Introduction
Inflammation is a physiological process in which
tissues within the vascular system are stimulated by various
factors, such as injury (1).
Inflammation is a protective response that eliminates damage caused
by stimuli and facilitates the repair of damaged tissues, thereby
serving a key role in immune regulation (2). However, excessive inflammation can
cause serious organ damage and dysfunction, potentially leading to
autoimmune diseases, neurodegenerative diseases or cancer and thus
significantly affecting the quality of life of patients (3,4). The
exploration of novel therapeutic approaches for treatment of the
inflammatory process has emerged as a focus for researchers.
Milk is a source of nutrition in the human diet,
comprising of a multitude of nutrients, growth regulators,
immune-related factors and other physiologically active substances,
such as oligosaccharides and active peptides (5). Milk possesses anti-inflammatory and
anti-oxidative properties (6,7).
Additionally, milk is typically inexpensive and widely available
body fluid that can be produced on an industrial scale in a manner
that retains exosomes (Exos), enabling the extraction of
milk-derived Exos (M-Exos). Due to the strong biocompatibility,
high stability, safety profile and other biological characteristics
of milk, as well as its anti-inflammatory, anti-apoptotic and
tissue repair functions, M-Exos have been investigated in medical
research (8-10).
M-Exos contain certain immune-related
physiologically active substances, such as microRNAs (miRNA) and
amino acids (11-14).
These substances can transmit biological information into target
cells in the body and serve a biological role in inflammatory
reactions by transporting factors between cells and regulating
biological pathways in recipient cells (15). Reif et al (16) reported that miRNAs or proteins,
such as TGF-β or DNA methyltransferases, found in M-Exos regulate
inflammation, induce cell proliferation and contribute to the
repair of colon tissue injury during colitis. The recovery effect
of colon tissue injury is superior compared with that of human milk
and it can be used as a premium nutritional supplement to human
milk and be incorporated into enteral nutrition formulas. Ocansey
et al (17) assessed the
impact of M-Exos on intestinal inflammation in an ulcerative
colitis (UC) mouse model and reported that M-Exos possess
cytoprotective and anti-inflammatory properties, indicating that
M-Exos may be important in the prevention and progression of UC.
However, the precise mechanisms of underlying process are yet to be
fully elucidated.
Macrophages are essential parts of the immune system
that significantly contribute to the inflammatory response
(18). LPS can bind to toll-like
receptor 4 (TLR4) on the surface of macrophages, resulting in a
cascade of events that disrupt cell-signaling pathways and cytokine
secretion, leading to inflammation (19,20).
The TLR4/NF-κB and PI3K/AKT inflammatory signaling pathways are
involved in the onset and progression of LPS-induced RAW 264.7
macrophage inflammation, which are strongly associated with the
production and release of pro-inflammatory factors. Crosstalk also
exists between the TLR4/NF-κB and PI3K/AKT signaling pathways
(21-23).
The present study used LPS to stimulate macrophages to establish a
model of cellular inflammation. The occurrence and development of
LPS-induced macrophage-related inflammation was investigated using
M-Exos, with the aim of clarifying the mechanism by which M-Exos
regulate inflammation. These results provided an experimental basis
for subsequent research and application of M-Exos, a natural
bioactive substance, and offered new insights into
anti-inflammatory nutrients in food.
Materials and methods
Materials
LPS (cat. no. L6529) extracted from Escherichia
coli O55:B5 was obtained from Sigma-Aldrich (Merck KGaA). FBS,
penicillin G, streptomycin and DMEM were obtained from Gibco
(Thermo Fisher Scientific, Inc.).
Methylthiazolyldiphenyl-tetrazolium bromide (MTT; ≥98%) was
obtained from Beijing Solarbio Science & Technology Co., Ltd.
DMSO (≥99%) was obtained from Sinopharm Chemical Reagent Co., Ltd.
The following rabbit antibodies were purchased from Beyotime
Institute of Biotechnology: Anti-TLR4 (cat. no. AF8187; 1:1,000),
anti-GAPDH (cat. no. AG8015; 1:3,000), anti-inducible nitric oxide
synthase (iNOS) (cat. no. AF7281; 1:1,000), anti-cyclooxygenase-2
(COX2) (cat. no. AF1924; 1:1,000) and Cy3-labeled goat anti-rabbit
IgG (H+L) (cat. no. A0516; 1:500). Rabbit anti-phosphorylated
(p)-p65, anti-p65 antibodies and HRP-conjugated goat anti-rabbit
IgG were purchased from Sangon Biotech. Rabbit anti-p-IκBα,
anti-IκBα, anti-tumor susceptibility 101 (TSG101), anti-CD63 and
anti-CD81 antibodies were purchased from Abcam. Rabbit anti-p-PI3K,
anti-PI3K, anti-p-AKT, anti-AKT, anti-Bax and anti-Bcl2 antibodies
were purchased from Cell Signaling Technology, Inc.
Isolation of exosomes in milk
M-Exos were isolated according to a previously
described method, in which M-Exos were obtained from bovine milk
through a combination of centrifugation and isoelectric-point
precipitation (24,25). A 720 ml bottle of non-fat
(<0.5%) pasteurized fresh milk was purchased from Jingdong
Supermarket (Nei Mongol, China). The milk was centrifuged at 13,000
x g for 30 min at 4˚C to remove the upper fat layer, part of the
casein and lower cellular remains. The pH of the milk was adjusted
to 4.6 (the isoelectric point of casein) with ~15 ml 2 mol/l
hydrochloric acid to precipitate casein. The protein precipitate
was removed by centrifugation at 10,000 x g for l h at 4˚C and the
residual proteins in the collected supernatant were removed by
filtration through 0.45 and 0.22 µm injection filters. The
supernatant was ultracentrifuged using a horizontal rotor at
135,000 x g for 1 h at 4˚C (ultra-centrifuge CP80NX; Hitachi,
Ltd.). The precipitate was obtained by washing with 4˚C precooled
sterile PBS solution three times and resuspended in PBS to obtain
the purified M-Exo sample. The protein content of M-Exo was
assessed using a BCA protein assay kit (cat. no. P0012S; Beyotime
Institute of Biotechnology).
Characterization of exosomes
The physical dimensions of M-Exos were evaluated
using a nanoparticle tracking analyzer (NTA; cat. no. NS300) and
Nanosight NTA3.2 software (Malvern Instruments, Ltd.). The
characterization of M-Exos was conducted using transmission
electron microscopy (TEM; cat. no. JEOL-2100F; JEOL Ltd.). Western
blotting was employed to assess the protein expression profile of
M-Exos by detecting TSG101 (cat. no. ab133586), CD63 (cat. no.
ab134045) and CD81 (cat. no. ab286173) expression levels at a
dilution of 1:1,000. Western blotting was performed as described
below.
Cell culture and treatment
RAW 264.7 cells were obtained from The Cell Bank of
Type Culture Collection of The Chinese Academy of Science. Cells
were cultured in DMEM supplemented with high glucose (4.5 g/l), 10%
FBS, 0.5% penicillin G and 0.5% streptomycin. Cells were incubated
at 37˚C in a humidified atmosphere with 5% CO2. Cells
were passaged every 2 days. Once cells reached 70-80% confluence,
the cells were scraped using a cell scraper and 1/4 of the cell
fluid was removed and added to DMEM high-glucose complete medium
(Gibco; Thermo Fisher Scientific, Inc.). In a T25 cell culture
bottle (Wuxi NEST Biotechnology Co., Ltd.), the cells were evenly
distributed by shaking horizontally and subsequently incubated at
37˚C and 5% CO2. To determine the role of M-Exos in
regulating inflammation and the underlying mechanisms of action,
the RAW 264.7 macrophages were categorized as follows: i) The
control group, which consisted of cells cultured in DMEM alone for
24 h; ii) the LPS group, which included cells cultured in DMEM
supplemented with 1 µg/ml LPS for 24 h at 37˚C; and iii) the LPS +
M-Exos group, which comprised cells cultured in DMEM containing 1
µg/ml LPS and 25, 50 or 100 µg/ml M-Exos for 24 h at 37˚C.
Cell viability evaluation by MTT
assay
The RAW 264.7 cells were seeded at a density of
1x104 cells/well in 96-well plates. The DMEM group was
cultured in DMEM medium, the Exo group was cultured in DMEM with
different M-Exos concentrations (10, 20, 50, 100, 200 and 400
µg/ml), the LPS group was cultured with 1 µg/ml LPS and the LPS +
M-Exos group was cultured with 1 µg/m LPS and different Exos
concentrations (10, 20, 50, 100 and 200 µg/ml). Following a 24 h
incubation period at 37˚C, a 1% MTT solution was added to each well
and cells were incubated in the dark for 4 h. Thereafter, the
medium was aspirated and replaced with 100 µl of DMSO in each well.
Following a 10 min incubation at 37˚C, the absorbance was
determined at a wavelength of 570 nm using a microplate reader
(Multiskan; Thermo Fisher Scientific, Inc.). Cell viability was
calculated using the following formula: Cell viability (%)=OD
absorbance of tested groups/OD absorbance of
control group x100.
ELISA assay
The RAW 264.7 cells were seeded at a density of
3x105 cells/well in 6-well plates for 24 h and each
group was treated as aforementioned. After 24 h of co-culture with
M-Exos, the cell medium was collected and centrifuged at 500 x g
for 5 min at room temperature. The serum of mice was collected by
centrifugation at 1,500 x g for 15 min at 4˚C. Subsequently, the
supernatant was analyzed using a Rat IL-6 Uncoated ELISA Kit (cat.
no. 88-50625-88; Thermo Fisher Scientific, Inc.) and Mouse TNF
alpha Uncoated ELISA Kit (cat. no. 88-7324-88; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was used to quantify the mRNA expression
levels of IL-6, TNF-α, iNOS and COX-2. Total RNA was extracted from
RAW 264.7 cells and colon tissues using a commercial RNeasy™ Total
RNA extraction kit (Beyotime Institute of Biotechnology) following
the manufacturer's instructions. RT was performed using a Revert
Aid First-Strand cDNA Synthesis Kit (Thermo Fisher Scientific,
Inc.) using the same concentration of RNA/sample of each group,
following the manufacturer's instructions. Gene expression was
quantified by RT-qPCR using an UltraSYBR mixture (Jiangsu CoWin
Biotech Co., Ltd.) using a LightCycler® 96 real-time
system (Roche Diagnostics GmbH). Initial denaturation was performed
at 95˚C for 10 min, denaturation at 95˚C for 15 sec, and annealing
and extension at 60˚C for 1 min (35 cycles). GAPDH was used as the
normalization control and the data were analyzed using the
2-ΔΔCq method (26). The primer sequences for each target
gene are shown in Table I.
 | Table IPCR primer sequences used in RAW264.7
cells. |
Table I
PCR primer sequences used in RAW264.7
cells.
| Gene | Sequence
(5'-3') |
|---|
| IL-6 forward |
ACAACCACGGCCTTCCCTACTT |
| IL-6 reverse |
CACGATTTCCCAGAGAACATGTG |
| TNF-α forward |
AAGCCTGTAGCCCACGTCGTA |
| TNF-α reverse |
GGCACCACTAGTTGGTTGTCTTTG |
| iNOS forward |
GAGCTCGGGTTGAAGTGGTATG |
| iNOS reverse |
GAAACTATGGAGCACAGCCACAT |
| COX-2 forward |
CTGGTGCCTGGTCTGATGATGTATG |
| COX-2 reverse |
AGCTGTACTCCTGGTCTTCAATGTTG |
| GAPDH forward |
GAGCCAAACGGGTCATCATCT |
| GAPDH reverse |
GAGGGGCCATCCACAGTCTT |
Measurement of nitric oxide (NO)
levels
RAW 264.7 cells were seeded at a density of
3x105 cells/well in 6-well plates for 24 h. The
supernatant of RAW 264.7 cells was collected at 500 x g for 5 min
at 4˚C for the measurement of NO using a commercial NO detection
kit (cat. no. S0021S; Beyotime Institute of Biotechnology) in
accordance with the manufacturer's instructions.
Measurement of glutathione (GSH)
levels
RAW 264.7 cells were seeded at a density of
3x105 cells/well in 6-well plates for 24 h. The
precipitate of RAW 264.7 cells was collected by centrifugation at
300 x g for 5 min and protein removal reagent S solution (Beyotime
Institute of Biotechnology) was added at three times the volume of
the cell precipitate. After vortexing thoroughly and incubating at
4˚C for 10 min, the homogenate was centrifuged at 10,000 x g for 10
min at 4˚C. The supernatant was collected for measurement of total
GSH using a commercial GSH assay kit (cat. no. S0053; Beyotime
Institute of Biotechnology) in accordance with the manufacturer's
instructions.
Western blotting
RAW 264.7 cells were seeded at a density of
3x105 cells/well in 6-well plates for 24 h. After 24 h
of co-culture with M-Exos, the cell medium was aspirated and 80 µl
of RIPA lysis buffer (Beyotime Institute of Biotechnology) and 1 mM
PMSF (Sangon Biotech Co., Ltd.) were added. Cells were subsequently
lysed on ice. Samples were centrifuged at 12,000 x g for 10 min at
4˚C and the supernatant was used for subsequent experiments.
Protein samples were measured using a Pierce™ BCA protein assay kit
(Beyotime Institute of Biotechnology) in accordance with the
manufacturer's instructions. After protein denaturation with
loading buffer (Beyotime Institute of Biotechnology), protein
samples (20 µg) were separated using 10% SDS-PAGE. Proteins were
subsequently transferred from the gel to a PVDF membrane
(MilliporeSigma). The PVDF membrane was treated with a blocking
solution (QuickBlock™ Western; cat. no. P0252; Beyotime Institute
of Biotechnology) to inhibit non-specific binding at room
temperature for 10 min, then the membranes were incubated at 4˚C
for 12-16 h with the primary antibodies. Following removal of the
primary antibodies, the membrane was incubated with secondary
antibodies for 1 h at room temperature. The membrane was rinsed
three times (10 min/rinse) with TBS-T solution (0.1% Tween 20)
after each step. Protein bands were visualized using a
chemiluminescence image-analysis system (Tanon-5200; Tanon Science
and Technology Co., Ltd.). The antibodies used were as follows:
Rabbit anti-iNOS (cat. no. AF7281; 1:1,000), anti-COX-2 (cat. no.
AF1924; 1:1,000), anti-TLR4 (cat. no. AF8187; 1:1,000), anti-p-p65
(cat. no. D155006; 1:1,000), anti-p65 (cat. no. D221030; 1:1,000),
anti-p-IκBα (cat. no. ab133462; 1:5,000), anti-IκBα (cat. no.
ab32518; 1:2,000), anti-p-PI3K (cat. no. 17366T; 1:1,000),
anti-PI3K (cat. no. 4249T; 1:1,000), anti-p-AKT (cat. no. 4060T;
1:1,000), anti-AKT (cat. no. 9271T; 1:1,000), anti-GAPDH (cat. no.
AG8015; 1:3,000), anti-Bax (cat. no. 2772T; 1:1,000), anti-Bcl2
(cat. no. 3498T; 1:1,000) and HRP-conjugated goat anti-rabbit IgG
(cat. no. D110058; 1:10,000). Gray scan analysis was performed
using ImageJ software (version 1.8.0; National Institutes of
Health).
Immunofluorescence (IF) staining
RAW 264.7 cells were seeded at a density of
2x104 cells/well onto confocal dishes for 24 h. After 24
h of co-culture with M-Exos, the cell medium was aspirated. Cells
were fixed using 4% paraformaldehyde (Beyotime Institute of
Biotechnology) at room temperature for 10 min and subsequently
subjected to blocking with the QuickBlock™ Blocking Buffer
(Beyotime Institute of Biotechnology) at room temperature for 1 h.
Permeabilization of the cells was performed using 0.5% Triton X-100
(Beyotime Institute of Biotechnology) for 15 min at room
temperature. Subsequently, cells were incubated overnight at 4˚C
with rabbit anti-iNOS antibodies (cat. no. AF7281; 1:300). The
primary antibodies were removed, and the cells were incubated with
Cy3-labeled secondary antibodies (cat. no. A0516; 1:500; Beyotime
Institute of Biotechnology) at room temperature for 60 min. Then,
the cell nucleus was stained using DAPI (Beyotime Institute of
Biotechnology) at room temperature for 15 min in the dark. The
samples were imaged using a fluorescence microscope (Ti-U; Nikon
Corporation).
Animal experiments
Female C57BL/6J mice (n=15) were obtained from
Jiangsu Jicui Yaokang Biotechnology Co., Ltd. and kept in the
experimental animal center of Wuxi Medical College at Jiangnan
University (Wuxi, China). The specific-pathogen-free conditions
were as follows: 20-26˚C, 45±10% humidity and 12 h/light-dark
cycle. Mice were allowed to acclimate for 1 week, and then the
experiment was conducted for 1 week. To establish the UC model, the
mice were administered drinking water containing 3.0% (w/v) dextran
sodium sulfate (DSS; 36-50 kDa; Dalian Meilun Biotechnology Co.,
Ltd.) for 7 days. The control group was provided ordinary drinking
water. Mice were randomly divided into 3 groups (n=5/group): i)
Control group; ii) DSS group; and iii) Exos-treated DSS group
(Exos). The control and DSS groups were administered 200 µl PBS
intragastrically daily, while the Exos group was administered
intragastrically 400 µg of M-Exos daily. All groups were provided
with standard maintenance feed for 7 days and euthanized on day 8.
The weight of the mice was measured daily, and the disease activity
index (DAI) was recorded (27).
Animals (n=15) were anesthetized using 1 ml of 4% isoflurane for
induction of anesthesia, followed by 1 ml of 1.5% isoflurane for
maintenance and then euthanized by cervical dislocation. To confirm
verify animal death following euthanasia, examination of cardiac
arrest and reflex absence were assessed. No mice died unexpectedly
during the experiment. The colon tissues and serum from each group
were collected and subsequently frozen at -80˚C. All animal
experimental protocols were approved by the Animal Ethics Committee
of Jiangnan University [approval no. 20230830c1201005(355); Wuxi,
China].
Statistical analysis
All experiments were performed in triplicate and the
data are expressed as mean ± SEM. Statistical analysis was
performed using the SPSS software (version 26.0; IBM Corp.) and
data were presented using the GraphPad Prism software (version 8;
Dotmatics). The Shapiro-Wilk and Levene tests were used to test the
data normality and homogeneity of variance. If P>0.05, the data
were considered to be normally distributed, whereas if P>0.05,
the data were considered to have equal variance. The difference
among groups was determined using a one-way ANOVA followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
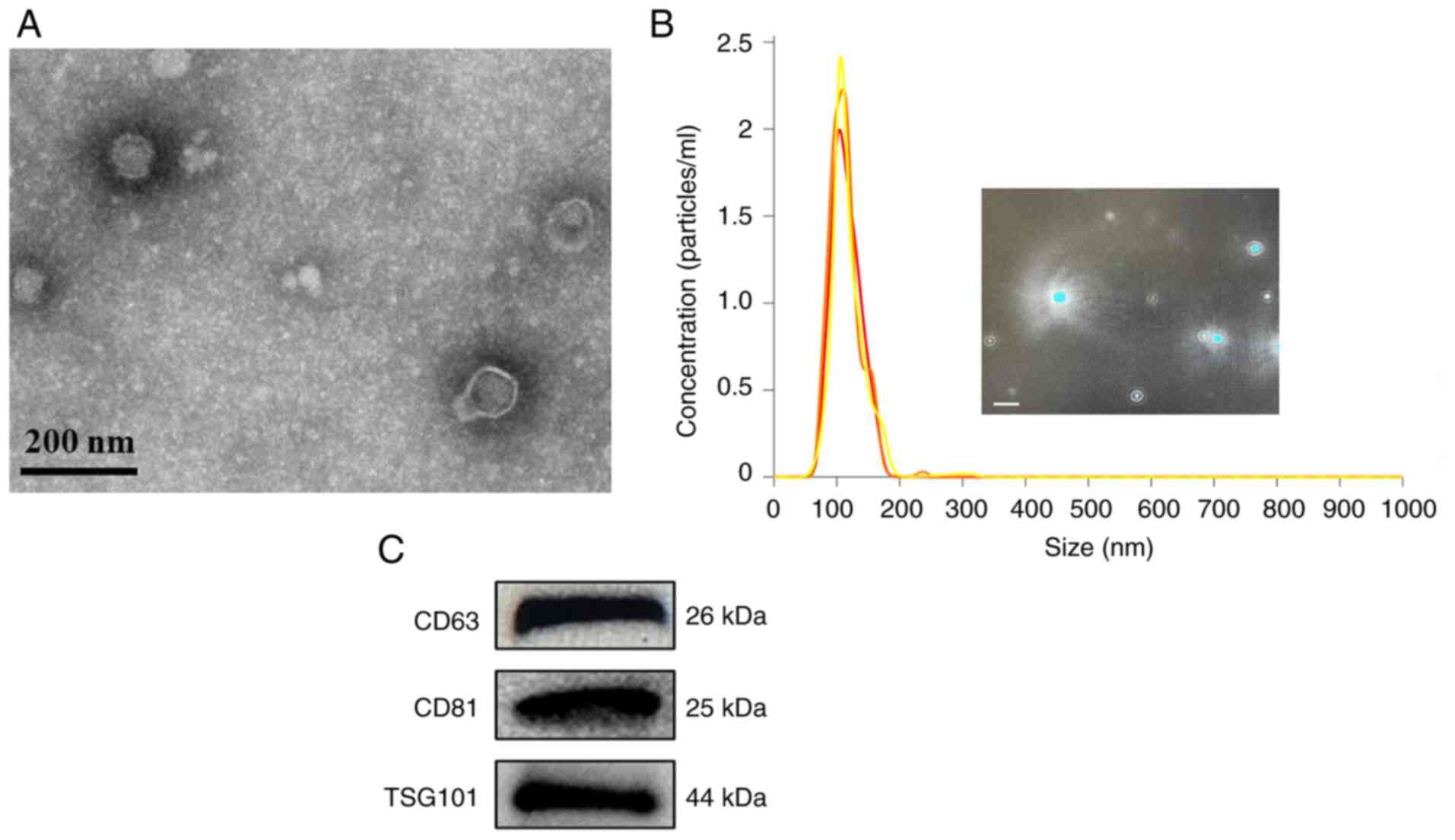
Preparation and characterization of
M-Exos
The protein concentration of the M-Exos extracted
was 1.0-1.5 mg/ml, as assessed using a BCA assay. TEM analysis
showed that the extracted M-Exos exhibited classical the Exo
structural feature of a round or elliptical cup-shaped morphology
(Fig. 1A). NTA demonstrated that
the diameter of the bovine M-Exos ranged from 20-150 nm, with a
particle size of 115.7±1.7 nm (Fig.
1B) and a particle concentration of 1.14x10-9
particles/ml. Western blot analysis demonstrated that the
Exo-enriched proteins CD63, CD81 and TSG101 were expressed in the
extracted M-Exos (Fig. 1C). These
results demonstrated the successful extraction of M-Exos, which
were used in subsequent experiments.
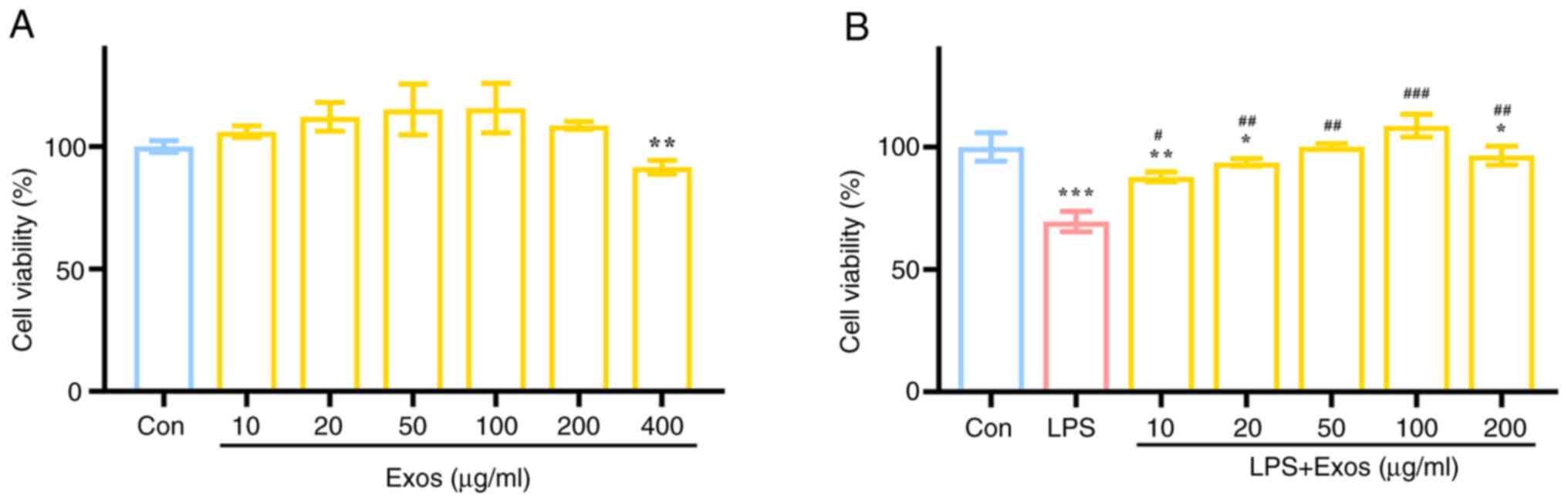
Effect of M-Exos on the proliferation
of RAW 264.7 cells
To assess the impact of M-Exos on the viability of
RAW 264.7 cells, a series of M-Exos concentrations (10, 20, 50,
100, 200 and 400 µg/ml) were added to the RAW 264.7 cell cultures
for 24 h. The objective was to evaluate the effect of M-Exos on the
viability of RAW 264.7 cells (Fig.
2A). Compared with the control group, the administration of
M-Exos from 0 to 200 µg/ml did not exert any significant adverse
effects on the viability of RAW 264.7 cells after 24 h of
co-culture. However, M-Exos at a concentration of 400 µg/ml caused
a significant decrease in the viability of RAW 264.7 cells.
Accordingly, the subsequent experiments were conducted using M-Exo
concentrations of 10, 20, 50, 100 and 200 µg/ml.
To evaluate the effect of M-Exos on the viability of
LPS-induced RAW 264.7 cells, different concentrations of M-Exos
(10, 20, 50, 100 and 200 µg/ml) were added to DMEM high-glucose
medium containing 1 µg/ml of LPS and cells were incubated for 24 h
and cell viability was assessed (Fig.
2B). Compared with the control group, the cell viability of the
LPS alone group was significantly reduced by ~40%. Significant
differences were observed in the cell viability of the groups
treated with different concentrations of M-Exos compared with the
LPS group (P<0.05). Increased M-Exos concentration caused an
increase in cell viability, with the highest level of cell
viability recorded at an M-Exo concentration of 100 µg/ml.
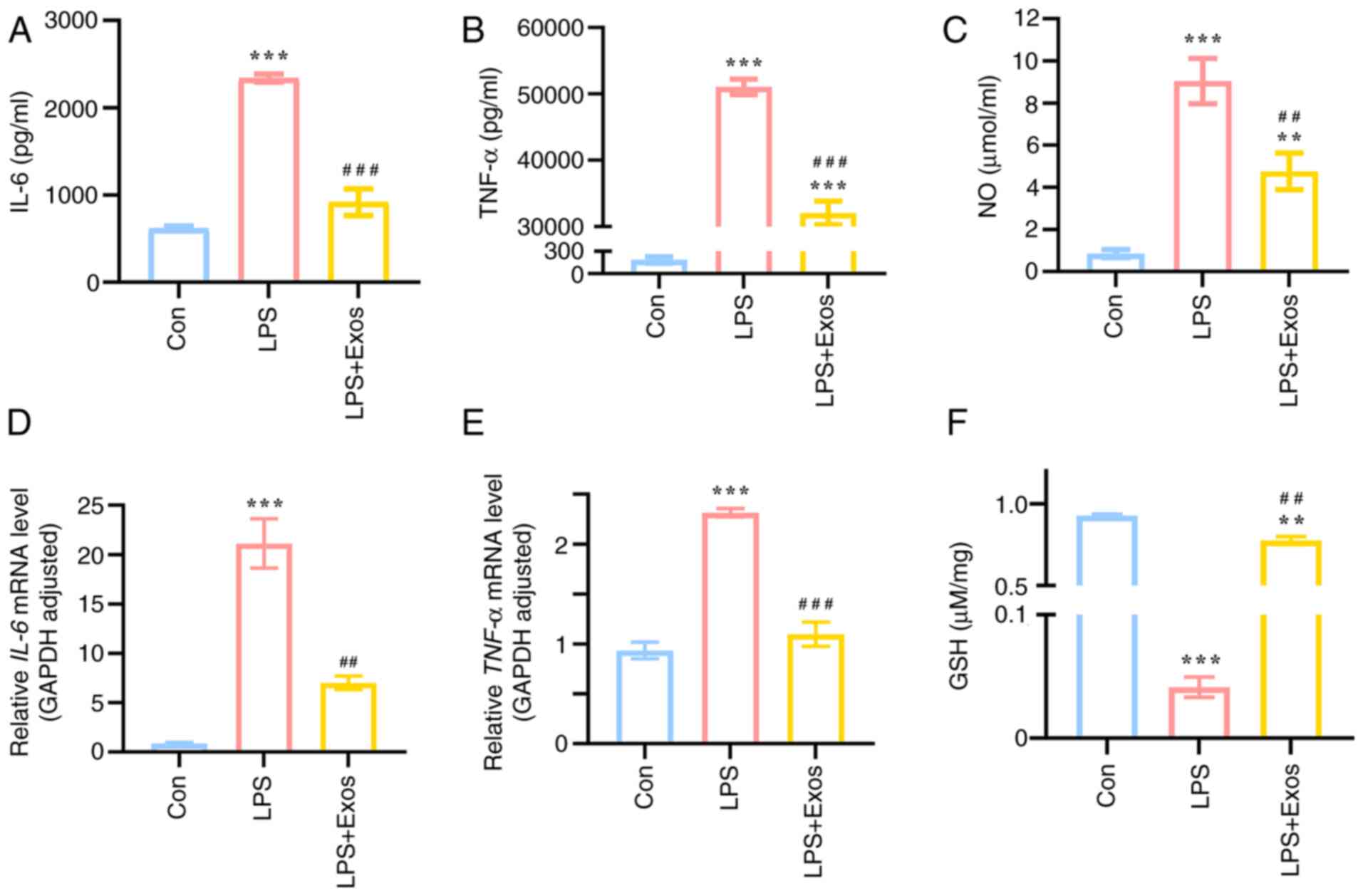
Effect of M-Exos on the expression of
inflammatory cytokines in RAW 264.7 cells
Compared with the control group, LPS stimulation
significantly increased the secretion of IL-6 and TNF-α
pro-inflammatory factors by RAW 264.7 cells (P<0.05; Fig. 3A and B). Following incubation with M-Exos, the
secretion of IL-6 and TNF-α decreased significantly, except in the
RAW 264.7 cells treated with the lowest concentration of M-Exos (25
µg/ml). Increased secretion of NO caused by LPS stimulation of RAW
264.7 cells was significantly reduced by M-Exo co-culture treatment
(P<0.05) (Fig. 3C). Following
LPS stimulation, the mRNA expression levels of IL-6 and
TNF-α in RAW264.7 cells increased significantly (Fig. 3D and E). Conversely, the expression levels of
IL-6 and TNF-α decreased significantly following
M-Exos treatment (P<0.05), except in lowest concentration group
(25 µg/ml), which was consistent with the ELISA results. Following,
M-Exo co-culture, the excessive consumption of GSH in RAW 264.7
cells stimulated by LPS was significantly recovered (P<0.05;
Fig. 3F). As the concentration of
M-Exos increased, the ability of M-Exos to inhibit the secretion of
inflammatory factors becomes more pronounced. This indicated that
M-Exos exert a dose-dependent effect on the cellular inflammatory
response. Accordingly, 100 µg/ml was selected as the optimal
concentration of M-Exos for subsequent studies on anti-inflammatory
activity. In conclusion, M-Exos may positively regulate the
expression levels of inflammatory factors in RAW 264.7 cells.
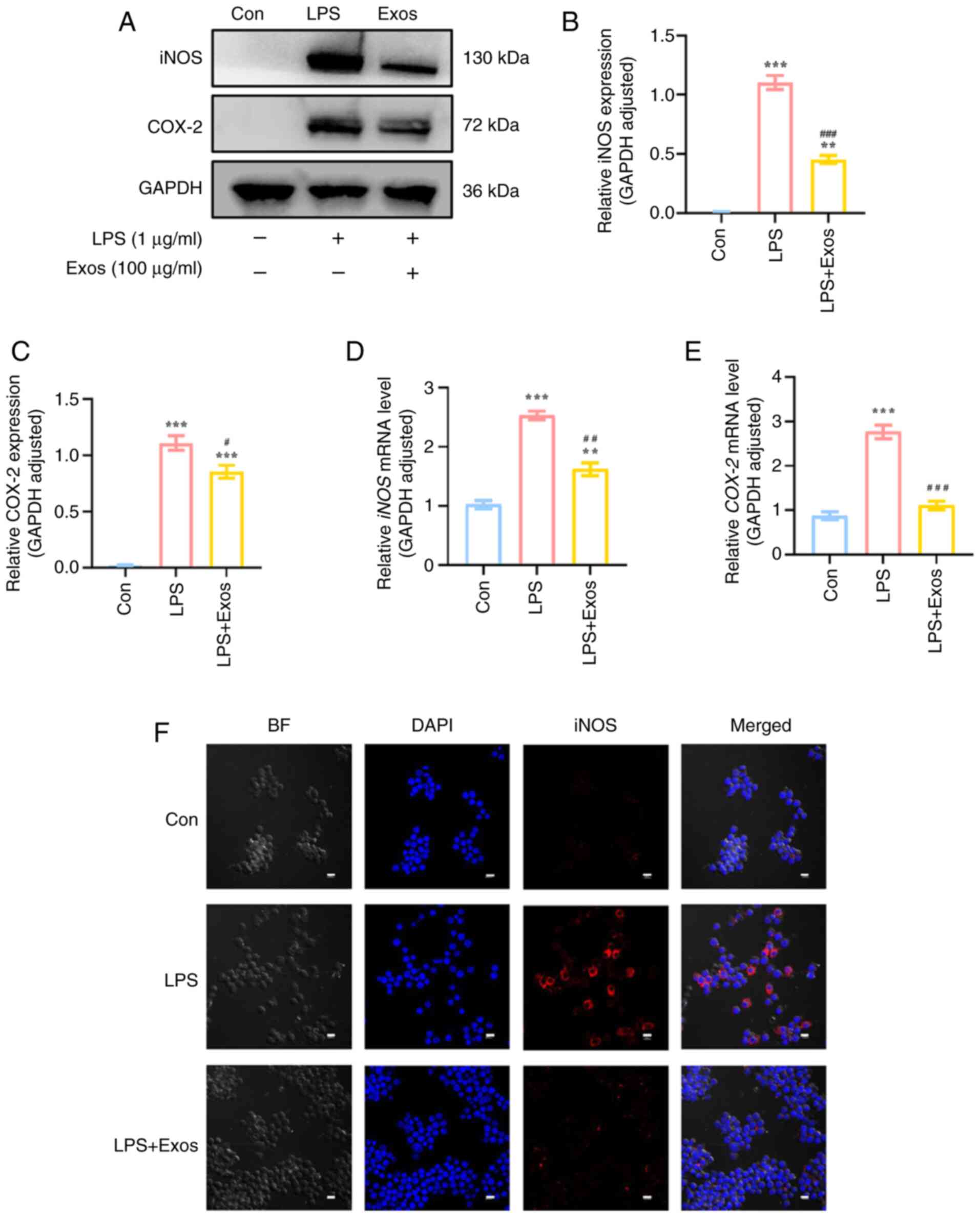
Effect of M-Exos on the expression of
oxidative stress factors in RAW 264.7 cells
The relative expression levels of iNOS and COX-2 in
RAW 264.7 cells were significantly increased in response to LPS
stimulation compared with the control group (P<0.05; Fig. 4). Compared with the LPS group, the
co-culture of M-Exos demonstrated a significant inhibitory effect
on the relative protein expression levels of iNOS and COX-2, with a
decrease of ~65 and 25%, respectively. Additionally, the mRNA
expression levels of iNOS and COX-2 were decreased
upon co-culture with M-Exos (P<0.05). After M-Exos co-culture,
the decreased expression of iNOS was verified using IF (Fig. 4F); these results were consistent
with the western blot and RT-qPCR results. These findings
demonstrated that M-Exos can inhibit the LPS-induced synthesis of
iNOS and COX-2 oxidative stress factors in RAW 264.7 cells.
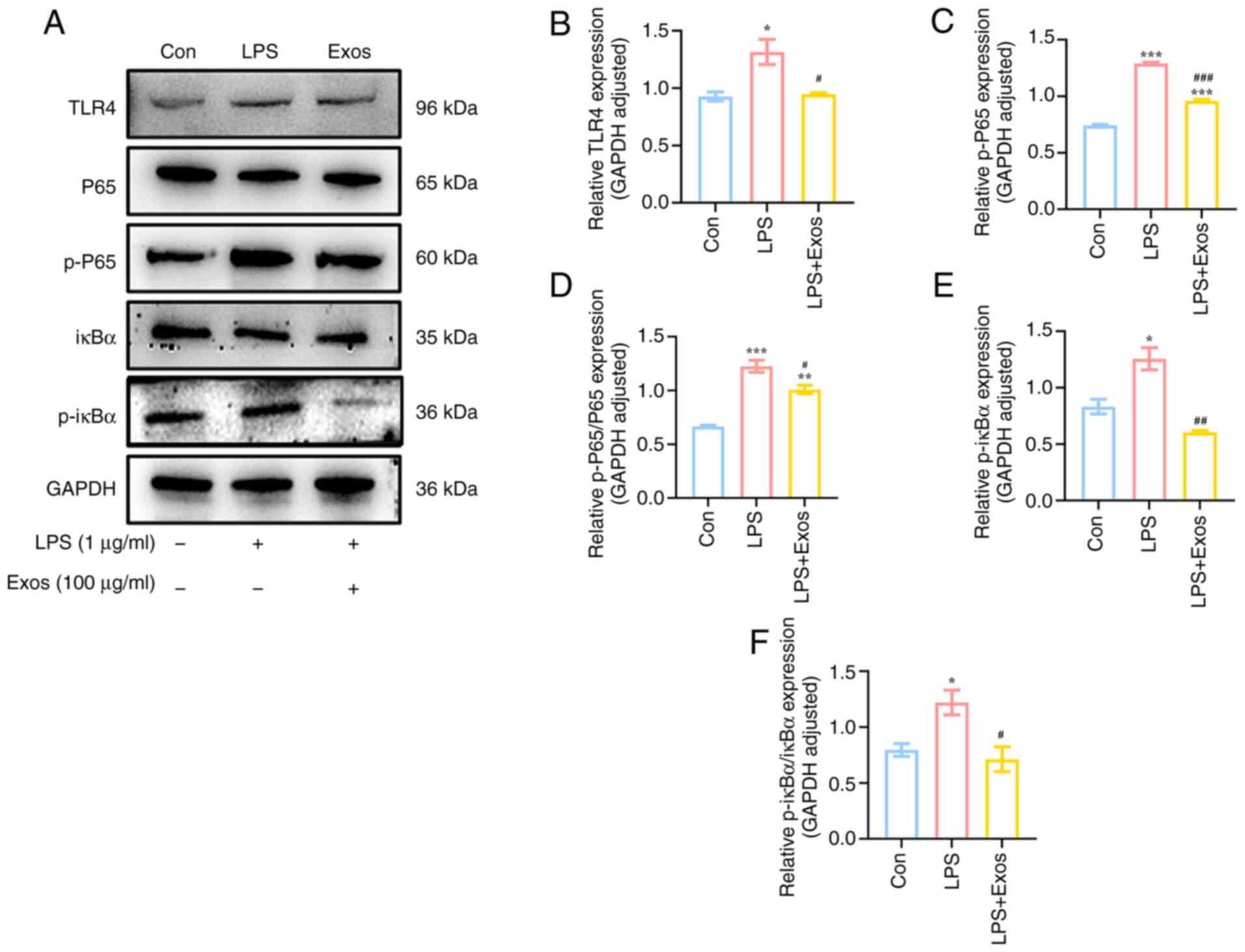
M-Exos decrease the LPS-induced
activation of the TLR4/NF-κB signaling pathway in RAW 264.7
cells
The TLR4/NF-κB signaling pathway serves a pivotal
role in the pathogenesis of LPS-induced inflammation in RAW 264.7
cells (21). Compared with the
control group, the TLR4 receptor on the surface of macrophages was
activated following LPS stimulation (Fig. 5), which was associated with the
significantly increased phosphorylation of downstream proteins p65
and IκBα. Conversely, following M-Exos co-culture, the expression
levels of related proteins (TLR4, p-p65 and p-iκBα) in this
signaling pathway were significantly decreased (P<0.05). These
findings demonstrated that M-Exos could impede the activation of
the TLR4/NF-κB signaling pathway, thereby exerting a role in
anti-inflammatory processes.
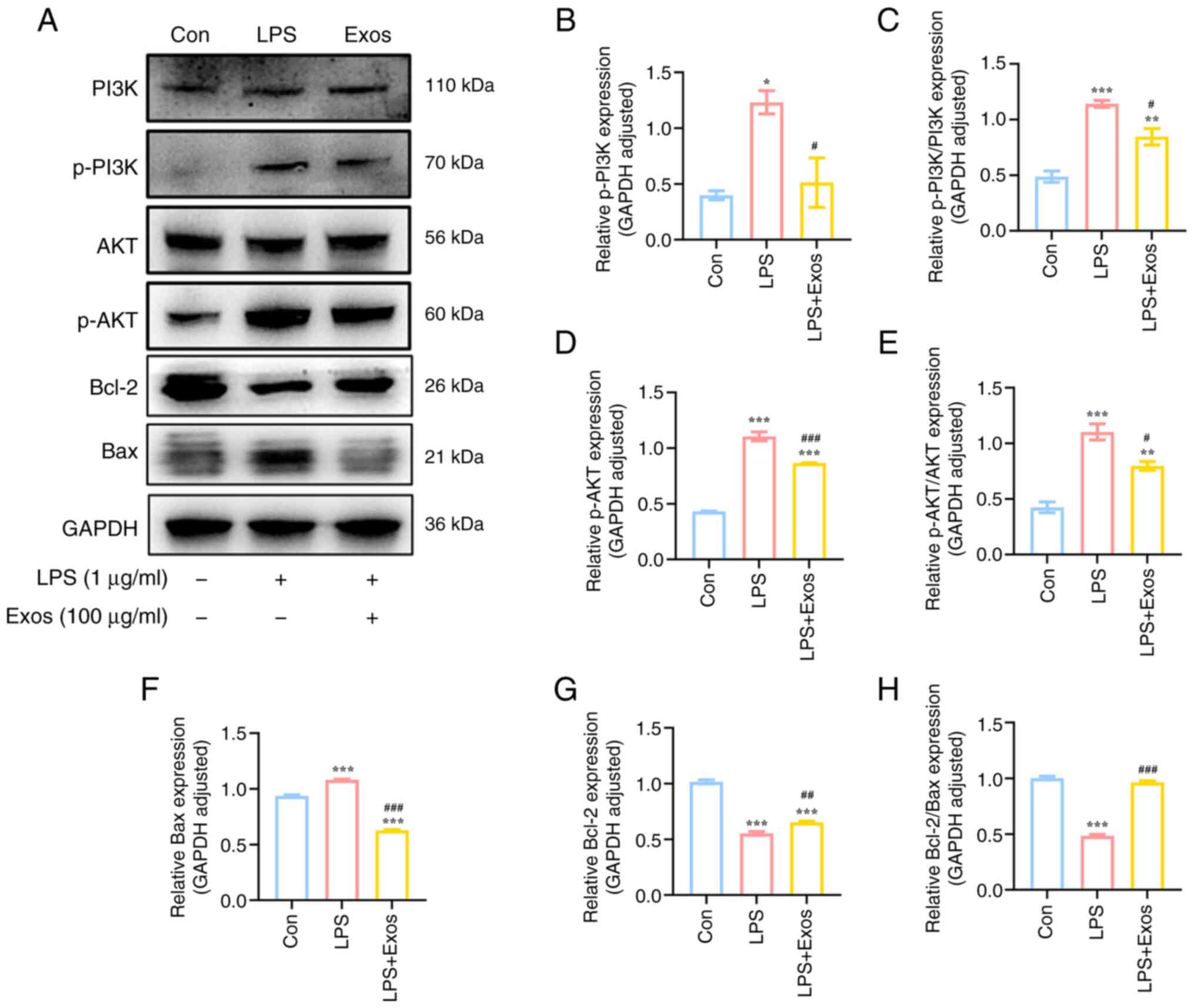
M-Exos inhibit the LPS-induced
activation of the PI3K/AKT signaling pathway in RAW 264.7
cells
The PI3K/AKT signaling pathway is key in regulating
the proliferation and apoptosis of macrophages. This pathway is
also associated with the development of inflammation (23). The activation state of the PI3K/AKT
signaling pathway in RAW264.7 cells was therefore analyzed in the
present study. Compared with the control group, LPS stimulation
significantly increased the expression levels of the phosphorylated
proteins PI3K and AKT (Fig. 6).
Conversely, the expression levels of the phosphorylated proteins
PI3K and AKT significantly decreased following co-culture with
M-Exos (P<0.05). These findings demonstrated that M-Exos may
control the advancement of the PI3K/AKT signaling pathway and
regulate the progression of inflammation.
M-Exos reduce LPS-induced apoptosis in
RAW 264.7 cells
LPS has been reported to stimulate RAW 264.7 cells
to secrete certain inflammatory factors, leading to the occurrence
of abnormal apoptosis. Proteins associated with apoptosis are also
regulated by the NF-κB and PI3K/AKT signaling pathways (28,29).
The ratio of Bcl-2/Bax protein expression levels in the LPS group
was significantly decreased compared with the control group
(P<0.05; Fig. 6). These
findings indicated that LPS stimulation resulted in increased
apoptosis. However, the co-culture with M-Exos reversed this trend.
Therefore, M-Exos could inhibit the apoptosis of RAW 264.7 cells
induced by LPS. M-Exos inhibited LPS-induced apoptosis in RAW 264.7
macrophages by restoring Bcl-2/Bax ratio and modulating
NF-κB/PI3K/AKT pathways, demonstrating therapeutic potential
against LPS-induced inflammation.
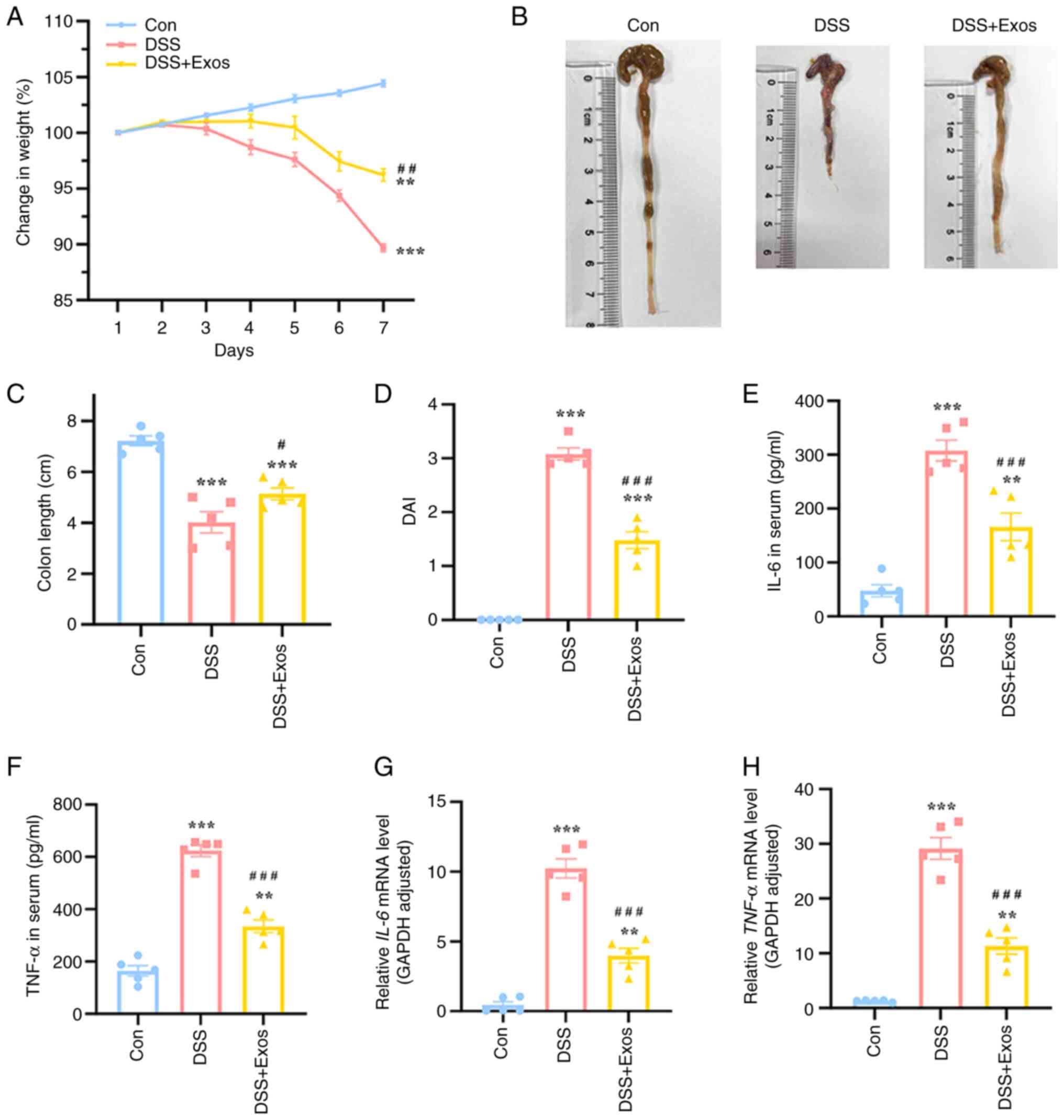
Effects of M-Exos on the inflammation
of DSS-induced colitis in mice
The body weight of mice in each treatment group was
measured and these results showed that the weight of mice in the
control group increased steadily and gradually over time (Fig. 7A). By contrast, the weight of mice
consuming DSS began to decline on the 3rd day of treatment. By the
7th day, the average weight of mice in the DSS group had decreased
by ~10% compared with their weight at the beginning of the
experiment, while the weight of mice in the Exos group, which
received treatment concurrently, decreased by ~5%, demonstrating a
significant improvement in body weight loss compared with the DSS
group. Furthermore, the colon length of mice administered with 3%
DSS was significantly reduced compared with the control group,
whereas the Exos group exhibited a notable increased in colon
length, counteracting the shortening caused by DSS (P<0.05;
Fig. 7B and C). Based on weight loss, the presence of
sticky stools and occult blood in the stool, the DAI scores of
DSS-fed mice were significantly increased compared with the
control, while those of M-Exos-fed mice were significantly
decreased compared with the DSS group (P<0.05; Fig. 7D). To investigate the secretion of
intestinal inflammatory factors, the levels of certain inflammatory
factors in the serum of mice were measured. The serum levels of
IL-6 and TNF-α in the DSS group and the expression levels of
IL-6 and TNF-α in colon tissue were also
significantly increased compared with the control (Fig. 7E-H). By contrast, the Exos group
exhibited a significant decrease in both the secretion and
transcription of the aforementioned inflammatory factors compared
with the DSS group (P<0.05). To summarize, M-Exos could
effectively reduce inflammation levels in a mouse colitis
model.
Discussion
Purified M-Exos were extracted using a protocol
combining the isoelectric-point precipitation protein method with
ultra-high-speed refrigerated centrifugation. The pH of the
defatted milk solution was adjusted to 4.6 using a diluted
hydrochloric acid solution to reach the isoelectric point of casein
(30), thereby precipitating and
removing protein. NTA is an emerging nanoscale identification
technology whereby particles are tracked and analyzed using
high-speed cameras and software (31). The results of the present study
demonstrated that the particle-size distribution of M-Exos was
narrow, which indicated a relatively uniform size, suggesting their
possible utilization for subsequent research. Western blotting is a
commonly used method for the identification of M-Exos. In the
present study, the expression of Exo-related marker proteins,
including the transmembrane proteins CD63 and CD81 and the
membrane-transport complex protein TSG101, were detected in the
extracted nanoparticles. Therefore, the nanoparticles were those of
M-Exos. A cup-shaped saucer structure was observed using TEM, which
confirmed the successful extraction of M-Exos and demonstrated that
the particle-size distribution was consistent with that detected by
NTA. These findings indicated that the M-Exos extracted in the
present study met the three-step identification criteria
established by the International Society for Extracellular Vesicles
(32). Consequently, M-Exos have
the potential to be utilized for subsequent research and
applications.
The inflammatory response is a vital mechanism for
combating infection and injury. This process is precisely regulated
to enable the body to resist pathogens and restore tissue
homeostasis. Macrophages, innate immune cells with a tissue
residence, serve a pivotal role in the regulation of inflammation
(33). It has been reported that
M-Exos can enhance macrophage activity, stimulate cell
proliferation and suppress the inflammatory response to pathogens
(34). Matic et al
(35) demonstrated that M-Exos
exhibit stability under both normal oxygen levels and hypoxia. In
the presence of sufficient oxygen, the proliferation of RAW 264.7
cells is markedly enhanced, whereas cisplatin-induced apoptosis is
effectively inhibited (36). The
administration of M-Exos under hypoxic conditions has been
demonstrated to markedly diminish the production of ROS by RAW264.7
cells, thereby facilitating the restoration of cellular activity
(37).
In the present study, macrophages were polarized to
a pro-inflammatory phenotype using 1 µg/ml of LPS. These results
demonstrated a notable decline in cell viability within the
LPS-induced inflammatory model group, which suggested that LPS
stimulation may potentially induce macrophage apoptosis.
Concurrently, the co-culture of M-Exos with RAW 264.7 cells
demonstrated the capacity to effectively restore and enhance cell
viability, which indicated that M-Exos could reverse the
LPS-induced decline in macrophage viability. The optimal
concentration of M-Exos for cell viability recovery was 100 µg/ml.
This concentration was subsequently used to investigate the impact
of M-Exos concentration on the macrophage inflammatory
response.
NO is a primary mediator of the oxidative stress
response and has the potential to participate in the inflammatory
response by exacerbating inflammation (36). The activation of macrophages by LPS
has been reported to enhance the development of pro-inflammatory
macrophages and increase the production of NO. An excess of NO
release reacts with superoxide anion to generate peroxynitrite,
which further encourages the production of inflammatory factors
such as IL-6 and TNF-α (37). The
outcomes of this process are damage to local tissue and an
exacerbated inflammatory response (37). The release of inflammatory factors
further leads to increased oxidative stress, which in turn
diminishes the management of the GSH antioxidant system.
Consequently, there is a disruption in the intracellular redox
system, which heightens the inflammatory response (38). The results of the present study
demonstrated that LPS stimulation increased NO levels. The addition
of M-Exos to RAW 264.7 macrophages significantly inhibited the
production of NO and the increase in expression levels of
pro-inflammatory factors TNF-α and IL-6, thereby restoring
intracellular GSH. The expression of iNOS directly determines the
secretion of NO and thus serves as an important indicator for
detecting oxidative stress in inflammatory reactions (39,40).
COX-2 is an inducible enzyme that serves a crucial role in
inflammatory reactions by catalyzing the conversion of arachidonic
acid into prostaglandins (41).
Therefore, the inflammatory response can be effectively controlled
through the reduction or inhibition of iNOS and COX-2 activation.
The results of the present study experiment demonstrated that the
expression levels of iNOS and COX-2 in cells of the M-Exos-treated
group were significantly lower compared with those of the
LPS-treated group. This indicated that M-Exos served an
anti-inflammatory role by restricting the expression of iNOS and
COX-2.
TLR4 is found in almost all cell lines, with a
particularly high abundance in cells involved in host defense
functions, such as macrophages (42). NF-κB is a principal transcriptional
regulator of the mammalian immune system. NF-κB facilitates the
migration of immune cells into inflammatory tissues, induces the
expression of iNOS in response to stimulation and produces certain
anti-apoptotic proteins to prevent apoptosis (43). Upon recognition of LPS by TLR4, the
IKKβ subunit within the intracellular heterotrimeric complex IκB
kinase is activated and phosphorylated, subsequently
phosphorylating IκBα. Following the degradation of IκBα, which
leads to the phosphorylation and proteasome degradation of IκB,
NF-κB translocates into the nucleus where NF-κB induces the
expression of IL-6, TNF-α and other inflammatory cytokines
(21,43). The present results indicated that
M-Exos could reduce TLR4 expression levels, NF-κB pathway
activation and signal transduction by inhibiting IκBα
phosphorylation and nuclear translocation of the NF-κB-p65 subunit
in LPS-primed macrophages. Furthermore, the secretion of IL-6,
TNF-α and NO was inhibited by the NF-κB signaling pathway (44).
PI3K is a cell-membrane-bound enzyme with
serine/threonine kinase and phosphatidylinositol kinase activities.
Upon binding to TLR4, LPS induces the phosphorylation of PI3K,
leading to its activation (45).
This activates downstream protein kinases, thereby initiating
downstream signaling pathways. The PI3K/AKT signaling pathway is a
common downstream signaling pathway, which serves an important
regulatory role in a range of cellular processes, including cell
proliferation, apoptosis, metabolism and migration (23). Upon activation, AKT phosphorylates
various target proteins such as the Bcl-2 family of proteins,
thereby exerting a wide range of effects on cells, including the
promotion of cell survival and inhibition of apoptosis (46,47).
AKT can also facilitate activation of IκB kinase, leading to IκB
degradation (48). This results in
the release of NF-κB from the cytoplasm for nuclear translocation,
thereby exacerbating the overactivation of the TLR4/NF-κB signaling
pathway. In the present study, following stimulation with LPS, the
TLR4 receptor on the surface of the macrophages was activated. This
caused phosphorylation of the downstream proteins P65 and IκBα,
which increased the expression levels of p-PI3K and p-AKT proteins.
Ultimately, these phenomena manifested as the upregulation of the
expression of a range of inflammatory cytokines.
The activation of the TLR4/NF-κB and PI3K/AKT
inflammatory signaling pathways results in the creation of an
abnormal physiological and metabolic environment within cells,
ultimately leading to apoptosis (28). It has been reported that in
inflammatory diseases, therapeutic agents can exert
anti-inflammatory effects by downregulating TLR4 expression levels
and inhibiting the PI3K/AKT signaling pathway (29). The results of the present study
showed that M-Exos reduced the phosphorylation levels of PI3K and
AKT in LPS-induced RAW 264.7 cells, which indicated that M-Exos
regulated inflammation by inhibiting activation of the PI3K/AKT
signaling pathway. This may be related to certain exosome
components, such as specific miRNAs and proteins. Notably, M-Exos
are enriched in immune-related miRNAs, which impact immunity and
regulate inflammatory signaling pathways (49). In a murine colitis model, miR-146b
alleviated intestinal inflammation and improved the intestinal
epithelial barrier via the NF-κB pathway, providing beneficial
effects in preventing colitis (50). It has been reported that
M-Exos-derived miR-155 and miR-148 can regulate the expression of
intestinal cytokines and the T cell immune response (51).
M-Exos also contain various proteins that
participate in the regulation of signaling pathways (52). CD63 and Alix influence the
activation of the NF-κB signaling pathway by modulating the
interaction between M-Exos and TLR4 receptors (53). Heat shock proteins (HSPs), such as
HSP70 and HSP90, regulate the PI3K/AKT signaling pathway, enabling
cells to adapt to environmental stress, suppress apoptosis and
exert anti-inflammatory effects (53). In summary, M-Exos exert
anti-inflammatory effects by regulating the TLR4/NF-κB and PI3K/AKT
signaling pathways through their specific miRNAs and proteins.
miRNAs control the activity of signaling pathways by regulating the
expression of target genes, while proteins affect various stages of
signal transduction by directly interacting with receptor cells
(49-52).
The synergistic effect of these components helps M-Exos to
alleviate inflammatory reactions (8).
In the present study, following treatment with
M-Exos, the expression levels of certain key proteins within the
TLR4/NF-κB and PI3K/AKT inflammatory signaling pathways were
markedly diminished, thereby considerably reducing the expression
levels of the pro-apoptotic protein Bax. By contrast, the
expression levels of the anti-apoptotic protein Bcl-2 were
increased. Future research should include a more in-depth analysis
of how specific miRNAs and proteins in M-Exos exert
anti-inflammatory effects by regulating target genes, in order to
enhance the current understanding of the anti-inflammatory
mechanisms of M-Exos. In addition, the present study used a UC
mouse model to demonstrate that M-Exos reversed inflammation and
the adverse consequences of colon shortening in UC mice. Colon
length is an important index to evaluate colitis diseases (54), indicating that M-Exo intervention
could alleviate colitis caused by DSS. Currently, commonly used
anti-inflammatory drugs in clinical practice include non-steroidal
anti-inflammatory drugs, corticosteroids and plant-derived
anti-inflammatory compounds. However, long-term use of certain
anti-inflammatory drugs (e.g. aspirin, ibuprofen and acetaminophen)
can lead to side effects such as gastrointestinal damage, renal
function damage and metabolic disorder, while others face
significant limitations in bioavailability and stability in
vivo (55,56).
M-Exos are food-derived, easy to prepare and exhibit
high biocompatibility and low immunogenicity while demonstrating
anti-inflammatory properties (57). M-Exos have emerged as ideal
candidates for anti-inflammatory therapies, particularly in chronic
inflammatory conditions requiring long-term treatment.
Additionally, M-Exos can serve as drug delivery vehicles to
transport anti-inflammatory molecules, miRNAs or small-molecule
drugs directly to inflammation sites. This targeted approach
improves bioavailability, reduces adverse effects and allows for
synergistic anti-inflammatory effects with co-administered
therapeutics (57). It has
previously been demonstrated that feeding mice a diet rich in
M-Exos can promote cell proliferation via the mTOR-AKT signaling
pathway. This diet can also alleviate growth inhibition caused by
nutritional deficiencies and fulfill the dietary needs of
malnourished individuals (58).
Furthermore, M-Exos have the potential to support the maturation of
the immune system and enhance the proliferation of immune cells
(59). Consequently, in the
treatment of inflammation-related diseases, M-Exos could
potentially be utilized as part of an adjuvant immunomodulatory
therapy in conjunction with nutritional interventions.
With further research into the molecular mechanisms
and clinical applications of M-Exos, these substances hold promise
as a component of therapeutic strategies for inflammatory diseases,
particularly in conditions such as LPS-induced systemic
inflammatory response syndrome, lung injury, UC and wound healing.
Proteomics and micronutrient analysis should be used to further
investigate M-Exos, as these techniques can offer additional
insights into their effects on the immune system and nutrition.
In conclusion, M-Exos were extracted by protein
isoelectric-point precipitation and ultracentrifugation. An
inflammatory model of RAW 264.7 cells was established by LPS
stimulation. The effects of M-Exos on LPS-induced inflammation and
oxidative stress in RAW 264.7 cells, in addition to the regulation
of associated signal pathways, were investigated. M-Exos inhibited
the secretion and expression of the pro-inflammatory factors IL-6
and TNF-α in RAW 264.7 cells. M-Exos reduced the level of NO, as
well as the expression levels of the oxidative stress factors iNOS
and COX-2. Additionally, M-Exos improved the function of
antioxidant enzymes, inhibited the initiation of the inflammatory
TLR4/NF-κB and PI3K/AKT signaling pathways and inhibited apoptosis
by regulating Bcl-2/Bax levels. Overall, the present study
contributed to the current understanding of M-Exos in
anti-inflammatory, anti-oxidative and anti-apoptotic processes,
thereby providing valuable insights into the potential
applications.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Postgraduate
Research & Practice Innovation Program of Jiangsu Province
(grant no. KYCX24_2650).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XC and CD conceived and designed the study. XC
performed data collection. XC, QS, RZ, YS, ZL and NL performed
analysis and interpretation of results. XC and CD undertook draft
manuscript preparation. All authors read and approved the final
version of the manuscript. XC and RZ confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
All animal experimental protocols were approved by
the Animal Ethics Committee of Jiangnan University [approval no.
20230830c1201005(355); Wuxi, China].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Karin M and Clevers H: Reparative
inflammation takes charge of tissue regeneration. Nature.
529:307–315. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Panigrahy D, Gilligan MM, Serhan CN and
Kashfi K: Resolution of inflammation: An organizing principle in
biology and medicine. Pharmacol Ther. 227(107879)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Grosso G, Laudisio D, Frias-Toral E,
Barrea L, Muscogiuri G, Savastano S and Colao A: Anti-inflammatory
nutrients and obesity-associated metabolic-inflammation: State of
the art and future direction. Nutrients. 14(1137)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Han R, Xiao Y, Bai Q and Choi CHJ:
Self-therapeutic metal-based nanoparticles for treating
inflammatory diseases. Acta Pharm Sin B. 13:1847–1865.
2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Khan IT, Nadeem M, Imran M, Ayaz M, Ajmal
M, Ellahi MY and Khalique A: Antioxidant capacity and fatty acids
characterization of heat treated cow and buffalo milk. Lipids
Health Dis. 16(163)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Khan IT, Nadeem M, Imran M, Ullah R, Ajmal
M and Jaspal MH: Antioxidant properties of milk and dairy products:
A comprehensive review of the current knowledge. Lipids Health Dis.
18(41)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hess JM, Stephensen CB, Kratz M and
Bolling BW: Exploring the links between diet and inflammation:
Dairy foods as case studies. Adv Nutr. 12 (Suppl 1):S1–S13.
2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhong Y, Wang X, Zhao X, Shen J, Wu X, Gao
P, Yang P, Chen J and An W: Multifunctional milk-derived small
extracellular vesicles and their biomedical applications.
Pharmaceutics. 15(1418)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Betker JL, Angle BM, Graner MW and
Anchordoquy TJ: The potential of exosomes from cow milk for oral
delivery. J Pharm Sci. 108:1496–505. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim NH, Kim J, Lee JY, Bae HA and Kim CY:
Application of milk exosomes for musculoskeletal health: Talking
points in recent outcomes. Nutrients. 15(4645)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
van Herwijnen MJC, Driedonks TAP, Snoek
BL, Kroon AMT, Kleinjan M, Jorritsma R, Pieterse CMJ, Hoen E and
Wauben MHM: Abundantly present miRNAs in milk-derived extracellular
vesicles are conserved between mammals. Front Nutr.
5(81)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Taketoshi H, Kosuke M, Hajime N, Yasunari
Y, Tsukasa M and Naohito A: Isolation of bovine milk-derived
microvesicles carrying mRNAs and microRNAs. Biochem Biophys Res
Commun. 396:528–533. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ngu A, Wang S, Wang H, Khanam A and
Zempleni J: Milk exosomes in nutrition and drug delivery. Am J
Physiol Cell Physiol. 322:C865–C874. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ghorbani S, Talebi F, Chan WF, Masoumi F,
Vojgani M, Power C and Noorbakhsh F: MicroRNA-181 variants regulate
T cell phenotype in the context of autoimmune neuroinflammation.
Front Immunol. 8(758)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yu Z, Teng Y, Yang J and Yang L: The role
of exosomes in adult neurogenesis: Implications for
neurodegenerative diseases. Neural Regen Res. 19:282–288.
2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Reif S, Elbaum-Shiff Y, Koroukhov N, Shilo
I, Musseri M and Golan-Gerstl R: Cow and human milk-derived
exosomes ameliorate colitis in DSS murine model. Nutrients.
12(2589)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ocansey DKW, Zhang L, Wang Y, Yan Y, Qian
H, Zhang X, Xu W and Mao F: Exosome-mediated effects and
applications in inflammatory bowel disease. Biol Rev Camb Philos
Soc. 95:1287–1307. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yurakova TR, Gorshkova EA, Nosenko MA and
Drutskaya MS: Metabolic adaptations and functional activity of
macrophages in homeostasis and inflammation. Biochemistry (Mosc).
89:817–138. 2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nomura F, Akashi S, Sakao Y, Sato S, Kawai
T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K and Akira
S: Cutting edge: Endotoxin tolerance in mouse peritoneal
macrophages correlates with down-regulation of surface toll-like
receptor 4 expression. J Immunol. 164:3476–3479. 2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bongartz H, Bradfield C, Gross J, Fraser
IDC, Nita-Lazar A and Meier-Schellersheim M: IL-10 dependent
adaptation allows macrophages to adjust inflammatory responses to
TLR4 stimulation history bioRxiv (Preprint): 2024.03.28.587272,
2024.
|
|
21
|
Zusso M, Lunardi V, Franceschini D,
Pagetta A, Lo R, Stifani S, Frigo AC, Giusti P and Moro S:
Ciprofloxacin and levofloxacin attenuate microglia inflammatory
response via TLR4/NF-kB pathway. J Neuroinflammation.
16(148)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Malemud CJ: Intracellular signaling
pathways in rheumatoid arthritis. J Clin Cell Immunol.
4(160)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Vanhaesebroeck B, Guillermet-Guibert J,
Graupera M and Bilanges B: The emerging mechanisms of
isoform-specific PI3K signalling. Nat Rev Mol Cell Biol.
11:329–341. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Li D, Yao S, Zhou Z, Shi J, Huang Z and Wu
Z: Hyaluronan decoration of milk exosomes directs tumor-specific
delivery of doxorubicin. Carbohydr Res. 493(108032)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wolf T, Baier SR and Zempleni J: The
intestinal transport of bovine milk exosomes is mediated by
endocytosis in human colon carcinoma caco-2 cells and rat small
intestinal IEC-6 cells. J Nutr. 145:2201–2206. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Deng C, Hu Y, Conceicao M, Wood MJA, Zhong
H, Wang Y, Shao P, Chen J and Qiu L: Oral delivery of
layer-by-layer coated exosomes for colitis therapy. J Control
Release. 354:635–650. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rex J, Lutz A, Faletti LE, Albrecht U,
Thomas M, Bode JG, Borner C, Sawodny O and Merfort I: IL-1β and
TNFα differentially influence NF-κB activity and fasl-induced
apoptosis in primary murine hepatocytes during LPS-induced
inflammation. Front Physiol. 10(117)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yuan X, Juan Z, Zhang R, Sun X, Yan R, Yue
F, Huang Y, Yu J and Xia X: Clemastine fumarate protects against
myocardial ischemia reperfusion injury by activating the
TLR4/PI3K/AKT signaling pathway. Front. Pharmacol.
11(28)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yelubaeva MY, Buralkhiev BA, Serikbayeva
AD, Narmuratova MH and Kenenbay SY: Electrophoretic identification
of casein in various types of milk. J Biol.Chem. 17:348–352.
2017.
|
|
31
|
Tian MY, Hao DX, Liu Y, He J, Zhao ZH, Guo
TY, Li X and Zhang Y: Milk exosomes: An oral drug delivery system
with great application potential. Food Funct. 14:1320–1337.
2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lai JJ, Chau ZL, Chen SY, Hill JJ, Korpany
KV, Liang NW, Lin LH, Lin YH, Liu JK, Liu YC, et al: Exosome
processing and characterization approaches for research and
technology development. Adv Sci (Weinh). 9(e2103222)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rodríguez-Morales P and Franklin RA:
Macrophage phenotypes and functions: Resolving inflammation and
restoring homeostasis. Trends Immunol. 44:986–998. 2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Salehi M, Negahdari B, Mehryab F and
Shekari F: Milk-derived extracellular vesicles: bomedical
applications, current challenges, and future perspectives. J Agric
Food Chem. 72:8304–8331. 2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Matic S, D'Souza DH, Wu T, Pangloli P and
Dia VP: Bovine milk exosomes affect proliferation and protect
macrophages against cisplatin-induced cytotoxicity. Immunol Invest.
49:711–725. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tsai CF, Chen GW, Chen YC, Shen CK, Lu DY,
Yang LY, Chen JH and Yeh WL: Regulatory effects of quercetin on
M1/M2 macrophage polarization and oxidative/antioxidative balance.
Nutrients. 14(67)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Torregrosa Paredes P, Esser J, Admyre C,
Nord M, Rahman QK, Lukic A, Rådmark O, Grönneberg R, Grunewald J,
Eklund A, et al: Bronchoalveolar lavage fluid exosomes contribute
to cytokine and leukotriene production in allergic asthma. Allergy.
67:911–919. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang M, Hu W, Cai C, Wu Y, Li J and Dong
S: Advanced application of stimuli-responsive drug delivery system
for inflammatory arthritis treatment. Mater Today Bio.
14(100223)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Malik R, Paudel KR, Manandhar B, De Rubis
G, Shen J, Mujwar S, Singh TG, Singh SK, Gupta G, Adams J, et al:
Agarwood oil nanoemulsion counteracts LPS-induced inflammation and
oxidative stress in RAW264.7 mouse macrophages. Pathol Res Pract.
251(154895)2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Izadparast F, Riahi-Zajani B, Yarmohammadi
F, Hayes AW and Karimi G: Protective effect of berberine against
LPS-induced injury in the intestine: A review. Cell Cycle.
21:2365–2378. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang R, Wang N, Han Y, Xu J and Xu Z:
Dulaglutide alleviates LPS-induced injury in cardiomyocytes. ACS
Omega. 6:8271–8278. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fitzgerald KA and Kagan JC: Toll-like
receptors and the control of immunity. Cell. 180:1044–1066.
2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Stierschneider A and Wiesner C: Shedding
light on the molecular and regulatory mechanisms of TLR4 signaling
in endothelial cells under physiological and inflamed conditions.
Front Immunol. 14(1264889)2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wu Z, Mehrabi Nasab E, Arora P and Athari
SS: Study effect of probiotics and prebiotics on treatment of
OVA-LPS-induced of allergic asthma inflammation and pneumonia by
regulating the TLR4/NF-kB signaling pathway. J Transl Med.
20(130)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhong J, Qiu X, Yu Q, Chen H and Yan C: A
novel polysaccharide from Acorus tatarinowii protects against
LPS-induced neuroinflammation and neurotoxicity by inhibiting
TLR4-mediated MyD88/NF-κB and PI3K/AKT signaling pathways. Int J
Biol Macromol. 163:464–475. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Acosta-Martinez M and Cabail MZ: The
PI3K/AKT pathway in meta-inflammation. Int J Mol Sci.
23(15330)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
He C, Wang K, Xia J, Qian D, Guo J, Zhong
L, Tang D, Chen X, Peng W, Chen Y and Tang Y: Natural exosomes-like
nanoparticles in mung bean sprouts possesses anti-diabetic effects
via activation of PI3K/AKT/GLUT4/GSK-3β signaling pathway. J
Nanobiotechnology. 21(349)2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhong R, Xia T, Wang Y, Ding Z, Li W, Chen
Y, Peng M, Li C, Zhang H and Shu Z: Physalin B ameliorates
inflammatory responses in lipopolysaccharide-induced acute lung
injury mice by inhibiting NF-κB and NLRP3 via the activation of the
PI3K/AKT pathway. J Ethnopharmacol. 284(114777)2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhou Q, Li M, Wang X, Li Q, Wang T, Zhu Q,
Zhou X, Wang X, Gao X and Li X: Immune-related microRNAs are
abundant in breast milk exosomes. Int J Biol Sci. 8:118–123.
2012.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Nata T, Fujiya M, Ueno N, Moriichi K,
Konishi H, Tanabe H, Ohtake T, Ikuta K and Kohgo Y: MicroRNA-146b
improves intestinal injury in mouse colitis by activating nuclear
factor-κB and improving epithelial barrier function. J Gene Med.
15:249–2460. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Melnik BC and Schmitz G: Exosomes of
pasteurized milk: Potential pathogens of Western diseases. J
Transl. Med. 17(3)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Yang M, Song D, Cao X, Wu R, Liu B, Ye W,
Wu J and Yue X: Comparative proteomic analysis of milk-derived
exosomes in human and bovine colostrum and mature milk samples by
iTRAQ-coupled LC-MS/MS. Food Res. Int. 92:17–25. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chang X, Wang SL, Zhao SB, Shi YH, Pan P,
Gu L, Yao J, Li ZS and Bai Y: Extracellular vesicles with possible
roles in gut intestinal tract homeostasis and IBD. Mediators
Inflamm. 2020(1945832)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Bacchi S, Palumbo P, Sponta A and
Coppolino MF: Clinical pharmacology of non-steroidal
anti-inflammatory drugs: A review. Antiinflamm Antiallergy Agents
Med Chem. 11:52–64. 2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yatoo MI, Gopalakrishnan A, Saxena A,
Parray OR, Tufani NA, Chakraborty S, Tiwari R, Dhama K and Iqbal
HMN: Anti-inflammatory drugs and herbs with special emphasis on
herbal medicines for countering inflammatory diseases and
disorders-A review. Recent Pat Inflamm Allergy Drug Discov.
12:39–58. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Liu J, Wang Y, Heelan WJ, Chen Y, Li Z and
Hu Q: . Mucoadhesive probiotic backpacks with ROS nanoscavengers
enhance the bacteriotherapy for inflammatory bowel diseases. Sci
Adv. 8(8798)2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S,
Liu C, Barnes S, Grizzle W, Miller D and Zhang HG: A novel
nanoparticle drug delivery system: the anti-inflammatory activity
of curcumin is enhanced when encapsulated in exosomes. Mol Ther.
18:1606–1614. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Garcia-Martinez J, Salto R, Giron MD,
Perez-Castillo IM, Bueno Vargas P, Vilchez JD, Linares-Perez A,
Manzano M, Garcia-Corcoles MT, Rueda R and López-Pedrosa JM:
Supplementation with a whey protein concentrate enriched in bovine
milk exosomes improves longitudinal growth and supports bone health
during catch-up growth in rats. Nutrients. 16(7)2024.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Yang H, Wuren T, Zhai BT, Liu Y and Er D:
Milk-derived exosomes in the regulation of nutritional and immune
functions. Food Sci. Nutr. 12:7048–7059. 2024.PubMed/NCBI View Article : Google Scholar
|