1. Introduction
Retinal neovascularization is defined as the
abnormal growth of blood vessels from pre-existing small retinal
veins under specific disease conditions. Specifically, these newly
formed vessels grow out of the pre-existing vascular system
(1,2). The retina is nourished by two
distinct vascular networks; the inner part of the retina relies
primarily on the retinal vascular system of tightly packed
endothelial cells (ECs) for nourishment, forming a blood-retinal
barrier (BRB) (3). By contrast,
the exterior part of the retina relies on a choroid plexus network
of highly permeable, fenestrated ECs and few pericytes; here, an
external retinal barrier is formed, through which oxygen is
transmitted to the photoreceptor cells. At both barriers, lesions
can lead to neovascularization and cause severe visual impairment
(4,5).
VEGF is widely recognized as a key molecule in the
pathogenesis of a variety of ocular diseases, such as proliferative
diabetic retinopathy (PDR), retinopathy of prematurity and
neovascular age-related macular degeneration (nvAMD); all of these
diseases are major causes of visual impairment (6-8),
and different ocular diseases associated with VEGF-driven abnormal
neovascularization have been reported. The advent of anti-VEGF
therapy has revolutionized the therapeutic outlook for fundus
neovascularization disease and has improved patient vision
(9). Although anti-VEGF therapy
has shown clinical feasibility, it still faces challenges, such as
short half-life and the need for repeated injections; these
challenges increase medical risks and decrease the quality of life
of patients to a certain extent. In addition, not all patients
benefit from a single anti-VEGF medication, and some problems
remain after long-term treatment, such as the possibility of other
ocular complications (including hemorrhage and infections) or
failure to achieve the desired vision restoration. A low response
to VEGF therapy has also been associated with a reduction in the
number of photoreceptor cells. Therefore, anti-VEGF monotherapy can
notably suppress fundus neovascularization to some extent but may
not be sufficient to restore visual function in the long term
(10). Considering the
aforementioned issues, the focus of the present review is to
explore molecular research advances based on the VEGF/VEGFR2
pathway in the field of fundus neovascularization, with the aim of
providing novel treatment concepts in this field.
2. Basics of the VEGF/VEGFR2 pathway
VEGF family members and their
biological functions
The VEGF family includes five protein members:
VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factor (PlGF)
(11,12). Among them, the main role of VEGF-A
is to induce endothelial cell activation and increase vascular
permeability by binding to its corresponding receptors (VEGFR1 and
VEGFR2) (13). VEGF-A has several
isoforms, such as VEGF111, VEGF121, VEGF145, VEGF165, VEGF189 and
VEGF206. For example, VEGF165 was the first isoform to be
identified and is considered to be the classic pro-angiogenic
VEGF-A subtype (14). VEGF-B has
potential for the treatment of coronary artery disease and heart
failure. Different from other proangiogenic factors of the VEGF
family, in the heart, VEGF-B has the highest expression in
cardiomyocytes and is involved in cardiac remodeling after
myocardial infarction. Thus, VEGF-B regulates myocardial
contraction and metabolism, and VEGF-B exerts cardioprotective
effects, protecting cardiomyocytes from ischemia through
physiological hypertrophy, Specifically, VEGF-B may activate the
downstream akt/mtorc1 pathway to mediate physiological hypertrophy
(15). VEGF-C has structural
homology with VEGF-D and serves a major role in lymphangiogenesis
(16). PLGF can bind to VEGFR1,
thereby preventing VEGF from binding to VEGFR2. It is also a factor
that promotes abnormal angiogenesis in the retina and subretina
(11).
Structure and function of VEGF
receptors
VEGFR2 is a tyrosine kinase receptor that consists
mainly of an extracellular, a transmembrane and an intracellular
structural domain (17). The
extracellular domain has seven immunoglobulin homologous structural
domain repeats (18). The
intracellular domain consists of a kinase structural domain
including several tyrosine residues, and VEGF binding to VEGFR2
leads to the phosphorylation of the tyrosine residues and
activation of the kinase domain. The key phosphorylation sites
include Y1054 and Y1059, whereas the coreceptor neuropilin-1
(NRP-1) is involved in developmental angiogenesis by binding to
VEGFR2 (19-21).
In addition, phosphorylation of VEGFR2 can activate signaling
pathways, such as those promoting endocytosis of the key EC
adhesion molecule vascular endothelial (VE)-cadherin, leading to
increased vascular permeability (22). The phosphorylation site Y949 of
VEGFR2 is targeted to limit vascular permeability in retinopathy
through downstream signaling. When the VEGFR2 Y949 signaling
pathway is impaired, phosphorylation of the vascular endothelial
VE-cadherin Y685 site is reduced. These results indicate that
targeting VEGFR2-regulated VE-cadherin phosphorylation may inhibit
edema (23). VEGFR1 and VEGFR3
also contain the same three domains as VEGFR2, but their biological
functions are different. VEGFR1 can act as a decoy receptor to
limit VEGFA/VEGFR2 activity in the physiological environment, while
VEGFR3 mainly binds VEGF-C/VEGF-D to regulate the formation of
lymphatic vessels (4). A graphical
representation of VEGF and its receptors is illustrated in Fig. 1.
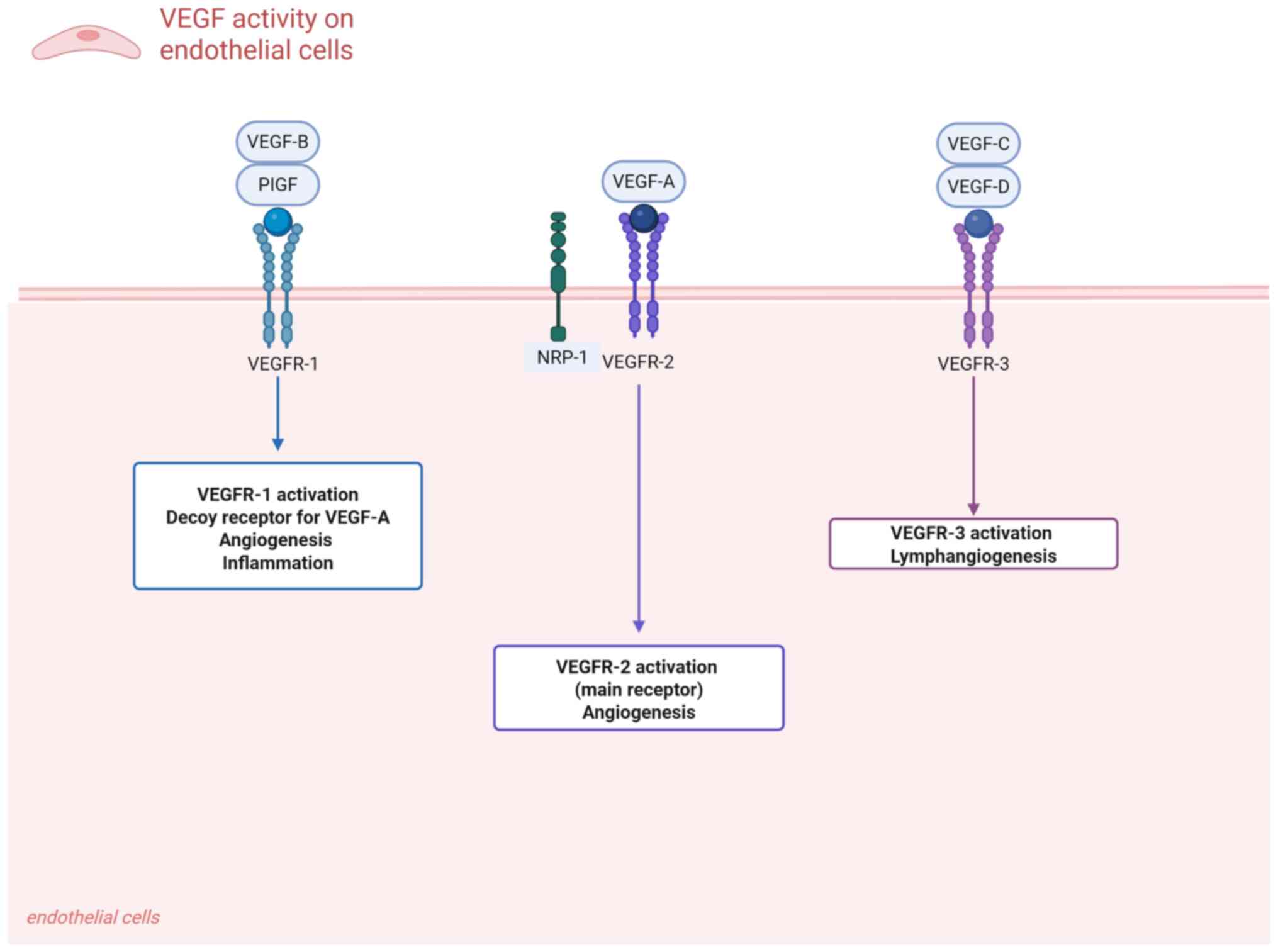 | Figure 1Schematic representation of VEGF
signaling and receptor interactions. VEGF family members, including
VEGF-A, VEGF-B, VEGF-C, VEGF-D and PlGF, mediate key angiogenic,
inflammatory and lymphangiogenic processes through binding to their
cognate receptors VEGFR-1, VEGFR-2 and VEGFR-3. Notably, the
coreceptor NRP-1 forms functional complexes with VEGFR-2,
amplifying downstream signal transduction during pathological
angiogenesis. PlGF, placental growth factor; NRP-1,
neuropilin-1. |
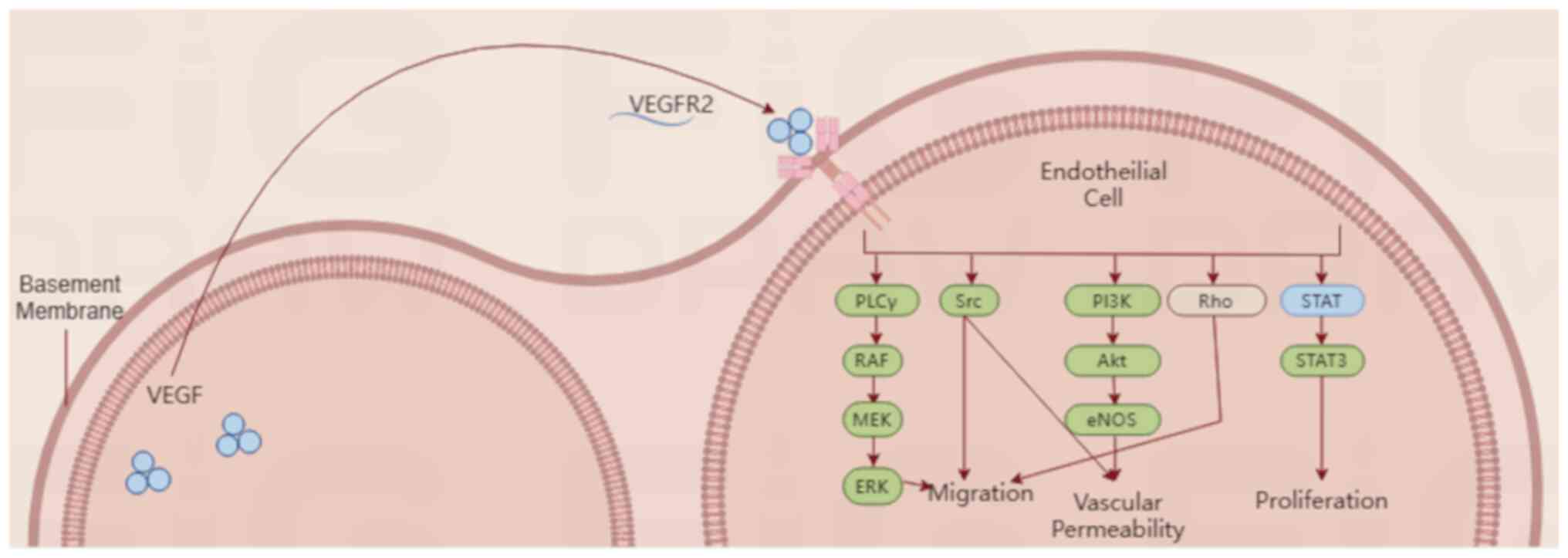
Overview of the VEGF/VEGFR2 signaling
pathway
VEGF and its receptor VEGFR play a role not only in
normal physiological blood vessel growth but also in pathological
angiogenesis, tumor growth and metastasis (24,25).
VEGF usually regulates angiogenesis by binding to VEGFR1 or VEGFR2.
Notably, VEGF mainly binds to VEGFR2, which has more potent
proangiogenic activity and higher tyrosine kinase activity than
VEGFR1(26), whereas VEGFR1 is
more commonly involved in inflammatory pathways (27).
VEGF binds to VEGFR2 and activates downstream
signaling, including the PI3K, Akt, Ras and MAPK pathways, to
promote cell proliferation, migration and differentiation (28). Among them, proliferation and
migration occur in ECs, thereby regulating vascular patterns by
controlling δ-notch signaling (29). VEGF also protects neurons from
ischemia via VEGFR2(30), and
VEGFR2 deficiency results in abnormal neuronal angiogenesis
(31).
3. Association between VEGF/VEGFR2 and
fundus neovascularization disease
Overview of major fundus
neovascularization diseases
Retinal neovascularization occurs in a group of
ischemic retinopathies resulting from damage to the retinal
vasculature, among which DR and retinal vein occlusion (RVO) are
the most common. Subretinal or choroidal neovascularization (CNV)
occurs in diseases involving the outer retina or Bruch's membrane,
and nvAMD is the most common type of such neovascularization
(32).
Overview of DR. DR is a tissue-specific
neurovascular complication caused by type 1 and type 2 diabetes
mellitus. It is estimated that 440 million individuals will have
diabetes mellitus by 2030, and DR affects ~29% of diabetic patients
(33). The two main categories are
the PDR and non-PDR types. The pathophysiological changes in DR
include several aspects of neurodegeneration, inflammation and
oxidative stress. Current treatments for DR, including anti-VEGF
therapy, steroids, laser photocoagulation and vitrectomy, have
limitations and side effects such as visual acuity and visual field
damage, and new therapeutic strategies need to be explored
(34). It has been shown that only
laser photocoagulation and anti-VEGF injections are effective
treatments in cases of severe retinopathy and that traditional
Chinese medicine may be promising in reducing VEGF levels,
inflammation, oxidative stress, apoptosis and angiogenesis in DR
(35). For example, control of
late glycosylation by intraocular injection of genipin may be a
strategy to prevent retinopathy (36).
Overview of RVO. RVO is one of the most
common retinal vascular diseases (37). Obstruction of the main retinal
veins is known as central RVO (CRVO), and obstruction of the
smaller veins is known as branch RVO (BRVO). The prevalence rates
of CRVO and BRVO are 0.1-0.4 and 0.6-1.2%, respectively, worldwide
(38). The pathogenesis of RVO is
multifactorial and involves complex interactions between multiple
vascular and inflammatory mediators. VEGF is a potent mediator of
vascular permeability and inflammation and plays a central role in
the pathogenesis of RVO; additionally, several cytokines, including
IL-6, IL-8 and C-C motif chemokine 2 (CCL2) (39,40),
have been reported to be involved.
nvAMD. nvAMD is an important cause of vision
loss in elderly individuals, the worldwide prevalence of the
disease is 8.7% (41) and it
mainly affects the deeper retinal layers of the macula and the
surrounding choroidal system. nvAMD pathogenesis is closely related
to age, oxidative stress and lipid metabolism. The molecular
pathways of macular neovascularization include angiogenesis and
arteriogenesis. For example, platelet-derived growth factor (PDGF),
angiopoietin (Ang)-1 and Ang-2 play crucial roles in regulating
angiogenesis and influencing vascular growth, maturation and
stabilization, and VEGF is an important factor in the development
of CNV and retinal leakage (42,43).
VEGF/VEGFR2 expression and its role in
neovascular disease. DR
Key pathological processes in DR include impaired
capillary perfusion and subsequent tissue ischemia; these processes
can lead to retinal microinfarcts and collateral vessel formation,
in which VEGF-A promotes neovascularization by activating VEGFR2
(44,45). Unlike physiological processes where
vascular growth is balanced by anti-angiogenic factors, in the
pathological process of DR, pathological vascular structural
disturbances caused by pericyte loss are associated with high VEGF
expression (46). VEGFR2 is highly
expressed in diabetic microvessels; therefore, it may be used as a
biomarker for the early diagnosis of DR. For example, a recent
study revealed that the application of VEGFR2 nanoprobes for in
vivo detection may have translational potential in the early
diagnosis of DR (47).
nvAMD. nvAMD is characterized by CNV, in
which the VEGF/VEGFR2 pathway is involved. Choroidal ECs secrete
CCL2, which attracts macrophages to CNV lesions and promotes
macrophage M1 polarization; additionally, M1-type
macrophages/microglia secrete IL-6 and TNF-α, which synergize with
VEGF to promote pathological angiogenesis and contribute to CNV
development (48,49). Although intravitreal injection of
anti-VEGF drugs has become the first-line treatment for nvAMD, this
treatment has a number of drawbacks, including the need for
repeated injections, poor or no response in some patients and
complications such as retinal fibrosis (41) Therefore, in addition to the
traditional VEGF/VEGFR2 pathway-targeted therapy, VEGF-C and VEGF-D
signaling also plays key roles. Hypoxia-induced retinal VEGF-C
expression induces pathological retinal neovascularization to a
similar extent as VEGF-A, and VEGF-D has been shown to promote
angiogenesis in corneal angiogenesis models (50).
4. Advances in molecular mechanisms
Role of VEGF/VEGFR2 in pathological
angiogenesis
Neovascularization begins with stimulation by
hypoxia-inducible factor (HIF). Under normoxia, HIF-1α is unstable
and rapidly degraded, whereas under hypoxic conditions, HIF-1α
dimerizes with HIF-1β, which translocates to the nucleus and
activates transcription of the target gene VEGF, leading to the
production of VEGF by stromal cells; VEGF then diffuses into
pre-existing blood vessels before binding to VEGFR2(51). The binding of VEGF to VEGFR2
results in disruption of the basement membrane of ECs, which are
transformed into tip cells with high migratory potential. When tip
cells form, the activation of VEGFR2 receptors follows VEGF
gradients or other proangiogenic stimuli to direct
neovascularization (52,53).
Gene regulatory mechanisms associated
with VEGF/VEGFR2. ERK/MAPK pathways
The ERK/MAPK mechanistic signaling pathway involves
a variety of kinases, and ERK1/2 are members of the classical MAPK
cascade (54). This pathway is
associated with a variety of diseases, including several types of
cancer and cardiovascular disease (55,56).
Moreover, the ERK/MAPK pathway is among the main targets of VEGFR2
activation. The binding of VEGF-A to VEGFR2 phosphorylates
phospholipase C-γ (PLC-γ) and activates the ERK/MAPK pathway;
subsequently, adipose mesenchymal stem cells differentiate into ECs
to promote neointima formation (13,57).
Fucoxanthin may play an important role in the
prevention of neovascularization. Fucoxanthin interacts with VEGF,
thereby impairing the ability of VEGF to activate VEGFR2 and its
associated downstream signaling, namely the phosphorylation of MEK
and ERK; thus, fucoxanthin acts as an anti-angiogenic agent
(58). For specific fundus
neovascularization diseases, such as nvAMD, the search for new
therapeutic targets is particularly important, since anti-VEGF
treatment still results in poor end-stage visual acuity. Zhou et
al (59) reported that
blockade of the VEGF signaling pathway in human retinal pigment
epithelial (RPE) cells increased IL-8 secretion through the
MEK/ERK1/2 axis, whereas overactivation of the VEGF pathway
decreased IL-8 production. IL-8 expression is upregulated in hRPE
cells after VEGF signaling inhibition, which is associated with
nvAMD. Therefore, IL-8 could serve as an alternative therapeutic
target for nvAMD (59). A recent
study revealed that elevated levels of YKL-40 and VEGF and
activation of the ERK pathway were observed in the neural retina
and RPE/choroidal tissues of laser-induced CNV mice. Moreover,
after intravitreal injection of the anti-YKL-40 antibody, the
levels of YKL-40 and phosphorylated proteins in the ERK pathway
decreased; these results indicated that the anti-YKL-40 antibody
inhibited the activation of the ERK pathway. These results have
indicated that YKL-40 could be a new target for the diagnosis and
treatment of CNV (60). The
association between the ERK/MAPK pathway and VEGFR2 activation
indicates an unprecedented role for this receptor in response to
stimulation by various mechanical factors. Specifically, ERK
activation is related to the phosphorylation of VEGFR-2 at Y1175
and Y1214(61). Although the MAPK
pathway is prevalent in numerous diseases, the specificity of
VEGFR2 as a mechanoreceptor could provide unique targets and
concepts for the angiogenic process.
c-Src pathway. c-Src is a cytoplasmic
tyrosine kinase associated with cell or endosomal membranes, which
also plays a crucial role in regulating VEGF signaling. c-Src is
similarly associated with a variety of diseases, including cancer
and cognitive disorders (62,63).
c-Src directly interacts with VEGFR2 in response to VEGF
stimulation, thereby acting as a mechanotransduction protein
downstream of VEGFR-2 signaling (64). c-Src serves a role in several
cellular processes, including adhesion, motility, proliferation and
differentiation (65). In
addition, c-Src activity is regulated, at least in part, by
mechanical factors such as shear stress and matrix adhesion. c-Src
lies upstream of other effectors, such as the ERK pathway, and has
been shown to phosphorylate tyrosine residues on VEGFR2, which
further activates both VEGFR2 and c-Src by creating a positive
feedback loop (66). A previous
study suggested that in the Akimba model of diabetic retinopathy,
the inhibition of c-Src family kinases with highly specific
inhibitors could be an attractive therapeutic intervention for
retinal vasculopathy (67).
Another study further demonstrated the potential relationship
between VEGFR2 and c-Src. This study indicated that PLC-γ is
induced downstream of VEGFR2 phosphorylation at Y1173 (pY1173). The
Y1173/PLC-γ/endothelial nitric oxide synthase (eNOS)/c-Src pathway
was examined in both the healthy and tumor vasculature of
VEGFR2Y1173F/+ mice; this pathway was associated with reduced PLC-γ
and eNOS activation and suppressed vascular leakage. The PLC-γ
pathway downstream of VEGFR2 py1173 can couple VEGFR2 to c-Src.
High PLC-γ expression is associated with angiogenic activity and
poor prognosis (68). Overall,
c-Src acts as a mechanotransduction factor to regulate VEGFR2
activation, which subsequently affects angiogenesis and could be a
target for future studies.
PI3K/Akt pathway. The activation of the
PI3K/Akt pathway stimulates EC proliferation, mainly by recruiting
Akt to the cell membrane and inhibiting apoptosis; however, the
activation of PLC-γ leads to the modulation of the intracellular
calcium concentration or the production of eNOS through the
activation of nuclear factor of activated T cells. This ultimately
increases vascular permeability and affects EC proliferation and
survival through the aforementioned processes (69,70).
The molecular chaperone heat shock protein 90 (HSP90) is a
promising molecular target, and HSP90 inhibitors have been
indicated to induce anti-angiogenic effects by affecting the
PI3K/Akt/eNOS signaling pathway in ECs and downregulating VEGFR2
expression (71). In another
study, a peptide (VGB3) mimicked the interaction between VEGF-B and
VEGFR1, and binding to VEGFR2 abolished the PI3K/AKT/mTOR pathway
and inhibited the proliferation and tube formation of human
umbilical vein ECs (HUVECs) (72).
Thus, inhibition of the PI3K/Akt axis by inhibitors of VEGFR2 could
reduce angiogenesis, and these inhibitors are expected to be
applied to further models of neovascular disease in the future.
Rho GTPase family pathway. The Rho family of
small GTPases are molecular switches capable of converting
extrinsic stimuli into cytoskeletal rearrangements. In vascular
ECs, Cdc42, Rac1 and RhoA control cell migration and cell-cell
junction formation downstream of angiogenic and inflammatory
cytokines. For example, in cell migration, Cdc42 leads to filopodia
formation by promoting actin linear extension. Rac1 promotes
lamellipodia formation through the WAVE2 complex and arp2/3. RhoA
regulates vascularization and permeability by promoting
actinomyosin contraction and serves an important role in EC
migration and vascular stabilization (73,74).
During angiogenesis, VEGF attracts ECs, whereas
semaphorin 3E (Sema3E) repels them. The small GTPase RhoJ plays a
multifaceted role in this EC-directed migration. In the GTP-bound
state, RhoJ interacts with the cytoplasmic structural domain of
plexin D1. RhoJ released from plexin D1 induces cell contraction
upon stimulation with Sema3E; this further mediates the
Sema3E-induced binding of plexin D1 to VEGFR2, transphosphorylation
of VEGFR2 at Y1214 and activation of p38, leading to reverse EC
migration. Upon stimulation with VEGF-A, RhoJ promotes the
formation of an all-receptor complex consisting of VEGFR2, plexin
D1 and NRP-1, which prevents the degradation of internalized VEGFR2
and maintains intracellular signaling. Upon conversion to the
GDP-bound state, RhoJ is diverted from plexin D1 to VEGFR2, which
terminates VEGFR2 signaling. As confirmed in an oxygen induced
retinopathy mouse model, RhoJ deficiency in the ECs of ischemic
retina effectively inhibits abnormal angiogenesis (75).
In addition, an increase in RhoA activity can
potentially cause anti-angiogenic effects, as shown by Hauke et
al (76). Hauke et al
(76) reported that, compared with
dominant-negative RhoA, active and wild-type RhoA markedly
inhibited EC proliferation, migration, tube formation and vascular
sprouting in vitro. In addition, active RhoA reduced
HUVEC-associated angiogenesis in vivo. These results
indicated that RhoA itself may have an anti-angiogenic effect
rather than being dependent on the RhoA/Rho-associated coiled-coil
kinase axis for its action. In another study, Katari et al
(77) focused on the
Rho/Yes-associated protein (YAP)/VEGFR2 mechanotransductional
pathway regulating the transient receptor potential cation channel
subfamily V member 4 (TRPV4) activity in the tumor
microenvironment, and identified endothelial TRPV4 as a new
alternative therapeutic target for tumor angiogenesis; however, its
role in fundus neovascularization disease is unknown, and more
studies are needed to confirm its role in vascular
normalization.
Based on the above studies, Rho GTPases could be
considered potential targets for the treatment of abnormal
angiogenesis and hyperpermeability in retinal vascular
diseases.
STAT pathway. STAT family members are
activated downstream of VEGFR2 and are particularly important for
EC survival and proliferation. Ramshekar et al (78) explored retinal endothelial STAT3 as
a downstream effector of VEGF-triggered signaling and examined its
ability to promote the development of vascularization in the
vitreous body and delay the expansion of intraretinal
vascularization. The study showed that subretinal delivery of
lentiviral vectors driven by a Cdh5 promoter-expressing short
hairpin RNA targeting STAT3 reduced vitreous vascularization but
did not prolong intraretinal vascularization; these results
indicated that VEGF-triggered activation of STAT3 in the retinal
endothelium was needed for intravitreal vasculature formation, but
not for intraretinal vascularization. In addition, Yu et al
(79) identified apatinib as a
potential target for alleviating neovascularization and fibrosis in
nvAMD; its mechanism of action was related to the inhibition of
VEGFR2 activation, which prevented angiogenesis and fibrosis
through the downregulation of STAT3 phosphorylation. In summary,
the STAT pathway plays a key role in inhibiting neovascularization,
and the discovery of new clinical inhibitors can aid in the
treatment of retinal and choroidal vascular diseases. The
downstream mediators of the VEGFR2 signaling pathway are
illustrated in Fig. 2.
5. Current treatment strategies
Anti-VEGF therapy: Drugs and their
mechanisms of action
Anti-VEGF therapy plays a crucial role in blocking
pathological angiogenesis. However, a large body of clinical data
indicates that VEGF-targeted therapies may be limited by decreased
visual acuity after repeated administration, with a loss of
efficacy after the initial response (80). Patients with disease recurrence may
develop resistance to anti-VEGF therapy through unknown mechanisms.
Therefore, a single anti-VEGF therapy does not adequately prevent
intraocular neovascularization, and other new molecular pathways
need to be targeted to further improve or effectively complement
the anti-VEGF effect. The following is an overview of the main
current therapeutic agents.
Bevacizumab. Bevacizumab
(Avastin®) is a humanized anti-VEGF antibody developed
by Genentech, Inc., which inhibits VEGF signaling by binding to
VEGF-A, VEGF-C and VEGF-D and preventing their interaction with
VEGFR2(81). Bevacizumab has been
reported to be effective in treating nvAMD when it is administered
intravitreally. Bevacizumab is also less costly than other drugs,
such as ranibizumab, pegaptanib and aflibercept, and is effective
in the long-term treatment of nvAMD (82,83).
However, patients whose eyes were treated with aflibercept were
almost three times more likely to stop treatment after 1 year than
patients whose eyes were treated with bevacizumab (43 vs. 15%).
These results reveal the superiority of aflibercept over
bevacizumab and have important clinical implications for treatment
selection in patients with nvAMD (84). Therefore, more prospective studies
focusing on the comparative impact and efficacy of anti-VEGF
therapeutics on neovascularization diseases are needed.
Despite some of the clinical benefits of anti-VEGF
therapy, monthly injections may lead to numerous ocular
complications, including hemorrhage, infection and endophthalmitis.
Reddy et al (85)
determined the efficacy of mesenchymal stem cell-derived small
extracellular vesicles (MSC-sEVs) loaded with the anti-VEGF drug
bevacizumab by measuring the efficacy of this drug in a rat model,
and the results indicated that bevacizumab-loaded MSC-sEVs reduced
the frequency of intravitreal injections needed to treat DR. The
reduction in the frequency of injections may reduce ocular
complications and improve patient compliance, providing the
possibility of improved patient treatment.
Ranibizumab. Ranibizumab (sold under the
brand name Lucentis® and developed by Genentech,
Inc./Novartis AG) is a recombinant humanized monoclonal fragment
antigen-binding (Fab) region that targets VEGF. Like bevacizumab,
ranibizumab prevents the activation of VEGFR1 and VEGFR2 by binding
with high affinity to all VEGF isoforms, thereby inhibiting
angiogenesis (86).
For different diseases, ranibizumab and aflibercept
have different effects. Because the differences between the effects
of aflibercept and ranibizumab on the choroid of patients with
BRVO-macular edema (ME) are not known, Kishishita et al
(87) included 36 patients with
BRVO-ME who were treated with intravitreal injections of
aflibercept or ranibizumab and observed changes in the choroidal
thickness of the subcentral concave area for a follow-up period of
12 months or longer. The results revealed that the effects of
ranibizumab and aflibercept on choroidal thickness in patients with
BRVO-ME were the same. However, in another study, ranibizumab
provided a complementary solution to the poor outcome of
aflibercept treatment. In patients with nvAMD who were >50 years
old and exhibited an inadequate response to aflibercept, treatment
with ranibizumab for 6 months improved visual acuity (88). To investigate the advantages and
disadvantages of the injection modalities reported in clinical
trials, a 2-year study by Debourdeau et al (89) examined 3,313 eyes with nvAMD; 1,243
eyes were categorized as ‘pro re nata’ (PRN) and 2,070 eyes were
initiated on the ‘treat-and-extend’ (T&E) program. The PRN
protocol applies to regular monthly visits after the lesion has
become quiescent, while the T&E protocol adds treatment
intervals after stabilization of the lesion to maintain therapeutic
stability. After treatment with ranibizumab and aflibercept, eyes
treated with the T&E program had better visual acuity (VA)
outcomes compared with eyes treated with PRN.
Owing to the high cost of current anti-VEGF
treatments, patients with nvAMD require frequent intravitreal
injections for optimal visualization, which may lead to
undertreatment for patients with a high financial burden. As a
result, the ranibizumab port delivery system (PDS) was developed,
and Eichenbaum et al (90)
proposed surgical implantation of this refillable device in the
vitreous cavity, which would enable the sustained release of
ranibizumab. Although ranibizumab is a widely used anti-VEGF drug,
the risk of adverse events and safety as well as the efficacy of
PDS need to be further investigated. A study by Lowater et
al (91) revealed that
patients receiving PDS had a high incidence of adverse events,
including 25% experiencing vitreous hemorrhage and 20% experiencing
hyphemia. Future studies include further improving the therapeutic
safety of PDS. An ongoing clinical trial (92) is further exploring the potential of
PDS with ranibizumab. Carlà et al (92) reported that the level of clinical
efficacy (the incidence of adverse reactions was determined
according to the examination of the eyes of the patients) of
ranibizumab given continuously with PDS was comparable to the
efficacy of the IVI treatment in nvAMD. However, a high incidence
of adverse effects remains; vitreous hemorrhage occurred in 68% of
patients and endophthalmitis occurred in 18% of patients.
Therefore, future studies are needed to better define the long-term
efficacy of PDS and improve patients' vision.
Aflibercept. Aflibercept was developed by
Regeneron Pharmaceuticals, Inc (93). It is a recombinant fusion protein
that fuses the extracellular immunoglobulin-like (Ig) structural
domain 2 of VEGFR1 and the extracellular Ig structural domain 3 of
VEGFR2 to the fragment crystallizable (Fc) portion of human IgG1;
this process enables stronger binding of aflibercept to VEGF-A and
VEGF-B than the previously used ranibizumab or bevacizumab
(94). Aflibercept was approved
for the treatment of AMD in 2011 and has subsequently been used to
treat certain DRs (95).
For the treatment of AMD, Cao et al (84) examined 122 eyes of 106 patients
with nvAMD who received injections of aflibercept for 3 consecutive
months (n=70) or bevacizumab (n=52), followed by a
treatment-extension interval regimen. The results revealed that
patients whose eyes were treated with aflibercept were three times
more likely to discontinue treatment after 1 year compared with
those whose eyes were treated with bevacizumab (43 vs. 15%). These
observations revealed that aflibercept was superior to bevacizumab
and had important clinical implications for patient treatment
selection. In another study, Kucukevcilioglu et al (96) focused on a multicenter comparison
of the 24-month efficacy of ranibizumab, aflibercept and the
ranibizumab-aflibercept switch. Their results indicated that visual
outcomes, including VA and central macular thickness, were similar
in non-switchers (aflibercept and ranibizumab groups) and switchers
(from ranibizumab to aflibercept) after 2 years of follow-up;
moreover, patients that received aflibercept required fewer
injections, office visits or additional treatments and the
treatment was beneficial for patients whose families were not
financially stable. To investigate the effects of different
anti-VEGF drugs on AMD, Kanadani et al (97) utilized the T&E protocol in an
observational study of 131 patients with exudative nvAMD and
compared four anti-VEGF drugs: Ranibizumab, aflibercept,
bevacizumab and aflibercept. The study revealed that intravitreal
aflibercept administration resulted in better visual and anatomical
improvements in the T&E protocol, with notably fewer
injections, compared with the other drugs tested. However, large
multicenter randomized clinical trials with longer follow-up
periods are needed to assess whether this therapeutic route is
effective and whether it provides better results in the treatment
of retinal vascular diseases.
Faricimab. The Ang/Tie pathway plays an
important role in maintaining vascular stability and synergizing
with VEGF. However, under pathological conditions, Ang-2 and VEGF-A
synergistically can cause vascular leakage and neoangiogenesis. In
addition, Ang-2 can increase vascular permeability by activating
FAK phosphorylation via β1 integrins, inducing cytoskeletal
remodeling and pericyte loss (98). Faricimab is a bispecific
anti-VEGF/Ang-2 antibody and exhibits improved vascular stability
and reduced retinal inflammation compared with single anti-VEGF
therapy (99). To evaluate the
safety and efficacy of faricimab, Khanani et al (100) conducted a phase III clinical
trial for the treatment of nvAMD, and concluded that a fixed dosing
regimen every 16 weeks was required in the first year based on the
patients' disease activity, while this was incorporated into the
second year in a personalized treatment interval (PTI) format. The
PTI approach aims to reduce patient burden by customizing treatment
intervals to meet the needs of individual patients. Moreover, Heier
et al (101) demonstrated
that extended treatment intervals with faricimab (every 16 weeks)
reduced the patient burden of treatment compared with aflibercept,
which inhibited the VEGF pathway alone and was given every 8 weeks,
and provided visual benefit for patients with nvAMD and diabetic
macular edema. To assess ocular anatomical and functional outcomes
in patients with nvAMD treated with vitreous faricimab, Pandit
et al (102) examined the
central fovea thickness (CFT), maximal fiber-vascular pigment
epithelial detachment (fvPED) and Snellen visual acuity and
compared them with those of patients who were previously switched
to faricimab after other anti-VEGF treatments. The proportion of
hydrops in the retina before conversion was 36.7%, which decreased
to 24.8% after conversion. The proportion of eyes with subretinal
fluid was 53.2% before conversion and decreased to 26.6% after
conversion. The results revealed that vitreous faricimab improved
anatomical outcomes compared with those of patients with previously
treated nvAMD while maintaining visual acuity in the short term.
Moreover, Szigiato et al (103) similarly observed the short-term
efficacy of faricimab and evaluated the CFT, fvPED, subretinal
fluid and intraretinal fluid of patients; their results indicated a
notable reduction in CFT and fvPED and stabilization of visual
acuity, as well as a better visual outcome, occurred in patients
that switched to faricimab after previous anti-VEGF therapy.
However, long-term clinical studies are needed to confirm the
long-term efficacy of faricimab. A comparison of different
anti-VEGF drugs is presented in Table
I (104-106).
 | Table IComparison of different anti-VEGF
drugs. |
Table I
Comparison of different anti-VEGF
drugs.
| Name | Target | Initial treatment
frequency | Maintenance
treatment frequency | Adverse events |
|---|
| Bevacizumab | VEGF-A | Weekly (first 4
injections) | Extended intervals
(4-8 weeks) | 9% intraocular
pressure elevation; 4% vitreous hemorrhage |
| Ranibizumab | VEGFR2 | Monthly (extended
after 3 injections) | T&E | 11% intraocular
pressure elevation; 3% vitreous hemorrhage |
| Aflibercept | VEGF-A; VEGF-B;
PlGF | Monthly (3
injections) | T&E | 14% intraocular
pressure elevation; 3% conjunctivitis; 2% vitreous hemorrhage |
| Faricimab | VEGF-A; Ang-2 | Every 4 weeks
(first 4 injections) | Every 16 weeks | 3% conjunctivitis;
3.9% RPE RAP |
Research progress in VEGFR2-targeted
therapy
In gene editing technology, the creation of
dominant-negative VEGFR2 using the multifunctional prime editing
system could block aberrant retinal angiogenesis in a mouse model
of oxygen-induced retinopathy (107). Additionally, advances have been
made in the development of molecular inhibitors that target VEGFR2.
CLK inhibitors (MU1210 and T3-CLK) have been shown to reduce the
mRNA and protein expression, as well as the downstream signaling of
VEGFR2. This was partly due to the reduced activity of the
WNT/β-catenin pathway, since the activation of this pathway induced
VEGFR2 expression, whereas the knockdown of β-catenin blocked
VEGFR2 expression. Notably, no alternative splicing of VEGFR2 was
detected. Therefore, C81 may be a promising compound for the
treatment of diseases that depend on angiogenesis and inflammation,
as they impair both processes (108). Furthermore, Tang et al
(109) reported that acrizanib,
which is a small-molecule inhibitor of VEGFR2, acts by
differentially inhibiting multiple phosphorylation sites of VEGFR2
in ECs, namely Y951, Y996, Y1059, Y1175 and Y1214. Among them, the
highest (Y1214) and lowest (Y996) inhibition differed by ~2.5-fold.
This has an important impact on physiological angiogenesis and
pathological neovascularization. In summary, inhibitors developed
on the basis of VEGFR2 may attenuate angiogenesis and provide
better treatment outcomes.
Inhibiting neoangiogenesis by interfering with the
VEGF and Ang-2/Tie2-related pathways is a novel concept. Using
in vitro and in vivo experiments, Lei et al
(110) demonstrated that
5α-hydroxycostic acid (isolated from the natural plant Viburnum
vulgare) had a therapeutic effect on neovascularization in a
rat CNV model by inhibiting cell proliferation and angiogenesis.
These results indicated that 5α-hydroxycostic acid may inhibit
neovascularization by interfering with the Ang-2/Tie2-related
pathway and may be a candidate for the treatment of CNV. In
addition, Liu et al (111)
suggested that intravitreal injection of borsub, which is a
reversible proteasome inhibitor, could ameliorate CNV by
antagonizing the VEGF-A/VEGFR2 and PDGF-D/PDGF receptor-β pathways.
These results indicate that the development of multitarget
therapies may hold potential in addressing the shortcomings of
inadequate single-target therapies.
Therapeutic approaches involving gene editing are
also being developed. Zeng et al (112) depleted VEGFA using a novel
CRISPR/Cas9 system, which inhibited the proliferation, migration
and tube formation of HUVECs in vitro and ultimately
inhibited corneal neovascularization in vivo. In addition,
Huang et al (113)
performed recombinant adeno-associated virus (AAV) serotype
1-mediated CRISPR/Cas9 editing of the genomic VEGFR2 locus, which
suppressed angiogenesis in mouse models of oxygen-induced
retinopathy and laser-induced CNV. These results indicate that gene
editing may be used in the future for the treatment of fundus
diseases.
6. Challenges and future research
directions
Limitations of the current study. High
cost
The drugs bevacizumab, ranibizumab and aflibercept
are expensive and require frequent intraocular injections;
therefore, they can be a heavy burden for economically
disadvantaged families (114).
Challenges also remain in the selection of specific anti-VEGF
drugs. From a practical point of view, ophthalmologists usually
choose a specific anti-VEGF drug because it improves vision and
reduces the cost or number of injections. However, numerous
challenges remain in the treatment of patients with nvAMD because
certain anti-VEGF drugs are limited to disease-specific
reimbursement (115).
Poor visual outcomes. While it has been
demonstrated that continued injections are needed to achieve better
vision, the financial situation of the patient's family and
compliance are key factors, and financial difficulties or a lack of
compliance may lead to inadequate treatment and increased medical
risk. Both can lead to suboptimal vision outcomes (116). Subretinal fibrosis is an
end-stage sequela of nvAMD and can lead to permanent central vision
loss in patients with nvAMD. Even when adequate anti-VEGF therapy
is given, ~1/3 of patients still develop irreversible visual
impairment due to subretinal fibrosis (117). Moreover, geographic atrophy
secondary to AMD is a progressive, irreversible loss of vision
involving photoreceptors, RPE cells and choroidal capillaries, and
its progression may be more rapid in patients receiving anti-VEGF
therapy (118). In addition,
normal retinal structures may be affected by certain treatments.
For example, Zhuang et al (119) further explored the short-term
changes in the normal retinal vasculature after anti-VEGF treatment
in patients with nvAMD; their results indicated that anti-VEGF
treatment transiently affected the relatively normal retinal
vasculature, which could lead to edema of the nerve fibers on the
nasal side of the optic disc. Therefore, anti-VEGF therapy may
affect both healthy and diseased tissues of the fundus, thereby
affecting the regression of patients' vision.
Mechanisms related to drug resistance:
Changes in VEGF isoform expression and epigenetic
regulation. The VEGF isoform VEGF165 is either in a diffusible
state (one half) or bound to heparan sulfate proteoglycan on the
cell surface (the other half), whereas VEGF121 lacks a
heparin-binding domain and diffuses freely in tissues (120). Serine/arginine protein kinase 2
binds and activates its downstream target serine/arginine splicing
factor 1 (SRSF1), which increases splicing and expression of
VEGFA165(121). Another study
showed that 30 kPa extracellular matrix rigidity inhibited the
expression and nuclear accumulation of YAP to regulate the
expression of SRSF1 via runt-related transcription factor 2, which
subsequently inhibited the expression and secretion of VEGF165 in
tumor cells (122).
In epigenetic regulation, the DNA
methylation-regulated VEGFR signaling pathway may be involved in
the development of diabetic cardiovascular disease. VEGF-B
hypomethylation may be a potential biomarker for early intervention
in patients with this disease. The expression of DNA
methyltransferases, such as DNA methyltransferase (DNMT)1, DNMT3a
and DNMT3b, may serve as potential biomarkers of the anti-VEGF
diabetic ME response (123).
Urgent issues, potential research
opportunities and the application of new technologies
Since anti-VEGF therapy is limited by the low
response rates of single drugs, drugs targeting multiple
neoangiogenic pathways are required. The application of existing
anti-VEGF drugs should be optimized and prognostic biomarkers
should be evaluated to identify those that predict effective
responses. Biomarkers need to be prospectively validated in
independent randomized trials; however, novel drug discovery and
the development of multitarget therapeutics are also important. In
this context, the bis-antibody-based drug RO-101 is an ideal
candidate for the treatment of retinal diseases. In preclinical
models, RO-101 has shown similar or greater effects than current
anti-VEGF treatments, including neointimal growth abrogation with
comparable or longer half-lives. In addition, RO-101 has shown
strong binding affinity for VEGF-A and Ang-2, and is biocompatible
with retinal tissue in animal studies, indicating potential
compatibility for use in humans (124). Therefore, further studies are
expected to validate its effectiveness and value in the clinic. In
addition, a new technology has been developed in which carbon
nanodonut (CND) with a donut-like structure is synthesized using
sodium alginate (SA) and 1,8-diaminooctane (DAO) as raw materials.
In human umbilical vein endothelial cells, CND can reduce the
levels of reactive oxygen species and proinflammatory cytokines
(such as IL-6 and IL-1β) five-fold compared with bevacizumab. In
addition, CND has a strong affinity for VEGFA165, with a
dissociation constant of 2.2x10-14 M; this value is
>1,600 times stronger than that of the commercial drug
bevacizumab (Avastin®). Therefore, SA/DAO-CNDs have the
potential to be used as drugs for the treatment of various
angiogenesis-related ocular diseases (125). Patient compliance and financial
pressure are equally challenging. The high cost of anti-VEGF
therapy and the need for multiple injections have led to decreased
compliance and financial stress for several patients. However,
investigating sustained-release materials that can prolong the
duration of drug action, minimize the number of intravitreal
injections, and improve patient compliance is a viable solution to
this problem and can lead to significant therapeutic benefits. Two
extended-release materials are currently undergoing clinical trials
(NCT03677934 and NCT03953079), including PDS and GB-102. PDS mainly
targets VEGF-A, while Gb-102 is a tyrosine kinase inhibitor that
targets VEGF-A and PDGF. Their role is to reduce the burden of
frequent intravitreal injections on patients and doctors. Although
basic research indicates that extended-release drugs have strong
therapeutic applications, the preparation of drug carriers with
good compatibility with drugs, long release times and prolonged
duration of action is still a major challenge at present (126,127).
Gene therapy applied directly to the eye has also
shown potential for long-lasting drug applications. In one study,
an AAV2 vector was used to fuse a truncated form of soluble VEGFR2
to the FC portion of human IgG1. AAV2-mediated gene delivery of
sVEGFR-2-Fc was effective in inhibiting laser-induced CNV in mice
and was considered a promising tool for developing therapeutic
tools for retina-related diseases and preventing neovascularization
(128). In addition, genes
encoding anti-VEGF Fab proteins have shown potential in the
treatment of retinal diseases. RGX-314 uses a novel recombinant
AAV8 vector to induce the production of anti-VEGF Fab proteins.
Various phase II and III studies are currently underway to evaluate
the efficacy of RGX-314 in patients with nvAMD (129,130). In a study, RGX-314 (currently
known as ABBV-RGX-314) expression consistently inhibited VEGF-A and
was safe and effective in treating patients with nvAMD (131). Another type of gene therapy
involves ixoberogene soroparvovec (ixo-vec), formerly known as
ADVM-022, which is administered by intravitreal injection. This
technology utilizes AAV.7m8 as a vector to generate an
aflibercept-carrying transgene for the treatment of nvAMD, and the
phase II LUNA trial assessing the safety and efficacy of ixo-vec is
currently in progress (132).
Finally, 4D-150, which contains transgenes that target aflibercept
and VEGF-C, is currently planned to enter a phase III clinical
trial for nvAMD (133). However,
gene therapy targets, routes of administration and potential safety
issues require further validation.
Understanding the structural biology of VEGFR2 has
markedly advanced drug design. As previously described, the
complete VEGFR2 receptor comprises an extracellular region, a
transmembrane domain and an intracellular kinase domain. Insights
from ligand-binding domain and coreceptor complex structures, such
as the VEGF-VEGFR2 complex and VEGFR1/VEGFR2 extracellular fusion
region, have guided the development of therapeutic agents such as
ranibizumab and aflibercept, which are now widely used to treat
AMD. Furthermore, by targeting the catalytic structural domain,
‘DFG-out’ (type II) VEGFR2 inhibitors effectively suppress VEGFR2
phosphorylation, reducing neoangiogenesis and overcoming drug
resistance (134). Future
research directions include capturing the transient states of
VEGFR2 structural activation to design conformation-specific
inhibitors, integrating genomic, proteomic and structural data for
precision-targeted therapies and developing nanomedicines capable
of penetrating the BRB based on structural insights. Thus,
structural biology serves not only as a fundamental tool for
elucidating VEGFR2 function but also as a cornerstone for
overcoming current therapeutic limitations and advancing
next-generation ophthalmic treatment.
Single-cell RNA sequencing (scRNA-seq) technology
offers novel insights into the cellular heterogeneity and molecular
pathways of retinal diseases (135). Gene expression profiles vary
across distinct retinal cell types and disease microenvironments.
In AMD, which predominantly affects the macula, pathological
alterations are most pronounced in macular neuroglial cells,
microglia and astrocytes during disease progression. Single-cell
sequencing may reveal gene expression changes in different cell
types (136). Transcriptomic
analysis of retinal samples from patients with DR at various stages
revealed dysregulated mechanisms, including the Hippo signaling
pathway and gap junction formation (137). scRNA-seq enables the
identification of disease-specific cellular subpopulations and
facilitates the prognosis of anti-VEGF therapeutic efficacy through
cell type-specific gene expression signatures, thereby guiding
personalized treatment strategies. Future directions include
integrating single-cell assay for transposase-accessible chromatin
with high-throughput-seq (to delineate epigenetic regulation) with
the phosphoproteomic profiling of VEGFR2 activation to dissect drug
resistance mechanisms, as well as establishing comprehensive
ophthalmic single-cell databases to inform individualized clinical
decision-making.
7. Conclusion
Anti-VEGF drugs for the treatment of
neovascularization fundus diseases focus mainly on clinical
efficacy, injection regimens and safety. However, problems, such as
the use of single therapeutic targets and low response rates,
remain; additionally, the high treatment cost and economic pressure
for the patients are the root causes of decreased patient
compliance. Therefore, future studies should focus on the
development of novel anti-VEGF drugs with multiple targets and drug
delivery systems, non-invasive drug delivery, gene therapy and
artificial intelligence technology, aiming to address current
therapeutic limitations, such as reducing the number of injections,
increasing patient compliance and improving the cure rate (138). Non-invasive drug delivery and
gene therapy can be used to solve the problems of anti-VEGF
treatment complications and low response rates. Although the
current cost of gene therapy is high, with the continuous progress
of science and technology, this is expected to decrease. The
economic burden on patients may be reduced in the future through
national policies, health insurance payment systems, commercial
insurance and the possibility of patients themselves jointly
bearing medical costs. Artificial intelligence technology could be
used to screen patients, treatment outcomes can be predicted using
targeted biomarkers and diagnostic accuracy may be improved.
Acknowledgements
Not applicable.
Funding
Funding: The present work was supported by the National Natural
Science Foundation of China (grant no. 82160199).
Availability of data and materials
Not applicable.
Authors' contributions
HSL analyzed the literature and wrote the original
draft. XGH designed the study and critically revised the
manuscript. Data authentication is not applicable. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Swift MR and Weinstein BM: Arterial-venous
specification during development. Circ Res. 104:576–588.
2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vishwakarma S and Kaur I: Molecular
mediators and regulators of retinal angiogenesis. Semin Ophthalmol.
38:124–133. 2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Díaz-Coránguez M, Ramos C and Antonetti
DA: The inner blood-retinal barrier: Cellular basis and
development. Vision Res. 139:123–137. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Uemura A, Fruttiger M, D'Amore PA, De
Falco S, Joussen AM, Sennlaub F, Brunck LR, Johnson KT, Lambrou GN,
Rittenhouse KD and Langmann T: VEGFR1 signaling in retinal
angiogenesis and microinflammation. Prog Retin Eye Res.
84(100954)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Selvam S, Kumar T and Fruttiger M: Retinal
vasculature development in health and disease. Prog Retin Eye Res.
63:1–19. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hu WH, Zhang XY, Leung KW, Duan R, Dong
TT, Qin QW and Tsim KW: Resveratrol, an inhibitor binding to VEGF,
restores the pathology of abnormal angiogenesis in retinopathy of
prematurity (ROP) in mice: Application by intravitreal and topical
instillation. Int J Mol Sci. 23(6455)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yan J, Deng J, Cheng F, Zhang T, Deng Y,
Cai Y and Cong W: Thioredoxin-interacting protein inhibited
vascular endothelial cell-induced HREC angiogenesis treatment of
diabetic retinopathy. Appl Biochem Biotechnol. 195:1268–1283.
2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Heloterä H and Kaarniranta K: A linkage
between angiogenesis and inflammation in neovascular age-related
macular degeneration. Cells. 11(3453)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bakri SJ, Thorne JE, Ho AC, Ehlers JP,
Schoenberger SD, Yeh S and Kim SJ: Safety and efficacy of
anti-vascular endothelial growth factor therapies for neovascular
age-related macular degeneration: A report by the american academy
of ophthalmology. Ophthalmology. 126:55–63. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xu X, Han N, Zhao F, Fan R, Guo Q, Han X,
Liu Y and Luo G: Inefficacy of anti-VEGF therapy reflected in
VEGF-mediated photoreceptor degeneration. Mol Ther Nucleic Acids.
35(102176)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Apte RS, Chen DS and Ferrara NL: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shaw P, Dwivedi SKD, Bhattacharya R,
Mukherjee P and Rao G: VEGF signaling: Role in angiogenesis and
beyond. Biochim Biophys Acta Rev Cancer.
1879(189079)2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling - in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Peach CJ, Mignone VW, Arruda MA, Alcobia
DC, Hill SJ, Kilpatrick LE and Woolard J: Molecular pharmacology of
VEGF-A isoforms: Binding and signalling at VEGFR2. Int J Mol Sci.
19(1264)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mallick R and Ylä-Herttuala S: Therapeutic
potential of VEGF-B in coronary heart disease and heart failure:
Dream or vision? Cells. 11(4134)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wada H, Suzuki M, Matsuda M, Ajiro Y,
Shinozaki T, Sakagami S, Yonezawa K, Shimizu M, Funada J, Takenaka
T, et al: Distinct characteristics of VEGF-D and VEGF-C to predict
mortality in patients with suspected or known coronary artery
disease. J Am Heart Assoc. 9(e015761)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sarabipour S, Ballmer-Hofer K and Hristova
K: VEGFR-2 conformational switch in response to ligand binding.
Elife. 5(e13876)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Simons M, Gordon E and Claesson-Welsh L:
Mechanisms and regulation of endothelial VEGF receptor signalling.
Nat Rev Mol Cell Biol. 17:611–625. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Koch S and Claesson-Welsh L: Signal
transduction by vascular endothelial growth factor receptors. Cold
Spring Harb Perspect Med. 2(a006502)2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kendall RL, Rutledge RZ, Mao X, Tebben AJ,
Hungate RW and Thomas KA: Vascular endothelial growth factor
receptor KDR tyrosine kinase activity is increased by
autophosphorylation of two activation loop tyrosine residues. J
Biol Chem. 274:6453–6460. 1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gelfand MV, Hagan N, Tata A, Oh WJ,
Lacoste B, Kang KT, Kopycinska J, Bischoff J, Wang JH and Gu C:
Neuropilin-1 functions as a VEGFR2 co-receptor to guide
developmental angiogenesis independent of ligand binding. Elife.
3(e03720)2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gavard J and Gutkind JS: VEGF controls
endothelial-cell permeability by promoting the
beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol.
8:1223–1234. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Smith RO, Ninchoji T, Gordon E, André H,
Dejana E, Vestweber D, Kvanta A and Claesson-Welsh L: Vascular
permeability in retinopathy is regulated by VEGFR2 Y949 signaling
to VE-cadherin. Elife. 9(e54056)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shibuya M: VEGF-VEGFR signals in health
and disease. Biomol Ther (Seoul). 22:1–9. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mabeta P and Steenkamp V: The VEGF/VEGFR
axis revisited: Implications for cancer therapy. Int J Mol Sci.
23(15585)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Melincovici CS, Boşca AB, Şuşman S,
Mărginean M, Mihu C, Istrate M, Moldovan IM, Roman AL and Mihu CM:
Vascular endothelial growth factor (VEGF)-key factor in normal and
pathological angiogenesis. Rom J Morphol Embryol. 59:455–467.
2018.PubMed/NCBI
|
|
27
|
Shibuya M: VEGF-VEGFR system as a target
for suppressing inflammation and other diseases. Endocr Metab
Immune Disord Drug Targets. 15:135–144. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Patel SA, Nilsson MB, Le X, Cascone T,
Jain RK and Heymach JV: Molecular mechanisms and future
implications of VEGF/VEGFR in cancer therapy. Clin Cancer Res.
29:30–39. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shibuya M and Claesson-Welsh L: Signal
transduction by VEGF receptors in regulation of angiogenesis and
lymphangiogenesis. Exp Cell Res. 312:549–560. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jin KL, Mao XO and Greenberg DA: Vascular
endothelial growth factor: Direct neuroprotective effect in in
vitro ischemia. Proc Natl Acad Sci USA. 97:10242–10247.
2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Okabe K, Kobayashi S, Yamada T, Kurihara
T, Tai-Nagara I, Miyamoto T, Mukouyama YS, Sato TN, Suda T, Ema M
and Kubota Y: Neurons limit angiogenesis by titrating VEGF in
retina. Cell. 159:584–596. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Campochiaro PA: Molecular pathogenesis of
retinal and choroidal vascular diseases. Prog Retin Eye Res.
49:67–81. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Abu Serhan H, Taha MJJ, Abuawwad MT,
Abdelaal A, Irshaidat S, Abu Serhan L, Abu Salim QF, Awamleh N,
Abdelazeem B and Elnahry AG: Safety and efficacy of brolucizumab in
the treatment of diabetic macular edema and diabetic retinopathy: A
systematic review and meta-analysis. Semin Ophthalmol. 39:251–260.
2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang Z, Zhang N, Lin P, Xing Y and Yang N:
Recent advances in the treatment and delivery system of diabetic
retinopathy. Front Endocrinol (Lausanne).
15(1347864)2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kaur A, Kumar R and Sharma A: Diabetic
retinopathy leading to blindness-a review. Curr Diabetes Rev.
20(e240124225997)2024.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhou YM, Cao YH, Guo J and Cen LS:
Potential prospects of Chinese medicine application in diabetic
retinopathy. World J Diabetes. 15:2010–2014. 2024.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Baseline and early natural history report.
The central vein occlusion study. Arch Ophthalmol. 111:1087–1095.
1993.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Vitiello L, Lixi F, Coppola A, Abbinante
G, Gagliardi V, Salerno G, De Pascale I, Pellegrino A and
Giannaccare G: Intravitreal dexamethasone implant switch after
anti-VEGF treatment in patients affected by retinal vein occlusion:
A review of the literature. J Clin Med. 13(5006)2024.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Minaker SA, Mason RH, Bamakrid M, Lee Y
and Muni RH: Changes in aqueous and vitreous inflammatory cytokine
levels in retinal vein occlusion: A systematic review and
meta-analysis. J Vitreoretin Dis. 4:36–64. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang B, Zhang X, Chen H, Koh A, Zhao C and
Chen Y: A review of intraocular biomolecules in retinal vein
occlusion: Toward potential biomarkers for companion diagnostics.
Front Pharmacol. 13(859951)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Finocchio L, Zeppieri M, Gabai A, Toneatto
G, Spadea L and Salati C: Recent developments in gene therapy for
neovascular age-related macular degeneration: A review.
Biomedicines. 11(3221)2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cheng S, Zhang S, Huang M, Liu Y, Zou X,
Chen X and Zhang Z: Treatment of neovascular age-related macular
degeneration with anti-vascular endothelial growth factor drugs:
progress from mechanisms to clinical applications. Front Med
(Lausanne). 11(1411278)2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Fragiotta S, Bassis L, Abdolrahimzadeh B,
Marino A, Sepe M and Abdolrahimzadeh S: Exploring current molecular
targets in the treatment of neovascular age-related macular
degeneration toward the perspective of long-term agents. Int J Mol
Sci. 25(4433)2024.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Nakao S, Zandi S, Hata Y, Kawahara S,
Arita R, Schering A, Sun D, Melhorn MI, Ito Y, Lara-Castillo N, et
al: Blood vessel endothelial VEGFR-2 delays lymphangiogenesis: An
endogenous trapping mechanism links lymph- and angiogenesis. Blood.
117:1081–1090. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Duh EJ, Sun JK and Stitt AW: Diabetic
retinopathy: Current understanding, mechanisms, and treatment
strategies. JCI Insight. 2(e93751)2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mrugacz M, Bryl A and Zorena K: Retinal
Vascular endothelial cell dysfunction and neuroretinal degeneration
in diabetic patients. J Clin Med. 10(458)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhang YL, Pirmardan ER, Jiang H, Barakat A
and Hafezi-Moghadam A: VEGFR-2 adhesive nanoprobes reveal early
diabetic retinopathy in vivo. Biosens Bioelectron.
237(115476)2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Liu X, Guo A, Tu Y, Li W, Li L, Liu W, Ju
Y, Zhou Y, Sang A and Zhu M: Fruquintinib inhibits VEGF/VEGFR2 axis
of choroidal endothelial cells and M1-type macrophages to protect
against mouse laser-induced choroidal neovascularization. Cell
Death Dis. 11(1016)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Xu J, Tu Y, Wang Y, Xu X, Sun X, Xie L,
Zhao Q, Guo Y, Gu Y, Du J, et al: Prodrug of
epigallocatechin-3-gallate alleviates choroidal neovascularization
via down-regulating HIF-1α/VEGF/VEGFR2 pathway and M1 type
macrophage/microglia polarization. Biomed Pharmacother.
121(109606)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Leitch IM, Gerometta M, Eichenbaum D,
Finger RP, Steinle NC and Baldwin ME: Vascular endothelial growth
factor C and D signaling pathways as potential targets for the
treatment of neovascular age-related macular degeneration: A
narrative review. Ophthalmol Ther. 13:1857–1875. 2024.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Cui K, Liu J, Huang L, Qin B, Yang X, Li
L, Liu Y, Gu J, Wu W, Yu Y and Sang A: Andrographolide attenuates
choroidal neovascularization by inhibiting the HIF-1α/VEGF
signaling pathway. Biochem Biophys Res Commun. 530:60–66.
2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang X, Bove AM, Simone G and Ma B:
Molecular Bases of VEGFR-2-Mediated physiological function and
pathological role. Front Cell Dev Biol. 8(599281)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Gomes E and Rockwell P: p38 MAPK as a
negative regulator of VEGF/VEGFR2 signaling pathway in serum
deprived human SK-N-SH neuroblastoma cells. Neurosci Lett.
431:95–100. 2008.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hendrikse CSE, Theelen PMM, van der Ploeg
P, Westgeest HM, Boere IA, Thijs AMJ, Ottevanger PB, van de Stolpe
A, Lambrechts S, Bekkers RLM and Piek JMJ: The potential of
RAS/RAF/MEK/ERK (MAPK) signaling pathway inhibitors in ovarian
cancer: A systematic review and meta-analysis. Gynecol Oncol.
171:83–94. 2023.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Gallo S, Vitacolonna A, Bonzano A,
Comoglio P and Crepaldi T: ERK: A key player in the pathophysiology
of cardiac hypertrophy. Int J Mol Sci. 20(2164)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Almalki SG and Agrawal DK: ERK signaling
is required for VEGF-A/VEGFR2-induced differentiation of porcine
adipose-derived mesenchymal stem cells into endothelial cells. Stem
Cell Res Ther. 8(113)2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Guo GX, Qiu YH, Liu Y, Yu LL, Zhang X,
Tsim KW, Qin QW and Hu WH: Fucoxanthin attenuates angiogenesis by
blocking the VEGFR2-mediated signaling pathway through binding the
vascular endothelial growth factor. J Agric Food Chem.
72:21610–21623. 2024.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zhou LB, Zhou YQ and Zhang XY: Blocking
VEGF signaling augments interleukin-8 secretion via MEK/ERK/1/2
axis in human retinal pigment epithelial cells. Int J Ophthalmol.
13:1039–1045. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Bin Y, Liu YY, Jiang SQ and Peng H:
Elevated YKL-40 serum levels may contribute to wet age-related
macular degeneration via the ERK1/2 pathway. FEBS Open Bio.
11:2933–2942. 2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Miller B and Sewell-Loftin MK:
Mechanoregulation of vascular endothelial growth factor receptor 2
in angiogenesis. Front Cardiovasc Med. 8(804934)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Jin H, Ko YS, Yun SP, Park SW and Kim HJ:
P2Y(2)R-mediated transactivation of VEGFR2 through Src
phosphorylation is associated with ESM-1 overexpression in
radiotherapy-resistant-triple negative breast cancer cells. Int J
Oncol. 62(73)2023.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Gao X, Chen J, Yin G, Liu Y, Gu Z, Sun R,
Sun X, Jiao X, Wang L, Wang N, et al: Hyperforin ameliorates
neuroinflammation and white matter lesions by regulating microglial
VEGFR(2)/SRC pathway in vascular cognitive impairment mice. CNS
Neurosci Ther. 30(e14666)2024.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Chen TT, Dong JL, Zhou HY, Deng X, Li R,
Chen N, Luo M, Li Y, Wu J and Wang L: Glycation of fibronectin
inhibits VEGF-induced angiogenesis by uncoupling VEGF
receptor-2-c-Src crosstalk. J Cell Mol Med. 24:9154–9164.
2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Moysenovich AM, Tatarskiy VV, Yastrebova
MA, Bessonov IV, Arkhipova AY, Kolosov AS, Davydova LI,
Khamidullina AI, Bogush VG, Debabov VG, et al: Akt and Src mediate
the photocrosslinked fibroin-induced neural differentiation.
Neuroreport. 31:770–775. 2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Rezzola S, Di Somma M, Corsini M, Leali D,
Ravelli C, Polli VAB, Grillo E, Presta M and Mitola S: VEGFR2
activation mediates the pro-angiogenic activity of BMP4.
Angiogenesis. 22:521–533. 2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Sergeys J, Van Hove I, Hu TT, Temps C,
Carragher NO, Unciti-Broceta A, Feyen JHM, Moons L and Porcu M: The
retinal tyrosine kinome of diabetic Akimba mice highlights
potential for specific Src family kinase inhibition in retinal
vascular disease. Exp Eye Res. 197(108108)2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Sjöberg E, Melssen M, Richards M, Ding Y,
Chanoca C, Chen D, Nwadozi E, Pal S, Love DT, Ninchoji T, et al:
Endothelial VEGFR2-PLCγ signaling regulates vascular permeability
and antitumor immunity through eNOS/Src. J Clin Invest.
133(e161366)2023.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Yang L, Guan H, He J, Zeng L, Yuan Z, Xu
M, Zhang W, Wu X and Guan J: VEGF increases the proliferative
capacity and eNOS/NO levels of endothelial progenitor cells through
the calcineurin/NFAT signalling pathway. Cell Biol Int. 36:21–27.
2012.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Huang TF, Wang SW, Lai YW, Liu SC, Chen
YJ, Hsueh TM, Lin CC, Lin CH and Chung CH: 4-Acetylantroquinonol B
suppresses prostate cancer growth and angiogenesis via a
VEGF/PI3K/ERK/mTOR-dependent signaling pathway in subcutaneous
xenograft and in vivo angiogenesis models. Int J Mol Sci.
23(1446)2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Qi S, Deng S, Lian Z and Yu K: Novel drugs
with high efficacy against tumor angiogenesis. Int J Mol Sci.
23(6934)2022.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Namjoo M, Ghafouri H, Assareh E, Aref AR,
Mostafavi E, Hamrahi Mohsen A, Balalaie S, Broussy S and Asghari
SM: A VEGFB-Based peptidomimetic inhibits VEGFR2-Mediated
PI3K/Akt/mTOR and PLCγ/ERK signaling and elicits apoptotic,
antiangiogenic, and antitumor activities. Pharmaceuticals (Basel).
16(906)2023.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Uemura A and Fukushima Y: Rho GTPases in
retinal vascular diseases. Int J Mol Sci. 22(3684)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Claesson-Welsh L and Welsh M: VEGFA and
tumour angiogenesis. J Intern Med. 273:114–127. 2013.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Fukushima Y, Nishiyama K, Kataoka H,
Fruttiger M, Fukuhara S, Nishida K, Mochizuki N, Kurihara H,
Nishikawa SI and Uemura A: RhoJ integrates attractive and repulsive
cues in directional migration of endothelial cells. EMBO J.
39(e102930)2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Hauke M, Eckenstaler R, Ripperger A, Ender
A, Braun H and Benndorf RA: Active RhoA exerts an inhibitory effect
on the homeostasis and angiogenic capacity of human endothelial
cells. J Am Heart Assoc. 11(e025119)2022.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Katari V, Dalal K, Adapala RK, Guarino BD,
Kondapalli N, Paruchuri S and Thodeti CK: A TRP to pathological
angiogenesis and vascular normalization. Compr Physiol.
14:5389–5406. 2024.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Ramshekar A, Bretz CA and Hartnett ME:
RNA-Seq Provides Insights into VEGF-Induced signaling in human
retinal microvascular endothelial cells: Implications in
retinopathy of prematurity. Int J Mol Sci. 23(7354)2022.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Yu E, Kim H, Park H, Hong JH, Jin J, Song
Y, Woo JM, Min JK and Yun J: Targeting the VEGFR2 signaling pathway
for angiogenesis and fibrosis regulation in neovascular age-related
macular degeneration. Sci Rep. 14(25682)2024.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Hara C, Wakabayashi T, Fukushima Y,
Sayanagi K, Kawasaki R, Sato S, Sakaguchi H and Nishida K:
Tachyphylaxis during treatment of exudative age-related macular
degeneration with aflibercept. Graefes Arch Clin Exp Ophthalmol.
257:2559–2569. 2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Ribatti D, Solimando AG and Pezzella F:
The Anti-VEGF(R) drug discovery legacy: Improving attrition rates
by breaking the vicious cycle of angiogenesis in cancer. Cancers
(Basel). 13(3433)2021.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Estarreja J, Mendes P, Silva C, Camacho P
and Mateus V: The efficacy, safety, and efficiency of the off-label
use of bevacizumab in patients diagnosed with age-related macular
degeneration: Protocol for a systematic review and meta-analysis.
JMIR Res. 12(e38658)2023.PubMed/NCBI View
Article : Google Scholar
|
|
83
|
Siktberg J, Kim SJ, Sternberg P Jr and
Patel S: Effectiveness of bevacizumab step therapy for neovascular
age-related macular degeneration. EYE (Lond). 37:1844–1849.
2023.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Cao X, Sanchez JC, Patel TP, Yang ZY, Guo
CY, Malik D, Sopeyin A, Montaner S and Sodhi A: Aflibercept more
effectively weans patients with neovascular age-related macular
degeneration off therapy with bevacizumab. J Clin Invest.
133(e159125)2023.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Reddy SK, Ballal AR, Shailaja S, Seetharam
RN, Raghu CH, Sankhe R, Pai K, Tender T, Mathew M and Aroor A: ,
et al: Small extracellular vesicle-loaded bevacizumab
reduces the frequency of intravitreal injection required for
diabetic retinopathy. Theranostics. 13:2241–2255. 2023.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Fong AH and Lai TY: Long-term
effectiveness of ranibizumab for age-related macular degeneration
and diabetic macular edema. Clin Interv Aging. 8:467–482.
2013.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Kishishita S, Sakanishi Y, Morita S,
Matsuzawa M, Usui-Ouchi A and Ebihara N: Effects of intravitreal
injection of ranibizumab and aflibercept for branch retinal vein
occlusion on the choroid: A retrospective study. BMC Ophthalmol.
22(458)2022.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Rouvas A, Datseris I, Androudi S,
Tsilimbaris M, Kabanarou SA, Pharmakakis N, Koutsandrea C, Charonis
A, Kousidou O and Pantelopoulou G: A real-world, multicenter,
6-month prospective study in greece of the effectiveness and safety
of ranibizumab in patients with age-related macular degeneration
who have inadequately responded to aflibercept: The ‘ELEVATE’
study. Clin Ophthalmol. 16:2579–2593. 2022.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Debourdeau E, Beylerian H, Nguyen V,
Barthelmes D, Gillies M, Gabrielle PH, Vujosevic S, Otoole L, Puzo
M, Creuzot-Garcher C, et al: Treat-and-Extend Versus Pro re nata
regimens of ranibizumab and aflibercept in neovascular age-related
macular degeneration: A comparative study from routine clinical
practice. Ophthalmol Ther. 13:2343–2355. 2024.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Eichenbaum DA, Ahmed A and Hiya F:
Ranibizumab port delivery system: A clinical perspective. BMJ Open
Ophthalmol. 7(e001104)2022.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Lowater SJ, Grauslund J, Subhi Y and
Vergmann AS: Clinical trials and future outlooks of the port
delivery system with ranibizumab: A narrative review. Ophthalmol
Ther. 13:51–69. 2023.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Carlà MM, Savastano MC, Boselli F,
Giannuzzi F and Rizzo S: Ranibizumab port delivery system in
neovascular age-related macular degeneration: Where do we stand?
Overview of pharmacokinetics, clinical results, and future
directions. Pharmaceutics. 16(314)2024.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Sharma T, Dhingra R, Singh S, Sharma S,
Tomar P, Malhotra M and Bhardwaj TR: Aflibercept: A novel VEGF
targeted agent to explore the future perspectives of
anti-angiogenic therapy for the treatment of multiple tumors. Mini
Rev Med Chem. 13:530–540. 2013.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Baybora H: Perifoveal retinal thickness
changes after intravitreal aflibercept injection for choroidal
neovascularization in age-related macular degeneration.
Photodiagnosis Photodyn Ther. 46(104028)2024.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Heier JS, Brown DM, Chong V, Korobelnik
JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos
GD, et al: Intravitreal aflibercept (VEGF trap-eye) in wet
age-related macular degeneration. Ophthalmology. 119:2537–2548.
2012.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Kucukevcilioglu M, Yesiltas YS, Durukan
AH, Unlu N, Onen M, Alp MN, Kalayci D, Acar MA, Sekeroglu MA,
Citirik M, et al: Real life multicenter comparison of 24-month
outcomes of anti-VEGF therapy in diabetic macular Edema in Turkey:
Ranibizumab vs. aflibercept vs. ranibizumab-aflibercept switch.
Medicina (Kaunas). 59(263)2023.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Kanadani T, Rabelo N, Takahashi D,
Magalhaes L and Farah M: Comparison of antiangiogenic agents
(ranibizumab, aflibercept, bevacizumab and ziv-aflibercept) in the
therapeutic response to the exudative form of age-related macular
degeneration according to the treat-and-extend protocol-true
head-to-head study. Int J Retina Vitreous. 10(13)2024.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Joussen AM, Ricci F, Paris LP, Korn C,
Quezada-Ruiz C and Zarbin M: Angiopoietin/Tie2 signalling and its
role in retinal and choroidal vascular diseases: A review of
preclinical data. Eye (Lond). 35:1305–1316. 2021.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Foxton RH, Uhles S, Grüner S, Revelant F
and Ullmer C: Efficacy of simultaneous VEGF-A/ANG-2 neutralization
in suppressing spontaneous choroidal neovascularization. EMBO Mol
Med. 11(e10204)2019.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Khanani AM, Guymer RH, Basu K, Boston H,
Heier JS, Korobelnik JF, Kotecha A, Lin H, Silverman D, Swaminathan
B, et al: TENAYA and LUCERNE: Rationale and design for the phase 3
clinical trials of faricimab for neovascular age-related macular
degeneration. Ophthalmol Sci. 1(100076)2021.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Heier JS, Khanani AM, Quezada Ruiz C, Basu
K, Ferrone PJ, Brittain C, Figueroa MS, Lin H, Holz FG, Patel V, et
al: Efficacy, durability, and safety of intravitreal faricimab up
to every 16 weeks for neovascular age-related macular degeneration
(TENAYA and LUCERNE): Two randomised, double-masked, phase 3,
non-inferiority trials. Lancet. 399:729–740. 2022.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Pandit SA, Momenaei B, Wakabayashi T,
Mansour HA, Vemula S, Durrani AF, Pashaee B, Kazan AS, Ho AC,
Klufas M, et al: Clinical outcomes of faricimab in patients with
previously treated neovascular age-related macular degeneration.
Ophthalmol Retina. 8:360–366. 2024.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Szigiato A, Mohan N, Talcott KE, Mammo DA,
Babiuch AS, Kaiser PK, Ehlers JP, Rachitskaya A, Yuan A, Srivastava
SK and Sharma S: Short-term outcomes of faricimab in patients with
neovascular age-related macular degeneration on prior anti-VEGF
Therapy. Ophthalmol Retina. 8:10–17. 2024.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Grimaldi G, Cancian G, Rizzato A, Casanova
A, Perruchoud-Ader K, Clerici M, Consigli A and Menghini M:
Intravitreal faricimab for neovascular age-related macular
degeneration previously treated with traditional anti-VEGF
compounds: A real-world prospective study. Graefes Arch Clin Exp
Ophthalmol. 262:1151–1159. 2024.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Mori R, Honda S, Gomi F, Tsujikawa A,
Koizumi H, Ochi H, Ohsawa S and Okada AA: TENAYA and LUCERNE
Investigators. Efficacy, durability, and safety of faricimab up to
every 16 weeks in patients with neovascular age-related macular
degeneration: 1-year results from the Japan subgroup of the phase 3
TENAYA trial. Jpn J Ophthalmol. 67:301–310. 2023.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Diabetic Retinopathy Clinical Research
Network. Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP,
Antoszyk AN, Arnold-Bush B, Baker CW, Bressler NM, et al:
Aflibercept, bevacizumab, or ranibizumab for diabetic macular
edema. N Engl J Med. 372:1193–1203. 2015.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Huang X, Wu W, Qi H, Yan X, Dong L, Yang
Y, Zhang Q, Ma G, Zhang G and Lei H: Exploitation of enhanced prime
editing for blocking aberrant angiogenesis. J Adv Res: Jul 10, 2024
(Epub ahead of print).
|
|
108
|
Zech TJ, Wolf A, Hector M, Bischoff-Kont
I, Krishnathas GM, Kuntschar S, Schmid T, Bracher F, Langmann T and
Fürst R: 2-Desaza-annomontine (C81) impedes angiogenesis through
reduced VEGFR2 expression derived from inhibition of CDC2-like
kinases. Angiogenesis. 27:245–272. 2024.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Tang X, Cui K, Wu P, Hu A, Fan M, Lu X,
Yang F, Lin J, Yu S, Xu Y and Liang X: Acrizanib as a novel
therapeutic agent for fundus neovascularization via inhibitory
phosphorylation of VEGFR2. Transl Vis Sci Technol.
13(1)2024.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Lei W, Xu H, Yao H, Li L, Wang M, Zhou X
and Liu X: 5α-Hydroxycostic acid inhibits choroidal
neovascularization in rats through a dual signalling pathway
mediated by VEGF and angiopoietin 2. Mol Med.
29(151)2023.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Liu Y, Feng M, Cai J, Li S, Dai X, Shan G
and Wu S: Repurposing bortezomib for choroidal neovascularization
treatment via antagonizing VEGF-A and PDGF-D mediated signaling.
Exp Eye Res. 204(108446)2021.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Zeng Z, Li S, Ye X, Wang Y, Wang Q, Chen
Z, Wang Z, Zhang J, Wang Q, Chen L, et al: Genome editing VEGFA
prevents corneal neovascularization in vivo. Adv Sci (Weinh).
11(e2401710)2024.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Huang X, Zhou G, Wu W, Duan Y, Ma G, Song
J, Xiao R, Vandenberghe L, Zhang F, D'Amore PA and Lei H: Genome
editing abrogates angiogenesis in vivo. Nat Commun.
8(112)2017.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Toutounchian S, Ahmadbeigi N and Mansouri
V: Retinal and choroidal neovascularization antivascular
endothelial growth factor treatments: The role of gene therapy. J
Ocul Pharmacol Ther. 38:529–548. 2022.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Yiu G, Gulati S, Higgins V, Coak E, Mascia
D, Kim E, Spicer G and Tabano D: Factors involved in anti-VEGF
treatment decisions for neovascular age-related macular
degeneration: Insights from real-world clinical practice. Clin
Ophthalmol. 18:1679–1690. 2024.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Wykoff CC, Garmo V, Tabano D, Menezes A,
Kim E, Fevrier HB, LaPrise A and Leng T: Impact of Anti-VEGF
treatment and patient characteristics on vision outcomes in
neovascular age-related macular degeneration: Up to 6-year analysis
of the AAO IRIS® Registry. Ophthalmol Sci. 4(100421)2023.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Liu D, Zhang C and Zhang J, Xu GT and
Zhang J: Molecular pathogenesis of subretinal fibrosis in
neovascular AMD focusing on epithelial-mesenchymal transformation
of retinal pigment epithelium. Neurobiol Dis.
185(106250)2023.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Fleckenstein M, Mitchell P, Freund KB,
Sadda S, Holz FG, Brittain C, Henry EC and Ferrara D: The
progression of geographic atrophy secondary to age-related macular
degeneration. Ophthalmology. 125:369–390. 2018.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Zhuang X, Su Y, Li M, Zhang L, Mi L, Ji Y,
Deng F, Xiao O, Zhang X and Zhou L: , et al: A prospective
observation of influence of anti-VEGF on optic disc vasculature in
nAMD patients. Photodiagnosis Photodyn Ther.
45(103863)2024.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Pérez-Gutiérrez L and Ferrara N: Biology
and therapeutic targeting of vascular endothelial growth factor A.
Nat Rev Mol Cell Biol. 24:816–834. 2023.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Wu Y, Wang J, Zhao J, Su Y, Li X, Chen Z,
Wu X, Huang S, He X and Liang L: LTR retrotransposon-derived LncRNA
LINC01446 promotes hepatocellular carcinoma progression and
angiogenesis by regulating the SRPK2/SRSF1/VEGF axis. Cancer Lett.
598(217088)2024.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Bao M, Chen Y, Liu JT, Bao H, Wang WB, Qi
YX and Lv F: Extracellular matrix stiffness controls VEGF(165)
secretion and neuroblastoma angiogenesis via the YAP/RUNX2/SRSF1
axis. Angiogenesis. 25:71–86. 2022.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Camacho P, Ribeiro E, Pereira B, Varandas
T, Nascimento J, Henriques J, Dutra-Medeiros M, Delgadinho M,
Oliveira K, Silva C and Brito M: DNA methyltransferase expression
(DNMT1, DNMT3a and DNMT3b) as a potential biomarker for anti-VEGF
diabetic macular edema response. Eur J Ophthalmol. 33:2267–2274.
2023.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Xu L, Prentice JR, Velez-Montoya R, Sinha
A, Barakat MR, Gupta A, Lowenthal R, Khanani AM, Kaiser PK, Heier
JS, et al: Bispecific VEGF-A and Angiopoietin-2 Antagonist RO-101
preclinical efficacy in model of neovascular eye disease.
Ophthalmol Sci. 4(100467)2024.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Jian HJ, Anand A, Lai JY, Huang CC, Ma DH,
Lai CC and Chang HT: Ultrahigh-Efficacy VEGF neutralization using
carbonized nanodonuts: Implications for intraocular anti-angiogenic
therapy. Adv Healthc Mater. 13(e2302881)2024.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Xu M, Fan R, Fan X, Shao Y and Li X:
Progress and challenges of Anti-VEGF agents and their
sustained-release strategies for retinal angiogenesis. Drug Des
Devel Ther. 16:3241–3262. 2022.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Zhou C, Lei F, Sharma J, Hui PC, Wolkow N,
Dohlman CH, Vavvas DG, Chodosh J and Paschalis EI: Sustained
inhibition of VEGF and TNF-α achieves multi-ocular protection and
prevents formation of blood vessels after severe ocular trauma.
Pharmaceutics. 15(2059)2023.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Puranen J, Koponen S, Nieminen T, Kanerva
I, Kokki E, Toivanen P, Urtti A, Ylä-Herttuala S and Ruponen M:
Antiangiogenic AAV2 gene therapy with a truncated form of soluble
VEGFR-2 reduces the growth of choroidal neovascularization in mice
after intravitreal injection. Exp Eye Res.
224(109237)2022.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Desideri LF, Vaccaro S, Vagge A, Nicolò M,
Scorcia V, Traverso CE and Giannaccare G: AAV8 gene therapy
encoding anti-VEGF Fab Treatment of wet age-related macular
degeneration Treatment of diabetic retinopathy. Drugs Future.
47:737–741. 2022.
|
|
130
|
Papaioannou C: Advancements in the
treatment of age-related macular degeneration: A comprehensive
review. Postgrad Med J. 100:445–450. 2024.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Campochiaro PA, Avery R, Brown DM, Heier
JS, Ho AC, Huddleston SM, Jaffe GJ, Khanani AM, Pakola S, Pieramici
DJ, et al: Gene therapy for neovascular age-related macular
degeneration by subretinal delivery of RGX-314: A phase 1/2a
dose-escalation study. Lancet. 403:1563–1573. 2024.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Poulsen K, Hanna K, Nieves J, Nguyen N,
Sharma P, Grishanin R, Corbau R and Kiss S: Nonclinical study of
ixo-vec gene therapy for nAMD supports efficacy for a human dose of
6E10 vg/eye and staggered dosing of fellow eyes. Mol Ther Methods
Clin Dev. 33(101430)2025.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Shirian JD, Shukla P and Singh RP:
Exploring new horizons in neovascular age-related macular
degeneration: Novel mechanisms of action and future therapeutic
avenues. Eye (Lond). 39:40–44. 2025.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Modi SJ and Kulkarni VM: Exploration of
structural requirements for the inhibition of VEGFR-2 tyrosine
kinase: Binding site analysis of type II, ‘DFG-out’ inhibitors. J
Biomol Struct Dyn. 40:5712–5727. 2022.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Zong Y, Xiao S, Lei D and Li H:
Discoveries in retina physiology and disease biology using
single-cell RNA sequencing. Front Biosci (Landmark Ed).
28(247)2023.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Lyu Y, Zauhar R, Dana N, Strang CE, Hu J,
Wang K, Liu S, Pan N, Gamlin P, Kimble JA, et al: Implication of
specific retinal cell-type involvement and gene expression changes
in AMD progression using integrative analysis of single-cell and
bulk RNA-seq profiling. Sci Rep. 11(15612)2021.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Becker K, Klein H, Simon E, Viollet C,
Haslinger C, Leparc G, Schultheis C, Chong V, Kuehn MH,
Fernandez-Albert F and Bakker RA: In-depth transcriptomic analysis
of human retina reveals molecular mechanisms underlying diabetic
retinopathy. Sci Rep. 11(10494)2021.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Deng J and Qin YH: Advancements and
emerging trends in ophthalmic anti-VEGF therapy: A bibliometric
analysis. Int Ophthalmol. 44(368)2024.PubMed/NCBI View Article : Google Scholar
|