Introduction
Airway inflammation is a central feature in the
pathogenesis of respiratory diseases such as asthma, chronic
obstructive pulmonary disease (COPD), bronchitis, and allergic
rhinitis, all of which contribute significantly to global morbidity
(1). Allergic asthma and rhinitis
alone affect approximately 300 million people worldwide, with
projections suggesting an additional 100 million cases by 2030 due
to escalating environmental exposures (2). Among various environmental triggers,
particulate matter (PM)-originating from both anthropogenic sources
(e.g., vehicle exhaust, industrial emissions) and natural phenomena
(e.g., wildfires, dust storms)-has emerged as a potent inducer of
airway inflammation and systemic injury (3,4).
Upon inhalation, PM can disrupt pulmonary
homeostasis by promoting allergen-specific immunoglobulin
production, complement activation, and recruitment of inflammatory
cells such as neutrophils, eosinophils, mast cells, and macrophages
(5,6). These immune responses contribute to
disease-specific pathologies, including bronchoconstriction and
airway hyperresponsiveness in asthma, mucus hypersecretion in
chronic bronchitis, and histamine-mediated nasal symptoms in
allergic rhinitis (7-9).
Although inhaled corticosteroids, leukotriene modifiers,
antihistamines, and bronchodilators can attenuate these
inflammatory pathways. However, their long-term use is often
limited by side effects such as mucosal dryness, drowsiness, and
high cost. Moreover, these treatments do not address the underlying
oxidative stress and cytokine-mediated tissue damage (10,11).
Given these limitations, there is growing interest in
phytochemicals that offer targeted anti-inflammatory action with
improved safety and affordability.
Natural products have gained increasing attention as
accessible, non-toxic alternatives to conventional therapies while
often offering comparable efficacy (12). Notably, combinations of natural
compounds frequently exhibit enhanced pharmacological activities
compared to a single agent (13).
Among these, Petasites japonicus (butterbur), a perennial
herb in the Asteraceae family, has long been used in East Asia for
its antipyretic, antitussive, and wound-healing properties and for
treating migraines, allergies, bronchial asthma, and
gastrointestinal ulcers (14).
Recent studies have further demonstrated butterbur's anti-tumor,
anti-allergic, anti-inflammatory, neuroprotective, and
anti-asthmatic effects (15).
Propolis is a natural substance used by bees to protect their
hives. It is an extract of a mixture of tree resin, honey, pollen,
and their own enzymes. Propolis is known to have a variety of
pharmacological properties, including antioxidant,
anti-inflammatory, antibacterial, wound healing, and
immunomodulatory effects (16-18).
However, the therapeutic potential of a P.
japonicus-propolis (PJP) mixture remains unexplored, especially
in the setting of PM10-induced airway inflammation.
Thus, this study aimed to evaluate the efficacy of
PJP extract in a mouse model of airway inflammation exacerbated by
particulate matter exposure. We focused on its ability to modulate
the IL-33/ST2/NF-κB signaling pathway. IL-33, an alarmin cytokine
released during cellular stress or damage, can bind to the ST2
receptor to activate NF-κB signaling, thereby driving the
production of pro-inflammatory cytokines and chemokines that
recruit immune cells to the airway (19). Our previous in vitro studies
demonstrated that a 1:1 mixture of P. japonicus and propolis
exhibited synergistic anti-inflammatory and antioxidant effects in
particulate matter-exposed cell models (20,21).
Based on these findings, we sought to investigate whether this
synergistic effect would be replicated in vivo. By targeting
this axis, PJP might offer a novel, natural strategy for mitigating
environmentally induced airway inflammation.
Materials and methods
Reagents
Ovalbumin (OVA, A5503), particulate matter 10
(PM10, MRMCZ110), aluminum hydroxide (239186),
dexamethasone (Dex, D4902) and decalcifying solution (HS-105) were
sourced from Sigma-Aldrich (St. Louis, MO, USA). H&E staining
kit (ab245880) and periodic acid-Schiff (PAS, ab150680),
Picro-Sirius Red stain kit (ab150681) and rabbit-specific HRP/DAB
detection IHC kit (ab64261) were obtained from Abcam (Cambridge,
UK). Toluidine blue O (198161) was sourced from Sigma-Aldrich (St.
Louis, MO, USA). Mouse IgE ELISA Kit (EMIGHE) and anti-ST2 antibody
(PA5-28383) were acquired from Thermo Fisher Scientific (Rockford,
IL, USA). Anti-Ovalbumin IgG1 (mouse) ELISA Kit Cayman (500830)
(Ann Arbor, MI, USA) and, IL-4 ELISA kits (M4000B) were sourced
from R&D system (Minneapolis, MN, USA). Histamine ELISA kit
(ENZ-KIT140A) was obtained from Enzo life sciences (Farmingdale,
NY, USA)., Donkey anti-rabbit IgG secondary antibody (31458) was
obtained from Thermo Fisher Scientific (Rockford, IL, USA). Goat
anti-Mouse IgG(H+L)-HRP (SA001) was sourced from GenDEPOT (Katy,
TX, USA)., IL-33 (88513) was obtained from Cell Signaling (Danvers,
MA, USA)., NF-κB (sc-8008), p-NF-κB (sc-136548), and β-actin
(sc-8432) were acquired from Santa Cruz Biotechnology (Dallas, TX,
USA).
Preparation of P. japonicus propolis
extract
In this study, P. japonicus was sourced from
(Jinan-gun, South Korea) and propolis powder was obtained from
Unique Biotech Co., Ltd. (Iksan-si, South Korea). Identification
and authentication of the plant were conducted by Professor
Hong-Jun Kim (College of Oriental Medicine, Woosuk University,
Jeonbuk, South Korea). A voucher specimen (#2024-02-01) was placed
in the Department of Health Management, College of Medical Science,
Jeonju University. P. japonicus samples were ground using a
blender and then extracted with 15 times their volume of 70%
ethanol at 50˚C under reflux for 6 hours. The resulting extract was
filtered through a cartridge filter using a Rotavapor R-210 (BUCHI
Labortechnik AG, Flawil, Switzerland). The filtered extract was
then concentrated using a vacuum rotary evaporator and subsequently
lyophilized with a freeze dryer. The P. japonicus extract
was then mixed with the propolis powder (1:1). The prepared sample
(PJP) was stored at -20˚C to maintain its stability until use.
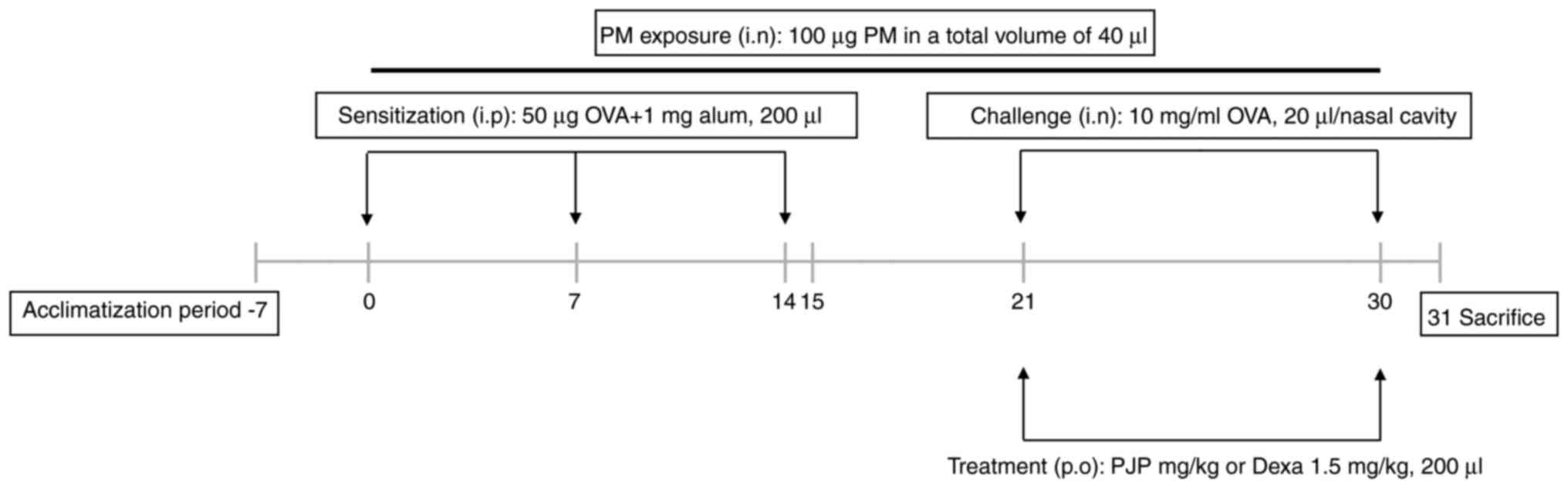
Animal studies and experimental
design
Animal studies were designed and conducted using
adult male BALB/c mice (6 weeks old, approximately 20 g) obtained
from Han-il Laboratory Animal Center (Wanju, South Korea). Mice
were housed under controlled environmental conditions (temperature:
22±2˚C, humidity: 50-60%, 12/12 h light/dark cycle) with ad libitum
access to a standard laboratory diet and water. The present study
was approved by Institutional Animal Care and Use Committee (IACUC)
of Jeonju University. The health status of each of the animals was
monitored daily. Humane experimental endpoints were established as
a 20% or greater loss of body weight, a decreased appetite for more
than two consecutive days, dyspnea, increased heart rate,
self-mutilation, jaundice, persistent diarrhea or vomiting, or a
decreased response to external stimuli. No animals reached these
endpoints, and no mortality or euthanasia occurred during the
study. Mice were acclimatized for one week before they were used
for experiments. The total duration of the animal experiment,
including the one-week acclimatization period and treatment
schedule, was 38 days. The sample size of six mice per group was
determined based on previous studies using
PM10/OVA-induced airway inflammation models (22), which commonly employ five to six
animals per group to detect biologically meaningful differences in
inflammatory markers with approximately 80% statistical power. This
number balances scientific rigor with ethical considerations
following the 3Rs principle (Reduction) to minimize animal use
while ensuring robust and reproducible results. While we
acknowledge that the limited sample size may affect statistical
power and generalizability, the current findings offer meaningful
preliminary insights. Future studies employing larger sample sizes
are planned to validate and expand upon these results. The
PM10/OVA-induced airway inflammation model used in this
study closely mimics key pathological features of human allergic
asthma, such as airway hyperresponsiveness, eosinophilic
inflammation, and mucus hypersecretion. After acclimation, mice
were randomly assigned to six groups, with each group consisting of
six mice. Group 1 (negative control group) was exposed to,
sensitized, treated, and challenged with saline. Group 2 (positive
control group) was exposed to 100 µg of PM10, sensitized
with 50 µg of OVA and 1 mg of alum and challenged with 10 mg/ml of
OVA, and treated with saline. Group 3 (PJP-50 group) was exposed to
100 µg of PM10, sensitized with 50 µg of OVA and 1 mg of
alum, challenged with 10 mg/ml of OVA, and treated with PJP at 50
mg/kg. Group 4 (PJP-100 group) was exposed to 100 µg of
PM10, sensitized with 50 µg of OVA and 1 mg of alum,
challenged with 10 mg/ml of OVA, and treated with PJP at 100 mg/kg.
Group 5 (PJP-200 group) was exposed to 100 µg of PM10,
sensitized with 50 µg of OVA and 1 mg of alum, challenged with 10
mg/ml of OVA, and treated with PJP at 200 mg/kg. Group 6
(dexamethasone group) was exposed to 100 µg of PM10,
sensitized with 50 µg of OVA and 1 mg of alum, challenged with 10
mg/ml of OVA, and treated with dexamethasone at 1.5 mg/kg. All test
compounds were administered via oral gavage. Groups 2-6 were
exposed to 100 µg PM10 in a total volume of 40 µl daily
via intranasal instillation and also sensitized on days 0, 7, and
14 with an intraperitoneal injection of 50 µg of OVA and 1 mg of
alum adjuvant solution. From day 15 to day 30, mice were treated
with either saline, 50-200 mg/kg PJP, or 1.5 mg/kg dexamethasone.
On days 21 and 30, all mice except those in the control group
received an intranasal challenge of 20 µl of OVA solution (10
mg/ml). On day 30, rubbing and sneezing were recorded for 15 min
for each mouse. Throughout the study, no noticeable adverse effects
related to PJP administration, such as body weight loss or reduced
appetite, were observed. Mice were sacrificed 24 h after the last
OVA challenge (day 31). Mice were deeply anesthetized with
inhalation of isoflurane at 2-6% for induction and maintained at
1-3% during sample collection. Euthanasia was performed by cervical
dislocation under deep anesthesia. Death was confirmed by the
absence of respiratory movement and heartbeat. Blood samples were
collected from their orbital venous plexus 24 h after the last OVA
challenge (day 31). Required mouse samples were then collected and
stored at -80˚C until use. A schematic of the experimental overview
is shown in Fig. 1.
Evaluation of nasal symptoms
After the final OVA challenge on day 30, each
mouse's behavior was recorded with a camera for 15 min to monitor
frequencies of nasal rubbing and sneezing. The scoring of sneezing
and nasal rubbing was performed by observers blinded to the
experimental groups to prevent bias.
Collection of serum, nasal lavage
fluid (NLF), and bronchoalveolar lavage fluid (BALF)
Serum, NLF, and BALF were collected using previously
established methods (23). At the
end of the experiment, mice were anesthetized with a 2-6%
isoflurane for induction and maintained at a 1-3% concentration.
Blood samples (600-800 µl per animal, collected once) were
collected from the orbital venous plexus. Subsequently, mice were
euthanized through cervical dislocation and, after confirming the
absence of respiratory and heartbeat, the trachea was opened, and 1
ml of sterile saline was injected into the nasal cavity using a
small tube to collect NLF. BALF was obtained by inserting an
18-gauge catheter into the partially exposed trachea and infusing 1
ml of saline, which was then gently aspirated. The collected fluid
was transferred to a microcentrifuge tube for centrifugation. The
supernatant obtained and serum were used for enzyme-linked
immunosorbent assays (ELISAs).
Histopathology
Nasal and lung tissues were collected immediately
after euthanasia on day 31. The tissues were carefully dissected
and rinsed with cold saline to remove blood prior to fixation.
Nasal and lung tissues were fixed in 10% formalin at 24±2˚C for
three days. Nasal tissues were then decalcified using a
decalcifying solution (HS-105) for histopathological examination.
After fixation, tissues were embedded in paraffin blocks and
sectioned at a thickness of 5 µm. The tissue sections were stained
with hematoxylin and eosin (H&E), Sirius red, Toluidine blue
and periodic acid-Schiff (PAS) according to the manufacturer's
guidelines to analyze general morphology, goblet cell hyperplasia,
and mast cell infiltration. The severity of lung inflammation was
assessed using a five-point scoring system as previously described
(24): 0 for normal, 1 for a few
cells, 2 for a ring of cells one-cell-layer deep, 3 for a ring of
cells two-to four-cells deep, and 4 for a ring of cells deeper than
four-cells deep. Areas of PAS-positive regions were measured using
Fiji software.
Evaluation of immunoglobulin and
cytokines
Levels of OVA-specific IgE and OVA-specific IgG1 in
serum were quantified using commercial ELISA kits following the
manufacturer's instructions. Similarly, levels of IL-4 and
histamine in both NLF and BALF were measured using ELISA kits
according to the manufacturer's guidelines.
Immunohistochemistry
Nasal tissue samples were collected and fixed in 10%
formalin at 24±2˚C for three days. The nasal tissues were then
decalcified using a decalcifying solution for histopathological
examination. After fixation, the tissues were embedded in paraffin
blocks and sectioned at a thickness of 4.5 µm. These sections were
analyzed using a rabbit-specific HRP/DAB detection IHC kit.
Western blotting
Lung tissues were homogenized, sonicated, and lysed
using radioimmunoprecipitation assay buffer. Protein quantification
was performed using the Bradford method, with bovine serum albumin
as the standard. Protein samples (50 µg) were separated by 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to polyvinylidene difluoride membranes. These membranes
were then blocked with freshly prepared 5% skim milk at 22±2˚C for
1 h. Each membrane was subsequently incubated overnight on an
orbital shaker with a primary antibody specific to the target
protein. After washing, the membranes were incubated with a
secondary antibody for approximately 2 h at 22±2˚C. Signals were
detected and visualized using an enhanced chemiluminescence
detection system (Alliance version 15.11; UVITEC). Bands were
analyzed using Image J software (NIH, Bethesda, MD, USA).
Statistical analysis
All data are presented as mean ± standard error of
the mean (SEM) (n=6). The Shapiro-Wilk test was used to assess the
normality of the data, and the results indicated that the data did
not meet the assumption of normality. Therefore, non-parametric
statistical analyses were performed. Group comparisons were
conducted using the Kruskal-Wallis test, followed by Dunn's
multiple comparison test for post hoc analysis. Statistical
analyses were performed using SPSS version 26.0 (IBM, Armonk, NY,
USA), and a P-value of <0.05 was considered statistically
significant.
Results
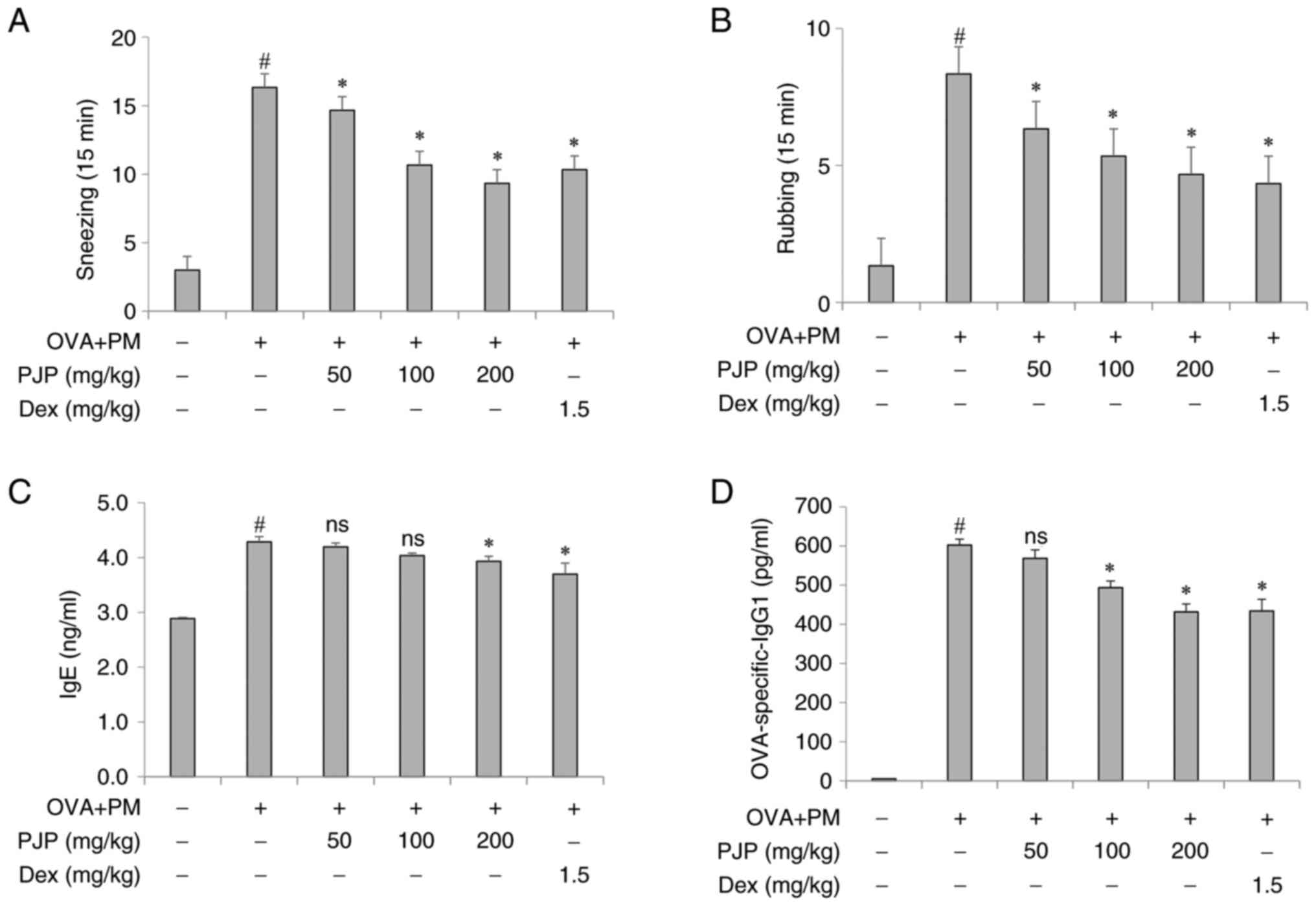
PJP reduces nasal symptoms of mice
with PM10/OVA-induced respiratory disease in mice
Exposure to PM10 and OVA throughout the
experimental period led to a significant increase in the frequency
of nasal rubbing and sneezing in mice of the positive control group
compared to the negative control group. However, treatment with PJP
at various doses resulted in a decrease in these symptoms. In
particular, mice administered PJP at 200 mg/kg exhibited
approximately a 42.9% reduction in sneezing and a 44.5% reduction
in nasal rubbing compared to the positive control group. These
effects were comparable to those observed in the
dexamethasone-treated group (1.5 mg/kg), with no significant
differences between the PJP 100 or 200 mg/kg groups and the
dexamethasone group (Fig. 2A and
B).
PJP reduces immunoglobulin levels in
serum of PM10/OVA-induced respiratory disease in
mice
Effects of PJP on immunoglobulin levels in mice with
respiratory disease were assessed. Exposure to PM10 and
OVA throughout the experimental period significantly increased
serum levels of OVA-specific IgE and IgG1 in the positive control
group compared to the negative control group. Administration of
various doses of PJP resulted in a decrease in these immunoglobulin
levels. In particular, mice administered 200 mg/kg of PJP showed
approximately 9.3 and 28.3% decrease in serum OVA-specific IgE and
IgG1 levels, respectively, compared to the positive control group.
There was no significant difference in these levels between mice
treated with PJP at 100 mg/kg or 200 mg/kg and those treated with
dexamethasone at 1.5 mg/kg (Fig.
2C and D).
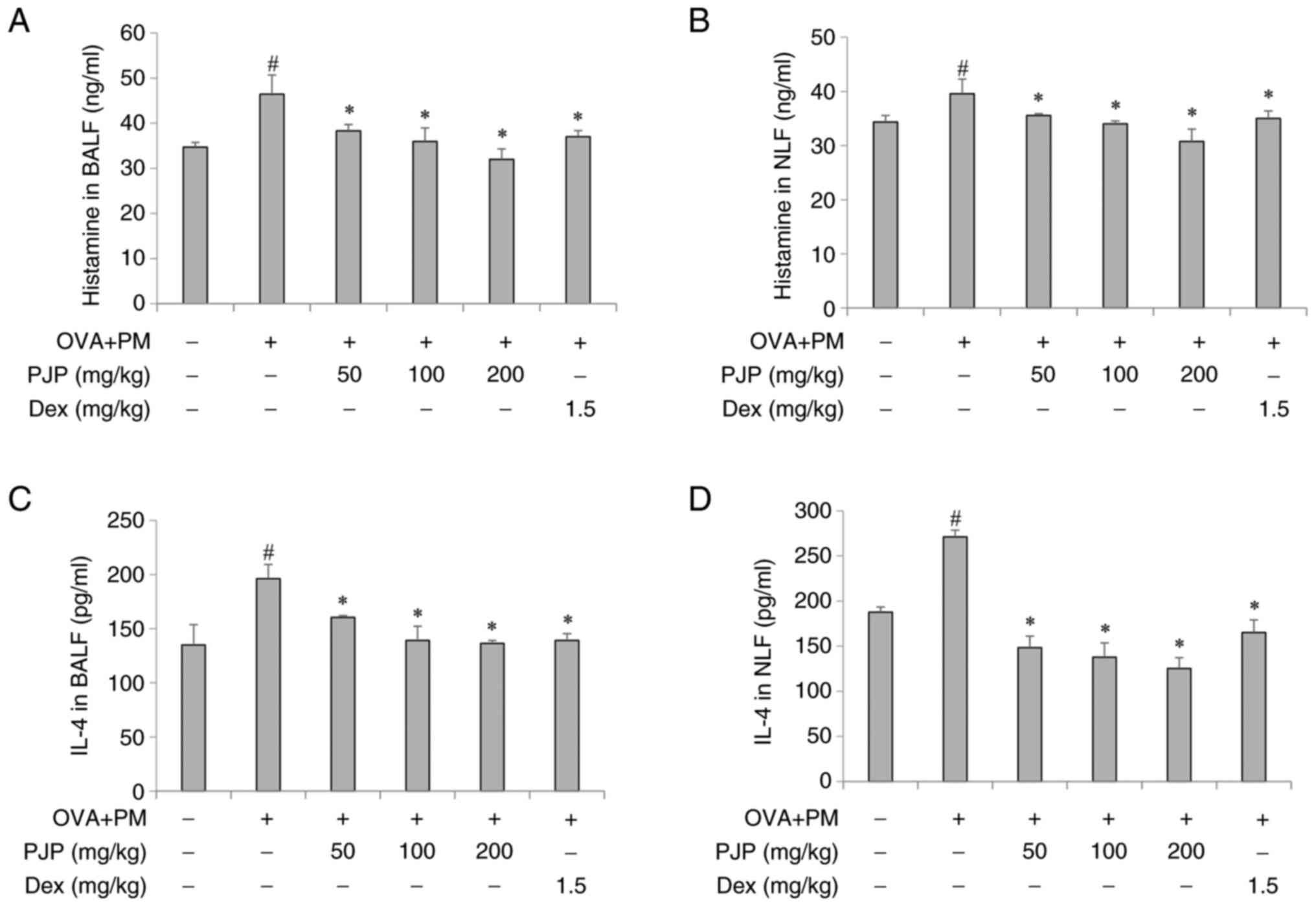
PJP reduces inflammatory mediators and
histamine release in BALF and NLF of PM10/OVA-induced
respiratory disease in mice
Effects of PJP on inflammatory mediators and
histamine release in BALF and NLF were investigated. Exposure to PM
and OVA throughout the experimental period significantly increased
levels of IL-4 and histamine in the BALF and NLF of the positive
control group compared to the negative control group. Treatment
with PJP at various doses resulted in a decrease in histamine
levels in both BALF and NLF, with significant reductions observed
at all doses. Interestingly, PJP at 200 mg/kg reduced histamine
release by approximately 31.3 and 22.4% in BALF and NLF, which was
more effective than the reduction achieved by dexamethasone at 1.5
mg/kg (Fig. 3A and B). In addition, PJP treatment
significantly decreased IL-4 secretion in both BALF and NLF. PJP at
200 mg/kg reduced IL-4 levels in BALF by approximately 30.4%
compared to the positive control group. Furthermore, in NLF, PJP at
200 mg/kg reduced IL-4 secretion by approximately 53.8% more than
the reduction observed with dexamethasone at 1.5 mg/kg (Fig. 3C and D).
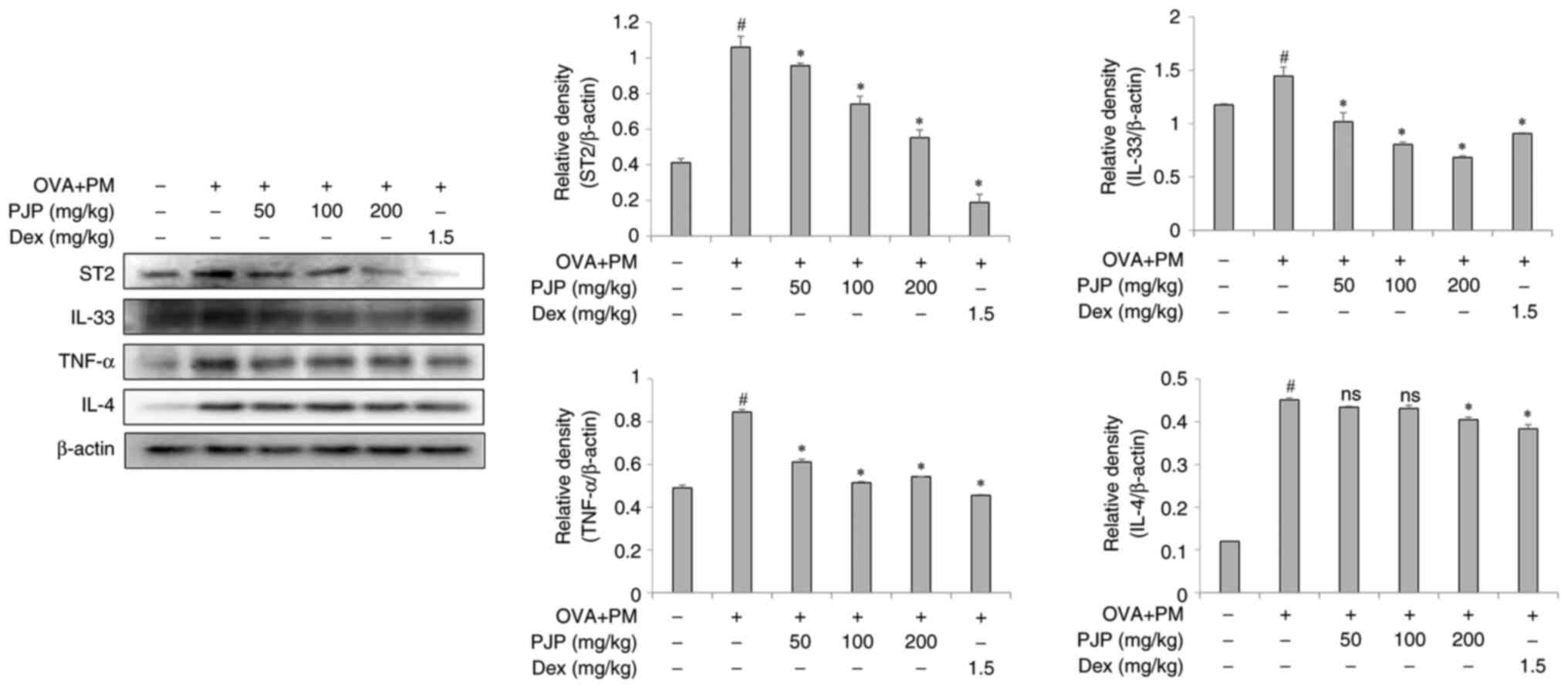
PJP reduces inflammatory mediators in
lung tissues of PM10/OVA-induced respiratory disease in
mice
Effects of PJP on inflammatory mediator expression
in lung tissues were examined. Exposure to PM10 and OVA
throughout the experimental period significantly increased the
expression of ST2, IL-33, TNF-α, and IL-4 in lung tissues of the
positive control group compared to the negative control group.
Treatment with various doses of PJP resulted in a reduction in the
expression of these mediators, with the greatest decreases observed
as follows: 52.8% for ST2, 45.7% for IL-33, 41.2% for TNF-α, and
11.1% for IL-4. These effects were comparable to those observed in
the dexamethasone-treated group (1.5 mg/kg) (Fig. 4).
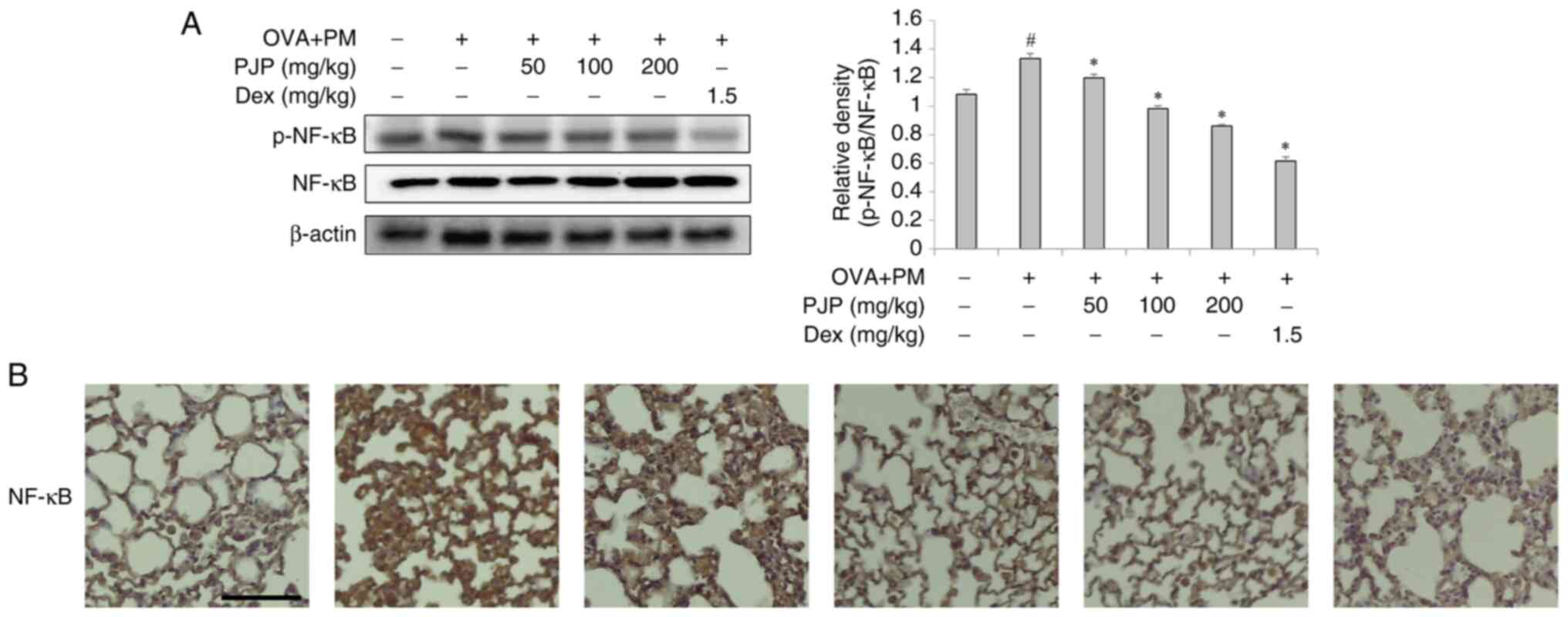
Effect of PJP on NF-κB signaling
pathway in lung tissues
Effects of PJP on activation of the MAPK/NF-κB
signaling pathway were analyzed. Both western blot and IHC analysis
of NF-κB phosphorylation in lung tissues revealed a significant
increase in p-NF-κB expression in the positive control group
compared to the negative control group. Treatment with various
doses of PJP decreased p-NF-κB expression compared to the positive
control group. Notably, PJP at 200 mg/kg reduced p-NF-κB expression
by approximately 35.3%. In addition, a significant difference was
observed between the PJP-treated group and the
dexamethasone-treated group (Fig.
5A and B).
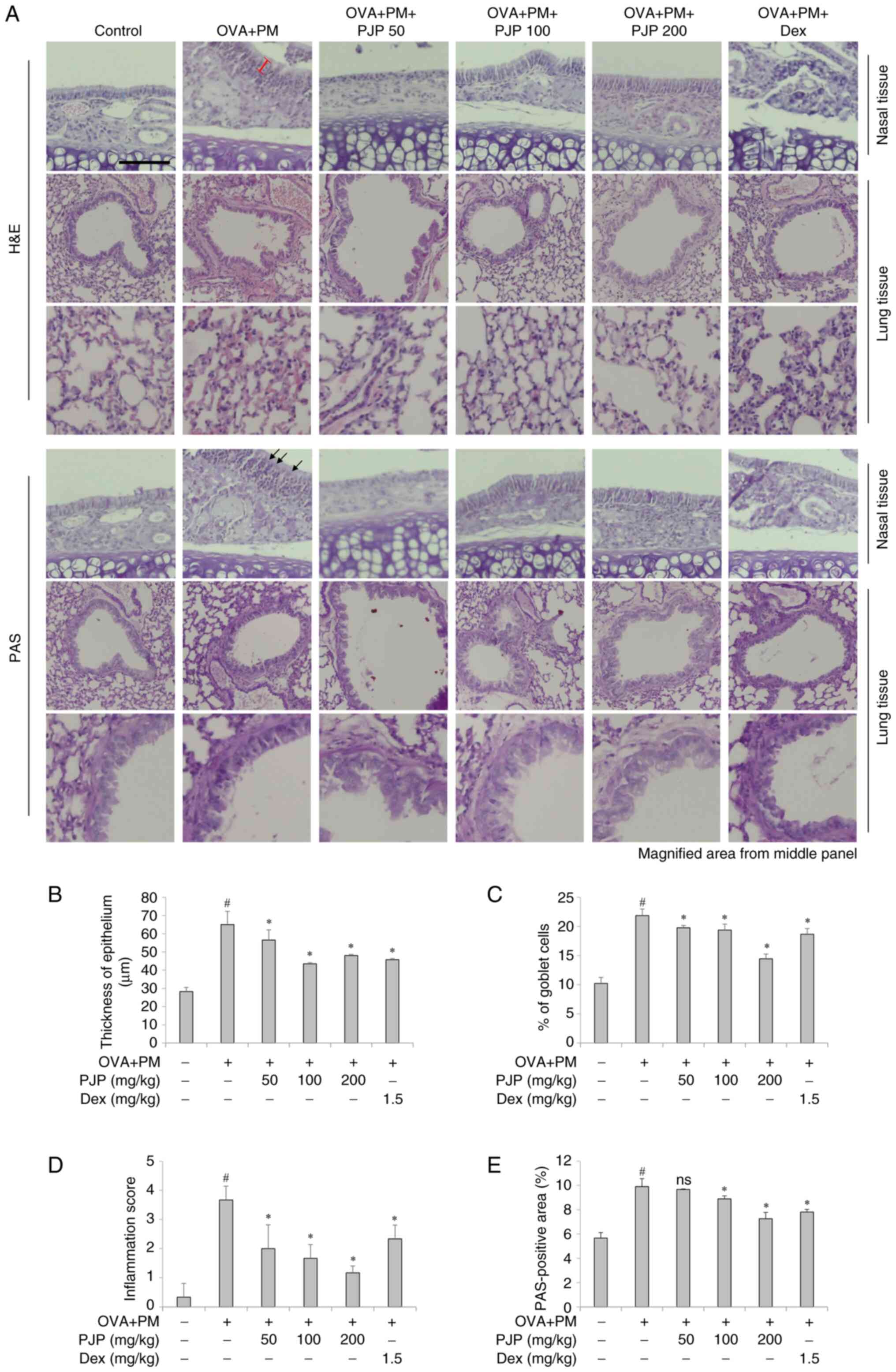
PJP ameliorates lung and nasal
histology in PM10/OVA-induced respiratory disease in
mice
Effects of PJP on the morphology and histology of
lung and nasal tissues were investigated. H&E staining revealed
that exposure to PM10 and OVA throughout the
experimental period significantly increased nasal mucosal thickness
in the positive control group compared to the negative control
group. Treatment with various doses of PJP resulted in a reduction
in epithelial thickness. Mice administered PJP exhibited a
significant decrease in epithelial thickness compared to the
positive control group. Notably, treatment with PJP at 100 mg/kg
reduced epithelial thickness by approximately 32.9%, and this
effect was not significantly different from that observed in the
dexamethasone-treated group (Fig.
6A and B). Additionally,
goblet cell hyperplasia was more pronounced in mice exposed to
PM10 and OVA compared to the negative control group.
However, the number of goblet cells was significantly reduced in
mice treated with PJP or dexamethasone compared to the positive
control group. In particular, PJP 200 mg/kg resulted in an
approximate 34.2% reduction in goblet cell numbers (Fig. 6C). Inflammatory cell infiltration
in lung tissues was also evaluated using H&E and PAS staining.
The inflammatory score was significantly higher in the
PM10/OVA-induced respiratory disease groups compared to
the control group. PJP supplementation significantly reduced the
inflammatory score compared to the positive control group.
Treatment with PJP at all doses significantly decreased the
inflammatory scores compared to the positive control group, with
the PJP 200 mg/kg showing an approximately 67.6% reduction in
inflammatory scores compared to the positive control group.
Notably, the anti-inflammatory effect observed in the PJP 200 mg/kg
group was greater than that observed in the dexamethasone-treated
group (Fig. 6D). Similarly, the
PAS-positive area was significantly higher in the
PM10/OVA-induced respiratory disease groups compared to
the negative control groups. PJP administration reduced the
PAS-positive area, indicating a decrease in mucus accumulation in
the airways. In particular, PJP at 200 mg/kg reduced the
PAS-positive area by approximately 26.3% compared to the positive
control group, demonstrating a comparable effect to dexamethasone
at 1.5 mg/kg (Fig. 6E).
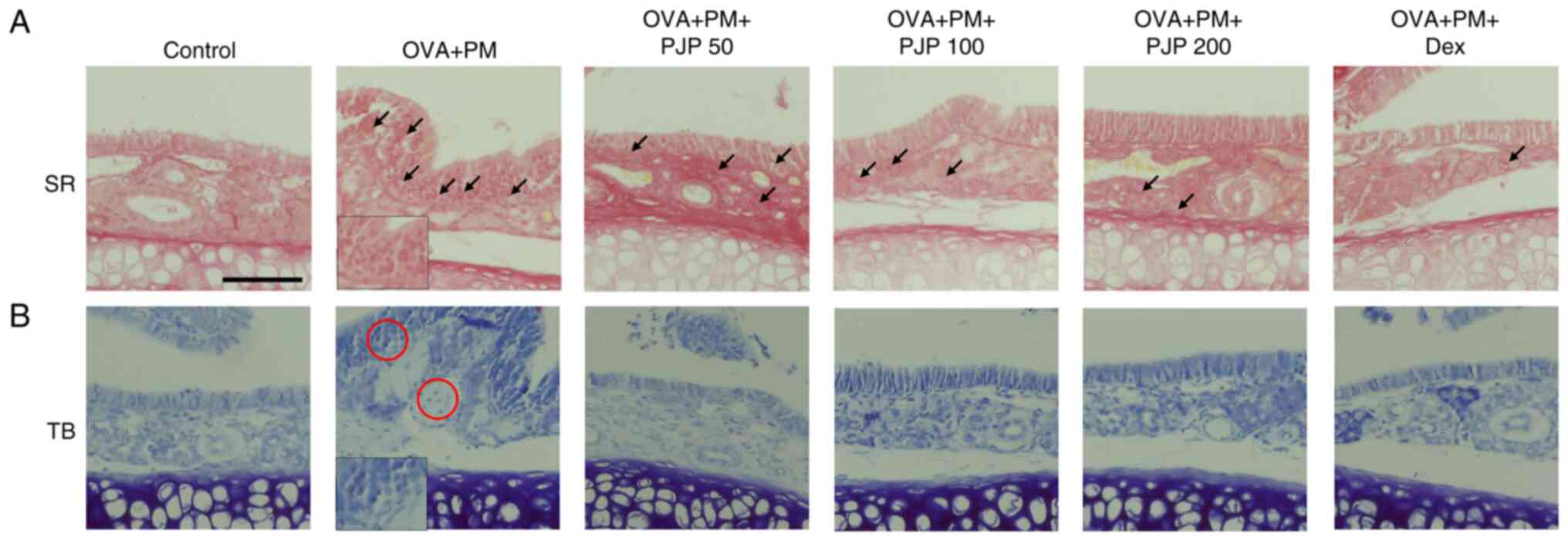
PJP reduces eosinophil and mast cell
infiltration in nasal tissues of PM10/OVA-induced
respiratory disease in mice
Effects of PJP on eosinophil and mast cell
infiltration in nasal tissues were investigated. Exposure to
PM10 and OVA throughout the experimental period
significantly increased eosinophil and mast cell infiltration in
nasal tissues of the positive control group compared to the
negative control group. This was demonstrated by Toluidine blue
staining of mast cells and Sirius red staining of eosinophils.
Treatment with PJP at various doses resulted in decreased
eosinophil and mast cell infiltration, with results similar to
those of the dexamethasone-treated group (Fig. 7A and B).
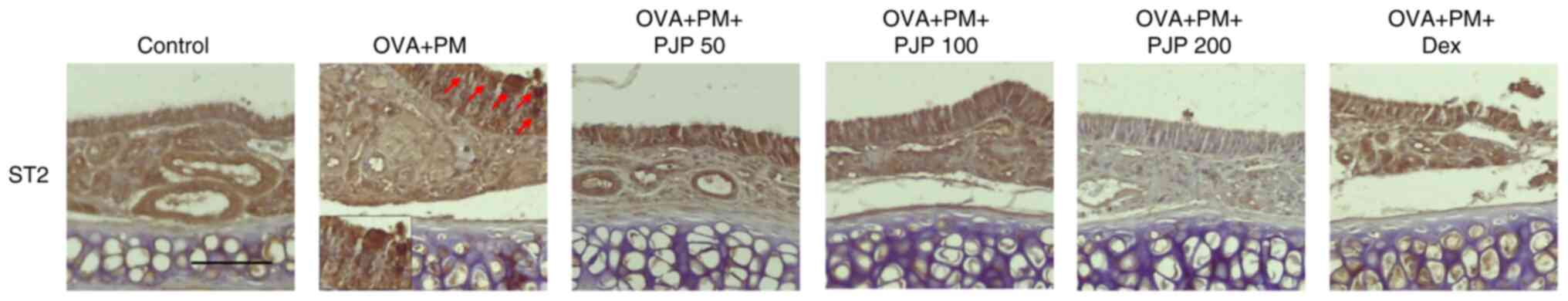
PJP reduces ST2 in nasal tissues of
PM10/OVA-induced respiratory disease in mice
ST2 expression in nasal tissues was evaluated using
IHC staining. Exposure to PM10 and OVA significantly
increased ST2 expression in nasal tissues of the positive control
group compared to the negative control group. Treatment with
various doses of PJP reduced ST2 expression, such, effects of PJP
were comparable to or more pronounced than those of dexamethasone
(Fig. 8).
Discussion
This study explored the effects of PM10
and OVA exposure on airway inflammation in a murine model of
allergic rhinitis and asthma, and evaluated the therapeutic
potential of PJP. Our findings demonstrated that PJP effectively
alleviated nasal symptoms, reduced immunoglobulin levels,
suppressed inflammatory mediators, and modulated key signaling
pathways involved in airway inflammation.
PM is a well-known exacerbating factor in
respiratory diseases, including asthma and allergic rhinitis
(3,25). PM exposure leads to increased
production of pro-inflammatory cytokines and recruitment of immune
cells such as mast cells, eosinophils, and macrophages, which
contribute to airway hyperresponsiveness and remodeling (26). Our PM10/OVA mouse model
successfully recapitulated these features, including enhanced nasal
rubbing, sneezing, epithelial thickening, goblet cell hyperplasia,
and elevated serum IgE and IgG1 levels, consistent with previous
reports using similar models (27,28).
The observed reduction of nasal symptoms and
immunoglobulin levels by PJP aligns with studies showing that
natural plant extracts and propolis components can modulate
allergic inflammation by regulating IgE-mediated responses and
immune cell activation (29,30).
Notably, PJP administration significantly decreased serum
OVA-specific IgG1 and IgE, which are critical in mast cell
sensitization and histamine release, thereby directly linking the
observed symptom relief to underlying immunomodulatory effects.
Furthermore, our results showed that PJP treatment
reduced levels of IL-4 and histamine in both BALF and NLF. IL-4 is
a central cytokine in Th2-mediated allergic responses that promotes
IgE class switching and mast cell recruitment (31,32).
The suppression of IL-4 and histamine release suggests that PJP
modulates the Th2 immune axis, a mechanism corroborated by previous
studies where propolis extracts inhibited Th2 cytokines and
associated inflammation (33).
Importantly, PJP's inhibitory effect on histamine release was more
pronounced than that of dexamethasone, highlighting its potent
anti-allergic properties.
In addition to Th2 cytokines, TNF-α and the
IL-33/ST2 axis are critical mediators of airway inflammation. TNF-α
amplifies allergic inflammation by enhancing Th2 cell migration and
cytokine production (34,35). The IL-33/ST2 pathway activates
downstream ERK1/2 signaling, leading to inflammatory gene
expression and recruitment of innate lymphoid cells (19,36).
PJP's ability to significantly downregulate TNF-α, IL-33, and ST2
expression underscores its broad-spectrum anti-inflammatory
potential, targeting multiple pathways implicated in allergic
airway disease pathogenesis.
The suppression of NF-κB phosphorylation in lung
tissues by PJP provides further insight into its mechanism of
action. NF-κB is a master regulator of inflammatory gene
transcription, and its activation is a hallmark of PM-induced
airway inflammation (37). By
inhibiting NF-κB activation, PJP may prevent the transcription of
pro-inflammatory cytokines and chemokines, thereby reducing immune
cell recruitment and inflammation. This molecular mechanism is
consistent with reports that P. japonicus and propolis
components can modulate NF-κB signaling in various inflammatory
models (38,39).
Histologically, PJP improved airway remodeling, as
evidenced by reduced epithelial thickness, goblet cell hyperplasia,
and inflammatory cell infiltration. These morphological
improvements are critical because chronic airway remodeling
contributes to disease severity and resistance to conventional
therapies (40). The notable
reduction in eosinophil and mast cell infiltration in nasal tissues
further validates PJP's anti-inflammatory efficacy.
In addition, to clarify whether the observed
cytokine-inhibitory effects are attributable solely to Petasites
japonicus, propolis, or their combination, our previous in
vitro studies using NCI-H292 lung epithelial cells and RAW264.7
macrophages demonstrated that the PJP mixture significantly
suppressed inflammatory cytokines (IL-6, IL-1β, TNF-α), restored
antioxidant enzyme levels (SOD, catalase, glutathione), and
inhibited NF-κB and MAPK signaling pathways under PM stimulation
(20,21). These findings suggest that both
Petasites japonicus and propolis individually exert
anti-inflammatory and antioxidant effects, but their combination
enhances these actions through potential synergistic mechanisms.
Although the current in vivo study did not include groups
treated with individual extracts, our prior in vitro data
support the hypothesis that each component contributes to the
overall efficacy of PJP. Future studies should include single
extract control groups to further delineate the individual and
combined effects of each component in vivo.
Despite these promising results, there are
limitations to consider. The study used an acute animal model, and
the long-term effects and safety profile of PJP remain to be
established. Moreover, the exact bioactive compounds responsible
for these therapeutic effects require further identification and
characterization. Future studies should also explore the
pharmacokinetics and potential synergistic effects of PJP
constituents, as well as validate these findings in clinical
trials. Additionally, the PJP used in this study lacked
standardization and quantification of key active compounds such as
petasin, flavonoids, and CAPE, which may affect reproducibility.
Although our previous in vitro studies confirmed
anti-inflammatory effects of each component, the present in
vivo study did not include propolis-only or P.
japonicus-only groups, limiting the ability to distinguish
their individual effects in vivo. Furthermore, while we
focused on NF-κB signaling, additional analysis of pathways such as
MAPK and ERK1/2 is needed to fully elucidate the therapeutic
mechanisms. Future studies will address these limitations.
In summary, our study demonstrates that PJP
mitigates PM10/OVA-induced airway inflammation through
modulation of immunoglobulin production, suppression of Th2
cytokines, inhibition of NF-κB signaling, and reduction of
inflammatory cell infiltration and airway remodeling. These
findings support the potential of PJP as a natural therapeutic
agent for allergic respiratory diseases exacerbated by air
pollution. Further research is warranted to fully elucidate its
mechanisms and clinical applicability.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Technology Development
Program (grant no. S3403654) funded by the Ministry of SMEs and
Startups (MSS, Korea).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JHP and JYS made major contributions to the study
design and manuscript writing, and made substantial contributions
to the acquisition, analysis and interpretation of the data. DNC
contributed to data interpretation, manuscript review, editing,
drafting and validation. MYK contributed to methodology development
and data visualization, and assisted with data analysis. YKH
provided essential resources, performed formal data analysis and
contributed to data interpretation. GSS contributed to data
visualization, assisted in data interpretation and performed
validation. BOC contributed to supervision, project administration
and study conceptualization. SIJ contributed to supervision,
project administration, study conceptualization and funding
acquisition. JHP and SIJ confirm the authenticity of all the raw
data. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by Jeonju University
Institutional Animal Care and Use Committee (approval no.
jjIACUC-20230602-2022-0505-A1; Jeonju, South Korea).
Patient consent for publication
Not applicable.
Competing interests
JHP, MYK and YKH are affiliated with Unique Biotech
Co., Ltd., which provided the propolis powder used in the present
study. The other authors declare that they have no competing
interests.
References
|
1
|
Jeffery PK and Haahtela T: Allergic
rhinitis and asthma: Inflammation in a one-airway condition. BMC
Pulm Med. 6 (Suppl 1)(S5)2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dharmage SC, Perret JL and Custovic A:
Epidemiology of asthma in children and adults. Front Pediatr.
7(246)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ramli NA, Md Yusof NFF, Shith S and Suroto
A: Chemical and biological compositions associated with ambient
respirable particulate matter: A review. Water Air Soil Pollut.
231(120)2020.
|
|
4
|
Bauer RN, Diaz-Sanchez D and Jaspers I:
Effects of air pollutants on innate immunity: The role of Toll-like
receptors and nucleotide-binding oligomerization domain-like
receptors. J Allergy Clin Immunol. 129:14–26. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cao TBT, Quoc QL, Jang JH and Park HS:
Immune cell-mediated autoimmune responses in severe asthma. Yonsei
Med J. 65:194–201. 2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Karagiannis SN, Karagiannis P, Josephs DH,
Saul L, Gilbert AE, Upton N and Gould HJ: Immunoglobulin E and
allergy: Antibodies in immune inflammation and treatment. Microbiol
Spectr. 1:2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Galli SJ, Tsai M and Piliponsky AM: The
development of allergic inflammation. Nature. 454:445–454.
2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yamauchi K and Ogasawara M: The role of
histamine in the pathophysiology of asthma and the clinical
efficacy of antihistamines in asthma therapy. Int J Mol Sci.
20(1733)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Costanzo G, Costanzo GAML, Del Moro L,
Nappi E, Pelaia C, Puggioni F, Canonica GW, Heffler E and Paoletti
G: Mast cells in upper and lower airway diseases: Sentinels in the
front line. Int J Mol Sci. 24(9771)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wise SK, Lin SY, Toskala E, Orlandi RR,
Akdis CA, Alt JA, Azar A, Baroody FM, Bachert C, Canonica GW, et
al: International consensus statement on allergy and rhinology:
Allergic rhinitis. Int Forum Allergy Rhinol. 8:108–352.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pascual RM and Peters SP: The irreversible
component of persistent asthma. J Allergy Clin Immunol.
124:883–892. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dzobo K: The role of natural products as
sources of therapeutic agents for innovative drug discovery. In:
Comprehensive Pharmacology. Kenakin T (ed). Elsevier, pp408-422,
2022.
|
|
13
|
Yang WJ, Li DP, Li JK, Li MH, Chen YL and
Zhang PZ: Synergistic antioxidant activities of eight traditional
Chinese herb pairs. Biol Pharm Bull. 32:1021–1026. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hiemori-Kondo M: Antioxidant compounds of
Petasites japonicus and their preventive effects in chronic
diseases: A review. J Clin Biochem Nutr. 67:10–18. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim EJ, Jung JI, Jeon YE and Lee HS:
Aqueous extract of Petasites japonicus leaves promotes
osteoblast differentiation via up-regulation of Runx2 and Osterix
in MC3T3-E1 cells. Nutr Res Pract. 15:579–590. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Acito M, Varfaj I, Brighenti V, Cengiz EC,
Rondini T, Fatigoni C, Russo C, Pietrella D, Pellati F, Bartolini
D, et al: A novel black poplar propolis extract with promising
health-promoting properties: Focus on its chemical composition,
antioxidant, anti-inflammatory, and anti-genotoxic activities. Food
Funct. 15:4983–4999. 2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bhatti N, Hajam YA, Mushtaq S, Kaur L,
Kumar R and Rai S: A review on dynamic pharmacological potency and
multifaceted biological activities of propolis. Discov Sustain.
5(185)2024.
|
|
18
|
Valverde TM, Soares BNGDS, Nascimento AMD,
Andrade ÂL, Sousa LRD, Vieira PMA, Santos VR, Seibert JB, Almeida
TCS, Rodrigues CF, et al: Anti-inflammatory, antimicrobial,
antioxidant and photoprotective investigation of red propolis
extract as sunscreen formulation in polawax cream. Int J Mol Sci.
24(5112)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Griesenauer B and Paczesny S: The
ST2/IL-33 axis in immune cells during inflammatory diseases. Front
Immunol. 8(475)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kang ES, Jeong SY, Cho BO, Shin JY, Kim
MY, Hur YK and Jang SI: Propolis and Petasites japonicus
leaf extract mixture attenuates PM2.5-induced inflammation in
NCI-H292 lung epithelial cells. Korean J Food Sci Technol.
56:458–464. 2024.
|
|
21
|
Kang ES, Shin JY, Jeong SY, Cho BO, Kim
MY, Hur YK and Jang SI: Antioxidant and anti-inflammatory effect of
Petasites japonicus leaf and propolis extract mixture in
RAW264.7 cells exposed to PM2.5. Korean J Food Sci Technol.
56:556–563. 2024.
|
|
22
|
Han H, Oh EY, Lee JH, Park JW and Park HJ:
Effects of particulate matter 10 inhalation on lung tissue RNA
expression in a murine model. Tuberc Respir Dis (Seoul). 84:55–66.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hofmann Bowman MA, Heydemann A, Gawdzik J,
Shilling RA and Camoretti-Mercado B: Transgenic expression of human
S100A12 induces structural airway abnormalities and limited lung
inflammation in a mouse model of allergic inflammation. Clin Exp
Allergy. 41:878–889. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tighe RM, Birukova A, Yaeger MJ, Reece SW
and Gowdy KM: Euthanasia-and lavage-mediated effects on
bronchoalveolar measures of lung injury and inflammation. Am J
Respir Cell Mol Biol. 59:257–266. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tiotiu AI, Novakova P, Nedeva D,
Chong-Neto HJ, Novakova S, Steiropoulos P and Kowal K: Impact of
air pollution on asthma outcomes. Int J Environ Res Public Health.
17(6212)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu K, Hua S and Song L: PM2.5 exposure
and asthma development: The key role of oxidative stress. Oxid Med
Cell Longev. 2022(3618806)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pang L, Yu P, Liu X, Fan Y, Shi Y and Zou
S: Fine particulate matter induces airway inflammation by
disturbing the balance between Th1/Th2 and regulation of GATA3 and
Runx3 expression in BALB/c mice. Mol Med Rep.
23(378)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Marín-Palma D, Tabares-Guevara JH, Taborda
N, Rugeles MT and Hernandez JC: Coarse particulate matter (PM10)
induce an inflammatory response through the NLRP3 activation. J
Inflamm Lond Engl. 21(15)2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Igarashi G, Segawa T, Akiyama N, Nishino
T, Ito T, Tachimoto H and Urashima M: Efficacy of Brazilian
propolis supplementation for Japanese lactating women for atopic
sensitization and nonspecific symptoms in their offspring: A
randomized, double-blind, placebo-controlled trial. Evid Based
Complement Alternat Med. 2019(8647205)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Azman S, Sekar M, Bonam SR, Gan SH,
Wahidin S, Lum PT and Dhadde SB: Traditional medicinal plants
conferring protection against ovalbumin-induced asthma in
experimental animals: A review. J Asthma Allergy. 14:641–662.
2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bao K and Reinhardt RL: The differential
expression of IL-4 and IL-13 and its impact on type-2 immunity.
Cytokine. 75:25–37. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Haas H, Falcone FH, Holland MJ, Schramm G,
Haisch K, Gibbs BF, Bufe A and Schlaak M: Early interleukin-4: Its
role in the switch towards a Th2 response and IgE-mediated allergy.
Int Arch Allergy Immunol. 119:86–94. 1999.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Missima F, Pagliarone AC, Orsatti CL,
Araújo JP Jr and Sforcin JM: The effect of propolis on Th1/Th2
cytokine expression and production by melanoma-bearing mice
submitted to stress. Phytother Res. 24:1501–1507. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Iwasaki M, Saito K, Takemura M, Sekikawa
K, Fujii H, Yamada Y, Wada H, Mizuta K, Seishima M and Ito Y:
TNF-alpha contributes to the development of allergic rhinitis in
mice. J Allergy Clin Immunol. 112:134–140. 2003.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jang DI, Lee AH, Shin HY, Song HR, Park
JH, Kang TB, Lee SR and Yang SH: The role of tumor necrosis factor
Alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in
therapeutics. Int J Mol Sci. 22(2719)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ding W, Zou GL, Zhang W, Lai XN, Chen HW
and Xiong LX: Interleukin-33: Its emerging role in allergic
diseases. Molecules. 23(1665)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang J, Huang J, Wang L, Chen C, Yang D,
Jin M, Bai C and Song Y: Urban particulate matter triggers lung
inflammation via the ROS-MAPK-NF-κB signaling pathway. J Thorac
Dis. 9:4398–4412. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Eo H, Lee S, Kim SH, Ju IG, Huh E, Lim J,
Park S and Oh MS: Petasites japonicus leaf extract inhibits
Alzheimer's-like pathology through suppression of
neuroinflammation. Food Funct. 13:10811–10822. 2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Búfalo MC, Ferreira I, Costa G, Francisco
V, Liberal J, Cruz MT, Lopes MC, Batista MT and Sforcin JM:
Propolis and its constituent caffeic acid suppress LPS-stimulated
pro-inflammatory response by blocking NF-κB and MAPK activation in
macrophages. J Ethnopharmacol. 149:84–92. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hough KP, Curtiss ML, Blain TJ, Liu RM,
Trevor J, Deshane JS and Thannickal VJ: Airway remodeling in
asthma. Front Med (Lausanne). 7(191)2020.PubMed/NCBI View Article : Google Scholar
|