Introduction
Lumbar interbody fusion (LIF) surgery is widely
employed in orthopaedic procedures to treat various spine-related
diseases, such as degenerative diseases, spondylolisthesis, lumbar
spinal stenosis (LSS) and spinal instability (1). The primary aim of this surgery is to
alleviate pain and increase spinal stability by fusing two or more
vertebrae (2). This is achieved by
implanting bone fusion materials (such as autologous bone,
polyether ether ketone or metal) and reinforcing the spinal
structure with metal devices (such as screws and rods) (3).
Full endoscopic spinal surgery represents an
innovation in the surgical field, allowing physicians to perform
spinal operations using an endoscope through smaller incisions
(4). This technique provides
clearer and broader visual fields, finer neural decompression and
improved intervertebral space handling. Using the trans-superior
articular process approach under endoscopy allows direct
decompression of the affected foramina, lateral recess stenosis and
central canal (5). Under
endoscopic observation, the cartilage endplate can be scraped
without excessive treatment of the bony endplate. This improvement
in surgical anatomy can minimize surgical trauma, reduce recovery
time and decrease the incidence of complications (6). This technique has been widely applied
in various spinal surgeries, such as decompression, spinal fixation
and spinal fusion surgeries. Despite its numerous advantages, it
faces some challenges. First, full endoscopic surgical techniques
require highly skilled surgeons, which can only be achieved through
specialized training. Second, for some complex conditions, such as
severe spinal scoliosis (7) or
severe spinal stenosis, this technique may not provide a sufficient
surgical view or operating space. Additionally, the long-term
efficacy and success rate of full endoscopic techniques in spinal
surgery require further research for confirmation.
Trans-superior articular process LIF surgery under
full endoscopy is an innovative surgical method designed to
overcome the limitations of traditional spinal surgical techniques.
Traditional spinal surgical methods, especially LIF surgery,
typically require large surgical incisions, extensive tissue
dissection and wide exposure of spinal structures (8). Furthermore, uncertainties in the bone
fusion process and potential failures of fixation devices could
affect the long-term success rate post-surgery (9). These factors may lead to significant
surgical trauma, prolonged recovery time and a high risk of
complications. Current studies almost unanimously agree that
endoscopic lumbar fusion surgery has advantages over traditional
surgical methods, such as smaller surgical incisions, reduced
muscle and soft tissue dissection, less bleeding and faster
postoperative recovery (10-16).
Therefore, developing a new surgical method is crucial to reduce
surgical trauma, shorten surgery and recovery times and lower the
risk of complications. In the present study, the primary goal of
transforaminal upper facet joint LIF (TSAP-LIF) under full
endoscopy was to use endoscopes and specialized surgical tools
through smaller incisions and with less tissue dissection. Unlike
the currently popular approaches, this technique enables LIF
surgery to be performed by only removing the superior articular
process, without removing the inferior articular process, resulting
in fewer steps and a reduced surgical duration for patients.
Materials and methods
Study design
TSAP-LIF under full endoscopy has similar
indications to traditional spinal fusion techniques for the
treatment of Grade 1-2 symptomatic spondylolisthesis and spinal
stenosis (Table I, classified by
the Meyerding system) (17). The
present study is a retrospective analysis aimed at evaluating the
early clinical efficacy and preliminary safety of TSAP-LIF
performed under full endoscopy in patients treated at Changzhi
Yunfeng Hospital (Changzhi, China). All surgeries were conducted by
the same experienced surgeon to ensure consistency in surgical
technique and its impact on patient outcomes. Follow-up data and
imaging studies from January 1, 2021, to December 31, 2022, were
retrospectively analyzed. The study data obtained from the
hospital's electronic medical records included patients'
preoperative baseline characteristics, operative time,
intraoperative blood loss, postoperative complications and
follow-up outcomes (with a follow-up period of 6 months). Clinical
outcomes were assessed using the Oswestry Disability Index (ODI)
(Table II) (18) and Visual Analog Scale (VAS)
(Table III) (19), while radiographic evaluations were
performed using postoperative X-rays and computed tomography (CT)
scans to determine the interbody fusion rate. Identifiable
information about individual participants (Table IV), such as name, sex, age,
address, identification number and facial image data, was obtained
during or after data collection.
 | Table IMeyerding classification of
spondylolisthesis. |
Table I
Meyerding classification of
spondylolisthesis.
| Meyerding
grade | Percentage of slip
(%) | Clinical
description |
|---|
| Grade I | 0-25 | Mild
spondylolisthesis |
| Grade II | 25-50 | Moderate
spondylolisthesis |
| Grade III | 50-75 | Severe
spondylolisthesis |
| Grade IV | 75-100 | Very severe
spondylolisthesis |
| Grade V | >100 | Spondyloptosis |
 | Table IIOswestry disability Index assessment
form. |
Table II
Oswestry disability Index assessment
form.
| Item | Scoring options
(0-5 points) |
|---|
| Pain intensity | No pain (0)-worst
imaginable pain (5) |
| Personal care | Normal self-care
(0)-bedridden requiring assistance (5) |
| Lifting
ability | Can lift heavy
weights without pain (0)-unable to lift anything (5) |
| Walking
ability | Unlimited walking
(0)-can only crawl (5) |
| Sitting
tolerance | Can sit comfortably
for any duration (0)-unable to sit at all (5) |
| Standing
ability | Can stand as needed
(0)-unable to stand (5) |
| Sleep quality | Uninterrupted sleep
(0)-complete insomnia due to pain (5) |
| Sex life | Normal sexual
activity (0)-unable to engage (5) |
| Social life | Unrestricted social
activities (0)-complete loss of social life (5) |
| Travel ability | Can travel long
distances (0)-only able to travel for medical care (5) |
 | Table IIIVisual analogue scale. |
Table III
Visual analogue scale.
| Pain level | Analogue scale
(cm) | Pain intensity | Symptom
description |
|---|
| 0 | 0 | No pain | Not applicable |
| 1 | 1-2 | Mild pain | Tolerable pain with
normal daily activities and sleep unaffected |
| 2 | 3-4 | Moderate pain | Pain moderately
affects sleep and requires analgesics |
| 3 | 5-6 | Severe pain | Severe pain
disrupting sleep, requiring narcotic analgesics |
| 4 | 7-8 | Intense pain | Significant sleep
disturbance with associated symptoms (e.g. Sweating,
tachycardia) |
| 5 | 9-10 | Unbearable
pain | Profound sleep
impairment with comorbidities or passive positioning |
 | Table IVSummary of patient statistics and
diagnostic data. |
Table IV
Summary of patient statistics and
diagnostic data.
| Patient no. | Sex | Age, years | Diagnosis | Level |
|---|
| 1 | F | 45 | L4-5 disc
herniation and degeneration (left-of-center type) combined with
mild posterior slippage of the L4 vertebral body. | L4/5 |
| 2 | F | 52 | L4-5 intervertebral
disc prolapse, degeneration leading to spinal canal stenosis
(right-of-center type), combined with degenerative changes of the
endplates. | L4/5 |
| 3 | F | 55 | L4-5 intervertebral
disc prolapse, degeneration leading to spinal canal stenosis. | L4/5 |
| 4 | M | 67 | L5-S1
intervertebral disc herniation combination of L5 isthmic fracture
of the arch with forward slip (first degree). | L5/S1 |
| 5 | F | 46 | L4-5 intervertebral
disc herniation, degeneration combined with mild forward slippage
of L4 vertebrae, bilateral vertebral tuberosity and hyperplasia
leading to spinal canal stenosis. | L4/5 |
| 6 | F | 54 | L4-5 disc
herniation, degeneration (right intervertebral foramina type)
leading to spinal stenosis combined with mild forward slip of the
L4 vertebrae, and spinal stenosis due to bilateral hypertrophy and
hyperplasia of the vertebral tuberculum. | L4/5 |
| 7 | M | 52 | L4-5 intervertebral
disc herniation, degeneration leading to spinal stenosis, combined
with vertebral body endplate inflammation. | L4/5 |
| 8 | M | 23 | L4-5 disc
herniation causing spinal stenosis. | L4/5 |
| 9 | M | 32 | L4-5 disc
herniation causing spinal stenosis (left radicular type). | L4/5 |
Preoperative planning
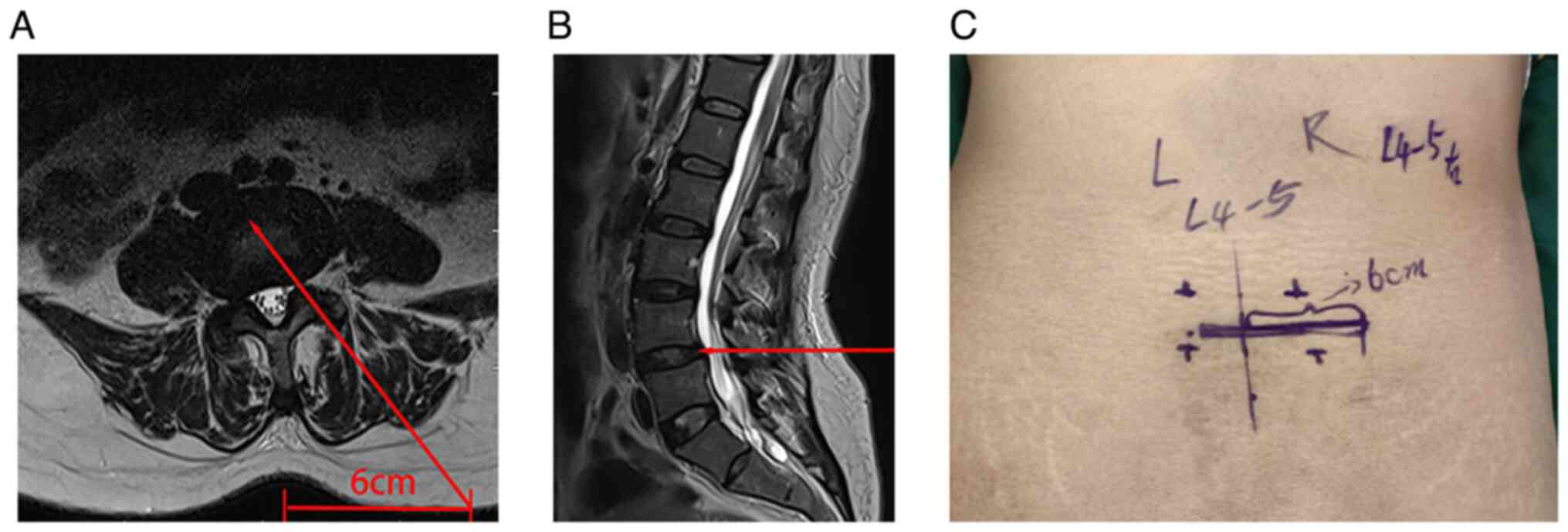
Preoperatively, the surgical approach trajectory was
planned using axial T2-weighted magnetic resonance imaging, with
the approach marked by continuous red lines reaching the lateral
recess through the SAP (Fig. 1A
and B).
Intraoperative procedures
Patients were positioned prone on an X-ray
translucent surgical table under general anesthesia with sedation
monitoring. The endoscopic video monitor and C-arm X-ray machine
were placed opposite the affected side of the patient, and the
surgeon operated from the affected side. By suspending a saline bag
1-1.5 meters above the patient's plane, sufficient flow was ensured
to maintain a clear endoscopic view. Saline irrigation was
performed without a pump to avoid increasing intracranial pressure
in the event of an iatrogenic dural tear. Active communication with
the anesthesia team maintained systolic pressure below 110 mmHg,
effectively controlling intraoperative bleeding.
Approach and exposure
During surgery, the endoscope was inserted from the
side with radiculopathy symptoms. Using the C-arm X-ray machine for
positioning, the skin entry point of the L4/L5 pedicle was marked,
and a 1- to 1.5-cm incision was made 6 cm lateral to the
interlaminar space (Fig. 1C) as a
puncture point for inserting the working cannula and interbody
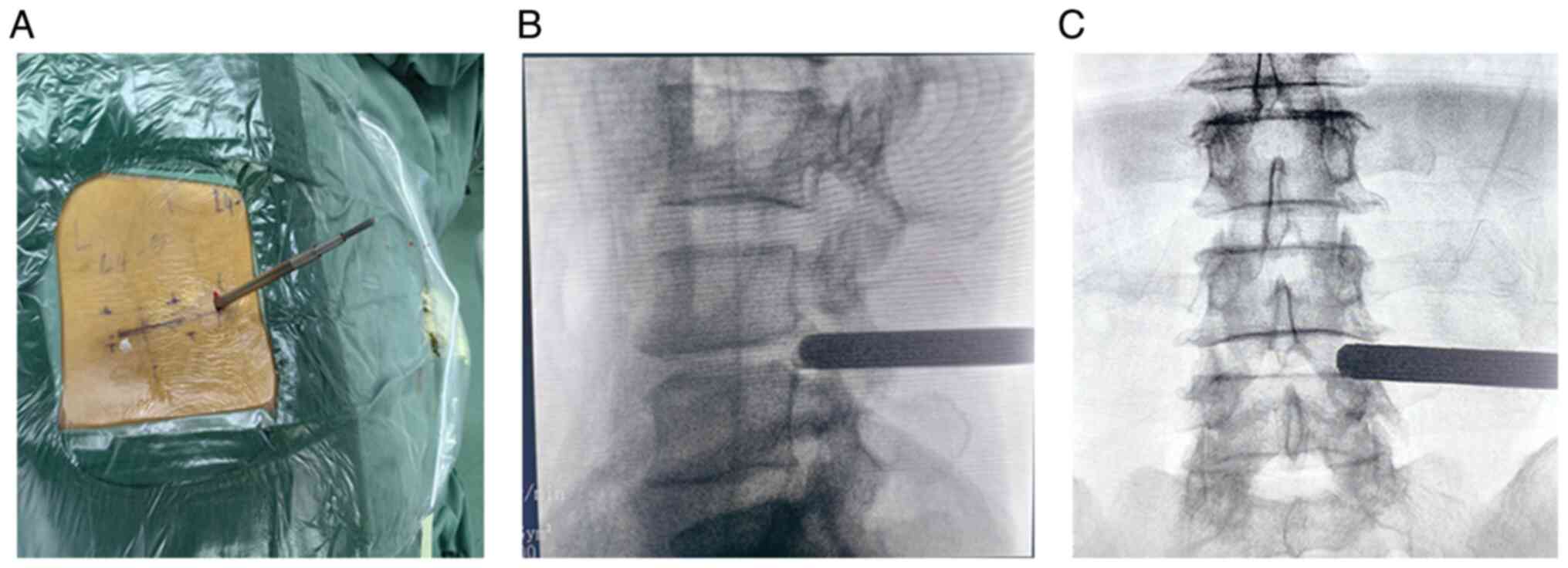
cage. Following skin disinfection and draping, a puncture was made
at the marked point, and a guide wire was percutaneously introduced
into the dorsolateral part of the L5 superior articular process,
guiding the insertion of the working cannula (Fig. 2A-C). Local anesthesia was
administered with 5% lidocaine (5 ml) layer by layer to the
shoulder of the L5 superior articular process.
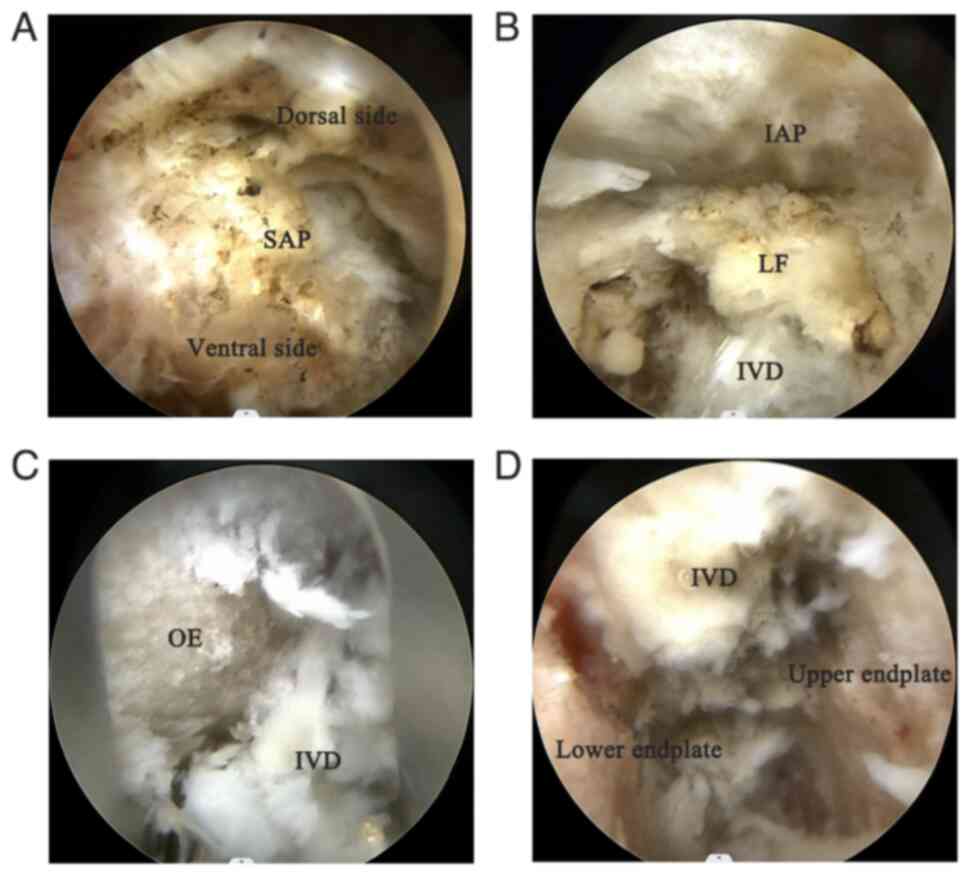
Bone resection and decompression
During the resection of the L5 superior articular
process, the target area was adequately exposed by gradual dilation
through the cannula, and an osteotome was used to progressively
resect the left superior articular process of L5 under endoscopic
view (Fig. 3A), safely exposing
the nerve root. The osteotome was operated slowly and steadily to
avoid damaging the spinal canal or nerves. Before performing the
discectomy and contralateral lateral recess decompression, the
ligamentum flavum (Fig. 3B) was
removed to expand the surgical field and expose the intervertebral
disc.
Interbody fusion
Upon completing bilateral lateral recess
decompression, a large working cannula was inserted, and under
direct endoscopic visualization, a discectomy was performed at
L4-5, relieving the compressed nerve root. After adequate
decompression, an endoscopic dilator was used to expand the
intervertebral space, and the endoscope sheath was rotated to
protect the endplates. Disc material and cartilage endplates were
cleaned using rongeurs and curettes (Fig. 3C and D), preparing the upper and lower
endplates for the cage insertion.
Grafting and instrumentation
For grafting and cage insertion, the intervertebral
space was progressively expanded using a spreader, and a trial cage
was used to determine the appropriate height of the interbody cage.
Based on the trial's tightness, a polyetheretherketone (PEEK)
interbody cage of suitable height was selected. The resected
articular process was trimmed into suitable bone graft blocks, and
a funnel-shaped graft inserter was used to initially place part of
the graft block anteriorly into the L4-5 intervertebral space,
followed by graft granules. Before inserting the cage, the position
of the exiting nerve root was rechecked under endoscopy, and the
cage was placed under X-ray fluoroscopy to confirm its satisfactory
position. Pedicle screws were inserted using a percutaneous
technique, and titanium rods were placed and secured with
compression fixation after confirming the fluoroscopic position. A
drain was placed on the rod side to avoid nerve irritation.
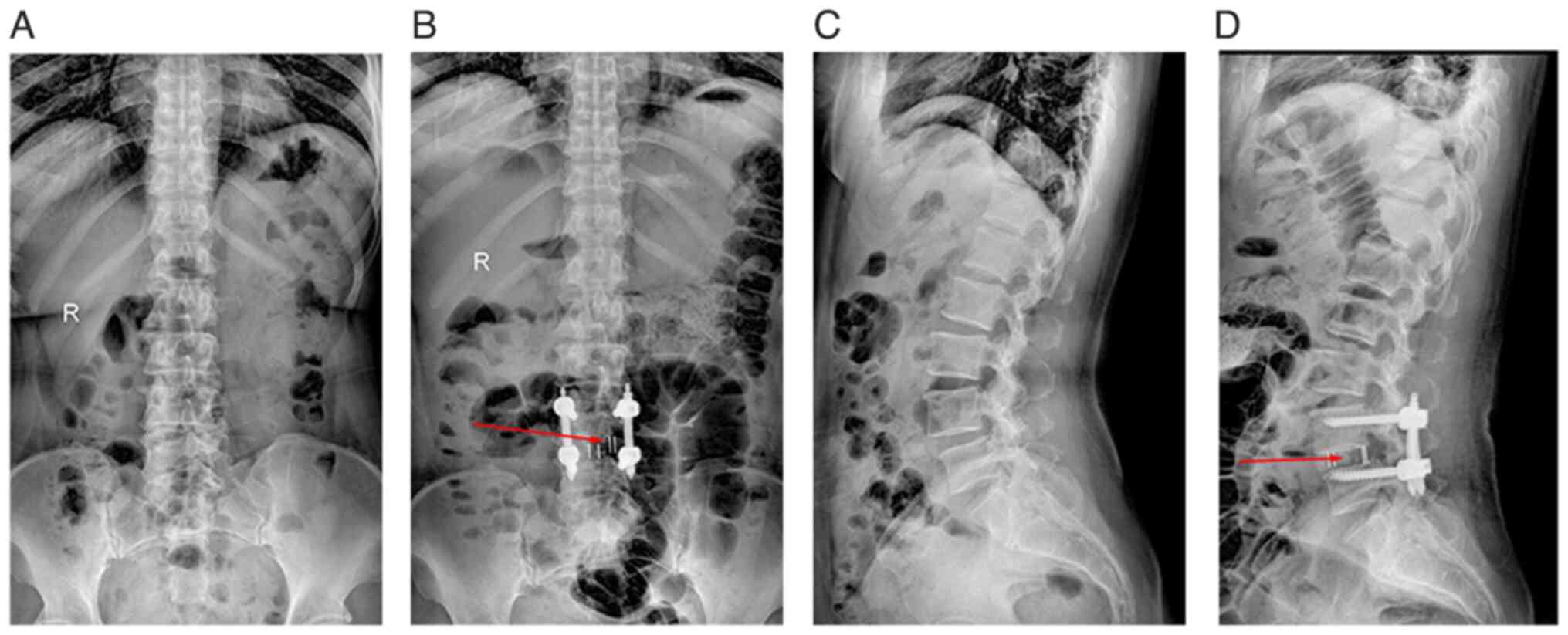
Postoperative management
Postoperatively, decompression work and interbody
graft size were assessed using plain films (Fig. 4) and CT (Fig. 5). Patients were discharged in good
condition, with proper wound care, effective pain control and
satisfactory mobility. Patients were required to wear a tight
thoracolumbar brace for 1.5 months. Short-term radiographic
outcomes were assessed, including a 6-month postoperative follow-up
and CT images beyond 6 months. Clinical outcomes were evaluated
using the ODI and VAS for back pain, as well as operative time,
intraoperative blood loss, hospitalization time and surgery-related
complications.
Outcome measures
Pain intensity was quantified using the Visual
Analogue Scale (VAS), a validated 10-cm horizontal line anchored by
‘no pain’ (0) and ‘worst imaginable pain’ (10). Patients marked their perceived pain
level, which was measured to the nearest millimeter (0-100 mm
scale). Pain severity was categorized as: 0 (no pain), 1-3 (mild
pain, no sleep disturbance), 4-6 (moderate pain, mild sleep
interference) and 7-10 (severe pain, sleep disruption). The
Oswestry Disability Index (ODI) assessed lumbar dysfunction through
10 domains: Pain intensity, personal care, lifting, walking,
sitting, standing, sleeping, sexual function, social life and
traveling. Each item contains six statements graded 0 (no
disability) to 5 (maximum disability). If all items were answered
(maximum 50 points), the denominator was 50; if any item was
omitted (e.g., sexual function), the denominator adjusted to 45.
Higher scores indicate greater disability. Assessments were
conducted preoperatively and postoperatively at 1, 3 and 6 months
and at the final follow-up.
Statistical analysis
Statistical analyses were conducted using IBM SPSS
Statistics v27.0 (IBM Corp). Continuous variables are expressed as
the mean ± standard deviation following confirmation of normality
via Shapiro-Wilk tests. Between-group comparisons utilized
repeated-measures ANOVA with Greenhouse-Geisser epsilon correction
for sphericity violations. Post-hoc pairwise comparisons applied
Bonferroni-adjusted α levels (0.05/3=0.0167 for three-group
comparisons). All tests were two-tailed, with P<0.05 considered
to indicate a statistically significant difference. Data were
presented as mean ± SD.
Results
The present retrospective study aimed to evaluate
the early clinical efficacy and preliminary safety of endoscopic
TSAP-LIF in patients with LSS. The study included 9 patients with
LSS, as detailed in Table I, which
presents their baseline characteristics. The average age of the
patients (5 women and 4 men) was 47.3±13.1 (range: 23-67) years.
All patients underwent single-segment fusion, with 8 receiving L4/5
segment fusion and 1 receiving L5/S1 segment fusion. Results showed
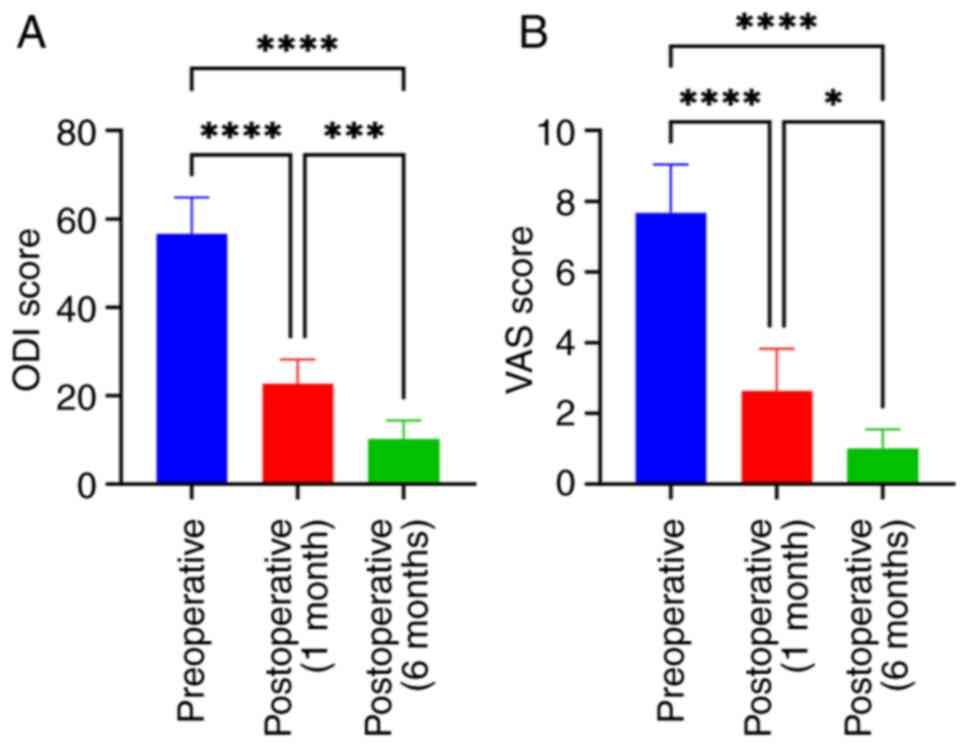
significant improvement in ODI and VAS scores at 1 and 6 months
post-operation compared with preoperative scores, with an
intervertebral fusion rate of 88% at 6 months (based on
postoperative CT imaging, which revealed 1 case of non-fusion) and
no postoperative complications (Table
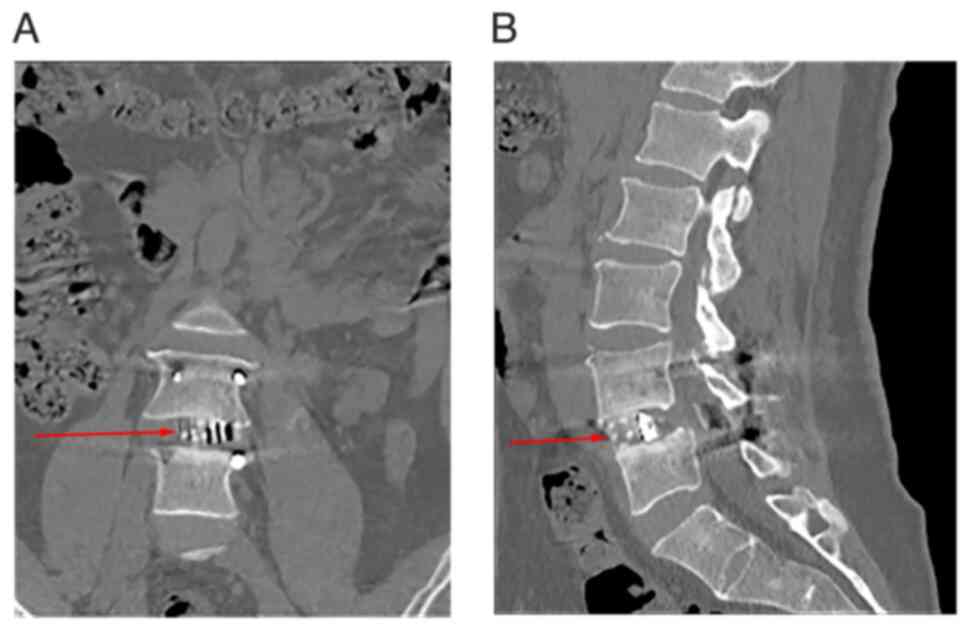
V). CT scans 6 months post-operation (Figs. 4B and D and 5)
showed adequate decompression of the affected side and central
spinal canal. Additionally, imaging revealed a larger graft contact
area compared with that of the entire intervertebral disc region.
X-rays (Fig. 4B and D) taken within 1 week post-operation
indicated well-prepared cartilage endplates with no gaps between
the intervertebral graft and vertebrae. Furthermore, CT scans at 6
months post-operation (Fig. 5)
showed continuous growth and remodeling of trabecular bone, with no
significant gaps observed between the graft, cage and endplates.
The average surgical time was 113.3±13.9 min, and the average
intraoperative blood loss was 101.6±13.8 ml (Table V). The average hospital stay was
12.7±3.2 days. No surgery-related complications occurred in the
present study (Table V). The
average VAS score improved from 7.7±1.6 preoperatively to 2.6±1.4
(P<0.0001) at 3 months post-operation and to 1.2±1.1 (P=0.1283)
at 6 months post-operation. The average ODI score improved from
56.7±8.2 preoperatively to 22.7±5.6 (P<0.0001) at 1-month
post-operation and to 10.2±4.2 (P<0.001) at 6 months
post-operation (Fig. 6).
 | Table VIntraoperative and postoperative
patient metrics. |
Table V
Intraoperative and postoperative
patient metrics.
| Patient no. | Surgical time,
min | Intra- operative
blood loss, ml | Duration of
hospital stay, days | Interver- tebral
fusion |
|---|
| 1 | 115 | 102 | 18 | Yes |
| 2 | 100 | 88 | 12 | Yes |
| 3 | 120 | 116 | 9 | Yes |
| 4 | 95 | 84 | 12 | Yes |
| 5 | 125 | 107 | 10 | No |
| 6 | 105 | 92 | 18 | Yes |
| 7 | 115 | 104 | 12 | Yes |
| 8 | 140 | 127 | 11 | Yes |
| 9 | 105 | 94 | 12 | Yes |
Discussion
Lumbar degenerative disease is a common condition in
spinal orthopaedics, often causing back and leg pain with
restricted movement. Lumbar fusion surgery has become the standard
procedure for treating such diseases. The origin of spinal
endoscopy dates back to the early 1930s when Burman used
arthroscopic instruments to perform the first ‘spinal endoscopy’ on
a cadaver, successfully displaying the spinal cord and nerve roots
(20). Soon after, Pool (21) began performing spinal endoscopy
through incisions ≤2.5 mm long, providing detailed observation of
the nerve roots. With the advancement of optical lens systems and
fiber optic technology, and the continuous development and
expansion of surgical techniques, Cloward (22) first proposed posterior LIF (PLIF)
in 1953. This technique offered clear surgical field exposure, high
neural decompression and a stable three-dimensional spine
structure, restoring the normal lumbar curvature. However, PLIF
also had significant drawbacks, such as damaging posterior spinal
structures (e.g. spinous processes, lamina and bilateral facet
joints) and causing nerve root traction injuries (22). In 1983, Kambin and Zhou (23) performed the first percutaneous
arthroscopic discectomy, and in 1991, Kambin (24) introduced the concept of a
triangular safety zone, a triangular area formed by the upper edge
of the lower vertebra, the outer edge of the dural sac or
traversing nerve root and the inner edge of the exiting nerve root.
This area is relatively safe for surgical operations and is the
pathway for endoscopic transforaminal neural decompression and
interbody fusion. To reduce iatrogenic injuries, Leu and Hauser
(25) first reported the use of
percutaneous endoscopic lumbar fusion in 1996, although this
surgery had a high overall complication rate, including
postoperative nerve root pain with dysesthesia, symptomatic
interbody cage displacement and the need for salvage surgery. Since
then, surgical methods and tools have gradually improved, allowing
for adequate discectomy, endplate preparation and the use of
appropriate lumbar interbody cages to avoid nerve injuries.
The indications for TSAP-LIF under full endoscopy
are similar to those for conventional spinal fusion surgery,
especially when spinal instability causes neural compression
(26). With regard to approach
selection, the SAP reshaping should ensure the safety of the
exiting nerve root, typically by gradually reshaping the dorsal
side of the SAP to create sufficient safe space and reduce the
probability of nerve root injury. The rational use of
foraminoplasty tools, such as power systems, trephine systems and
protective sleeves under full endoscopy, can effectively prevent
injury to the exiting nerve root and dural sac (27).
According to the location and characteristics of
stenosis, a reasonable decompression method should be selected. The
TSAP approach can cover most of the intervertebral foramen's
internal and external ranges and the affected-side lateral recess.
It is also suitable for severe central canal stenosis dorsal
decompression and L5/S1 segment decompression (28), but cannot perform bilateral
decompression through a unilateral approach, which is a limitation
of this technique (29).
Therefore, the indications of the patient should be clarified
preoperatively.
Proper graft bed preparation is a prerequisite for
achieving bony fusion, which can be performed under direct vision
or endoscopic vision. The former is similar to traditional methods,
using endplate chisels and curettes to scrape the cartilage
endplate, but it is prone to damaging the bony endplate.
High-quality endoscopic treatment of the cartilage endplate can
avoid excessive damage to the bony endplate, although it is less
efficient. Previous reports (30-32)
used modified instruments, such as specially designed endplate
chisels and L-shaped reverse curettes, to handle the intervertebral
space safely and efficiently, preparing the graft bed through a
combination of direct and endoscopic vision (33).
After years of clinical screening and verification,
PEEK cages are the most widely used clinically (34). Although smaller cages can safely
pass through the working path, they may not effectively restore
intervertebral height, and PEEK is an inert bone-inducing material,
unfavorable for LIF. Clinical refinements to the shape and size of
the cages have been made to address this problem, and their hollow
centers have been designed to accommodate osteoinductive material
implants to promote inward growth of bone to induce fusion where
rigid stabilization is required. Graft material selection includes
autogenous bone from decompression, autogenous iliac bone,
allograft bone and BMP-2. In theory, autogenous bone from
decompression is the best choice. If affected by bone quantity,
composite grafting with autogenous bone from local decompression
and other graft materials can be used (35).
Bilateral pedicle screw fixation is currently
advocated, providing a more stable biomechanical environment than
unilateral fixation, bilateral lamina facet screws or facet screws,
reducing complications such as cage displacement, graft non-union
and internal fixation failure (36).
TSAP-LIF has significant advantages in terms of a
shorter operation time compared with other minimally invasive LIF
surgeries. Zhang et al (37) conducted a retrospective study of 62
patients, where the operation time for endoscopic transforaminal
LIF (Endo-TLIF) was 202.6±31.4 min, and that for minimally invasive
transforaminal LIF was 192.1±18.9 min. In the present study, the
operation time was 113.3±13.9 min. Unlike Endo-TLIF, which requires
the removal of both superior and inferior articular processes,
TSAP-LIF only involves the removal of the superior articular
process. The use of ring saw tools allows for rapid excision of the
superior articular process, thus shortening the operation time. Fan
et al (38) retrospectively
analyzed the data of 69 patients with LSS, finding that the
operation time for unilateral lateral interbody fusion was
112.78±19.29 min and that for Endo-TLIF was 174.58±18.41 min.
According to a recent meta-analysis, percutaneous endoscopic lumbar
interbody fusion (PE-LIF) compared with unilateral biportal
endoscopic transforaminal lumbar interbody fusion (UBE-TLIF), the
TSAP-LIF procedure showed similar operative time; however, TSAP-LIF
exhibited advantages in reduced tissue trauma (supported by lower
intraoperative blood loss and postoperative CRP levels), which
accelerated wound healing (39).
Nevertheless, due to limitations in instrument maneuverability, the
working and viewing channels of TSAP-LIF are integrated into a
single portal, precluding simultaneous bilateral decompression of
the intervertebral foramina and lateral recesses. The mean hospital
stay for patients in this study was 12.7±3.2 days, influenced by
China's universal healthcare system (40), which reduces hospitalization costs,
thereby enabling patients to discharge upon clinical improvement or
to undergo in-hospital rehabilitation prior to discharge.
Additionally, the preoperative and postoperative ODI
and VAS scores from the present study were compared with those
reported in other studies on endoscopic LIF techniques. Brusko and
Wang (10) reported a significant
improvement in the average ODI score by 12.3 points at the 1-year
follow-up of 100 patients. Morimoto et al (28) showed improvements in VAS scores for
back pain to 4.4-6.0 and for leg pain to 4.3-6.9, with ODI score
improvements ranging from 19.5 to 41.5. In the study by Xie et
al (41), the VAS scores
decreased from 7.43±0.50 preoperatively to 3.20±0.48 at the first
postoperative week, 2.97±0.41 at 1 month and 2.80±0.41 at 1 year.
The improvements in ODI and VAS scores from the present study are
generally consistent with those achieved by other minimally
invasive lumbar spine surgical techniques (42-45),
indicating significant relief of preoperative symptoms of lower
back and leg pain, as well as favorable outcomes in interbody
fusion.
There are certain limitations to the present study.
As TSAP-LIF is a novel surgical technique evaluated in a
single-center institutional study, the retrospective analysis is
limited by a relatively small sample size. Although paired sample
tests revealed significant differences (P<0.05) in the changes
in VAS scores for back pain and ODI indices in TSAP-LIF patients,
the limited sample size could result in broad confidence intervals,
increasing uncertainty in the outcomes and potentially affecting
inferences about the effects of TSAP-LIF. A small sample size may
lead to unstable effect size estimates, potentially resulting in an
inaccurate assessment of the superiority of TSAP-LIF over other
techniques. Additionally, selection bias may influence the results
due to factors such as patient selection criteria and willingness
to participate, limiting the generalizability of the findings.
Future prospective studies should employ strategies such as
randomization and stratified sampling, and involve larger
multicenter cohorts to verify these results and enhance their
applicability to a broader population.
The primary aim of this retrospective study was to
evaluate the initial safety and short-term efficacy of TSAP-LIF. A
6-month follow-up period is commonly used in similar studies to
assess early postoperative recovery and functional improvements. In
clinical practice, significant recovery and symptom improvement
typically occur within the first 6 months post-surgery, providing
sufficient data to evaluate the direct effects of the surgery.
Research by Uçar et al (46) showed that patients could be allowed
to engage in full activity and return to work 6 months after lumbar
spine fusion surgery. Woods et al (47) conducted a retrospective study on
137 patients who underwent LIF, and the CT fusion rate at all
fusion levels was 97.9% at 6 months after surgery. A 6-month
follow-up period is adequate for early efficacy and safety
assessments, and it can provide some insight into long-term
outcomes, such as fusion durability and sustained clinical
improvement. However, it is essential to extend the follow-up
duration beyond 12 months to thoroughly evaluate the long-term
success of TSAP-LIF. This is particularly important due to the
progressive nature of LSS and the potential for recurrence of
symptoms over time.
The technical and instrumentation limitations of
TSAP-LIF are important considerations, particularly for patients
with unilateral or central stenosis. The lumbar spine features
substantial upper and lower laminar spaces, which allow for the
removal of the upper articular process to decompress the
contralateral affected side. Due to the specific anatomy of the
lumbar spine, there is often ipsilateral blockage from the upper
and lower articular eminences or lamina, making it difficult to
decompress the lateral recess or foramen on both sides
simultaneously via the superior articular eminence. Consequently,
the intervention primarily addresses the affected side and the
central region through this approach. In cases where there is
bilateral compression in the lumbar spine, it may be necessary to
remove portions of the upper and lower articular processes or even
the vertebral plate on the same side for ipsilateral decompression.
This demands a high level of skill on the part of the surgeon in
manipulating the instrumentation. Furthermore, the option exists to
perform simultaneous surgeries using a dual- or multichannel
system, which facilitates enhanced decompression of both sides of
the intervertebral foramen and lateral recess. The integration of
more flexible and maneuverable endoscopes and surgical instruments
can better accommodate diverse anatomical structures, thus
overcoming some limitations in instrument maneuverability.
Additionally, the utilization of real-time imaging and navigation
technologies, along with robotic-assisted systems, can enhance the
precision and adaptability of surgical instruments. This
advancement not only aids surgeons in accurately localizing the
surgical site but also enables them to execute more complex
procedures effectively.
In conclusion, TSAP-LIF represents a promising
minimally invasive technique for addressing lumbar spine
pathologies. The technique offers significant advantages, including
reduced operative time, minimized intraoperative blood loss and the
ability to achieve effective decompression of the affected and
central regions through a unilateral approach. This allows for the
efficient completion of discectomy and endplate preparation.
Despite the challenges posed by the limited maneuverability of
current instruments, advancements in surgical tools and the growing
expertise of surgeons in endoscopic spinal procedures enhance the
potential for improved clinical outcomes. Future efforts will focus
on extending patient follow-up to 12 months, employing
postoperative imaging techniques such as plain films and CT scans
to thoroughly evaluate long-term fusion success. By addressing the
current limitations and with ongoing innovations in surgical
technology, TSAP-LIF has the potential to deliver superior clinical
and radiological outcomes, thereby extending the capabilities of
the Kambin approach.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Changzhi Key
Laboratory of Biomechanical Research and Application of Spinal
Degenerative Diseases (grant no. 2022sy008).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HL and JL conceptualized the study design, performed
surgical interventions, analyzed data and drafted the manuscript.
YG and SQ conducted data acquisition and postoperative follow-up
evaluations. PH and YX supervised the study, validated analytical
methods and critically revised the manuscript. PH and YX confirm
the authenticity of all the raw data. All authors participated in
drafting/revising the manuscript, provided final approval of the
published version and agree to be accountable for all aspects of
the work. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Yunfeng Hospital (approval no. YFHETH2024006). All
patients provided written informed consent to participate in the
study.
Patient consent for publication
Written informed consent for publication of their
clinical details and/or clinical images was obtained from the
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim YH, Ha KY, Rhyu KW, Park HY, Cho CH,
Kim HC, Lee HJ and Kim SI: Lumbar interbody fusion: Techniques,
pearls and pitfalls. Asian Spine J. 14:730–741. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Su SF, Wu MS, Yeh WT and Liao YC: Effects
of lumbar fusion surgery with ISOBAR devices versus posterior
lumbar interbody fusion surgery on pain and disability in patients
with lumbar degenerative diseases: A meta-analysis. J Invest Surg.
33:79–93. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gao Y, Li J, Cui H, Zhang F, Sun Y, Li Z,
Ding W, Shen Y and Zhang W: Comparison of intervertebral fusion
rates of different bone graft materials in extreme lateral
interbody fusion. Medicine (Baltimore). 98(e17685)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lokhande PV: Full endoscopic spine
surgery. J Orthop. 40:74–82. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
McGrath LB, White-Dzuro GA and Hofstetter
CP: Comparison of clinical outcomes following minimally invasive or
lumbar endoscopic unilateral laminotomy for bilateral
decompression. J Neurosurg Spine. 30:491–499. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ju CI and Lee SM: Complications and
management of endoscopic spinal surgery. Neurospine. 20:56–77.
2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Du JW, Zhang LM, Yan YQ, Zhang YN, Rong
XQ, Xiao SH and Zhang XF: Case report: Adult degenerative scoliosis
in two patients treated with percutaneous spinal
endoscopic-assisted lumbar interbody fusion and percutaneous
pedicle screw fixation. Front Surg. 9(730504)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim CH, Easley K, Lee JS, Hong JY, Virk M,
Hsieh PC and Yoon ST: Comparison of minimally invasive versus open
transforaminal interbody lumbar fusion. Global Spine J. 10 (2
Suppl):143S–150S. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sayari AJ, Patel DV, Yoo JS and Singh K:
Device solutions for a challenging spine surgery: Minimally
invasive transforaminal lumbar interbody fusion (MIS TLIF). Expert
Rev Med Devices. 16:299–305. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Brusko GD and Wang MY: Endoscopic lumbar
interbody fusion. Neurosurg Clin N Am. 31:17–24. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fan W, Chen Y, Zhou T, Xu Y and Gu Y:
Comparison of percutaneous transforaminal endoscopic surgery (PTES)
with MIS-TLIF for treating lumbar degenerative disease in obese
patients. J Pain Res. 18:555–561. 2025.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hao J, Chen R, Zheng J, Xu S, Xue H and
Yao Y: Clinical outcomes of unilateral biportal endoscopic
discectomy (UBE) compared with conventional open lumbar discectomy
with 3D microscope (OLDM) assisted. Medicine (Baltimore).
104(e41440)2025.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ma T, Tu X, Li J, Geng Y, Wu J, Chen S,
Yan D, Jiang M, Gao G and Nong L: Comparative analysis of clinical
efficacy of unilateral biportal endoscopic and open transforaminal
lumbar interbody fusion in the treatment of lumbar degenerative.
Front Surg. 12(1487168)2025.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lee MH, Jang HJ, Moon BJ, Kim KH, Chin DK,
Kim KS and Park JY: Comparative outcomes of biportal endoscopic
decompression, conventional subtotal laminectomy, and minimally
invasive transforaminal lumbar interbody fusion for lumbar central
stenosis. Neurospine. 21:1178–1189. 2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
You KH, Hyun JT, Park SM, Kang MS, Cho SK
and Park HJ: Comparison of clinical and radiologic outcomes between
biportal endoscopic transforaminal lumbar interbody fusion and
posterior lumbar interbody fusion. Sci Rep.
14(29652)2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
He Y, Cheng Q and She J: Unilateral
biportal endoscopic lumbar interbody fusion versus minimally
invasive transforaminal lumbar interbody fusion for single-segment
lumbar degenerative disease: A meta-analysis. BMC Musculoskelet
Disord. 25(938)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Meyerding HW: Spondylolisthesis. Surg
Gynecol Obstet. 54:371–377. 1932.
|
|
18
|
Cook CE, Garcia AN, Wright A, Shaffrey C
and Gottfried O: Measurement properties of the oswestry disability
index in recipients of lumbar spine surgery. Spine (Phila Pa 1976).
46:E118–E125. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Faiz KW: VAS-visual analog scale. Tidsskr
Nor Laegeforen. 134(323)2014.PubMed/NCBI View Article : Google Scholar : (In Norwegian).
|
|
20
|
Burman MS: Myeloscopy or the direct
visualization of the spinal canal and its contents. J Bone Joint
Surg. 13:695–696. 1931.
|
|
21
|
Pool JL: Direct visualization of dorsal
nerve roots of the cauda equina by means of a myeloscope. Arch
Neurol. Psychiatr. 39:1308–1312. 1938.
|
|
22
|
Cloward R: The treatment of ruptured
lumbar intervertebral discs by vertebral body fusion. I.
Indications, operative technique, after care. J Neurosurg.
10:154–168. 1953.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kambin P and Zhou L: History and current
status of percutaneous arthroscopic disc surgery. Spine (Phila Pa
1976). 21 (24Suppl):S57–S61. 1996.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kambin P: Arthroscopic microdiskectomy. Mt
Sinai J Med. 58:159–164. 1991.PubMed/NCBI
|
|
25
|
Leu HF and Hauser RK: Percutaneous
endoscopic lumbar spine fusion. Neurosurg Clin N Am. 7:107–717.
1996.PubMed/NCBI
|
|
26
|
Spiker WR, Goz V and Brodke DS: Lumbar
interbody fusions for degenerative spondylolisthesis: Review of
techniques, indications, and outcomes. Global Spine J. 9:77–84.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ito K, Ito Z, Nakamura S, Ito F, Shibayama
M and Miura Y: Minimization of lumbar interbody fusion by
percutaneous full-endoscopic lumbar interbody fusion (PELIF), and
its minimally invasiveness comparison with minimally invasive
surgery-transforaminal lumbar interbody fusion (MIS-TLIF).
Interdiscip Neurosurg. 34(101794)2023.
|
|
28
|
Morimoto M, Wada K, Tamaki S, Soeda S,
Sugiura K, Manabe H, Tezuka F, Yamashita K and Sairyo K: Clinical
outcome of full endoscopic trans kambin's triangle lumbar interbody
fusion: A systematic review. World Neurosurg. 178:317–329.
2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hasan S, White-Dzuro B, Barber JK, Wagner
R and Hofstetter CP: The endoscopic trans-superior articular
process approach: A novel minimally invasive surgical corridor to
the lateral recess. Oper Neurosurg (Hagerstown). 19:E1–E10.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hirase T, Ling JF, Haghshenas V and Weiner
BK: Instrumented versus noninstrumented spinal fusion for
degenerative lumbar spondylolisthesis: A systematic review. Clin
Spine Surg. 35:213–221. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chien KT, Feng HW, Chang TK, Liu YC, Chen
LP, Huang YC, Lian YS and Li JY: Optimizing disc and cartilage
endplate preparation in full-endoscopic lumbar interbody fusion: An
in-depth exploration of surgical instruments with a technique note
and narrative review. World Neurosurg. 189:228–247. 2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen Y, Qin H and Zhong Y: Percutaneous
lumbar interbody fusion with novel self-developed instruments for
lumbar spondylolisthesis. Orthop J China. 30:1410–1413.
2022.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
33
|
Lin GX, Chen CM, Rui G and Kim JS: A pilot
study of endoscope-assisted MITLIF with fluoroscopy-guided
technique: Intraoperative objective and subjective evaluation of
disc space preparation. BMC Surg. 22(109)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Canseco JA, Karamian BA, DiMaria SL, Patel
PD, Divi SN, Chang M, Timmons T, Grewal L, Hallman H, Lee JK, et
al: Static versus expandable polyether ether ketone (PEEK)
interbody cages: A comparison of one-year clinical and radiographic
outcomes for one-level transforaminal lumbar interbody fusion.
World Neurosurg. 152:e492–e501. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kim YH, Ha KY, Kim YS, Kim KW, Rhyu KW,
Park JB, Shin JH, Kim YY, Lee JS, Park HY, et al: Lumbar interbody
fusion and osteobiologics for lumbar fusion. Asian Spine J.
16:1022–1033. 2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang S, Liu Z, Lu C, Zhao L, Feng C, Wang
Y and Zhang Y: Oblique lateral interbody fusion combined with
different internal fixations for the treatment of degenerative
lumbar spine disease: A finite element analysis. BMC Musculoskelet
Disord. 23(206)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang H, Zhou C, Wang C, Zhu K, Tu Q, Kong
M, Zhao C and Ma X: Percutaneous endoscopic transforaminal lumbar
interbody fusion: Technique note and comparison of early outcomes
with minimally invasive transforaminal lumbar interbody fusion for
lumbar spondylolisthesis. Int J Gen Med. 14:549–558.
2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fan Z, Wu X, Guo Z, Shen N, Chen B and
Xiang H: Unilateral biportal endoscopic lumbar interbody fusion
(ULIF) versus endoscopic transforaminal lumbar interbody fusion
(Endo-TLIF) in the treatment of lumbar spinal stenosis along with
intervertebral disc herniation: A retrospective analysis. BMC
Musculoskelet Disord. 25(186)2024.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li X, Qu Y, Zhou L, Zhou Y, Peng B and Duo
J: Meta-analysis of the clinical efficacy and safety of unilateral
biportal endoscopic lumbar interbody fusion versus endoscopic
lumbar interbody fusion for the treatment of lumbar degenerative
diseases. World Neurosurg. 195(123662)2025.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li Y, Zhang C, Zhan P, Fu H and Yip W:
Trends and projections of universal health coverage indicators in
China, 1993-2030: An analysis of data from four nationwide
household surveys. Lancet Reg Health West Pac.
31(100646)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xie YZ, Shi Y, Zhou Q, Feng CQ, Zhou Y, Li
T, Yu Y and Fan XH: Comparison of the safety and efficacy of
unilateral biportal endoscopic lumbar interbody fusion and
uniportal endoscopic lumbar interbody fusion: A 1-year follow-up. J
Orthop Surg Res. 17(360)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gunjotikar S, Pestonji M, Tanaka M,
Komatsubara T, Ekade SJ, Heydar AM and Hieu HK: Evolution, current
trends, and latest advances of endoscopic spine surgery. J Clin
Med. 13(3208)2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu Z, Wang S, Li T, Chen S, Li Y, Xie W
and Tang J: Clinical efficacy of percutaneous endoscopic posterior
lumbar interbody fusion and modified posterior lumbar interbody
fusion in the treatment of lumbar degenerative disease. J Orthop
Surg Res. 19(70)2024.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wagner R and Haefner M: Uniportal
endoscopic lumbar interbody fusion. Neurospine. 17 (Suppl
1):S120–S128. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hu X, Yan L, Jin X, Liu H, Chai J and Zhao
B: Endoscopic lumbar interbody fusion, minimally invasive
transforaminal lumbar interbody fusion, and open transforaminal
lumbar interbody fusion for the treatment of lumbar degenerative
diseases: A systematic review and network meta-analysis. Global
Spine J. 14:295–305. 2024.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Uçar BY, Özcan Ç, Polat Ö and Aman T:
Transforaminal lumbar interbody fusion for lumbar degenerative
disease: Patient selection and perspectives. Orthop Res Rev.
11:183–189. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Woods KRM, Billys JB and Hynes RA:
Technical description of oblique lateral interbody fusion at L1-L5
(OLIF25) and at L5-S1 (OLIF51) and evaluation of complication and
fusion rates. Spine J. 17:545–553. 2017.PubMed/NCBI View Article : Google Scholar
|