Introduction
Solid pseudopapillary neoplasm of the pancreas (SPN)
is a rare low-grade malignant tumor, accounting for ~0.2-2.7% of
all pancreatic neoplasms (1-3).
As morphological and pathological studies on SPN have advanced in
both domestic and international research settings, the lesion has
been referred to using several different names, including
pancreatic papillary-solid tumor, pancreatic papillary-cystic
tumor, pancreatic cystic-solid tumor and pancreatic cystic-solid
papillary acinar cell tumor (4-6).
In 1996, the World Health Organization (WHO) officially designated
SPN based on its characteristic solid pseudopapillary architecture
and classified it as a borderline malignant tumor with
indeterminate biological behavior (7). By 2010, the WHO further defined SPN
as a low-grade malignant neoplasm (8). SPN predominantly occurs in young
females and its clinical presentation is typically nonspecific.
Preoperative diagnosis primarily depends on imaging modalities such
as computed tomography (CT) and magnetic resonance imaging (MRI).
Complete surgical resection remains the treatment of choice. The
postoperative prognosis for SPN is generally favorable, with the
reported 5-year survival rate exceeding 95% (1).
Case report
The patient was a 20-year-old female with no
significant clinical symptoms. A mass in the pancreatic tail was
incidentally detected during a routine physical examination and the
patient was subsequently admitted to Xingtai People's Hospital
(Xingtai, China) in March 2025 for further evaluation and
management. The patient's laboratory findings, including complete
blood count, cytokine analysis, comprehensive biochemical assays,
neutrophil apolipoprotein measurement and thymidine kinase 1
testing, demonstrated no clinically significant abnormalities.
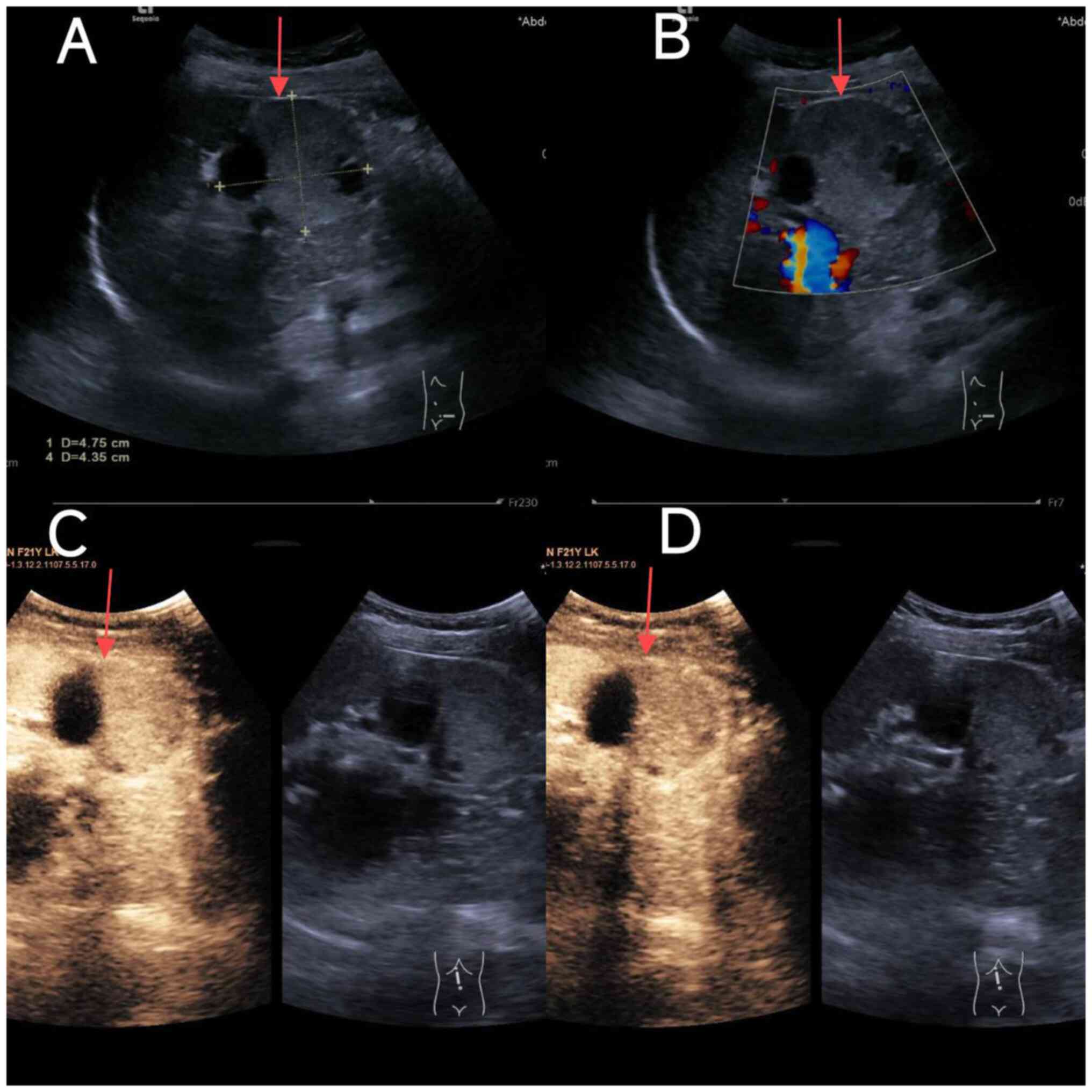
Conventional ultrasound demonstrated an irregularly shaped,
well-circumscribed cystic-solid lesion measuring ~48x44 mm in the
tail of the pancreas (Fig. 1A).
Color Doppler flow imaging revealed no detectable blood flow within
the lesion (Fig. 1B).
Contrast-enhanced ultrasound showed synchronous enhancement between
the lesion and the pancreas beginning at 10 sec, characterized by
heterogeneous moderate enhancement with visible non-enhancing
areas, followed by persistent hypo-enhancement (Fig. 1C and D). The ultrasound diagnosis indicated a
cystic-solid mass in the pancreatic tail, likely benign and of
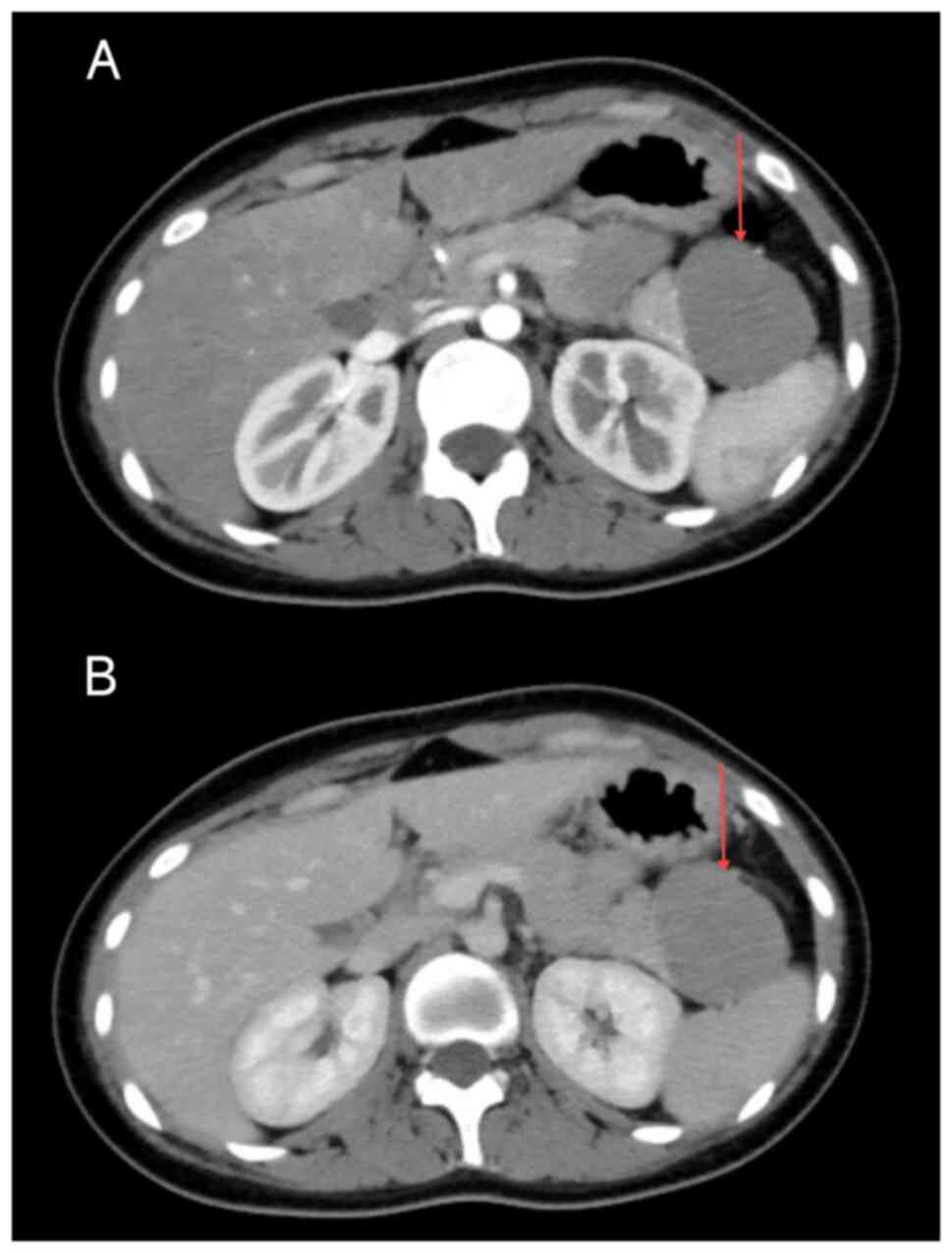
pancreatic origin, with SPN not entirely excluded. Enhanced CT scan
revealed an irregular mass measuring ~49x42 mm in the pancreatic
tail, showing CT attenuation values of 28-55 HU in the arterial
phase (Fig. 2A) and 36-68 HU in
the venous phase (Fig. 2B). The
lesion exhibited gradual mild enhancement, with visible
calcifications and internal septations, as well as an intact
capsule and well-defined margins. Radiological diagnosis suggested
a space-occupying lesion in the pancreatic tail, with SPN being a
probable diagnosis. Given the definitive diagnosis and absence of
comorbid conditions, tumor marker testing was not performed.
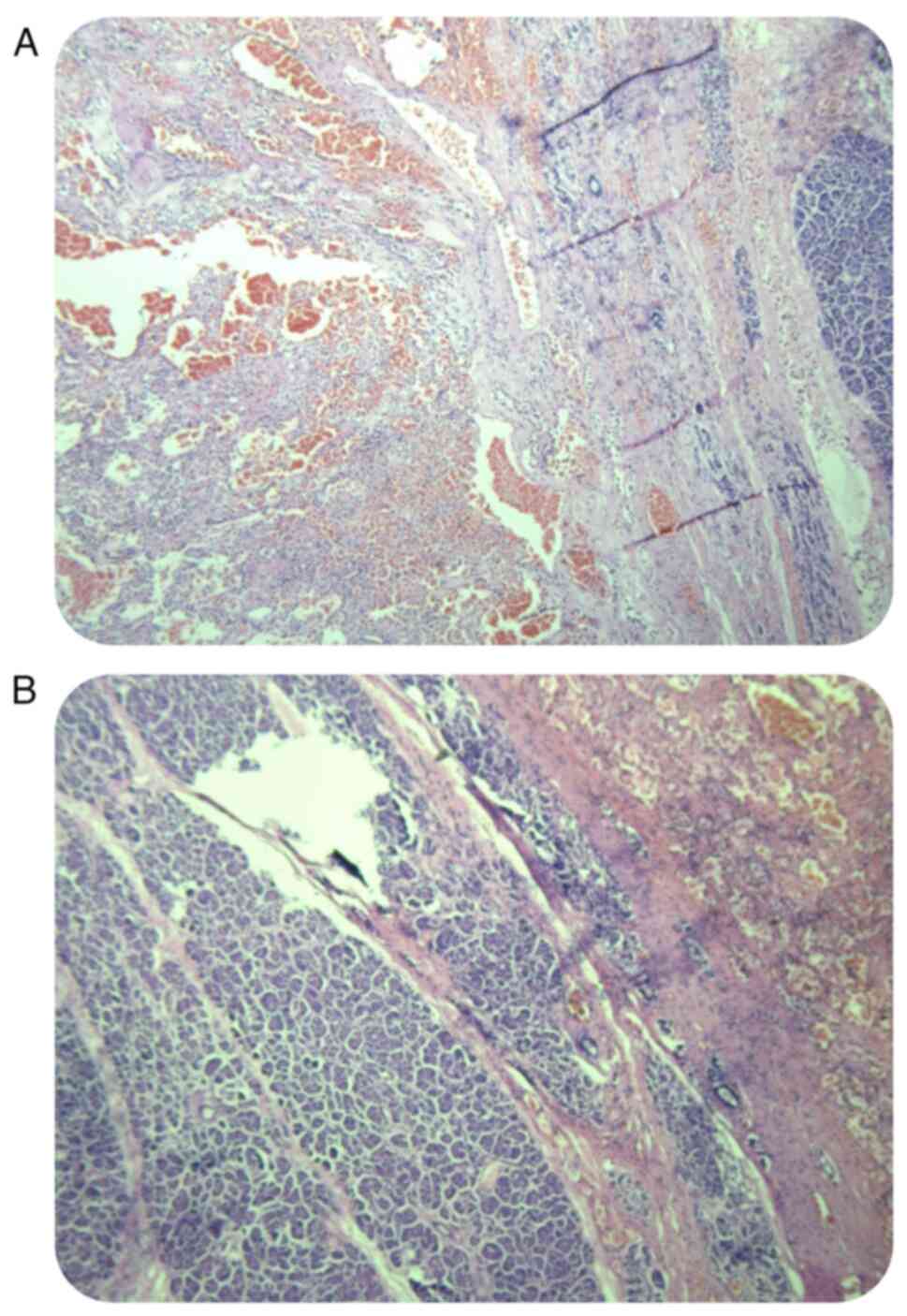
Following complete surgical resection, histopathological analyses
confirmed the diagnosis of SPN with focal cystic degeneration and
hemorrhage (Fig. 3A and B). H&E staining was performed as
follows: The tumor tissues were fixed in 10% formalin for >24 h
at 25˚C (Beijing BioDee Biotechnology Co., Ltd.), followed by
gradient alcohol dehydration and embedding in paraffin.
Subsequently, 3-µm thick sections were prepared and stained with
H&E. The ICD-10 classification code for this disease is
D37.700x003(7). According to the
ICD-10 classification system, borderline tumors are assigned codes
within the range D37 to D48. Within this range, codes D37 to D44
are used for tumors categorized as ‘uncertain malignant potential’
(dynamic undetermined), whereas codes D45 to D48 are assigned to
tumors classified as ‘unspecified behavior’ (dynamic unknown). The
specific ICD-10 code for borderline pancreatic tumors is D37.7,
which is classified under the category ‘tumors of other digestive
organs, uncertain malignant potential or unspecified behavior’. The
patient completed the initial follow-up examination within one
month following surgery, with clinical observations confirming
satisfactory wound healing and the restoration of normal
gastrointestinal function. Subsequent follow-up evaluations should
be performed at regular intervals of 3 to 6 months. Postoperative
follow-up demonstrated no abnormal abdominal masses or symptoms and
enhanced CT imaging revealed no radiological evidence of tumor
recurrence or metastasis. It is essential to closely monitor the
patient's psychological condition and provide timely psychological
counseling and support to alleviate negative emotions, such as
anxiety and depression. Simultaneously, the patient should be
encouraged to maintain a positive and optimistic outlook in order
to strengthen their confidence in the recovery process.
Discussion
SPN is a rare low-grade malignant tumor
characterized by solid and pseudopapillary architectural features
(4). Since its initial description
by Frantz (9) in 1959, the
understanding of SPN has progressively advanced. In recent years,
with advancements in imaging modalities such as CT and MRI, along
with improvements in clinical diagnostic and therapeutic
capabilities, the reported incidence of SPN has increased (10). The histogenesis and underlying
pathogenetic mechanisms of SPN remain incompletely understood.
Kosmahl et al (11)
proposed that SPN originates from primordial cells of the
reproductive ridge and ovarian anlagen, which may integrate with
the developing pancreatic primordium during embryogenesis. This
hypothesis aligns with the clinical observation that SPN
predominantly affects young females (2). Current evidence suggests that SPN
development is associated with mutations in exon 3 of the β-catenin
gene and reduced E-cadherin-mediated signal transduction within the
Wnt signaling pathway (12,13).
Furthermore, emerging studies indicate potential involvement of
other molecular pathways, including the Notch and Hedgehog
signaling pathways, in the pathogenesis of SPN (14,15).
A study conducted by Law et al (16) enrolled a total of 2,744 patients
diagnosed with SPN. The findings indicated that the mean age of the
patient cohort was 28.5 years, with females comprising as high as
87.8% of the study population. Although SPN can arise in any region
of the pancreas, it most frequently occurs in the pancreatic body
and tail. Yu et al (17)
reported a mean tumor diameter of 7.87 cm, with 54.8% of cases
located in the body and tail of the pancreas, while Song et
al (18) observed a mean tumor
size of 6.4 cm, with 60.4% of tumors arising in the same anatomical
region.
SPN lacks specific clinical manifestations.
Approximately one-third of patients are asymptomatic and may be
identified incidentally during routine physical examinations
(6). The most frequently observed
clinical presentations include abdominal pain and discomfort, while
other common symptoms comprise nausea, vomiting, back pain and the
detection of palpable abdominal masses. Laboratory findings in
patients with SPN typically fall within normal ranges and the
majority of tumor markers yield negative results (19,20).
Although tumor markers demonstrate limited diagnostic specificity
for SPN, they serve a valuable role in differential diagnosis,
particularly in distinguishing SPN from malignancies such as
pancreatic cancer.
Given the lack of specificity in laboratory tests
for SPN, imaging modalities such as CT, MRI and ultrasound play a
crucial role in its diagnosis (11). With continuous advancements in
imaging technologies, the accuracy of the preoperative diagnosis of
SPN has significantly improved. CT remains the most widely utilized
imaging method for the preoperative evaluation of SPN, typically
revealing a well-defined, heterogeneous cystic-solid mass that may
be associated with hemorrhage or calcification (6,21). A
limitation of the present study is the absence of MRI evaluation.
Compared to CT, MRI offers superior soft-tissue contrast, enabling
more precise visualization of the tumor's relationship with
adjacent bile ducts and pancreatic ducts, which is of considerable
importance for surgical planning (22). Pancreatic duct dilation is uncommon
in SPN (17). Endoscopic
ultrasound-guided fine-needle aspiration (EUS-FNA) serves as a
valuable technique for obtaining histological confirmation prior to
surgery. Studies have demonstrated that EUS-FNA achieves a
preoperative diagnostic accuracy exceeding 80% for SPN (23). Nevertheless, due to its invasive
nature and potential complications, including bleeding, infection
and tumor seeding along the needle tract, its clinical application
remains limited. A nationwide study based on Japanese SPN cases
indicated that fluorodeoxyglucose positron emission tomography may
provide additional diagnostic value for small-diameter tumors
(<2 cm) (24). SPN can be
differentiated from pancreatic cancer, pancreatic cystadenoma,
intraductal papillary mucinous neoplasm (IPMN), and pancreatic
pseudocyst based on patient age at onset and CT imaging findings.
Pancreatic cancer typically presents as a hypodense lesion during
the contrast-enhanced phase of CT imaging, with poorly defined
margins relative to adjacent normal pancreatic parenchyma, and is
commonly associated with hepatic and lymph node metastases.
Mucinous cystadenoma is characterized by multilocular cystic
structures with low attenuation, frequently accompanied by
eggshell-like peripheral calcifications, which constitute a
hallmark radiological feature. Serous cystadenoma typically appears
as unilocular or multilocular cystic lesions with fluid-equivalent
density on CT scans, featuring a pathognomonic central stellate
scar and calcification. IPMN of the pancreas manifests as a
low-attenuation mass, often associated with pancreatic ductal
dilatation. Pancreatic pseudocysts are usually depicted as
well-circumscribed, homogeneously attenuated, round or ovoid
lesions on CT imaging, with enhancement limited to the cyst wall
during the contrast-enhanced phase (25).
Surgical resection remains the primary and preferred
treatment modality for SPN (26).
The selection of the surgical approach should be based on tumor
size, anatomical location and intraoperative rapid pathological
evaluation of frozen sections. Several studies have indicated that
surgical resection should still be considered even in cases with
preoperative evidence of local organ invasion or distant metastasis
(27-29).
Emerging evidence suggests that gemcitabine-based chemotherapy
regimens may offer a clinical benefit in patients with metastatic
or unresectable SPN (30,31). The patient of the present study
underwent radiotherapy or chemotherapy. Considering the limited
evidence regarding the application of radiotherapy or chemotherapy
in the treatment of SPN, which is primarily derived from case
reports, the effectiveness of these therapeutic modalities in
managing SPN remains to be fully elucidated and corroborated. The
definitive diagnosis of SPN primarily depends on histopathological
and immunohistochemical analyses. The characteristic pathological
features of SPN include the presence of solid, pseudopapillary and
cystic components, with tumor cells arranged around fibrovascular
cores in a pseudopapillary configuration. Currently, no specific
immunophenotypic profile is unique to SPN; however, several markers
demonstrate consistent expression patterns. In most studies, high
expression levels of β-catenin, progesterone receptor (PR),
synaptophysin (Syn), α-1-antichymotrypsin (AACT), vimentin (Vim)
and CD56 have been observed, whereas chromogranin A (CgA) typically
shows low or negative expression (32,33).
The overexpression of β-catenin aligns with the molecular mechanism
involving mutations in the β-catenin gene and its role in SPN
pathogenesis via the WNT signaling pathway (34). As an immunophenotype associated
with sex hormones, the high expression of PR suggests a potential
involvement of hormonal factors in the development of SPN (35,36).
Wang et al (37) reported
that loss of PR expression was significantly associated with poorer
recurrence-free survival and disease-specific survival.
Immunohistochemical profiling enables differentiation of SPN from
pancreatic ductal adenocarcinoma and neuroendocrine tumors. Markers
such as β-catenin, lymphoid enhancer-binding factor 1 (LEF-1) and
transcription factor E3 (TFE3) have demonstrated utility in
distinguishing SPN from these mimics (34,38).
Several studies recommend the use of combined immunophenotypic
panels to enhance diagnostic accuracy for SPN (38,39).
Kim et al (39)
demonstrated that a panel consisting of β-catenin, LEF-1 and TFE3
achieved a sensitivity of 100% and a specificity of 91.9% in
differentiating SPN from pancreatic cancer and neuroendocrine
tumors. Ki-67 serves as a proliferation marker reflecting tumor
cell growth activity. Given that SPN is a low-grade malignant tumor
with an indolent biological behavior, Ki-67 expression is generally
low. Yang et al (40)
indicated that a Ki-67 labeling index ≥4% was associated with
adverse postoperative outcomes in patients with SPN.
Given the low-grade malignant potential of SPN and
its generally favorable prognosis, definitive conclusions regarding
risk factors for benign vs. malignant behavior, recurrence or
metastasis remain elusive. Several studies have investigated the
association between tumor size and malignant potential. Kang et
al (41) reported that a tumor
diameter >5 cm may indicate malignant transformation in SPN. By
contrast, De Robertis et al (42) observed no significant association
between tumor size and malignancy grade. Additionally, the presence
of an incomplete tumor capsule and calcification has been linked to
poorer clinical outcomes in patients with SPN (43,44).
Patients diagnosed with SPN generally demonstrate a
favorable prognosis following surgical resection. A study by Liu
et al (45) followed 243
patients with SPN for an average of almost four years and their
five-year survival rate was even higher at 98.4%. Even if SPN
recurred or spread after the first surgery, numerous individuals
still survived for a long time if they had another operation.
In summary, SPN is a low-grade malignant tumor of
the pancreas that predominantly affects young women between the
ages of 20 and 30 years. It is most frequently located in the
pancreatic body or tail. Due to its indolent clinical course, SPN
often presents without specific symptoms and is commonly detected
incidentally during routine physical examinations. When symptoms
occur, they may include nonspecific abdominal discomfort such as
pain or distension. Laboratory findings are typically unremarkable,
with minimal abnormalities observed in routine blood tests.
Preoperative diagnosis primarily depends on imaging modalities,
particularly contrast-enhanced CT, while definitive diagnosis
relies on histopathological evaluation combined with
immunohistochemical profiling. Accumulating evidence suggests that
an incomplete tumor capsule serves as an independent prognostic
indicator for more aggressive biological behavior in SPN. Complete
surgical resection remains the cornerstone of treatment, offering
favorable long-term outcomes in the majority of cases. Notably,
even in instances of preoperative metastasis or postoperative
recurrence, aggressive surgical intervention can still yield
prolonged survival. Postoperatively, regular follow-up is strongly
recommended for all patients with SPN, particularly those with
high-risk features, to enable early detection and timely management
of potential recurrences or metastases.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DJ conceived and designed the study. LZ analyzed and
summarized the data and wrote the manuscript. KL, LZ and DJ
collected the laboratory examination data and images of the case.
LZ critically revised the manuscript. KL and LZ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
principles expressed in the Declaration of Helsinki.
Patient consent for publication
The patient involved in the present study was
subjected to standard clinical practice and provided written
informed consent for the publication of medical data and
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Papavramidis T and Papavramidis S: Solid
pseudopapillary tumors of the pancreas: Review of 718 patients
reported in English literature. J Am Coll Surg. 200:965–972.
2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wu J, Mao Y, Jiang Y, Song Y, Yu P, Sun S
and Li S: Sex differences in solid pseudopapillary neoplasm of the
pancreas: A population-based study. Cancer Med. 9:6030–6041.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Elta GH, Enestvedt BK, Sauer BG and Lennon
AM: ACG clinical guideline: Diagnosis and management of pancreatic
cysts. Am J Gastroenterol. 113:464–479. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
La Rosa S and Bongiovanni M: Pancreatic
solid pseudopapillary neoplasm: Key pathologic and genetic
features. Arch Pathol Lab Med. 144:829–837. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Boor PJ and Swanson MR: Papillary-cystic
neoplasm of the pancreas. Am J Surg Pathol. 3:69–76.
1979.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Schlosnagle DC and Campbell WG Jr: The
papillary and solid neoplasm of the pancreas:a report of two cases
with electron microscopy,one containing neurosecretory granules.
Cancer. 47:2603–2610. 1981.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Klöppel G, Solcia E, Sobin LH, Longnecker
DS and Capella C (eds): Histological typing of tumours of the
exocrine pancreas. 2nd edition. Springer Berlin, Heidelberg,
1996.
|
|
8
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA:
WHO classification of tumours editorial board. The 2019 WHO
classification of tumours of the digestive system. Histopathology.
76:182–188. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Frantz VK: Tumors of the Pancreas. Armed
Forces Institute of Pathology, Washington, DC, pp32-33, 1959.
|
|
10
|
Jena SS, Ray S, Das SAP, Mehta NN, Yadav A
and Nundy S: Rare pseudopapillary neoplasm of the pancreas:
A10-year experience. Surg Res Pract. 2021(7377991)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kosmahl M, Seada LS, Jänig U, Harms D and
Klöppel G: Solid-pseudopapillary tumor of the pancreas: Its origin
revisited. Virchows Arch. 436:473–480. 2000.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ghio M and Vijay A: Molecular alterations
in solid pseudopapillary neoplasm of the pancreas: The achilles
heel in conquering pancreati tumorigenesis. Pancreas. 50:1343–1347.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cavard C, Audebourg A, Letourneur F,
Audard V, Beuvon F, Cagnard N, Radenen B, Varlet P, Vacher-Lavenu
MC, Perret C and Terris B: Gene expression profiling provides
insights into the pathways involved in solid pseudopapillary
neoplasm of the pancreas. J Pathol. 218:201–209. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wils LJ and Bijlsma MF: Epigenetic
regulation of the Hedgehog and Wnt pathways in cancer. Crit Rev
Oncol Hematol. 121:23–44. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Park M, Kim M, Hwang D, Park M, Kim WK,
Kim SK, Shin J, Park ES, Kang CM, Paik YK and Kim H:
Characterization of gene expression and activated signaling
pathways in solid-pseudopapillary neoplasm of pancreas. Mod Pathol.
27:580–593. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Law JK, Ahmed A, Singh VK, Akshintala VS,
Olson MT, Raman SP, Ali SZ, Fishman EK, Kamel I, Canto MI, et al: A
systematic review of solid-pseudopapillary neoplasms: Are these
rare lesions? Pancreas. 43:331–337. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yu PF, Hu ZH, Wang XB, Guo JM, Cheng XD,
Zhang YL and Xu Q: Solid pseudopapillary tumor of the pancreas: A
review of 553 cases in Chinese literature. World J Gastroenterol.
16:1209–1214. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Song H, Dong M, Zhou J, Sheng W, Zhong B
and Gao W: Solid pseudopapillary neoplasm of the pancreas:
Clinicopathologic feature, risk factors of malignancy, and survival
analysis of 53 cases from a single center. Biomed Res Int.
2017(5465261)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yao J and Song H: A review of
clinicopathological characteristics and treatment of solid
pseudopapillary tumor of the pancreas with 2450 cases in Chinese
population. Biomed Res Int. 2020(2829647)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yang F, Wu W, Wang X, Zhang Q, Bao Y, Zhou
Z, Jin C, Ji Y, Windsor JA, Lou W and Fu D: Grading solid
pseudopapillary tumors of the pancreas:the fudan prognostic index.
Ann Surg Oncol. 28:550–559. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yu CC, Tseng JH, Yeh CN, Hwang TL and Jan
YY: Clinicopathological study of solid and pseudopapillary tumor of
pancreas:emphasis on magnetic resonance imaging findings. World J
Gastroenterol. 13:1811–1815. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
El Nakeeb A, Abdel Wahab M, Elkashef WF,
Azer M and Kandil T: Solid pseudopapillary tumour of the pancreas:
Incidence,prognosis and outcome of surgery (single center
experience). Int J Surg. 11:447–457. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kumar NAN, Bhandare MS, Chaudhari V, Sasi
SP and Shrikhande SV: Analysis of 50 cases of solid pseudopapillary
tumor of pancreas: Aggressive surgical resection provides excellent
outcomes. Eur J Surg Oncol. 45:187–191. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Law JK, Stoita A, Wever W, Gleeson FC,
Dries AM, Blackford A, Kiswani V, Shin EJ, Khashab MA, Canto MI, et
al: Endoscopic ultrasound-guided fine needle aspiration improves
the pre-operative diagnostic yield of solid pseudopapillary
neoplasm of the pancreas: An international multicenter case series
(with video). Surg Endosc. 28:2592–2598. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Scott J, Martin I, Redhead D, Hammond P
and Garden OJ: Mucinous cystic neoplasms of the pancreas: Imaging
features and diagnostic difficulties. Clin Radiol. 55:187–192.
2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kurihara K, Hanada K, Serikawa M, Ishii Y,
Tsuboi T, Kawamura R, Sekitou T, Nakamura S, Mori T, Hirano T, et
al: Investigation of fluorodeoxyglucose positron emission
tomography for the diagnosis of solid pseudopapillary neoplasm of
the pancreas: A study associated with a national survey of solid
pseudopapillary neoplasms. Pancreas. 48:1312–1320. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
European Study Group on Cystic Tumours of
the Pancreas. European evidence-based guidelines on pancreatic
cystic neoplasms. Gut. 67:789–804. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kang CM, Choi SH, Kim SC, Lee WJ, Choi DW
and Kim SW: Korean Pancreatic Surgery Club. Predicting recurrence
of pancreatic solid pseudopapillary tumors after surgical
resection:a multicenter analysis in Korea. Ann Surg. 260:348–355.
2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Martin RC, Klimstar DS, Brennan MF and
Conlon KC: Solid-pseudopapillary tumor of the pancreas:a surgical
enigma? Ann Surg Oncol. 9:35–40. 2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Prasad TV, Madhusudhan KS, Srivastava DN,
Dash NR and Gupta AK: Transarterial chemoembolization for liver
metastases from solid pseudopapillary epithelial neoplasm of
pancreas: A case report. World J Radiol. 7:61–65. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Maffuz A, Bustamante Fde T, Silva JA and
Torres-Vargas S: Preoperative gemcitabine for unresectable, solid
pseudopapillary tumour of the pancreas. Lancet Oncol. 6:185–186.
2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Serra S and Chetty R: Revision 2: An
immunohistochemical approach and evaluation of solid
pseudopapillary tumour of the pancreas. J Clin Pathol.
61:1153–1159. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lubezky N, Papoulas M, Lessing Y, Gitstein
G, Brazowski E, Nachmany I, Lahat G, Goykhman Y, Ben-Yehuda A,
Nakache R and Klausner JM: Solid pseudopapillary neoplasm of the
pancreas: Management and long-term outcome. Eur J Surg Oncol.
43:1056–1060. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Din NU, Rahim S, Abdul-Ghafa J, Ahmed A
and Ahmad Z: Clinicopathological and immunohistochemical study of
29 cases of solid-pseudopapillary neoplasms of the pancreas in
patients under 20 years of age along with detailed review of
literature. Diagn Pathol. 15(139)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Naar L, Spanomichou DA, Mastoraki A,
Smyrniotis V and Arkadopoulos N: Solid pseudopapillary neoplasms of
the pancreas:A surgical and genetic enigma. World J Surg.
41:1871–1881. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tang LH, Aydin H, Brennan MF and Klimstra
DS: Clinically aggressive solid pseudopapillary tumors of the
pancreas:a report of two cases with components of undifferentiated
carcinoma and a comparative clinicopathologic analysis of 34
conventional cases. Am J Surg Pathol. 29:512–519. 2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang F, Meng Z, Li S, Zhang Y and Wu H:
Prognostic value of progesterone receptor in solid pseudopapillary
neoplasm of the pancreas:evaluation of a pooled case series. BMC
Gastroenterol. 18(187)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Harrison G, Hemmerich A, Guy C, Perkinson
K, Fleming D, McCall S, Cardona D and Zhang X: Overexpression of
SOX11 and TFE3 in solid-pseudopapillary neoplasms of the pancreas.
Am J Clin Pathol. 149:67–75. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kim EK, Jang M, Park M and Kim H: LEF1,
TFE3, and AR are putative diagnostic markers of solid
pseudopapillary neoplasms. Oncotarget. 8:93404–93413.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yang F, Yu X, Bao Y, Du Z, Jin C and Fu D:
Prognostic value of Ki-67 in solid pseudopapillary tumor ofthe
pancreas:Huashan experience and systematic review of the literatur.
Surgery. 159:1023–1031. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kang CM, Kim KS, Choi JS, Kim H, Lee WJ
and Kim BR: Solid pseudopapillary tumor of the pancreas suggesting
malignant potential. Pancreas. 32:276–280. 2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
De Robertis R, Marchegiani G, Catania M,
Ambrosetti MC, Capelli P, Salvia R and D'Onofrio M: Solid
pseudopapillary neoplasms of the pancreas:Clinicopathologic and
radiologic features according to size. AJR Am J Roentgenol.
213:1073–1080. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chung YE, Kim MJ, Choi JY, Lim JS, Hong
HS, Kim YC, Cho HJ, Kim KA and Choi SY: Differentiation of benign
and malignant solid pseudopapillary neoplasms of the pancreas. J
Comput Assist Tomogr. 33:689–694. 2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kim HH, Yun SK, Kim JC, Park EK, Seoung
JS, Hur YH, Koh YS, Cho CK, Shin SS, Kweon SS, et al: Clinical
features and surgical outcome of solid pseudopapillary tumor of the
pancreas: 30 consecutive clinical cases. Hepatogastroenterology.
58:1002–1008. 2011.PubMed/NCBI
|
|
45
|
Liu M, Liu J, Hu Q, Liu W, Zhang Z, Sun Q,
Qin Y, Yu X, Ji S and Xu X: Management of solid pseudopapillary
neoplasms of pancreas:A single center experience of 243 consecutive
patients. Pancreatology. 19:681–685. 2019.PubMed/NCBI View Article : Google Scholar
|