Introduction
Neurodegenerative diseases can result in the gradual
loss of neuronal function or structure in the central nervous
system (CNS), and include Alzheimer's disease (AD) and motor neuron
disease (1). A pathological
feature of these diseases is the abnormal deposition of proteins,
such as amyloid β (Aβ) and tau, in the brain and spinal cord
(2). Various neurodegenerative
diseases display specific differences, but they all exhibit common
clinical features; namely, the progressive loss of cognitive
function, motor coordination deficits and various symptoms
resulting from the loss of specific neuronal groups (3). Neurodegenerative diseases affect
millions of individuals worldwide (4). The prevalence and mortality rates
have also shown a growing trend over time, and these diseases are
considered one of the main threats to personal and social
well-being (4). Oxidative stress
(OS) serves an important role in the pathogenesis of
neurodegenerative diseases (5,6). The
CNS is particularly susceptible to OS because of its high oxygen
utilization rate and large polyunsaturated fatty acid contents
(7). Therefore, the antioxidant
inhibition of OS is considered a therapeutic strategy for
neurodegenerative diseases due to its ability to neutralize
reactive oxygen species (ROS), which is of therapeutic relevance
for reducing the progression of OS.
Neurodegenerative diseases have become one of the
largest health problems worldwide, and there is still a shortage of
appropriate treatment methods (8).
Traditional Chinese medicine (TCM) displays multi-component and
multi-target characteristics, providing a promising method for
preventing neurodegenerative diseases (9). The compounds and extracts derived
from TCM, such as evodiamine from Tetradium ruticarpum and
ginsenoside compound K from Panax ginseng, have received
widespread attention due to their possible applications as
therapeutic agents for AD and Parkinson's disease (10). In clinical practice, TCM has been
extensively utilized for treating age-related conditions, including
memory loss and cognitive decline (11,12).
Furthermore, bioactive compounds in plants, such as genipin from
Gardenia jasminoides fruit extract, had been proven to
possess the ability to prevent and stop the progression of AD and
Parkinson's disease (8). They can
regulate crosstalk between pathways through multiple targets, thus
improving chronic inflammatory interactions and inhibiting OS
damage (13). The leaves, stems
and rhizomes of Epimedium, also known as barrenwort, can be
used as therapeutic drugs (14).
Pharmacological studies have shown that Epimedium can
markedly regulate and improve the human immune system, and the
chemicals in Epimedium have great potential as plant drugs
for guarding against and treating chronic diseases such as AD
(15,16). Notably, >260 compounds have been
extracted from Epimedium (16), and research has suggested that the
compound icariin possesses strong neuroprotective properties and
has the potential as a drug for preventing neurological disorders
such as AD (17). However, the
main bioactive chemicals and their specific mechanisms that exert
antioxidative, anti-aging and neuroprotective effects in
Epimedium still require further exploration.
The median effect concentration value of
Wushanicaritin in PC12 cells is 3.87 µM, making it a promising
neuroprotective agent (16). In
addition, Wushanicaritin has been shown to maintain mitochondrial
activity and the enzymatic antioxidant defense system,
demonstrating marked intercellular antioxidant and neuroprotective
effects (16). Epimedin C was used
as the indicator component in the quality control for assessing the
quality of Wushan Epimedium in the Chinese Pharmacopoeia
(2020 edition) (18). A previous
study confirmed that total flavonoids of Epimedium can
prevent dopaminergic neuron death and cellular neurotoxicity in
vivo and in vitro, exerting neuroprotective effects
(19). Epimedin C is a
triterpenoid component extracted from the water extract of
Epimedium, containing high levels of flavonol glycosides
(20). The chemical structure of
Epimedin C is similar to that of icariin, indicating that they may
have similar pharmacological effects (21). Moreover, Epimedin C has been
reported to exert anti-inflammatory properties (21), and to have potential therapeutic
efficacy in angiogenesis, antioxidant damage and the inhibition of
apoptosis (22). However, it is
currently unclear whether Epimedin C can reduce neuronal cell
apoptosis by inhibiting oxidative damage, thereby preventing and/or
treating neurodegenerative diseases such as AD.
The PC12 rat adrenal pheochromocytoma cell line is
often adopted as a model neuron-like cell line and applied to study
neurodegenerative diseases (23).
Exogenous H2O2 can trigger OS damage and
cause apoptosis of PC12 cells (24). Our previous research indicated that
Tiaogeng Decoction (TGD) may be a potential therapeutic agent for
the treatment of neurodegenerative diseases, as it is involved in
mediating the nuclear factor erythroid 2-related factor 2 (Nrf2)
and JNK pathways (25). Notably,
TGD comprises 10 TCMs, including Epimedium. A core component
of neuronal response to ROS is the activation of JNK (26); JNK belongs to the mitogen-activated
protein kinase (MAPK) family, and JNK phosphorylation is associated
with diverse apoptotic transcription factors (27). Nrf2 participates in maintaining
cellular redox homeostasis and regulating the inflammatory response
of the body, and Nrf2 activation has a cellular protective effect
on neurodegenerative diseases (28). Heme oxygenase-1 (HO-1) serves as a
protective protein during the OS response (29). Nrf2/HO-1 pathway activation
accelerates the upregulation of antioxidant and cell protective
genes, thereby reducing OS and inflammatory damage (30). Ultra-high performance liquid
chromatography-quadrupole-Exactive Orbitrap high resolution mass
spectrometry (UHPLC-Q-Exactive Orbitrap HRMS) can be utilized to
rapidly identify complex compound mixtures in plants (31). The present study aimed to assess
the active ingredients of Epimedium using UHPLC-Q-Exactive
Orbitrap HRMS. In addition to mass spectrometry results, network
pharmacological analysis was performed to explore whether Epimedin
C mediated the JNK/Nrf2/HO-1 pathway, and prevented the
H2O2-related OS damage and apoptosis of PC12
cells. Moreover, the present study aimed to clarify the possible
effect of Epimedin C on managing neurodegenerative diseases.
Materials and methods
Identification of chemical components
in Epimedium by UHPLC-Q-Exactive Orbitrap HRMS
In our previous research, UHPLC-Q-Exactive Orbitrap
HRMS analysis was conducted on TGD (11). By comparing and analyzing the
chemical composition of each herb detected in the viscera and serum
of rats after oral administration of TGD, the effective chemical
components of each TCM that worked in the formula were explored.
Epimedium is an important component of TGD. A total of 250
mg Epimedium sample (Sichuan Neo-Green Pharmaceutical
Technology Development Co., Ltd.) was taken and placed in a 2ml
centrifuge tube. Thereafter, 20% methanol (4 ml) was added for
centrifugation (4˚C, 10,000 x g, 15 min). Subsequently, 200 µl
supernatant was taken as the experimental sample. Chromatographic
separation was implemented through a Waters ACQUITY UPLC BEH C18
column (2.1x100 mm, 1.7 µm; Waters Corporation) at a column
temperature of 40˚C. The mobile phase included two solvents; mobile
phase A consisted of methanol whereas mobile phase B comprised a
0.1% formic acid aqueous solution. Elution was completed according
to the following schedule: 4% A from 0 to 4.0 min, 4-12% A at
4.0-10.0 min, 12-70% A at 10.0-30.0 min, 70-95% A at 30.0-35.0 min,
95% A at 35.0-38.0 min, and 4% A at 42.0-45.0 min. The flow rate
was 0.3 ml/min and the injection volume was 2 µl. The resolution
was 70,000 full width at half maximum and the cumulative time was
300 msec. Data collection and analysis of the results of
UHPLC-Q-Exactive Orbitrap HRMS analysis (Thermo Fisher Scientific,
Inc.) were performed using Xcalibur 4.1 software (Thermo Fisher
Scientific, Inc.).
Network pharmacological analysis:
Target prediction of Epimedium for improving AD
Drug targets were retrieved using the key word
‘Epimedium’ in the TCM systems pharmacology (TCMSP)
(https://www.91tcmsp.com/), SwissTarget
(http://swisstargetprediction.ch/),
PharmMapper (http://lilab-ecust.cn/pharmmapper/) and HERB databases
(http://herb.ac.cn/), utilizing the criteria oral
bioavailability ≥30% and drug similarity ≥0.18. Subsequently, with
‘Alzheimer's disease’ being used as the key word, drug targets were
searched in the GeneCards (https://www.genecards.org/), DisGeNet (https://disgenet.com) and Online Mendelian Inheritance
In Man (OMIM) databases (https://www.omim.org/), and Venn diagrams (http://bioinformatics.psb.ugent.be/webtools/Venn/)
were used to screen and generate overlapping targets between
diseases and drugs. The ‘drug disease target’ was visualized with
Cytoscape 3.8.2 (https://cytoscape.org/download.html), while common
targets of drugs were imported in STRING database (https://string-db.org/) for protein-protein
interaction (PPI) analysis. In the PPI network, proteins were
denoted by nodes, whereas protein interactions were signified by
edges. Nodes of various colors and sizes represented diverse degree
values, and larger nodes and darker red colors declared greater
degree values and more important targets. Gene Ontology (GO) and
Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses
were performed on the top 20 core targets in Metascape database
(https://metascape.org/gp/index.html).
Subsequently, the exported clustering network was sorted in
accordance with the P-value using R 4.1.2 (https://www.r-project.org/) and a bubble chart was
generated.
Chemicals and reagents
Epimedin C was supplied by Chengdu Biopurify
Phytochemicals Ltd. (CAS no. 110642-44-9), and its purity was
detected by HPLC-Diode Array Detection to be 95-99%. In addition,
17β-estradiol (17β-E2) was provided by Sigma-Aldrich;
Merck KGaA (cat. no. E2758). All samples were stored in the
laboratory at 4˚C for future use.
Modeling and intervention
PC12 cells were obtained from The Cell Bank of Type
Culture Collection of The Chinese Academy of Sciences and were
cultured in the Dulbecco's Modified Eagle medium (cat. no.
C11995500BT; Gibco; Thermo Fisher Scientific Inc.) with 10% FBS
(cat. no. 10099-141C; Gibco; Thermo Fisher Scientific Inc.) at 37˚C
and 5% CO2. The compound 17β-E2 can be
produced in the brain and it participates in regulating the
reproductive axis (32). In
addition, it can act on synaptic plasticity, and improve neural
pathways and neurodegenerative diseases (32-34).
Therefore, the present study used 17β-E2 in the positive
control group. PC12 cells were classified into the normal control,
H2O2 model, 17β-E2 and Epimedin C
(1, 5 and 10 µM) groups. A concentration of 150 µM
H2O2 (25)
(cat. no. 7722-84-1; Sigma-Aldrich; Merck KGaA) was applied for 4 h
at 37˚C and 5% CO2 to induce OS within PC12 cells in the
model and treatment groups. A total of 24 h before
H2O2 treatment, Epimedin C (1, 5 and 10 µM)
was introduced into the TCM groups, whereas 17β-E2 (1
nM) was added into the positive control group for 24 h at 37˚C and
5% CO2. To determine whether the JNK pathway affected
Epimedin C-induced Nrf2 activation, PC12 cells from the model and
treatment groups were also treated with the JNK agonist anisomycin
(5 µM; cat. no. S7409; Selleck Chemicals). Following 4 h of
H2O2 treatment, the culture medium was
removed, and the cells were further incubated with anisomycin for
24 h at 37˚C and 5% CO2.
Cell viability and toxicity
assays
The cultured cells were digested, centrifuge at
1,200 x g for 5 min at 25˚C and counted. Subsequently, cells
(2x104/well) were seeded into a 96-well plate and were
cultured for 12 h under 37˚C, 5% CO2 and 90% humidity
conditions. The cells were then treated with different
concentrations of Epimedin C (0-160 µM) for 24 h at 37˚C and 5%
CO2. Subsequently, cell viability was assessed according
to the instructions of the Cell Counting Kit-8 (cat. no. GK3607;
Gen-View Scientific Inc.) and the optimal drug administration
concentration was screened. In addition, according to the
manufacturer's instructions, a lactate dehydrogenase (LDH)
detection kit (cat. no. C0016; Beyotime Institute of Biotechnology)
was used to assess the cytotoxicity of Epimedin C at different
concentrations (0-160 µM) to evaluate their safety. Six replicates
were set for each sample and the absorbance was measured at 450 nm
for the CCK-8 assay and at 490 nm for the LDH assay using a
microplate reader (BioTek; Agilent Technologies, Inc.).
ROS and malondialdehyde (MDA) level
measurements
The ROS assay kit (cat. no. S0033; Beyotime
Institute of Biotechnology) was adopted to detect cellular ROS
levels. PBS was introduced to dilute DCFH-DA to 10 µmol/l. After
removing the culture medium, the diluted DCFH-DA at 10 µM was added
and the cells were incubated for 20 min at 37˚C. The cells were
then rinsed three times with PBS to thoroughly remove the DCFH-DA
that had not entered the cells. A microplate reader (BioTek;
Agilent Technologies, Inc.) was adopted for testing ROS levels. The
fluorescence was measured at an excitation wavelength of 485 nm and
an emission wavelength of 525 nm.
The lipid peroxidation level in PC12 cells was
evaluated using the MDA assay kit (cat. no. S0131S; Beyotime
Institute of Biotechnology). PC12 cells (1x104/well)
were collected and lysed using RIPA lysis buffer (cat. no. P0013K;
Beyotime Institute of Biotechnology). The cell lysate was then
centrifuged at 12,000 x g for 5 min at 4˚C to remove insoluble
debris. Finally, the absorbance of the resulting supernatant was
measured at 532 nm using a microplate reader. The MDA concentration
in the samples was calculated based on a standard curve prepared
concurrently.
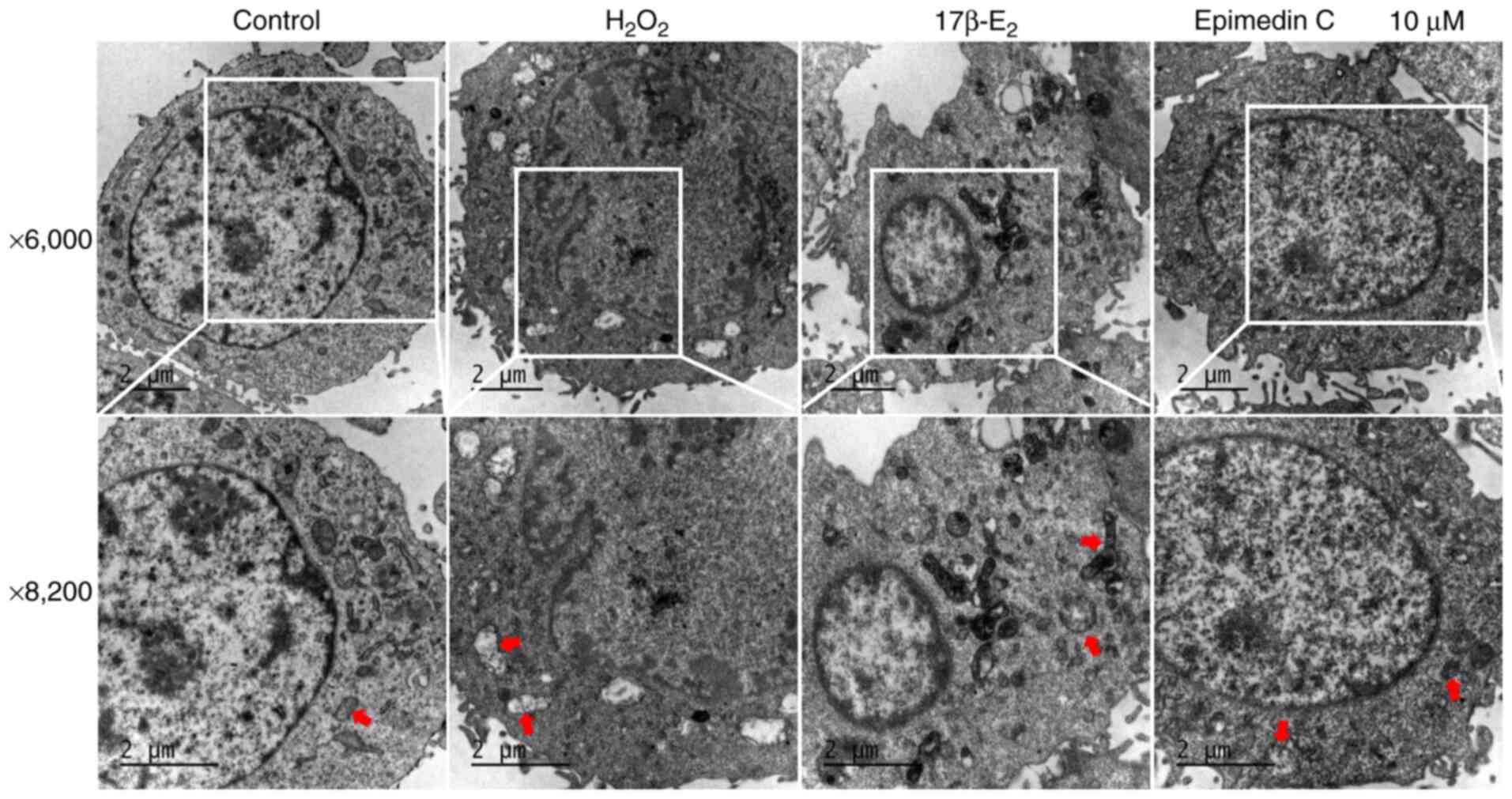
Transmission electron microscopy
(TEM)
The PC12 cells were scraped and digested into a cell
suspension, and were immediately fixed with 2.5% glutaraldehyde in
0.1 M phosphate buffer (pH 7.4) for 24 h at 4˚C. Following primary
fixation, the cells were post-fixed in 1% OsO4 tetroxide
for 2 h at 4˚C, then dehydrated through a graded series of ethanol
(50, 70, 90 and 100%). The dehydrated cells were embedded in epoxy
resin. Ultrathin sections were cut using an ultramicrotome at a
thickness of 70-90 nm and collected on copper grids. The sections
were then stained with 1% uranyl acetate at room temperature for 10
min, and the samples were observed using a Talos L120C transmission
electron microscope (Thermo Fisher Scientific, Inc.) operated at an
accelerating voltage of 80-120 kV. Digital images were acquired for
analysis.
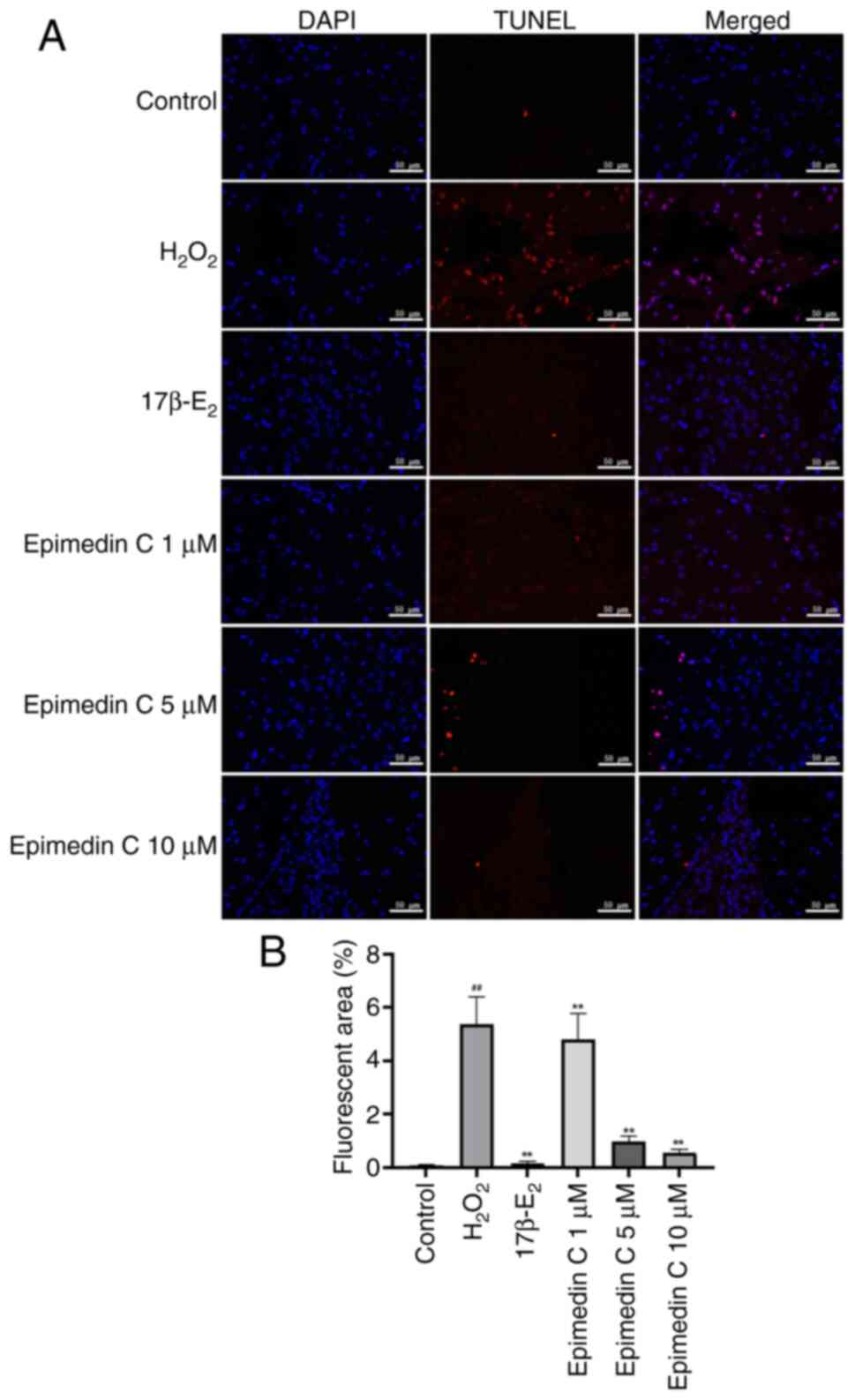
TUNEL analysis
After intervention with different groups of drugs,
PC12 cells were immersed in 4% paraformaldehyde for 30 min at 4˚C,
after which the cells were rinsed three times with PBS and
permeabilized using 0.1% Triton X-100 for 3 min at 25˚C.
Subsequently, the TUNEL reaction solution (cat. no. C1170S;
Beyotime Institute of Biotechnology) was added to the cells and
incubated for 1 h in the dark at 37˚C. The cells were then rinsed
with PBS and later subjected to nuclear counterstaining with DAPI
(cat. no. C1006; Beyotime Institute of Biotechnology) at room
temperature for 5 min. The number of TUNEL-positive cells from five
random regions in three individual samples was quantified using a
fluorescence microscope (Ti-E; Nikon Corporation), and
TUNEL-positive cell rate (%) was calculated as: TUNEL-positive cell
area/total cell area x100.
Flow cytometry
Flow cytometry was applied for the quantitative
analysis of apoptosis and mitochondrial membrane potential (MMP) in
PC12 cells, using the CytExpert software (version 2.5; Beckman
Coulter, Inc.) for analysis. The Annexin V-FITC/PI cell apoptosis
detection kit (cat. no. 556547; BD Biosciences) was utilized for
detecting cell apoptosis. Briefly, cells (2x105/well)
were harvested and resuspended in 400 µl binding buffer.
Thereafter, Annexin V-FITC and PI (5 µl each) were added and mixed,
and the cells were incubated in the dark for 15 min at room
temperature. Afterwards, flow cytometry (CytoFLEX SRT; Beckman
Coulter Inc.) was used to analyze early and late apoptotic cell
proportion.
The level of MMP can reflect the status of
apoptosis. Briefly, the cells (2x105/well) were cultured
in a 6-well plate and rinsed once with PBS, Subsequently, 1 ml JC-1
(cat. no. C2006; Beyotime Institute of Biotechnology) staining
solution was introduced, mixed sufficiently with the media and
incubated at 37˚C in an incubator for 20 min. Afterwards, the
supernatant was discarded, and the sample was rinsed twice with
JC-1 staining buffer (1X). An Altra flow cytometer (CytoFLEX SRT;
Beckman Coulter Inc.) was used to verify the changes in MMP in each
group of cells. The JC-1 aggregates (healthy mitochondria) were
detected in the PE channel (575 nm emission), while the JC-1
monomers (depolarized mitochondria) were detected in the FITC
channel (530 nm emission). The ratio of the geometric mean
fluorescence intensity (PE/FITC) was calculated to quantify the
MMP.
Western blotting (WB)
After treatment, PC12 cells were subjected to lysis
with RIPA buffer that contained 1% PMSF (cat. no. ST506; Beyotime
Institute of Biotechnology). The BCA protein detection kit (cat.
no. P0011; Beyotime Institute of Biotechnology) was used to measure
protein concentration. Subsequently, PBS and 4X loading buffer were
added according to protein concentration, mixed evenly and boiled
at 95˚C for 10 min. Proteins (20 µg/lane) were separated by
SDS-PAGE on 10% gels and were then transferred to polyvinylidene
fluoride membranes (cat. no. ISEQ00010; Merck KGaA). After blocking
for 1 h at room temperature in 5% BSA (Beyotime Institute of
Biotechnology), the membranes were subjected to primary antibody
incubation overnight at 4˚C using antibodies against: GAPDH
(1:1,000; cat. no. 5174; CST Biological Reagents Co., Ltd.), JNK
(1:1,000; cat. no. 9252; CST Biological Reagents Co., Ltd.),
phosphorylated (p)-JNK (1:1,000; cat. no. 4668; CST Biological
Reagents Co., Ltd.), HO-1 (1:1,000; cat. no. 43966; CST Biological
Reagents Co., Ltd.), Bcl-2 (1:1,000; cat. no. 2870; CST Biological
Reagents Co., Ltd.), Bax (1:1,000; cat. no. 14796; CST Biological
Reagents Co., Ltd) and Nrf2 (1:500; cat. no. ab137550; Abcam). The
next day, the membranes were washed six times with TBS-0.01% Tween
and were incubated with a HRP-conjugated Goat Anti-Rabbit IgG (H+L)
secondary antibody (1:10,000; cat. no. 33101ES60; Shanghai Yeasen
Biotechnology Co., Ltd) at room temperature for 1 h. After
incubation, the membranes were washed six times with TBST.
Subsequently, the membranes were visualized using Omni-ECL
(Epizyme; Ipsen Pharma). The gel imaging system (Chemiscope6300;
Clinx Science Instruments Co., Ltd.) was used for imaging and
semi-quantification was performed using ImageJ (version 1.6.0;
National Institutes of Health).
Statistical analysis
Data are presented as the mean ± SD. Unpaired
Student's t-tests were used for pairwise comparisons between two
groups. For comparisons across more than two groups, the
homogeneity of variances was first assessed using Levene's test.
Following a significant Levene's test (P<0.05) which indicated
heterogeneity of variances, a Welch's ANOVA was conducted in place
of the standard one-way ANOVA. Post hoc comparisons against the
control or H2O2 group were then performed
using Dunnett's T3 test to account for the unequal variances. In
cases where Levene's test was not significant (P>0.05),
indicating homogenous variances, a standard one-way ANOVA was
performed, followed by Bonferroni's post hoc test for all
comparisons against the control or H2O2
group. GraphPad Prism version 9.3 (Dotmatics) was employed for data
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Identification of active ingredients
and potential targets of Epimedium
Comparing the active ingredients in TGD that can
enter the bloodstream, as identified by UHPLC-Q-Exactive Orbitrap
HRMS in our previous study (11),
a total of 20 active ingredients in Epimedium that could
enter the rat bloodstream and exert their effects were identified,
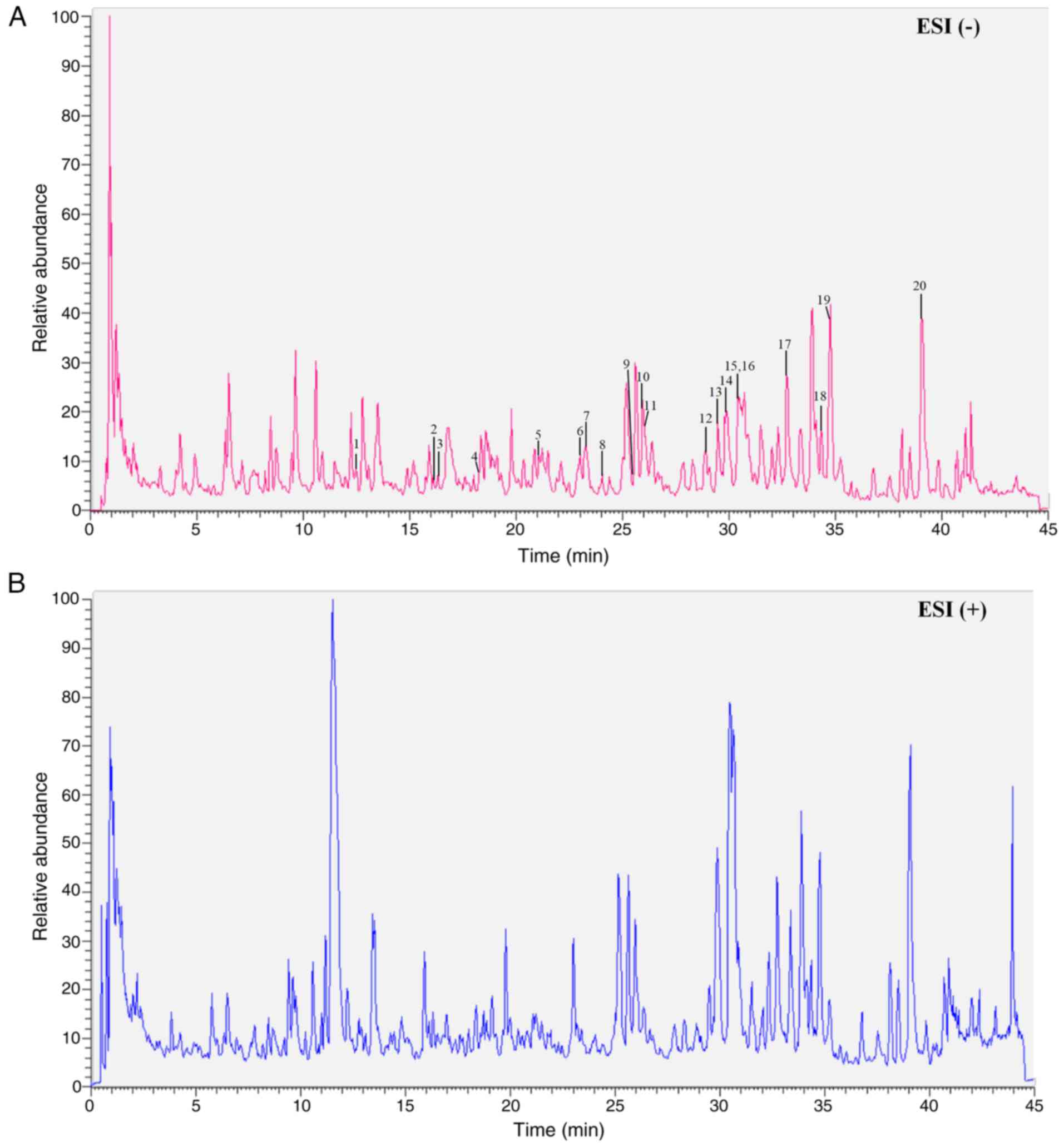
including Epimedin C, Epimidin B and others (Table I). The total ion chromatograms of
active ingredients within Epimedium compound are displayed
in Fig. 1.
 | Table IIdentification of active ingredients
in Epimedium that can enter the bloodstream. |
Table I
Identification of active ingredients
in Epimedium that can enter the bloodstream.
| Number | RT, min | Precursor ion | Measured mass,
Da | Calculated mass,
Da | Error, ppm | Formula | Identification |
|---|
| 1 | 12.24 | (M-H)- | 337.09348 | 337.09179 | 1.793 |
C16H18O8 |
3-O-p-coumaroylquinic acid |
| 2 | 16.09 | (M-H)- | 479.08377 | 479.08201 | 0.987 |
C21H20O13 | Isoyangmei bark
glycoside |
| 3 | 16.45 | (M-H)- | 447.09393 | 447.09218 | 1.235 |
C21H20O11 | Trifolin |
| 4 | 18.38 | (M-H)- | 464.09064 | 463.08710 | 6.321 |
C21H20O12 | Hyperin |
| 5 | 21.34 | (M-H)- | 576.17704 | 577.15518 | 0.163 |
C27H30O14 | Kaempferitrin |
| 6 | 22.94 | (M-H)- | 465.23335 | 465.21190 | -1.173 |
C24H34O9 |
(4-(β-D-glucopyranosyloxy)-2,6-bis(3-methyl-2-buten-1-yl)phenyl)(hydroxy)acetic
acid |
| 7 | 23.39 | (M-H)- | 677.2199 | 677.20761 | 2.394 |
C32H38O16 | Hexandraside E |
| 8 | 24.03 | (M-H)- | 678.21332 | 678.20761 | 0.032 |
C32H38O16 | Epimedium B |
| 9 | 25.6 | (M-H)- | 661.21472 | 661.21269 | 1.960 |
C32H38O15 | Epimedium A |
| 10 | 25.95 | (M-H)- | 807.27338 | 807.27060 | 0.166 |
C38H48O19 | Epimidin B |
| 11 | 26.06 | (M-H)- | 691.22565 | 691.22326 | 2.223 |
C33H40O16 |
Anhydroicaritin-3,7-di-O-glucoside |
| 12 | 28.95 | (M-H)- | 515.15625 | 515.15478 | 0.237 |
C26H28O11 | Epimedin C |
| 13 | 29.55 | (M-H)- | 675.23077 | 675.22834 | 2.342 |
C33H40O15 | Baohuoside VII |
| 14 | 29.94 | (M-H)- | 645.22003 | 645.21778 | 2.636 |
C32H38O14 | Sagittatoside
B |
| 15 | 30.47 | (M-H)- | 529.17163 | 529.17043 | 0.287 |
C27H30O11 | Baohuoside C |
| 16 | 30.47 | (M-H)- | 721.23596 | 721.23382 | 3.895 |
C34H42O17 | Icariin |
| 17 | 32.88 | (M-H)- | 529.17194 | 529.17043 | 0.060 |
C27H30O11 | Icariin I |
| 18 | 34.36 | (M-H)- | 631.20447 | 631.20213 | 0.132 |
C31H36O14 | Icariin F |
| 19 | 34.71 | (M-H)- | 499.16156 | 499.15987 | 0.454 |
C26H28O10 | Icariside |
| 20 | 39.09 | (M-H)- | 513.18706 | 513.17552 | -0.338 |
C27H30O10 | Baohuoside I |
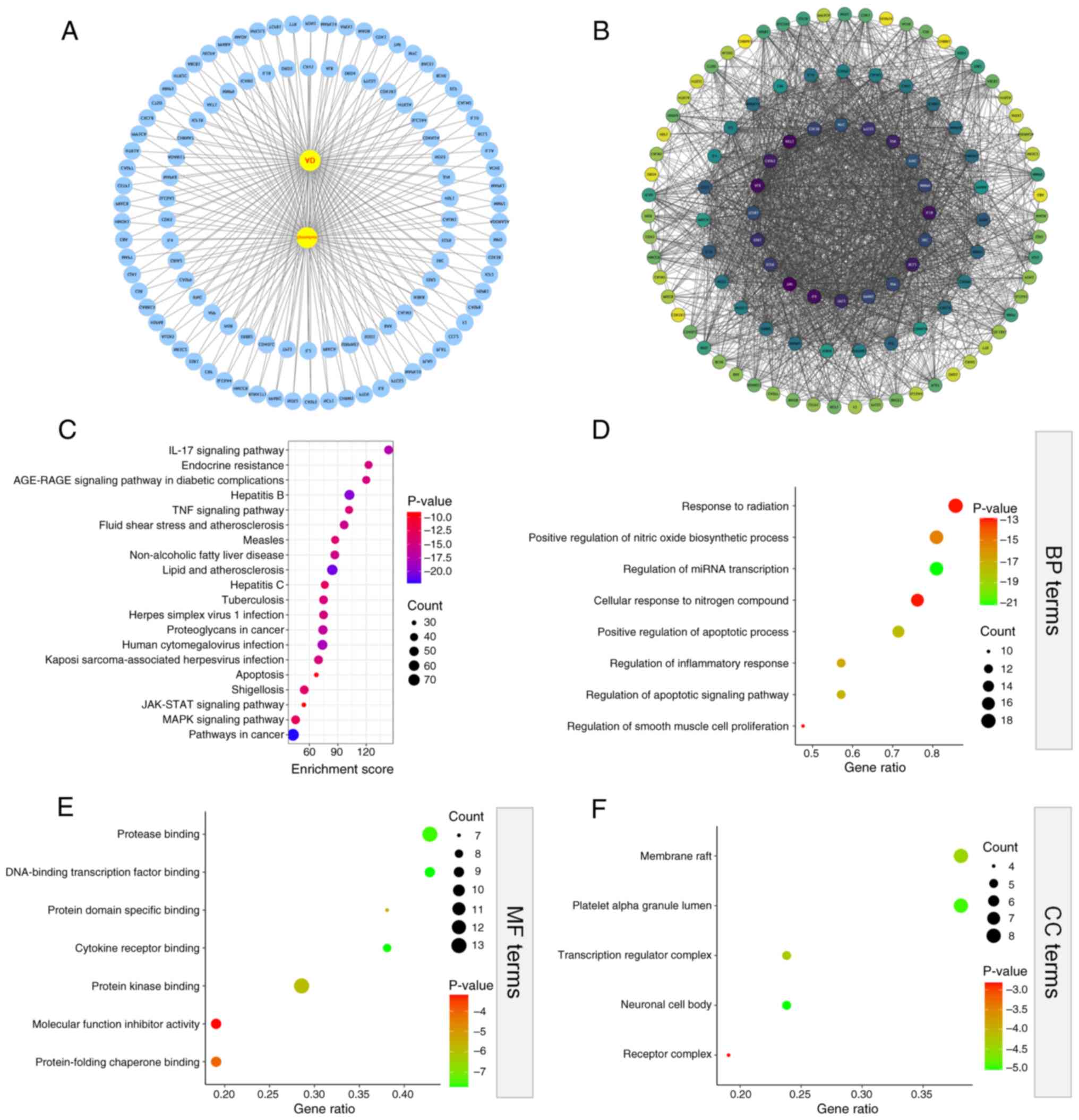
To compensate for the lack of recent updates in drug
databases, which may result in incomplete inclusion of components,
network pharmacological analysis (using AD as an example) was
conducted in conjunction with MS. Using ‘Epimedium’ as the
key word, and oral bioavailability ≥30% and drug similarity ≥0.18
as the screening criteria in TCMSP, SwissTarget, PharmMapper and
HERB databases, a total of 23 components of Epimedium were
obtained, and 379 drug targets were retrieved. Using ‘Alzheimer's
disease’ as the key word, a total of 1,165 drug targets were
obtained from the GeneCards, DisGeNet and OMIM databases after
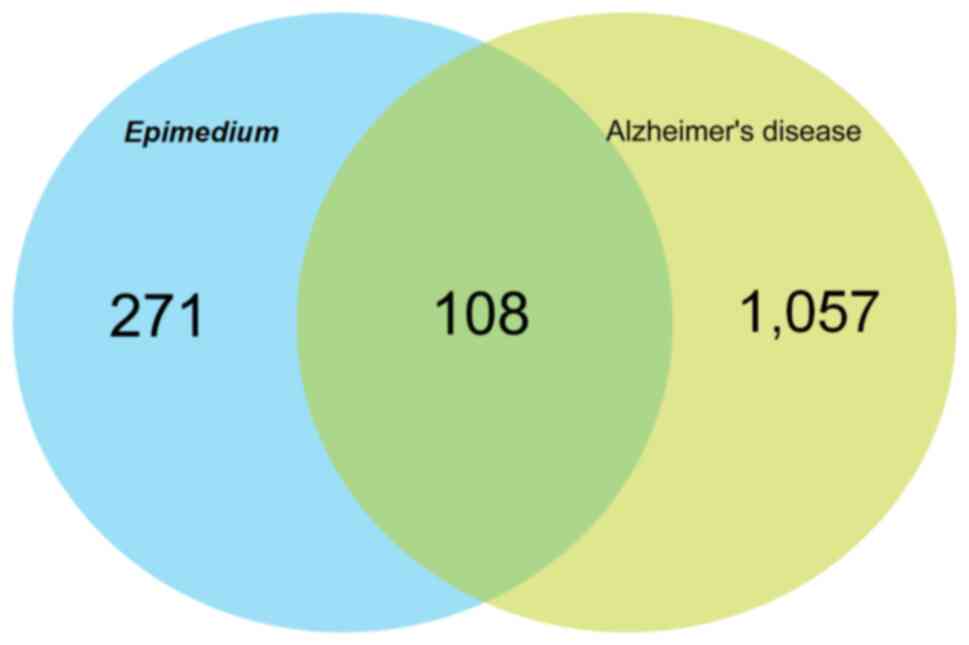
deduplication. Based on the Venn diagram, 108 common targets of
Epimedium and AD were identified (Fig. 2).
Preventive effect of Epimedin C on
H2O2-induced oxidative damage in PC12
cells
H2O2 stimulation can cause OS
damage, leading to apoptosis or necrosis in PC12 cells (35,36).
Except for the control group, all the other groups of PC12 cells
were exposed to 150 µM H2O2 treatment for 4 h
for modeling OS (25). Before
treatment with H2O2, these cells were
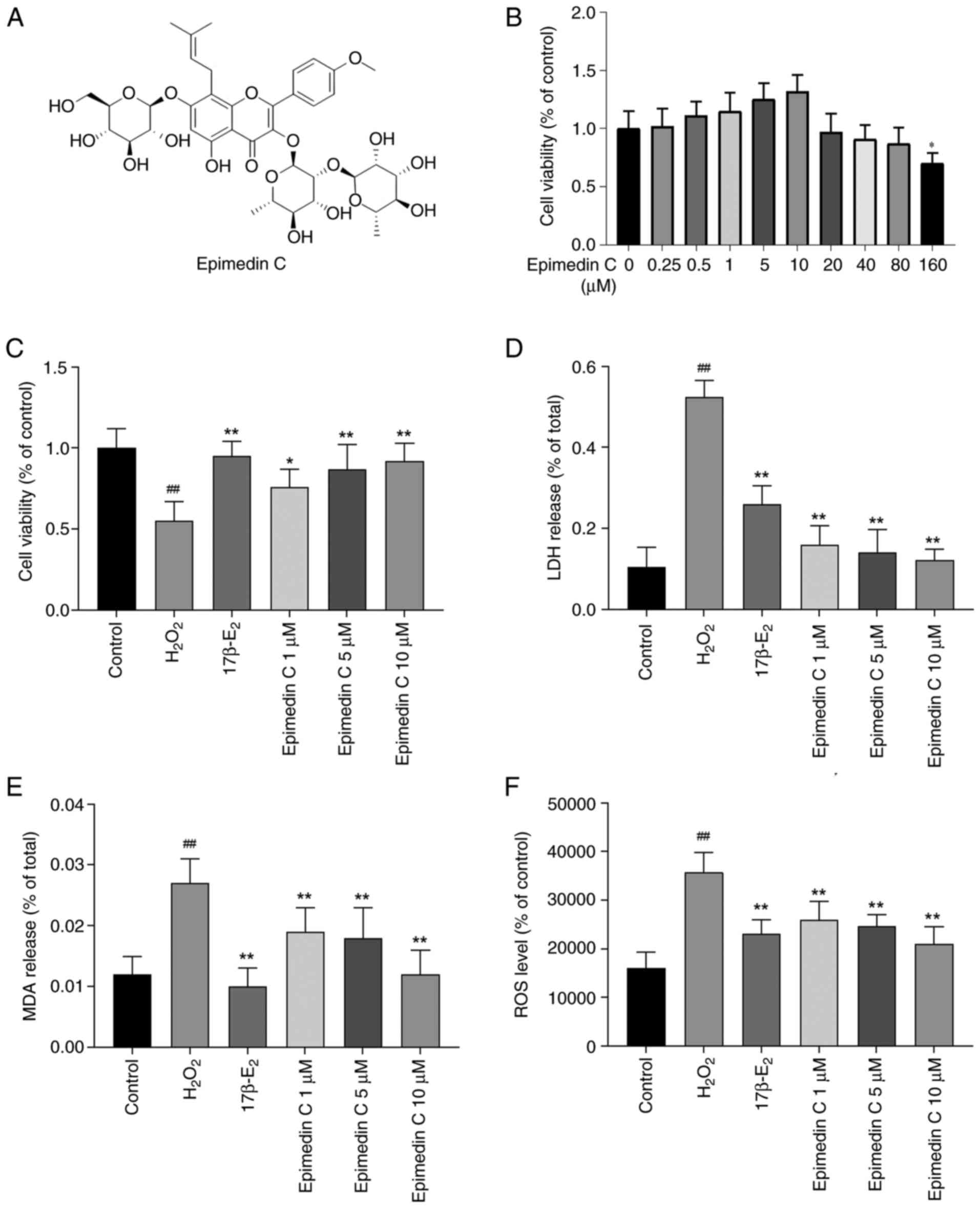
pretreated with 0-160 µM Epimedin C (Fig. 3A) for 24 h to evaluate the
protective effects of Epimedin C on PC12 cells and to identify its
optimal concentration range for improving OS status (Fig. 3B). The results showed that 0-80 µM
Epimedin C pretreatment in PC12 cells did not have an effect on the
viability of PC12 cells compared with the control. Among these
concentrations, Epimedin C at concentrations of 1, 5 and 10 µM had
an improved cell survival rate than other concentrations; however,
this was not statistically significant. Therefore, the
concentrations of 1, 5 and 10 µM were used for subsequent
experiments. Relative to the OS model group, after 24 h of
intervention with 17β-E2 and Epimedin C, the viability of PC12
cells was significantly improved (Fig.
3C). Among them, the cell survival rate significantly rose in a
dose-dependent manner in response to pretreatment with 1, 5 and 10
µM Epimedin C. LDH analysis was applied to detect the toxicity of
different treatments on PC12 cells in each group; according to the
results, relative to the control group, the
H2O2-induced group showed a significant
increase in LDH release (Fig. 3D).
Alternatively, the cells pretreated with Epimedin C and
17β-E2 displayed a significant decrease in LDH secretion
and cytotoxicity compared with those in the
H2O2-induced group. These comprehensive
results indicated that intervention with 10 µM Epimedin C had the
best therapeutic efficacy in PC12 cell survival and the least toxic
side effects.
MDA and ROS levels
MDA and ROS are involved in producing OS free
radicals and are key indicators for measuring oxidative and
antioxidant capacity (37,38). The results of the present study
revealed that relative to the control group, MDA and ROS contents
in PC12 cells induced by H2O2 were
significantly increased (Fig. 3E
and F). In cells that had been
pretreated with 17β-E2 or 1, 5 and 10 µM Epimedin C, the MDA and
ROS contents were reduced to varying degrees compared with those in
the H2O2 group, indicating that Epimedin C is
beneficial for alleviating the OS status of PC12 cells. Notably, 10
µM Epimedin C was the most effective concentration.
Epimedin C can inhibit the
H2O2-mediated apoptosis of PC12 cells
OS can induce cell apoptosis associated with
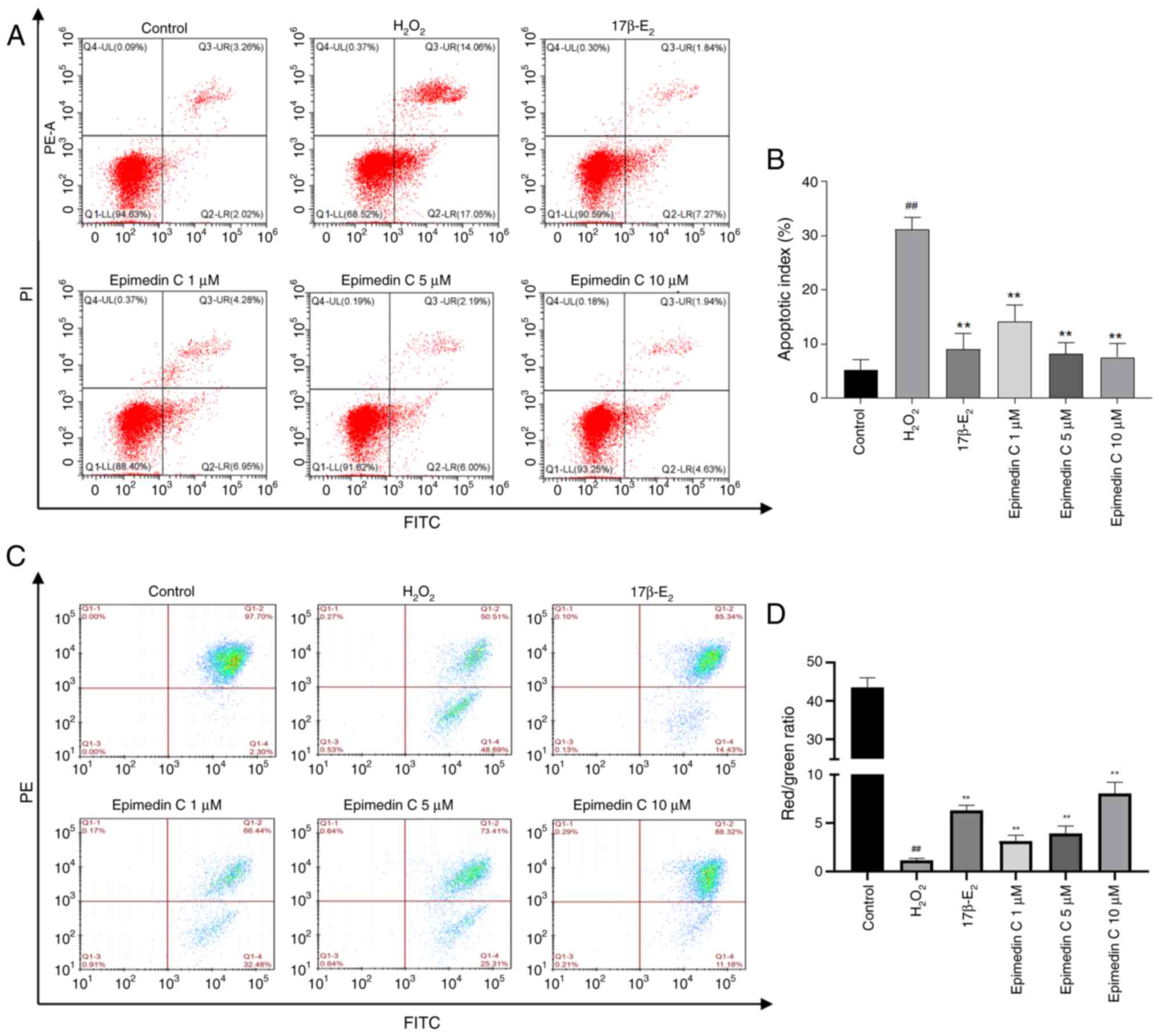
increased ROS production and decreased MMP levels (39). Therefore, the cells were stained
with Annexin V-FITC and PI, and the apoptosis rate was measured
using flow cytometry (Fig. 4A and
B). The present results showed
that, after treatment with 150 µM H2O2 for 4
h, the PC12 cell apoptosis rate was significantly increased. By
contrast, intervention with Epimedin C resulted in a decline in
apoptosis rate compared with that in the H2O2
group, suggesting that Epimedin C can inhibit cell apoptosis.
A reduction in MMP represents a hallmark event
during early cell apoptosis, which is evident by the transition of
JC-1 from red fluorescence to green fluorescence (40). To further evaluate the apoptosis
status of each group of cells, the MMP loss within
H2O2-treated PC12 cells was observed through
JC-1 staining. As shown in Fig. 4C
and D, the MMP of
H2O2-treated PC12 cells was decreased,
whereas pretreatment with Epimedin C (1, 5 and 10 µM) resulted in a
dose-dependent improvement in MMP, with varying degrees of
upregulation.
TUNEL staining can be conducted to detect apoptotic
cells with large amounts of DNA degradation during the late stage
of apoptosis (41). Therefore,
TUNEL staining was performed on each group of cells in the current
study (Fig. 5A). The results of
the TUNEL assay revealed that relative to the control group,
following H2O2 induction modeling, the
apoptosis of PC12 cells in the model group (level of red
fluorescence) was significantly increased (Fig. 5B). Relative to the model group,
after drug intervention, the apoptosis rates of PC12 cells in the
17β-E2 and Epimedin C groups were significantly
improved. The degree of apoptosis was ameliorated to varying
degrees in response to different concentrations of Epimedin C, with
the largest improvement observed in the 10 µM Epimedin C group.
Ultrastructural changes of PC12
cells
Within the present study ultrastructural changes of
PC12 cells in each group was observed using TEM (Fig. 6). Under physiological conditions,
the mitochondria are present with prominent cristae and intact
membranes (42). The control group
cells had clear nuclear membranes and uniform chromatin. After
H2O2 induction, marked mitochondrial damage
was observed in PC12 cells, evident as mitochondrial membrane
rupture and chromatin condensation, accompanied by vacuolization.
As aforementioned, 10 µM Epimedin C had the largest effect on
improving H2O2-mediated OS damage to PC12
cells. Accordingly, TEM was performed on PC12 cells treated with 10
µM Epimedin C to detect ultrastructural changes. The results
indicated that treatment with 10 µM Epimedin C could inhibit
mitochondrial damage and alleviate mitochondrial swelling in PC12
cells, indicating that 10 µM Epimedin C can improve oxidative
damage and inhibit cell apoptosis.
Roles of Epimedin C in activating the
JNK/Nrf2/HO-1 pathway within H2O2-mediated
PC12 cells
The ‘drug-disease-target’ network was visualized
with Cytoscape 3.8.2 software, and common targets between drugs
(Epimedium) and diseases (AD) were imported in STRING
database for PPI analysis. The PPI results included 110 nodes and
2,102 edges (Fig. 7A and B). Thereafter, the top 20 core genes of
Epimedium for treating AD were screened using R language (R
4.1.2), including BCL2, APP, JUN, CASP3, ESR1, IL1B and TNF. The
top 20 core targets underwent GO analysis in Metascape database. As
a result, the shared targets were involved in various GO biological
processes (Fig. 7D), including
‘positive regulation of apoptotic process’, ‘regulation of
apoptotic signaling pathway’, ‘regulation of inflammatory response’
and ‘cellular response to nitrogen compound’. Moreover, the GO
molecular functional results (Fig.
7E) showed that the shared targets involved in ‘DNA-binding
transcription factor binding’, ‘cytokine receptor binding’,
‘protease binding’ and ‘protein kinase binding’. While GO cellular
component results (Fig. 7F)
indicated that the shared targets were closely related to ‘platelet
alpha granule lumen’, ‘neuronal cell body’, ‘transcription
regulator complex’ and ‘receptor complex’. Additionally, as
revealed by KEGG enrichment analysis, the shared targets were
enriched in pathways such as ‘MAPK signaling pathway’, ‘endocrine
resistance’, cell clearance and ‘apoptosis’ (Fig. 7C).
JNK belongs to the MAPK family and has a crucial
effect on cell proliferation, differentiation, apoptosis, immune
response and embryonic development (43). From the network pharmacological
analysis, KEGG enrichment analysis showed that the pathway through
which Epimedium improves AD was significantly enriched with
the ‘MAPK signaling pathway’. In addition, the PPI network revealed
that the top 20 core targets included APP, JUN and BCL2. Notably,
JUN (also known as C-JUN) is involved in the JNK pathway (44), and activated JUN promotes the
expression of various pro-apoptotic proteins (45). The Nrf2 pathway is an important
regulatory factor for cellular antioxidant response (46), which is involved in triggering the
expression of antioxidant and cell protective genes, and enhancing
cellular antioxidant capacity (47); therefore, activation of Nrf2 can
reduce neuronal damage caused by OS and inflammation (48). The free Nrf2 then transfers to the
nucleus and binds to antioxidant-related elements, leading to the
expression of various antioxidant and detoxifying genes, such as
HO-1(49). Research has shown that
MAPKs are closely interrelated with Nrf2 nuclear translocation in
eliminating OS (50).
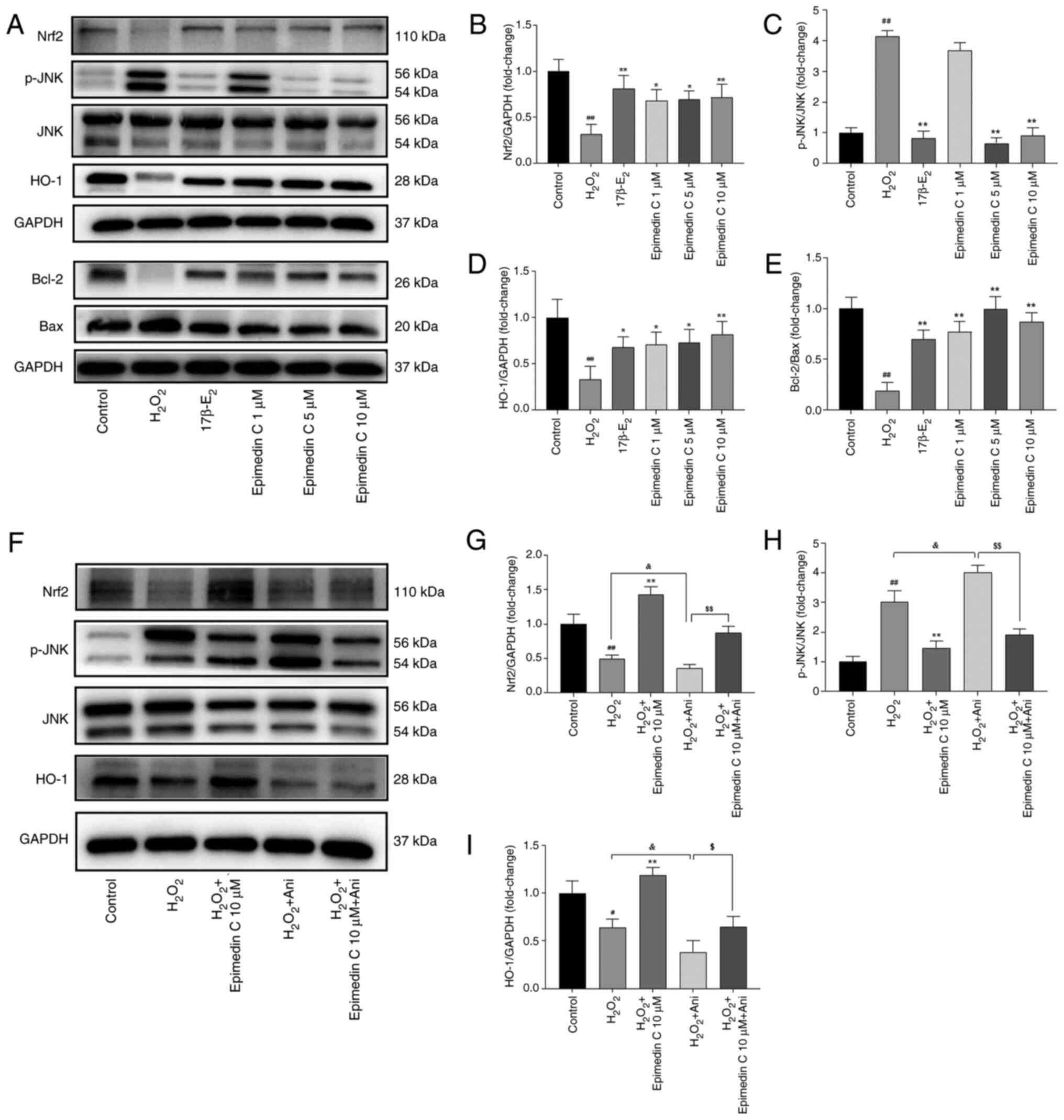
Therefore, to study the neuroprotective role
mediated by Epimedin C, WB was conducted for verifying p-JNK, Nrf2
and HO-1 protein levels. Relative to the control group,
H2O2 increased p-JNK levels but decreased
Nrf2 levels, whereas Epimedin C downregulated p-JNK expression, and
upregulated Nrf2 and HO-1 levels induced by
H2O2 (Fig.
8A-D). Furthermore, it was observed that compared with in the
H2O2 model group, Epimedin C decreased Bax
expression while increasing Bcl-2 expression (Fig. 8A and E).
To confirm that Epimedin C mediated protection via
the JNK pathway, the present study used a JNK agonist (anisomycin)
for supplementary validation. The results revealed that p-JNK was
activated and significantly upregulated in PC12 cells co-cultured
with JNK agonist (Fig. 8F and
H). By contrast, after treatment
with Epimedin C and anisomycin, p-JNK expression decreased compared
with following treatment with anisomycin alone, whereas Nrf2 and
HO-1 levels were significantly increased (Fig. 8F-I).
Discussion
With the aging population, neurodegenerative
diseases are receiving increasing attention from the fields of
science and medicine (51).
According to statistics, in 2016, 5.4 million Americans suffered
from AD (52), and based on
further data published in 2021, it is estimated that >12 million
Americans may develop neurodegenerative diseases over the next 30
years (52). Neurodegenerative
diseases are the main chronic progressive diseases that affect
individual physical health (53),
which exhibit the typical features of gradual selective neuronal
system loss (54). OS can damage
the blood-brain barrier (BBB), and as a result, neurotoxic
substances can enter the brain and ultimately result in the
accumulation of ROS (55).
Upregulation of ROS levels has been confirmed to be a common major
feature within the brains of patients with neurodegenerative
disease (56), with excessive
production of ROS causing ATP loss, decreased MMP and increased
apoptosis (57). TCM has been used
for treating neurodegenerative diseases for thousands of years
(58), and according to a number
of modern pharmacological studies, prescriptions, herbs, bioactive
ingredients and monomeric compounds of TCM are effective at
treating neurodegenerative diseases (59-61).
Epimedium is a TCM that is mainly applied in
treating Parkinson's disease and AD (62). Research has confirmed that
Epimedium has a protective effect on neurodegenerative
diseases such as AD (19), and the
active ingredients of Epimedium are flavonoids, which
regulate various biological effects in vitro and in
vivo, such as angiogenesis, antioxidant damage and inhibition
of apoptosis (22,63). According to relevant research,
icariin possesses antioxidant and immunomodulatory effects, and can
regulate neuroendocrine function (64). It has been determined that 100 g/l
Epimedin C is found to yield ~34.24 g/l icariin within 8 h
(65). Compared with icariin,
Epimedin C has a lower cost and comparable efficacy (21,65),
and is an effective antioxidant. At present, to the best of our
knowledge, no studies have yet been performed on Epimedin C and its
specific mechanism in preventing and/or treating neurodegenerative
diseases, including AD.
There is evidence to suggest that in AD, a vicious
cycle revolves around the production of Aβ, Aβ aggregation, plaque
formation, microglia/immune response, inflammation and ROS
production. In this cycle, ROS serves a central role and
H2O2 is considered an important second
messenger of ROS (66). The
excessive production of H2O2 can lead to OS
and inflammation in the brain of patients with AD (67); therefore, it is considered that
H2O2 can simulate the disease manifestations
of AD-related oxidative damage, and some studies have confirmed
this (68,69). The present study confirmed that
Epimedin C intervention enhanced cell viability and survival rate,
improved OS damage caused by H2O2 exposure
and reduced the H2O2-mediated LDH leakage in
PC12 cells. In the body, oxygen free radicals can be generated via
both enzymatic and non-enzymatic systems, and the antioxidant
defense system balances their levels; notably, if the biochemical
balance between oxidants and antioxidants is disrupted, OS will
occur (70). The increase in ROS
content can disrupt the basic structures of proteins, nucleic acids
and lipids, resulting in structural damage and functional changes,
ultimately resulting in cell apoptosis (71). MDA is the final product of the
oxidative decomposition of lipid peroxides, and its content can be
measured to identify the degree of lipid peroxidation on the cell
membrane (72). Therefore, the
activities of superoxide dismutase and MDA are considered to be
indicative of the degree of OS. The experimental results of the
present study indicated that Epimedin C effectively reduced ROS
accumulation mediated by H2O2 within PC12
cells and lowered the levels of MDA after
H2O2 treatment, demonstrating its ability to
resist OS. In addition, flow cytometry, TUNEL staining and MMP
results all showed that PC12 cells underwent significant apoptosis
after H2O2 induction, and Epimedin C
intervention had an inhibitory effect on cell apoptosis, with the
best effect observed in respones to 10 µM. Under TEM, the PC12 cell
ultrastructure also confirmed this: The mitochondrial membrane of
PC12 cells was ruptured and chromatin was condensed, showing
vacuolization and apoptosis after H2O2
intervention. However, mitochondrial damage was notably reduced
after intervention with 10 µM Epimedin C. Therefore, it was
hypothesized that Epimedin C has potential therapeutic effects on
preventing and improving neurodegenerative diseases.
To seek the key targets and potential mechanisms of
Epimedin C in improving neurodegenerative diseases, the present
study first identified the active ingredients of Epimedium
through UHPLC-Q-Exactive Orbitrap HRMS analysis and conducted
network pharmacological analysis using AD as an example. A total of
108 shared targets were screened, and the top 20 core targets
included APP, JUN and BCL2. From GO and KEGG analyses, the
potential mechanism of Epimedium in improving AD was
significantly enriched in ‘neuronal cell body’, ‘regulation of
apoptotic signaling pathway’ and ‘MAPK signaling pathway’. MAPKs
are conserved signaling proteins responsible for regulating
numerous eukaryotic processes (73), and members of the MAPK family,
including ERK1/2, JNK/SAPK, p38 and ERK5. are known to participate
in neuron growth, differentiation and survival (74). JNK has an important effect on
different diseases such as AD and Parkinson's disease caused by
inflammation and OS (75), and
research has confirmed that JNK pathway activation can exacerbate
OS, leading to neurotoxic effects (76). Nrf2 is considered the main
regulator of redox balance, which has a crucial role in cellular
defense against OS (77,78). Research has shown that Nrf2/HO-1
expression is directly influenced by the JNK pathway, and
activation of Nrf2/HO-1 signaling is crucial for reducing oxidative
damage (79). Notably, our
previous studies (80,81) have focused on validating the
mechanism of action of the JNK pathway in response to TGD,
particularly linking the JNK pathway to
neuroprotection/neurodegeneration and OS response. One of these
previous studies (25) confirmed
that TGD can provide neuroprotective effects on
H2O2-mediated oxidative damage and apoptosis
of PC12 cells through the Nrf2 and JNK pathways. Epimedium
is the main drug component of TGD, and the aforementioned results
offer a biological background and scientific foundation for the
present study. Therefore, the present study focused on the JNK
pathway to verify whether Epimedin C exerted neuroprotective
effects through this pathway.
TGD has also been validated to improve premature
menopause-associated cognitive dysfunction by increasing estrogen
levels (11). In addition, other
in vitro and in vivo studies have supported the
neuroprotective effects of estrogen and its effects on
neurotransmitter systems related to cognition (25,80-82).
When treating neurological diseases, the efficacy of oral TCM is
determined by the ability of the components to reach target organs
via the BBB (80). It is important
to comprehensively understand serum pharmacokinetics of oral TCM to
elucidate the absorption, distribution, metabolism and excretion of
such active ingredients (83).
According to research, active ingredients in TCM, such as
Epimedium, can potentially improve BBB function, elevate
cerebral blood flow and avoid cognitive impairment through
suppressing the activation of astrocytes and microglia, protecting
the myelin function of oligodendrocytes and reducing neuronal
apoptosis (84). Furthermore,
there is evidence suggesting that icariin exerts neuroprotection
through crossing the BBB and regulating pathways (85). In our previous study,
UHPLC-Q-Exactive Orbitrap HRMS was used to identify the components
absorbed by mouse brain tissue after oral administration of TGD, in
order to clarify the specific components that exert their effects
on the brain. The results showed that icariin C could penetrate the
BBB and exert therapeutic effects on the brain (11). A previous study reported that
Epimedin C can be converted into icariin in vivo and
gradually hydrolyzed into metabolic small molecules such as icariin
C to exert its effects (86). Wong
et al (87) explored the
pharmacokinetics of isoprenoid flavonoids after ingestion of
standardized Epimedium extract and assessed their
relationship with serum estrogenic kinetics. The results of this
previous study revealed the potential estrogenic effects of
Epimedium extract and suggested that isoprenoid flavonoids
might be used to treat menopause and other diseases requiring
estrogenic effects. Epimedin C has been confirmed to be a mature
plant extract with estrogen-like activity (88). Estradiol has been proven to have
neuroprotective effects on excitatory neurotoxicity, OS and
apoptosis (89-91).
Therefore, similar to previous studies, the present study used
17β-E2 as a positive control drug and compared its
efficacy with Epimedin C.
In the present study, WB results showed that
relative to the control group, the JNK pathway was significantly
activated following H2O2 exposure, and Nrf2
and HO-1 levels were significantly reduced. However, after Epimedin
C intervention, p-JNK expression evidently decreased, whereas Nrf2
and HO-1 levels increased. At the same time, Epimedin C decreased
the levels of Bax and upregulated Bcl-2 levels, indicating that
Epimedin C may alleviate OS damage and inhibit the apoptosis of
PC12 cells through suppressing JNK pathway phosphorylation and
activating Nrf2/HO-1. To further confirm this, the JNK agonist
anisomycin was employed in the present study to further activate
the JNK pathway and observe the improvement results after Epimedin
C intervention. The results signified that compared with in the
control and H2O2 groups, JNK was activated
and markedly upregulated in PC12 cells co-cultured with JNK agonist
(H2O2 + anisomycin group). After treatment
with Epimedin C (H2O2 + anisomycin + Epimedin
C group), p-JNK expression was decreased, and Nrf2 and HO-1 levels
were increased, which further confirmed the present hypothesis.
Notably, according to the network pharmacological analysis
performed in the current study, Epimedin C has the characteristics
of targeting multiple molecules and complex signal transduction. In
this exploration, only the inhibitory effect of Epimedin C on JNK
pathway phosphorylation was investigated, which may activate
Nrf2/HO-1 and suppress the OS-induced apoptosis of PC12 cells.
However, whether Epimedin C has other preventive mechanisms against
neurodegenerative diseases still needs to be explored. According to
a previous cell experiment, the medicinal components of Epimedin C
can be effectively utilized and also converted into icariin
(21). Notably, the difference in
solubility between Epimedin C and icariin results in a higher
Epimedin C initial concentration (9.72 mg/l) than icariin at the
same dose (7.86 mg/l), and the residual concentration of Epimedin C
in cells is also higher than that of icariin (21). At present, there is still a lack of
research on the absorption of Epimedin C in the human body after
ingestion of Epimedium. Therefore, it is crucial to optimize
and explore the optimal drug concentration in cells, and drug
absorption into target cells is a prerequisite for the subsequent
therapeutic effects (92).
According to the results of the present study, Epimedin C at 160 µM
exerted neurotoxicity: Considering that Epimedium contains
~5 µM/g Epimedin C (93), it is
speculated the human dosage of Epimedium should not exceed
32 g. A limitation of the present study is that only in
vitro PC12 cell experiments were conducted. Due to the
complexity and heterogeneity of human pathology, translating these
findings into human therapies requires careful and robust
validation through in-depth molecular research and comprehensive
clinical trials (91). Future
research will further conduct relevant clinical trials, focusing on
the pharmacological mechanisms of Epimedin C in preventing
neurodegenerative diseases in vivo and in vitro,
aiming to offer a reliable scientific foundation for applying
Epimedin C to exert neuroprotective effects and prevent
neurodegenerative diseases.
In conclusion the present study revealed the
neuroprotective mechanism of Epimedin C. To the best of our
knowledge, the current findings are the first to indicate that
Epimedin C can mediate the JNK/Nrf2/HO-1 pathway, providing
protection from H2O2-mediated OS and
apoptosis in PC12 cells. These findings suggest that Epimedin C may
become a candidate drug for preventing neurodegenerative diseases.
In the future, further exploration of the potential preventive
mechanisms of Epimedin C in neurodegenerative diseases is needed to
provide more reliable and direct scientific evidence.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Natural Science
Foundation of China (grant nos. 82174427 and 82305290).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CC and XLL were involved in writing the original
draft, and reviewing and editing the manuscript. CC was also
responsible for data curation, software application and data
analysis. XLL conceptualized and supervised the project. CC, XLL,
and GYL performed the experiments. GYL was responsible for data
analysis and software application. LWX was responsible for
conceptualization, project administration and funding acquisition.
GYL and LWX ultimately reviewed and edited the manuscript. CC, XLL,
GYL and LWX confirm the authenticity of all the raw data, and read
and approved the final manuscript. All authors agree to be
accountable for all aspects of work ensuring integrity and
accuracy.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li R, Robinson M, Ding X, Geetha T,
Al-Nakkash L, Broderick TL and Babu JR: Genistein: A focus on
several neurodegenerative diseases. J Food Biochem.
46(e14155)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bell SM, Burgess T, Lee J, Blackburn DJ,
Allen SP and Mortiboys H: Peripheral glycolysis in
neurodegenerative diseases. Int J Mol Sci. 21(8924)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kovacs GG: Molecular pathology of
neurodegenerative diseases: Principles and practice. J Clin Pathol.
72:725–735. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Varela L and Garcia-Rendueles MER:
Oncogenic pathways in neurodegenerative diseases. Int J Mol Sci.
23(3223)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Orfali R, Alwatban AZ, Orfali RS, Lau L,
Chea N, Alotaibi AM, Nam YW and Zhang M: Oxidative stress and ion
channels in neurodegenerative diseases. Front Physiol.
15(1320086)2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chang KH, Cheng ML, Chiang MC and Chen CM:
Lipophilic antioxidants in neurodegenerative diseases. Clin Chim
Acta. 485:79–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li Y, Li L and Hölscher C: Therapeutic
potential of genipin in central neurodegenerative diseases. CNS
Drugs. 30:889–897. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen Q, Chen G and Wang Q: Application of
network pharmacology in the treatment of neurodegenerative diseases
with traditional Chinese medicine. Planta Med. 91:226–237.
2025.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang SF, Wu MY, Cai CZ, Li M and Lu JH:
Autophagy modulators from traditional Chinese medicine: Mechanisms
and therapeutic potentials for cancer and neurodegenerative
diseases. J Ethnopharmacol. 194:861–876. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li XL, Lin ZH, Chen SR, Ni S, Lin GY, Wang
W, Lin JY, Zhao Q, Cong C and Xu LW: Tiaogeng decoction improves
mild cognitive impairment in menopausal APP/PS1 mice through the
ERs/NF-κ b/AQP1 signaling pathway. Phytomedicine.
138(156391)2025.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang X, Chen J, Huang W, Zhang Y, Yan X,
Zhou Z and Wang Y: Synthesis of icariin in tobacco leaf by
overexpression of a glucosyltransferase gene from Epimedium
sagittatum. Ind Crop Prod. 156(112841)2020.
|
|
13
|
Li J, Yu Y, Zhang Y, Zhou Y, Ding S, Dong
S, Jin S and Li Q: Flavonoids derived from Chinese Medicine:
Potential neuroprotective agents. Am J Chin Med. 52:1613–1640.
2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhou M, Zheng W, Sun X, Yuan M, Zhang J,
Chen X, Yu K, Guo B and Ma B: Comparative analysis of chemical
components in different parts of Epimedium Herb. J Pharm Biomed
Anal. 198(113984)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang HF, Yang XH, Zhao LD and Wang Y:
Ultrasonic-assisted extraction of epimedin C from fresh leaves of
Epimedium and extraction mechanism. Innovative Food Science &
Emerging Technologies. 54-60:1466–8564. 2009.
|
|
16
|
Luo D, Shi D and Wen L: From epimedium to
neuroprotection: Exploring the potential of wushanicaritin. Foods.
13(1493)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li XA, Ho YS, Chen L and Hsiao WL: The
protective effects of icariin against the homocysteine-induced
neurotoxicity in the primary embryonic cultures of rat cortical
neurons. Molecules. 21(1557)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chinese Pharmacopoeia Commission:
Pharmacopoeia of the People's Republic of China. Vol 1. China
Medical Science Press, Beijing, 2020.
|
|
19
|
Wu L, Du ZR, Xu AL, Yan Z, Xiao HH, Wong
MS, Yao XS and Chen WF: Neuroprotective effects of total flavonoid
fraction of the Epimedium koreanum Nakai extract on dopaminergic
neurons: In vivo and in vitro. Biomed Pharmacother. 91:656–663.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang HF, Yang TS, Li ZZ and Wang Y:
Simultaneous extraction of epimedin A, B, C and icariin from Herba
Epimedii by ultrasonic technique. Ultrason Sonochem. 15:376–385.
2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Huang X, Wang X, Zhang Y, Shen L, Wang N,
Xiong X, Zhang L, Cai X and Shou D: Absorption and utilisation of
epimedin C and icariin from Epimedii herba, and the regulatory
mechanism via the BMP2/Runx2 signalling pathway. Biomed
Pharmacother. 118(109345)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wei DH, Deng JL, Shi RZ, Ma L, Shen JM,
Hoffman R, Hu YH, Wang H and Gao JL: Epimedin C protects
H2O2-induced peroxidation injury by enhancing the function of
endothelial progenitor HUVEC populations. Biol Pharm Bull.
42:1491–1499. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ohnuma K, Hayashi Y, Furue M, Kaneko K and
Asashima M: Serum-free culture conditions for serial subculture of
undifferentiated PC12 cells. J Neurosci Methods. 151:250–261.
2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cheng B, Lu H, Bai B and Chen J:
d-β-Hydroxybutyrate inhibited the apoptosis of PC12 cells induced
by H2O2 via inhibiting oxidative stress. Neurochem Int. 62:620–625.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gao X, Li S, Liu X, Cong C, Zhao L, Liu H
and Xu L: Neuroprotective effects of Tiaogeng decoction against
H2O2-induced oxidative injury and apoptosis in PC12 cells via Nrf2
and JNK signaling pathways. J Ethnopharmacol.
279(114379)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhao Y, Kuca K, Wu W, Wang X, Nepovimova
E, Musilek K and Wu Q: Hypothesis: JNK signaling is a therapeutic
target of neurodegenerative diseases. Alzheimers Dement.
18:152–158. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cho H and Hah JM: A perspective on the
development of c-Jun N-terminal Kinase inhibitors as therapeutics
for Alzheimer's disease: Investigating structure through docking
studies. Biomedicines. 9(1431)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Osama A, Zhang J, Yao J, Yao X and Fang J:
Nrf2: A dark horse in Alzheimer's disease treatment. Ageing Res
Rev. 64(101206)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang M, Tong K, Chen Z and Wen Z:
Mechanisms of 15-Epi-LXA4-Mediated HO-1 in cytoprotection following
inflammatory injury. J Surg Res. 281:245–255. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Atabaki MM, Ghotbeddin Z, Rahimi K and
Tabandeh MR: The potential of alphapinene as a therapeutic agent
for maternal hypoxia-induced cognitive impairments: A study on HO-1
and Nrf2 gene expression in rats. Metab Brain Dis.
40(112)2025.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li J, Kuang G, Chen X and Zeng R:
Identification of chemical composition of leaves and flowers from
paeonia rockii by UHPLC-Q-exactive orbitrap HRMS. Molecules.
21(947)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lozan E, Shinkaruk S, Al Abed SA, Lamothe
V, Potier M, Marighetto A, Schmitter JM, Bennetau-Pelissero C and
Buré C: Derivatization-free LC-MS/MS method for estrogen
quantification in mouse brain highlights a local metabolic
regulation after oral versus subcutaneous administration. Anal
Bioanal Chem. 409:5279–5289. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Guglielmotto M, Reineri S, Iannello A,
Ferrero G, Vanzan L, Miano V, Ricci L, Tamagno E, De Bortoli M and
Cutrupi S: E2 regulates epigenetic signature on neuroglobin
enhancer-promoter in neuronal cells. Front Cell Neurosci.
10(147)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Singh P and Paramanik V: Neuromodulating
roles of estrogen and phytoestrogens in cognitive therapeutics
through epigenetic modifications during aging. Front Aging
Neurosci. 14(945076)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lin J, Wu J, Xu Y, Zhao Y and Ye S:
RhFGF21 protected PC12 cells against mitochondrial apoptosis
triggered by H2O2 via the AKT-mediated ROS signaling pathway. Exp
Cell Res. 445(114417)2025.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li Y, Long J, Li L, Yu Z, Liang Y, Hou B,
Xiang L and Niu X: Pioglitazone protects PC12 cells against
oxidative stress injury: An in vitro study of its antiapoptotic
effects via the PPARγ pathway. Exp Ther Med. 26(522)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cheng Y, Huang X, Tang Y, Li J, Tan Y and
Yuan Q: Effects of evodiamine on ROS/TXNIP/NLRP3 pathway against
gouty arthritis. Naunyn Schmiedebergs Arch Pharmacol.
397:1015–1023. 2024.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Abedi A, Ghobadi H, Sharghi A, Iranpour S,
Fazlzadeh M and Aslani MR: Effect of saffron supplementation on
oxidative stress markers (MDA, TAC, TOS, GPx, SOD, and
pro-oxidant/antioxidant balance): An updated systematic review and
meta-analysis of randomized placebo-controlled trials. Front Med
(Lausanne). 10(1071514)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Satoh T, Enokido Y, Aoshima H, Uchiyama Y
and Hatanaka H: Changes in mitochondrial membrane potential during
oxidative stress-induced apoptosis in PC12 cells. J Neurosci Res.
50:413–420. 1997.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Perelman A, Wachtel C, Cohen M, Haupt S,
Shapiro H and Tzur A: JC-1: Alternative excitation wavelengths
facilitate mitochondrial membrane potential cytometry. Cell Death
Dis. 3(e430)2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kyrylkova K, Kyryachenko S, Leid M and
Kioussi C: Detection of apoptosis by TUNEL assay. Methods Mol Biol.
887:41–47. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Protasoni M and Zeviani M: mitochondrial
structure and bioenergetics in normal and disease conditions. Int J
Mol Sci. 22(586)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang G, Zhao Z, Ren B, Yu W, Zhang X, Liu
J, Wang L, Si D and Yang M: Exenatide exerts a neuroprotective
effect against diabetic cognitive impairment in rats by inhibiting
apoptosis: Role of the JNK/c-JUN signaling pathway. Mol Med Rep.
25(111)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kong R, Shi J, Xie K, Wu H, Wang X, Zhang
Y and Wang Y: A study of JUN's promoter region and its regulators
in chickens. Genes (Basel). 15(1351)2024.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Huang HM and Liu JC: c-Jun blocks cell
differentiation but not growth inhibition or apoptosis of chronic
myelogenous leukemia cells induced by STI571 and by histone
deacetylase inhibitors. J Cell Physiol. 218:568–574.
2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chen F, Xiao M, Hu S and Wang M:
Keap1-Nrf2 pathway: A key mechanism in the occurrence and
development of cancer. Front Oncol. 14(1381467)2024.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Galan-Cobo A, Sitthideatphaiboon P, Qu X,
Poteete A, Pisegna MA, Tong P, Chen PH, Boroughs LK, Rodriguez MLM,
Zhang W, et al: LKB1 and KEAP1/NRF2 pathways cooperatively promote
metabolic reprogramming with enhanced glutamine dependence in
KRAS-mutant lung adenocarcinoma. Cancer Res. 79:3251–3267.
2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Buendia I, Michalska P, Navarro E, Gameiro
I, Egea J and León R: Nrf2-ARE pathway: An emerging target against
oxidative stress and neuroinflammation in neurodegenerative
diseases. Pharmacol Ther. 157:84–104. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Xiao Q, Piao R, Wang H, Li C and Song L:
Orientin-mediated Nrf2/HO-1 signal alleviates H2O2-induced
oxidative damage via induction of JNK and PI3K/AKT activation. Int
J Biol Macromol. 118 (Pt A):747–755. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Feng L, Wu Y, Wang J, Han Y, Huang J and
Xu H: Neuroprotective effects of a novel tetrapeptide SGGY from
Walnut against H2O2-Stimulated oxidative stress in SH-SY5Y cells:
Possible involved JNK, p38 and Nrf2 signaling pathways. Foods.
12(1490)2023.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kuntić M, Hahad O, Münzel T and Daiber A:
Crosstalk between oxidative stress and inflammation caused by noise
and air pollution-implications for neurodegenerative diseases.
Antioxidants (Basel). 13(266)2024.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Downs BW, Kushner S, Bagchi M, Blum K,
Badgaiyan RD, Chakraborty S and Bagchi D: Etiology of
neuroinflammatory pathologies in neurodegenerative diseases: A
treatise. Curr Psychopharmacol. 10:123–137. 2021.
|
|
53
|
Li Y, Zhang W, Zhang Q, Li Y, Xin C, Tu R
and Yan H: Oxidative stress of mitophagy in neurodegenerative
diseases: Mechanism and potential therapeutic targets. Arch Biochem
Biophys. 764(110283)2025.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Pardillo-Díaz R, Pérez-García P, Castro C,
Nunez-Abades P and Carrascal L: Oxidative stress as a potential
mechanism underlying membrane hyperexcitability in
neurodegenerative diseases. Antioxidants (Basel).
11(1511)2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kim S, Jung UJ and Kim SR: Role of
oxidative stress in blood-brain barrier disruption and
neurodegenerative diseases. Antioxidants (Basel).
13(1462)2024.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Tarozzi A: Oxidative stress in
neurodegenerative diseases: From preclinical studies to clinical
applications. J Clin Med. 9(1223)2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Shams Ul Hassan S, Ishaq M, Zhang WD and
Jin HZ: An overview of the mechanisms of marine Fungi-derived
anti-inflammatory and anti-tumor agents and their novel role in
drug targeting. Curr Pharm Des. 27:2605–2614. 2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Cai Z, Liu M, Zeng L, Zhao K, Wang C, Sun
T, Li Z and Liu R: Role of traditional Chinese medicine in
ameliorating mitochondrial dysfunction via non-coding RNA
signaling: Implication in the treatment of neurodegenerative
diseases. Front Pharmacol. 14(1123188)2023.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Mohd Sairazi NS and Sirajudeen KNS:
Natural products and their bioactive compounds: Neuroprotective
potentials against neurodegenerative diseases. Evid Based
Complement Alternat Med. 2020(6565396)2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Chen L, Liu Y and Xie J: The beneficial
pharmacological effects of Uncaria rhynchophylla in
neurodegenerative diseases: Focus on alkaloids. Front Pharmacol.
15(1436481)2024.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wang ZY, Liu J, Zhu Z, Su CF,
Sreenivasmurthy SG, Iyaswamy A, Lu JH, Chen G, Song JX and Li M:
Traditional Chinese medicine compounds regulate autophagy for
treating neurodegenerative disease: A mechanism review. Biomed
Pharmacother. 133(110968)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Chen XL, Li SX, Ge T, Zhang DD, Wang HF,
Wang W, Li YZ and Song XM: Epimedium Linn: A comprehensive review
of phytochemistry, pharmacology, clinical applications and quality
control. Chem Biodivers. 21(e202400846)2024.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Li C, Li Q, Mei Q and Lu T:
Pharmacological effects and pharmacokinetic properties of icariin,
the major bioactive component in Herba Epimedii. Life Sci.
126:57–68. 2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Zong N, Li F, Deng Y, Shi J, Jin F and
Gong Q: Icariin, a major constituent from Epimedium brevicornum,
attenuates ibotenic acid-induced excitotoxicity in rat hippocampus.
Behav Brain Res. 313:111–119. 2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Liu F, Wei B, Cheng L, Zhao Y, Liu X, Yuan
Q and Liang H: Co-Immobilizing two glycosidases based on
cross-linked enzyme aggregates to enhance enzymatic properties for
achieving high titer Icaritin biosynthesis. J Agric Food Chem.
70:11631–11642. 2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Yang J, Yang J, Liang SH, Xu Y, Moore A
and Ran C: Imaging hydrogen peroxide in Alzheimer's disease via
cascade signal amplification. Sci Rep. 6(35613)2016.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Gharai PK, Khan J, Mallesh R, Garg S, Saha
A and Ghosh S and Ghosh S: Vanillin benzothiazole derivative
reduces cellular reactive oxygen species and detects amyloid
fibrillar aggregates in Alzheimer's disease brain. ACS Chem
Neurosci. 14:773–786. 2023.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Sajjad N, Wani A, Sharma A, Ali R, Hassan
S, Hamid R, Habib H and Ganai BA: Artemisia amygdalina Upregulates
Nrf2 and protects neurons against oxidative stress in Alzheimer
disease. Cell Mol Neurobiol. 39:387–399. 2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Zakharova IO, Sokolova TV, Furaev VV,
Rychkova MP and Avrova NF: (Effects of oxidative stress inducers,
neurotoxins, and ganglioside GM1 on Na+, K+-ATPase in PC12 and
brain synaptosomes). Zh Evol Biokhim Fiziol. 43:148–154.
2007.PubMed/NCBI(In Russian).
|
|
70
|
Demirci-Çekiç S, Özkan G, Avan AN, Uzunboy
S, Çapanoğlu E and Apak R: Biomarkers of oxidative stress and
antioxidant defense. J Pharm Biomed Anal.
209(114477)2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Pisoschi AM and Pop A: The role of
antioxidants in the chemistry of oxidative stress: A review. Eur J
Med Chem. 97:55–74. 2015.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Zhang R, Guo X, Zhang Y and Tian C:
Influence of modified atmosphere treatment on post-harvest reactive
oxygen metabolism of pomegranate peels. Nat Prod Res. 34:740–744.
2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
González-Rubio G, Sellers-Moya Á, Martín H
and Molina M: A walk-through MAPK structure and functionality with
the 30-year-old yeast MAPK Slt2. Int Microbiol. 24:531–543.
2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Miloso M, Scuteri A, Foudah D and Tredici
G: MAPKs as mediators of cell fate determination: An approach to
neurodegenerative diseases. Curr Med Chem. 15:538–548.
2008.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Du J, Wang G, Luo H, Liu N and Xie J:
JNK-IN-8 treatment alleviates lipopolysaccharide-induced acute lung
injury via suppression of inflammation and oxidative stress
regulated by JNK/NF-κB signaling. Mol Med Rep. 23(150)2021.
|
|
76
|
Li R, Yang W, Yan X, Zhou X, Song X, Liu
C, Zhang Y and Li J: Folic acid mitigates the developmental and
neurotoxic effects of bisphenol A in zebrafish by inhibiting the
oxidative stress/JNK signaling pathway. Ecotoxicol Environ Saf.
288(117363)2024.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Shakya A, McKee NW, Dodson M, Chapman E
and Zhang DD: Anti-ferroptotic effects of Nrf2: Beyond the
antioxidant response. Mol Cells. 46:165–175. 2023.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Hallis SP, Kim JM and Kwak MK: Emerging
role of NRF2 signaling in cancer stem cell phenotype. Mol Cells.
46:153–164. 2023.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Huang SY, Chang SF, Chau SF and Chiu SC:
The protective effect of hispidin against hydrogen peroxide-induced
oxidative stress in ARPE-19 cells via Nrf2 signaling pathway.
Biomolecules. 9(380)2019.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Li S, Cong C, Liu Y, Liu X, Liu H, Zhao L,
Gao X, Gui W and Xu L: Tiao Geng decoction inhibits tributyltin
chloride-induced GT1-7 neuronal apoptosis through ASK1/MKK7/JNK
signaling pathway. J Ethnopharmacol. 269(113669)2021.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Li S, Cong C, Liu Y, Liu X, Kluwe L, Shan
X, Liu H, Gao M, Zhao L, Gao X and Xu L: Tiao Geng decoction for
treating menopausal syndrome exhibits anti-aging effects likely via
suppressing ASK1/MKK7/JNK mediated apoptosis in ovariectomized
rats. J Ethnopharmacol. 261(113061)2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Ryan J, Scali J, Carriere I, Ritchie K and
Ancelin ML: Hormonal treatment, mild cognitive impairment and
Alzheimer's disease. Int Psychogeriatr. 20:47–56. 2008.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Li C, Jia WW, Yang JL, Cheng C and Olaleye
OE: Multi-compound and drug-combination pharmacokinetic research on
Chinese herbal medicines. Acta Pharmacol Sin. 43:3080–3095.
2022.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Chen L, Zhen Y, Wang X, Wang J and Zhu G:
Neurovascular glial unit: A target of phytotherapy for cognitive
impairments. Phytomedicine. 119(155009)2023.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Sharma S, Mehan S, Khan Z, Tiwari A, Kumar
A, Gupta GD, Narula AS and Kalfin R: Exploring the neuroprotective
potential of icariin through modulation of neural pathways in the
treatment of neurological diseases. Curr Mol Med: Sep 26, 2024
(Epub ahead of print).
|
|
86
|
Zhang Y, Wang Y, Zhang X, Liu Y, Li J and
Wang J: Metabolic profiling of epimedin C in Rats: In vivo and in
vitro studies. Front. Pharmacol. 9(456)2018.
|
|
87
|
Wong SP, Shen P, Lee L, Li J and Yong EL:
Pharmacokinetics of prenylflavonoids and correlations with the
dynamics of estrogen action in sera following ingestion of a
standardized Epimedium extract. J Pharm Biomed Anal. 50:216–223.
2009.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Xie L, Zhao S, Zhang X, Huang W, Qiao L,
Zhan D, Ma C, Gong W, Dang H and Lu H: Wenshenyang recipe treats
infertility through hormonal regulation and inflammatory responses
revealed by transcriptome analysis and network pharmacology. Front
Pharmacol. 13(917544)2022.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Hara Y, Waters EM, McEwen BS and Morrison
JH: estrogen effects on cognitive and synaptic health over the
lifecourse. Physiol Rev. 95:785–807. 2015.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Uddin MS, Rahman MM, Jakaria M, Rahman MS,
Hossain MS, Islam A, Ahmed M, Mathew B, Omar UM, Barreto GE and
Ashraf GM: Estrogen signaling in Alzheimer's disease: Molecular
Insights and therapeutic targets for Alzheimer's dementia. Mol
Neurobiol. 57:2654–2670. 2020.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Dubal DB, Zhu H, Yu J, Rau SW, Shughrue
PJ, Merchenthaler I, Kindy MS and Wise PM: Estrogen receptor alpha,
not beta, is a critical link in estradiol-mediated protection
against brain injury. Proc Natl Acad Sci USA. 98:1952–1957.
2001.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Li B, Lima MRM, Nie Y, Xu L, Liu X, Yuan
H, Chen C, Dias AC and Zhang X: HPLC-DAD fingerprints combined with
multivariate analysis of epimedii folium from major producing areas
in eastern Asia: Effect of geographical origin and species. Front
Pharmacol. 12(761551)2021.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Lee D, Jeong HC, Kim SY, Chung JY, Cho SH,
Kim KA, Cho JH, Ko BS, Cha IJ, Chung CG, et al: A comparison study
of pathological features and drug efficacy between Drosophila
models of C9orf72 ALS/FTD. Mol Cells. 47(100005)2024.PubMed/NCBI View Article : Google Scholar
|