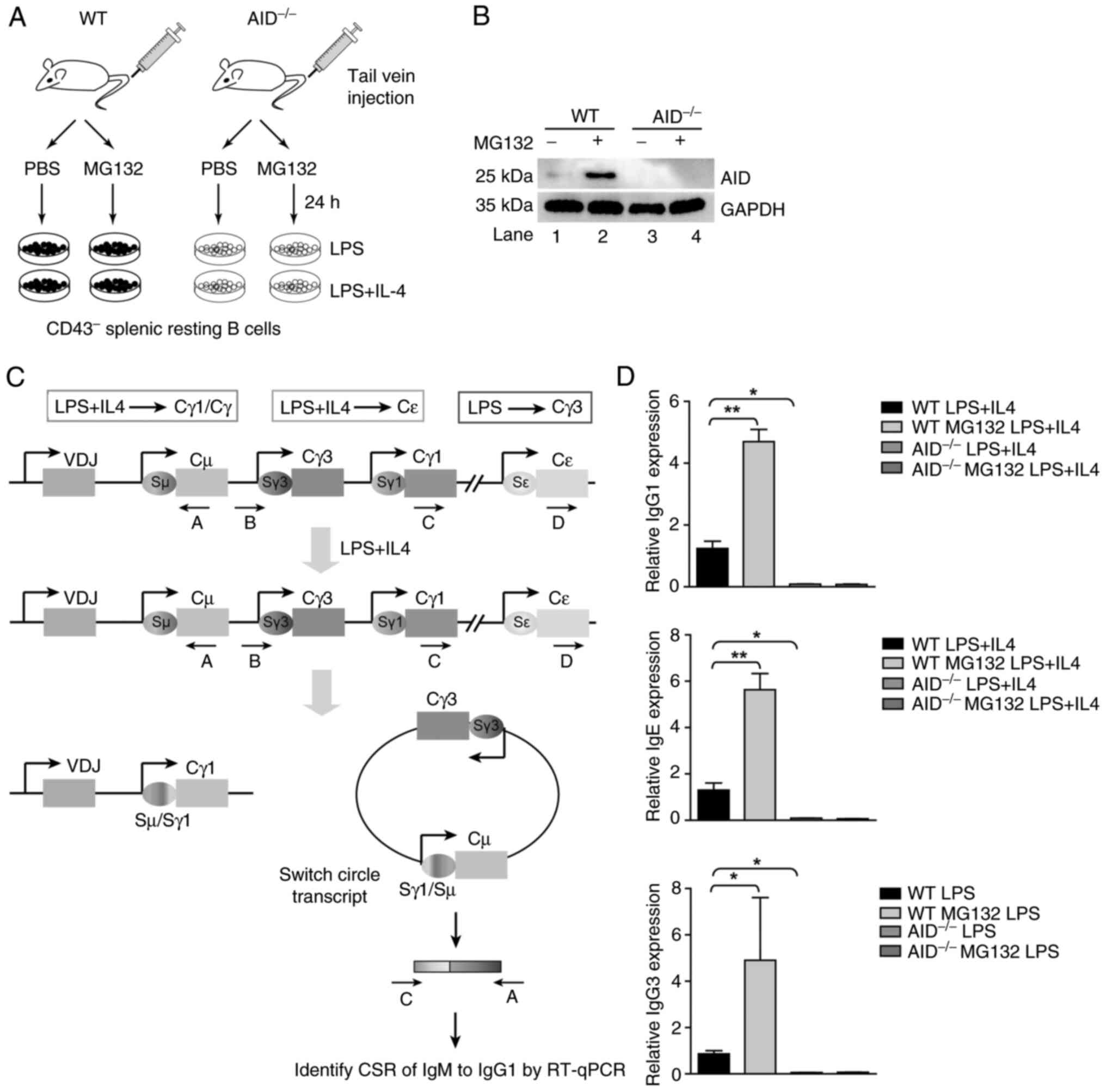
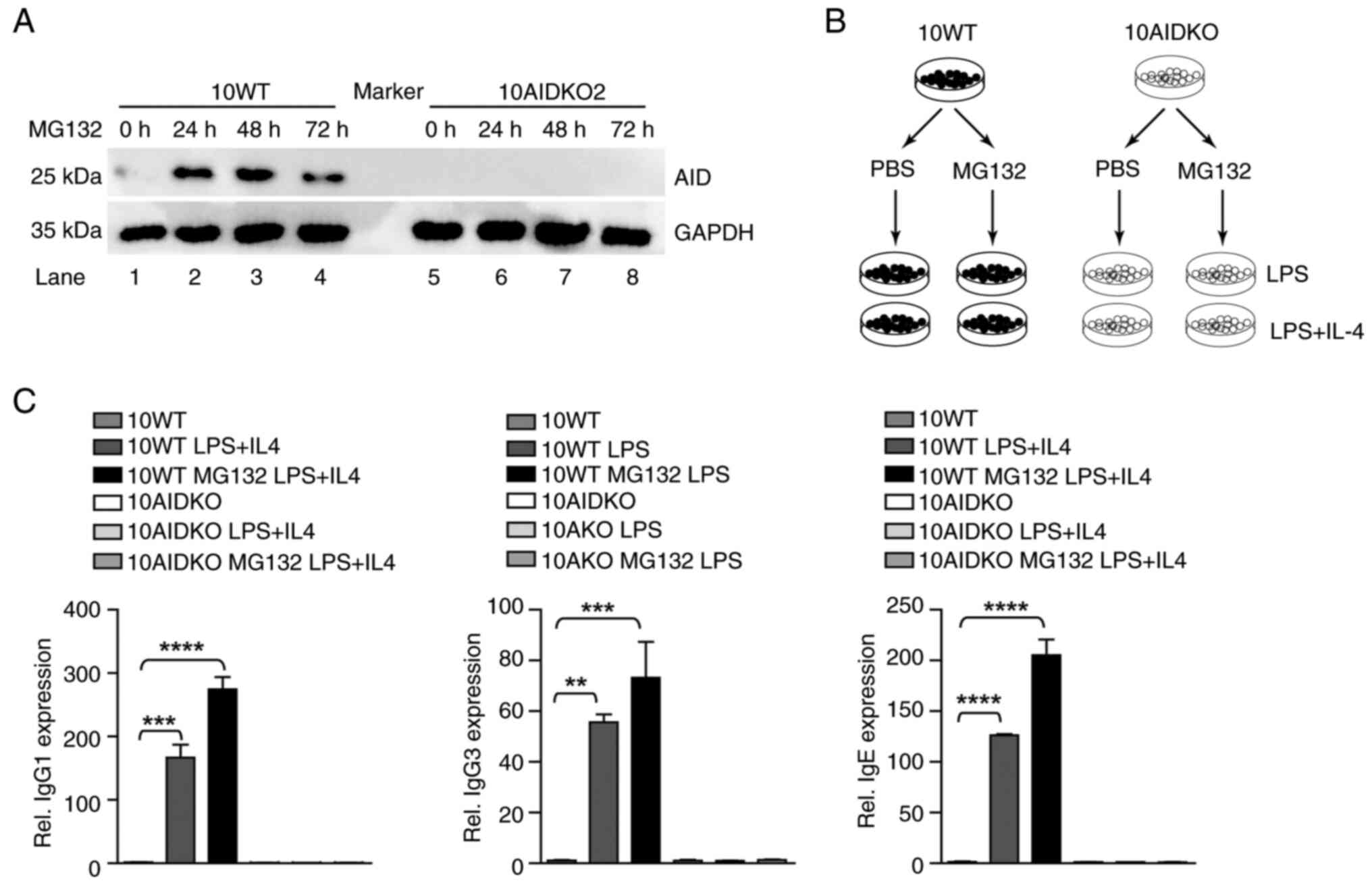
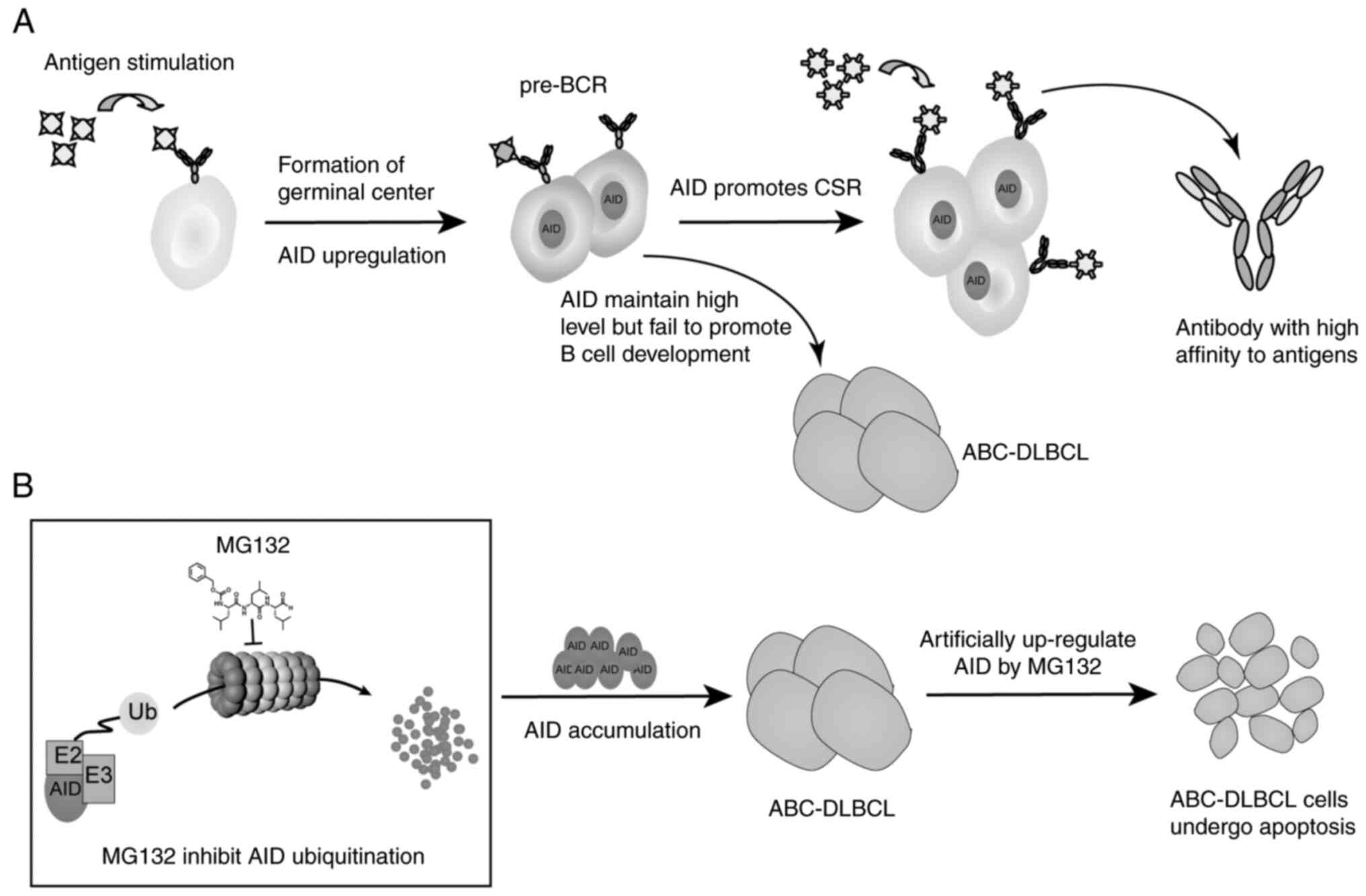
|
1
|
Li S, Young KH and Medeiros LJ: Diffuse
large B-cell lym-phoma. Pathology. 50:74–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schmitz R, Wright GW, Huang DW, Johnson
CA, Phelan JD, Wang JQ, Roulland S, Kasbekar M, Young RM, Shaffer
AL, et al: Genetics and pathogenesis of diffuse large B-cell
lymphoma. N Engl J Med. 378:1396–1407. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Crombie J: Classifying DLBCL subtypes for
optimal treatment. Oncology (Williston Park).
33(686504)2019.PubMed/NCBI
|
|
4
|
Takahara T, Nakamura S, Tsuzuki T and
Satou A: The immunology of DLBCL. Cancers (Basel).
15(835)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Alaggio R, Amador C, Anagnostopoulos I,
Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D,
Calaminici M, et al: The 5th edition of the world health
organization classification of haematolymphoid tumours: Lymphoid
neoplasms. Leukemia. 36:1720–1748. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Coiffier B and Sarkozy C: Diffuse large
B-cell lymphoma: R-CHOP failure-what to do? Hematology Am Soc
Hematol Educ Program. 2016:366–378. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nowakowski GS, Chiappella A, Witzig TE,
Spina M, Gascoyne RD, Zhang L, Flament J, Repici J and Vitolo U:
ROBUST: Lenalidomide-R-CHOP versus placebo-R-CHOP in previously
untreated ABC-type diffuse large B-cell lymphoma. Future Oncol.
12:1553–1563. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Frick M, Bettstetter M, Bertz S,
Schwarz-Furlan S, Hartmann A, Richter T, Lenze D, Hummel M,
Dreyling M, Lenz G and Gaumann A: Mutational frequencies of CD79B
and MYD88 vary greatly between primary testicular DLBCL and
gastrointestinal DLBCL. Leuk Lymphoma. 59:1260–1263.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Davis RE, Ngo VN, Lenz G, Tolar P, Young
RM, Romesser PB, Kohlhammer H, Lamy L, Zhao H, Yang Y, et al:
Chronic active B-cell-receptor signalling in diffuse large B-cell
lymphoma. Nature. 463:88–92. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lenz G, Nagel I, Siebert R, Roschke AV,
Sanger W, Wright GW, Dave SS, Tan B, Zhao H, Rosenwald A, et al:
Aberrant immunoglobulin class switch recombination and switch
translocations in activated B cell-like diffuse large B cell
lymphoma. J Exp Med. 204:633–643. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kumar R, DiMenna LJ, Chaudhuri J and Evans
T: Biological function of activation-induced cytidine deaminase
(AID). Biomed J. 37:269–283. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
An L, Chen C, Luo R, Zhao Y and Hang H:
Activation-induced cytidine deaminase aided in vitro antibody
evolution. Methods Mol Biol. 1707:1–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Teater M, Dominguez PM, Redmond D, Chen Z,
Ennishi D, Scott DW, Cimmino L, Ghione P, Chaudhuri J, Gascoyne RD,
et al: AID drives epigenetic heterogeneity and accelerates germinal
center-derived lymphomagenesis. Nat Commun. 9(222)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jiao J, Lv Z, Zhang P, Wang Y, Yuan M, Yu
X, Otieno Odhiambo W, Zheng M, Zhang H, Ma Y and Ji Y: AID assists
DNMT1 to attenuate BCL6 expression through DNA methylation in
diffuse large B-cell lymphoma cell lines. Neoplasia. 22:142–153.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jiao J, Jin Y, Zheng M, Zhang H, Yuan M,
Lv Z, Odhiambo W, Yu X, Zhang P, Li C, et al: AID and TET2
co-operation modulates FANCA expression by active demethylation in
diffuse large B cell lymphoma. Clin Exp Immunol. 195:190–201.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kawamura K, Wada A, Wang JY, Li Q, Ishii
A, Tsujimura H, Takagi T, Itami M, Tada Y, Tatsumi K, et al:
Expression of activation-induced cytidine deaminase is associated
with a poor prognosis of diffuse large B cell lymphoma patients
treated with CHOP-based chemotherapy. J Cancer Res Clin Oncol.
142:27–36. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Varshavsky A: The ubiquitin system,
autophagy, and regulated protein degradation. Annu Rev Biochem.
86:123–128. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Thibaudeau TA and Smith DM: A practical
review of proteasome pharmacology. Pharmacol Rev. 71:170–197.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Guo N and Peng Z: MG132, a proteasome
inhibitor, induces apoptosis in tumor cells. Asia Pac J Clin Oncol.
9:6–11. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Schenkein D: Proteasome inhibitors in the
treatment of B-cell malignancies. Clin Lymphoma. 3:49–55.
2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Aoufouchi S, Faili A, Zober C, D'Orlando
O, Weller S, Weill JC and Reynaud CA: Proteasomal degradation
restricts the nuclear lifespan of AID. J Exp Med. 205:1357–1368.
2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
National Research Council: Committee for
the Update of the Guide for the Care and Use of Laboratory Animals.
Guide for the Care and Use of Laboratory Animals. 8th edition.
National Academies Press, Washington, DC, 2011.
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Muramatsu M, Kinoshita K, Fagarasan S,
Yamada S, Shinkai Y and Honjo T: Class switch recombination and
hypermutation require activation-induced cytidine deaminase (AID),
a potential RNA editing enzyme. Cell. 102:553–563. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Caron AZ, Haroun S, Leblanc E, Trensz F,
Guindi C, Amrani A and Grenier G: The proteasome inhibitor MG132
reduces immobilization-induced skeletal muscle atrophy in mice. BMC
Musculoskelet Disord. 12(185)2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rupniewska ZM, Roliński J and
Bojarska-Junak A: Universal CD43 molecule. Postepy Hig Med Dosw.
54:619–638. 2000.PubMed/NCBI(In Polish).
|
|
27
|
Szydłowski M, Garbicz F, Jabłońska E,
Górniak P, Komar D, Pyrzyńska B, Bojarczuk K, Prochorec-Sobieszek
M, Szumera-Ciećkiewicz A, Rymkiewicz G, et al: Inhibition of PIM
kinases in DLBCL targets MYC transcriptional program and augments
the efficacy of anti-CD20 antibodies. Cancer Res. 81:6029–6043.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Advani R, Flinn I, Popplewell L, Forero A,
Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP,
et al: CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin's
lymphoma. N Engl J Med. 379:1711–1721. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Schmitt A, Xu W, Bucher P, Grimm M,
Konantz M, Horn H, Zapukhlyak M, Berning P, Brändle M, Jarboui MA,
et al: Dimethyl fumarate induces ferroptosis and impairs
NF-κB/STAT3 signaling in DLBCL. Blood. 138:871–884. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xu W, Berning P and Lenz G: Targeting
B-cell receptor and PI3K signaling in diffuse large B-cell
lymphoma. Blood. 138:1110–1119. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ruan J, Martin P, Furman RR, Lee SM,
Cheung K, Vose JM, Lacasce A, Morrison J, Elstrom R, Ely S, et al:
Bortezomib plus CHOP-rituximab for previously untreated diffuse
large B-cell lymphoma and mantle cell lymphoma. J Clin Oncol.
29:690–697. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Davies AJ, Barrans S, Stanton L, Caddy J,
Wilding S, Saunders G, Mamot C, Novak U, McMillan A, Fields P, et
al: Differential efficacy from the addition of bortezomib to R-CHOP
in diffuse large B-Cell lymphoma according to the molecular
subgroup in the REMoDL-B study with a 5-year follow-up. J Clin
Oncol. 41:2718–2723. 2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lin Z, Chen X, Li Z, Zhou Y, Fang Z, Luo
Y, Zhao J and Xu B: The role of bortezomib in newly diagnosed
diffuse large B cell lymphoma: A meta-analysis. Ann Hematol.
97:2137–2144. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Victora GD and Nussenzweig MC: Germinal
centers. Annu Rev Immunol. 40:413–442. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bao K, Zhang J, Scherl A, Ziai J,
Hadadianpour A, Xu D, Dela Cruz C, Liu J, Liang Y, Tam L, et al:
Activation-induced cytidine deaminase impacts the primary antibody
repertoire in naive mice. J Immunol. 208:2632–2642. 2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhou J, Zuo M, Li L, Li F, Ke P, Zhou Y,
Xu Y, Gao X, Guan Y, Xia X, et al: PIM1 and CD79B
mutation status impacts the outcome of primary diffuse large B-Cell
lymphoma of the CNS. Front Oncol. 12(824632)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yu K: AID function in somatic
hypermutation and class switch recombination. Acta Biochim Biophys
Sin (Shanghai). 54:759–766. 2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Çakan E and Gunaydin G: Activation induced
cytidine deaminase: An old friend with new faces. Front Immunol.
13(965312)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Rios LAS, Cloete B and Mowla S:
Activation-induced cytidine deaminase: In sickness and in health. J
Cancer Res Clin Oncol. 146:2721–2730. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jiao J, Lv Z, Wang Y, Fan L and Yang A:
The off-target effects of AID in carcinogenesis. Front Immunol.
14(1221528)2023.PubMed/NCBI View Article : Google Scholar
|