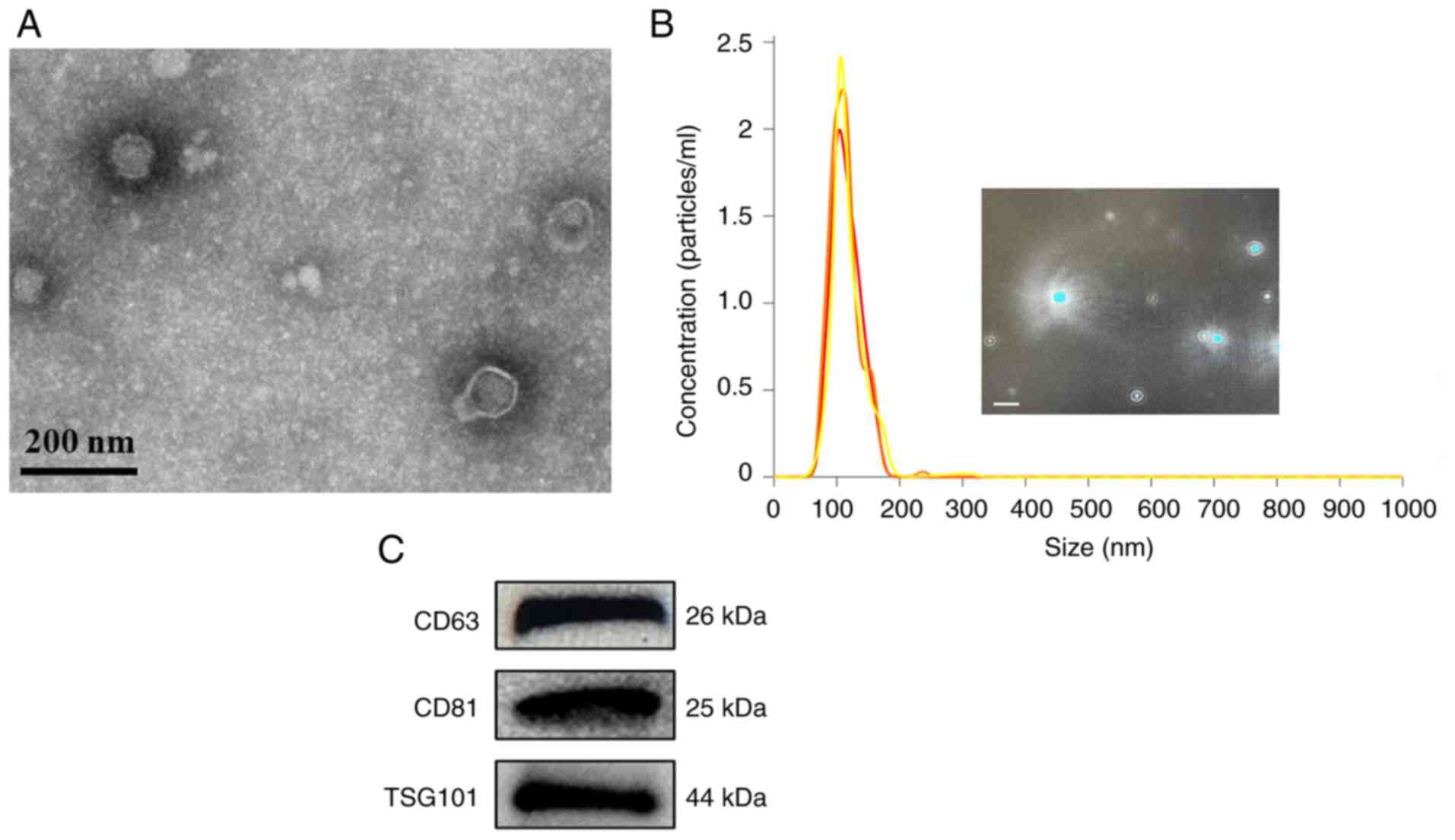
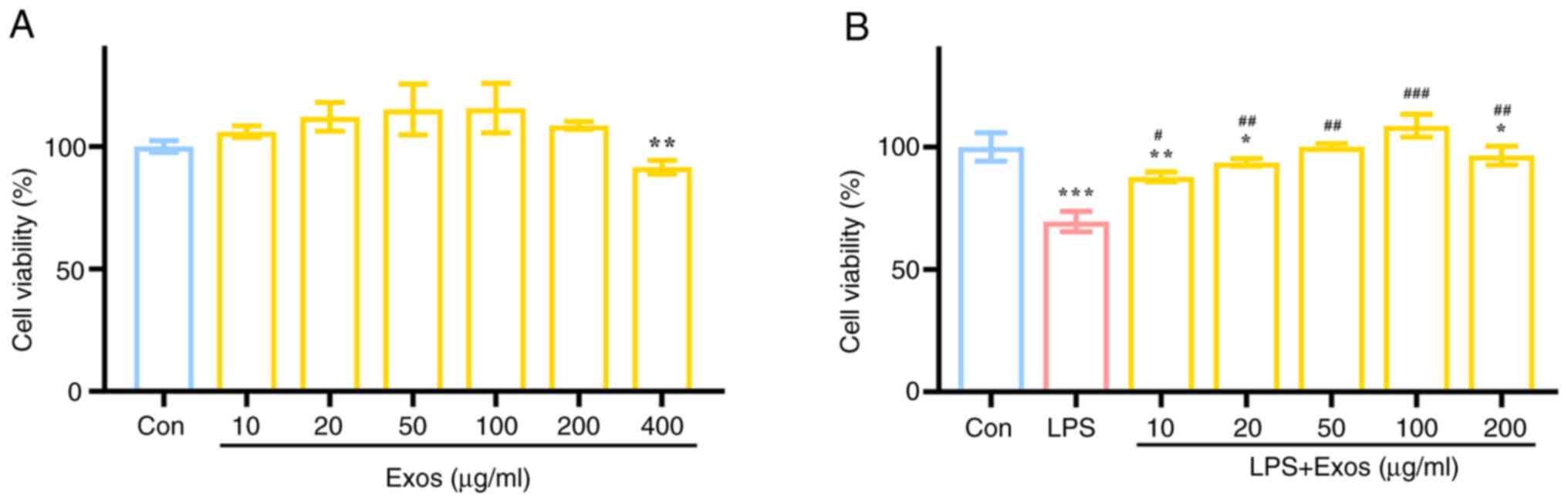
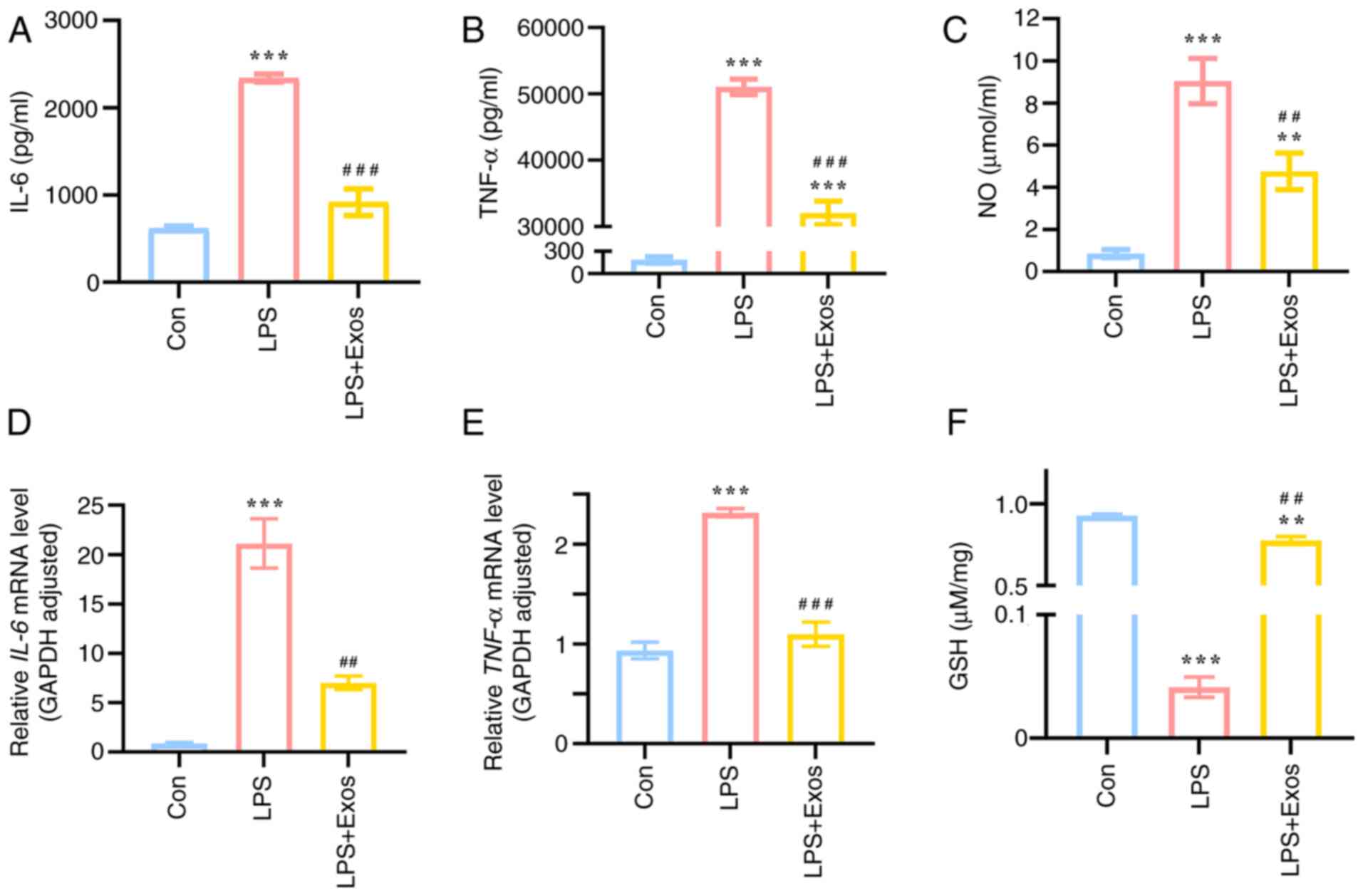
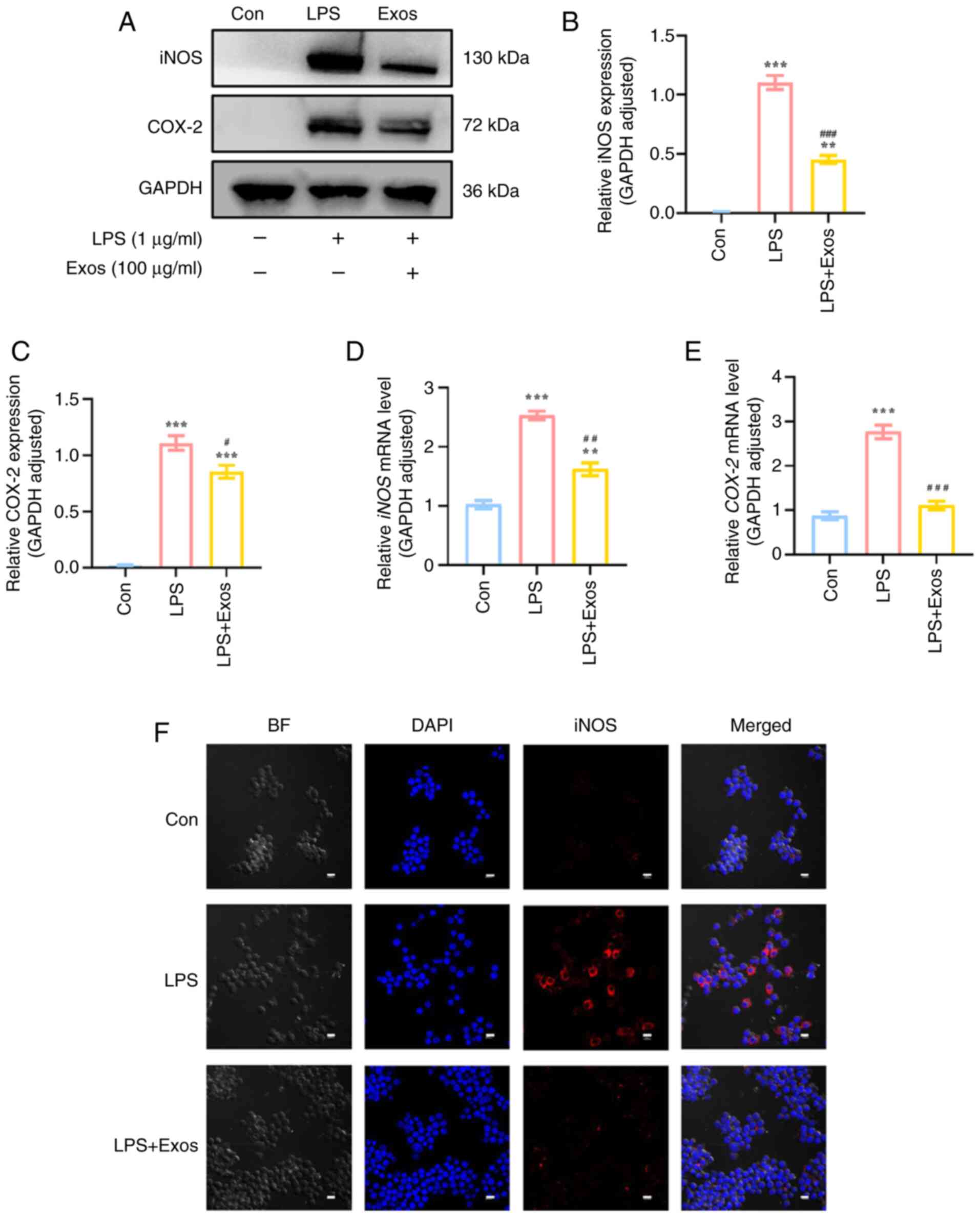
|
1
|
Karin M and Clevers H: Reparative
inflammation takes charge of tissue regeneration. Nature.
529:307–315. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Panigrahy D, Gilligan MM, Serhan CN and
Kashfi K: Resolution of inflammation: An organizing principle in
biology and medicine. Pharmacol Ther. 227(107879)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Grosso G, Laudisio D, Frias-Toral E,
Barrea L, Muscogiuri G, Savastano S and Colao A: Anti-inflammatory
nutrients and obesity-associated metabolic-inflammation: State of
the art and future direction. Nutrients. 14(1137)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Han R, Xiao Y, Bai Q and Choi CHJ:
Self-therapeutic metal-based nanoparticles for treating
inflammatory diseases. Acta Pharm Sin B. 13:1847–1865.
2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Khan IT, Nadeem M, Imran M, Ayaz M, Ajmal
M, Ellahi MY and Khalique A: Antioxidant capacity and fatty acids
characterization of heat treated cow and buffalo milk. Lipids
Health Dis. 16(163)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Khan IT, Nadeem M, Imran M, Ullah R, Ajmal
M and Jaspal MH: Antioxidant properties of milk and dairy products:
A comprehensive review of the current knowledge. Lipids Health Dis.
18(41)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hess JM, Stephensen CB, Kratz M and
Bolling BW: Exploring the links between diet and inflammation:
Dairy foods as case studies. Adv Nutr. 12 (Suppl 1):S1–S13.
2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhong Y, Wang X, Zhao X, Shen J, Wu X, Gao
P, Yang P, Chen J and An W: Multifunctional milk-derived small
extracellular vesicles and their biomedical applications.
Pharmaceutics. 15(1418)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Betker JL, Angle BM, Graner MW and
Anchordoquy TJ: The potential of exosomes from cow milk for oral
delivery. J Pharm Sci. 108:1496–505. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim NH, Kim J, Lee JY, Bae HA and Kim CY:
Application of milk exosomes for musculoskeletal health: Talking
points in recent outcomes. Nutrients. 15(4645)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
van Herwijnen MJC, Driedonks TAP, Snoek
BL, Kroon AMT, Kleinjan M, Jorritsma R, Pieterse CMJ, Hoen E and
Wauben MHM: Abundantly present miRNAs in milk-derived extracellular
vesicles are conserved between mammals. Front Nutr.
5(81)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Taketoshi H, Kosuke M, Hajime N, Yasunari
Y, Tsukasa M and Naohito A: Isolation of bovine milk-derived
microvesicles carrying mRNAs and microRNAs. Biochem Biophys Res
Commun. 396:528–533. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ngu A, Wang S, Wang H, Khanam A and
Zempleni J: Milk exosomes in nutrition and drug delivery. Am J
Physiol Cell Physiol. 322:C865–C874. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ghorbani S, Talebi F, Chan WF, Masoumi F,
Vojgani M, Power C and Noorbakhsh F: MicroRNA-181 variants regulate
T cell phenotype in the context of autoimmune neuroinflammation.
Front Immunol. 8(758)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yu Z, Teng Y, Yang J and Yang L: The role
of exosomes in adult neurogenesis: Implications for
neurodegenerative diseases. Neural Regen Res. 19:282–288.
2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Reif S, Elbaum-Shiff Y, Koroukhov N, Shilo
I, Musseri M and Golan-Gerstl R: Cow and human milk-derived
exosomes ameliorate colitis in DSS murine model. Nutrients.
12(2589)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ocansey DKW, Zhang L, Wang Y, Yan Y, Qian
H, Zhang X, Xu W and Mao F: Exosome-mediated effects and
applications in inflammatory bowel disease. Biol Rev Camb Philos
Soc. 95:1287–1307. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yurakova TR, Gorshkova EA, Nosenko MA and
Drutskaya MS: Metabolic adaptations and functional activity of
macrophages in homeostasis and inflammation. Biochemistry (Mosc).
89:817–138. 2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nomura F, Akashi S, Sakao Y, Sato S, Kawai
T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K and Akira
S: Cutting edge: Endotoxin tolerance in mouse peritoneal
macrophages correlates with down-regulation of surface toll-like
receptor 4 expression. J Immunol. 164:3476–3479. 2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bongartz H, Bradfield C, Gross J, Fraser
IDC, Nita-Lazar A and Meier-Schellersheim M: IL-10 dependent
adaptation allows macrophages to adjust inflammatory responses to
TLR4 stimulation history bioRxiv (Preprint): 2024.03.28.587272,
2024.
|
|
21
|
Zusso M, Lunardi V, Franceschini D,
Pagetta A, Lo R, Stifani S, Frigo AC, Giusti P and Moro S:
Ciprofloxacin and levofloxacin attenuate microglia inflammatory
response via TLR4/NF-kB pathway. J Neuroinflammation.
16(148)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Malemud CJ: Intracellular signaling
pathways in rheumatoid arthritis. J Clin Cell Immunol.
4(160)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Vanhaesebroeck B, Guillermet-Guibert J,
Graupera M and Bilanges B: The emerging mechanisms of
isoform-specific PI3K signalling. Nat Rev Mol Cell Biol.
11:329–341. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Li D, Yao S, Zhou Z, Shi J, Huang Z and Wu
Z: Hyaluronan decoration of milk exosomes directs tumor-specific
delivery of doxorubicin. Carbohydr Res. 493(108032)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wolf T, Baier SR and Zempleni J: The
intestinal transport of bovine milk exosomes is mediated by
endocytosis in human colon carcinoma caco-2 cells and rat small
intestinal IEC-6 cells. J Nutr. 145:2201–2206. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Deng C, Hu Y, Conceicao M, Wood MJA, Zhong
H, Wang Y, Shao P, Chen J and Qiu L: Oral delivery of
layer-by-layer coated exosomes for colitis therapy. J Control
Release. 354:635–650. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rex J, Lutz A, Faletti LE, Albrecht U,
Thomas M, Bode JG, Borner C, Sawodny O and Merfort I: IL-1β and
TNFα differentially influence NF-κB activity and fasl-induced
apoptosis in primary murine hepatocytes during LPS-induced
inflammation. Front Physiol. 10(117)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yuan X, Juan Z, Zhang R, Sun X, Yan R, Yue
F, Huang Y, Yu J and Xia X: Clemastine fumarate protects against
myocardial ischemia reperfusion injury by activating the
TLR4/PI3K/AKT signaling pathway. Front. Pharmacol.
11(28)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yelubaeva MY, Buralkhiev BA, Serikbayeva
AD, Narmuratova MH and Kenenbay SY: Electrophoretic identification
of casein in various types of milk. J Biol.Chem. 17:348–352.
2017.
|
|
31
|
Tian MY, Hao DX, Liu Y, He J, Zhao ZH, Guo
TY, Li X and Zhang Y: Milk exosomes: An oral drug delivery system
with great application potential. Food Funct. 14:1320–1337.
2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lai JJ, Chau ZL, Chen SY, Hill JJ, Korpany
KV, Liang NW, Lin LH, Lin YH, Liu JK, Liu YC, et al: Exosome
processing and characterization approaches for research and
technology development. Adv Sci (Weinh). 9(e2103222)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rodríguez-Morales P and Franklin RA:
Macrophage phenotypes and functions: Resolving inflammation and
restoring homeostasis. Trends Immunol. 44:986–998. 2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Salehi M, Negahdari B, Mehryab F and
Shekari F: Milk-derived extracellular vesicles: bomedical
applications, current challenges, and future perspectives. J Agric
Food Chem. 72:8304–8331. 2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Matic S, D'Souza DH, Wu T, Pangloli P and
Dia VP: Bovine milk exosomes affect proliferation and protect
macrophages against cisplatin-induced cytotoxicity. Immunol Invest.
49:711–725. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tsai CF, Chen GW, Chen YC, Shen CK, Lu DY,
Yang LY, Chen JH and Yeh WL: Regulatory effects of quercetin on
M1/M2 macrophage polarization and oxidative/antioxidative balance.
Nutrients. 14(67)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Torregrosa Paredes P, Esser J, Admyre C,
Nord M, Rahman QK, Lukic A, Rådmark O, Grönneberg R, Grunewald J,
Eklund A, et al: Bronchoalveolar lavage fluid exosomes contribute
to cytokine and leukotriene production in allergic asthma. Allergy.
67:911–919. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang M, Hu W, Cai C, Wu Y, Li J and Dong
S: Advanced application of stimuli-responsive drug delivery system
for inflammatory arthritis treatment. Mater Today Bio.
14(100223)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Malik R, Paudel KR, Manandhar B, De Rubis
G, Shen J, Mujwar S, Singh TG, Singh SK, Gupta G, Adams J, et al:
Agarwood oil nanoemulsion counteracts LPS-induced inflammation and
oxidative stress in RAW264.7 mouse macrophages. Pathol Res Pract.
251(154895)2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Izadparast F, Riahi-Zajani B, Yarmohammadi
F, Hayes AW and Karimi G: Protective effect of berberine against
LPS-induced injury in the intestine: A review. Cell Cycle.
21:2365–2378. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang R, Wang N, Han Y, Xu J and Xu Z:
Dulaglutide alleviates LPS-induced injury in cardiomyocytes. ACS
Omega. 6:8271–8278. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fitzgerald KA and Kagan JC: Toll-like
receptors and the control of immunity. Cell. 180:1044–1066.
2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Stierschneider A and Wiesner C: Shedding
light on the molecular and regulatory mechanisms of TLR4 signaling
in endothelial cells under physiological and inflamed conditions.
Front Immunol. 14(1264889)2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wu Z, Mehrabi Nasab E, Arora P and Athari
SS: Study effect of probiotics and prebiotics on treatment of
OVA-LPS-induced of allergic asthma inflammation and pneumonia by
regulating the TLR4/NF-kB signaling pathway. J Transl Med.
20(130)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhong J, Qiu X, Yu Q, Chen H and Yan C: A
novel polysaccharide from Acorus tatarinowii protects against
LPS-induced neuroinflammation and neurotoxicity by inhibiting
TLR4-mediated MyD88/NF-κB and PI3K/AKT signaling pathways. Int J
Biol Macromol. 163:464–475. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Acosta-Martinez M and Cabail MZ: The
PI3K/AKT pathway in meta-inflammation. Int J Mol Sci.
23(15330)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
He C, Wang K, Xia J, Qian D, Guo J, Zhong
L, Tang D, Chen X, Peng W, Chen Y and Tang Y: Natural exosomes-like
nanoparticles in mung bean sprouts possesses anti-diabetic effects
via activation of PI3K/AKT/GLUT4/GSK-3β signaling pathway. J
Nanobiotechnology. 21(349)2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhong R, Xia T, Wang Y, Ding Z, Li W, Chen
Y, Peng M, Li C, Zhang H and Shu Z: Physalin B ameliorates
inflammatory responses in lipopolysaccharide-induced acute lung
injury mice by inhibiting NF-κB and NLRP3 via the activation of the
PI3K/AKT pathway. J Ethnopharmacol. 284(114777)2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhou Q, Li M, Wang X, Li Q, Wang T, Zhu Q,
Zhou X, Wang X, Gao X and Li X: Immune-related microRNAs are
abundant in breast milk exosomes. Int J Biol Sci. 8:118–123.
2012.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Nata T, Fujiya M, Ueno N, Moriichi K,
Konishi H, Tanabe H, Ohtake T, Ikuta K and Kohgo Y: MicroRNA-146b
improves intestinal injury in mouse colitis by activating nuclear
factor-κB and improving epithelial barrier function. J Gene Med.
15:249–2460. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Melnik BC and Schmitz G: Exosomes of
pasteurized milk: Potential pathogens of Western diseases. J
Transl. Med. 17(3)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Yang M, Song D, Cao X, Wu R, Liu B, Ye W,
Wu J and Yue X: Comparative proteomic analysis of milk-derived
exosomes in human and bovine colostrum and mature milk samples by
iTRAQ-coupled LC-MS/MS. Food Res. Int. 92:17–25. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chang X, Wang SL, Zhao SB, Shi YH, Pan P,
Gu L, Yao J, Li ZS and Bai Y: Extracellular vesicles with possible
roles in gut intestinal tract homeostasis and IBD. Mediators
Inflamm. 2020(1945832)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Bacchi S, Palumbo P, Sponta A and
Coppolino MF: Clinical pharmacology of non-steroidal
anti-inflammatory drugs: A review. Antiinflamm Antiallergy Agents
Med Chem. 11:52–64. 2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yatoo MI, Gopalakrishnan A, Saxena A,
Parray OR, Tufani NA, Chakraborty S, Tiwari R, Dhama K and Iqbal
HMN: Anti-inflammatory drugs and herbs with special emphasis on
herbal medicines for countering inflammatory diseases and
disorders-A review. Recent Pat Inflamm Allergy Drug Discov.
12:39–58. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Liu J, Wang Y, Heelan WJ, Chen Y, Li Z and
Hu Q: . Mucoadhesive probiotic backpacks with ROS nanoscavengers
enhance the bacteriotherapy for inflammatory bowel diseases. Sci
Adv. 8(8798)2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S,
Liu C, Barnes S, Grizzle W, Miller D and Zhang HG: A novel
nanoparticle drug delivery system: the anti-inflammatory activity
of curcumin is enhanced when encapsulated in exosomes. Mol Ther.
18:1606–1614. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Garcia-Martinez J, Salto R, Giron MD,
Perez-Castillo IM, Bueno Vargas P, Vilchez JD, Linares-Perez A,
Manzano M, Garcia-Corcoles MT, Rueda R and López-Pedrosa JM:
Supplementation with a whey protein concentrate enriched in bovine
milk exosomes improves longitudinal growth and supports bone health
during catch-up growth in rats. Nutrients. 16(7)2024.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Yang H, Wuren T, Zhai BT, Liu Y and Er D:
Milk-derived exosomes in the regulation of nutritional and immune
functions. Food Sci. Nutr. 12:7048–7059. 2024.PubMed/NCBI View Article : Google Scholar
|