Introduction
Geriatric syndromes such as cognitive decline, bone
fragility, and mood disturbances often co-occur in older adults and
may share overlapping pathophysiological pathways (1-5).
For example, dementia, as typified by Alzheimer's disease (AD), and
bone fragility are common among the elderly, and the causal
relationship in their development has started to attract attention
(1-3,5).
In addition, cognitive impairment and bone fragility in the elderly
have been linked to mental health conditions such as apathy and
depression (2,6). Because these conditions are
associated with severe morbidity, long-term disability, mortality,
and significant socioeconomic impact, there is an urgent global
need to develop effective strategies to address them.
Despite extensive research over the past 30 years,
the development of fundamental treatments for geriatric diseases
remains elusive. Consequently, interest is growing in comprehensive
approaches to prevent geriatric diseases and extend healthy life
expectancy through lifestyle modification, including daily diet
(7,8). A variety of foods containing certain
naturally occurring ingredients have the potential to prevent
several diseases (9-11).
For the past decade, we have been investigating functional foods
that can be easily incorporated into the daily diet and may help
prevent or manage various geriatric diseases (12-15).
In this study, we focused on the functional properties of rice, a
staple food for more than half of the global population. Generally,
the rice consumed daily is white rice (WR) milled from brown rice
(BR). The bran layer on BR is rich in various nutrients, including
minerals, vitamins, and dietary fibers, and contains many
substances that are considered effective against geriatric
diseases, including γ-oryzanol, γ-aminobutyric acid (GABA), and
ferulic acid (16-19).
However, from a consumer perspective, regular BR has many
drawbacks, such as a distinctive flavor and taste, hard texture,
and low water absorbency, which makes it difficult to cook and hard
to digest.
An ultrahigh hydrostatic pressure apparatus capable
of applying water pressure of up to 6,000 atm (600 MPa) was
recently developed (20,21). The ultra-high hydrostatic
pressurized brown rice (UBR) obtained by processing with this
equipment overcomes various drawbacks of BR. Compared with regular
BR, UBR has improved water absorption, making it easier to cook, a
less distinctive flavor, better texture, and is more easily
digested (20,21). In addition, the ultrahigh
hydrostatic pressure process confers an advantage in rice
preservation by reducing the number of bacteria in the BR (20,21).
We previously conducted a 24-month intervention trial of UBR intake
in elderly participants, which was associated with the preservation
or maintenance of cognitive function (22). A 12-month intake of UBR was
associated with the maintenance of bone health in the elderly
(23). Although previous findings
have suggested that UBR may contribute to maintaining cognitive
function and bone density, the interrelationship among these
domains and the effects of UBR on mental health remain unclear.
Therefore, we conducted a 12-month, randomized controlled trial to
evaluate the effects of UBR intake on cognitive function, apathy,
and bone health in older adults and to explore possible
correlations among these outcomes.
Materials and methods
Study design and participants
This study was a 12-month, randomized controlled
trial conducted in community-dwelling older adults aged 65-85
years. The intervention was carried out between October 2015 and
March 2017 in Iinan Town, Shimane Prefecture, Japan. Although the
study was conducted prior to trial registration, it was
retrospectively registered in the UMIN Clinical Trials Registry
(registration no. UMIN000053587, date: February 9th, 2024) before
submission. The delay in registration occurred due to a lack of
awareness of prospective trial registration requirements at the
time of study initiation. We acknowledge this as a limitation and
affirm that all future clinical trials will be prospectively
registered in accordance with international standards. The study
was approved by the Ethics Committee of the Shimane University
School of Medicine (approval no. 1940-2504). The study adhered to
the ethical principles outlined in the Declaration of Helsinki.
Prior to study participation, all volunteers provided written
informed consent. In this study, 54 healthy volunteers
participated, and underwent physical examinations including
anthropometry, medical interview by a physician and blood
biochemical tests. Volunteers completed a lifestyle questionnaire
covering medical and medication history. The following volunteers
were excluded from the study: volunteers with any medical
disorders, including cardiac, hepatic, renal, gastrointestinal,
respiratory disease, diabetes mellitus, neurological disorders, and
osteoporosis, metabolic, cancer, endocrine, or hematological
disorders; those eating BR daily; those consuming
medications/supplements to treat bone disorders or osteoporosis
and/or improve cognitive and mental function that could affect the
results of the study; those with allergies and hypersensitivity;
smokers. Of the 54 volunteers screened for eligibility, 44
participants (mean age, 73.1±5.6 years) were enrolled into the
study and assigned to either the WR-intake group (n=22) or the UBR
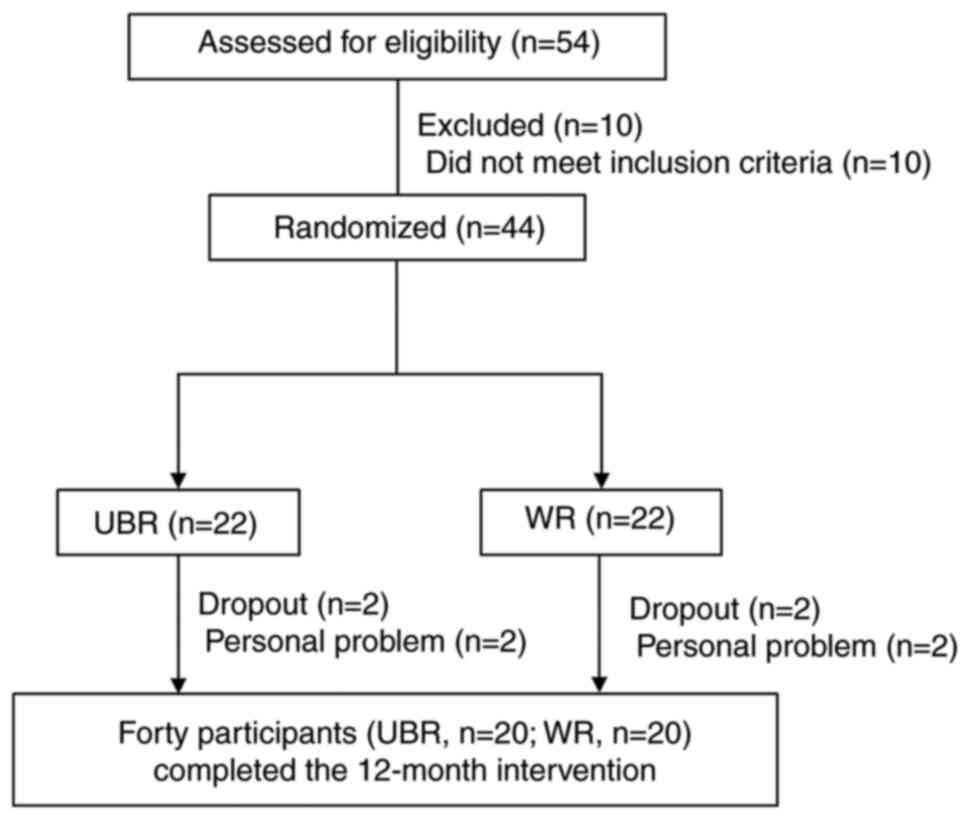
intake group (n=22) (Fig. 1).
Participants were diagnosed by a clinician to ensure they had no
neuropsychiatric or other disorders. No volunteers had participated
in any other clinical studies within the past year. Group
allocation was conducted by stratified random assignment, as
described previously (24,25). The randomization code lists were
prepared by an independent clinical research advisor and concealed
from the investigators, participants, outcome assessors, and data
analysts. All assessments and data analyses were performed in a
blinded manner, and group assignments were disclosed only after
completion of the study. Although participants were not informed
which intervention (UBR or WR) was considered active, the
noticeable differences in color, texture, and flavor between the
two made complete blinding of participants infeasible. Participants
in the WR-intake group received 200 g of WR daily for 12 months;
those in the UBR-intake group received 100 g of UBR and 100 g of WR
daily for 12 months. The rice intake was voluntary for the
participants. During the intervention period, participants were
advised to avoid other BR products and functional foods potentially
influencing cognitive or bone health. This request was made to
reduce confounding factors, and adherence was encouraged without
disrupting participants' usual lifestyle. Participants' adherence
to rice consumption was daily investigated using a
self-administered questionnaire.
Test foods
Both UBR and WR were supplied by NPO Satoyama
Commission (Iinan-cho). To prepare UBR, BR was exposed to water at
a hydrostatic pressure of 600 MPa for 5 s using a hydrostatic
pressurizer (21). UBR and WR were
both cultivated in Iinan-cho, Shimane, Japan, and produced from the
same variety of rice. The nutrient content of WR and UBR was
measured at Shimane Environment & Health Public Corporation and
Shimane Institute for Industrial Technology and is summarized in
Table I. Compared with WR, UBR was
richer in lipids and dietary fiber and has a higher content of
minerals, including magnesium (Mg), calcium (Ca), inorganic
phosphorus (Pi), and iron (Fe). In addition, UBR is rich in
vitamins B1, B6, and niacin, as well as
bioactive substances such as GABA, inositol, and ferulic acid
(Table I).
 | Table INutrients content of WR and UBR. |
Table I
Nutrients content of WR and UBR.
| Nutrients | WR | UBR |
|---|
| Protein, g | 6.80 | 7.70 |
| Carbohydrate,
g | 75.30 | 76.60 |
| Dietary fiber,
g | 0.30 | 7.10 |
| Lipid, g | 1.30 | 3.00 |
| GABA, mg | 2.00 | 9.10 |
| Inositol, mg | ND | 202.00 |
| Ferulic acids,
mg | ND | 12-50 |
| Calcium, mg | 2.00 | 9.00 |
| Magnesium, mg | 20.00 | 110.00 |
| Phosphate, mg | 140.00 | 290.00 |
| Potassium, mg | 110.00 | 230.00 |
| Sodium, mg | 2.00 | 1.89 |
| Iron, mg | 0.50 | 2.10 |
| Zinc, mg | 4.00 | 1.80 |
| Vitamin B1, mg | 0.12 | 0.51 |
| Vitamin B2, mg | 0.03 | 0.04 |
| Vitamin B6, mg | 0.05 | 0.32 |
| Vitamin E, mg | 0.40 | ND |
| Niacin, mg | 1.40 | 7.46 |
Anthropometry
Height, waist circumference, and blood pressure were
measured by trained nurses. Body weight and body fat were measured
using a bioelectrical impedance analyzer (WB-150; TANITA Co.), as
described previously (26). After
these measurements, participants completed two self-administered
questionnaires: a general lifestyle questionnaire, which included
items on educational background and medical/medication history, and
a brief diet history questionnaire, as described previously
(22,26).
Blood biochemistry
At baseline and after 12 months of the intervention,
blood samples were drawn by nurses in the morning after confirming
whether participants had fasted. Serum was separated from whole
blood samples and stored at -80˚C until use. Serum biochemical
parameters were measured using an automated clinical chemistry
analyzer TBA-c16000 (TOSHIBA); serum HbA1c level was determined
with high-performance liquid chromatography (HPLC) using an
HLC-723G9 (TOSOH); and blood sugar was measured using the GA08III
automatic analyzer (A&T Co.), as described previously (7).
Safety assessment
Adverse events were monitored throughout the
12-month study period using structured monthly interviews and
questionnaires. Participants were asked about specific symptoms
including gastrointestinal issues (e.g., bloating, flatulence,
diarrhea), allergic reactions, appetite changes, and bowel habit
alterations. Any reported events were recorded and evaluated by the
study physician to determine their relationship to the
intervention.
Serum monoamine levels
The concentrations of monoamines, i.e., epinephrine
(Epi), norepinephrine (NE), dopamine (DA), and serotonin (5-HT), in
the serum were measured using a previously described HPLC method
(22). Briefly, the serum was
pretreated with a clean EG column (EICOM). The HPLC equipment
consisted of an EICOM HTEC-500 (EICOM) equipped with a data
processor (EICOM EPC-500 PowerChrom) and an automatic injector
(EICOM M-514). Chromatographic separation was performed using an
EICOMPAK CA-5ODS column (2.1x150 mm ID) linked to a precolumn
(EICOM PREPAK PC-03-CA). PowerChrom software was used for data
collection and analysis (EICOM). The mobile phase consisted of 0.1
M phosphate buffer (pH 5.7) containing 700 mg/l sodium
1-octanesulfonate, 12% methanol, and 50 mg/l EDTA disodium salt.
The flow rate was set to 0.23 ml/min, and the applied potential was
+450 mV with respect to an Ag/AgCl reference electrode. The column
temperature was maintained at 25˚C.
Cognitive function and mental health
assessment
Cognitive function was assessed using the
Mini-Mental State Examination (MMSE) (27), the Revised Hasegawa's Dementia
Scale (HDS-R) (28), the Frontal
Assessment Battery (FAB) (29),
and the Cognitive Assessment for Dementia, iPad version (CADi)
(30). The MMSE is a cognitive
function test consisting of 11 subitems. MMSE can be used to screen
cognitive impairment, estimate the severity of cognitive impairment
at a given point in time, track an individual's cognitive changes
over time, and document an individual's response to treatment
(27). A total MMSE score of 24 to
27 points is considered indicative of mild cognitive impairment
(MCI), while a score below 23 suggests possible dementia (27). The HDS-R is commonly used for
dementia screening in Japan and includes 9 items assessing
orientation, memory, attention, and verbal fluency (28). Generally, a score below 20 is
considered suggestive of dementia (28). The FAB, consisting of 6 subtests,
is a cognitive test that incorporates several clinical assessments
to screen for frontotemporal dementia, including S-word generation,
similarities, Luria's test, grasp reflex, and the Go-No-Go test
(29). The CADi consists of 10
items and is a useful tool for population screening for dementia
and is known to correlate significantly with MMSE scores (30). In CADi, the time spent performing a
task is also evaluated and used as an indicator of cognitive
processing ability (30).
Furthermore, mental condition, i.e., apathy and depression, was
assessed using the Japanese version of the Starkstein apathy scale
(31) and the Zung Self-Rating
Depression Scale (SDS) (32),
respectively. The Starkstein apathy scale is a subjective rating
scale with scores ranging from 0 to 42; higher scores indicate less
motivation and a greater apathy severity (31). The cutoff for the apathy scale is
16 points, and apathy is suspected in those who score 16 points or
higher. The SDS is also a self-rating scale, comprising responses
to 20 questions on a 4-point scale; a higher total score indicates
a tendency toward depression (32). These tests are commonly used in
clinical and interventional studies as they are relatively quick
and can easily assess an individual's cognitive and mental function
(12-15,22,25,26).
All assessments were conducted by trained clinicians.
Quantitative ultrasound measurements
of the calcaneum
To assess bone status, we used an ultrasonic bone
densitometer (Venus-α; Nihon Kohden, Tokyo, Japan) to perform
quantitative ultrasound (QUS) of the right calcaneus, as previously
described (23). The device uses a
combination of ultrasound pulse reflection and ultrasound pulse
transmission methods to measure the speed of sound (SOS) and the
bone width of the calcaneus and subsequently calculates the bone
area ratio (BAR). QUS offers a quick and noninvasive method for
evaluating bone health without the radiation exposure associated
with dual-energy X-ray absorptiometry (DXA). The BAR values
obtained with this device have been reported to correlate
significantly with bone mineral density (BMD) measurements by DXA
at the lumbar spine (r=0.77, P<0.01) and calcaneus (r=0.83,
P<0.01) (33). For each
participant, the BAR of the right calcaneus was measured at least
three times and converted to the percentage of the young adult mean
(%YAM), using reference data from Japanese adults aged 20-44, in
which 100% represents the average bone density for that age group.
%YAM is widely used in clinical practice in Japan as an index of
bone status and allows for standardized comparisons across
individuals (34,35).
Sample size power calculation
The sample size was calculated using PS: Power &
Sample Size Calculation Software Version 3.1.2 (Vanderbilt
University, Nashville, TN, USA). The primary outcomes were the
change in MMSE scores after 12 months of intervention. Based on
data from a previous randomized controlled trial involving a
similar population (15,22), a mean difference of 1.4 points
(SD=2.0) between groups was expected. Assuming two-sided α=0.05 and
90% power, a minimum of 20 participants per group was required
using an independent t-test. To account for an anticipated dropout
rate of 10%, we enrolled 22 participants per group.
Statistical analysis
All statistical analyses were conducted using IBM
SPSS Statistics for Windows, version 26.0 (IBM Corp.). A
per-protocol approach was adopted for efficacy analyses. Data were
first assessed for normality using the Shapiro-Wilk test. All
variables met normality assumptions and are presented as mean ±
standard error of the mean (SEM). The primary outcomes were the
MMSE score and Starkstein apathy scale score at 12 months.
Secondary outcomes included other cognitive measures (CADi, HDS-R,
FAB), additional mental health indicators (SDS), and bone health
index (%YAM). To assess between-group differences at 12 months
while controlling for potential baseline imbalances, analysis of
covariance (ANCOVA) was employed. In the ANCOVA models, the
12-month score was entered as the dependent variable, group (UBR
vs. WR) as the fixed factor, and baseline score and age as
covariates. To examine time-by-group interaction effects across
baseline and 12 months, two-way repeated measures ANOVA were used.
When a significant interaction was found, post hoc comparisons were
performed using Tukey's test. Between-group comparisons of change
scores (Δ=12 months-baseline) were conducted using independent
samples t-tests as supplementary analyses. While change scores are
reported for descriptive purposes, interpretation of primary
effects was based on ANCOVA results. Effect sizes for between-group
comparisons were calculated using Cohen's d, with thresholds of
0.2, 0.5, and 0.8 representing small, medium, and large effects,
respectively. Partial correlation analyses were conducted to
explore the associations among cognitive, mental, and bone-related
outcomes at 12 months, adjusting for age and baseline scores. All
tests were two-tailed, and statistical significance was set at
P<0.05.
Results
Adherence
Fig. 1 shows a flow
diagram of this intervention study. During the 12-month
intervention, four subjects (two in the WR-intake group and two in
the UBR-intake group) retired for personal reasons (Fig. 1). Therefore, the 12-month
intervention trial was completed by twenty participants both in the
WR-intake group and UBR-intake group (Fig. 1). Participants demonstrated
excellent adherence to the intervention protocol for 12 months
(UBR: 95.5%, WR: 96.8%). Data from general questionnaires on
lifestyle habits and medical/medication history showed no
significant differences during 12 months of intervention (data not
shown). Adverse effects disturbing participants' daily lives such
as palpitation, anorexia, diarrhea, constipation, allergic
reactions, and irritated stomach were not observed in both groups.
The BDHQ survey showed no significant difference in mean dietary
nutrient intake between the UBR-intake and WR-intake groups at 12
months (data not shown).
Body composition, blood pressure,
blood biochemistry, and serum monoamine levels
Table II shows the
values for body composition, blood pressure, blood biochemistry,
and serum monoamine levels of the participants at baseline and at
12 months after the intervention, as well as the changes during the
intervention period. At baseline, there were no significant
differences between the WR-intake and UBR intake groups in body
weight, height, fat mass, BMI, waist circumference, or blood
pressure (Table II). Similarly,
no significant differences were observed between the groups for
blood biochemical variables, aspartate transaminase (AST), alanine
transaminase (ALT), γ-glutamyl transpeptidase (γ-GTP), albumin
(ALB), total cholesterol (T-cho), triglyceride (TG), blood urea
nitrogen (BUN), creatinine (CRE), LDL-cholesterol (LDL-C),
HDL-cholesterol (HDL-C), blood sugar, or HbA1c levels at baseline
(Table II). After 12 months of
the intervention, the two groups did not differ in height, body
weight, body fat mass, BMI, waist circumference, or blood pressure
(Table II), and there were no
significant differences in blood biochemistry parameters, blood
sugar, or HbA1c. Furthermore, for each parameter, the change from
baseline to 12 months was not significantly different (Table II). Serum EPi, NE, DA, and 5-HT
levels did not differ between the groups at baseline, and long-term
UBR intake had no effect. From baseline to 12 months, D5-HT levels
were slightly increased in the UBR intake group, but the change was
not statistically significant (P=0.081, Table II).
 | Table IIParticipants' characteristics and
anthropometric biochemical variables. |
Table II
Participants' characteristics and
anthropometric biochemical variables.
| | Baseline | Month 12 | Change amount
(Δ) | |
|---|
| Characteristic | WR | UBR | WR | UBR | WR | UBR | P-value |
|---|
| N | 22 | 22 | 20 | 20 | - | - | - |
| Sex
(male/female) | 10/12 | 10/12 | 8/12 | 10/10 | - | - | 0.532 |
| Age | 75.2±1.3 | 70.8±1.2 | 75.7±1.4 | 72.4±1.3 | - | - | 0.038 |
| Anthropometry | | | | | | | |
|
Height,
cm | 153.3±1.7 | 157.5±2.5 | 153.8±1.7 | 159.1±2.6 | 0.6±0.5 | 0.7±0.6 | 0.779 |
|
Body weight,
kg | 53.7±1.9 | 60.7±3.2 | 53.8±1.9 | 60.3±3.5 | 0.1±0.3 | -0.4±0.5 | 0.094 |
|
BMI,
kg/m2 | 22.8±0.6 | 23.9±0.7 | 22.6±0.5 | 23.8±0.7 | 1.7±3.9 | 4.7±4.6 | 0.924 |
|
Body fat,
% | 28.4±1.7 | 28.9±2.1 | 29.4±1.3 | 28.8±1.2 | 1.0±0.3 | 0.1±0.4 | 0.621 |
|
WC, cm | 86.1±1.7 | 87.1±2.1 | 83.8±1.9 | 86.3±2.4 | -2.4±0.9 | -0.8±0.6 | 0.421 |
| Blood pressure | | | | | | | |
|
SBP,
mmHg | 143.6±6.6 | 141.6±3.0 | 144.9±5.9 | 147.4±4.0 | 0.8±2.2 | 2.9±2.1 | 0.864 |
|
DPB,
mmHg | 76.4±3.6 | 78.7±2.1 | 78.6±3.2 | 82.2±2.3 | 1.1±0.2 | 2.0±0.2 | 0.119 |
| Biochemistry | | | | | | | |
|
AST,
IU/l | 24.9±1.4 | 27.2±0.9 | 26.4±1.7 | 27.9±1.5 | 1.5±1.4 | 0.7±1.0 | 0.779 |
|
ALT,
IU/l | 16.1±1.9 | 20.8±1.9 | 18.7±2.1 | 21.3±1.5 | 1.6±1.6 | 0.4±1.3 | 0.383 |
|
γ-GTP,
IU/l | 31.9±8.6 | 41.9±10.4 | 32.8±9.3 | 45.1±11.9 | 0.8±1.6 | 2.5±2.9 | 0.461 |
|
ALB,
g/dl | 4.1±0.1 | 4.1±0.1 | 4.2±0.1 | 4.2±0.1 | 0.1±0.1 | 0.1±0.0 | 0.620 |
|
T-cho,
mg/dl | 192.2±5.3 | 191.6±6.0 | 196.6±6.6 | 197.5±5.4 | 4.3±6.0 | 5.9±2.8 | 0.998 |
|
TG,
mg/dl | 125.3±8.6 | 121.3±14.2 | 129.1±10.5 | 144.1±19.8 | 4.5±11.0 | 23.6±15.2 | 0.758 |
|
BUN,
mg/dl | 17.0±1.0 | 15.4±0.5 | 15.6±1.1 | 15.0±0.6 | -1.3±0.8 | -0.3±0.6 | 0.289 |
|
BS,
mg/dl | 113.9±6.3 | 100.9±6.2 | 118.6±7.0 | 109.1±4.9 | 4.9±8.7 | 8.1±4.3 | 0.968 |
|
CRE,
mg/dl | 0.7±0.0 | 0.7±0.0 | 0.7±0.0 | 0.7±0.0 | 0.0±0.0 | 0.0±0.0 | 0.814 |
|
HDL-C,
mg/dl | 65.1±2.0 | 62.8±2.3 | 66.2±3.9 | 64.2±2.8 | 1.8±1.9 | 1.9±1.5 | 0.779 |
|
LDL-C,
mg/dl | 102.1±5.4 | 106.2±6.1 | 104.7±5.3 | 104.5±6.5 | 1.7±7.0 | -0.7±3.2 | 0.947 |
|
HbA1c,
% | 5.8±0.1 | 5.9±0.1 | 5.9±0.1 | 6.0±0.1 | 0.2±0.4 | 0.1±0.1 | 0.121 |
| Serum
monoamines | | | | | | | |
|
Epi,
ng/ml | 6.7±6.0 | 1.8±1.0 | 7.7±2.7 | 4.9±1.2 | 1.0±5.7 | 3.2±1.0 | 0.353 |
|
NE,
ng/ml | 45.9±6.9 | 55.5±4.2 | 102.8±6.8 | 112.6±5.4 | 67.0±10.3 | 56.9±4.9 | 0.752 |
|
DA,
ng/ml | 3.1±1.5 | 6.0±3.1 | 2.6±1.7 | 4.9±1.1 | 2.6±1.7 | 0.5±0.3 | 0.208 |
|
5-HT,
ng/ml | 10,470±986 | 11,313±1184 | 12,416±1274 | 14,448±810 | 1,690±631 | 3,305±650 | 0.087 |
Safety assessment
Adverse events were monitored monthly using
structured interviews. Participants were asked about common
symptoms potentially associated with dietary fiber intake, such as
bloating, diarrhea, and allergic reactions. No participants
reported any adverse symptoms during the 12-month study period.
These findings, together with the absence of unfavorable changes in
clinical parameters (e.g., liver enzymes, kidney function, blood
pressure), support the safety of long-term UBR intake.
Cognitive and mental health
assessments
MMSE, CADi, HSD-R, and FAB were used to assess
participants' cognitive function, and the results were summarized
in Table III. In this study, all
participants had MMSE scores of 24 or higher, Apathy scores of 16
or lower, and SDS scores of 40 or lower during the intervention
period, suggesting that they were not suffering from dementia,
apathy, or depression. At baseline, there were no significant
differences between the groups for all measures of cognitive
outcomes (Table III). At 12
months, MMSE and CADi total scores in the UBR-intake group were
higher than in the WR-intake group. A two-way ANOVA revealed a
significant group x time interaction for MMSE total scores,
F(1,78)=7.67, P=0.021. Although the mean change in MMSE from
baseline to 12 months was not statistically significant between the
groups (P=0.142), ANCOVA adjusting for baseline value and age
revealed that the 12-month MMSE score was significantly higher in
the UBR group than in the WR group [F(1,36)=4.21, P=0.046, Cohen's d=0.72].
Sensitivity analyses using a simple comparison without covariates
(P=0.034) and an alternative ANCOVA adjusting for age and sex
(P=0.040) confirmed these results (Table SI). The absolute difference of 1.4
points, while statistically significant, was below the commonly
cited in Minimal Clinically Important Difference (MCID) of 2-3
points, suggesting cognitive preservation rather than true
improvement. Subitem analysis of the MMSE revealed no significant
between-group difference in the change in ‘Recall 3 words’, a
measure of recent memory (P=0.098, Table III). Regarding CADi, significant
group x time interactions were observed for total score
[F(1,78)=5.59, P=0.048] and execution time [F(1,78)=16.46,
P=0.014]. At 12 months, CADi total score increased (P=0.038) and
execution time decreased (P=0.008) in the UBR group compared to WR
(Table III). The change in
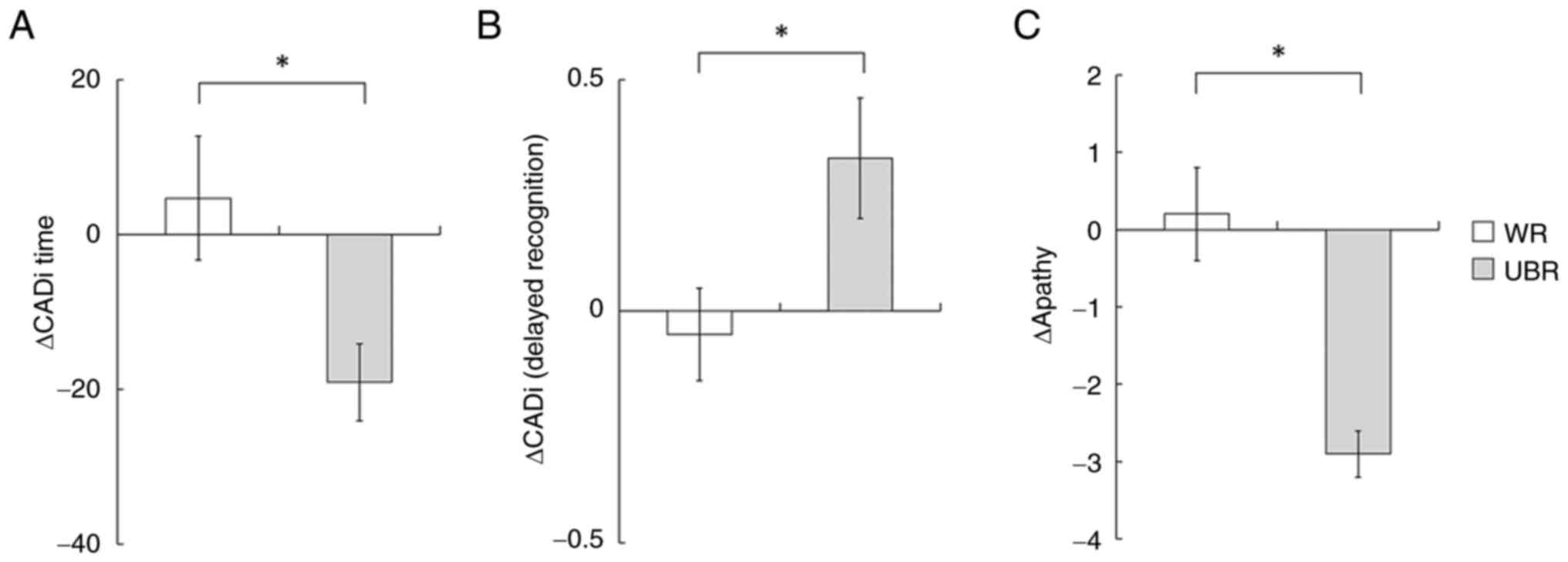
execution time (ΔCADi time) was significantly greater in the UBR
group (Fig. 2A, P=0.026), and
subitem analysis revealed a significant difference in ‘Delayed
Recognition’ (Fig. 2B, P=0.027).
For apathy, ANCOVA adjusting for baseline score and age showed a
significantly lower 12-month score in the UBR group [F(1,36)=7.92, P=0.008; Cohen's d=0.89]. A
two-way ANOVA also revealed a significant group x time interaction
[F(1,78)=11.13, P=0.007], and Δapathy scores were significantly
lower in the UBR group compared to the control (Fig. 2C, P=0.015), suggesting a potential
benefit of UBR intake on motivational state. SDS values showed a
non-significant trend toward reduction in the UBR group at 12
months (P=0.142), and between-group differences in change scores
were not significant (P=0.294). HDS-R and FAB scores showed no
significant differences. Sensitivity analyses for apathy and CADi
confirmed consistent results across models (P=0.010-0.012 for
apathy; P<0.05 for CADi), with moderate to large effect sizes
(apathy: d=0.89; CADi: d=0.75-1.02). For HDS-R, FAB, and SDS,
exploratory sensitivity analyses revealed small to moderate effect
sizes (e.g., HDS-R: d=0.38) despite non-significant P-values
(Table SII).
 | Table IIICognitive and mental health
assessments. |
Table III
Cognitive and mental health
assessments.
| | Baseline | Month 12 | Change amount
(Δ) | |
|---|
| Assessment | WR | UBR | WR | UBR | WR | UBR | P-value |
|---|
| MMSE | 28.2±0.2 | 28.5±0.1 | 27.4±0.2 |
28.7±0.2a | -0.6±0.4 | 0.2±0.3 | 0.142 |
| ‘Recall 3
Words’ | 2.5±0.1 | 2.4±0.2 | 2.4±0.1 | 2.6±0.1 | -0.1±0.1 | 0.2±0.1 | 0.098 |
| CADi total | 7.6±0.3 | 8.0±0.3 | 7.9±0.3 |
8.8±0.2a | 0.3±0.3 | 0.6±0.2 | 0.439 |
| ‘Delayed
Recognition’ | 0.63±0.1 | 0.61±0.1 | 0.60±0.1 | 0.91±0.1 | -0.05±0.2 | 0.31±0.2 | 0.024 |
| CADi time | 141.0±8.2 | 127.6±10.3 | 151.7±11.2 |
108.6±6.7b | 4.7±8.0 | -19.1±5.0 | 0.026 |
| ‘Delayed
Recognition’ | 18.6±2.3 | 16.7±1.3 | 18.3±2.3 |
12.2±1.2a | 0.0±1.2 | -4.0±1.2 | 0.016 |
| FAB | 14.7±0.3 | 15.2±0.5 | 15.1±0.4 | 15.6±0.4 | 0.5±0.3 | 0.4±0.3 | 0.775 |
| HDS-R | 27.7±0.5 | 28.9±0.2 | 27.5±0.5 | 28.9±0.4 | -0.2±0.4 | 0.0±0.1 | 0.581 |
| Apathy | 11.1±1.0 | 9.8±1.2 | 11.3±1.2 |
6.6±1.0b,c | 0.2±0.6 | -2.9±0.3 | 0.015 |
| SDS | 33.6±1.5 | 32.8±1.6 | 32.5±1.4 | 29.0±1.6 | -1.0±1.3 | -3.6±1.1 | 0.294 |
Assessment of bone status using
quantitative ultrasound
Table IV presents
the values of SOS and %YAM at baseline and after the 12-month
intervention, along with the corresponding changes over time. At
baseline, no significant differences were observed between the UBR
and WR groups in SOS or %YAM. After 12 months, both SOS and %YAM
values were higher in the UBR group compared to the WR group. A
two-way ANOVA revealed a significant main effect of group for both
SOS [F(1,78)=4.22, P=0.046] and %YAM [F(1,78)=4.64, P=0.043],
indicating that participants in the UBR group maintained
significantly greater bone status than those in the WR group. No
significant group x time interaction effects were found for either
SOS or %YAM (P>0.05). Post hoc comparisons showed that the %YAM
at 12 months was significantly higher in the UBR group compared to
the WR group (P=0.032). Similarly, SOS was significantly greater in
the UBR group at 12 months (P<0.05). No significant difference
in calcaneal bone width was detected between groups (data not
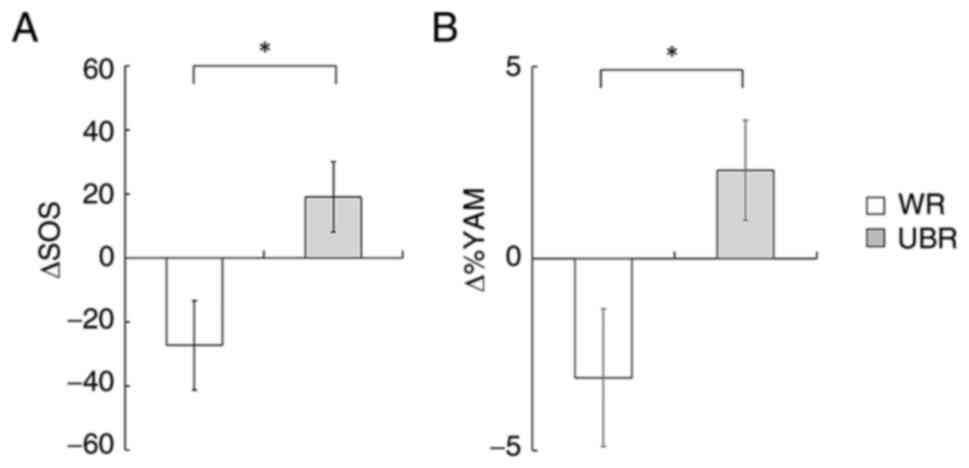
shown). Fig. 3 illustrates the
changes in SOS (ΔSOS) and %YAM (Δ%YAM) from baseline to 12 months.
Both ΔSOS (P=0.035) and Δ%YAM (P=0.034) were significantly greater
in the UBR group compared to the WR group, further supporting a
potential benefit of UBR intake on bone health in older adults.
 | Table IVBone health assessment using
quantitative ultrasound measurements. |
Table IV
Bone health assessment using
quantitative ultrasound measurements.
| | Baseline | Month 12 | Change amount
(Δ) | |
|---|
| Measurement | WR | UBR | WR | UBR | WR | UBR | P-value |
|---|
| SOS, m/sec | 1,799.5±14.3 | 1,830.8±20.5 | 1,774.5±16.7 |
1,855.1±22.9a | -27.3±14.1 | 19.7±11.6 | 0.035 |
| %YAM | 85.6±2.4 | 88.6±2.4 | 82.4±2.0 |
91.5±2.7a | -3.1±1.8 | 2.3±1.3 | 0.034 |
Correlation analysis
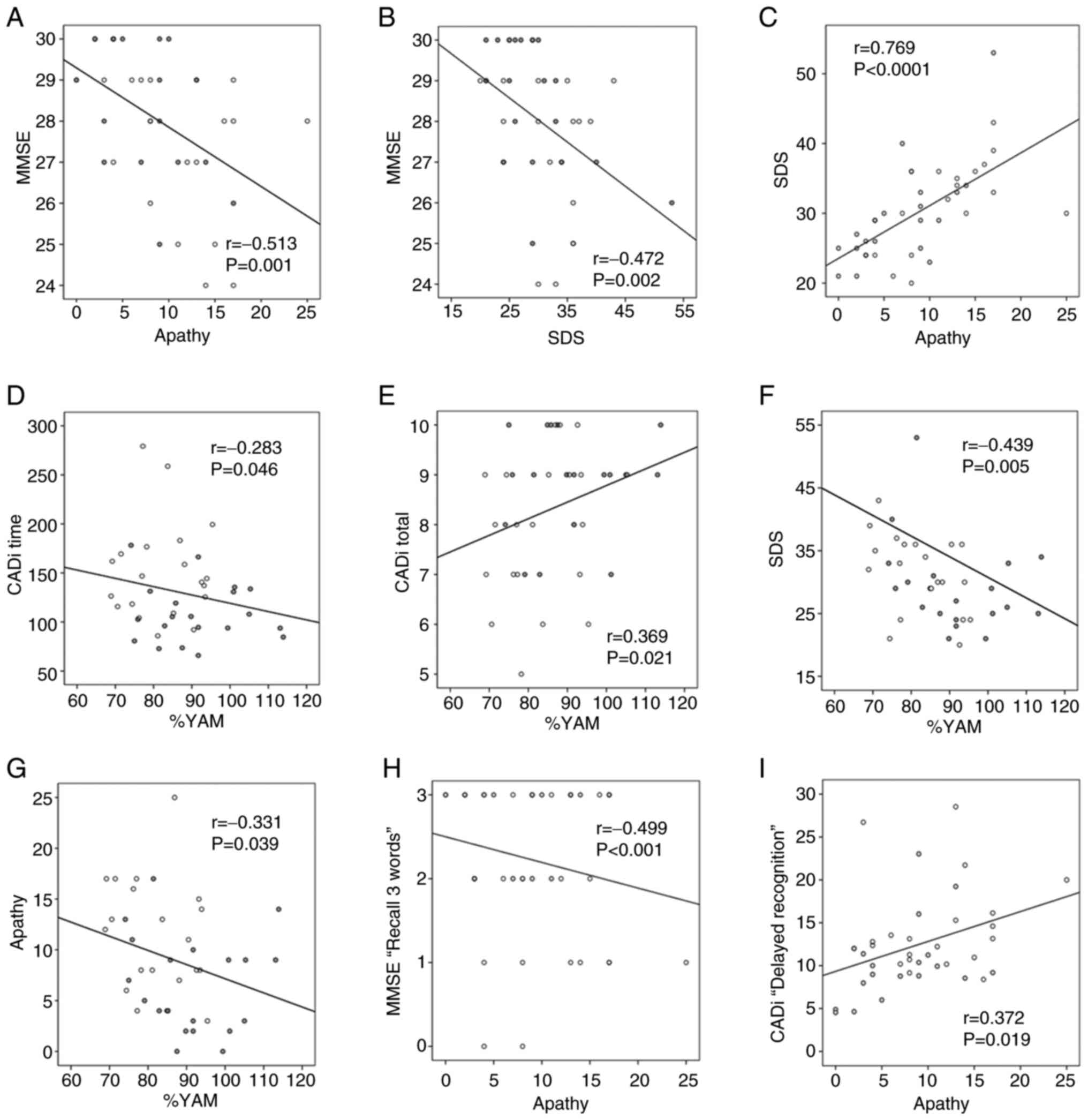
Partial correlation analyses adjusting for age and
baseline values revealed significant associations between cognitive
and mental health indicators. After 12 months of intervention, MMSE
score had significant negative correlations with the apathy score
(Fig. 4A, r=-0.513, P=0.001) and
SDS (Fig. 4B, r=-0.472, P=0.002).
A strong positive correlation was observed between apathy and
depression levels (Fig. 4C,
r=0.769, P<0.0001). Moreover, %YAM had a significant negative
correlation with CADi time (Fig.
4D, r=-0.283, P=0.046) and a positive correlation with CADi
total (Fig. 4E, r=0.369, P=0.021).
The %YAM had significant negative correlations with SDS (Fig. 4F, r=-0.439, P=0.005) and apathy
score (Fig. 4G, r=-0.331, P=0.039)
after 12 months of intervention. The apathy score showed a
significant negative correlation with MMSE subitem ‘Recall 3 words’
(Fig. 4H, r=-0.499, P<0.001),
and a significant positive correlation with CADi time subitem
‘Delayed Recognition’ (Fig. 4I,
r=0.372, P=0.019). A full matrix of partial correlation
coefficients is presented in Table
SIII.
Discussion
This study investigated the effects of daily UBR
consumption over a 12-month period on cognitive performance, mental
health, and bone status in community-dwelling older adults. The
results indicated that MMSE scores at 12 months were significantly
higher in the UBR group compared to the WR group, although the
change from baseline was not statistically significant. In
contrast, UBR intake was associated with the preservation of CADi
total scores and a slower execution time, suggesting a potential
benefit in cognitive processing speed and memory-related tasks.
Additionally, apathy scores significantly decreased in the UBR
group over the intervention period, while no significant changes
were observed in depression scores (SDS). Importantly, CADi and
apathy scores were significantly correlated with bone health
indices (%YAM), highlighting a potential interplay between
cognitive-motivational function and bone density. Although causal
relationships cannot be inferred from the present analyses, the
observed partial correlations between cognitive/apathy scores and
%YAM may indicate a potential association among cognitive,
motivational, and skeletal health in older adults. These findings
warrant further investigation in studies with longitudinal
mediation models. No serious adverse events were reported, and UBR
consumption did not result in notable changes in hepatic or renal
markers, body composition, or metabolic parameters, suggesting that
the intervention was well tolerated and safe for long-term
intake.
Cognitive impairment and fragility fractures mainly
affect the elderly population and significantly increase the
prevalence and incidence of dementia and osteoporosis. Because of
the severe morbidity, long-term disability, and mortality
associated with these conditions, as well as their socioeconomic
impact, there is an urgent global need to develop strategies to
prevent these diseases (5).
Although the causal relationship between dementia and bone
fragility is still under debate, several clinical and
epidemiological reports have shown that patients with dementia,
including AD, exhibit lower BMD, higher susceptibility to falls,
and an increased propensity for fracture compared with their
healthy counterparts (5,36). Retrospective and prospective
studies consistently highlight cognitive decline as a risk factor
for bone fracture, with both sexes experiencing dementia and
osteoporosis as the most common condition (5,37-39).
In a longitudinal study (the Rotterdam Study), Xiao et al.
(3) found that individuals with
lower BMD were more likely to develop dementia later in life,
suggesting that bone fragility may be a risk factor for dementia.
The relationship between dementia and osteoporosis in treatment can
be seen with raloxifene, an osteoporosis drug shown to reduce the
risk of vertebral fractures (40).
The analysis of cognitive function in postmenopausal women,
raloxifene was found to reduce the risk of dementia, especially in
women with MCI (40,41). Furthermore, a systematic review
also showed that raloxifene reduced the risk of MCI and lowered the
risk of AD (42). Thus,
ingredients that improve bone density may also have beneficial
effects on cognition. Although a causal relationship cannot be
established, the observed associations suggest that UBR intake may
contribute to the preservation of both cognitive and bone health.
However, further research is needed to fully understand the complex
relationship between cognitive impairment, BMD decline, and the
mechanism of action of UBR.
Typically, even healthy older adults without MCI or
dementia experience various changes in cognitive abilities as they
age, one of which is decline in recent memory (43). In this study, CADi analysis showed
that UBR intake was associated with an increase in total scores and
a significant reduction in execution time. Furthermore, the CADi
subitem analysis shows a significant increase in ‘Delayed
Recognition’ in the UBR group compared to the WR group. A trend
toward improvement with UBR-intake was also observed in changes in
the MMSE subitem ‘Recall 3 words’ (recent memory scale). These
findings suggest that UBR intake may support recent memory in older
individuals. It is also of interest that UBR intake was associated
with a significant reduction in apathy scores in the elderly;
moreover, these results were significantly correlated with
cognitive abilities and %YAM. In the elderly, several brain areas
(including the prefrontal cortex, anterior cingulate cortex,
temporal lobe and hippocampus) atrophy with age, leading to MCI and
the subsequent onset of AD, the most common form of
neurodegenerative disease (44).
The earliest pathogenesis of AD is the accumulation of β-amyloid
(Aβ) plaques in the brain, which begin to form more than 20 years
before onset of the disease (44).
Aβ deposition and corticolimbic dysfunction have also been shown to
be positively correlated with the development of apathy and
depression (45,46). In addition, apathy and depression
have been linked with frailty and lower BMD, suggesting that a
comprehensive approach is required to combat dementia (47,48).
In a preclinical study, Okuda et al. reported that chronic
administration of UBR in a mouse model of AD
(senescence-accelerated mouse prone-8) suppressed Aβ accumulation
in the brain and improved cognitive function (49). Therefore, the observed preservation
of cognitive function and reduction in apathy in the UBR intake
group may, at least in part, be associated with the potential role
of UBR in attenuating amyloid burden.
The molecular mechanisms through which UBR may
contribute to cognitive, motivational, and bone-related benefits
remain unclear; however, several bioactive components in UBR are
hypothesized to play a role in these effects. For example, UBR is
richer in minerals (e.g., Ca, Mg, Pi, and Fe) than WR (Table I). Mineral deficiencies can affect
memory function and are a likely cause of age-related cognitive
impairment and mental illness (50-55).
Deficiencies in nutrients also pose serious problems for bone
health in the elderly (56). In
addition, UBR is richer in lipids and dietary fiber than WR and
contains bioactive substances such as ferulic acid, GABA and
g-Oryzanol (Table I). Ferulic acid
has been reported to induce an increase in blood estradiol and
alkaline phosphatase activity in castrated female rats,
consequently preventing a decrease in BMD (54). Ferulic acid has also been reported
to have beneficial effects in dementia and depression and to
inhibit Ab aggregation, oxidative stress and inflammatory responses
(19,55). GABA, a major inhibitory
neurotransmitter, is known for its neuroprotective properties and
potential to preserve cognitive function (17,57-59).
g-Oryzanol has been shown to exert neuroprotective effects by
reducing oxidative stress and neuroinflammation, thereby supporting
cognitive function, and may also contribute to bone health by
inhibiting osteoclast differentiation and reducing bone resorption
through its anti-inflammatory properties (60,61).
Together, these components could synergistically contribute to
preserving cognitive function, reducing apathy via neurotransmitter
modulation, and supporting bone quality through anti-inflammatory
and antioxidant mechanisms. In addition, B vitamins, which are
abundant in UBR (Table I), are
known to be closely involved in the formation of collagen
crosslinks in bone, and a high correlation has been reported
between insufficient vitamin B6 intake and decreased bone density
(62,63). Recently, elevated serum
homocysteine levels due to vitamin B6 deficiency have been shown to
have a negative impact on bone metabolism and result in an
increased fracture risk (64,65).
Increased homocysteine levels due to a deficiency of B vitamins
increase the risk of dementia and other related diseases (66). Various functional components in
UBR, along with their potential synergistic interactions, may help
prevent cognitive decline and bone loss. These findings underscore
the need for further mechanistic research to clarify the biological
pathways involved.
The benefits of regular intake of BR for human
physiology and disease prevention have been well studied (10,67,68).
However, continuous intake of regular BR is often difficult for
various reasons, and in some cases, it has been associated with
health concerns such as digestive malabsorption (69). In this study, long-term consumption
of UBR showed no difference between the two groups in terms of
changes in liver function, renal function, lipid metabolism, and
carbohydrate metabolism before and after intervention (Table II). Furthermore, participant
adherence was high, and no physical complaints related to chronic
intake were reported during the intervention period. These results
demonstrate that the long-term consumption of UBR does not
interfere with daily life in the elderly individuals and that UBR
is a sustainable and safe staple food. Given its low cost, wide
availability, and ease of daily consumption, UBR may serve as a
feasible dietary approach for older adults. Compared to
pharmaceutical interventions, UBR may offer a more accessible and
sustainable strategy for maintaining cognitive and bone health,
particularly in community-dwelling populations.
Despite the encouraging findings, this study has
several limitations. First, the relatively small sample size may
have limited the power to detect subtle effects and increases the
risk of type II error. For example, the MMSE difference, while
statistically significant, did not exceed the established MCID (2-3
points), suggesting limited clinical relevance. Similarly, some
secondary outcomes showed non-significant results despite small to
moderate effect sizes. These findings should be interpreted
cautiously, and future studies with larger samples are needed to
validate these effects. Second, the trial was retrospectively
registered, which we acknowledge as a limitation. Future studies
should ensure prospective registration to enhance transparency,
reduce reporting bias, and strengthen the credibility of the
findings. Third, the assessment of bone health relied solely on
calcaneal QUS, using %YAM as the endpoint. While %YAM is widely
used in Japan for screening purposes, it is not a direct measure of
BMD and lacks internationally standardized diagnostic thresholds.
Unlike DXA, which is the gold standard for diagnosing osteoporosis,
QUS primarily reflects bone quality and structural integrity.
Furthermore, the QUS device used in this study did not generate
validated T-scores, which are essential for classification based on
WHO criteria. As such, clinical interpretation of bone status and
risk based solely on %YAM should be approached with caution. This
limitation underscores the need for future studies to incorporate
DXA or QUS devices that provide validated T-scores to allow for
more clinically meaningful assessment of bone health. Fourth,
although the sample size limited stratified analysis, the effects
of UBR intake may vary according to baseline characteristics such
as age, sex, physical activity, vitamin D exposure, and dietary
patterns. These potential effect modifiers should be considered in
future studies with larger and more diverse populations. Fifth,
although complete participant blinding was not feasible due to
perceptible differences between UBR and WR, all outcome assessments
and data analyses were conducted by staff who were blinded to group
allocation, minimizing assessment bias. Finally, the lack of
mechanistic investigation limits our ability to draw conclusions
about the underlying biological effects of UBR.
In conclusion, daily intake of UBR over 12 months
was associated with preservation of cognitive function, reduced
apathy, and maintenance of bone-related parameters in older adults,
without adverse effects. While causality cannot be established,
these findings support the potential of UBR as a functional staple
food to help mitigate age-related declines in cognition and bone
health. Further large-scale, mechanistically informed studies are
needed to confirm and expand upon these observations.
Supplementary Material
Sensitivity analyses of between-group
differences in MMSE endpoint under alternative models.
Exploratory sensitivity analyses and
corresponding effect sizes for secondary outcomes at 12 months
Partial correlation coefficients
between cognitive, emotional and bone-related outcomes at 12
months.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported in part by a Grant-in-Aid for
Scientific Research (C) from the Ministry of Education Culture,
Sports, Science and Technology of Japan (grant no. 26500008).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Author's contributions
MH contributed to the conceptualization, design of
the study and was responsible for project administration, including
ethical approvals and coordination among research sites. KM, SY, YK
and MH contributed to formal analysis, data interpretation, and
data curation. KM, SY, YK and MH were involved in clinical
assessments. KM and MH contributed to the development of the study
methodology, including the design of outcome measures and
statistical analysis plans. SY, OS, KY, HKis and MH provided
essential experimental equipment and resources required for the
conduct of the study. HN, TM, and HKin managed rice processing and
the provision of test food. TM, HKin and MH coordinated participant
recruitment. KM, YK, HN, TM, HKin and MH contributed to data
acquisition through participant support and intervention
management. TM, HKin, KY and MH contributed to the design and
interpretation of nutritional analysis. KM wrote the first draft of
the manuscript and performed data visualization. SY, OS, HKis and
MH contributed to the interpretation of results and critical review
of the manuscript. MH secured the research funding. SY, OS, KY,
HKis and MH supervised the study. KM, YK and MH confirmed the
authenticity of all raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Shimane University School of Medicine (approval no. 1940-2504).
All procedures were conducted in accordance with the Declaration of
Helsinki. Written informed consent was obtained from all
participants prior to their inclusion in the study.
Patient consent for publication
All participants provided written informed consent
for participation in the study and for the publication of
anonymized data.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
ORCID: Kentaro Matsuzaki, 0000-0002-5942-8840; Shozo
Yano, 0000-0002-9210-2949; Hiroko Kishi, 0000-0003-2896-7355;
Michio Hashimoto, 0000-0002-3726-4347.
References
|
1
|
Loskutova N, Honea RA, Vidoni ED, Brooks
WM and Burns JM: Bone density and brain atrophy in early
Alzheimer's disease. J Alzheimers Dis. 18:777–785. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mehta K, Thandavan SP, Mohebbi M, Pasco
JA, Williams LJ, Walder K, Ng BL and Gupta VB: Depression and bone
loss as risk factors for cognitive decline: A systematic review and
meta-analysis. Ageing Res Rev. 76(101575)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xiao T, Ghatan S, Mooldijk SS, Trajanoska
K, Oei L, Gomez MM, Ikram MK, Rivadeneira F and Ikram MA:
Association of bone mineral density and dementia: The Rotterdam
study. Neurology. 100:e2125–e2133. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Filler J, Georgakis MK and Dichgans M:
Risk factors for cognitive impairment and dementia after stroke: A
systematic review and meta-analysis. Lancet Healthy Longev.
5:e31–e44. 2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ruggiero C, Baroni M, Xenos D, Parretti L,
Macchione IG, Bubba V, Laudisio A, Pedone C, Ferracci M, Magierski
R, et al: Dementia, osteoporosis and fragility fractures: Intricate
epidemiological relationships, plausible biological connections,
and twisted clinical practices. Ageing Res Rev.
93(102130)2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mehta K, Mohebbi M, Pasco JA, Williams LJ,
Walder K, Ng BL and Gupta VB: Impact of mood disorder history and
bone health on cognitive function among men without dementia. J
Alzheimers Dis. 96:381–393. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ichinose T, Kato M, Matsuzaki K, Tanabe Y,
Tachibana N, Morikawa M, Kato S, Ohata S, Ohno M, Wakatsuki H, et
al: Beneficial effects of docosahexaenoic Acid-enriched milk
beverage intake on cognitive function in healthy elderly Japanese:
A 12-month randomized, double-blind, placebo-controlled trial. J
Funct Foods. 74(104195)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lisco G, Disoteo OE, De Tullio A, De
Geronimo V, Giagulli VA, Monzani F, Jirillo E, Cozzi R,
Guastamacchia E, De Pergola G and Triggiani V: Sarcopenia and
diabetes: A detrimental liaison of advancing age. Nutrients.
16(63)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hashimoto M, Hossain S, Al Mamun A,
Matsuzaki K and Arai H: Docosahexaenoic acid: One molecule diverse
functions. Crit Rev Biotechnol. 37:579–597. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hashimoto M, Hossain S, Matsuzaki K, Shido
O and Yoshino K: The journey from white rice to ultra-high
hydrostatic pressurized brown rice: An excellent endeavor for ideal
nutrition from staple food. Crit Rev Food Sci Nutr. 62:1502–1520.
2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Matsuzaki K, Nakajima A, Guo Y and Ohizumi
Y: A narrative review of the effects of citrus peels and extracts
on human brain health and metabolism. Nutrients.
14(1847)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hashimoto M, Yamashita K, Kato S, Tamai T,
Tanabe Y, Mitarai M, Matsumoto I and Ohno M: Beneficial effects of
daily dietary omega-3 polyunsaturated fatty acid supplementation on
age-related cognitive decline in elderly Japanese with very mild
dementia: A 2-year randomized, double-blind, placebo-controlled
trial. J Aging Res Clin Pract. 1:193–201. 2012.
|
|
13
|
Hashimoto M, Matsuzaki K, Maruyama K,
Hossain S, Sumiyoshi E, Wakatsuki H, Kato S, Ohno M, Tanabe Y,
Kuroda Y, et al: Perilla seed oil in combination with
nobiletin-rich ponkan powder enhances cognitive function in healthy
elderly Japanese individuals: A possible supplement for brain
health in the elderly. Food Funct. 13:2768–2781. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hashimoto M, Matsuzaki K, Maruyama K,
Sumiyoshi E, Hossain S, Wakatsuki H, Kato S, Ohno M, Tanabe Y,
Kuroda Y, et al: Perilla frutescens seed oil combined with Anredera
cordifolia leaf powder attenuates age-related cognitive decline by
reducing serum triglyceride and glucose levels in healthy elderly
Japanese individuals: A possible supplement for brain health. Food
Funct. 13:7226–7239. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ichinose T, Matsuzaki K, Kato M, Tanabe Y,
Tachibana N, Morikawa M, Kato S, Ohata S, Ohno M, Wakatsuki H, et
al: Intake of docosahexaenoic Acid-enriched milk beverage prevents
Age-related cognitive decline and decreases serum bone resorption
marker levels. J Oleo Sci. 70:1829–1838. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kaup RM, Khayyal MT and Verspohl EJ:
Antidiabetic effects of a standardized Egyptian rice bran extract.
Phytother Res. 27:264–271. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Savage K, Firth J, Stough C and Sarris J:
GABA-modulating phytomedicines for anxiety: A systematic review of
preclinical and clinical evidence. Phytother Res. 32:3–18.
2018.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Kokumai T, Ito J, Kobayashi E, Shimizu N,
Hashimoto H, Eitsuka T, Miyazawa T and Nakagawa K: Comparison of
blood profiles of γ-oryzanol and ferulic acid in rats after oral
intake of γ-oryzanol. Nutrients. 11(1174)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jalali A, Firouzabadi N and Zarshenas MM:
Pharmacogenetic-based management of depression: Role of traditional
Persian medicine. Phytother Res. 35:5031–5052. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hayashi R: High pressure in bioscience and
biotechnology: pure science encompassed in pursuit of value.
Biochim Biophys Acta. 1595:397–399. 2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yoshino K and Inoue T: Ultra high pressure
and food processing technology. J Highly Water Pressurized Food
Eng. 1:5–14. 2016.
|
|
22
|
Kuroda Y, Matsuzaki K, Wakatsuki H, Shido
O, Harauma A, Moriguchi T, Sugimoto H, Yamaguchi S, Yoshino K and
Hashimoto M: Influence of ultra-High hydrostatic pressurizing brown
rice on cognitive functions and mental health of elderly Japanese
individuals: A 2-year randomized and controlled trial. J Nutr Sci
Vitaminol (Tokyo). 65 (Suppl 1):S80–S87. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Matsuzaki K, Yano S, Sumiyoshi E, Shido O,
Katsube T, Tabata M, Okuda M, Sugimoto H, Yoshino K and Hashimoto
M: Long-term ultra-high hydrostatic pressurized brown rice intake
prevents bone mineral density decline in elderly Japanese
individuals. J Nutr Sci Vitaminol (Tokyo). 65 (Suppl 1):S88–S92.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sumiyoshi E, Matsuzaki K, Sugimoto N,
Tanabe Y, Hara T, Katakura M, Miyamoto M, Mishima S and Shido O:
Sub-chronic consumption of dark chocolate enhances cognitive
function and releases nerve growth factors: A parallel-group
randomized trial. Nutrients. 11(2800)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hashimoto M, Matsuzaki K, Hossain S, Ito
T, Wakatsuki H, Tanabe Y, Ohno M, Kato S, Yamashita K and Shido O:
Perilla Seed oil enhances cognitive function and mental health in
healthy elderly Japanese individuals by enhancing the biological
antioxidant potential. Foods. 10(1130)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hashimoto M, Matsuzaki K, Kato S, Hossain
S, Ohno M and Shido O: Twelve-month studies on perilla oil intake
in Japanese adults-possible supplement for mental health. Foods.
9(530)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Folstein MF, Folstein SE and McHugh PR:
‘Mini-mental state’. A practical method for grading the cognitive
state of patients for the clinician. J Psychiatr Res. 12:189–198.
1975.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Imai Y and Hasegawa K: The revised
Hasegawa's dementia scale (HDS-R)-evaluation of its usefulness as a
screening test for dementia. J.H.K.C Psych. 4:2–24. 1994.
|
|
29
|
Dubois B, Slachevsky A, Litvan I and
Pillon B: The FAB: A frontal assessment battery at bedside.
Neurology. 55:1621–1626. 2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Onoda K, Hamano T, Nabika Y, Aoyama A,
Takayoshi H, Nakagawa T, Ishihara M, Mitaki S, Yamaguchi T, Oguro
H, et al: Validation of a new mass screening tool for cognitive
impairment: Cognitive Assessment for Dementia, iPad version. Clin
Interv Aging. 8:353–360. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Okada K, Kobayashi S, Aoki K, Suyama N and
Yamaguchi S: Assessment of motivational loss in poststroke patients
using the Japanese version of Starkstein's Apathy Scale. Jpn J
Stroke. 20:318–327. 1998.
|
|
32
|
Zung WWK: A Self-rating depression scale.
Arch Gen Psychiatry. 12:63–70. 1965.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yamazaki K, Kushida K, Ohmura A, Sano M
and Inoue T: Ultrasound bone densitometry of the os calcis in
Japanese women. Osteoporosis Int. 4:220–225. 1994.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Soen S, Fukunaga M, Sugimoto T, Sone T,
Fujiwara S, Endo N, Gorai I, Shiraki M, Hagino H, Hosoi T, et al:
Diagnostic criteria for primary osteoporosis: Year 2012 revision. J
Bone Miner Metab. 31:247–257. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Matsuzaki K, Hossain S, Wakatsuki H,
Tanabe Y, Ohno M, Kato S, Shido O and Hashimoto M: Perilla seed oil
improves bone health by inhibiting bone resorption in healthy
Japanese adults: A 12-month, randomized, double-blind,
placebo-controlled trial. Phytother Res. 37:2230–2241.
2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhao Y, Shen L and Ji HF: Alzheimer's
disease and risk of hip fracture: A meta-analysis study. Sci World
J. 2012:1–5. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Baker NL, Cook MN, Arrighi HM and Bullock
R: Hip fracture risk and subsequent mortality among Alzheimer's
disease patients in the United Kingdom, 1988-2007. Age Ageing.
40:49–54. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lai SW, Chen YL, Lin CL and Liao KF:
Alzheimer's disease correlates with greater risk of hip fracture in
older people: A cohort in Taiwan. J Am Geriatr Soc. 61:1231–1232.
2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang HK, Hung CM, Lin SH, Tai YC, Lu K,
Liliang PC, Lin CW, Lee YC, Fang PH, Chang LC and Li YC: Increased
risk of hip fractures in patients with dementia: A nationwide
population-based study. BMC Neurol. 14(175)2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yaffe K, Krueger K, Cummings SR, Blackwell
T, Henderson VW, Sarkar S, Ensrud K and Grady D: Effect of
raloxifene on prevention of dementia and cognitive impairment in
older women: The Multiple Outcomes of Raloxifene Evaluation (MORE)
randomized trial. Am J Psychiatry. 162:683–690. 2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jacobsen DE, Melis RJF, Verhaar HJJ and
Olde Rikkert MGM: Raloxifene and tibolone in elderly women: A
randomized, double-blind, double-dummy, placebo-controlled trial. J
Am Med Dir Assoc. 13:189.e1–e7. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yang ZD, Yu J and Zhang Q: Effects of
raloxifene on cognition, mental health, sleep and sexual function
in menopausal women: A systematic review of randomized controlled
trials. Maturitas. 75:341–348. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Golomb J, de Leon MJ, Kluger A, George AE,
Tarshish C and Ferris SH: Hippocampal atrophy in normal aging. An
association with recent memory impairment. Arch Neurol. 50:967–973.
1993.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Jack CR Jr, Knopman DS, Jagust WJ,
Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ,
Weigand SD, et al: Tracking pathophysiological processes in
Alzheimer's disease: An updated hypothetical model of dynamic
biomarkers. Lancet Neurol. 12:207–216. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Mori T, Shimada H, Shinotoh H, Hirano S,
Eguchi Y, Yamada M, Fukuhara R, Tanimukai S, Zhang MR, Kuwabara S,
et al: Apathy correlates with prefrontal amyloid β deposition in
Alzheimer's disease. J Neurol Neurosurg Psychiatry. 85:449–455.
2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Moriguchi S, Takahata K, Shimada H, Kubota
M, Kitamura S, Kimura Y, Tagai K, Tarumi R, Tabuchi H, Meyer JH, et
al: Excess tau PET ligand retention in elderly patients with major
depressive disorder. Mol Psychiatry. 26:5856–5863. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Parrotta I, Maltais M, Rolland Y,
Spampinato DA, Robert P, de Souto Barreto P and Vellas B: MAPT/DSA
group. The association between apathy and frailty in older adults:
A new investigation using data from the Mapt study. Aging Ment
Health. 24:1985–1989. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Voorend CGN, van Buren M, Berkhout-Byrne
NC, Kerckhoffs APM, van Oevelen M, Gussekloo J, Richard E, Bos WJW
and Mooijaart SP: Apathy symptoms, physical and cognitive function,
health-related quality of life, and mortality in older patients
with CKD: A longitudinal observational study. Am J Kidney Dis.
83:162–172.e1. 2024.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Okuda M, Fujita Y, Katsube T, Tabata H,
Yoshino K, Hashimoto M and Sugimoto H: Highly water pressurized
brown rice improves cognitive dysfunction in senescence-accelerated
mouse prone 8 and reduces amyloid beta in the brain. BMC Complement
Altern Med. 18(110)2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Morris MC, Schneider JA and Tangney CC:
Thoughts on B-vitamins and dementia. J Alzheimers Dis. 9:429–433.
2006.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gil Martínez V, Avedillo Salas A and
Santander Ballestín S: Vitamin supplementation and dementia: A
systematic review. Nutrients. 14(1033)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Fekete M, Lehoczki A, Tarantini S,
Fazekas-Pongor V, Csípő T, Csizmadia Z and Varga JT: Improving
cognitive function with nutritional supplements in aging: A
comprehensive narrative review of clinical studies investigating
the effects of vitamins, minerals, antioxidants, and other dietary
supplements. Nutrients. 15(5116)2023.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Dai Z and Koh WP: B-vitamins and bone
health-a review of the current evidence. Nutrients. 7:3322–3346.
2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Sassa S, Kikuchi T, Shinoda H, Suzuki S,
Kudo H and Sakamoto S: Preventive effect of ferulic acid on bone
loss in ovariectomized rats. In Vivo. 17:277–280. 2003.PubMed/NCBI
|
|
55
|
Di Giacomo S, Percaccio E, Gullì M, Romano
A, Vitalone A, Mazzanti G, Gaetani S and Di Sotto A: Recent
advances in the neuroprotective properties of ferulic acid in
Alzheimer's disease: A narrative review. Nutrients.
14(3709)2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Fujii K, Kajiwara T and Kurosu H: Effect
of vitamin B6 deficiency on the crosslink formation of collagen.
FEBS Lett. 97:193–195. 1979.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Murari G, Liang DR, Ali A, Chan F,
Mulder-Heijstra M, Verhoeff NPLG, Herrmann N, Chen JJ and Mah L:
Prefrontal GABA levels correlate with memory in older adults at
high risk for Alzheimer's disease. Cereb Cortex Commun.
1(tgaa022)2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Inotsuka R, Uchimura K, Yamatsu A, Kim M
and Katakura Y: γ-Aminobutyric acid (GABA) activates neuronal cells
by inducing the secretion of exosomes from intestinal cells. Food
Funct. 11:9285–9290. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Meng N, Pan P, Hu S, Miao C, Hu Y, Wang F,
Zhang J and An L: The molecular mechanism of γ-aminobutyric acid
against AD: the role of CEBPα/circAPLP2/miR-671-5p in regulating
CNTN1/2 expression. Food Funct. 14:2082–2095. 2023.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Rungratanawanich W, Cenini G, Mastinu A,
Sylvester M, Wilkening A, Abate G, Bonini SA, Aria F, Marziano M,
Maccarinelli G, et al: γ-Oryzanol improves cognitive function and
modulates hippocampal proteome in mice. Nutrients.
11(753)2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Muhammad SI, Maznah I, Mahmud R, Zuki AB
and Imam MU: Upregulation of genes related to bone formation by
γ-amino butyric acid and γ-oryzanol in germinated brown rice is via
the activation of GABAB-receptors and reduction of serum IL-6 in
rats. Clin Interv Aging. 8:1259–1271. 2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Welan R: Effect of vitamin B6 on
osteoporosis fracture. J Bone Metab. 30:141–147. 2023.PubMed/NCBI View Article : Google Scholar
|
|
63
|
van Meurs JBJ, Dhonukshe-Rutten RAM,
Pluijm SMF, van der Klift M, de Jonge R, Lindemans J, de Groot
LCPGM, Hofman A, Witteman JCM, van Leeuwen JPTM, et al:
Homocysteine levels and the risk of osteoporotic fracture. N Engl J
Med. 350:2033–2041. 2004.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Fratoni V and Brandi ML: B vitamins,
homocysteine and bone health. Nutrients. 7:2176–2192.
2015.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Seshadri S, Beiser A, Selhub J, Jacques
PF, Rosenberg IH, D'Agostino RB, Wilson PWF and Wolf PA: Plasma
homocysteine as a risk factor for dementia and Alzheimer's disease.
N Engl J Med. 346:476–483. 2002.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Tanaka K, Ao M and Kuwabara A:
Insufficiency of B vitamins with its possible clinical
implications. J Clin Biochem Nutr. 67:19–25. 2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Itoh M, Nishibori N, Sagara T, Horie Y,
Motojima A and Morita K: Extract of fermented brown rice induces
apoptosis of human colorectal tumor cells by activating
mitochondrial pathway. Phytother Res. 26:1661–1666. 2012.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Praengam K, Sahasakul Y, Kupradinun P,
Sakarin S, Sanitchua W, Rungsipipat A, Rattanapinyopituk K,
Angkasekwinai P, Changsri K, Mhuantong W, et al: Brown rice and
retrograded brown rice alleviate inflammatory response in dextran
sulfate sodium (DSS)-induced colitis mice. Food Funct. 8:4630–4643.
2017.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Saleh ASM, Wang P, Wang N, Yang L and Xiao
Z: Brown rice versus white rice: nutritional quality, potential
health benefits, development of food products, and preservation
technologies. Compr Rev Food Sci Food Saf. 18:1070–1096.
2019.PubMed/NCBI View Article : Google Scholar
|