Introduction
Pseudomonas bacteria, which belong to the
family Pseudomonadaceae (1), are
gram-negative rods and are commonly found in diverse environments,
including soil, water and vegetation (2). Their ability to survive in extreme
conditions is accompanied by their pathogenicity in
immunocompromised individuals, such as those suffering from cystic
fibrosis (CF) or acquired immunodeficiency syndrome (3). Patients with CF often develop high
levels of circulating Exotoxin A antibodies, which have been
associated with increased mortality (4).
Pseudomonas infections are multifactorial due
to the numerous virulence factors these bacteria produce, and can
result in a variety of diseases, including septicemia, urinary
tract infections, pneumonia, chronic lung infections, endocarditis,
dermatitis and osteochondritis (5). Clinical isolates of P.
aeruginosa are often multidrug-resistant, posing considerable
challenges for infection management (6). These infections typically progress in
three stages: Bacterial adhesion and colonization, local invasion,
and disseminated systemic disease (7).
P. aeruginosa secretes various virulence
factors, including pyocyanin, elastase, exoenzyme S, phospholipase
C, Exotoxin A and siderophores, all of which contribute to tissue
damage and bacteremia (5). Among
these, Exotoxin A is the most virulent, causing direct tissue
damage and necrosis (8). It
functions by enzymatically transferring a nicotinamide adenine
dinucleotide molecule onto elongation factor 2, thereby halting
polypeptide chain elongation and leading to cell death (9). A number of exotoxin genes are carried
by mobile genetic elements such as bacteriophages, and are
associated with a number of human diseases (10). Transduction between phages and
bacteria can result in the rapid evolution of new pathogens, which
may have major consequences for public health (9).
Exotoxin A, encoded by the toxA gene, is produced as
a 71-kDa precursor and secreted as a 66-kDa toxin via the type II
secretion system. Its expression is regulated by environmental
factors such as temperature, amino acids and aeration, with optimal
production observed at 32˚C in iron-deficient media (11). The toxin exerts its effects through
a three-step mechanism: i) Binding to the α2-macroglobulin
receptor, a member of the low-density lipoprotein receptor family;
ii) internalization via endocytosis; and iii) cleavage by a
protease, followed by translocation to the cytosol, where it
ADP-ribosylates elongation factor 2, thereby blocking protein
synthesis and causing cell death (12).
The potential of microbial-derived proteins,
particularly bacterial toxins, has garnered interest as an
innovative approach to cancer therapy. Bacterial toxins can
selectively target and disrupt tumor cells, offering a promising
alternative to current cancer therapies (13). Exotoxin A produced by P.
aeruginosa has been shown to exhibit notable specificity for
cancer cells by inhibiting protein synthesis and inducing
apoptosis. This specificity is attributed to the altered metabolic
and signaling pathways characteristic of malignant cells, which
increase their susceptibility to bacterial toxins compared with
that of normal cells (11). Recent
advances in genetic engineering have enhanced the therapeutic
potential of bacterial toxins. Through targeted mutations, the
efficacy and selectivity of Exotoxin A can be improved, reducing
potential side effects and improving cancer cell specificity.
Furthermore, understanding the regulatory mechanisms governing
Exotoxin A expression and secretion is crucial for the development
of a safe and precisely controlled therapeutic approach (13).
Despite the promise of bacterial toxins in oncology,
several challenges remain, particularly those associated with
biosafety, immunogenicity and delivery mechanisms (14). The introduction of Exotoxin A as a
cancer therapeutic requires a thorough investigation of its
pharmacokinetics, dosage optimization and potential immune
responses in human subjects. Furthermore, ethical considerations
regarding the use of bacterial toxins in clinical settings must be
addressed to ensure their safe and effective application in cancer
therapy (15).
Despite the potency of current cancer treatments,
treatment failure in certain patients may occur, necessitating
alternative therapeutic strategies. Bacterial toxins, such as
Exotoxin A, have demonstrated promising anticancer effects by
specifically targeting cancer cells, including MCF-7 breast cancer
cells (16). In addition, Exotoxin
A has been found to inhibit the formation of pre-cancerous lesions
induced by potent carcinogens (17). The present study aimed to explore
the potential of P. aeruginosa-derived Exotoxin A as an
anticancer agent by inducing genetic modifications to enhance its
production and cytotoxic efficacy. By presenting preliminary
insights into the genetic variations that influence Exotoxin A
synthesis and evaluating the cytotoxicity of Exotoxin A in a breast
cancer cell line, the present study contributes to the growing body
of knowledge regarding microbial-based cancer therapeutics.
Materials and methods
Isolation and bacterial
identification
A total of 20 clinical P. aeruginosa isolates
were collected from Qassim University Hospital and King Fahad
Specialist Hospital (both Al-Qassim, Saudi Arabia) between October
2023 and January 2024. These isolates were donated by the hospitals
for research purposes. Samples were obtained under aseptic
conditions prior to antibiotic treatment or 3 days after the
cessation of antibiotic therapy. The isolates were cultured on
MacConkey agar (Oxoid, Ltd.; Thermo Fisher Scientific, Inc.) and
incubated at 37˚C for 24 h. Colonies were subsequently subcultured
on cetrimide agar (Oxoid, Ltd.; Thermo Fisher Scientific, Inc.) to
assess pyocyanin pigment production (18). Bacterial identification was
performed based on morphological characteristics (Gram staining)
and biochemical tests, including the triple sugar iron (TSI)
(19), glucose fermentation,
citrate utilization and urease tests (20). The identities of the
isolates were confirmed using the Sensititre™ Complete Automated
Antimicrobial Susceptibility Testing System (Thermo Fisher
Scientific, Inc.), which includes 32 biochemical tests for
gram-negative bacteria.
Molecular characterization. SDS-PAGE
analysis
Total cellular proteins were extracted from all
isolates and analyzed via SDS-PAGE (Bio-Rad Laboratories, Inc.).
Bacterial pellets were suspended in protein extraction buffer
consisting of 62.5 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 5%
2-mercaptoethanol and 0.01% bromophenol blue (Sigma-Aldrich; Merck
KGaA), then boiled at 95˚C for 5 min to lyse the cells and denature
proteins. Protein concentration was determined using the
bicinchoninic acid (BCA) assay kit (Thermo Fisher Scientific,
Inc.), following the manufacturer's instructions. Equal amounts of
protein (50 µg) were loaded onto a 12% polyacrylamide gel and then
subjected to electrophoresis at 175 V for 1 h. The gels were
stained with 0.2% Coomassie Blue R-250 (Uptima; Interchim) 1 h at
room temperature. Densitometric analysis of the gel bands was
performed using Image Lab software 6.1 (Bio-Rad Laboratories,
Inc.).
Detection of Exotoxin A gene by PCR. PCR was
conducted to amplify the Exotoxin A gene using specific primers
(Table I). DNA was extracted using
a DNeasy Kit (Qiagen, Inc.) following the manufacturer's
instructions. Each 25-µl reaction mixture contained 1X PCR buffer,
2.5 mM MgCl2, 0.2 mM dNTP mix, 1 µM each primer, 1-unit
Taq DNA polymerase (Thermo Fisher Scientific, Inc.), and 50 ng
genomic DNA. The thermocycling conditions consisted of an initial
denaturation step at 94˚C for 5 min, followed by 35 cycles of
denaturation at 94˚C for 1 min, annealing at 60˚C for 45 sec, and
extension at 72˚C for 3 min, with a final extension at 72˚C for 5
min. Amplified PCR products were resolved by 1.5% agarose gel
electrophoresis (21), stained
with ethidium bromide, and visualized under UV
transillumination.
 | Table IPrimer sequences used in PCR
analysis. |
Table I
Primer sequences used in PCR
analysis.
| Primer | Sequence
(5'-3') | Target size
(Ref.) | GenBank accession
no. |
|---|
| S1-F | GAC AAC GCC CTC AGC
ACC AGC | 367 bp (21) | NC_002516.2 |
| S1-R | CGC TGG CCC ATT CGC
TCC AGC GCT | | |
Mutation induction
Six wild-type Pseudomonas isolates (numbers
1, 4, 8, 14, 16 and 20) were selected based on dendrogram analysis
and suspended in 5 ml M9 minimal medium (Sigma-Aldrich; Merck
KGaA). To induce genetic variability, UV irradiation at 260 nm was
applied, as this wavelength is able to introduce point mutations
and small deletions by promoting the formation of pyrimidine
dimers, which can lead to errors in DNA repair (22,23).
This method is widely used for bacterial mutagenesis as it
generates a broad spectrum of mutations while maintaining cell
viability under optimized exposure conditions (14). The isolates were exposed to UV
light at 260 nm for 5 sec from a height of 15 cm at room
temperature (22-25˚C) in the absence of daylight or ambient light.
After irradiation, the cells were incubated in the dark at room
temperature for 1 h to allow DNA repair processes to occur.
Following this, 15 ml nutrient agar (Oxoid, Ltd.; Thermo Fisher
Scientific, Inc.) was poured onto the cells on glass plates, which
were then incubated at 37˚C for 21 h. The surviving colonies
(putative mutants) were screened for the exotoxin A gene by PCR. To
evaluate the impact of UV-induced mutations on exotoxin A
production, 20 mutants from each isolate (120 mutants in total)
were randomly selected for evaluation.
Purification of Exotoxin A
The isolates were cultivated in trypticase soy broth
(TSB; BD Difco™; Becton, Dickinson and Company). Exotoxin A was
purified by precipitation using 70% saturated ammonium sulfate.
Following dialysis, the proteins were purified by Sephadex G-150
column chromatography (GE Healthcare Technologies, Inc.). A
pre-packed column (HiPrep™ 16/60 Sephadex G-150; GE Healthcare Life
Sciences) with a bed volume of 120 ml was equilibrated with sodium
citrate buffer (0.05 M, pH 6.5), and the purification was carried
out at room temperature (22-25˚C). Proteins were eluted with the
same buffer at a flow rate of 1 ml/min. A total of fifteen
fractions (2 ml/fraction) were then collected at 15-min intervals
and the optical density (OD) at 280 nm was measured to monitor
protein elution. Fractions containing Exotoxin A were pooled,
concentrated, and dialyzed against 0.05 M sodium citrate buffer, pH
6.5. The concentration of purified protein was determined using BCA
assay and the protein was analyzed by SDS-PAGE to confirm a
molecular weight of 66 kDa (24).
MCF-7 cell culture
The MCF-7 breast cancer cell line was obtained from
the American Tissue Culture Collection (ATCC). Cells were
maintained in Dulbecco's Modified Eagle's Medium with high glucose
and 1% L-glutamine HEPES buffer (ATCC) supplemented with
heat-inactivated 10% fetal bovine serum (v/v) (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc.). Cells were incubated at
37˚C in a humidified atmosphere containing 5% CO2. Cells
were regularly sub-cultured and passaged every 3 days to maintain
exponential growth.
Cytotoxicity assay
Cell toxicity was monitored by determining the
effect of the test samples on cell morphology and viability. The
MCF-7 breast cancer cell line was seeded at a density of
1x104 cells per well in 96-well plates and incubated at
37˚C in a humidified incubator with 5% CO2. After 24 h,
various concentrations of crude and purified Exotoxin A (1.56-50
µg/ml) were added to the wells. Following an additional 24-h
incubation, MTT reagent (Elabscience Bionovation Inc.) was added,
and the plates were incubated for 2 h. After removing the medium,
DMSO (Thermo Fisher Scientific, Inc.) was added to dissolve the
formazan crystals, and the absorbance was measured at 540 nm using
a microplate reader (BioTek; Agilent Technologies, Inc.).
Half-maximal inhibitory concentration (IC50) values were
determined using nonlinear regression analysis, and Exotoxin A
concentrations are presented on a logarithmic scale (µg/ml)
(25,26).
Statistical analysis
Protein concentrations and IC50 values
were calculated using GraphPad Prism 10 software (Dotmatics). Data
are presented as the mean ± SEM. The IC50 values for
Exotoxin A in the MTT cytotoxicity assay were calculated using
non-linear regression (log Exotoxin A concentration vs. normalized
cell viability). The inhibitory responses of different
concentrations of crude and pure isolated Exotoxin A proteins were
compared using two-way ANOVA followed by Bonferroni's multiple
comparisons test.
Results
Identification of P. aeruginosa
isolates
The 20 P. aeruginosa isolates were confirmed
based on their morphological, biochemical and molecular
characteristics. Gram staining showed that all isolates were
gram-negative rods (Fig. 1A).
Biochemical characterization confirmed that these isolates were
P. aeruginosa, based on standard tests, including the
urease, citrate and TSI tests (Fig.
1B and C). In addition, the
isolates produced the characteristic pyocyanin pigment on cetrimide
agar (Fig. 1D), and PCR
amplification verified the presence of the Exotoxin A gene in all
isolates.
 | Figure 1Identification of P. aeruginosa
isolates. (A) Gram-negative P. aeruginosa observed as
pink-red rods under a microscope after Gram staining
(magnification, x1,000; oil immersion). (B) Reference biochemical
identification tests for P. aeruginosa (from left to right):
Urease, citrate and TSI control agar slants. (C) Representative
experimental results for P. aeruginosa isolates: Urease test
(+, pink), citrate test (+, blue), and TSI (-) with no gas
production. (D) Characteristic blue-green pigment (pyocyanin)
production on cetrimide agar slant, indicating a positive result
for P. aeruginosa. P. aeruginosa, Pseudomonas
aeruginosa; TSI, triple sugar iron. |
Protein profiling and classification
of isolates
SDS-PAGE analysis of total cellular proteins
revealed distinct protein banding patterns among the isolates, with
protein sizes ranging from 10 to 260 kDa. Variations in band
intensity and distribution indicate genetic diversity among the
isolates (Fig. 2A), which was
further analyzed using numerical clustering.
 | Figure 2Protein profiling and dendrogram of
the P. aeruginosa isolates. (A) SDS-PAGE protein profiles of
selected P. aeruginosa isolates. (B) Dendrogram representing
the classification of 20 P. aeruginosa isolates into six
groups based on SDS-PAGE band patterns: Group 1, isolates 1-3;
group 2, isolates 4-7; group 3, isolates 8-11, group 4, isolates
12-15; group 5, isolates 16 and 17; group 6, isolates 18-20. The
SDS-PAGE analysis revealed protein sizes ranging from 10-260 kDa,
with variations in band intensity and distribution. Among these,
isolate 4 was selected for further mutation studies due to its
strong protein expression of Exotoxin A. P. aeruginosa,
Pseudomonas aeruginosa; M, protein molecular weight marker. |
The 20 isolates were classified into six distinct
groups based on their protein banding profiles. A dendrogram was
constructed, in which the isolates were grouped based on band
similarities (Fig. 2B). Isolates
within the same cluster exhibited nearly identical protein
expression patterns, suggesting shared genetic characteristics. The
clusters were defined as follows: Group 1, isolates 1-3; group 2,
isolates 4-7; group 3, isolates 8-11; group 4, isolates 12-15;
group 5, isolates 16 and 17; and group 6, isolates 18-20. Isolate 4
was identified a strong candidate for further mutation analysis,
based on its strong protein expression of Exotoxin A revealed by
the SDS-PAGE analysis.
Detection of Exotoxin A gene by
PCR
PCR was performed to detect the Exotoxin A gene in
six selected wild-type isolates (1, 4, 8, 14, 16 and 20; Fig. 3A), one from each group. A 367-bp
PCR amplicon, characteristic of the Exotoxin A gene, was identified
in all tested wild-type isolates. Following UV mutagenesis, PCR
analysis revealed that mutant 4* exhibited additional DNA bands,
which are likely attributable to modifications in the primer
binding sites (Fig. 3B). Based on
the findings of the SDS-PAGE and PCR analyses, mutant 4* and
wild-type isolate 4 were selected for exotoxin A purification and
cytotoxicity assay.
 | Figure 3Detection of Exotoxin A gene by PCR.
PCR products obtained using primer S1 for (A) six wild-type
isolates (1, 4, 8, 14, 16, and 20) and (B) six corresponding
mutated isolates (1*, 4*, 8*, 14*, 16*, 20*). PCR analysis
confirmed the presence of a 367-bp amplicon in all tested isolates,
characteristic of the Exotoxin A gene. Following mutagenesis,
additional DNA bands were observed in mutant 4*, suggesting
modifications in primer binding sites due to genetic variations. M,
lambda DNA marker. |
Purification and molecular weight
conformation of Exotoxin A
Wild-type isolate 4 and mutant 4* were subjected to
cultivation, precipitation and dialysis, followed by the
purification of Exotoxin A by column chromatography. Protein
concentration was estimated by UV absorbance at 280 nm and verified
using the BCA protein assay (Thermo Fisher Scientific, Inc.). The
highest yield was observed between fractions 4 and 12 based on
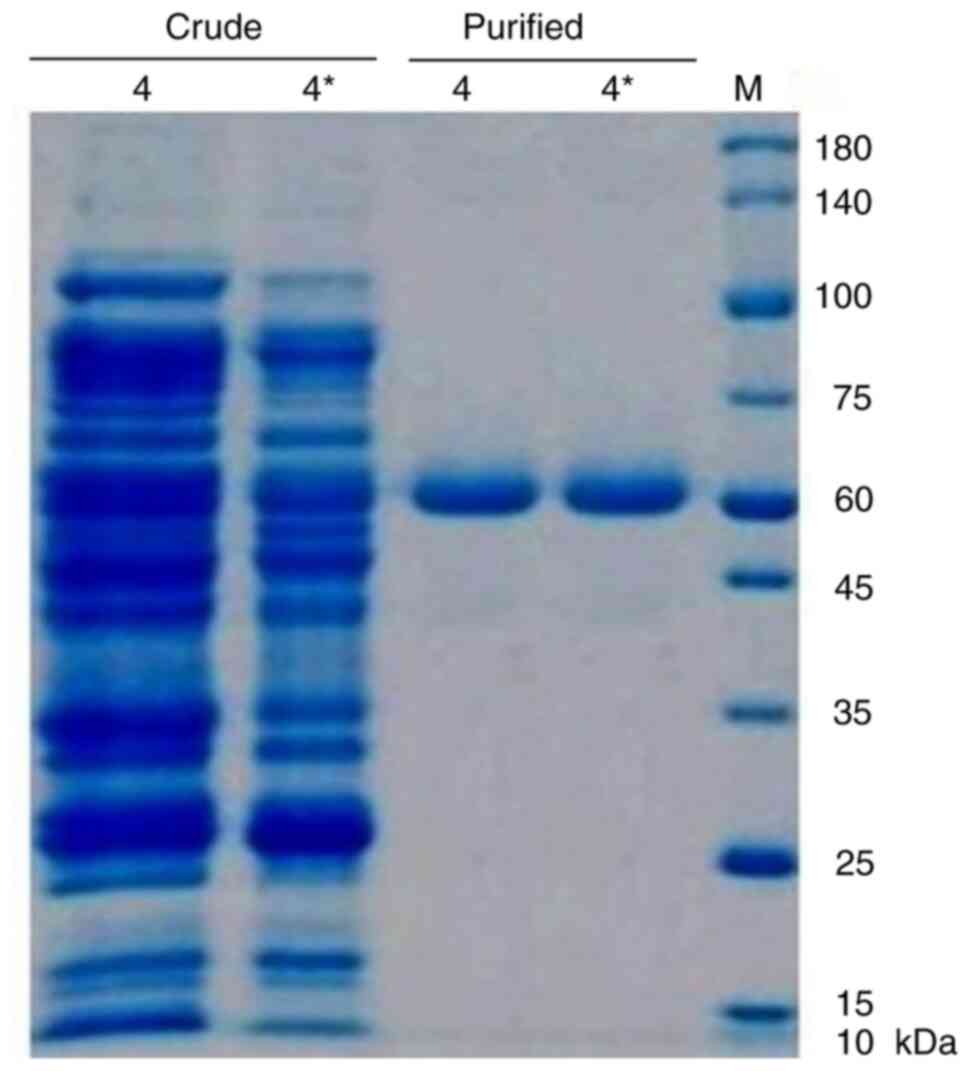
OD280 measurements, corresponding to the Exotoxin A peak (Table II). As shown in Fig. 4, SDS-PAGE analysis revealed a
distinct 66 kDa protein band in the purified fractions, confirming
successful isolation. Notably, mutant isolate 4* exhibited a higher
protein yield than wild-type isolate 4 as determined by optical
density at 280 nm and confirmed by SDS-PAGE densitometric
analysis.
 | Table IIProtein concentration of crude and
purified Exotoxin A from isolates 4 and 4*. |
Table II
Protein concentration of crude and
purified Exotoxin A from isolates 4 and 4*.
| | Crude Exotoxin
A | Purified Exotoxin
A |
|---|
| Isolate no. | OD 280 nm, mean ±
SEM | Concentration,
µg/ml | OD 280 nm, mean ±
SEM | Concentration,
µg/ml |
|---|
| 4 | 0.48±0.01 | 15.40 | 0.47±0.01 | 15.10 |
| 4* | 0.49±0.01 | 15.70 | 0.48±0.01 | 15.30 |
Cytotoxicity of Exotoxin A on MCF-7
cells
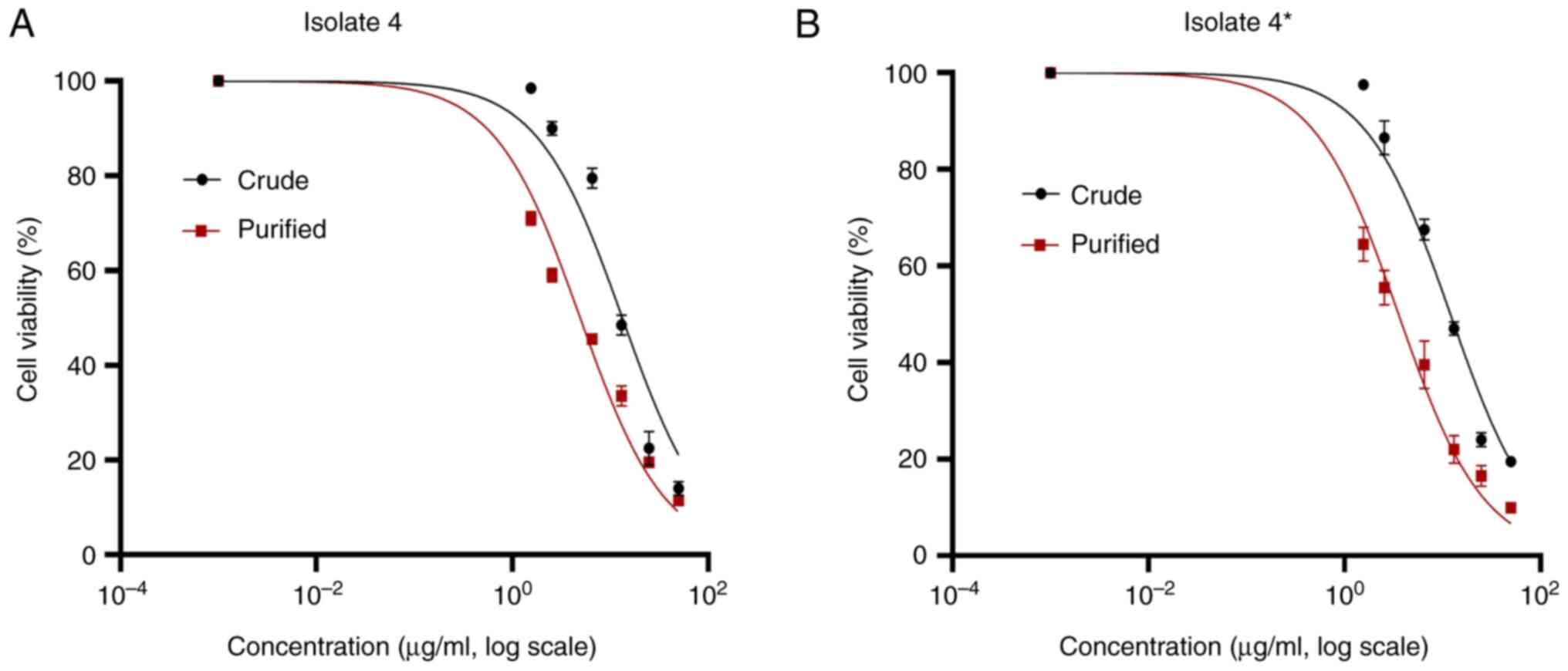
The cytotoxic effect of Exotoxin A on MCF-7 cells
was assessed using the MTT assay (Fig.
5). Both crude and purified Exotoxin A demonstrated marked
dose-dependent cytotoxicity against the MCF-7 cells. The
IC50 values for the crude toxin were for 13.1 and 12.0
for isolate 4 and mutant 4*, respectively. After purification, the
cytotoxicity increased substantially, with IC50 values
of 4.9 and 3.6 µg/ml for isolate 4 and mutant 4*, respectively
(Fig. 5B). Further comparison of
the MTT assay results at various toxin concentrations confirmed
that purified Exotoxin A was significantly more cytotoxic than its
crude counterpart (Fig. 6). A
clear dose-dependent cytotoxic effect was observed, with
concentrations ≥6.56 µg/ml leading to a marked reduction in
viability, particularly for the purified isolates. The differences
between crude and purified Exotoxin A were statistically
significant at multiple concentrations (P<0.05). Notably, mutant
4* (Fig. 6B) exhibited a stronger
cytotoxic effect than isolate 4, demonstrated by a more pronounced
decline in cell viability with increased concentration (Fig. 6A). These findings suggest that
purified Exotoxin A derived from mutant 4 is a particularly potent
inhibitor of MCF-7 cell growth. These findings highlight the
potential of purified Exotoxin A, particularly that derived from
mutant 4*, as a promising antitumor agent.
Discussion
Malignancy is a complicated disease characterized by
interconnected dysregulated metabolic pathways that promote tumor
development and the evasion of immune surveillance. Genetic
mutations contribute to the formation of tumors, which are rapidly
proliferating cells that bypass normal regulatory mechanisms
(13). The search for effective
anticancer drugs remains challenging due to the ability of cancer
cells to resist apoptosis and escape immune detection. Secondary
metabolites derived from microorganisms have played a critical role
in the discovery of new chemotherapeutics, with bacterial toxins
showing promise due for the selective targeting of cancer cells
(27).
Exotoxin A, a well-characterized virulence factor of
P. aeruginosa, has shown marked potential for anticancer
applications due to its ability to inhibit protein synthesis and
induce apoptosis in cancer cells (28). The present study confirmed the
presence of Exotoxin A in all tested P. aeruginosa isolates,
supporting the findings of Aljebory (29), which demonstrated the widespread
presence of the exotoxin A gene in clinical isolates. However,
lower prevalences of Exotoxin A have been noted, for example, by
Ismail et al (30), who
reported a prevalence of 72%. These variations in prevalence are
likely due to differences in sampling locations and infection
control measures.
In the present study, molecular characterization of
the isolates using SDS-PAGE revealed clear differences in protein
banding patterns, which were used to classify the isolates into six
distinct groups. The purification of Exotoxin A was achieved by
ammonium sulfate precipitation followed by Sephadex G-150
chromatography, yielding a purified protein with a molecular mass
of ~66 kDa, in agreement with previous studies (15,31).
Gallant et al (32) demonstrated that optimizing culture
conditions, such as by supplementing the medium with glycerol and
monosodium glutamate, enhances Exotoxin A production. While the
present study employed UV-induced mutagenesis rather than media
optimization, the increased Exotoxin A production observed in
mutant P. aeruginosa isolates highlights the potential of
experimental modifications to enhance toxin yield.
The introduction of mutations via UV exposure led to
a marked increase in Exotoxin A production by mutant 4* compared
with that by the corresponding wild-type isolate, suggesting that
the mutation may have upregulated the gene responsible for toxin
production or altered regulatory elements involved in its
expression. This is consistent with previous research indicating
that bacterial virulence genes are subject to environmental and
genetic regulation (33). The PCR
analysis of mutant 4* revealed additional DNA bands that were not
present in the corresponding wild-type isolate, which may reflect
genetic rearrangements that affect gene regulation or expression
efficiency.
The cytotoxicity assay results demonstrated that the
potency of purified Exotoxin A against MCF-7 breast cancer cells
was greater than that of its crude counterpart. The IC50
values indicated that mutant 4* exhibited a 1.4-fold increase in
cytotoxic activity compared with the wild-type isolate, reinforcing
the hypothesis that mutations enhanced toxin production. These
findings are consistent with previous research demonstrating that
bacterial toxins can be engineered for improved specificity and
potency in cancer therapy (34)
Bacterial toxins such as Exotoxin A are being
explored as potential alternatives to conventional chemotherapy,
which is often associated with systemic toxicity and the
non-specific targeting of healthy tissues (35). It is hypothesized that conjugating
Exotoxin A to monoclonal antibodies or tumor-specific ligands may
lead to the development of targeted cancer therapies that
selectively kill tumor cells while sparing normal tissues. The use
of bacterial toxins in oncology is gaining traction, with current
research focusing on structural modifications to enhance their
stability and reduce potential immunogenicity (30).
Despite the promising results obtained in the
present study, several challenges remain to be addressed before
Exotoxin A can be considered a viable cancer therapeutic. The study
was conducted entirely in vitro; therefore, further
validation in animal models is required to assess pharmacokinetics,
systemic toxicity and long-term efficacy. In addition, while
UV-induced mutation increased toxin production, the exact genetic
modifications responsible remain unknown. Future studies should
employ whole-genome sequencing to identify the specific mutations
contributing to increased expression and activity. In addition, the
potential of site-directed mutagenesis to refine toxin production
and improve its clinical applicability also warrants
investigation.
Structural analyses, such as X-ray crystallography,
may provide insights into how genetic changes influence toxin
conformation and function. Furthermore, strategies for targeted
delivery, such as nanoparticle conjugation, should be investigated
to improve therapeutic efficacy and minimize off-target effects. It
is also important to note that the current study relied solely on
SDS-PAGE to verify the specific purification of Exotoxin A from the
isolates. In future studies, complementary methods, such as mass
spectrometry or immunodetection assays, should be used to identify
and validate the purified toxin.
In conclusion, the present study highlights the
potential of Exotoxin A from P. aeruginosa as a promising
anticancer agent. The results demonstrate that certain genetic
mutations can enhance Exotoxin A production and cytotoxicity,
making it a strong candidate for further development as an
anticancer therapeutic. However, additional research is necessary
to optimize its application, address biosafety concerns and explore
targeted delivery approaches for clinical use. While these findings
provide a strong foundation for microbial toxin-based cancer
therapies, future studies must prioritize in vivo validation
and translational research to bring Exotoxin A-based treatments
closer to clinical application.
Acknowledgements
The author wishes to thank the Deanship of Graduate
Studies and Scientific Research at Qassim University for financial
support. The author also expresses gratitude to Dr Nahla Azab and
Dr Medhat Rehan (Qassim University) for their invaluable support,
assistance with laboratory materials and guidance.
Funding
Funding: Financial support was provided by the Deanship of
Graduate Studies and Scientific Research at Qassim University
(grant no. QU-APC-2025-2/1).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ISA was responsible for conceptualization,
methodology and data analysis, and for writing, reviewing and
editing the manuscript. ISA confirms the authenticity of all the
raw data. The author has read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declares that they have no competing
interests.
Authors' information
Dr Ibtesam S. Almami, ORCID ID: https://orcid.org/0000-0001-7876-560X.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript, and subsequently, the author revised
and edited the content produced by the artificial intelligence
tools as necessary, taking full responsibility for the ultimate
content of the present manuscript.
References
|
1
|
Washington JA: Principles of Diagnosis in
Baron's Medical Microbiology (Baron S), 4th Edition, Chapter 10,
1996. Available from: http://www.ncbi.nlm.nih.gov/books/NBK8014/.
|
|
2
|
Palleroni NJ and Moore ERB: Taxonomy of
pseudomonads: Experimental approaches. In: Pseudomonas, 2004.
|
|
3
|
Qin S, Xiao W, Zhou C, Pu Q, Deng X, Lan
L, Liang H, Song X and Wu M: Pseudomonas aeruginosa: Pathogenesis,
virulence factors, antibiotic resistance, interaction with host,
technology advances and emerging therapeutics. Signal Transduct
Target Ther. 7(199)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lu S, Chen K, Song K, Pilewski JM, Gunn
BM, Poch KR, Rysavy NM, Vestal BE, Saavedra MT and Kolls JK:
Systems serology in cystic fibrosis: Anti-Pseudomonas IgG1
responses and reduced lung function. Cell Rep Med.
4(101210)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Do Rego H and Timsit JF: Management
strategies for severe Pseudomonas aeruginosa infections. Curr Opin
Infect Dis. 36:585–595. 2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ren Y, Zhu R, You X, Li D, Guo M, Fei B,
Liu Y, Yang X, Liu X and Li Y: Quercetin: A promising virulence
inhibitor of pseudomonas aeruginosa LasB in vitro. Appl Microbiol
Biotechnol. 108(57)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ryan KJ, Ray CG, Ahmad N, et al: Sherris
Medical Microbiology 6th edition, 2014.
|
|
8
|
Liao C, Huang X, Wang Q, Yao D and Lu W:
Virulence factors of pseudomonas aeruginosa and antivirulence
strategies to combat its drug resistance. Front Cell Infect
Microbiol. 12(926758)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Casas V and Maloy S: Role of
bacteriophage-encoded exotoxins in the evolution of bacterial
pathogens. Future Microbiol. 6:1461–1473. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gellatly SL and Hancock REW: Pseudomonas
aeruginosa: New insights into pathogenesis and host defenses.
Pathog Dis. 67:159–173. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Balasubramanian D, Schneper L, Kumari H
and Mathee K: A dynamic and intricate regulatory network determines
Pseudomonas aeruginosa virulence. Nucleic Acids Res. 41:1–20.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gholami A, Minai-Tehrani D, Mahdizadeh SJ,
Saenz-Mendez P and Eriksson LA: Structural insights into
pseudomonas aeruginosa exotoxin A-elongation factor 2 interactions:
A molecular dynamics study. J Chem Inf Model. 63:1578–1591.
2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yarahmadi A, Zare M, Aghayari M, Afkhami H
and Jafari GA: Therapeutic bacteria and viruses to combat cancer:
double-edged sword in cancer therapy: New insights for future. Cell
Commun Signal. 22(239)2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Guo L, Ding J and Zhou W: Harnessing
bacteria for tumor therapy: Current advances and challenges.
Chinese Chemical Letters. 35(108557)2024.
|
|
15
|
Morgan RN, Saleh SE, Farrag HA and
Aboshanab KM: New insights on Pseudomonas Aeruginosa
exotoxin A-based immunotoxins in targeted cancer therapeutic
delivery. Ther Deliv. 14:31–60. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gao M, Zhou J, Su Z and Huang Y: Bacterial
cupredoxin azurin hijacks cellular signaling networks:
Protein-protein interactions and cancer therapy. Protein Science.
26:2334–2341. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Chakrabarty AM: Microbial pathogenicity: A
new approach to drug development. Adv Exp Med Biol. 808:41–49.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Benson RF, Tang PW and Fields BS:
Evaluation of the Binax and Biotest urinary antigen kits for
detection of Legionnaires' disease due to multiple serogroups and
species of Legionella. J Clin Microbiol. 38:2763–2765.
2000.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bannoehr J, Franco A, Iurescia M, Battisti
A and Fitzgerald JR: Koneman. Koneman's color atlas and textbook of
diagnostic microbiology. J Clin Microbiol. 47:469–471. 2009.
|
|
20
|
Greenwood D, Slack R and Peutherer JF: A
guide to microbial infections, pathogenesis, immunity, laboratory
diagnosis and control. In: Medical Microbiology.
ThriftBooks-Phoenix, Tolleson, AZ, 2006.
|
|
21
|
Khan AA and Cerniglia CE: Detection of
pseudomonas aeruginosa from clinical and environmental samples by
amplification of the exotoxin A gene using PCR. Appl Environ
Microbiol. 60:3739–3745. 1994.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Trovão M, Schüler LM, Machado A, Bombo G,
Navalho S, Barros A, Pereira H, Silva J, Freitas F and Varela J:
Random mutagenesis as a promising tool for microalgal strain
improvement towards industrial production. Mar Drugs.
20(440)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bose JL: Chemical and UV mutagenesis.
Methods Mol Biol. 1373:111–115. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vijayan P, Raghu C, Ashok G, Dhanaraj SA
and Suresh B: Antiviral activity of medicinal plants of Nilgiris.
Indian J Med Res. 120:24–29. 2004.PubMed/NCBI
|
|
27
|
Yang Q, Wang B, Zheng Q, Li H, Meng X,
Zhou F and Zhang L: A review of gut microbiota-derived metabolites
in tumor progression and cancer therapy. Adv Sci (Weinh).
10(e2207366)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Masuyer G: Crystal structure of exotoxin A
from aeromonas pathogenic species. Toxins (Basel).
12(397)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Aljebory IS: PCR detection of some
virulence genes of pseudomonas aeruginosa in Kirkuk city, Iraq. J
Pharm Sci Res. 10:1068–1071. 2018.
|
|
30
|
Ismail YM, Fayed SM, Elesawy FM, El-Halim
NZA and El-Shimi OS: Phenotypic and molecular characteristics of
pseudomonas aeruginosa isolated from burn unit. Egyptian Journal of
Medical Microbiology (Egypt). 30:19–28. 2021.
|
|
31
|
Bourdenet S, Doyonnas R, Vacheron MJ,
Guinand M, Fasciotto B, Ristic A, Michel G, Cozzone AJ, Durkin JP
and Whitfield JF: The cytotoxicity of Pseudomonas exotoxin A,
inactivated by modification of the cell-binding domain I, is
restored when conjugated to an erythroid cell-specific targeting
agent. Cancer Lett. 50:121–127. 1990.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gallant CV, Raivio TL, Olson JC, Woods DE
and Storey DG: Pseudomonas aeruginosa cystic fibrosis clinical
isolates produce exotoxin A with altered ADP-ribosyltransferase
activity and cytotoxicity. Microbiology (NY). 146:1891–1899.
2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Prinsloo S, Pieters R and Bezuidenhout CC:
A cell viability assay to determine the cytotoxic effects of water
contaminated by microbes. S Afr J Sci. 109:1–4. 2013.
|
|
34
|
Wolf P, Alt K, Bühler P, Katzenwadel A,
Wetterauer U, Tacke M and Elsässer-Beile U: Anti-PSMA immunotoxin
as novel treatment for prostate cancer? High and specific antitumor
activity on human prostate xenograft tumors in SCID mice. Prostate.
68:129–138. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Panahi Z, Owrang M and Goli HR:
Significant role of pyocyanin and exotoxin A in the pathogenesis of
pseudomonas aeruginosa isolated from hospitalized patients. Folia
Med (Plovdiv). 66:88–96. 2024.PubMed/NCBI View Article : Google Scholar
|