Introduction
Inflammatory myofibroblastic tumours (IMT) are
distinctive intermediate-grade soft-tissue neoplasms belonging to
the group of inflammatory spindle cell lesions (1). The worldwide incidence of these
tumours is low at 0.04-0.7% (2)
and clinical data has shown that even though the local recurrence
rate after surgical excision is 25%, they fortunately rarely
metastasize (3). IMTs can occur in
any location, predominantly in the mesentery, retroperitoneum, and
pelvis, frequently remaining entirely asymptomatic until they
attain a size that leads to complications (4). IMTs involving the spleen, or the
pancreas can exert pressure on the surrounding anatomic forms;
splenic vein compression is a dire manifestation since it can lead
to a localized type of portal hypertension known as ‘left-sided.’
In this condition, collateral venous blood flow develops, resulting
in localized dilation of the submucosal venous reticulum of the
gastric fundus, which connects the short and posterior gastric
veins to the coronary veins, potentially leading to the development
of gastric varices, splenomegaly, and hypersplenism (5). These varices may lead to
life-threatening upper gastrointestinal bleeding presenting with
hematemesis and/or melena. Although IMTs have been described in
various sites, including the spleen (6-8)
and the pancreas (9,10), a synchronous occurrence in both
organs is exceptionally rare. Herein, we report the first case, to
our knowledge, of a patient with upper GI bleeding from isolated
gastric varices (IGV) and hypersplenism due to left-sided portal
hypertension caused by an IMT involving both the spleen and the
pancreas.
Case report
A 33-year-old male of urban origin, presented to the
emergency department following multiple episodes of melena. The
patient described abdominal cramping and urgency of defecation,
followed by the passage of black tarry stool. He denied any
associated concerning symptoms in the months prior to the current
presentation, including fatigue, weight loss, abdominal or joint
pain.
This patient reports a previous admission in a
different hospital (within the past six months) for
gastrointestinal bleeding and associated haemoglobin drop requiring
blood transfusions. During this prior hospitalization he underwent
multiple upper endoscopies that revealed isolated gastric varices
(IGV) type 1 located in the fundus that were actively bleeding.
Variceal bleeding was treated with endoscopic obturation using
tissue adhesive. The patient's melena ceased after the endoscopic
intervention and his haemoglobin remained within the normal range.
He was discharged with a referral to a specialized gastroenterology
centre if symptoms recurred. During the same admission, an
abdominal ultrasound examination revealed a 16.5 cm long
splenomegaly and a large intrasplenic cyst (6 cm). Based on
previous abdominal imaging, this was a known asymptomatic splenic
pseudocyst secondary to a car accident injury five years ago.
Notably, the patient was a non-smoker and reported alcohol
consumption of less than 15 units per week. He was not undergoing
any pharmacological treatment and had no significant medical
history, including systemic diseases or a relevant family
history.
On initial presentation at our hospital, the patient
was hemodynamically stable. The physical examination found
splenomegaly, with a spleen palpable 3 cm below the left costal
margin. His abdomen was soft and non-tender, but the digital rectal
exam confirmed black stool. Laboratory data on admission revealed
pancytopenia, with white blood cell count 1.8 k/mcl, haemoglobin
7.5 g/dl, mean corpuscular volume (MCV) 69 fl, and platelets 105
k/mcl. Prothrombin time and biochemistry profile, including liver
function tests, as well as bilirubin and albumin levels showed no
abnormalities. He was admitted to our clinic for monitoring and
further investigation.
After the patient was stabilized with intravenous
fluid resuscitation and blood transfusion, an immediate endoscopy
was performed on this patient, who was rushed to our hospital for
upper gastrointestinal bleeding with normal liver function and
splenomegaly. Endoscopy revealed the presence of varices localized
in the gastric fundus. At that time, no active bleeding was found
and thus no intervention was required, with bleeding resolving on
its own. Transabdominal ultrasonography (US) with Doppler excluded
the presence of systemic portal hypertension and showed no
indications of liver cirrhosis. Abdominal computed tomography (CT)
confirmed the known splenomegaly (cephalocaudal diameter 16.5 cm)
and the 6 cm intrasplenic cyst, which remained unchanged in
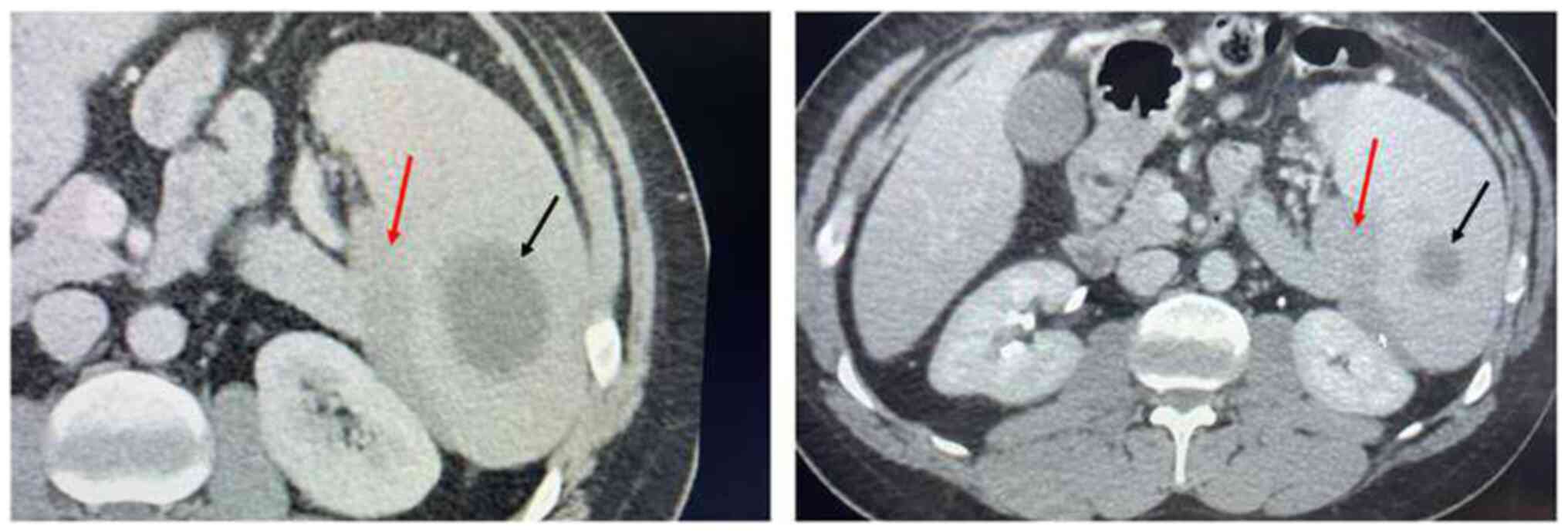
comparison to previous imaging. Interestingly, a subcapsular
hypodense splenic mass of 7x2 cm was described, extending from the
portal of the spleen to its inferior pole, with a peripheral
localization, showing no contrast enhancement (Fig. 1). Of note, the mass compressed
externally the splenic vein, but neither portal, nor splenic vein
thrombosis were found. The above-mentioned radiological description
did not make it possible to evoke a conclusive diagnosis about the
nature of the mass. Therefore, a Positron Emission Tomography (PET)
scan was performed, in order to better differentiate this splenic
mass as benign or malignant. According to the PET/CT the lesion of
interest in the lower medial margin of the spleen displayed
moderate 18F-FDG uptake (SUVmax=4.3). These findings were
inconclusive for diagnosis.
Concurrently, our management focused on identifying
other causative conditions of cytopenias. Characteristically, low
ferritin levels were identified due to recent blood loss, whereas
no B12 or folic acid deficiency was detected. Iron was repleted
with intravenous ferric carboxymaltose. The peripheral blood smear
revealed no morphological abnormalities. Bone marrow biopsy and
myelogram were performed to investigate the emerging pancytopenia,
revealing a cellular bone marrow without evidence of
infiltration/replacement or failure. In order to eliminate
autoimmune destruction, screening tests for autoantibodies,
including antinuclear Ab, anti-Ro/SS-A Ab, anti-La/SS-B Ab,
anti-cardiolipin Ab, myeloperoxidase-anti-neutrophil-cytoplasmic Ab
(MPO-ANCA) and proteiase-3-ANCA, were carried out, which gave
negative results. We also excluded preceding infections such as
those of brucellosis, tuberculosis, Epstein-Barr virus,
cytomegalovirus, parvovirus, human immunodeficiency virus,
varicella zoster virus, hepatitis B and C viruses. Next, chronic
myeloproliferative disorders were excluded, with negative BCR-ABL,
JAK2 V617F, CARL and MPL W515 mutations. Finally, thrombophilia
screening test results, including lupus anticoagulant, aPl, aCL and
anti-β2-GPI IgM/IgG isotypes, Protein C and S, along with Factor V
Leiden mutation and prothrombin G20210A mutation, were all
negative. Since all the aforementioned conditions were ruled out,
the diagnosis of pancytopenia attributed to hypersplenism due to
left-sided portal hypertension, possibly as a result of the mass
compressing and causing partial occlusion of the splenic vein, was
taken into consideration.
Our patient was stable until his condition
deteriorated with sudden onset haematochezia and subsequent
development of hypovolemic shock. He received three units of packed
red blood cells (pRBC), tranexamic acid, and was started on
intravenous infusion of omeprazole and crystalloids. An emergency
upper GI endoscopy was performed, but due to the large amount of
bleeding and poor endoscopic field of vision, haemostasis was not
achieved. The patient was considered too unstable to proceed with
additional imaging evaluation. Consequently, he underwent an urgent
laparotomy, where a 6cm mass was found on the inferior pole of the
spleen, infiltrating and exceeding the splenic capsule.
Unexpectedly, a similar 7.5 cm scleroelastic solid mass was
discovered, originating from the pancreatic tail. Given the
involvement of both the spleen and pancreas, a splenectomy and
distal pancreatectomy were performed. The splenic artery and vein
were carefully isolated, ligated, and divided to minimize blood
loss. The spleen was then mobilized by dissecting the short gastric
vessels and separating adhesions from the surrounding structures.
The organ, along with the infiltrating mass, was excised en bloc.
For the pancreatic resection, the tail of the pancreas was
mobilized by incising the peritoneal attachments along the splenic
hilum. The lesion was sharply dissected, and a partial distal
pancreatectomy was performed, ensuring a negative resection margin.
A closed-suction drain was placed near the pancreatic stump to
monitor postoperative fluid collection. Both resected specimens
were immediately sent for histopathological examination. The
abdominal cavity was irrigated with warm saline, and the incision
was closed in layers. The procedure was completed without
intraoperative complications.
The diagnosis was consistent with inflammatory
myofibroblastic tumour, which is a rare mesenchymal tumour of
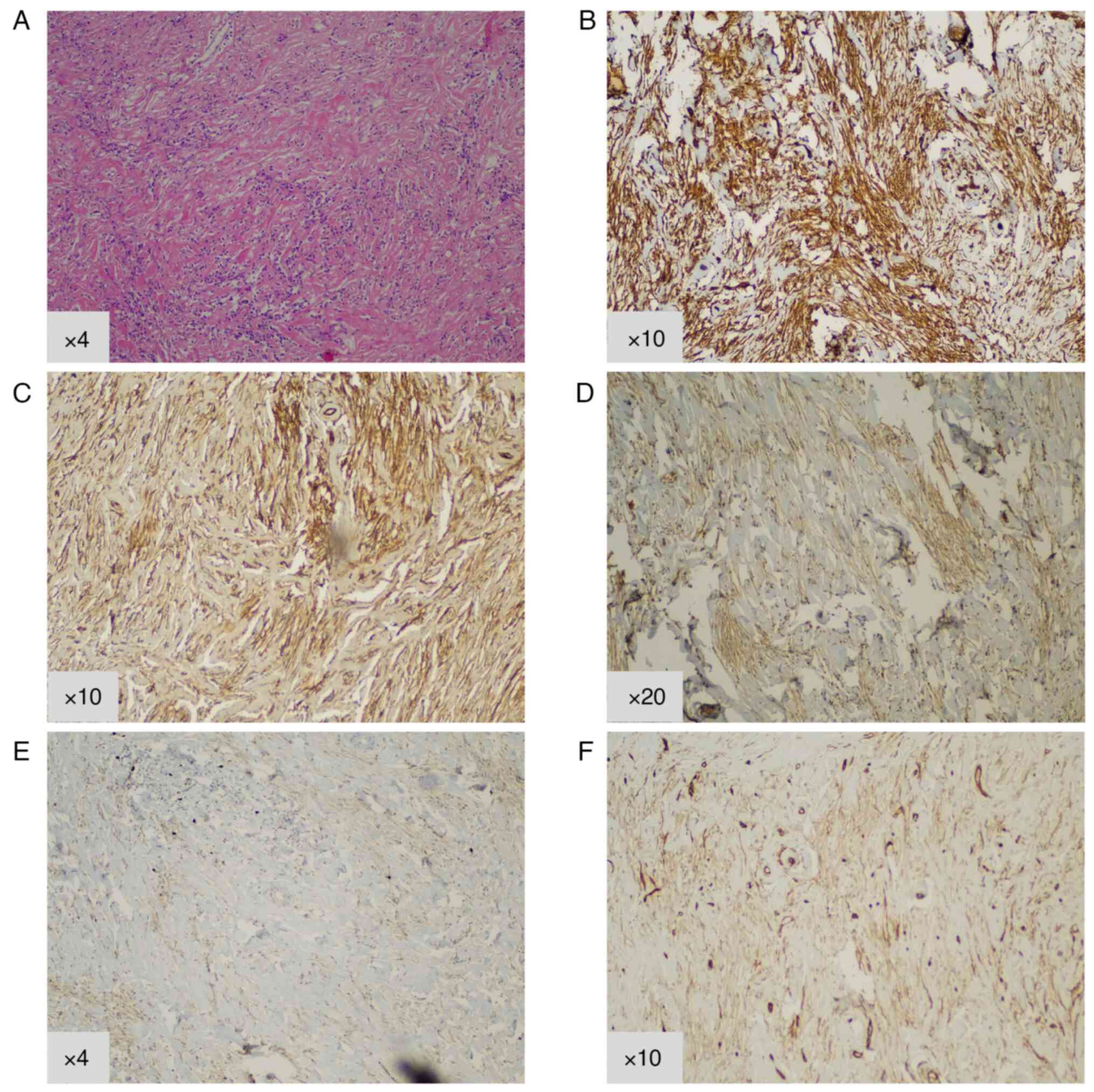
intermediate malignant potential. The microscopic examination
revealed the proliferation of spindle-shaped myofibroblasts,
showing swirling and fusiform growth patterns and inflammatory
lymphocytic infiltration (Fig. 2).
The immunohistochemical analysis was performed by manual
immunostaining procedures as per Ventana's protocol. According to
the histologic description, neither overt cytologic atypia nor
mitotic activity were observed, with a low Ki67 marker for
proliferation of <1%. Immunohistological staining was positive
for vimentin (1:300 dilution with PBS; Dako, Carpinteria, CA, USA)
and smooth muscle actin (SMA) (1:150 dilution with PBS; Cell Marque
Corp, Rocklin, CA, USA), displayed weaker expression of Desmin
(1:100 dilution with PBS; Zeta Corp., Sierra Madre, CA, USA) and
CD34 (1/100 dilution with PBS; Deer Park, IL, USA), whereas stains
were negative for S100, AE1/3, CK8/18, CD99, ALK and C-KIT.
Complete resection with R0 margins was achieved.
Over the following days, the patient did not
experience episodes of melena, his haemoglobin remained stable, and
stool colour returned to normal. Notably, a gastroscopy was
performed two months post-operatively that confirmed the resolution
of the gastric varices, and follow-up blood tests showed that
pancytopenia had also resolved. The patient was assessed by medical
oncology; close follow-up was recommended, and no adjuvant therapy
was suggested since complete surgical resection was achieved.
Written informed consent for publication was obtained from the
patient, ensuring ethical compliance and patient acknowledgment of
case reporting.
Discussion
IMTs are tumours of myofibroblast origin which,
despite the presence of an inflammatory immunohistochemical
background and an apparently benign morphological pattern, have
shown malignant potential (11).
IMTs are exceptionally rare, affecting only 150-200 people annually
in the United States (12). They
have been described in patients of all ages, although occurrence is
more frequent in children and young adults (13). Concerning the site and clinical
presentation, IMTs can be found in a variety of body sites and
organs, including the lung, mediastinum, head and neck, liver,
retroperitoneum, and abdominopelvic region, causing a wide array of
manifestations (1,4,14).
They are known for their resistance to standard chemotherapy and
radiation. As a result, surgical removal, when possible, remains
the primary treatment approach.
Our patient presented to the emergency department
with ongoing melena; there have been other cases where patients
harbouring IMTs presented with upper gastrointestinal bleeding, but
their tumours were located in different anatomical locations, such
as the oesophagus (15), the
stomach (16), the duodenum
(17), or even the liver via the
formation of oesophageal varices (18). There have also been IMTs in adults
originating in the pancreas, the majority of them in the head of
the organ, causing obstructive jaundice (19,20),
or even stenosis of the descending duodenum (21). Occurrence in the pancreatic tail,
as observed in our case, is rather unusual, as shown in a recent
review reporting a total of 26 adult cases of pancreatic IMT in the
literature (22). In this series,
none of the patients presented had a synchronous splenic tumour,
making our case the first one. Usually, splenic IMTs in adults are
incidental findings (23-25),
although they may present with pain in the left upper abdomen
(6,7,26,27),
left-sided abdominal distension (28), weight loss, malaise, and fever
(7,27). Another finding is splenomegaly, and
some patients can present with anaemia and signs of hypersplenism
(29), as witnessed in our
patient.
Particularly in our case, the tumour originating
from the pancreas and the spleen apparently obstructed the splenic
vein, leading to left gastric vein dilation and localized
splenoportal hypertension, also known as left-sided portal
hypertension. This occlusion causes the venous drainage of the
spleen to occur via low-pressure collaterals that include the short
and posterior gastric veins to the coronary veins, and the
gastroepiploic veins to the superior mesenteric vein (5). This leads to the formation of
isolated gastric varices (IGV) located in the fundus of the
stomach, commonly termed as type 1 (IGV1) (30). IGVs type 1 are usually attributed
to splenic or pancreatic disease that blocks the splenic venous
flow either by thrombus formation or by neighbouring mass effect,
with splenomegaly and gastrointestinal bleeding being two common
clinical features (31). In our
case, the external compression of the splenic vein by the
inflammatory myofibroblastic tutor demonstrates how even a partial
splenic vein occlusion can alter venous drainage patterns, despite
normal Doppler ultrasound findings. This obstruction can contribute
to variceal formation in the short gastric and left gastroepiploic
veins, with anatomical variations in the portal system and
collateral vessel branching potentially influencing this diversity.
The precise cause of our patient's splenomegaly remains unclear,
but it may stem from increased congestion in the splenic veins and
heightened arterial inflow through the splenic arteries (31). The precise cause of splenomegaly
remains unclear, but it might be associated with heightened
congestion in the splenic veins and increased blood flow and
pressure through the splenic arteries (31). Although splenectomy is the
preferred approach for managing cases with variceal bleeding
complications, there is a lack of agreement in the literature
regarding the treatment of asymptomatic patients.
Due to the tumour-related complications, complete
surgical resection is also the recommended treatment for localized
IMTs, since it ensures a favourable prognosis for most patients
(3). Diagnostic splenic biopsies
are not routinely recommended due to potentially high risk of
haemorrhage and poor specificity (32). Therefore, the final diagnosis of
this intricate entity characterized by myofibroblastic
differentiation is usually based on pathologic findings obtained
after surgery, due to its nonspecific symptoms and imaging
findings. Characteristically, radiological tests, including
ultrasound and CT imaging, are not considered pathognomonic for
IMTs, but can help narrow down the differential diagnosis (33). Even PET/CT findings cannot provide
a definitive diagnosis, besides indicating the biological behaviour
of the tumour cells or detecting distant metastases. In fact, IMTs
have shown high variability of FDG uptake in PET-CTs, with the
SUVmax ranging from 3.3 to 20.8, depending on tumour composition
and the level of inflammatory activity (33). In our patient, low Ki67 expression
of <1% and lack of mitotic activity could correlate with the
failure of the PET-CT to detect the pancreatic mass and the low
FDG-uptake of the splenic tumour, possibly suggesting a benign and
non-inflammatory tendency.
The immunohistochemical profile of the present case
revealed the presence of a myofibroblastic cell type, scattered
among inflammatory cells, mostly lymphocytes. Our patient's tumour
exhibited positive expression of SMA, desmin and cytokeratin after
staining, as do the majority of IMT cases (21,34).
Concerning the molecular changes in IMT, anaplastic lymphoma kinase
(ALK) gene rearrangements leading to uncontrolled cell
proliferation via constitutive tyrosine kinase activation are
extremely common (4,34,35).
Immunohistochemical ALK positivity reliably correlates with ALK
rearrangement, being present in 50 to 60% of IMT cases (36). The ALK gene fuses with
various partner genes, such as TPM3, TPM4, CLTC, and others,
through chromosomal translocations, resulting in the formation of
fusion proteins (1). These ALK
fusion proteins possess constitutive kinase activity, driving
tumorigenesis by activating downstream signalling pathways that
promote cell proliferation and survival (1). However, in patients over the age of
25, ALK positivity is less frequent (26). This was the case with our
33-year-old patient, in whom the tumour was immunohistochemically
negative for ALK expression. The absence of this oncogenic driver
alteration could be a possible indication of the low potential for
malignant transformation (4).
Still, it is difficult to extract safe conclusions since a clear
connection between the histological characteristics of IMT and its
clinical behaviour has not been established (3). Notably, molecular cytogenetic
analysis or next generation sequencing was not performed in our
patient's tumour sample to definitively exclude ALK gene
rearrangements in the inflammatory myofibroblastic tumour cells
(1). Identification of distinct
molecular changes is crucial in cases of recurrent, metastatic, or
unresectable IMT, as revisiting molecular testing upon disease
progression can help determine eligibility for targeted therapies,
which may influence treatment decisions and prognosis for patients
with ALK mutated IMTs. Specifically, tyrosine kinase inhibitors
that disrupt mutant signalling pathways, such as crizotinib, have
demonstrated efficacy in locally advanced or metastatic
ALK-positive IMTs, exhibiting high overall response rates in case
reports and Phase 1b/2 studies (37-39).
Based on data from two multicentre, single-arm, open-label
studies-including 14 paediatric cases from clinical trial
NCT00939770(40) and seven adult
cases from trial NCT01121588(38)-crizotinib was approved in July 2022
for the treatment of adult and paediatric patients with
unresectable, recurrent, or refractory ALK-positive IMT. Overall,
long-term follow-up is essential due to the uncertain and
unpredictable course of some cases harbouring this rare soft-tissue
tumour.
In conclusion, mesenchymal neoplasms represent a
diagnostic challenge with diverse clinical presentations. The
current case was a rare incidence of IGV1 bleeding and
hypersplenism, induced by IMT-associated splenic vein stenosis. Our
patient responded well to surgical resection; however, his rapid
deterioration that required urgent surgery highlights the
importance of early recognition of this rare tumour. In this
context, thorough recording of symptoms and presentation of case
reports will eventually contribute towards the refinement of
diagnostic criteria and, consequently, of therapeutic options for
IMT.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
EZ, AP and MK drafted the manuscript and made
substantial contributions to the conception of the case report, as
well as to the collection of patient data. IT, AM, MP, AA and CGZ
significantly contributed to data analysis and interpretation,
offering domain-specific expertise. GK was responsible for the
immunohistochemical analysis and pathological evaluation of tissue
samples. EM, MAD and FZ supervised the progress of the study,
contributed to its design, and critically reviewed the manuscript.
FZ and EM confirm the authenticity of all the raw data. All authors
participated in drafting or critically revising the manuscript for
important intellectual content, approved the final version for
publication, and agreed to be accountable for all aspects of the
work, ensuring the accuracy and integrity of the study are
appropriately addressed. All authors have read and approved the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for publication was
obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siemion K, Reszec-Gielazyn J, Kisluk J,
Roszkowiak L, Zak J and Korzynska A: What do we know about
inflammatory myofibroblastic tumors?-A systematic review. Adv Med
Sci. 67:129–138. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Panagiotopoulos N, Patrini D, Gvinianidze
L, Woo WL, Borg E and Lawrence D: Inflammatory myofibroblastic
tumour of the lung: A reactive lesion or a true neoplasm? J Thorac
Dis. 7:908–911. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sbaraglia M, Bellan E and Dei Tos AP: The
2020 WHO classification of soft tissue tumours: News and
perspectives. Pathologica. 113:70–84. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Coffin CM, Hornick JL and Fletcher CD:
Inflammatory myofibroblastic tumor: Comparison of
clinicopathologic, histologic, and immunohistochemical features
including ALK expression in atypical and aggressive cases. Am J
Surg Pathol. 31:509–520. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Butler JR, Eckert GJ, Zyromski NJ,
Leonardi MJ, Lillemoe KD and Howard TJ: Natural history of
pancreatitis-induced splenic vein thrombosis: A systematic review
and meta-analysis of its incidence and rate of gastrointestinal
bleeding. HPB (Oxford). 13:839–845. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lv ML, Zhong JQ and Feng H: Inflammatory
myofibroblastic tumor of spleen: A case report. Asian J Surg.
45:2111–2112. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bettach H, Alami B, Boubbou M, Chbani L,
Maâroufi M and Lamrani MA: Inflammatory myofibroblastic tumor of
the spleen: A case report. Radiol Case Rep. 16:3117–3119.
2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang B, Xu X and Li YC: Inflammatory
myofibroblastic tumor of the spleen: A case report and review of
the literature. Int J Clin Exp Pathol. 12:1795–1800.
2019.PubMed/NCBI
|
|
9
|
Wreesmann V, Van Eijck CH, Naus DC, Van
Velthuysen ML, Jeekel J and Mooi WJ: Inflammatory pseudotumour
(inflammatory myofibroblastic tumour) of the pancreas: A report of
six cases associated with obliterative phlebitis. Histopathology.
38:105–110. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen ZT, Lin YX, Li MX, Zhang T, Wan DL
and Lin SZ: Inflammatory myofibroblastic tumor of the pancreatic
neck: A case report and review of literature. World J Clin Cases.
9:6418–6427. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Biselli R, Boldrini R, Ferlini C, Boglino
C, Inserra A and Bosman C: Myofibroblastic Tumours: Neoplasias with
divergent behaviour. Ultrastructural and flow cytometric analysis.
Pathol Res Pract. 195:619–632. 1999.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Walsh EM, Xing D, Lippitt MH, Fader AN,
Wethington SL, Meyer CF and Gaillard SL: Molecular tumor board
guides successful treatment of a rare, locally aggressive, uterine
mesenchymal neoplasm. JCO Precis Oncol.
5(PO.20.00189)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
McDermott M: Inflammatory myofibroblastic
tumour. Semin Diagn Pathol: August 31, 2016 (Epub ahead of
print).
|
|
14
|
Meis JM and Enzinger FM: Inflammatory
fibrosarcoma of the mesentery and retroperitoneum. A tumor closely
simulating inflammatory pseudotumor. Am J Surg Pathol.
15:1146–1156. 1991.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jayarajah U, Bulathsinghala RP, Handagala
DMS and Samarasekera DN: Inflammatory myofibroblastic tumor of the
esophagus presenting with hematemesis and melaena: A case report
and review of literature. Clin Case Rep. 6:82–85. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shi H, Wei L, Sun L and Guo A: Primary
gastric inflammatory myofibroblastic tumor: A clinicopathologic and
immunohistochemical study of 5 cases. Pathol Res Pract.
206:287–291. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
González MG, Vela D, Álvarez M and Caramés
J: Inflammatory myofibroblastic duodenal tumor: A rare cause of
massive intestinal bleeding. Cancer Biomark. 16:555–557.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shang J, Wang YY, Dang Y, Zhang XJ, Song Y
and Ruan LT: An inflammatory myofibroblastic tumor in the
transplanted liver displaying quick wash-in and wash-out on
contrast-enhanced ultrasound: A case report. Medicine (Baltimore).
96(e9024)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jang EJ, Kim KW, Kang SH, Pak MG and Han
SH: Inflammatory myofibroblastic tumors arising from pancreas head
and peri-splenic area mimicking a malignancy. Ann Hepatobiliary
Pancreat Surg. 25:287–292. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lim K, Cho J, Pak MG and Kwon H:
Inflammatory myofibroblastic tumor of the pancreas: A case report
and literature review. Taehan Yongsang Uihakhoe Chi. 81:1497–1503.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ding D, Bu X and Tian F: Inflammatory
myofibroblastic tumor in the head of the pancreas with anorexia and
vomiting in a 69-year-old man: A case report. Oncol Lett.
12:1546–1550. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
A G H, Kumar S, Singla S and Kurian N:
Aggressive inflammatory myofibroblastic tumor of distal pancreas: A
diagnostic and surgical challenge. Cureus.
14(e22820)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gašljević G and Lamovec J: Malignant
lymphoma of the stomach in association with inflammatory
myofibroblastic tumor of the spleen. A case report. Pathol Res
Pract. 199:745–749. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ugalde P, García Bernardo C, Granero P,
Miyar A, González C, González-Pinto I, Barneo L and Vazquez L:
Inflammatory pseudotumor of spleen: A case report. Int J Surg Case
Rep. 7C:145–148. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ma ZH, Tian XF, Ma J and Zhao YF:
Inflammatory pseudotumor of the spleen: A case report and review of
published cases. Oncol Lett. 5:1955–1957. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Koechlin L, Zettl A, Koeberle D, von Flüe
M and Bolli M: Metastatic inflammatory myofibroblastic tumor of the
spleen: A case report and review of the literature. Case Rep Surg.
2016(8593242)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yan J, Peng C, Yang W, Wu C, Ding J, Shi T
and Li H: Inflammatory pseudotumour of the spleen: Report of 2
cases and literature review. Can J Surg. 51:75–76. 2008.PubMed/NCBI
|
|
28
|
Chen WC, Jiang ZY, Zhou F, Wu ZR, Jiang
GX, Zhang BY and Cao LP: A large inflammatory myofibroblastic tumor
involving both stomach and spleen: A case report and review of the
literature. Oncol Lett. 9:811–815. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Moriyama S, Inayoshi A and Kurano R:
Inflammatory pseudotumor of the spleen: Report of a case. Surg
Today. 30:942–946. 2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Luo X, Nie L, Wang Z, Tsauo J, Tang C and
Li X: Transjugular endovascular recanalization of splenic vein in
patients with regional portal hypertension complicated by
gastrointestinal bleeding. Cardiovasc Intervent Radiol. 37:108–113.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Köklü S, Yüksel O, Arhan M, Çoban Ş, Başar
Ö, Yolcu ÖF, Uçar E, Ibiş M, Ertuǧrul I and Şahin B: Report of 24
left-sided portal hypertension cases: A single-center prospective
cohort study. Dig Dis Sci. 50:976–982. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Alimoglu O and Cevikbas U: Inflammatory
Pseudotumor of the Spleen: Report of a case. Surg Today.
33:960–964. 2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dong A, Wang Y, Dong H, Gong J, Cheng C,
Zuo C and Lu J: Inflammatory myofibroblastic tumor: FDG PET/CT
findings with pathologic correlation. Clin Nucl Med. 39:113–121.
2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Coffin CM, Watterson J, Priest JR and
Dehner LP: Extrapulmonary inflammatory myofibroblastic tumor
(inflammatory pseudotumor). A clinicopathologic and
immunohistochemical study of 84 cases. Am J Surg Pathol.
19:859–872. 1995.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sukov WR, Cheville JC, Carlson AW, Shearer
BM, Piatigorsky EJ, Grogg KL, Sebo TJ, Sinnwell JP and Ketterling
RP: Utility of ALK-1 protein expression and ALK rearrangements in
distinguishing inflammatory myofibroblastic tumor from malignant
spindle cell lesions of the urinary bladder. Mod Pathol.
20:592–603. 2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cook JR, Dehner LP, Collins MH, Ma Z,
Morris SW, Coffin CM and Hill DA: Anaplastic lymphoma kinase (ALK)
expression in the inflammatory myofibroblastic tumor: A comparative
immunohistochemical study. Am J Surg Pathol. 25:1364–1371.
2001.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Butrynski JE, D'Adamo DR, Hornick JL, Dal
Cin P, Antonescu CR, Jhanwar SC, Ladanyi M, Capelletti M, Rodig SJ,
Ramaiya N, et al: Crizotinib in ALK-Rearranged inflammatory
myofibroblastic tumor. N Engl J Med. 363:1727–1733. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gambacorti-Passerini C, Orlov S, Zhang L,
Braiteh F, Huang H, Esaki T, Horibe K, Ahn JS, Beck JT, Edenfield
WJ, et al: Long-term effects of crizotinib in ALK-positive tumors
(Excluding NSCLC): A phase 1b open-label study. Am J Hematol.
93:607–614. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Schöffski P, Sufliarsky J, Gelderblom H,
Blay JY, Strauss SJ, Stacchiotti S, Rutkowski P, Lindner LH, Leahy
MG, Italiano A, et al: Crizotinib in patients with advanced,
inoperable inflammatory myofibroblastic tumours with and without
anaplastic lymphoma kinase gene alterations (European Organisation
for Research and Treatment of Cancer 90101 CREATE): A multicentre,
single-drug, prospective, non-randomised phase 2 trial. Lancet
Respir Med. 6:431–441. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Foster JH, Voss SD, Hall DC, Minard CG,
Balis FM, Wilner K, Berg SL, Fox E, Adamson PC, Blaney SM, et al:
Activity of crizotinib in patients with ALK-Aberrant
relapsed/refractory neuroblastoma: A children's oncology group
study (ADVL0912). Clin Cancer Res. 27:3543–3548. 2021.PubMed/NCBI View Article : Google Scholar
|