Introduction
Currently, rectal cancer ranks third among all
common malignant tumors worldwide and is primarily treated with
laparoscopic surgery. Anastomotic fistulas are a serious
postoperative complication of rectal cancer surgery, which
adversely affects patient recovery and prognosis (1). A key contributing factor to
anastomotic fistulas is insufficient blood supply to the
anastomotic site (2,3). However, reducing the incidence of
postoperative anastomotic fistulas in laparoscopic surgery for
rectal cancer remains challenging. At present, surgeons mainly rely
on subjective judgement when assessing intestinal blood supply
during laparoscopic surgery, such as whether the tissue around the
anastomosis appears reddish, if the small arteries are pulsating or
if there is bleeding at the incisal edge. This approach carries a
risk of error, which can lead to the occurrence of anastomotic
fistulas (4). This is particularly
critical in radical surgery for rectal cancer, wherein blood supply
to the proximal colon, following high ligation of the inferior
mesenteric artery, depends on the marginal artery arc. If blood
supply to the proximal colon is inaccurately assessed and
anastomosis is performed, postoperative anastomotic fistulas may
develop.
Indocyanine green (ICG) fluorescence imaging is a
simple and effective method for evaluating tissue perfusion during
laparoscopic surgery. It is safe and feasible, demonstrating
promising potential for clinical application (5,6). ICG
is a near-infrared contrast agent known for its biocompatibility
(7,8). When exposed to external light with
wavelengths ranging from 750 to 800 nm, ICG emits near-infrared
light at longer wavelengths. Due to the inherent property of ICG to
emit light within the near-infrared spectrum, it is typically
unaffected by potential spontaneous fluorescence arising from blood
components, such as hemoglobin and water. Leveraging on this
characteristic, the ICG molecular fluorescence imaging system
integrates fluorescence reception and imaging with fluorescence
excitation. It applies a computer image processing system that is
coupled to a highly sensitive near-infrared fluorescence camera and
a near-infrared excitation light source to produce ICG fluorescence
images. Upon administration into the human body, ICG undergoes
metabolism in the liver and is excreted in its intact molecular
form through the bile duct, ultimately being eliminated through the
feces. Notably, it does not participate in the enterohepatic
circulation and exhibits relatively rapid metabolic clearance. The
present study aimed to investigate the efficacy of ICG fluorescence
imaging by retrospectively analyzing its application in
laparoscopic radical resection for rectal cancer and examining the
clinical and pathological characteristics, surgical outcomes,
postoperative recovery and complications. The feasibility and
safety of ICG fluorescence imaging for assessing the blood supply
at the anastomotic site are discussed, providing
evidence-based support for its future application in
colorectal surgeries.
Patients and methods
Study participants
This is an opportunistic cohort study. The clinical
and pathological data of 12 patients with rectal cancer treated at
the Department of Gastrointestinal Surgery, the Second Affiliated
Hospital of Fujian Medical University (Quanzhou, China) between
January and May 2024 were retrospectively analyzed. The inclusion
criteria were preoperative colonoscopy and pathology confirming
rectal cancer, patients who underwent laparoscopic radical
resection of rectal cancer, with normal liver function prior to
surgery, no history of allergy to ICG and aged between 18 and 75
years. The exclusion criteria were preoperative liver dysfunction,
history of allergy to ICG or iodine, uncontrolled comorbidities and
incomplete clinical and pathological data. The present study was
approved by the Ethics Committee of the Second Affiliated Hospital
of Fujian Medical University (approval no. 2021368).
Surgical procedures
Laparoscopic surgery was performed by following the
standard procedure for laparoscopic radical resection for rectal
cancer. After tumor resection, 25 mg ICG [Eisai (Liaoning)
Pharmaceutical Co., Ltd.] was dissolved in 10 ml sterile water for
injection before anastomosis of the proximal and distal intestinal
segments. ICG was administered at 0.2 mg/kg through a peripheral or
central vein. The intraoperative laparoscopic procedure utilized
the OptoMedic fluorescence laparoscope system (Guangdong OptoMedic
Technologies, Inc.). The optimal timing for intraoperative blood
supply assessment occurs within 3-5 min following the injection of
ICG into the bloodstream. During this period, both the completeness
and intensity of fluorescence in the anastomosis and adjacent
intestinal wall are evaluated. Given that ICG has a half-life of
3-4 min, it is almost completely metabolized within 10-20 min
following injection into the bloodstream. Consequently, if
required, re-administration of ICG can be performed. However, it
was advisable that there be a minimum interval of ≥15 min between
successive administrations. The blood supply assessment system
devised by Sherwinter et al (9) was used in the fluorescence mode: A
score of ≥3 (uniform fluorescence distribution at the intestinal
transection or anastomotic site) indicated good blood supply, 2
(uneven fluorescence distribution) indicated poor blood supply and
1 (no fluorescence observed) indicated no blood supply. If
intraoperative evaluation revealed poor intestinal blood supply,
the resection range would then be extended appropriately to ensure
sufficient blood supply to the proximal and distal intestinal
segments at the anastomosis site. After anastomosis, the same dose
of ICG solution was injected under fluorescence mode to observe the
blood supply at the anastomotic site. In this study, ICG was
utilized intraoperatively, with all procedures monitored by life
support equipment and anesthesiologists. Due to the rapid
metabolism of ICG within the body, any acute adverse reactions,
such as allergies that may arise following its intraoperative use,
can be addressed promptly (10).
Outcome measures
The following parameters were recorded: i) General
characteristics, namely sex, age, body mass index (BMI), maximum
tumor diameter, distance from tumour to anal verge and
postoperative tumour-nodes-metastasis (TNM) staging; ii) surgical
parameters, namely initial imaging time after ICG injection,
duration of imaging, duration of operation, intraoperative blood
loss and length of resected bowel; and iii) (3) postoperative recovery, specifically
time to first postoperative flatus, time to first intake of liquid
food, postoperative length of hospital stay and occurrence of
postoperative complications. The time of the first postoperative
flatus is primarily related to the recovery of intestinal function
following surgery. The time to first intake of liquid food is
typically two days after the first postoperative flatus. The
standardized discharge criteria for rectal cancer surgery include
the following: Absence of fever for >48 h, restoration of
intestinal function, no infection at the incision site and
independent ambulation. Therefore, these indicators are not
influenced by factors other than the utilization of fluorescence
imaging. All patients were monitored for a period of 1 year
following their discharge. After the surgery, it is recommended
that blood routine tests, biochemical analyses, CEA and CA19-9
levels, as well as other relevant indicators are re-evaluated every
three months. Additionally, chest and abdominal CT scans should be
conducted every six months, while a colonoscopy is advised to take
place one year post-operation.
Statistical analysis
The aforementioned indicators were statistically
analyzed using the Excel software 16.0 (Microsoft Corp.), employing
descriptive statistical methods. For measurement data, the median
(with range) was utilized for representation. By contrast, for
categorical data, the frequency of cases was reported.
Results
General characteristics
The 12 patients included 10 male and 2 female
patients, with a median age of 61 years (range, 53-74 years). The
BMI was 22.5 kg/m² (range, 18.0-31.2 kg/m²), the median maximum
tumour diameter was 3.9 cm (range: 1.7-6.5 cm) and the distance
from the tumour to the anal verge was 8.5 cm (range, 7.0-13.0 cm).
Postoperative TNM staging revealed that one patient was in stage I,
three patients were in stage II and eight were in stage III
(Table I). The table contains the
data for each individual patient.
 | Table IGeneral patient characteristics. |
Table I
General patient characteristics.
| Patient no. | Sex | Age, years | BMI,
kg/m2 | Max tumour diameter,
cm | Distance from the
tumour to the anal verge, cm | Postoperative TNM
stage |
|---|
| 1 | Male | 74 | 21.484 | 5.5 | 8 | II |
| 2 | Male | 61 | 21.971 | 5 | 7 | III |
| 3 | Male | 73 | 17.959 | 6.5 | 7 | II |
| 4 | Male | 56 | 19.355 | 4.5 | 8 | III |
| 5 | Female | 53 | 24.877 | 3.6 | 10 | III |
| 6 | Male | 59 | 25.712 | 2.1 | 10 | II |
| 7 | Male | 55 | 23.437 | 5.5 | 8 | III |
| 8 | Male | 66 | 21.358 | 2.7 | 10 | I |
| 9 | Male | 55 | 28.72 | 3.3 | 8 | III |
| 10 | Male | 73 | 31.221 | 3 | 13 | III |
| 11 | Male | 67 | 20.55 | 4.2 | 9 | III |
| 12 | Male | 61 | 22.985 | 1.7 | 10 | III |
Surgical outcomes
The patients completed the surgery and showed no
allergic reactions during the procedure. None of the patients
underwent prophylactic ileostomy. Before anastomosis, the patients
were assessed visually and intestinal blood supply was deemed
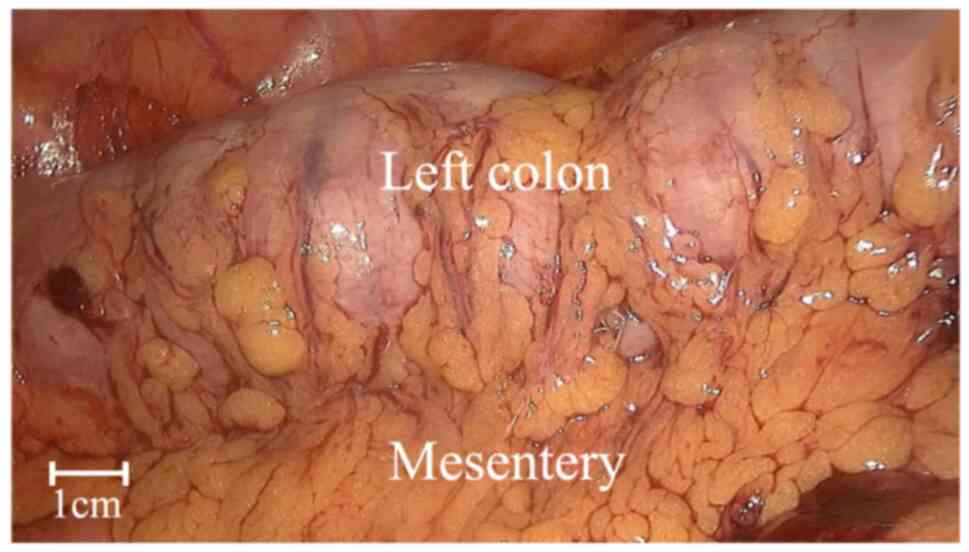
sufficient. In two patients, conventional laparoscopy showed good
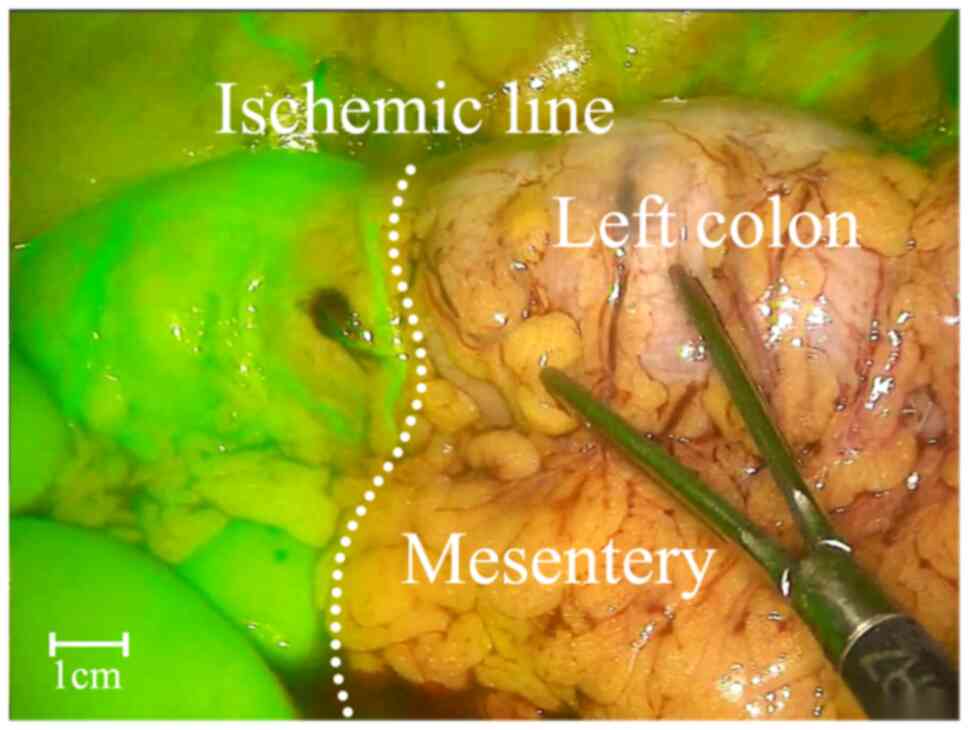
blood supply to the intestines before anastomosis (Fig. 1). However, ICG fluorescence imaging
demonstrated poor blood supply in the proximal intestinal segment,
necessitating extended resection (Sherwinter score, 1; Fig. 2). After resection, repeat
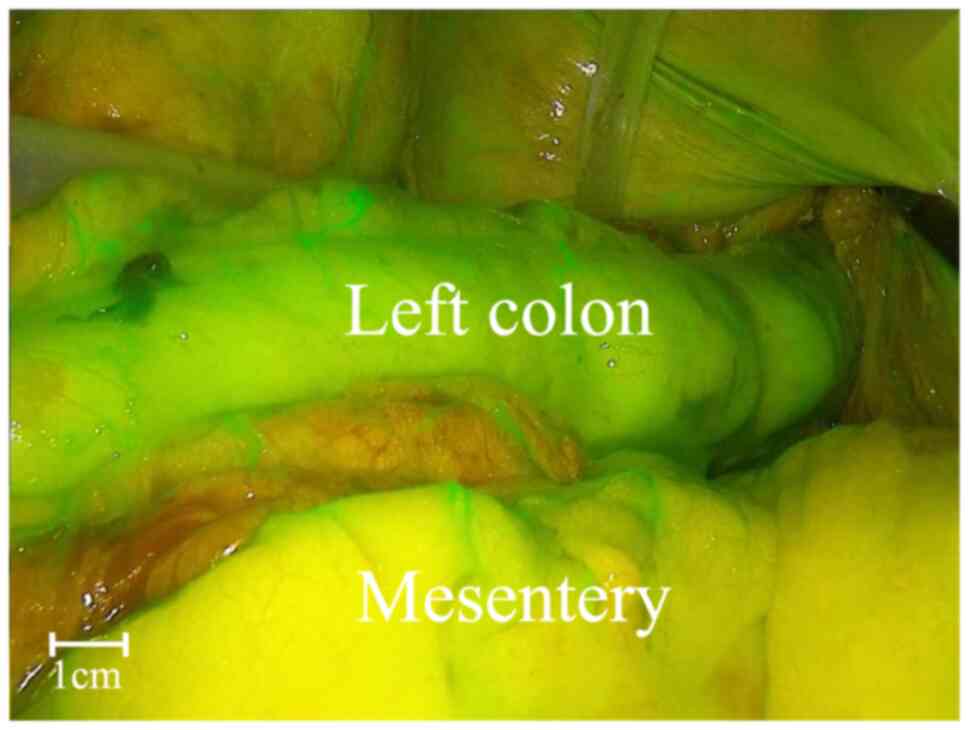
fluorescence imaging displayed good blood supply. Following
anastomosis, the same dose of ICG was injected and fluorescence
imaging confirmed adequate blood supply to the anastomosis site
(Sherwinter score, ≥3; Fig. 3).
Representative images are presented in the figures. In the
remaining patients, ICG fluorescence imaging before and after
anastomosis showed good blood supply (Sherwinter score ≥3), meaning
no extended resections were performed. The initial imaging time
after ICG injection for all patients was 44 sec (range, 31-69 sec),
the duration of imaging was 4 min (range, 3-6 min) and the duration
of the operation was 146 min (range: 112-193 min). The median
volume of intraoperative blood loss was 26.5 ml (range: 21.0-39.0
ml) and the length of resected bowel was 18 cm (range, 9.0-25.5 cm)
(Table II). The table contains
the data for each individual patient.
 | Table IIGeneral characteristics. |
Table II
General characteristics.
| Patient no. | Initial imaging
time after indocyanine green injection | Duration of
imaging, min | Duration of
operation, min | Intraoperative
blood loss, ml | Length of resected
bowel, cm |
|---|
| 1 | Male | 74 | 21.484 | 5.5 | 8 |
| 2 | Male | 61 | 21.971 | 5 | 7 |
| 3 | Male | 73 | 17.959 | 6.5 | 7 |
| 4 | Male | 56 | 19.355 | 4.5 | 8 |
| 5 | Female | 53 | 24.877 | 3.6 | 10 |
| 6 | Male | 59 | 25.712 | 2.1 | 10 |
| 7 | Male | 55 | 23.437 | 5.5 | 8 |
| 8 | Male | 66 | 21.358 | 2.7 | 10 |
| 9 | Male | 55 | 28.72 | 3.3 | 8 |
| 10 | Male | 73 | 31.221 | 3 | 13 |
| 11 | Male | 67 | 20.55 | 4.2 | 9 |
| 12 | Male | 61 | 22.985 | 1.7 | 10 |
Postoperative recovery
The time to first postoperative flatus was 2 days
(range, 1-3 days), the time to first intake of liquid food was 4
days (range, 3-5 days) and the postoperative length of hospital
stay was 8 days (range, 7-9 days). No postoperative complications,
such as anastomotic bleeding, anastomotic fistulas,
intra-abdominal bleeding, intra-abdominal infection
or intestinal obstruction, could be observed in all patients. The
aforementioned data are provided in Table III. The table contains the data
for each individual patient. No instances of tumour recurrence,
metastasis or long-term complications, such as intestinal
obstruction or anastomotic stricture, were observed in any of the
patients 1 year following the surgery.
 | Table IIIPostoperative recovery. |
Table III
Postoperative recovery.
| Patient no. | Time to first
postoperative flatus, days | Time to first
intake of liquid food, days | Postoperative
length of hospital stay, days | Occurrence of
postoperative complications |
|---|
| 1 | 3 | 5 | 9 | None |
| 2 | 1 | 3 | 7 | None |
| 3 | 1 | 3 | 7 | None |
| 4 | 2 | 4 | 8 | None |
| 5 | 2 | 4 | 8 | None |
| 6 | 2 | 4 | 8 | None |
| 7 | 1 | 3 | 7 | None |
| 8 | 2 | 4 | 8 | None |
| 9 | 1 | 3 | 7 | None |
| 10 | 1 | 3 | 7 | None |
| 11 | 2 | 4 | 8 | None |
| 12 | 2 | 4 | 8 | None |
Discussion
Anastomotic fistulas are a severe complication
following rectal cancer surgery, which leads to prolonged hospital
stays, increased costs and higher mortality rates, adversely
affecting short- and long-term outcomes of patients
(11-13).
Despite advances in neoadjuvant therapy and the implementation of
total mesorectal excision, as well as the development of
laparoscopic surgical instruments and improvements in surgical
techniques, the incidence of anastomotic fistula in colorectal
cancer surgery has not significantly decreased (14). Anastomotic fistulas can be caused
by various factors and are highly dependent on anastomotic blood
supply. Following inferior mesenteric artery ligation at its root
during radical resection of rectal carcinoma, the blood supply to
the residual distal intestine depends on the middle and inferior
rectal arteries (15). Blood
supply to the proximal colon is provided by the middle colic
artery, marginal arterial arcades or Riolan's arch (16,17).
If the marginal arterial arcades remain intact, the blood supply to
the descending colon is typically sufficient. It has been reported
that anastomotic fistulas may occur when blood flow is <70% of
normal levels, although the exact percentage required for reliable
intestinal healing remains elusive (18). Compared with postoperative
prevention and management, intraoperative real-time
evaluation of anastomotic perfusion is considered to be a more
reliable approach for preventing anastomotic fistulas (19). At present, intraoperative
assessment of blood supply to the anastomosis greatly relies on the
experience of the surgeon, which involves observing the degree of
intestinal peristalsis, colour, mesenteric fat appendage and
mesenteric arterial pulsation. In addition, surgeons may check for
active bleeding by cutting the marginal vessels or fat appendages.
However, this method is prone to errors. Previous reports revealed
that the sensitivity of subjective assessment is only 61.3%, with a
specificity of 88.5% (4). In obese
patients, wherein blood vessels are buried deep in the fat tissue
or in cases where the colon does not exhibit clear ischaemic
boundaries within a short time, it is challenging to accurately
evaluate blood supply (20).
Therefore, in rectal cancer surgery, accurately assessing blood
supply to the intestinal segments and anastomosis intraoperatively
whilst promptly modifying the surgical approach in cases of poor
blood supply may help reduce or prevent the occurrence of
anastomotic fistulas.
ICG fluorescence imaging is a simple and effective
method for real-time intraoperative visualisation. It is
primarily used clinically for tumor localisation, lymph node
tracing and tissue perfusion monitoring, for which it has been
proven to be safe and feasible (21). ICG is a non-radioactive
near-infrared imaging agent, meaning that no protective
measures are required during surgery (22). Considering that stained tissues are
not observable under visible light, ICG does not affect the
surgical field, prolong operative time or increase complexity
(23). In addition, ICG is a
cost-effective imaging agent. Despite all these advantages,
the use of ICG fluorescence imaging requires specialized
laparoscopic equipment, which may limit its widespread application.
After entering the bloodstream and reaching the target tissue, ICG
can be excited using lasers or light in the near-infrared
wavelength, emitting fluorescence within 1 min in regions with good
perfusion (24). This fluorescence
is then observed using a laparoscopic fluorescence imaging system.
During surgery, the Sherwinter scoring system can be used to
semi-quantitatively assess intestinal perfusion. In 2010,
Kudszus et al (25) were
the first to apply ICG fluorescence imaging in evaluating
anastomotic perfusion during colorectal cancer surgery, where the
results were positive. In another study by Ris et al
(26), ICG fluorescence imaging
was used in 30 patients during colorectal resection and
reconstruction, with successful imaging in 29 patients.
Specifically, ~35 sec after ICG injection, fluorescence appeared,
but no anastomotic fistula was observed postoperatively. A
meta-analysis by Blanco-Colino and
Espin-Basany (27), which
included 1,302 patients, concluded that the use of ICG
significantly reduced postoperative anastomotic fistula incidence
(ICG group vs. non-ICG group, 1.1 vs. 6.1%; P=0.02). In another
2020 Japanese multi-center retrospective study involving 422
patients with rectal cancer, where ICG fluorescence imaging was
used intraoperatively, surgical decisions were altered in 5.7%
patients owing to ICG imaging findings. This resulted in a ~6%
reduction in the rate of postoperative anastomotic fistulas, a
decrease in the reoperation rate and shortened hospital stays
(6).
Therefore, ICG fluorescence imaging is efficient and
precise in evaluating intestinal blood supply. This technique
eliminates the need for repeated intraoperative assessments,
significantly reducing operative time and accurately delineating
the resection margins, thereby minimising anastomotic complications
caused by poor perfusion. The core cost-effectiveness of ICG
fluorescence imaging is primarily attributed to its ability to
reduce complication risks. The single-use cost associated with ICG
is significantly lower compared with the expenses incurred from
treating postoperative complications (28). Traditional staining agents, such as
methylene blue and nano-carbon, exhibit various disadvantages,
including local adhesion issues, suboptimal visualization and even
the potential for abdominal cavity infections (29). Consequently, in comparison to
conventional staining techniques, ICG offers enhanced advantages in
terms of real-time performance, safety and accuracy in assessing
microcirculation. As a result, it has emerged as one of the most
cost-effective dynamic assessment tools for evaluating blood
perfusion. In the present study, two cases were observed in whom,
despite the proximal intestinal blood supply appearing normal under
white light, clear ischemia was determined under fluorescence
imaging. The accurate assessment provided by ICG fluorescence
imaging enabled the intraoperative resection of ischemic bowel
segments, preventing postoperative anastomotic ischemia and
fistulas. The ischemia in these two cases may have been due to poor
rotation of the descending colon, resulting in mesocolon thickening
and sigmoid colon adhesions. During surgery, mesocolon trimming may
have damaged the marginal artery, where ligating the inferior
mesenteric artery without preserving the left colic artery possibly
worsened the blood supply to the proximal colon. No obvious signs
of ischemia were detected during surgery. Without intraoperative
ICG fluorescence imaging, postoperative anastomotic fistulas due to
ischaemia may have occurred. If fluorescence intensity diminishes
during surgery, additional ICG can be administered intravenously to
enhance imaging quality. Although the present study did not include
a control group, the conclusions drawn are consistent with findings
from other studies, all of which suggest that ICG fluorescence
imaging can effectively assess the blood supply of anastomoses,
thereby reducing the incidence of postoperative anastomotic leakage
(30-32).
Inflammatory and nutritional biomarkers serve as
critical indicators for assessing the prognosis of patients with
colorectal cancer. Preoperative malnutrition and systemic
inflammatory status significantly impair the tissue repair capacity
and immune defence mechanisms of these patients, thereby elevating
the risk of poor anastomotic tissue healing post-surgery, which in
turn increases the incidence of anastomotic fistula (33). Once an anastomotic fistula occurs,
it triggers a severe local and systemic inflammatory response that
exacerbates catabolism and nutritional depletion within the body,
creating a vicious cycle that severely compromises both short-term
recovery and long-term oncological outcomes for patients (34). Consequently, close monitoring of
these inflammatory and nutritional biomarkers during the
perioperative period is invaluable for predicting the risk of
anastomotic fistula formation in addition to evaluating overall
prognosis. Shu et al (35)
previously indicated that low serum Ca2+ levels on the
first postoperative day are an independent risk factor affecting
overall survival and progression-free survival in patients with
colorectal cancer. Additionally, Li et al (36) previously identified that the
comprehensive immune-inflammation index can serve as a reliable
biomarker for prognostication in non-metastatic colorectal cancer
cases. The predictive scoring model developed from this index can
effectively forecast survival duration following surgery in
patients with non-metastatic colorectal cancer whilst aiding
clinical decision-making. Similar studies have further corroborated
the utility of both the comprehensive immune-inflammation index and
albumin-globulin ratio as dependable indicators for predicting
overall survival among patients with colorectal cancer. Notably,
elevated comprehensive immunoinflammatory indices coupled with a
reduced albumin-globulin ratio was found to be associated with
poorer prognostic outcomes (37).
A previous report has proposed a novel prognostic model, which
integrates the (neutrophils x platelets)/(lymphocytes x hemoglobin)
ratio along with the absolute monocyte count. This model
demonstrated superior predictive accuracy compared with that of
individual markers and exhibited high predictive performance for
1-, 3- and 5-year survival rates (38). Zhang et al (39) discovered that by assessing
preoperative serum nutritional biomarkers, such as the Onodera
Prognostic Nutritional Index and inflammatory indicators (such as
the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte
ratio), it was possible to effectively predict the risk of
anastomotic fistula in patients undergoing rectal cancer surgery.
Furthermore, the intraoperative application of ICG fluorescence
imaging technology allowed for the real-time assessment of
intestinal blood perfusion. This technique has been shown to reduce
the incidence of anastomotic fistulas, thereby minimizing this
serious complication and preventing subsequent inflammatory storms
and nutritional status deterioration triggered by such events.
Consequently, this approach significantly enhanced postoperative
recovery processes and improved long-term survival outcomes for
patients.
The present study has several limitations. The
inclusion of only 12 patients resulted in a limited sample size and
the potential biases were not sufficiently controlled for, which
somewhat reduced the statistical power. In addition, the present
study presented a single-group case series and did not include
comparisons with traditional intraoperative blood supply
assessments, making it challenging to fully demonstrate the
advantages of ICG technology. The postoperative follow-up period
was relatively short, preventing an evaluation of long-term
complications, where it remains unclear whether the short-term
benefits observed in the present study can be translated into
long-term outcomes. Therefore, supplementary follow-up is necessary
in subsequent stages. Future research will need to focus on
increasing the sample size, extending the follow-up duration,
establishing a randomized control group and initiating prospective
multi-center studies to clearly define endpoints and control for
various confounding factors or potential biases, thereby further
validating the findings of the present study.
In conclusion, the present study demonstrated that
ICG fluorescence imaging is safe, reliable and convenient for
evaluating intraoperative blood flow during laparoscopic surgery
for rectal cancer. This technique allowed the real-time
assessment of blood supply, reducing the risk of postoperative
anastomotic fistulas.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Fujian Provincial
Health and Youth Research Project (grant no. 2022QNA066) and the
Quanzhou Science and Technology Plan Project (grant no.
2021N034S).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
CYW contributed to conceptualization, data curation,
funding acquisition, investigation, methodology and
writing-original draft. QMH contributed to data curation, funding
acquisition, methodology, software and writing-original draft
preparation. KY contributed to methodology and writing-review and
editing. CHX contributed to conceptualization, supervision and
writing-review and editing. All authors read and approved the final
manuscript. All authors checked and confirmed the authenticity of
the raw data.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Second Affiliated Hospital of Fujian Medical University.
Because of its retrospective nature, the requirement of informed
patient consent was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zimmermann MS, Wellner U, Laubert T,
Ellebrecht DB, Bruch HP, Keck T, Schlöricke E and Benecke CR:
Influence of anastomotic leak after elective colorectal cancer
resection on survival and local recurrence: A propensity score
analysis. Dis Colon Rectum. 62:286–293. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nordholm-Carstensen A, Rolff HC and Krarup
PM: Differential impact of anastomotic leak in patients with stage
IV colonic or rectal cancer: A nationwide cohort study. Dis Colon
Rectum. 60:497–507. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Walz J, Burnett AL, Costello AJ, Eastham
JA, Graefen M, Guillonneau B, Menon M, Montorsi F, Myers RP, Rocco
B and Villers A: A critical analysis of the current knowledge of
surgical anatomy related to optimization of cancer control and
preservation of continence and erection in candidates for radical
prostatectomy. Eur Urol. 57:179–192. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Karliczek A, Harlaar NJ, Zeebregts CJ,
Wiggers T, Baas PC and van Dam GM: Surgeons lack predictive
accuracy for anastomotic leakage in gastrointestinal surgery. Int J
Colorectal Dis. 24:569–576. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hamabe A, Ogino T, Tanida T, Noura S,
Morita S and Dono K: Indocyanine green fluorescence-guided
laparoscopic surgery, with omental appendices as fluorescent
markers for colorectal cancer resection: A pilot study. Surg
Endosc. 33:669–678. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Watanabe J, Ishibe A, Suwa Y, Suwa H, Ota
M, Kunisaki C and Endo I: Indocyanine green fluorescence imaging to
reduce the risk of anastomotic leakage in laparoscopic low anterior
resection for rectal cancer: A propensity score-matched cohort
study. Surg Endosc. 34:202–208. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Swamy MMM, Murai Y, Monde K, Tsuboi S and
Jin T: Shortwave-infrared fluorescent molecular imaging probes
based on π-conjugation extended indocyanine green. Bioconjug Chem.
32:1541–1547. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gao C, Deng ZJ, Peng D, Jin YS, Ma Y, Li
YY, Zhu YK, Xi JZ, Tian J, Dai ZF, et al: Near-infrared dye-loaded
magnetic nanoparticles as photoacoustic contrast agent for enhanced
tumor imaging. Cancer Biol Med. 13:349–359. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sherwinter DA, Gallagher J and Donkar T:
Intra-operative transanal near infrared imaging of colorectal
anastomotic perfusion: A feasibility study. Colorectal Dis.
15:91–96. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Megaritis D, Echevarria C and Vogiatzis I:
Respiratory and locomotor muscle blood flow measurements using
near-infrared spectroscopy and indocyanine green dye in health and
disease. Chron Respir Dis. 21(14799731241246802)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shen Z, An Y, Shi Y, Yin M, Xie Q, Gao Z,
Jiang K, Wang S and Ye Y: The aortic calcification index is a risk
factor associated with anastomotic leakage after anterior resection
of rectal cancer. Colorectal Dis. 21:1397–1404. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ramphal W, Boeding JRE, Gobardhan PD,
Rutten HJT, de Winter LJMB, Crolla RMPH and Schreinemakers JMJ:
Oncologic outcome and recurrence rate following anastomotic leakage
after curative resection for colorectal cancer. Surg Oncol.
27:730–736. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Damen N, Spilsbury K, Levitt M, Makin G,
Salama P, Tan P, Penter C and Platell C: Anastomotic leaks in
colorectal surgery. ANZ J Surg. 84:763–768. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Paun BC, Cassie S, MacLean AR, Dixon E and
Buie WD: Postoperative complications following surgery for rectal
cancer. Ann Surg. 251:807–818. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Boxall TA, Smart PJ and Griffiths JD: The
blood-supply of the distal segment of the rectum in anterior
resection. BrJ Surg. 50:399–404. 1963.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Michels NA, Siddharth P, Komblith PL and
Parke WW: The variant blood supply to the descending colon,
rectosigmoid and rectum basedon 400 dissections. its importance in
regional resections: A review of medical literature. Dis Colon
Rectum. 8:251–278. 1965.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nano M, Dal Corso H, Ferronato M, Solej M,
Hornung JP and Dei Poli M: Ligation of the inferior mesenteric
artery in the surgery of rectal cancer: Anatomical considerations.
Dig Surg. 21:123–127. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shogan BD, Carlisle EM, Alverdy JC and
Umanskiy K: Do we really know why colorectal anastomoses leak? J
Gastrointest Surg. 17:1698–1707. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huh YJ, Lee HJ, Kim TH, Choi YS, Park JH,
Son YG, Suh YS, Kong SH and Yang HK: Efficacy of assessing
intraoperative bowel perfusion with near-infrared camera in
laparoscopic gastric cancer surgery. J Laparoendosc Adv Surg Tech
A. 29:476–483. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Spencer M, Unal R, Zhu B, Rasouli N,
McGehee RE Jr, Peterson CA and Kern PA: Adipose tissue
extracellular matrix and vascular abnormalities in obesity and
insulin resistance. J Clin Endocrinol Metab. 96:E1990–E1998.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xi S, Zheng X and Wang X, Jiang B, Shen Z,
Wang G, Jiang Y, Fang X, Qian D, Muhammad DI and Wang X: Initial
application of fluorescence imaging for intraoperative localization
of small neuroendocrine tumors in the pancreas: Case report and
review of the literature. J Gastrointest Cancer.
56(23)2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sosnowska-Sienkiewicz P, Kowalewski G,
Garnier H, Wojtylko A, Murawski M, Szczygieł M, Al-Ameri M,
Czauderna P, Godzinski J, Kalicinski P and Mankowski P: Practical
guidelines for the use of indocyanine green in different branches
of pediatric surgery: A polish nationwide multi-center
retrospective cohort study. Health Sci Rep.
8(e70586)2025.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Satoyoshi T, Okita K, Ishii M, Hamabe A,
Usui A, Akizuki E, Okuya K, Nishidate T, Yamano H, Nakase H and
Takemasa I: Timing of indocyanine green injection prior to
laparoscopic colorectal surgery for tumor localization: A
prospective case series. Surg Endosc. 35:763–769. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Martínek L, Pazdírek F, Hoch J and Kocián
P: Intra-operative fluorescence angiography of colorectal
anastomotic perfusion: A technical aspects. Rozhl Chir. 97:167–171.
2018.PubMed/NCBI(In Czech).
|
|
25
|
Kudszus S, Roesel C, Schachtrupp A and
Höer JJ: Intraoperative laser fluorescence angiography in
colorectal surgery: A noninvasive analysis to reduce the rate of
anastomotic leakage. Langenbecks Arch Surg. 395:1025–1030.
2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ris F, Hompes R, Cunningham C, Lindsey I,
Guy R, Jones O, George B, Cahill RA and Mortensen NJ:
Near-infrared(NIR) perfusion angiography in minimally invasive
colorectal surgery. Surg Endosc. 28:2221–2226. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Blanco-Colino R and Espin-Basany E:
Intraoperative use of ICG fluorescence imaging to reduce the risk
of anastomotic leakage in colorectal surgery: A systematic review
and meta-analysis. Tech Coloproctol. 22:15–23. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Brackney CK, Pestana IA, Hoffler HL and
Blazek CD: Assessment of flap viability for complex transmetatarsal
amputation using indocyanine green fluorescent angiography: A case
study. J Am Podiatr Med Assoc. 112:20–198. 2022.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Lee YN, Moon JH and Choi HJ: Role of
image-enhanced endoscopy in pancreatobiliary diseases. Clin Endosc.
51:541–546. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
De Nardi P, Elmore U, Maggi G, Maggiore R,
Boni L, Cassinotti E, Fumagalli U, Gardani M, De Pascale S, Parise
P, et al: Intraoperative angiography with indocyanine green to
assess anastomosis perfusion in patients undergoing laparoscopic
colorectal resection: Results of a multicenter randomized
controlled trial. Surg Endosc. 34:53–60. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jafari MD, Wexner SD, Martz JE, McLemore
EC, Margolin DA, Sherwinter DA, Lee SW, Senagore AJ, Phelan MJ and
Stamos MJ: Perfusion assessment in laparoscopic left-sided/anterior
resection (PILLAR II): A multi-institutional study. J Am Coll Surg.
220:82–92.e1. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chan DKH, Lee SKF and Ang JJ: Indocyanine
green fluorescence angiography decreases the risk of colorectal
anastomotic leakage: Systematic review and meta-analysis. Surgery.
168:1128–1137. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shi J, Wu Z, Li Z and Ji J: Roles of
macrophage subtypes in bowel anastomotic healing and anastomotic
leakage. J Immunol Res. 2018(6827237)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Foppa C, Ng SC, Montorsi M and Spinelli A:
Anastomotic leak in colorectal cancer patients: New insights and
perspectives. Eur J Surg Oncol. 46:943–954. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shu Y, Li KJ, Sulayman S, Zhang ZY,
Ababaike S, Wang K, Zeng XY, Chen Y and Zhao ZL: Predictive value
of serum calcium ion level in patients with colorectal cancer: A
retrospective cohort study. World J Gastrointest Surg.
17(102638)2025.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li K, Zeng X, Zhang Z, Wang K, Pan Y, Wu
Z, Chen Y and Zhao Z: Pan-immune-inflammatory values predict
survival in patients after radical surgery for non-metastatic
colorectal cancer: A retrospective study. Oncol Lett.
29(197)2025.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li K, Chen Y, Zhang Z, Wang K, Sulayman S,
Zeng X, Ababaike S, Guan J and Zhao Z: Preoperative
pan-immuno-inflammatory values and albumin-to-globulin ratio
predict the prognosis of stage I-III colorectal cancer. Sci Rep.
15(11517)2025.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang K, Li K, Zhang Z, Zeng X, Sulayman S,
Ababaike S, Wu Z, Pan Y, Chu J, Guan J, et al: Prognostic value of
combined NP and LHb index with absolute monocyte count in
colorectal cancer patients. Sci Rep. 15(8902)2025.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhang ZY, Li KJ, Zeng XY, Wang K, Sulayman
S, Chen Y and Zhao ZL: Early prediction of anastomotic leakage
after rectal cancer surgery: Onodera prognostic nutritional index
combined with inflammation-related biomarkers. World J Gastrointest
Surg. 17(102862)2025.PubMed/NCBI View Article : Google Scholar
|