Introduction
PDRN, an abbreviation of polydeoxyribonucleotide,
denotes a family of DNA fragments with low molecular weights or
otherwise a mixture of polydisperse deoxyribonucleotide
heteropolymers with different chain lengths and diverse nucleotide
sequences. PDRNs, originally isolated from human placentas through
a proprietary extraction protocol, were found to exhibit
therapeutic roles against various diseases, particularly tissue
wounding (1,2). As a result of cost limitation and
ethical reason, its source was switched to the sperm cells of some
salmon fishes, Oncorhynchus mykiss (salmon trout) and
Oncorhynchus keta (chum salmon), which could provide
relatively pure genomic DNA with less impurities such as lipids,
peptides and proteins, and have been further extending to other
marine organisms, such as laver (3), sea cucumber sperm (4), and starfish (5). In recent years, PDRNs have been
prepared and characterized from botanic sources, including aloe
(6), ginseng (7) and roselle (8).
PDRNs possess different pharmacological properties
such as wound healing (9,10), anti-inflammatory (11,12),
skin whitening (13),
anti-apoptotic (14),
anti-ischemic (15),
anti-ulcerative (16),
anti-osteoporotic (16),
anti-allodynic (16),
anti-osteonecrotic (16), and bone
regenerative activities (16).
They were also identified to have protective effects against
cadmium-induced toxicity (17) and
carbon tetrachloride-induced acute liver injury (18). PDRN, lately prepared from sea
cucumber, was found to attenuate H2O2-induced
oxidative stress through multiple pathways, which suggests its
plausible application in the prevention and treatment of diverse
oxidative stress-induced diseases (4).
The two action mechanisms of PDRNs have been known
for last a few decades. The first one is that PDRN, acting as an
agonist, selectively stimulates adenosine A2A receptor, which leads
to the regulation of various pharmacological properties (19). PDRN was basically recognized to
have wound healing and anti-inflammatory properties through the
activation of adenosine A2A receptor (19). PDRN plays a mesenchymal stem
cell-based therapeutic role against osteoarthritis through the
activation of adenosine A2A receptor (20). PDRNs alleviate inflammatory
response by the inhibition of Janus kinase/signal transducer and
activator of transcription pathway via the mediation of adenosine
A2A receptor expression and by the inhibition of neuronal cell
death in the model of ischemia/reperfusion injury (19). A second relevant action mechanism
of PDRNs is that their pharmacological properties are mediated by
nucleotide salvage pathways. PDRNs are likely cleaved by cell
membrane enzymes, supply a source for purines and pyrimidines to
different tissues, and are then converted to deoxyribonucleotides
which incorporate into DNA, then activating the proliferation and
growth of various cells, including fibroblasts, preadipocytes,
osteoblasts and chondrocytes (21). The wound healing and
anti-inflammatory properties of PDRNs have been estimated to be
mediated by both the activation of adenosine A2A receptor and the
nucleotide salvage pathways (21).
Regardless of biological sources, the most current
protocols utilized for the preparation of PDRNs include high
temperature treatment, for the purpose of proteins removal, DNA
fragmentation and/or sterilization, which, however, can disrupt the
double-helical DNA conformation then forming the single-stranded
shapes. It is considered that the most of the final PDRN products
usually contain ssDNA forms at the different ratios between dsDNA
and ssDNA, depending on the detailed processes used. But it remains
to clarify whether of the two DNA forms, dsDNA and ssDNA forms, is
directly responsible for the pharmacological efficacies of
PDRNs.
In this work, by thermal denaturation, a commercial
PDRN, named PDRN, was transformed to PDRN-H with substantially
higher ssDNA forms. When skin in vitro beneficial properties
of PDRN and PDRN-H were comparatively analyzed, PDRN-H showed much
higher skin beneficial properties than PDRN, which was supposedly
based on the increased ssDNA proportion in PDRN-H. Collectively,
the ssDNA random coils of PDRNs, as an active player, are
responsible for their in vitro pharmacological
efficacies.
Materials and methods
Chemicals
Ethidium bromide (EtBr), ascorbic acid (AA), DPPH
(2,2-diphenyl-1-picrylhydrazyl), ABTS
[2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)], ammonium
persulfate, sodium nitrite, NADH, nitroblue tetrazolium, phenazine
methosulfate, L-tyrosine, L-3,4-dihydroxyphenylalanine (L-DOPA),
mushroom tyrosinase type I, kojic acid (KA), Griess reagent,
porcine pancreatic elastase, collagenase type I,
N-succinyl-(L-Ala)3-p-nitroanilide
(STANA), epigallocatechin gallate (EGCG), and
N-[3-(2-furyl) acryloyl]-Leu-Gly-Pro-Ala (FALGPA) were from
Sigma-Aldrich Chemical Co.. QuantiFluor® dsDNA System
was obtained from Promega Corporation. A PDRN product, denoted as
PDRN throughout this work, was obtained from Mastelli s.r.l.
Officina Bio-Farmaceutica (Placentex, biological source/salmon
sperm). Additional chemicals used were purchased from global
commercial chemical companies.
Preparation of thermal-treated PDRN
(PDRN-H)
PDRN solution was subjected to 94˚C for 30 min,
appropriately diluted with phosphate-buffered saline, and kept on
ice-water bath. If necessary, the thermal-treated PDRN solution,
named PDRN-H, was frozen in the refrigerator.
Characterization of thermal-treated
PDRN solution (PDRN-H)
Whether PDRN-H contained increased ssDNA portions
than PDRN or not was determined through the comparisons of the
absorbance at 260 nm, dsDNA levels and EtBr-stained DNA portions
after agarose gel electrophoresis. The dsDNA levels in PDRN and
PDRN-H were quantified using QuantiFluor® dsDNA System.
EtBr-stained portions of PDRN and PDRN-H were compared through 2%
agarose electophoresis, EtBr staining and the image analysis with
ImageJ version 1.54h (NIH).
DPPH radical scavenging assay
The scavenging activities of PDRN and PDRN-H against
stable DPPH radical were determined as earlier described (22). In a whole volume of 250 µl, the
reaction mixture containing 120 µl of 1.8 mg/ml PDRN or PDRN-H and
130 µl of 0.1 mM DPPH was kept for 30 min at room temperature (RT)
under the darkness. Ascorbic acid (AA, 1 µg/ml) was employed as a
positive control. The absorbance at 517 nm was detected using a
microplate reader and the percent scavenging was calculated using
the formula, Percent Scavenging (%)=[(Control-Test)/Control]
x100.
ABTS radical scavenging activity
assay
The ABTS radical scavenging activities of PDRN and
PDRN-H were determined as previously described (23) with a slight modification. ABTS
radical cations (ABTS•+), generated by reacting ABTS
stock solution (0.07 mM) with 0.12 mM ammonium persulfate, were
kept standing for 16 h in the dark at RT before use. In a whole
volume of 300, 100 µl of 1.8 mg/ml PDRN or PDRN-H was mixed with
200 µl of ABTS•+ solution, and then the reaction mixture
was incubated for 15 min at RT under the darkness. AA (1 µg/ml) was
used as a positive control. The absorbance was measured at 745 nm
and the percent scavenging was calculated as described above.
Superoxide radical scavenging
assay
As previously described (24), the in vitro superoxide
radical scavenging activities of PDRN and PDRN-H were determined.
In a whole volume of 200, 20 µl of 1.2 mg/ml PDRN or PDRN-H was
mixed with 180 µl of 1 mM Tris buffer (pH 8.0) with 100 µM
nitroblue tetrazolium, 156 µM NADH, and 20 µM phenazine
methosulfate. The reaction mixture was stood for 5 min at RT. AA
(20 µg/ml) was used as a positive control. Absorbance at 560 nm was
measured at a microplate reader. The percent scavenging was
calculated as described above.
Nitrite scavenging assay
The in vitro nitrite scavenging activities of
PDRN and PDRN-H were determined as previously described (25). In a whole volume of 150, 60 µl of
1.2 mg/ml PDRN or PDRN-H was mixed with 30 µl of 0.1 mM citrate
buffer (pH 3.0), 6 µl of 50 µg/ml sodium nitrite and 54 µl of
distilled water. The reaction mixture was stored for 60 min at
37˚C, and mixed with the equal volume of Griess reagent. After
incubation for 10 min, absorbance at 538 nm was measured at a
microplate reader. AA (20 µg/ml) was used as a positive control.
The percent scavenging was calculated as described above.
Tyrosinase inhibition activity
assay
As previously described (26), the inhibitory activities of PDRN
and PDRN-H on both L-tyrosine hydroxylase and L-DOPA oxidase
activities of tyrosinase were determined. For the measurement of
the hydroxylase activity, each reaction mixture (200 µl) contained
80 µl of 0.04 mM phosphate buffer (pH 6.8), 40 µl of 0.16 mg/ml
PDRN or PDRN-H, 40 µl of 0.5 mM L-tyrosine in 0.04 mM phosphate
buffer (pH 6.8), and 40 µl of 6 units/ml mushroom tyrosinase type
I, and was then stored for 10 min at 37˚C. The amount of L-DOPA
generated was quantitated by absorbance at 475 nm using a
microplate reader. Kojic acid (KA, 2 µg/ml) was employed a positive
control. For the L-DOPA oxidase activity, 0.5 mM L-DOPA was used,
instead of L-tyrosine, as a substrate, and dopaquinone generated
was quantified by absorbance at 450 nm. Kojic acid (KA, 10 µg/ml)
was used a positive control. The percent inhibition was calculated
using the formula, Percent Inhibition (%)=[(Control-Test)/Control]
x100.
Elastase inhibition activity
assay
Elastase inhibition activity was determined by
measuring an attenuation in elastase activity in the presence of
PDRN or PDRN-H. Elastase activity was quantified based upon the
generation of p-nitroaniline from STANA utilized as a
chromogenic substrate (27). The
reaction mixture consisting of 100 µl of 0.2 M Tris buffer (pH 8.0)
and 50 µl of 1.2 mg/ml PDRN or PDRN-H was pre-incubated with 100 µl
STANA (0.8 mM) for 20 min at 37˚C, and the enzymatic reaction was
started by adding 50 µl porcine pancreatic elastase (0.1 U/ml) in
0.2 M Tris buffer (pH 8.0). Absorbance at 410 nm was measured at a
microplate reader. EGCG (20 µg/ml) was used as a positive control,
and the percent inhibition was calculated as described above.
Collagenase inhibition activity
assay
Collagenase inhibition activities of PDRN and PDRN-H
were determined by measuring a diminishment in collagenase
activity, based on previously described spectrophotometric assay
(28) with a slight modification.
Clostridium histolyticum collagenase type I (1 mg/ml, 20 µl)
was allowed to react with 1.8 mg/ml PDRN or PDRN-H (20 µl) in a
96-well microtiter plate containing 20 µl of 50 mM Tris buffer (pH
7.4) with 0.36 mM CaCl2 for 20 min in the dark at 37˚C.
After the pre-incubation, 40 µl FALGPA (2.4 mM) was added to each
well and the mixture was further incubated for 30 min in the dark
at 37˚C. Absorbance at 335 nm was measured at a microplate reader.
EGCG (20 µg/ml) was used as a positive control, and the percent
inhibition was calculated as described above.
Statistical analysis
Experiments, in this work, were repeated at least
three times. The data were presented as mean ± SD. The differences
between experimental groups were analyzed using one-way ANOVA
subsequently with post hoc Tukey HSD test for multiple comparisons.
A P<0.05 was recognized to be statistically significant.
Results
The increase of ssDNA portion in
PDRN-H
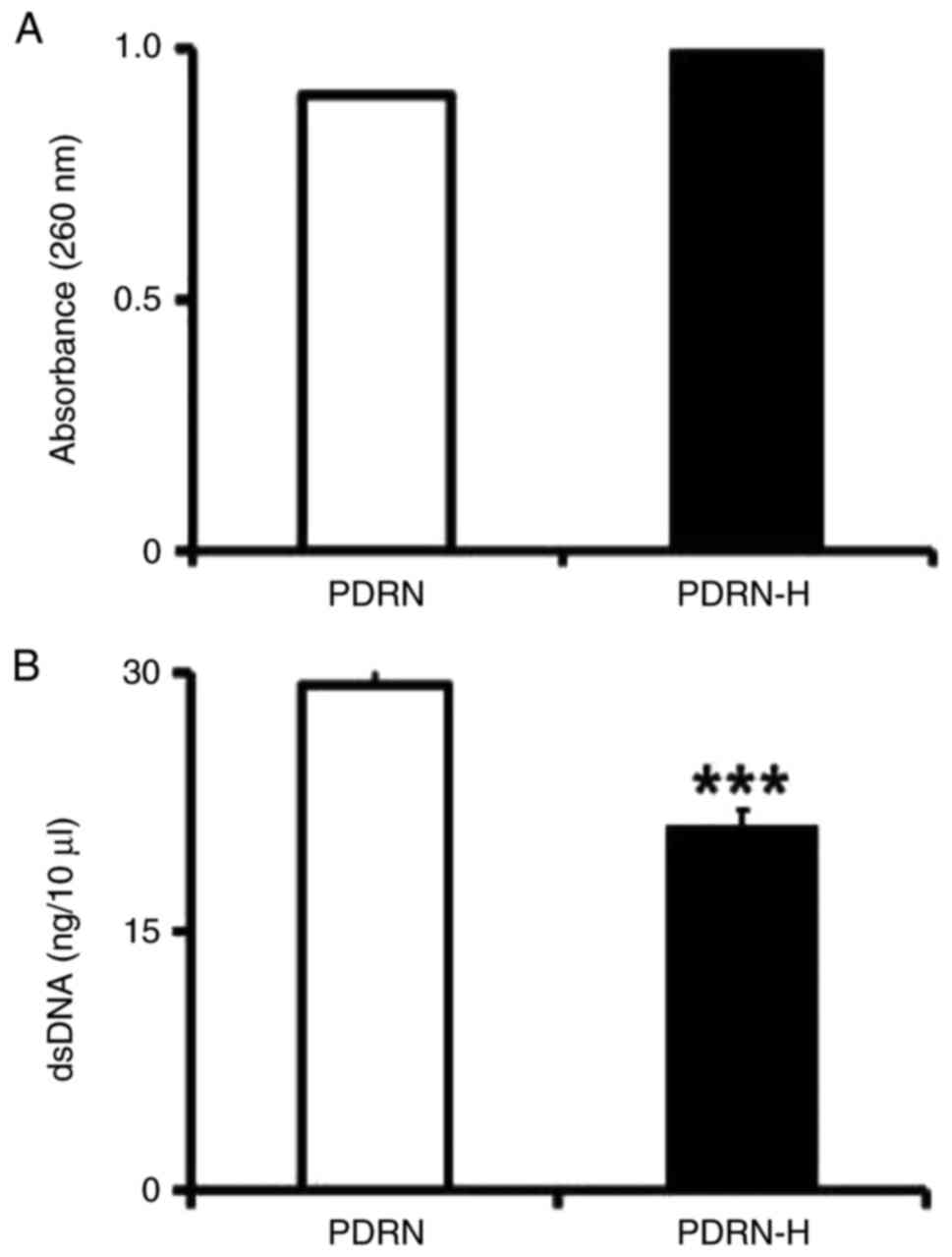
The UV light absorbances of PDRN and PDRN-H at 260
nm, shown in Fig. 1A, indicated
that PDRN-H exerted ~10% higher absorbance than PDRN. If PDRN was
presumed to be full of dsDNA, the treatment was thought to induce
~26.3% denaturation, since the complete denaturation of dsDNA
causes ~38% increase in absorbance at 260 nm. However, since
commercial PDRNs are believed to already have certain degrees of
ssDNA portions due to their manufacturing protocols, the percentage
of new thermal denaturation from the dsDNA portion might be
>26.3%. As shown in Fig. 1B,
dsDNA content was reduced to 71.6% in PDRN-H, compared to that of
PDRN, suggesting that ~28.4% of the existing dsDNA in PDRN would be
transformed to ssDNA in PDRN-H.
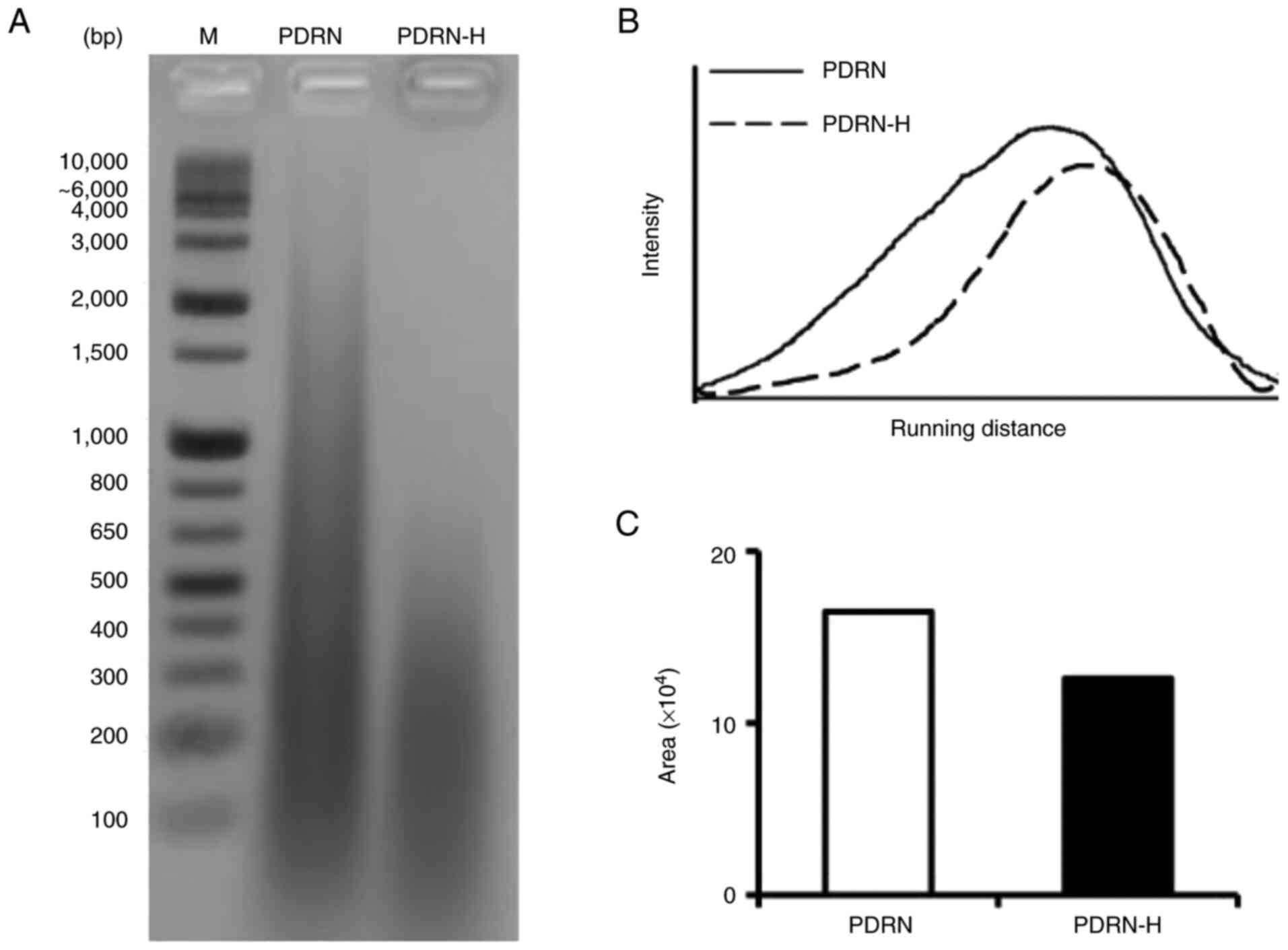
The transformation of dsDNA to ssDNA due to the
thermal denaturation was further examined by EtBr staining after
agarose gel electrophoresis. As shown in Fig. 2A, the EtBr-stained area of PDRN-H
became smaller and migrated faster than that of PDRN, suggesting
the increased portion of ssDNA in PDRN-H. Using ImageJ software,
the intensity profiles as a function of gel distance were drawn as
shown in Fig. 2B. When profile
areas of the lanes of PDRN and PDRN-H were calculated by
comparative densitometric quantification, the profile area of the
PDRN-H lane appeared to be 76.4% of that of the PDRN lane (Fig. 2C). This diminishment in PDRN-H
proposes that 24.6% of dsDNA in PDRN were transformed to ssDNA in
PDRN-H, which was not sufficiently stained with EtBr. Based upon
the mean of the three estimated values, the about 26.4% of the
existing dsDNA in PDRN was determined to denature to ssDNA in
PDRN-H.
Total antioxidant capacity
ROS, regarded as chemically reactive chemical
species containing oxygen, present in the body are mostly of
endogenous origin, and can also be produced in response to external
stimuli, such as ionizing radiation, UV light, pollution, alcohol
and tobacco consumption, toxic agents, and drugs (29). Although ROS play essential
biological roles, such as cell survival, proliferation, growth,
signaling and differentiation and immune response, oxidative stress
is induced from an imbalance between production and elimination of
ROS in cells and tissues. Oxidative stress is closely linked with
many disorders, such as chronic obstructive pulmonary disease,
cancer, atherosclerosis, Alzheimer's disease, inflammatory
diseases, and others (30).
Scavenging activities against free radicals have been thought as an
antioxidant strategy in the prevention of oxidative stress-related
disorders.
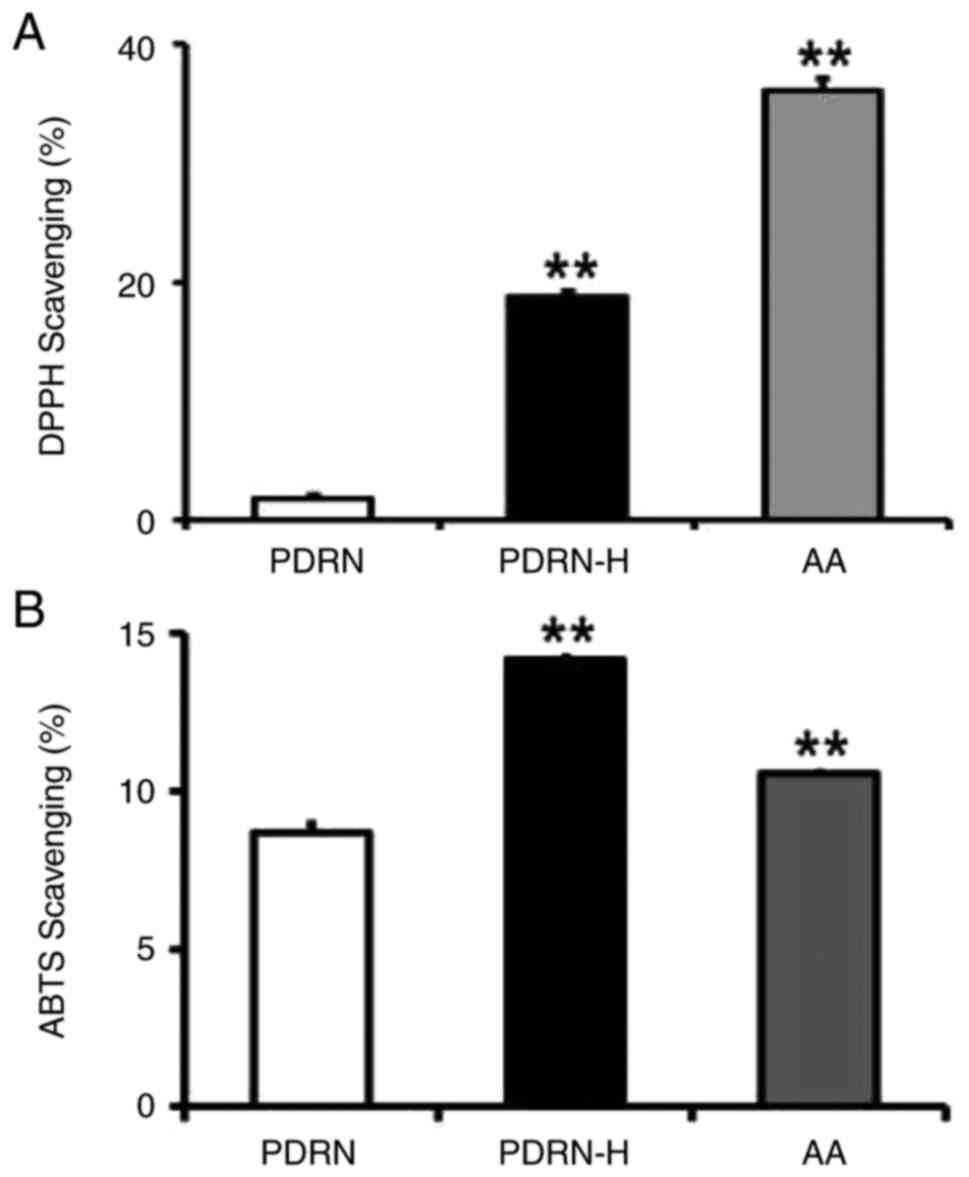
Total antioxidant activities of PDRN and PDRN-H were
evaluated using DPPH and ABTS scavenging assays. As shown in
Fig. 3A, PDRN and PDRN-H exhibited
1.8 and 18.8% scavenging activities in DPPH scavenging assay,
respectively. In ABTS scavenging assay, PDRN and PDRN-H gave rise
to 8.7 and 14.2% scavenging activities, respectively (Fig. 3B). The results show that PDRN-H
contains 10.4- and 1.6-fold higher scavenging activities over PDRN
in DPPH and ABTS scavenging assays, respectively. AA, used as a
positive control, exhibited 36.1 and 10.6% scavenging activities in
DPPH (Fig. 3A) and ABTS (Fig. 3B) scavenging assays, respectively.
In brief, PDRN-H possesses significantly higher total antioxidant
activities than PDRN, due to the increased ssDNA random coils.
Superoxide scavenging activity
Superoxide radical anion
(O2•−) is a primary ROS which is the first
species formed in the enzymatic respiratory chain by the reduction
of oxygen by the transfer of an electron. It is converted to
hydrogen peroxide by superoxide dismutase and further to hydroxyl
radical. Although its cellular level is in vivo controlled
by superoxide dismutase and superoxide reductase, their
insufficiency becomes responsible for oxidative stress in organisms
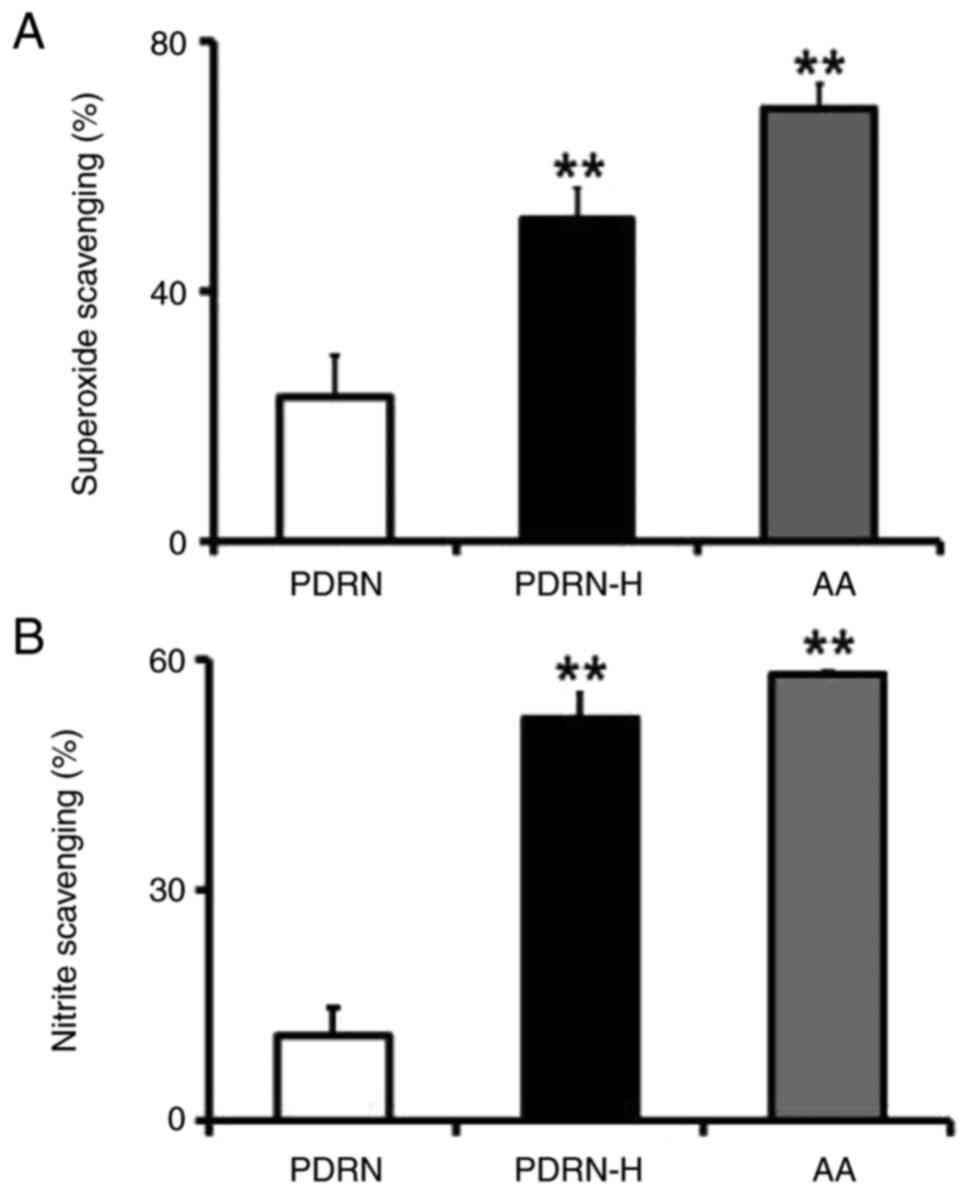
(29). In the scavenging assay
against superoxide radical, PDRN and PDRN-H displayed 23.0 and
51.5% scavenging activities, respectively (Fig. 4A). It suggests that PDRN-H has
2.2-fold higher scavenging activity over PDRN, which is based upon
the enhanced ssDNA portion of PDRN-H. AA, used as a positive
control, showed 69.2% scavenging activity in superoxide scavenging
assay (Fig. 4A).
Nitrite scavenging activity
Although nitric oxide (NO) displays an
anti-inflammatory activity under normal physiological situations,
it also acts as a key mediator in the induction of
inflammation based upon its inappropriate or excessive production
in diverse cells (31). Low-level
constitutive generation of NO is involved in in the maintenance of
barrier function, whereas NO synthetase activity, stimulated by
skin wounding or UV light, results in high-level NO through complex
reactions among diverse skin cells, and causes serious cutaneous
diseases such as atopic dermatitis and psoriasis (32). The generated NO undergoes the
oxidative breakdown metabolism to nitrite, a stable NO storage
form, and nitrite is reversely recycled to NO through
nitrate-nitrite-NO axis under inflamed skin conditions, such as UV
exposure and other inflammation-related diseases (33). Accordingly, anti-inflammatory
capacities of certain substances have been widely evaluated using
in vitro nitrite scavenging activity assay.
When the nitrite scavenging activities of PDRN and
PDRN-H were analyzed using the in vitro assay, they brought
about 11.1 and 52.3% scavenging activities, respectively (Fig. 4B). The result indicates that PDRN-H
has 4.7-fold higher scavenging activity on nitrite than PDRN. This
increment is thought to arise from the enhanced ssDNA random coils
in PDRN-H. Accordingly, PDRN-H was presumed to have significantly
higher anti-inflammatory property over PDRN. AA, used as a positive
control, showed 58.1% scavenging activity in nitrite scavenging
assay (Fig. 4B).
Tyrosinase inhibition activity
Although melanin protects the skin against the
harmful effects of UV irradiation, DNA damage and oxidative stress,
its overproduction leads to severe physical and psychological
distress, including skin darkening and aging, requiring the
approaches to preserve skin homeostasis (34). Tyrosinase, a copper-containing
rate-limiting enzyme located in the membrane of melanocytes,
catalyzes the rate limiting reactions of melanin synthesis: the
hydroxylation of L-tyrosine to 3,4-dihydroxy-L-phenylalanine
(L-DOPA) and the oxidation of L-DOPA to o-dopaquinone. The
inhibition of tyrosinase activity diminishes melanin synthesis,
then whitening the skin.
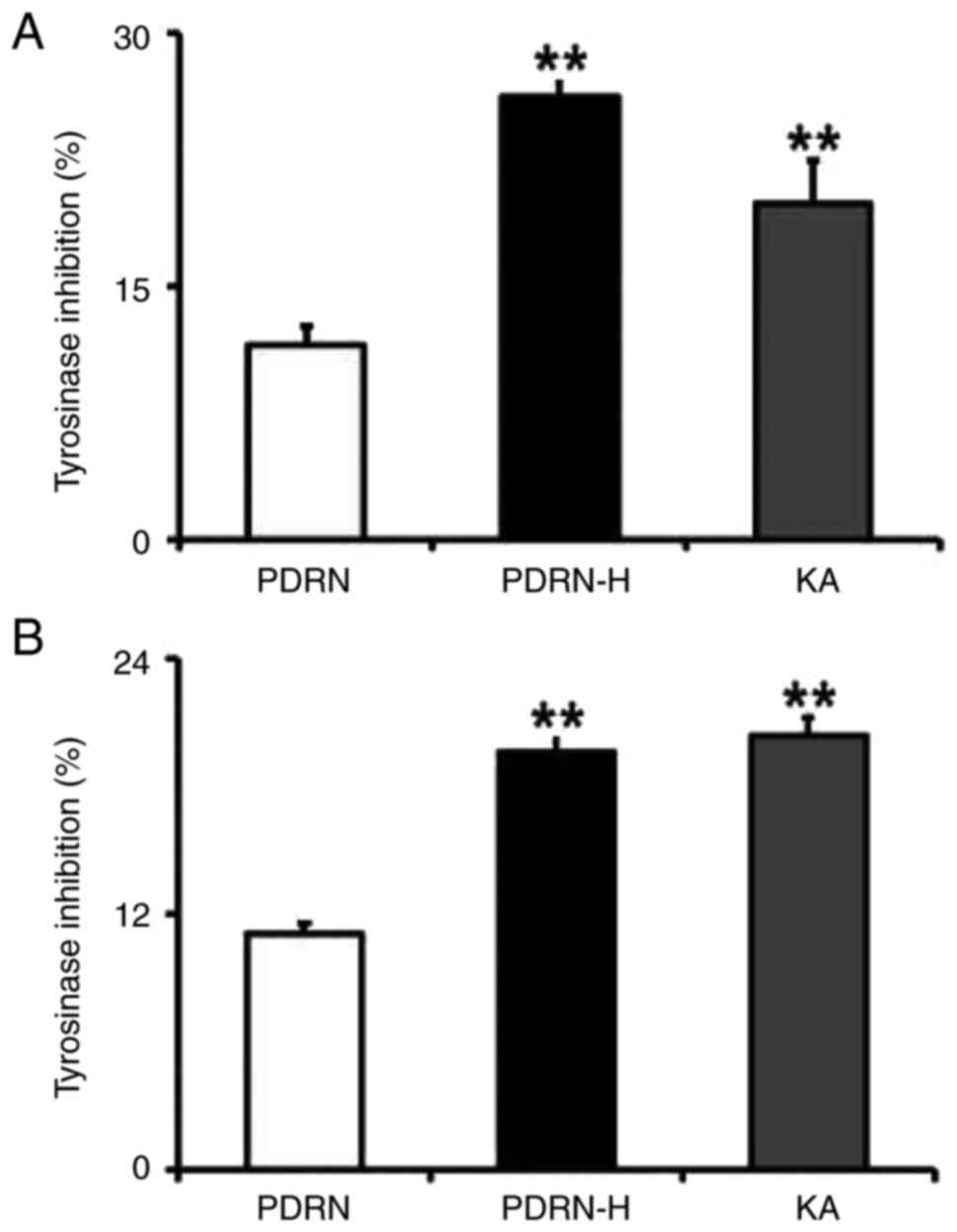
The inhibition activities of both PDRN and PDRN-H
were analyzed separately on the tyrosine hydroxylase and L-DOPA
oxidase activities of tyrosinase (Fig.
5). As shown in Fig. 5A, PDRN
and PDRN-H exhibited 11.5 and 26.2% inhibition activities on the
tyrosine hydroxylase activity, implying the 2.2-fold increased
inhibition activity of PDRN-H. In the L-DOPA oxidase inhibition
assay (Fig. 5B), they gave rise to
11.0 and 19.6% inhibition activity, respectively. This results in
the 1.8-fold higher inhibition activity of PDRN-H. Kojic acid (KA),
used as a positive control, displayed 19.8 and 20.4% inhibition
activities on the tyrosine hydroxylase (Fig. 5A) and the L-DOPA oxidase (Fig. 5B) activities of tyrosinase,
respectively. Collectively, PDRN-H has the significantly increased
inhibition activities against both tyrosine hydroxylase and L-DOPA
oxidase activities of tyrosinase and is subsequently estimated to
have much improved whitening activity.
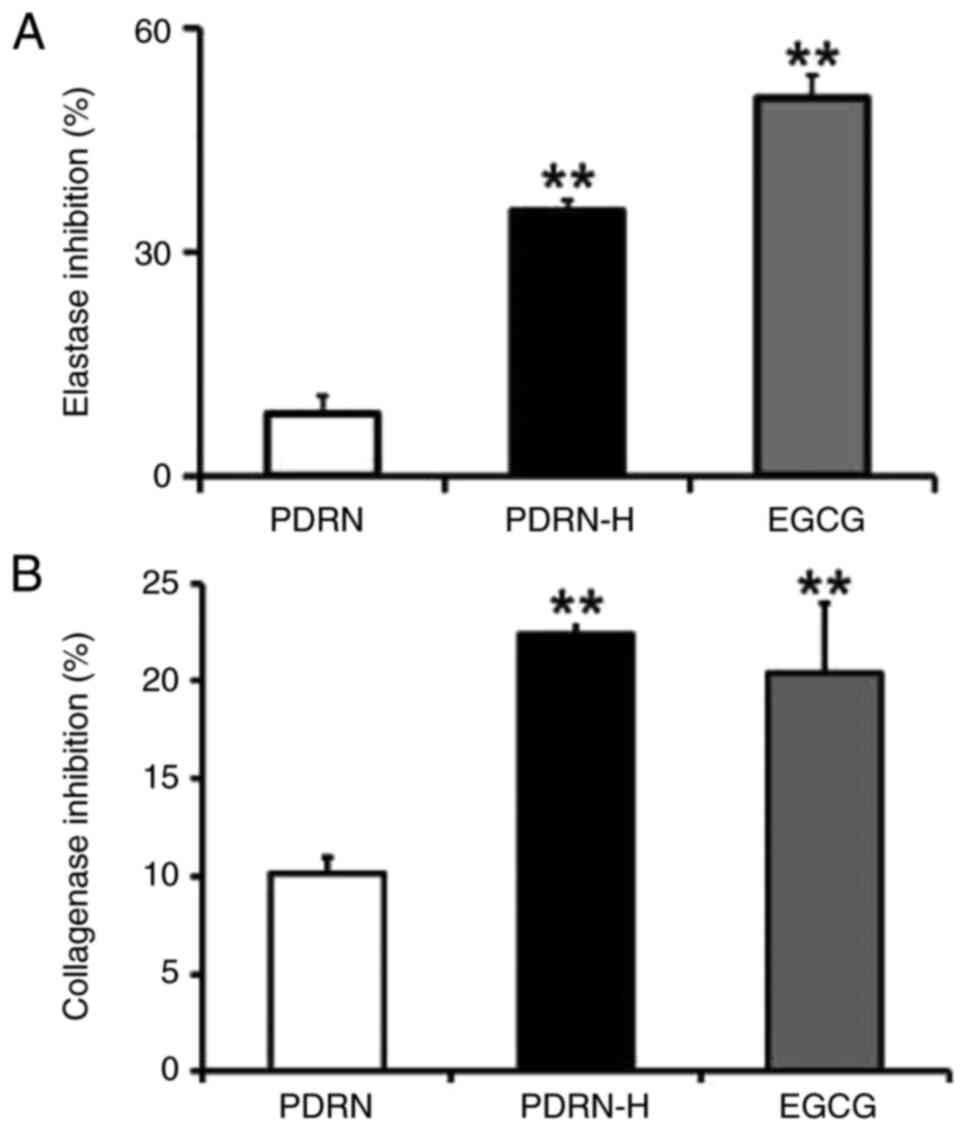
Elastase and collagenase inhibition
activities
Elastin and collagen, produced by fibroblasts, are
predominantly rich components in skin dermis extracellular matrix
(ECM). Breakdown and disorganization of elastin and collagen, two
main ECM proteins, bring about major characteristics of skin aging,
such as wrinkles, sagging, pigmentation and skin thinning, due to
the enhanced activation of elastase and collagenases (27). If the substances of certain origin
inhibit elastase and collagenase activities, they are presumed to
delay skin aging.
The inhibition activities of both PDRN and PDRN-H
were evaluated on elastase and collagenase activities. As shown in
Fig. 6A, PDRN and PDRN-H showed
9.0 and 35.1% inhibition activities against elastase activity,
respectively. As shown in Fig. 6B,
PDRN and PDRN-H showed 10.2 and 22.4% inhibition activities against
collagenase activity, respectively. From the results, PDRN-H was
found to have the 3.9- and 2.2-fold higher inhibition activities
against elastase and collagenase activities over PDRN,
respectively. EGCG, used as a positive control, displayed 50.5 and
20.4% inhibition activities on the elastase (Fig. 6A) and collagenase (Fig. 6B) activities, respectively. Based
upon the increased level of ssDNA portion in PDRN-H, it retains
significantly high inhibition activities against the two wrinkling
enzymes, which further suggests its strong skin anti-aging
properties.
Discussion
PDRNs have been proved to possess skin beneficial
properties using various experimental techniques. PDRN exhibits
antioxidant activities which suppress oxidative stress in skin
cells (35). It directly inhibits
mushroom and cellular tyrosinase activities, then lowering the
cellular melanin content in the melanocytes, and has attenuating
ability on the gene expression of tyrosinase-related protein 1,
another enzyme involved in the synthesis of melanin (35). It inhibits in vitro elastase
activity and suppresses matrix metalloproteinase-1 gene expression
in human skin fibroblast cells (35). In human dermal fibroblasts under
ultraviolet-B radiation, PDRN was identified to bring about the
attenuation of cyclobutane pyrimidine dimers, the enhancement of
DNA repair and the activation of p53 protein, suggesting its
protective effect against UV-induced DNA damage (36,37).
It has a treatment effect against psoriasis associated with chronic
inflammation, which is linked to its dual mode of action (38). Taken together, PDRNs retain
potential roles in preserving healthy skin homeostasis and
protecting against skin photoaging.
When thermal denaturation was carried out to augment
the ssDNA content in a commercial PDRN, the thermal-denatured PDRN
(PDRN-H) was determined to have the significantly enhanced ssDNA
content, as a result of the denaturation from about 26.4% of the
existing dsDNA in the non-thermal PDRN. When several skin in
vitro pharmacological properties of PDRN-H and PDRN were
comparatively measured, PDRN-H was found to have considerably
higher activities than PDRN, within the increasing range of 1.6- to
10.3-fold (Table I). This finding
strongly assures that the ssDNA random coils, markedly enriched in
PDRN-H, but not the dsDNA helical forms, relatively rich in the
non-thermal PDRN, are actually responsible for the pharmacological
activities tested. This work also demonstrates that non-thermal
PDRN contains the tested activities to a certain degree. It could
be thought that PDRN itself contains some degree of the ssDNA
portions which are presumed to be formed during the preparation
processes. The notion that ssDNA random coil acts as an active and
functional player of PDRNs is assisted indirectly by defibrotide
which is a polydisperse mixture of single-stranded
oligodeoxyribonucleotides prepared by controlled depolymerization
of purified porcine intestinal mucosa genomic DNA (39). Defibrotide was originally begun to
use to cure veno-occlusive disease, and has been found to contain
plasmin activating, anti-atherosclerotic, anti-inflammatory,
anti-ischemic and anti-thrombotic properties (39,40).
The pharmacological properties of defibrotide, chemically as
single-stranded oligodeoxyribonucleotides, have been naturally
attributed by the ssDNA forms. However, diverse PDRNs and
PDRN-based products, in the absence of the detailed information on
the proportions of dsDNA and ssDNA, have been utilized for the
purposes of academic research and medical and industrial
application. In the advanced research, the various types of
next-phase works, such as the validation in cellular in
vitro models (for example, keratinocytes, skin fibroblasts,
etc.), large-scale preparation of PDRNs in ssDNA forms,
optimization of size and composition of single-stranded PDRNs,
elucidation of mechanistic mechanism, application to animal and
human skin, and so on, can be accomplished.
 | Table IA summary on the thermal-induced
enhancements in in vitro skin beneficial properties of a
commercial PDRN product. |
Table I
A summary on the thermal-induced
enhancements in in vitro skin beneficial properties of a
commercial PDRN product.
| Beneficial
properties | Enhancement
(PDRN-H/PDRN)a |
|---|
| Antioxidant
properties | |
|
DPPH
scavenging activity | 10.3 |
|
ABTS
scavenging activity | 1.6 |
|
Superoxide
scavenging activity | 2.2 |
| Anti-inflammatory
properties | |
|
Nitrite
scavenging activity | 4.7 |
| Skin whitening
properties | |
|
Tyrosinase
inhibition activityb | 2.3 |
|
Tyrosinase
inhibition activityc | 1.8 |
| Antiwrinkle
properties | |
|
Elastase
inhibition activity | 3.9 |
|
Collagenase
inhibition activity | 2.2 |
The insight, obtained from the non-celled in
vitro assays used, strongly implies that the ssDNA forms of
PDRNs, without metabolic alteration, can interact with their
reacting molecules, such as protein molecules, such as tyrosinase,
elastase and collagenase, and small molecules such as DPPH and ABTS
radicals, superoxide radical ion and nitrite ion. That is, the
primary hydrolytic metabolites of PDRNs inside and/or outside
cells, such as deoxyribonucleotides, deoxyribonucleosides and free
bases, are not directly involved in their skin in vitro
properties. Direct contact between the ssDNA forms of PDRNs and
corresponding protein molecules can be more convinced by binding
studies, such as molecular docking and modified mobility shift
assay.
Based on this work, it is thought that the ssDNA
form, but not the dsDNA form, of PDRN acts as an active player in
performing its various pharmacological properties, including skin
in vitro properties. Also based on the use of the non-celled
in vitro assays, it is presumed that the skin in
vitro properties of PDRNs are displayed in a manner which is
independent on adenosine A2A receptor activation and nucleotide
salvage pathway, the two current mechanisms of PDRNs. Through the
coming studies, it can be decided whether the functional role of
the ssDNA forms of PDRNs is linked with their known mechanisms or
not. Considering that the free bases of PDRNs are necessarily
required for their action as an agonist of adenosine A2A receptor,
the ssDNA forms of PDRNs, based upon the fact that ssDNA only
retains unpaired, exposed bases, are thought to be much more
adequate than the dsDNA forms.
In conclusion, the ssDNA forms of PDRNs act as an
active and functional player in the performance of their in
vitro pharmacological properties, including skin in
vitro beneficial properties. Non-celled in vitro assay
system used implies that PDRNs are effective without metabolic
alteration and that their ssDNA random coils make a direct contact
with the appropriate reacting molecules. It seems that the in
vitro pharmacological properties of PDRNs are not mediated
through adenosine A2A receptor activation and nucleotide salvage
pathways. This is the novel finding on the functional action
mechanism of PDRNs, which greatly influence their academic research
and production. In the future, PDRNs in single-stranded DNA forms
but not double-stranded DNA forms are expected to be more desirable
in the generation of PDRNs and PDRN-based products for cosmetic and
pharmaceutical uses.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HL and CL conceptualized this study. HL, KK and CL
designed the experiments of this study. YH, HB and HK performed the
experiments. HK analyzed the data. KK and CL wrote the manuscript.
All authors have read and approved the final version of manuscript.
HK and CL confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tonello G, Daglio M, Zaccarelli N,
Sottofattori E, Mazzei M and Balbi A: Characterization and
quantitation of the active polynucleotide fraction (PDRN) from
human placenta, a tissue repair stimulating agent. J Pharm Biomed
Anal. 14:1555–1560. 1996.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pan SY, Chan MKS, Wong MBF, Klokol D and
Chernykh V: Placental therapy: An insight to their biological and
therapeutic properties. J Med Ther. 1:1–6. 2017.
|
|
3
|
Kim TH, Heo SY, Han JS and Jung WK:
Anti-inflammatory effect of polydeoxyribonucleotides (PDRN)
extracted from red alga (Porphyra sp.) (Ps-PDRN) in RAW 264.7
macrophages stimulated with Escherichia coli
lipopolysaccharides: A comparative study with commercial PDRN. Cell
Biochem Funct. 41:889–897. 2023.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Shu Z, Ji Y, Liu F, Jing Y, Jiao C, Li Y,
Zhao Y, Wang G and Zhang J: Proteomics analysis of the protective
effect of polydeoxyribonucleotide extracted from sea cucumber
(Apostichopus japonicus) sperm in a hydrogen
peroxide-induced RAW264.7 cell injury model. Mar Drugs.
22(325)2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim TH, Kim SC, Park WS, Choi IW, Kim HW,
Kang HW, Kim YM and Jung WK: PCL/gelatin nanofibers incorporated
with starfish polydeoxyribonucleotides for potential wound healing
applications. Mater Des. 229(111912)2023.
|
|
6
|
Kim M, Kim WJ, Lee HK, Kwon YS and Choi
YM: Efficacy in vitro antioxidation and in vivo skin barrier
recovery of composition containing mineral-cation-phyto DNA
extracted from Aloe vera adventitious root. Asian J Beauty
Cosmetol. 21:231–246. 2023.
|
|
7
|
Lee KS, Lee S, Wang H, Lee G, Kim S, Ryu
YH, Chang NH and Kang YW: Analysis of skin regeneration and
barrier-improvement efficacy of polydeoxyribonucleotide isolated
from Panax ginseng (C.A. Mey.) adventitious root. Molecules.
28(7240)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim E, Choi S, Kim SY, Jang SJ, Lee S, Kim
H, Jang JH, Seo HH, Lee JH, Choi SS and Moh SH: Wound healing
effect of polydeoxyribonucleotide derived from Hibiscus
sabdariffa callus via Nrf2 signaling in human keratinocytes.
Biochem Biophys Res Commun. 728(150335)2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shin J, Park G, Lee J and Bae H: The
effect of polydeoxyribonucleotide on chronic non-healing wound of
an amputee: A case report. Ann Rehabil Med. 42:630–633.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yun J, Park S, Park HY and Lee KA:
Efficacy of polydeoxyribonucleotide in promoting the healing of
diabetic wounds in a murine model of streptozotocin-induced
diabetes: A pilot experiment. Int J Mol Sci.
24(1932)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Picciolo G, Mannino F, Irrera N, Altavilla
D, Minutoli L, Vaccaro M, Arcoraci V, Squadrito V, Picciolo G,
Squadrito F and Pallio G: PDRN, a natural bioactive compound,
blunts inflammation and positively reprograms healing genes in an
‘in vitro’ model of oral mucositis. Biomed Pharmacother.
138(111538)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hwang L, Jin JJ, Ko IG, Kim S, Cho YA,
Sung JS, Choi CW and Chang BS: Polydeoxyribonucleotide attenuates
airway inflammation through A2AR signaling pathway in PM10-exposed
mice. Int Neurourol J. 25 (Suppl 1):S19–S26. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Noh TK, Chung BY, Kim SY, Lee MH, Kim MJ,
Youn CS, Lee MW and Chang SE: Novel anti-melanogenesis properties
of polydeoxyribonucleotide, a popular wound healing booster. Int J
Mol Sci. 17(1448)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ko IG, Jin JJ, Hwang L, Kim SH, Kim CJ,
Han JH, Lee S, Kim HI, Shin HP and Jeon JW: Polydeoxyribonucleotide
exerts protective effect against CCl4-induced acute
liver injury through inactivation of NF-κB/MAPK signaling pathway
in mice. Int J Mol Sci. 21(7894)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim SE, Ko IG, Jin JJ, Hwang L, Kim CJ,
Kim SH, Han JH and Jeon JW: Polydeoxyribonucleotide exerts
therapeutic effect by increasing VEGF and inhibiting inflammatory
cytokines in ischemic colitis rats. Biomed Res Int.
2020(2169083)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Khan A, Wang G, Zhou F, Gong L, Zhang J,
Qi L and Cui H: Polydeoxyribonucleotide: A promising skin
anti-aging agent. Chin J Plast Reconstr Surg. 4:187–193. 2022.
|
|
17
|
Marini HR, Puzzolo D, Micali A, Adamo EB,
Irrera N, Pisani A, Pallio G, Trichilo V, Malta C, Bitto A, et al:
Neuroprotective effects of polydeoxyribonucleotide in a murine
model of cadmium toxicity. Oxid Med Cell Longev.
2018(4285694)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee S, Won KY and Joo S: Protective effect
of polydeoxyribonucleotide against CCl4-induced acute
liver injury in mice. Int Neurourol J. 24 (Suppl 2):S88–S95.
2020.
|
|
19
|
Jo S, Baek A, Cho Y, Kim SH, Baek D, Hwang
J, Cho SR and Kim HJ: Therapeutic effects of
polydeoxyribonucleotide in an in vitro neuronal model of
ischemia/reperfusion injury. Sci Rep. 13(6004)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Baek A, Baek D, Kim SH, Kim J, Notario GR,
Lee DW, Kim HJ and Cho SR: Polydeoxyribonucleotide ameliorates
IL-1β-induced impairment of chondrogenic differentiation in human
bone marrow-derived mesenchymal stem cells. Sci Rep.
14(26076)2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Galeano M, Pallio G, Irrera N, Mannino F,
Bitto A, Altavilla D, Vaccaro M, Squadrito G, Arcoraci V, Colonna
MR, et al: Polydeoxyribonucleotide: A promising biological platform
to accelerate impaired skin wound healing. Pharmaceuticals (Basel).
14(1103)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jung HJ, Cho YW, Lim HW, Choi H, Ji DJ and
Lim CJ: Anti-inflammatory, antioxidant, anti-angiogenic and skin
whitening activities of Phryma leptostachya var. asiatica
Hara extract. Biomol Ther (Seoul). 21:72–78. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Thring TSA, Hili P and Naughton DP:
Anti-collagenase, anti-elastase and anti-oxidant activities of
extracts from 21 plants. BMC Complement Altern Med.
9(27)2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mandal S, Hazra B, Sarkar R, Biswas S and
Mandal N: Assessment of the antioxidant and reactive oxygen species
scavenging activity of methanolic extract of Caesalpinia
crista leaf. Evid Based Complement Alternat Med.
2011(173768)2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gharibzahedi SMT, Razavi SH and Mousavi M:
Characterizing the natural
canthaxanthin/2-hydroxypropyl-β-cyclodextrin inclusion complex.
Carbohydr Polym. 10:1147–1153. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Masuda T, Yamashita D, Takeda Y and
Yonemori S: Screening for tyrosinase inhibitors among extracts of
seashore plants and identification of potent inhibitors from
Garcinia subelliptica. Biosci Biotechnol Biochem.
69:197–201. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wittenauer J, Mäckle S, Sußmann D,
Schweiggert-Weisz U and Carle R: Inhibitory effects of polyphenols
from grape pomace extract on collagenase and elastase activity.
Fitoterapia. 101:179–187. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chattuwatthana T and Okello E:
Anti-collagenase, anti-elastase and antioxidant activities of
Pueraria candollei var. mirifica root extract and
Coccinia grandis fruit juice extract: An in vitro study. Eur
J Med Plants. 5:318–327. 2015.
|
|
29
|
Andrés CMC, Pérez de la Lastra JM, Andrés
Juan C, Plou FJ and Pérez-Lebeña E: Superoxide anion chemistry-its
role at the core of the innate immunity. Int J Mol Sci.
24(1841)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Forman HJ and Zhang H: Targeting oxidative
stress in disease: Promise and limitations of antioxidant therapy.
Nat Rev Drug Discov. 20:689–709. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sharma JN, Al-Omran A and Parvathy SS:
Role of nitric oxide in inflammatory diseases.
Inflammopharmacology. 15:252–259. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Akdeniz N, Aktaş A, Erdem T, Akyüz M and
Özdemir S: Nitric oxide levels in atopic dermatitis. The Pain
Clinic. 16:401–405. 2004.
|
|
33
|
Suschek CV, Feibel D, von Kohout M and
Opländer C: Enhancement of nitric oxide bioavailability by
modulation of cutaneous nitric oxide stores. Biomedicines.
10(2124)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Masum MN, Yamauchi K and Mitsunaga T:
Tyrosinase inhibitors from natural and synthetic sources as
skin-lightening agents. Rev Agric Sci. 7:41–58. 2019.
|
|
35
|
Kim YJ, Kim MJ, Kweon DK, Lim ST and Lee
SJ: Polydeoxyribonucleotide activates mitochondrial biogenesis but
reduces MMP-1 activity and melanin biosynthesis in cultured skin
cells. Appl Biochem Biotechnol. 191:540–554. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Squadrito F, Bitto A, Irrera N, Pizzino G,
Pallio G, Minutoli L and Altavilla D: Pharmacological activity and
clinical use of PDRN. Front Pharmacol. 8(224)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Belletti S, Uggeri J, Gatti R, Govoni P
and Guizzardi S: Polydeoxyribonucleotide promotes cyclobutane
pyrimidine dimer repair in UVB-exposed dermal fibroblasts.
Photodermatol Photoimmunol Photomed. 23:242–249. 2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Irrera N, Bitto A, Vaccaro M, Mannino F,
Squadrito V, Pallio G, Arcoraci V, Minutoli L, Ieni A, Lentini M,
et al: PDRN, a bioactive natural compound, ameliorates
imiquimod-induced psoriasis through NF-κB pathway inhibition and
Wnt/β-catenin signaling modulation. Int J Mol Sci.
21(1215)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Echart CL, Graziadio B, Somaini S, Ferro
LI, Richardson PG, Fareed J and Iacobelli M: The fibrinolytic
mechanism of defibrotide: Effect of defibrotide on plasmin
activity. Blood Coagul Fibrinolysis. 20:627–634. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Baker DE and Demaris K: Defibrotide. Hosp
Pharm. 51:847–854. 2016.PubMed/NCBI View Article : Google Scholar
|