Introduction
Hepatic mucinous cystic neoplasm (MCN), historically
classified as ‘biliary cystadenoma’ or ‘biliary
cystadenocarcinoma’, is a rare cystic liver tumor with an enigmatic
pathogenesis. According to the 2019 World Health Organisation (WHO)
classification of digestive system tumors, the diagnostic terms
‘bile duct adenoma’ and ‘biliary cystadenocarcinoma’ are no longer
used. Instead, the term ‘hepatic mucinous cystic neoplasm’ is
adopted, which is divided into three types: Mucinous cystic
neoplasm with low-grade dysplasia, mucinous cystic neoplasm with
high-grade dysplasia and invasive carcinoma associated with
mucinous cystic neoplasm (1).
Current hypotheses suggest that the origin of this tumor may
involve aberrant remodeling of the embryonic ductal plate (2), or the trans-differentiation of
primitive germ cells into ovarian-like stromal (OLS) cells within
the periductal fetal mesenchyme, while OLS occur exclusively in
females (3). MCN of the liver is
rare, comprising <5% of all hepatic cystic neoplasms. Notably,
this is predominantly diagnosed in females in the fourth or fifth
decade of life (4). The profound
disparity in incidence between biological sex, coupled with the
absence of established diagnostic biomarkers, highlights the
clinical challenge faced in the management of male patients with
cystic liver lesions.
Case report
Patient information and clinical
evaluation
A 74-year-old male patient presented to Xixi
Hospital (Hangzhou, China) with a two-week history of abdominal
distension and discomfort. Laboratory investigations demonstrated
notable serum tumor markers, including carcinoembryonic antigen and
carbohydrate antigen 19-9, and parameters indicative of healthy
liver function, such as alanine aminotransferase 17 U/l (normal
range, 7-40 U/l), aspartate aminotransferase 24 U/l (normal range,
13-35 U/l), alkaline phosphatase 88 U/l (normal range, 30-120 U/l)
and total bilirubin 0.9 mg/dl (normal range, 0.2-1.2 mg/dl).
Gastroscopic examination revealed an extrinsic compressive bulge in
the gastric antrum, while findings obtained via colonoscopy were
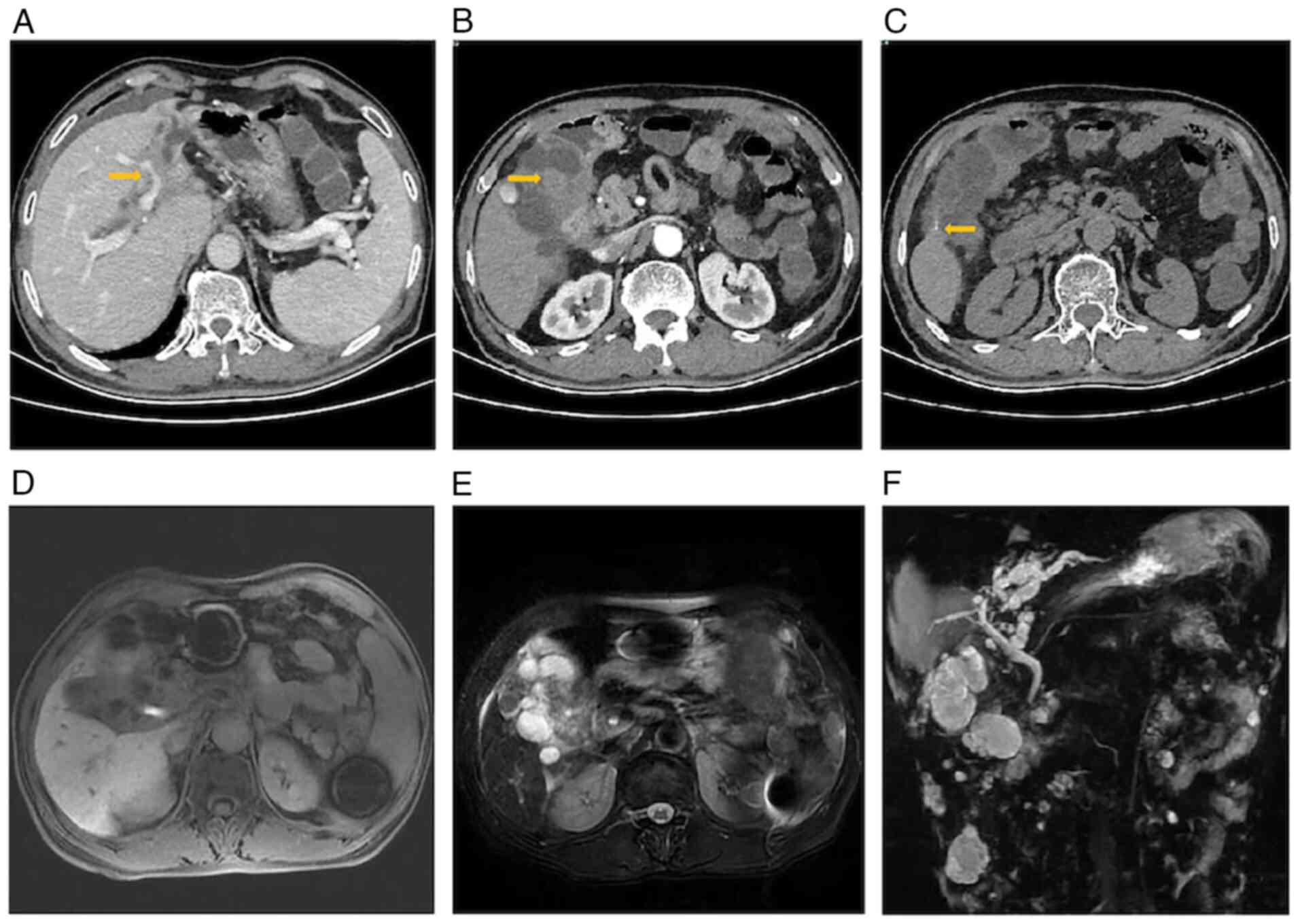
within healthy limits. Contrast-enhanced abdominal computed
tomography identified a multilocular cystic lesion (8.5x5.0 cm) in
the left hepatic lobe, with involvement of the common bile duct and
the left portal vein (Fig. 1A).
The lesion exhibited two key imaging hallmarks: i) Thickening of
the nodular wall (Fig. 1B) and ii)
curvilinear calcifications along the cyst wall (Fig. 1C). Upper abdominal magnetic
resonance imaging (MRI) demonstrated a heterogeneous signal
intensity within the hepatic parenchyma and an obscured anatomical
demarcation of the hilar region (Fig.
1D). The T2-weighted sequences revealed mixed hyperintense
signals (Fig. 1E). Dynamic
contrast-enhanced imaging revealed mild arterial-phase enhancement
of the hilar region, progressive enhancement during the portal
venous phase and persistent delayed-phase enhancement. Magnetic
resonance cholangiopancreatography further delineated a hilar mass
causing stricture of the proximal left intrahepatic bile ducts,
resulting in post-stenotic dilation of the distal biliary branches
(Fig. 1F).
Gastrointestinal endoscopy did not reveal any
primary tumors within the digestive tract, ruling out the potential
for liver metastasis from gastrointestinal tumors. Through imaging,
a multilocular cystic lesion was observed in the left liver,
characterized by thickened cyst walls and septa, in addition to
nodules (Fig. 1B) and
calcifications (Fig. 1C) on the
walls of the cyst. However, whether the lesion was directly
connected to the bile duct remained unclear, necessitating
differentiation from intraductal papillary neoplasm of the bile
duct (IPNB) and cholangiocarcinoma with cystic changes.
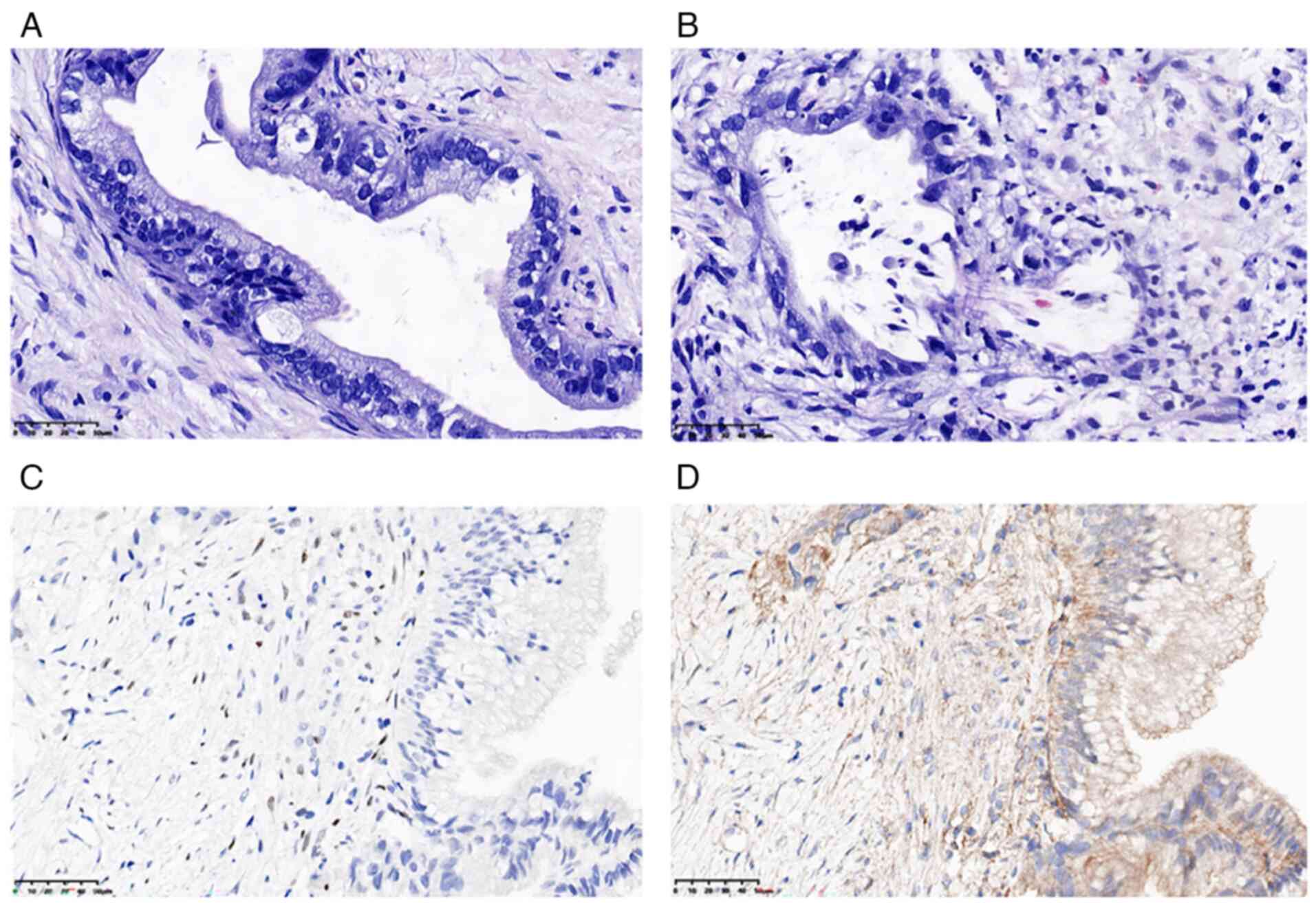
To further verify the initial diagnosis, a liver
biopsy was performed. Architectural features of the cyst included a
wall composed of hypocellular collagenous stroma, lined by
low-grade columnar mucinous epithelium without OLS (Fig. 2A). Irregular infiltration of
glandular structures with a desmoplastic reaction were indicative
of stromal invasion (Fig. 2B),
meeting the WHO 2022 criteria for invasive MCN (1).
Tissue analysis
For a more supportive diagnosis of MCN, further
immunohistochemical tests were conducted. The tissue
immunohistochemistry test was performed using specific antibodies
against caudal type homeobox 2 (CDX-2; Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.,), α-inhibin (Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.), estrogen receptor (ER; JOINN Biologics),
progesterone receptor (PR; JOINN Biologics), CK20 (Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd.), c-erbB-2 (Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd.), cytokeratin (CK)7
(Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.), Ki-67 (Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd.), p53 (Beijing Zhongshan
Jinqiao Biotechnology Co., Ltd.) and postmeiotic segregation
increased 2 (PMS2; Beijing Zhongshan Jinqiao Biotechnology Co.,
Ltd.) (cat. nos. ZA-0520, ZM-0430, Kit-0012, Kit-0013, ZM-0148,
ZM-0065, ZM0071, ZM0166, ZM0408 and ZM0407, respectively),
following the manufacturer's instructions. The final
immunohistochemical results were as follows: CDX-2(+), c-erbB-2(-),
CK7(-), CK20(+), Ki-67(30%+), p53(-), PMS2(+), ER(-), PR(+) and
α-inhibin(+). Notably, the co-expression of PR and α-inhibin
(Fig. 2C and D), despite the absence of morphologically
identifiable OLS, met the diagnostic criteria for MCN defined by
the WHO 2022 classification (1).
Treatment and prognosis
Given the confirmed diagnosis of invasive MCN with
portal vein involvement, curative intention hepatectomy was
recommended. However, the patient, concerned about financial
restrictions and potential side effects of
surgery/chemoradiotherapy, and with a lack of confidence in
treatment efficacy, declined antitumor therapy and was discharged
with symptomatic medications, including digestive enzymes. However,
during a telephone follow-up 6 months later, the patient was found
to have passed away.
Discussion
MCN is a rare cystic malignant tumor of the liver
that produces mucus and exhibits slow growth, with the potential to
progress to invasive carcinoma over several years. Prior to 2005,
<200 cases of MCN had been documented. At present, there are
~250 known cases of MCN; however, publications are limited due to
small sample sizes and uncertainty regarding associated risk
factors (5). MCNs predominantly
affect the female population, while cases in male patients are
considered rare (6). In the
largest study to date on pancreatic MCNs, 155 of 163 patients (95%)
were female, between 16 and 82 years of age, which indicates that
MCNs predominantly affect women (4). Over the past decade, sporadic cases
of hepatic MCN have been documented in male patients (7,8).
Although a few cases of invasive pancreatic MCN in male patients
have been reported, to date, no well-documented cases of invasive
hepatic MCN in males have been identified in the literature
(9). MCN is often asymptomatic in
the early stages of the disease; however, it may cause abdominal
distension, pain, dyspepsia and nausea (5).
MCN often exhibits a ‘cyst within cyst’ structure,
with contents primarily consisting of mucus and thickened cyst
walls, which may or may not be accompanied by calcification. MCN
typically presents as a solitary, large, well-demarcated
multilocular cystic mass, with the minority of the affected area
being unilocular (6-10%). It is more commonly located in the left
lobe of the liver and does not communicate directly with the bile
ducts (1). MRI often demonstrates
that MCN is hypointense on T1-weighted images and hyperintense on
T2-weighted images, although the intensity of the signal can vary
depending on the composition of the cystic fluid and the amount of
mucin present. Key features of MCN include cyst wall calcification,
thickened and improved septa, dilation of the upstream bile duct
and mural nodules (10). Notably,
a mural nodule exceeding 1 cm in size may be indicative of
malignant transformation (11).
Since 2010, the WHO has separated the classification of
intrahepatic cystic neoplasms into MCN and IPNB. The primary
distinction between these two conditions is the presence of OLS and
direct communication with the bile ducts (9). The precision of preoperative imaging
in diagnosing hepatic cystic lesions can be as low as 30% (12), leading to complexities in
distinguishing MCN from IPNB. This underscores the critical
importance of pathological analysis in accurately diagnosing
hepatic MCN.
Histologically, MCN is a cystic epithelial tumor
with a wall composed of three layers. The inner epithelial layer is
typically columnar or cuboidal in shape (13). The middle layer consists of
subepithelial OLS, which is diffusely present in ~50% of cases.
This layer is composed of densely packed spindle-shaped cells with
elongated nuclei and cytoplasm surrounded by collagen. Notably, the
outermost layer is a fibrous capsule (5). In cases of malignant transformation,
the histological appearance of these three layers changes. The
epithelium forms large pseudostratified nuclei with hyperchromasia.
The cells lose polarity and there are numerous mitotic figures in
cases of high-grade dysplasia. Furthermore, polypoid or papillary
projections are often observed (4). The intermediate layer of the stroma
undergoes degeneration, exhibiting hemorrhage, calcification and
necrosis. The external fibrous capsule may exhibit focal erosion
that is indicative of invasive behavior. Regarding the origin of
the OLS in the liver, certain researchers propose that the close
proximity of the liver and gonads during embryonic development
allows the migration of gonadal cells to the liver surface, leading
to the formation of OLS (14). In
addition, results of a previous study revealed that the peritoneal
surface epithelium of the embryonic gonads was covered with bulging
cells, as opposed to the typical flat epithelium; the examination
of embryos suggested that during the embryonic stage, these bulging
cells detach and migrate to the surfaces of nearby organs, such as
the liver (15).
When MCN is a benign cystic lesion, the cyst
epithelium exhibits characteristics of biliary-type epithelium,
specifically CK7(+)/CK20(-)/mucin 2 (MUC2)(-)/MUC5AC(-)/MUC6(-). At
this stage, the immunohistochemical characteristics of MCN are
comparable with those of cysts. As the tumor progresses, the cystic
epithelium exhibits gastrointestinal epithelial characteristics.
Furthermore, when MCN is considered borderline or malignant,
positivity rates for CK20, MUC2, MUC5AC and MUC6 are markedly
increased (3). This aids in
differentiating MCN from benign cysts; however, distinguishing MCN
from IPNB remains challenging. IPNB also exhibits gastrointestinal
epithelial characteristics, often exhibiting levels of positivity
for CK20, MUC2, MUC5AC and MUC6. However, positivity for MUC1 is
often only observed in the malignant phases of both tumors. ER, PR
and α-inhibin are often positively expressed in the OLS of MCN;
however, positive expression levels of these proteins are not
observed in IPNB (16,17).
According to the results of the imaging analysis,
the patient of the present study presented with a solid cystic
liver tumor that invaded the hepatic portal area, accompanied by
secondary dilation of the intrahepatic bile ducts in the left
liver. However, the tumor exhibited no direct communication with
the bile duct and no cystic dilation or nodular growth were
observed within the bile duct. Furthermore, septal thickening,
enhancement and wall nodules were observed in the mass, and this
was accompanied by cyst wall calcification. Notably, the
aforementioned radiological features are typical of MCN.
The diagnostic accuracy of preoperative imaging for
hepatic cystic lesions may be as low as 30% (10); thus, further diagnostic
confirmation is required using pathological analysis. Although the
presence of OLS is considered key for differentiating MCN from
IPNB, OLS is only present in female patients and is replaced by
hyaline stroma in male patients (9). Although OLS was not observed in the
present case, PR(+) and α-inhibin(+) are used to distinguish OLS
from IPNB. Furthermore, the patient showed positivity for CDX2 and
CK20, which is consistent with the gastrointestinal epithelial
characteristics observed in MCN with an associated invasive
carcinoma (3). The hallmark of
IPNB is the growth of the intraductal atypical epithelial papillary
and spread along the duct. Microscopic examination may reveal
adjacent bile ducts with intraepithelial neoplasia or peribiliary
glands within the cyst wall (17).
As these factors were not observed in the present case, the
diagnosis of MCN was confirmed.
Diagnostic imaging of hepatic MCN is considered to
be associated with low levels of accuracy; thus, histological
evaluation remains the primary method for definitive diagnosis.
Immunohistochemical analysis is therefore required when there are
complexities in identifying the stromal layer, mucin production is
limited or the diagnosis remains inconclusive (1).
In conclusion, the diagnosis of MCN should not rely
on biological sex. When evaluating patients with suspected cystic
liver disease, the potential for MCN should be considered. A
comprehensive assessment that integrates clinical characteristics,
imaging findings and pathological features is required for accurate
diagnoses in clinical practice. Furthermore, a multi-disciplinary
approach involving effective communication between pathologists and
clinicians is required for improved patient outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
ZZ and BW designed the study and coordinated the
clinical evaluation. YZ, WP and QH participated in the diagnosis
and treatment of the patient. YZ, WP and XZ were responsible for
data collection, clinical interpretation and histological
assessments. ZZ, WP and QH contributed to the analysis and
interpretation of the laboratory and imaging data, and supervised
the study. All authors participated in the writing of the original
draft and figure preparation. ZZ and BW critically reviewed and
revised the manuscript. XZ and QH confirm the authenticity of all
the raw data. All authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The present study was approved by Ethics Committee
Subcommittee for Scientific Research of Xixi Hospital (Hangzhou,
China; approval no. 2023-090).
Patient consent for publication
Written informed consent was obtained from the
patient and the patient's family for publication of this case
report and any accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript, and subsequently, the authors revised
and edited the content produced by the artificial intelligence
tools as necessary, taking full responsibility for the ultimate
content of the present manuscript.
References
|
1
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA:
WHO Classification of Tumours Editorial Board. The 2019 WHO
classification of tumours of the digestive system. Histopathology.
76:182–188. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
European Association for the Study of the
Liver. EASL clinical practice guidelines on the management of
cystic liver diseases. J Hepatol. 77:1083–1108. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Van Treeck BJ, Lotfalla M, Czeczok TW,
Mounajjed T, Moreira RK, Allende DS, Reid MD, Naini BV, Westerhoff
M, Adsay NV, et al: Molecular and immunohistochemical analysis of
mucinous cystic neoplasm of the liver. Am J Clin Pathol.
154:837–847. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Crippa S, Salvia R, Warshaw AL, Domínguez
I, Bassi C, Falconi M, Thayer SP, Zamboni G, Lauwers GY,
Mino-Kenudson M, et al: Mucinous cystic neoplasm of the pancreas is
not an aggressive entity: Lessons from 163 resected patients. Ann
Surg. 247:571–579. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hutchens JA, Lopez KJ and Ceppa EP:
Mucinous cystic neoplasms of the liver: Epidemiology, diagnosis,
and management. Hepat Med. 15:33–41. 2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shi B, Yu P, Meng L, Li H, Wang Z, Cao L,
Yan J, Shao Y, Zhang Y and Zhu Z: A paradigm shift in diagnosis and
treatment innovation for mucinous cystic neoplasms of the liver.
Sci Rep. 14(16507)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rizza Mae LABADAN and Jeff FONTANILLA:
Biliary mucinous cystic neoplasm in a 23-year old male: A case
report. Ann Hepatobiliary Pancreat Surg. 27 (Suppl
1)(S301)2023.
|
|
8
|
Yamada Y, Imai N, Morioka K, Matumoto K,
Yoshida N, Fuhisawa M, Kojima K, Fukasawa M, Beppu T, Nobukawa B,
et al: The senior male case of the mucinous cystic tumor (MCT) of
the liver. Tando. 17:51–56. 2003.
|
|
9
|
Yoon MH, Yoon JW and Han BH: Mucinous
cystadenoma of the liver with ovarian-like stroma: The need for
complete resection. J Korean Surg Soc. 81 (Suppl 1):S51–S54.
2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mortelé KJ and Ros PR: Cystic focal liver
lesions in the adult: Differential CT and MR imaging features.
Radiographics. 21:895–910. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Klompenhouwer AJ, Ten Cate DWG, Willemssen
FEJA, Bramer WM, Doukas M, de Man RA and Ijzermans JNM: The impact
of imaging on the surgical management of biliary cystadenomas and
cystadenocarcinomas; a systematic review. HPB (Oxford).
21:1257–1267. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Teoh AY, Ng SS, Lee KF and Lai PB: Biliary
cystadenoma and other complicated cystic lesions of the liver:
Diagnostic and therapeutic challenges. World J Surg. 30:1560–1566.
2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chan JH, Tsui EY, Luk SH, Fung AS, Yuen
MK, Szeto ML, Cheung YK and Wong KP: Diffusion-weighted MR imaging
of the liver: Distinguishing hepatic abscess from cystic or
necrotic tumor. Abdom Imaging. 26:161–165. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Albores-Saavedra J, Córdova-Ramón JC,
Chablé-Montero F, Dorantes-Heredia R and Henson DE: Cystadenomas of
the liver and extrahepatic bile ducts: Morphologic and
immunohistochemical characterization of the biliary and intestinal
variants. Ann Diagn Pathol. 19:124–129. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Soni S, Pareek P, Narayan S and Varshney
V: Mucinous cystic neoplasm of the liver (MCN-L): A rare
presentation and review of the literature. Med Pharm Rep.
94:366–371. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wheeler DA and Edmondson HA: Cystadenoma
with mesenchymal stroma (CMS) in the liver and bile ducts. A
clinicopathologic study of 17 cases, 4 with malignant change.
Cancer. 56:1434–1445. 1985.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zen Y, Pedica F, Patcha VR, Capelli P,
Zamboni G, Casaril A, Quaglia A, Nakanuma Y, Heaton N and Portmann
B: Mucinous cystic neoplasms of the liver: A clinicopathological
study and comparison with intraductal papillary neoplasms of the
bile duct. Mod Pathol. 24:1079–1089. 2011.PubMed/NCBI View Article : Google Scholar
|