Introduction
Premature ovarian insufficiency (POI) is an ovarian
dysfunction that mainly occurs in women aged <40 years (1) and is characterized by menstruation
abnormalities, elevated levels of follicle-stimulating hormone
(FSH) and fluctuating estrogen hormone levels (2). The final stage of POI, premature
ovarian failure, is associated with different degrees of
perimenopausal symptoms, such as mood changes, hot flashes and
trouble sleeping (3,4). POI affects ~1% of women worldwide
(3) and, if not treated, may cause
several long-term health conditions, such as osteoporosis,
cardiovascular disease, anxiety and depression (4,5).
Presently, a standard treatment for POI is
estrogen/progesterone combination therapy. However, this hormone
replacement therapy (HRT) is associated with increased health
risks, including breast cancer, stroke and cardiovascular diseases
(5). According to Traditional
Chinese Medicine, ovarian reserve hypofunction and infertility are
caused by various conditions, such as kidney malfunction.
Therefore, in Traditional Chinese Medicine, ovarian reserve
hypofunction and infertility can be treated with alternative
therapies (6).
Previous research has demonstrated certain
therapeutic effects of the Kuntai capsule on certain symptoms
associated with POI, which are related to decreased hormone levels
(7). The Kuntai capsule has been
used as a Chinese patent medicine for low ovarian reserve. The
capsule is based on Huanglian Ejiao decoction consisting of six
Chinese medicines: Coptis, Ejiao, Rehmannia glutinosa,
Scutellaria baicalensis Georgi, Poria cocos and white
peony root, which has been previously reported to improve
peri-menopausal and menopausal symptoms (8). A meta-analysis found that in patients
with POI, HRT combined with the Kuntai capsule is associated with a
higher number of basal antral follicles, an increased number of
eggs and a reduced cycle cancellation rate (9). Another 1-year study reported that
adding the Kuntai capsule to conventional HRT improved the
pregnancy rate in patients with POI (10). However, this regimen was associated
with some adverse reactions (10).
The present study aimed to critically analyze the
existing randomized clinical trials (RCTs) on the efficiency of the
Kuntai capsule for POI treatment and assess whether the Kuntai
capsule combined with HRT was more effective compared with HRT
alone. These results may provide an evidence-based evaluation of
the Kuntai capsule's efficacy for the treatment of POI.
Materials and methods
Data sources and search strategy
A systematic literature search of the China National
Knowledge Infrastructure (https://www.cnki.net), WanFang (https://med.wanfangdata.com.cn/), Chinese Biomedical
Literature PubMed (https://www.sinomed.ac.cn/index.jsp) (https://pubmed.ncbi.nlm.nih.gov), Embase
(http://embase.com) and Cochrane Library databases
(https://www.cochranelibrary.com/central/about-central)
was performed from the inception of the databases to 25th October
2020. RCTs that reported using the Kuntai Capsule treatment for POI
and were written in English or Chinese were included. The following
keywords were used: ‘Kuntai capsule’ AND (‘premature ovarian
insufficiency’ OR ‘decline in ovarian reserve’ OR ‘ovarian reserve
dysfunction’ OR ‘low ovarian reserve’) AND ‘randomized controlled
trial’. A total of two reviewers conducted the searches and both
reviewers agreed on the selected studies to be included in the
present manuscript. In case of differences in the selection of
studies, a third author was contacted to resolve the issue.
Inclusion and exclusion criteria
The inclusion criteria used were as follows: i)
RCTs; ii) POI diagnosis based on The European Society of Human
Reproduction and Embryology, Guideline Group on POI (2); iii) patients aged <40 years with
amenorrhea or oligomenorrhea for ≥4 months and serum
follicle-stimulating hormone (FSH) levels >25 IU/l on ≥2
separate measurements (>4 weeks apart); iv) studies that
reported data on the control group of patients treated with HRT
alone; and v) studies that reported on outcomes of serological
indices, such as levels of serum anti-Müllerian hormone (AMH), FSH,
luteinizing hormone (LH), estradiol (E2), antral follicle count
(AFC), Kupperman score (11) and
imaging indicators, such as ovarian volume, endometrial thickness
and peak systolic velocity (PSV) of the ovarian artery.
The exclusion criteria were as follows: i) Repeated
published literature; ii) studies written in languages other than
Chinese and English; iii) a lack of access to the full text; and
iv) retrospective, cohort and qualitative studies, case reports or
series, experience summaries and non-human studies.
Data extraction
The relevant data, including title, first author
name, publication year, patient grouping, age, course of the
disease, treatment, control group type, indicators such as AMH,
AFC, Kupperman score, FSH, LH, E2, ovarian volume, endometrial
thickness and PSV were independently collected by two authors.
Risk of bias
The risk of bias in the included studies was
assessed using the Cochrane Risk of Bias Tool (12).
Statistical analysis
RevMan Manage (version 5.3; The Cochrane
Collaboration) was used for the meta-analysis of each intervention
pair (Kuntai capsule combined with HRT treatment vs. HRT alone).
Treatment effectiveness was expressed as weighted mean differences
(MDs) and 95% confidence intervals (CIs) for continuous outcomes.
Heterogeneity was assessed using a χ2 test and
I2 statistics. The random-effects model was used
irrespective of the inter-study heterogeneity measured using the
I2 statistic. The Preferred Reporting Items for
Systematic reviews and Meta-Analyses guidelines were strictly
followed (13), and the
requirements for ethics approval and patient consent were waived
since the study used previously published data.
Results
Study selection and
characteristics
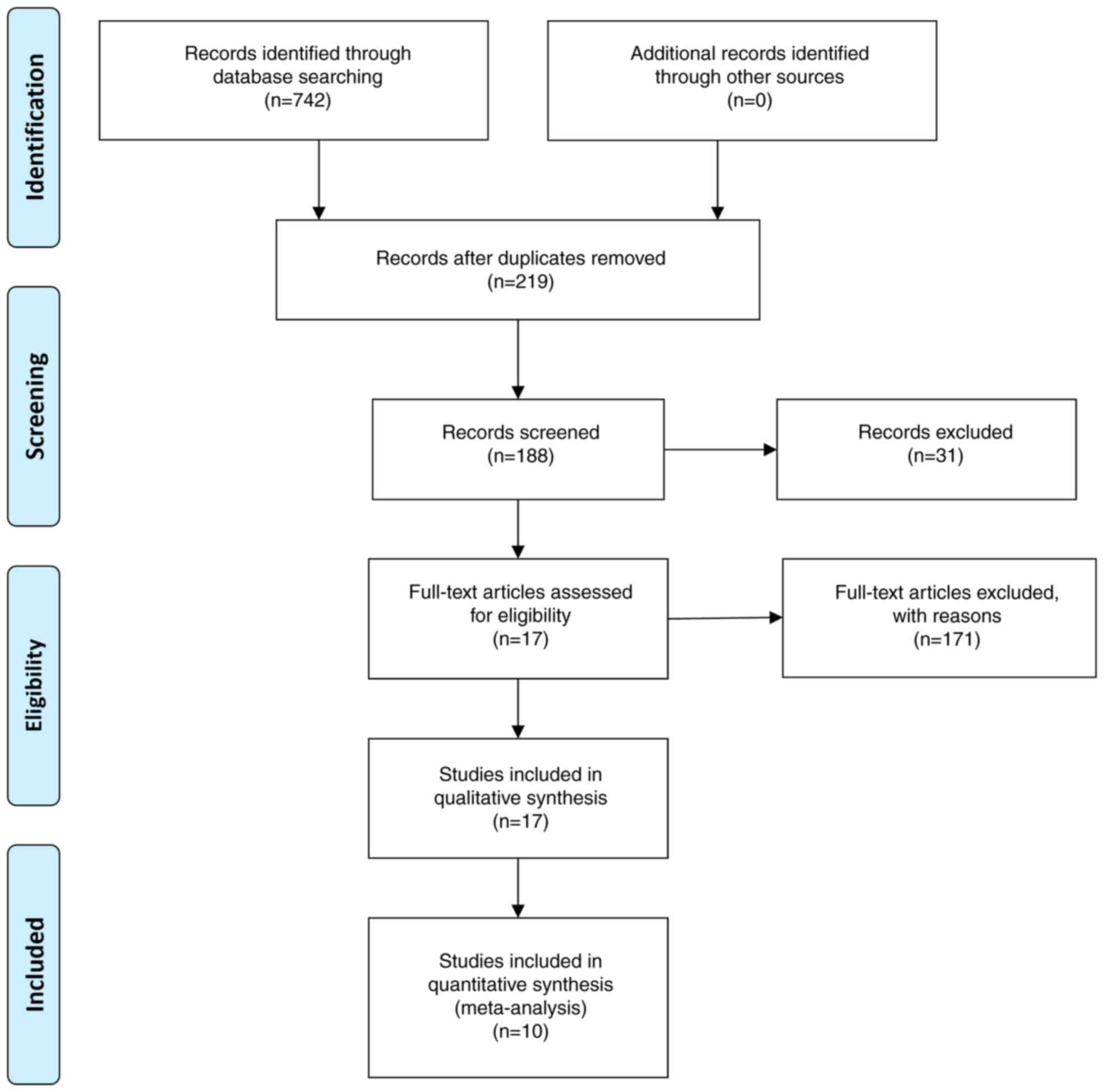
Screening identified a total of 742 articles. Of
these articles, 10 met the criteria for standard RCTs and were
included after the layer-by-layer screening (14-23).
The included studies reported data on 925 patients. Of these
patients, 462 received the Kuntai capsule combined with HRT
treatment and 463 were treated with HRT alone. HRT treatment
protocols included fenmetone, cremon and estrogen and progesterone
therapy. The A similar HRT protocol was used in both the study and
control groups. All patients were treated for 3 months and the
changes in outcome indices before and after the treatment were
compared (Table I). The flowchart
of the screening is shown in Fig.
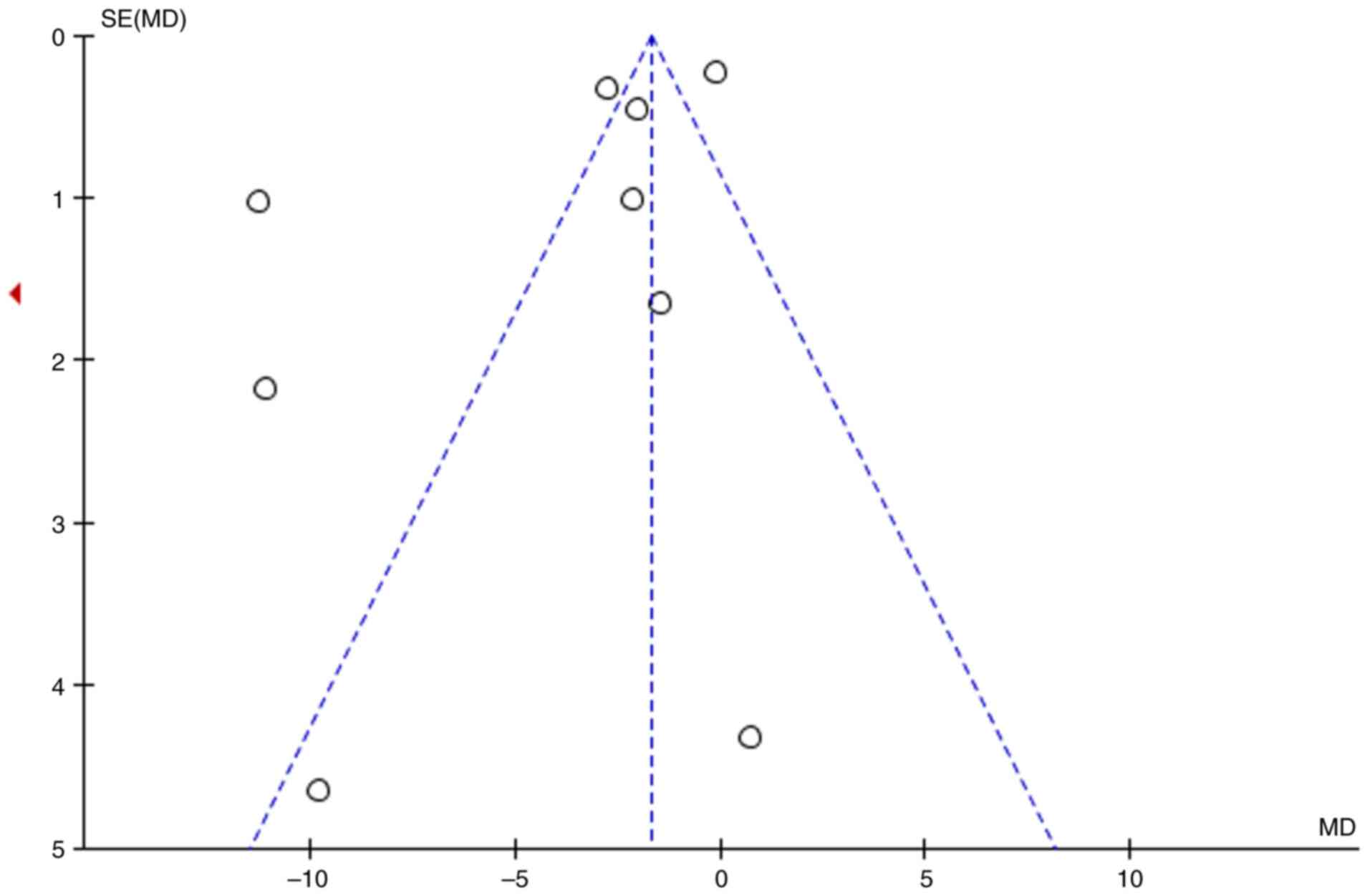
1. The Cochrane Risk of Bias Tool assessment showed that all 10
studies described the method of random grouping but did not detail
the blinding method and allocation concealment, which could have
affected the quality of the research. All RCTs were identified as
having a ‘high’ or ‘unclear’ risk of bias (Fig. 2).
 | Table IFeatures of the included studies. |
Table I
Features of the included studies.
| First author,
year | Cases (Kuntai + HRT
group/HRT group), n | Age, years (Kuntai +
HRT group/HRT group; mean ± SD) | Kuntai + HRT group
drug regimen | HRT group drug
regimen | Outcome measured | (Refs.) |
|---|
| Luan et al,
2017 | 58/60 |
42.3±2.2/41.2±3.1 | Kuntai capsule (2,000
mg three times daily) and fenmeton (one dose per day) | Fenmetone (one dose
per day) | AMH, AFC and serum
sex hormone levels | (14) |
| Li et al,
2014 | 20/20 |
34.2±6.9/34.0±7.2 | Kuntai capsule (2,000
mg three times daily) and cremon (one dose per day) | Cremon (one dose per
day) | AMH, AFC and serum
sex hormones levels and imaging examination | (15) |
| Jia et al,
2019 | 46/46 |
30.2±2.1/30.4±2.1 | Kuntai capsule (2,000
mg three times daily) and fenmetone (one dose per day) | Fenmetone (one dose
per day) | Serum sex hormone
levels | (16) |
| Wu et al,
2019 | 50/50 |
35.0±3.3/34.6±3.5 | Kuntai capsule (2,000
mg three times daily) and cremon (one dose per day) | Cremon (one dose per
day) | Serum sex hormone
levels and Kupperman score | (17) |
| Yuan et al,
2019 | 40/40 |
34.2±2.8/34.1±3.0 | Kuntai capsule
(2,000 mg three times daily) and cremon (one dose per day) | Cremon (one dose
per day) | AMH and serum sex
hormone levels | (18) |
| Yuan et al,
2018 | 40/40 |
35.2±2.6/35.8±2.1 | Kuntai capsule
(2,000 mg three times daily) and cremon (one dose per day) | Cremon (one dose
per day) | AMH, AFC and serum
sex hormone levels | (19) |
| Hong et al,
2018 | 30/30 |
31.3±6.6/34.6±3.5 | Kuntai capsule
(2,000 mg three times daily) and cremon (one dose per day) | Cremon (one dose
per day) | AMH, AFC and serum
sex hormone levels, Kupperman score and imaging examination | (20) |
| Hu et al,
2018 | 100/100 |
40.2±1.3/38.3±2.0 | Kuntai capsule
(2,000 mg three times daily) and fenmetone (one dose per day) | Fenmetone (one dose
per day) | AMH, AFC and serum
sex hormone levels and imaging examination | (21) |
| Xiao et al,
2015 | 45/45 |
36.2±3.9/36.2±4.1 | Kuntai capsule
(2,000 mg three times daily) and cremon (one dose per day) | Cremon (one dose
per day) | Serum sex hormone
levels, Kupperman score and imaging examination | (22) |
| Yao et al,
2013 | 33/32 |
32.5±3.4/31.4±3.7 | Kuntai capsule
(2,000 mg three times daily) and cremon (one dose per day) | Cremon (one dose
per day) | Serum sex hormone
levels and Kupperman score | (23) |
Serum gonadotrophin and estradiol
hormone levels
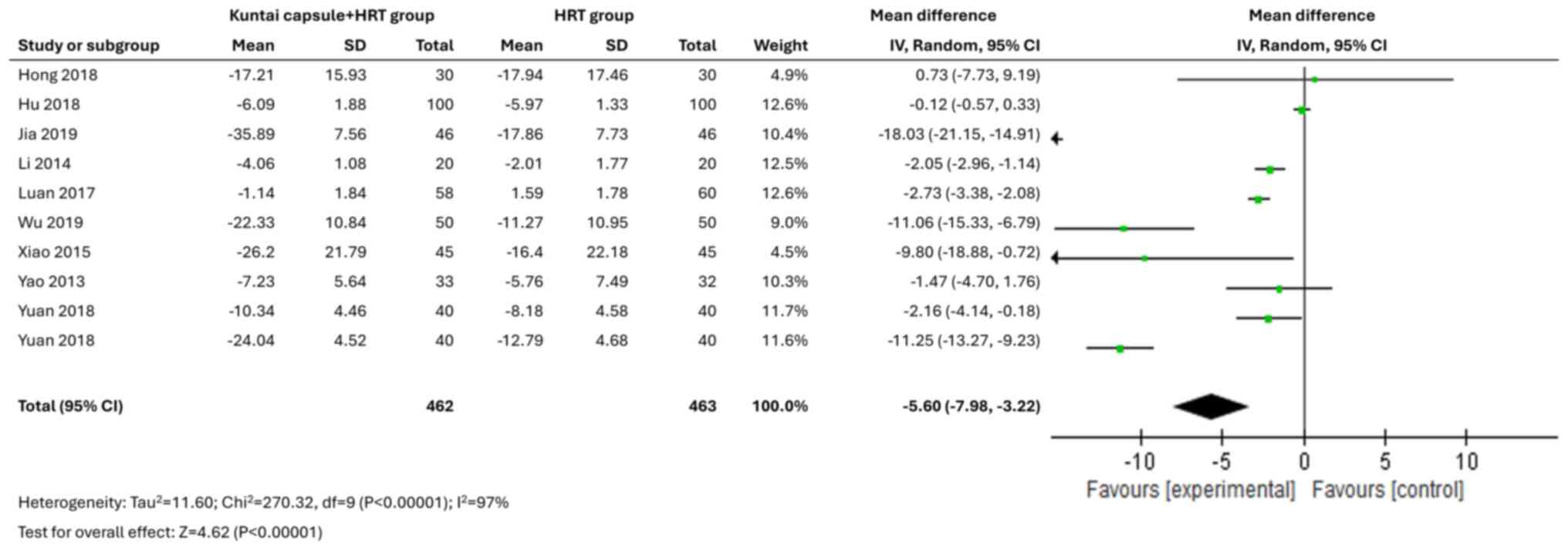
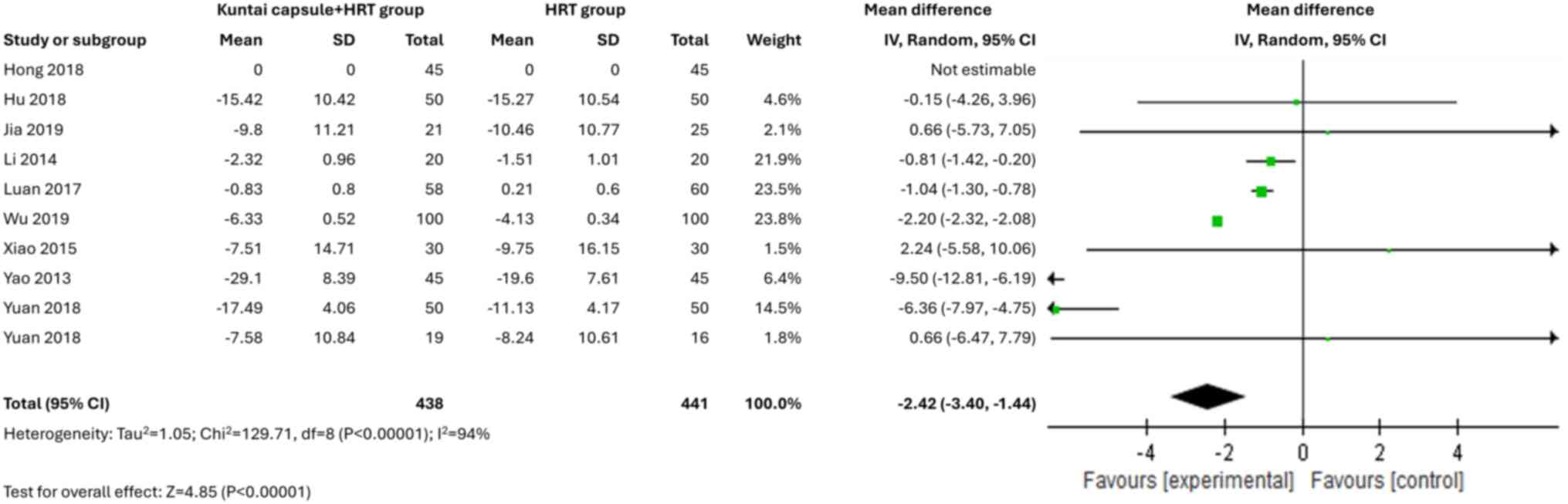
All 10 studies reported data on sex hormone levels.
The Kuntai capsule combined with HRT treatment was associated with
significantly reduced levels of FSH [MD=-5.60; 95% CI,
-7.98-(-3.22); P<0.00001; I2=97%] and LH [MD=-2.42;
95% CI, -3.40-(-1.44); P<0.00001; I2=94%] (Figs. 3 and 4). Moreover, the Kuntai capsule, in
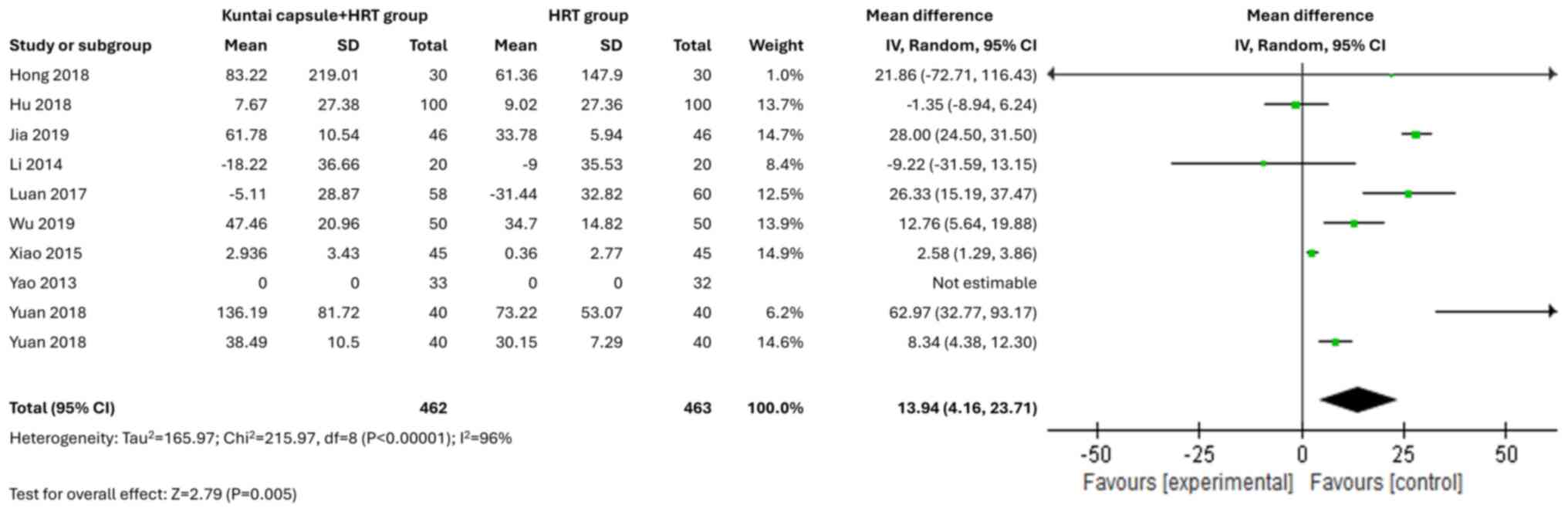
combination with HRT treatment, significantly increased E2 levels
(MD=13.94; 95% CI, 4.16-23.71; P<0.00001; I2=96%) when compared
with HRT only (Fig. 5).
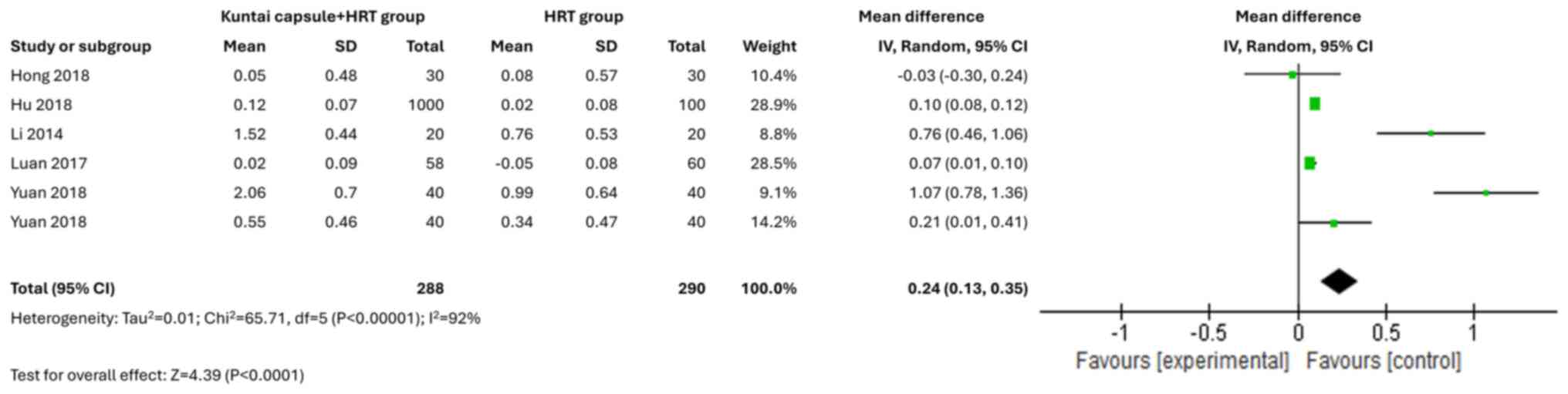
AMH levels
A total of six studies (14,15,18-21)
assessed the AMH levels. The Kuntai capsule treatment led to
significantly increased levels of AMH (MD=0.24; 95% CI, 0.13-0.35;
P<0.00001; I2=92%) (Fig.
6).
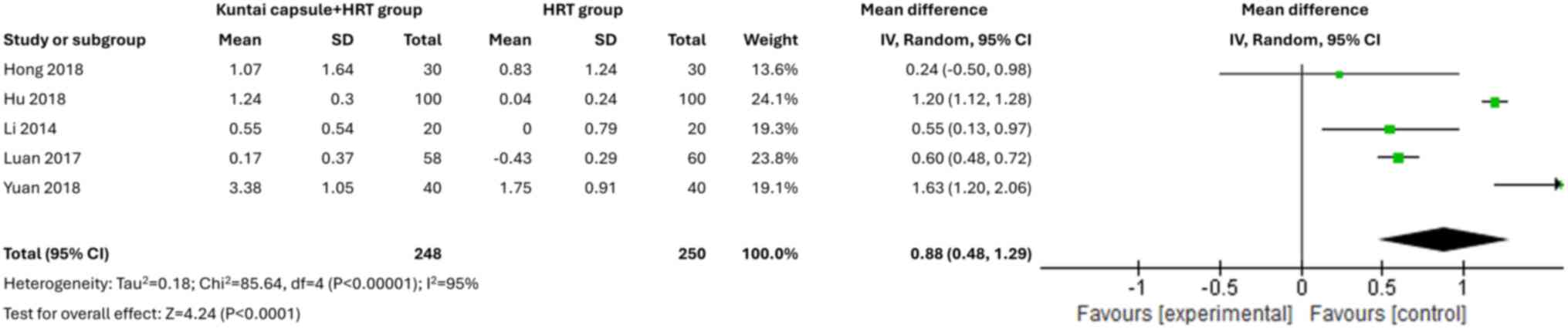
Number of AFCs
A total of five studies (14,15,19-21)
assessed the numbers of AFC. The pooled results showed that the
numbers of AFC were significantly greater in the combined treatment
group compared with the HRT alone group (MD=0.88; 95% CI,
0.48-1.29; P<0.00001; I2=95%) (Fig. 7).
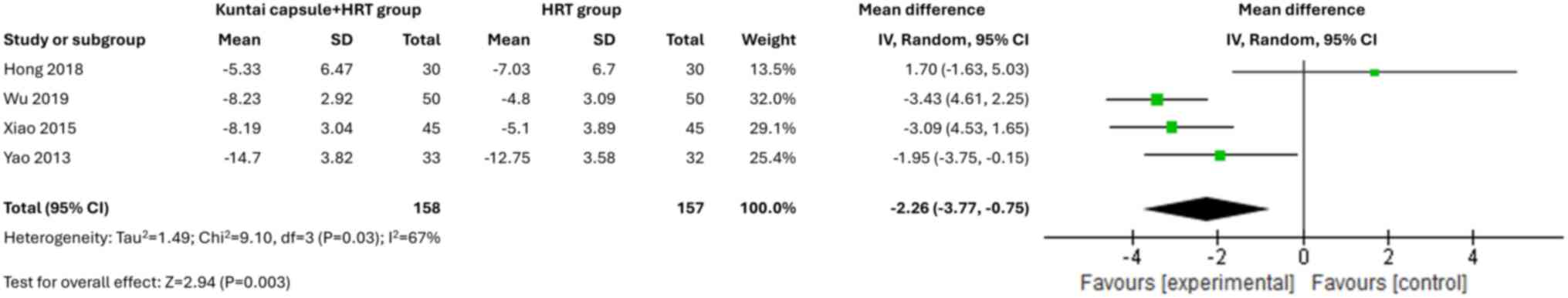
Improvement in perimenopausal
symptoms
A total of four studies (17,20,22,23)
used the Kupperman index to evaluate the improvement in
perimenopausal symptoms. The pooled results suggested that the
Kuntai capsule combined with HRT significantly improved the
perimenopausal symptoms in patients with POI [MD=-2.26; 95% CI,
-3.77(-0.75); P=0.003; I2=67%] compared with the HRT
regimen alone (Fig. 8).
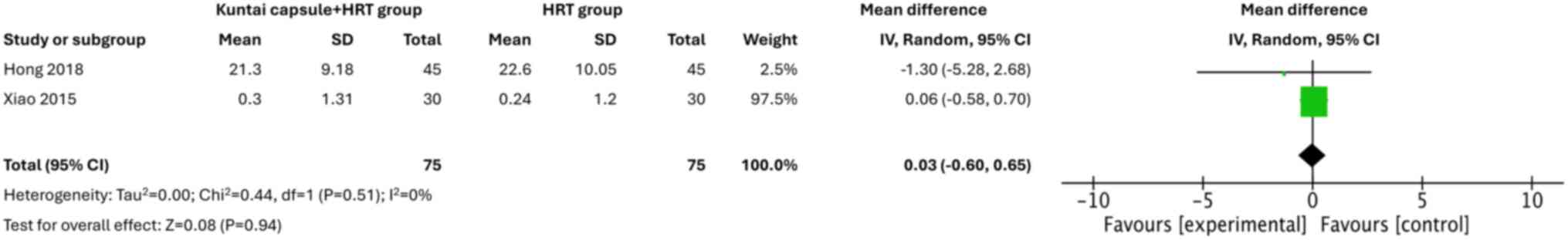
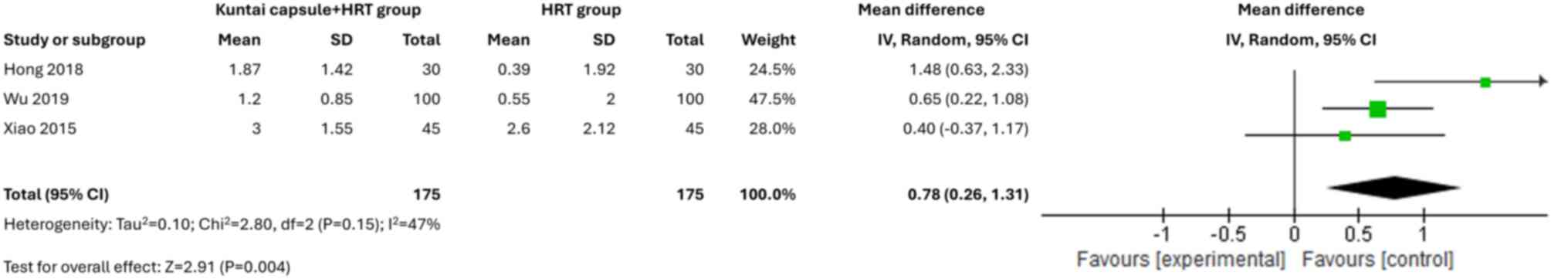
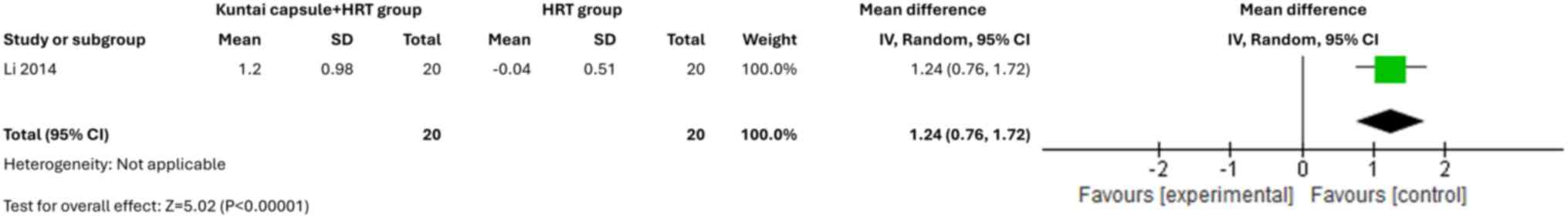
Effects on the ovaries and uterus
A total of two studies (20,22)
assessed ovarian volume, three (20-22)
assessed endometrial thickness and one study (15) assessed PSV. The pooled analysis
indicated that ovarian volumes were comparable in both groups of
patients (MD=0.03; 95% CI, -0.60-0.65; P=0.94; I2=0%)
(Fig. 9). However, the Kuntai
capsule combined with HRT significantly improved the endometrial
thickness (MD=0.78; 95% CI, 0.26-1.31; P=0.004; I2 =47%)
(Fig. 10) and PSV (MD=1.24; 95%
CI, 0.76-1.72; P<0.00001) (Fig.
11) in patients with POI.
Discussion
The present study showed that combining the Kuntai
capsule and HRT treatment significantly improved AMH levels and the
number of AFCs in patients with POI compared with those treated
with HRT alone. Kuntai capsule treatment also significantly reduced
perimenopausal symptoms, reduced FSH and LH serum levels and
increased serum levels of E2. These results suggested that the
Kuntai capsule and HRT combination had an improved curative effect
on improving ovarian reserve function during POI treatment compared
with monotherapy with HRT alone.
POI has a profound negative impact on the quality of
life (24). While HRT can relieve
symptoms and mitigate the impact of prolonged estrogen deficiency,
it mimics the normal physiological and endocrinological status,
with no evidence of improvement in ovarian function (2). Moreover, HRT increases various health
risks, including the risk for breast cancer, stroke and
cardiovascular diseases (5).
Several traditional medical therapies, such as
acupuncture and Chinese medicine, have been reported as being
effective for the treatment of POI. Pharmacological studies have
reported that the Kuntai capsule inhibits the apoptosis of ovarian
granulosa cells in mice with POI and improves the secretion of sex
hormones, thereby improving impaired ovarian function (25). Results of an animal study showed
that the Kuntai capsule activates the PI3K/AKT/mTOR signaling
pathway to improve the ovarian reserve function of mice with POI
and protect their fertility (26).
The aforementioned studies demonstrated the potential clinical
value of the Kuntai capsule for POI treatment (27). A previous systematic review
reported the superior efficacy and safety of the Kuntai capsule
combined with HRT for POI treatment compared to HRT alone and
showed that the Kuntai capsule combined with HRT can regulate serum
estrogen and progesterone levels and reduce the adverse effects of
hormone application such as nausea, vomiting, abdominal distension,
headache, dysmenorrhea, breast tenderness, vaginal spotting,
premenstrual syndrome and abnormal hepatic function. (28). A double-blind, randomized,
controlled clinical study by Chen et al (29) investigated the clinical
effectiveness and safety of the Kuntai capsule on peri-menopausal
symptoms in patients with endometriosis who undergo postoperative
gonadotropin-releasing hormone agonist (GnRH-a) treatment. The
study reported that the main reported side effects of the combined
Kuntai capsule and GnRH-a treatment included vaginal bleeding or
spotting and breast-distending pain and showed that this regimen
was associated with a significantly lower incidence of adverse
effects compared with the regimen of GnRH-a + tibolone, a selective
tissue-estrogenic-activity regulator.
In agreement with previous reports, the present
study confirmed that the combined regimen of the Kuntai capsule
with HRT effectively reduced hot flushes and related symptoms,
reduced FSH and LH serum levels and increased serum levels of E2.
Ma et al (30) showed that
the Kuntai capsule was more effective in reducing the levels of FSH
and LH and increasing the level of E2 compared with HRT only in the
treatment of patients with premature ovarian failure. The results
of the present study further confirmed these observations.
Furthermore, the present study showed that the
Kuntai capsule combined with HRT increased endometrial thickness
and peak systolic velocity of the ovarian artery, which indicated
improved uterine and ovarian condition. It could be suggested that
such changes may manifest in potentially increased pregnancy rates
(28). On the other hand, combined
therapy did not significantly affect the ovarian volume, which is
considered as a marker of ovarian reserve (3,4).
Furthermore, a meta-analysis by Zhang et al (31) reported that the Kuntai capsule
improved the ovulation and pregnancy rates of patients with
ovulatory disorders who underwent assisted reproduction
treatment.
Additionally, the present study demonstrated that
the combined regimen of the Kuntai capsule and HRT significantly
improved AMH levels and the number of basic antral follicles in
patients with POI. A study by Lian and Jiang (32) showed that the Kuntai capsule
increased the number of retrieved oocytes and improved the quality
of oocytes and embryos in patients undergoing fertility treatments.
The Kuntai capsule combined with routine HRT could potentially be
used before ovulation induction to increase the number of oocytes
and reduce the cycle cancellation rate. Future studies should focus
on the potential value of the Kuntai capsule in reproductive
medicine.
While there have been two previous meta-analyses
(33,34) that provided evidence of the
increased effectiveness of the Kuntai capsule combined with HRT vs.
HRT alone in improving outcomes of POI, the present study was
unique in that it used a more robust inclusion criteria to include
high quality data. Firstly, only studies which diagnosed POI based
on The European Society of Human Reproduction and Embryology,
Guideline Group on POI were included (2). Additionally, studies with patients
<40 years in age were selected, with amenorrhea or
oligomenorrhea for ≥4 months and serum FSH >25 IU/l on at least
two separate measurements (>4 weeks apart). This provided a more
robust diagnosis of POI, thereby providing higher-quality
evidence.
The present study had certain limitations. First,
the ‘unclear’ risk of bias in most incorporated studies reduced the
credibility of the conclusions. Therefore, RCTs that comply with
the Consolidated Standards of Reporting Trials reporting system are
required to confirm our observations (32). Second, all included studies were
conducted in China and reported data exclusively on patients of
Chinese origin, thus limiting the generalizability of these
results. Further trials of Traditional Chinese Medicines must
include diverse populations and regions to provide more accurate
and reliable data. Third, only articles written in Chinese and
English were included because of language restrictions. Fourth,
none of the included studies reported data on adverse reactions
caused by treatment with the Kuntai capsule, hence a separate
meta-analysis for this could not be conducted. Finally, the present
study was limited by the quality of the included literature.
Additional higher-quality studies with extensive sample sizes are
needed to verify these observations and provide evidence supporting
the clinical use of the Kuntai capsule.
In summary, the Kuntai capsule combined with HRT
treatment could alleviate menstrual disorders and peri-menopausal
symptoms and reduce serum sex hormone levels in patients with POI.
This treatment also significantly increased the number of antral
follicles in patients with POI compared with HRT alone. However,
the present study was limited by the quality of the literature
included. Additional high-quality studies with extensive sample
sizes are required to verify these findings. However, given the
limited side-effects of Kuntai capsule and potential benefits
demonstrated through the present meta-analysis, it may be
considered as a future supplemental therapy in patients using HRT
with POI.
Acknowledgements
Not applicable.
Funding
Funding: The Fujian Provincial Health and Youth Research
Projects Funding Scheme (grant no. 2019-1-13) and Startup Fund for
scientific research Fujian Medical University (grant no.
2021QH1201) funded the present study.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FL conceived and designed the study. FL, JL and LW
collected the data, performed the literature search and analyzed
the data. FL was involved in the writing of the manuscript. FL
edited the manuscript. All authors have read and approved the final
version of the manuscript. FL and JL confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maclaran K and Panay N: Current concepts
in premature ovarian insufficiency. Womens Health (Lond).
11:169–182. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
European Society for Human Reproduction
and Embryology (ESHRE) Guideline Group on POI. Webber L, Davies M,
Anderson R, Bartlett J, Braat D, Cartwright B, Cifkova R, de Muinck
Keizer-Schrama S, Hogervorst E, et al: ESHRE guideline: Management
of women with premature ovarian insufficiency. Hum Reprod.
31:926–937. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sadrzadeh S, Painter RC, van Kasteren YM,
Braat DDM and Lambalk CB: Premature ovarian insufficiency and
perinatal parameters: A retrospective case-control study.
Maturitas. 96:72–76. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Podfigurna-Stopa A, Czyzyk A, Grymowicz M,
Smolarczyk R, Katulski K, Czajkowski K and Meczekalski B: Premature
ovarian insufficiency: The context of long-term effects. J
Endocrinol Invest. 39:983–990. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sullivan SD, Sarrel PM and Nelson LM:
Hormone replacement therapy in young women with primary ovarian
insufficiency and early menopause. Fertil Steril. 106:1588–1599.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chi J and Zhang Y: Observations on the
efficacy of auricular pressure therapy combined with kuntai capsule
in the treatment of uterine fibroids combined with perimenopausal
syndrome. Hainan Med J. 27:3032–3034. 2016.
|
|
7
|
Xu J and Sun J: Experimental study on the
effect of prevention and treatment of Kuntai capsule on
osteoporosis in ovariectomized rats-CNKI. Mode J Inte Trad Chin
West Med. 24:2873–2875. 2015.
|
|
8
|
Zhu J and Wang X: Meta-analysis of
efficacy and safety of Kuntai capsule combined with hormone therapy
in treatment of climacteric syndrome. Chin Arch Trad Chin. 58–65.
2021.
|
|
9
|
Zhang B, Zhang L, Yang Y, Sun X, Yang L,
Wang L and Zhang X: Efficacy of Kuntai capsule for treating ovarian
reserve dysfunction: A meta-analysis. Chin J Reprod Contracep.
40:750–755. 2020.
|
|
10
|
Xu L: Clinical effect analysis of
dehydroepiandrosterone combined with Kuntai capsule in the
treatment of infertility due to hypo-ovarian reserve. Mater Child
Heal Care Chin. 35:3851–3854. 2020.
|
|
11
|
Cao H, Li H, Lin G, Li X, Liu S, Li P,
Cong C and Xu L: The clinical value of acupuncture for women with
premature ovarian insufficiency: A systematic review and
meta-analysis of randomized controlled trials. Front Endocrinol
(Lausanne). 15(1361573)2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Higgins JPT, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group. Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. PLoS Med.
6(e1000097)2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Luan S, Cui Q, Zhang Y and Ma H: Clinical
efficacy of Kuntai capsule in the treatment of premature ovarian
failure. Chin Tradit Pat Med. 39:1318–1320. 2017.
|
|
15
|
Li W, An L, Zhang G, et al: The combined
application of estradiol valerate-cproterone acetate and Kuntai
capsule in poor ovarian responders of in vitro fertilization. J
Pract Obstet Gynecol. 30:681–685. 2014.
|
|
16
|
Jia X: Clinical effect of Kuntai capsule
combined with estradiol tablet/estradiol didroxyprogesterone tablet
in the treatment of infertility with low ovarian reserve function.
Pract Clin Med. 20:42–43. 2019.
|
|
17
|
Wu S: Study on the value of Kuntai and HRT
treating premature ovarian failure. Pract Gynecol Endocr.
6:114–119. 2019.
|
|
18
|
Yuan H and Hu Y: Effect of Kuntai capsule
combined with hormone replacement therapy on premature ovarian
failure. Hubei J TCM. 41:16–18. 2019.
|
|
19
|
Yuan H and Hu Y: Clinical observation of
climen combined with kuntai capsule in treatment of diminished
ovarian reserve. Hubei J TCM. 40:7–9. 2018.
|
|
20
|
Hong: Clinical study on treatment of
premature ovarian insufficiency with integrated Chinese and western
medicine (unpublished thesis). Hubei University of Traditional
Chinese Medicine, 2018.
|
|
21
|
Hu Y: The application of kuntai combined
with femoston in patients with premature ovarian insufficiency
complicated with infertility. Chin Med Mod Distance Edu China.
16:107–109. 2018.
|
|
22
|
Xiao PM, Xu YY and Shi YH: Clinical
efficacy of Climen combined with Kuntai capsule for premature
ovarian failure. Chin J Gener Pract. 13:774–775, 787. 2015.
|
|
23
|
Yao Y, Xia M, Wang C and Wan D: Clinical
observation of Kuntai capsule combined with climen in treatment of
diminished ovarian reserve. In: Proceedings of the 13th National
Conference on traditional Chinese Medicine and Gynecology, Guiyang,
pp611-612, 2013.
|
|
24
|
Torrealday S, Kodaman P and Pal L:
Premature ovarian insufficiency-an update on recent advances in
understanding and management. F1000Res. 6(2069)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang J, Fang L, Shi L, Lai Z, Lu Z, Xiong
J, Wu M, Luo A and Wang S: Protective effects and mechanisms
investigation of Kuntai capsule on the ovarian function of a novel
model with accelerated aging ovaries. J Ethnopharmacol.
195:173–181. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang H, Qin F, Liu A, Sun Q, Wang Q, Li
Q, Lu S, Zhang D and Lu Z: Kuntai capsule attenuates premature
ovarian failure through the PI3K/AKT/mTOR pathway. J
Ethnopharmacol. 239(111885)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhou X, Wang L, Pan W and Li J: Clinical
Observation on the treatment of premature ovarian failure with
Kuntai capsule combined with hormone. J New Chin Med. 49:79–81.
2017.
|
|
28
|
Liu W, Nguyen TN, Tran Thi TV and Zhou S:
Corrigendum to ‘Kuntai capsule plus hormone therapy vs hormone
therapy alone in patients with premature ovarian failure: A
systematic review and meta-analysis’. Evid Based Complement
Alternat Med. 2021(5737914)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen JM, Gao HY, Ding Y, Yuan X, Wang Q,
Li Q and Jiang GH: Efficacy and safety investigation of Kuntai
capsule for the add-back therapy of gonadotropin releasing hormone
agonist administration to endometriosis patients: A randomized,
double-blind, blank- and tibolone-controlled study. Chin Med J
(Engl). 128:427–432. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ma Q, Tan Y and Mo G: Effectiveness of
cotreatment with Kuntai capsule and climen for premature ovarian
failure: A meta-analysis. Evid Based Complement Alternat Med.
2020(4367359)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang X, Zhang L, Xiong L, Liu X, Zhang J,
Yu F, Li Y, Chang W and Chen W: Kuntai capsule for the treatment of
diminished ovarian reserve: A systematic review and meta-analysis
of randomized controlled trials. J Ethnopharmacol.
329(118167)2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lian F and Jiang XY: Effect of kuntai
capsule on the number of retrieved oocytes, high-quality oocytes
and embryos in in vitro fertilization of poor ovarian response
patients. Zhongguo Zhong Xi Yi Jie He Za Zhi. 34:917–921.
2014.PubMed/NCBI(In Chinese).
|
|
33
|
Liu W, Nguyen TN, Tran Thi TV and Zhou S:
Kuntai capsule plus hormone therapy vs hormone therapy alone in
patients with premature ovarian failure: A systematic review and
meta-analysis. Evid Based Complement Alternat Med.
2019(2085804)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lin W, Zeng XP, Deng J, Liu Y, Cai RH,
Chen HY and Shi DH: Kuntai capsules in treatment of premature
ovarian failure: A systematic review and meta-analysis. J Herb Med.
31(100524)2022.
|