Introduction
Periodontal bone regeneration is a major clinical
challenge. Current surgical strategies, such as autologous bone
grafting combined with guided tissue regeneration, achieve
long-term success rates of ~58% in restoring functional attachment
(1-4).
These approaches are further constrained by donor site morbidity,
unpredictable graft resorption and diminished efficacy in patients
with systemic conditions such as diabetes mellitus (5-8).
Collectively, these limitations underscore the need for biological
therapies that directly modulate bone metabolism.
Sclerostin (Scl; encoded by the SOST gene) is a
22-kDa secreted glycoprotein that has emerged as a key regulator of
skeletal homeostasis, particularly in bone remodelling and
craniofacial biology. It serves as a key mediator of the
interaction between osteoblasts and osteoclasts, maintaining the
balance between bone formation and resorption (8). Mechanistically, Scl exerts its
effects through a number of interrelated pathways (9-12).
By antagonizing the canonical Wnt/β-catenin signaling cascade, Scl
binds to low-density lipoprotein receptor-related proteins (LRPs)
including LRP5/6 on osteoblast precursors, thereby suppressing
osteoblast proliferation, differentiation and matrix
mineralization. This inhibition decreases the capacity for new bone
formation (13-15).
Scl indirectly promotes osteoclastogenesis through bone
morphogenetic protein (BMP)/Wnt signaling interactions. It
increases the receptor activator of NF-κB ligand (RANKL) to
osteoprotegerin (OPG) ratio by stimulating RANKL secretion from
osteocytes while decreasing OPG expression, favoring osteoclast
differentiation and activity. Thus, Scl not only limits bone
formation but also actively enhances bone resorption (16-19).
Beyond local effects in bone, Scl may also act
systemically. Evidence suggests (16,17)
that it can influence distant organs such as the kidney or vascular
system, linking bone metabolism to broader physiological processes.
In periodontal disease, these regulatory networks become
dysregulated. The alveolar bone undergoes rapid turnover and is
subject to continuous mechanical loading from mastication and
occlusal forces (16-18).
When combined with chronic biofilm-induced inflammation, these
stressors amplify disruption of the Scl-Wnt-RANKL axis,
accelerating alveolar bone resorption and undermining periodontal
tissue stability (19).
Given its dual role in suppressing osteoblast
activity and stimulating osteoclastogenesis, therapeutic inhibition
of Scl has attracted extensive research (14-19),
particularly following the clinical success of romosozumab in
osteoporosis. However, whether these benefits extend to
biofilm-driven periodontitis, remains uncertain. The regulatory
pathways by which Scl influences alveolar bone remodeling,
including inhibition of canonical Wnt/β-catenin signaling,
modulation of the RANKL/OPG pathway, interactions with BMP
signaling and amplification through inflammatory cytokines are
illustrated in Fig. S1.
Research into therapeutic inhibition of Scl has
progressed since the approval of romosozumab for osteoporosis and
preclinical studies have confirmed its anabolic effects in
long-bone defect models (20-22).
Whether these effects extend to polymicrobial biofilm-induced
periodontitis remains unclear, given the unique challenges of the
periodontal environment, including alveolar bone turnover,
estimated to be 200-300% faster compared with that of tibial bone
(23-27),
constant bidirectional loading from mastication (28,29)
and chronic biofilm-driven inflammation (30,31).
To the best of our knowledge, the present
meta-analysis is the first to synthesize preclinical evidence on
Scl inhibition in experimental periodontitis. Existing studies
(14-19)
demonstrate limited insight into how treatment effects vary by
dose, anatomical site or systemic conditions. By integrating
available data, the present analysis aimed to outline the
dose-response association between SOST suppression and alveolar
regeneration, as well as potential differences across defect types
such as interproximal and furcation lesions and interactions with
systemic bone-modifying factors. Together, these findings may
provide a more comprehensive understanding of the therapeutic
potential of Scl inhibition in periodontal regeneration.
Materials and methods
Protocol and registration
The present protocol was registered on PROSPERO
(https://www.crd.york.ac.uk/PROSPERO;
registration ID: CRD42023388345). The present review aimed to
evaluate whether inhibition of Scl activity promotes alveolar bone
repair and regeneration in animal models of periodontal disease,
based on the recommendations from the Cochrane Handbook for
Systematic Reviews (32) and
PRISMA statement for reporting systematic reviews and meta-analysis
(33).
Inclusion criteria
A population, intervention, comparison, outcomes and
study design framework was applied to define the criteria.
Inclusion criteria were as follows: i) Animal models of
experimental periodontal disease; ii) evaluated the inhibition or
neutralization of Scl activity; iii) used animal models with
periodontal disease but without Scl inhibition as the control
group; iv) encompassed primary outcomes associated with decreased
alveolar bone loss (ABL) and secondary outcomes including changes
in bone volume fraction (BVF), bone mineral density (BMD),
bone-specific serum markers and the number of SOST-positive cells;
and v) in vivo randomized controlled trials.
Exclusion criteria
Studies that did not replicate periodontal
pathology, in vitro experiments and studies lacking a
control group or using non-periodontitis animals as controls were
excluded.
Search strategy
Studies were identified through a systematic s earch
of the following electronic databases: PubMed (https://pubmed.ncbi.nlm.nih.gov/), the Cochrane
Library (https://www.cochranelibrary.com/), Embase (https://www.embase.com/) and Web of Science (all
databases, https://www.webofscience.com/). Studies published from
inception to January 2025 were included, with no language
restrictions. The search combined medical subject headings with
free-text terms applied to titles, keywords and abstracts. In
addition, a manual search was performed by screening the reference
lists of articles and related reviews for additional relevant
studies. The complete search strategy is provided in Table SI.
Study selection
All retrieved records were imported into EndNote
software (version X9; Clarivate Plc) to remove duplicates. A total
of two reviewers then independently screened the remaining articles
against the inclusion and exclusion criteria. First, titles and
abstracts were reviewed to exclude irrelevant studies. Full text of
potentially eligible studies was assessed to confirm their
relevance. Any discrepancies were resolved by discussion and
consensus or consultation with a third reviewer.
Data extraction
Study publication date, author name, sample size,
animal species, the sex and diet of the animals, construction of
the periodontal disease model, the adopted interventions, route and
dose of administration, the duration of administration, outcomes
and types of detection (e.g., histological, radiographic or
molecular analyses) were collected.
If data were insufficient or presented only
graphically, attempts were made to contact the authors and obtain
the numerical values. If the information could not be retrieved,
WebPlotDigitizer (V5), a digital ruler software, was used to
measure graphical data (automeris.io/WebPlotDigitizer/) (34). If studies reported outcomes at
multiple time points, only data from the most commonly reported
time point across studies (e.g., 3 or 6 weeks) were acquired for
meta-analysis. If the experiments included a number of groups, only
periodontitis animal models were taken as the experimental and
control group for meta-analysis. The data were extracted
independently by two authors, with any disagreements being resolved
through discussion and consensus.
Quality assessment
Risk of bias was independently evaluated by two
reviewers using the Systematic Review Centre for Laboratory Animal
Experimentation (SYRCLE) risk of bias tool (35), which consists of ten domains
adapted from the Cochrane Collaboration tool (36). According to the criteria
recommended by Hooijmans et al (35), each domain was rated as low risk
(score=1) if sufficient methodological details were reported, high
risk (score=-1) if clear methodological shortcomings were present
or unclear risk (score=0) when information was insufficient to
permit judgement. The assessed domains included sequence
generation, baseline characteristics, allocation concealment,
random housing, blinding of caregivers/experimenters, random
outcome assessment, blinding of outcome assessors, incomplete
outcome data, selective outcome reporting and other potential
sources of bias. Discrepancies between reviewers were resolved by
discussion or consultation with a third reviewer.
Data synthesis and statistical
analysis
As only morphometric results, such as the distance
between the cementoenamel junction and alveolar bone crest
(CEJ-ABC), tissue mineral density (TMD) and BVF, yielded sufficient
data, these continuous variables were extracted for meta-analysis.
Analysis was conducted using REVMAN (version 5.3; The Cochrane
Collaboration) software to determine pooled effects. To identify
and measure heterogeneity in the results, χ2 tests and
I2 statistics were implemented. χ2 with a
significance level of α=0.1 was considered to indicate
heterogeneity.
As all outcome measures were continuous variables,
the standardized mean difference (SMD) with 95% CI was used to
describe the efficacy of the intervention effect. SMD was used
instead of weighted mean difference because the studies included
reported heterogeneous outcomes (BMD, BVF and ABL) in different
units and scales. The use of SMD allowed for standardization and
ensured comparability across studies. In accordance with the
Cochrane Handbook for Systematic Reviews of Interventions, a
random-effects model was applied for all meta-analyses,
irrespective of the I2 value, since heterogeneity is
expected across experimental studies.
Subgroup analyses were conducted to explore
potential sources of heterogeneity, such as type of intervention
(direct vs. indirect inhibition), species, sex, dosage, route of
administration, model construction and whether the ligature was
removed (most models were ligature-induced). To minimize potential
bias from individual studies, sensitivity analysis was conducted by
sequentially excluding each trial to evaluate its influence on the
pooled results.
Level of evidence
A total of two investigators independently assessed
the certainty (level) of evidence for each outcome using the
Grading of Recommendations Assessment, Development and Evaluation
(GRADE) system (GRADEPRO.org), based primarily on
five domains, including type of study design, risk of bias,
inconsistency, indirectness, imprecision and other comprehensive
consideration of sample size and confounding factors. Any
disagreements were resolved through discussion or by a third
researcher.
Results
Search outcomes
The literature search yielded a total of 99 studies
(Fig. S2). After removing the
duplicates and screening the remaining publications, 39 articles
were eligible for full-text evaluation. Of these, 31 were excluded.
A total of eight studies was retrieved for systematic review and
assessed by meta-analysis (37-44)
Study characteristics
The present review included articles published from
2013-2023. Although there were no restrictions on the type of
animal or publication language, all periodontal disease models
identified were rat or mouse studies published in English. The
number of animals ranged from 14-40, with a total of 119 males and
64 females [sex not reported in one study (41)]. Models included Sprague-Dawley rats
(37,38,42),
Wistar albino rats (44), inbred
F344 rats (39), OPG-deficient
mice (40) and periostin-knockout
mice (41). Diet primarily
comprised standard chow and tap water, except for the high-sugar
water provided to all groups by Chen et al (37) and the 10%
sucrose given to the experimental group by Yang et al
(43).
For interventions targeting Scl activity, Scl
antibody (Scl-Ab) was used in five studies (37,38,41,42,44),
SOST gene knockout was performed in two studies (41,43)
and specific pharmacological agents were tested in three studies,
namely caffeic acid phenethyl ester (CAPE) (44), low-dose doxycycline (LDD) (44), infliximab (39) and WP9QY (40).
In addition to ligature-induced periodontitis, five
studies also applied additional conditions unfavourable for bone
regeneration, such as ovariectomy (37,38),
periostin knockout (41), OPG
deficiency (40) and
streptozotocin-induced type I diabetes (37-41,43).
A total of one study used silk ligatures saturated with
Porphyromonas gingivalis (43).
Systemic subcutaneous injection of Scl-Ab was
employed in three studies (37,40,42),
while intraperitoneal injection was used in two studies (38,41).
The dosage and duration included 25 mg/kg twice weekly for 2-8
weeks in four studies (37,41,42,44)
and 5 mg/kg weekly for 22 weeks in one study (38). Local Scl-Ab application (125 µg)
was tested in one study (42) but
proved less effective compared with systemic administration. In
addition, Yiğit et al (44)
applied CAPE and LDD at 10 mg/kg for 14 days, delivered by
intraperitoneal injection and oral gavage, respectively. CAPE was
more effective compared with LDD at decreasing Scl expression. In
the other pharmacological studies, infliximab was administered
intraperitoneally at 5 mg/kg once or twice before euthanasia
(39) and WP9QY was given
subcutaneously at 10 mg/kg three times daily for 5 days (40).
The primary outcome assessment methods included
micro-computed tomography (CT) and histological examination to
evaluate ABL, BVF and BMD. Immunohistochemistry was used to assess
the number of SOST-positive cells and osteoclasts. In addition,
serum analysis, ELISA and reverse transcription-quantitative PCR
were performed to measure serum biomarkers, such as procollagen
type I N-terminal propeptide (P1NP), osteocalcin (OCN),
tartrate-resistant acid phosphatase 5b (TRAP5b), RANKL, C-terminal
telopeptide of type I collagen (CTx-1) and ALP. The characteristics
of the included studies are summarized in Table I.
 | Table ICharacteristics of included
studies. |
Table I
Characteristics of included
studies.
| | First author,
year |
|---|
| Characteristic | Taut et al,
2013 | Ren et al,
2015 | Chen et al,
2015 | Hadaya et
al, 2019 | Yang et al,
2016 | Yiğit et al,
2022 | Kim et al,
2016 | Ozaki et al,
2017 |
|---|
| Sample size | 16 | 16 | 24 | 40 | 20 | 20 | 16 | 14 |
| Animal | SD rat | Periostin knockout
mouse | SD rat | SD rat | C57BL/6 mouse | Wistar albino
rat | Inbred F344
rat | OPG-/- C57BL/6
mouse |
| Sex | Male | N/A | Female | Female | Male | Male | Male | Male |
| Diet | Regular rodent chow
diet | N/A | Food and high-sugar
drinking water | Standard diet
NIH-31 modified | Standard solid
mouse chow, 10% sucrose for EX group and sterile water for CON
group | Standard rat food
and tap water ad libitum | Standard rat chow
and water ad libitum | Sterilized water
and diet ad libitum |
| Construction of
periodontal disease model | Ligature | Periostin
knockout | Ligature; OVX | Ligature; OVX | Ligature of silk
saturated with P.g | Ligature | Ligature;
streptozoto- cin | OPG deficiency |
| Intervention,
EX/CON | Scl-Ab/PBS | Scl-Ab, SOST and
periostin double knockout/ PBS | Scl-Ab/ empty
vehicle | Scl-Ab/empty
vehicle | SOST gene
knockout/none | CAPE; LDD/ PBS | Infliximab/
PBS | WP9QY/PBS |
| Route of
administration | s.c. | i.p. | s.c. | i.p. | N/A | CAPE, i.p.; LDD,
oral gavage | i.p. | s.c. |
| Dose | 25 mg/kg | 25 mg/kg | 25 mg/kg | 5 mg/kg Scl-ab | N/A | CAPE, 10 µmol/
kg/day; LDD, 10 mg/kg/day | 5 mg/kg | 10 mg/kg |
| Duration | Twice/week for 3 or
6 weeks | Twice/week for 8
weeks | Twice/week for 6
weeks | Weekly for 12 weeks
before OVX and 10 weeks after OVX | N/A (constructive
knockout); 2 months | Daily for 14
days | Once for the 3 day
group (on day 0); twice for the 20 day group (on days 7 and
14) | 3 times/day for 5
days |
| Secondary
outcomes | BVF, TMD. OCN,
P1NP, TRAP5b | Bone deposition
rate, mor- phological changes of osteocytes, BV/TV | TRAP5b, CTx-1, BVF,
BMD | P1NP, TRAP5b | OPG, RANKL, JNK,
p38MAPK, ERK1/2 | PNMLS, BMP2 and
Scl-positive expression | IL-1β, Scl,
cathepsin K-, RANKL- and Scl-positive cells | BV/TV; number of
TRAP-5b-, sterix- orScl- positive cells; serum ALP |
| Detection
technique | Bone-specific serum
biomar- kers; micro-CT; histology immunohisto- chemistry;
fluorescent calcian labeling | Electron
microscopy; micro-CT; histology/ immunohis- tochemistry; FITC
staining and Imaris analysis | Bone- specific
serum biomarkers; micro-CT; histology | Serum analysis;
micro-CT; histology | Immunohisto-
chemistry; micro- CT | Histomorpho- metry,
histopa- thology and immunohisto- chemistry | Histology,
histomorpho- logy and immunohisto chemistry; RT-PCR | Micro-CT;
histomorpho- logy and immunohisto- chemistry; ELISA (serum
marker) |
| (Refs.) | (42) | (41) | (37) | (38) | (43) | (44) | (39) | (40) |
Quantitative synthesis and data
analysis
The outcome variables with sufficient data,
including changes in the CEJ-ABC distance, ABL, BMD, BVF, number of
Scl-positive cells and bone-specific serum biomarkers such as OCN,
P1NP and TRAP5b, were pooled for further analysis (Table SII).
Primary outcomes. ABL
ABL was evaluated by measuring the linear distance
or volume between the CEJ and ABC before and after treatment. This
parameter directly reflects periodontal tissue changes as assessed
by histological or micro-CT examination. All studies (37-44)
reported quantitative changes in ABL, therefore this was used as
the primary outcome variable of the present meta-analysis.
Extracted data were expressed as linear distance, surface area or
volume.
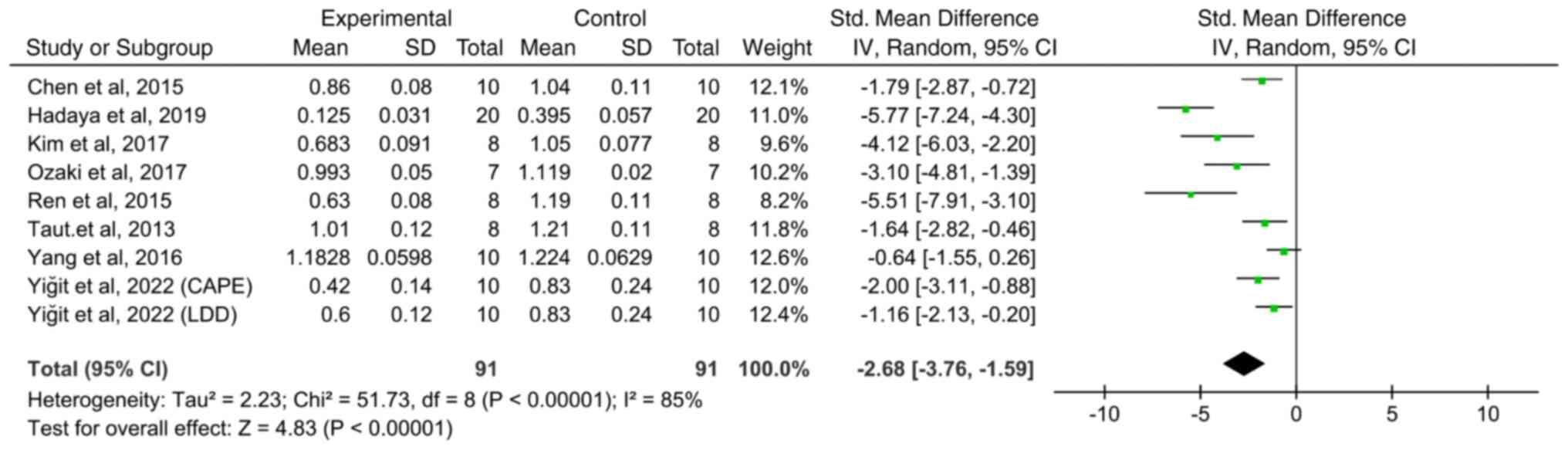
Pooled analysis revealed a significant decrease in
the CEJ-ABC distance in the Scl inhibition group compared with the
control group (Fig. 1; SMD: -2.68;
95% CI: -3.76 to -1.59; I2=85%; P<0.0001). In a study
by Yiğit et al (44), two
drugs (LDD and CAPE) with Scl-inhibiting potential were tested and
results were analysed separately. While numerous ABL data were
derived from micro-CT measurements, two studies employed
histological assessment (39,44),
resulting in different measurement units (linear distance, area or
volume).
Subgroup analysis was performed according to
species, sex, dose, route of administration, type of Scl inhibition
and periodontitis model. With the exception of dose and route of
administration, no significant subgroup differences were observed
(Fig. 2). Low-dose (5 mg/kg;
Z=7.70) and subcutaneous (Z=5.26) treatments yielded improved
outcomes compared with high-dose (25 mg/kg; Z=3.00; P=0.006;
Fig. 2B) and intraperitoneal
(Z=4.06; P=0.04) treatments (Fig.
2E).
A number of studies used a therapeutic design
initiated after ligature removal (37,41-43).
Taut et al (42) also
incorporated a preventive arm, but no quantitative data were
reported and this subgroup could not be included in the present
meta-analysis. By contrast, Hadaya et al (38), in which ligatures were not removed
and chronic inflammation persisted during treatment, exhibited a
larger effect size compared with ligature-removed models (Fig. S3). These findings suggested that
sustained inflammation may modify the response to Scl inhibition
and decrease its net regenerative benefit.
Sensitivity analysis was conducted using a
leave-one-out approach. After sequentially removing each included
study, the pooled effect size and its 95% CI did not notably change
(Fig. S4). This indicated that no
single study had a decisive influence on the overall results,
supporting the validity of the present meta-analysis.
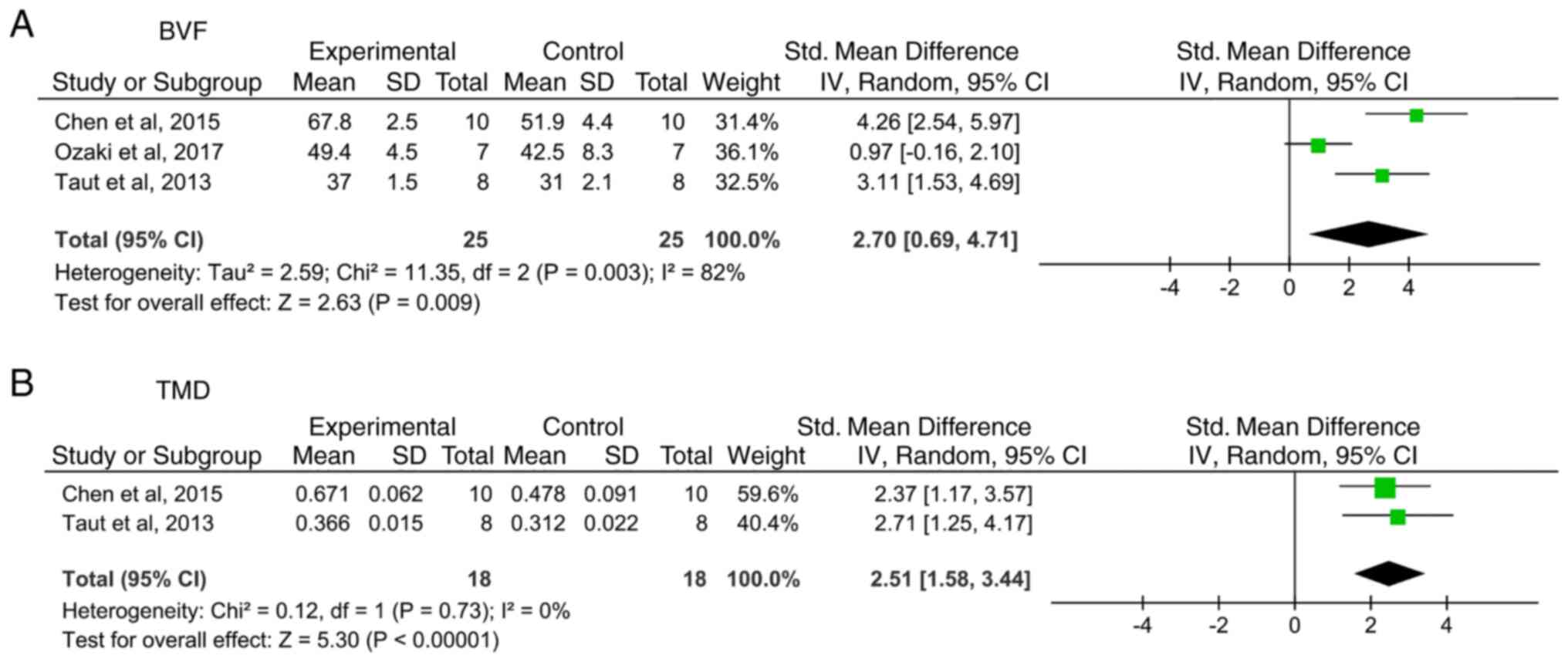
Secondary outcomes. BVF
BVF is a volumetric parameter calculated as the
ratio of BV to tissue volume based on micro-CT scanning. A total of
three studies (37,40,42)
reported the BVF and the newly formed bone area was significantly
larger in the experimental groups compared with the control groups
(Fig. 3A; SMD: 2.70; 95% CI:
0.69-4.71; I2=82%; P=0.009). In a study by Taut et al
(42), both systemic and local administration were tested, but
only systemic data were extracted for meta-analysis to ensure
consistency.
BMD/TMD. A total of two studies (37,42)
reported BMD/TMD and revealed that compared with control treatment,
systemic Scl-Ab treatment significantly increased BMD (Fig. 3B; SMD: 2.51; 95% CI: 1.58-3.44;
I2=0%; P<0.00001).
Other bone-specific serum biomarkers.
Bone-associated serum markers (P1NP, OCN, OPG, RANKL, TRAP5b, CTx-1
and ALP) were evaluated in four studies (37,38,42,43).
Except for OCN in two studies (37,42),
no significant pooled results were obtained (Fig. 4A). Hadaya et al (38) reported that P1NP levels were
notably higher in the Scl-Ab group at 6 and 12 weeks
post-ovariectomy but decreased below baseline at the end of
treatment (38). Taut et al
(42) reported that OCN levels
were notably elevated in the Scl-Ab group at 3 and 6 weeks. P1NP
was also notably higher at 3 but not at 6 weeks (42). Chen et al (37) observed marked increases in OCN and
OPG in the experimental group after 6 weeks, accompanied by
decreases in TRAP5b and CTx-1(37). Ozaki et al (40) reported that WP9QY did not reduce
ALP levels in OPG-deficient mice but increased osteoblast
differentiation in the M1 interradicular septum.
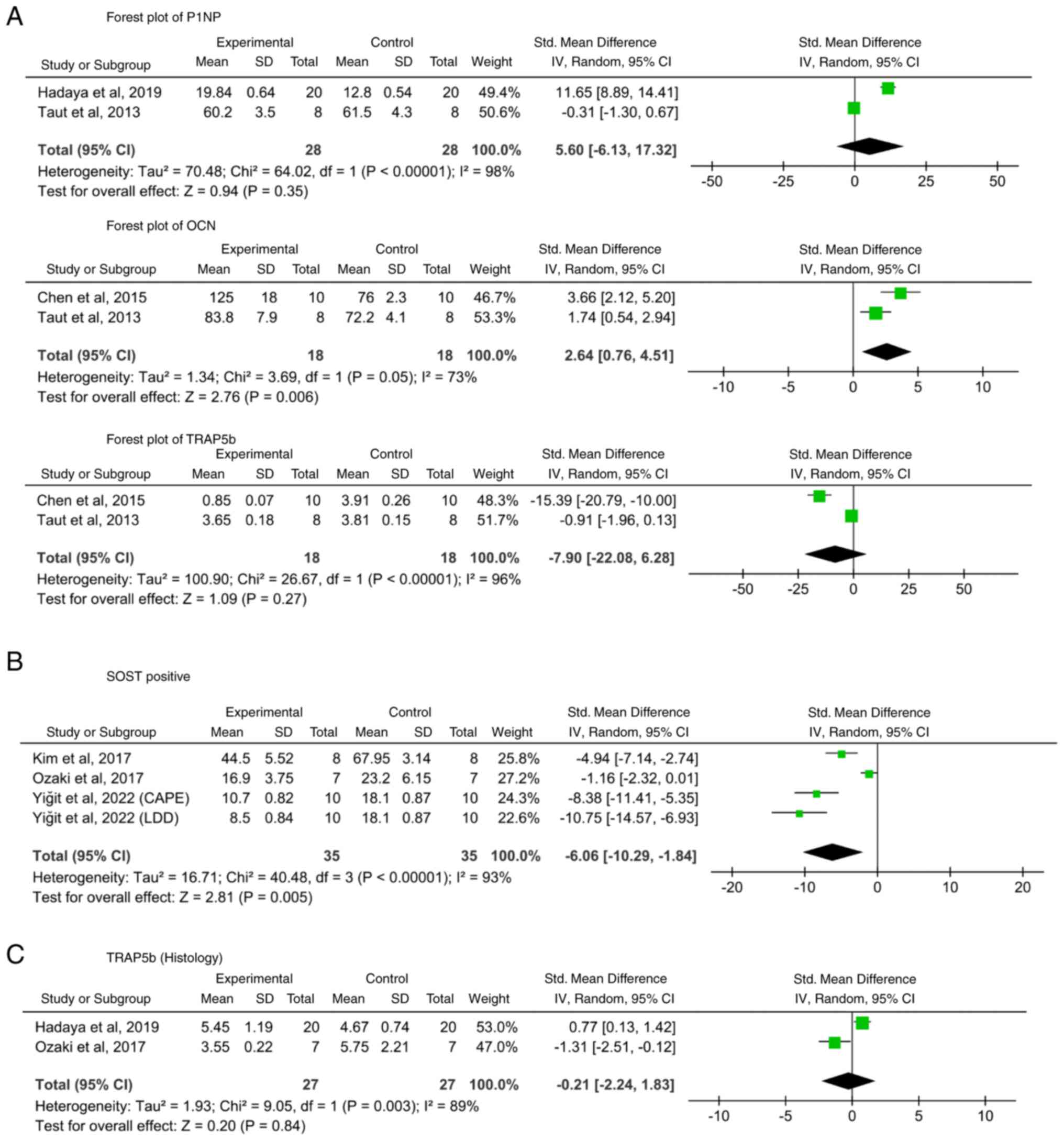 | Figure 4Forest plots of bone-specific
biomarkers and SOST-positive cells. (A) Bone-specific serum
biomarkers (P1NP, OCN and TRAP5b). (B) Histological test for
TRAP5b. (C) SOST-positive cells. Scl, sclerostin; df, degrees of
freedom; LDD, low-dose doxycycline; CAPE, caffeic acid phenethyl
ester; SMD, standardized mean difference; TRAP5b,
tartrate-resistant acid phosphatase 5b; SOST, sclerostin; IV,
Inverse Variance Model; Pp1NP, procollagen type I N-terminal
propeptide; OCN, osteocalcin; TRAP5b, tartrate-resistant acid
phosphatase 5b. Supplementary figure legends |
For serum TRAP5b, no notable differences were
observed between the groups. The detection methods varied: Two
studies (37,42) assessed TRAP5b expression through
Scl-associated assays, while two others (38,40)
used histological staining to quantify the number of
osteoclasts/unit area (Fig. 4C).
Although Taut et al (42)
concluded that Scl inhibition had no effect on TRAP5b expression,
the remaining studies indicated that Scl-Ab therapy decreased
TRAP5b expression, which was consistent with increases in the
expression of other osteogenic markers.
Number or ratio of SOST-positive cells. A
total of three studies (39,40,44)
using specific drugs assessed SOST-positive cells, consistently
showing that the number of Scl-positive cells increased
significantly in untreated periodontitis models but decreased
markedly after treatment (Fig. 4B;
SMD: -6.06, 95% CI: -10.29 to -1.84; I2=93%;
P=0.005).
Risk of bias. The risk of bias across ten
domains was assessed using SYRCLE (Table SIII). Overall, the methodological
quality of the included studies was moderate, with a number of
domains judged as unclear or high risk. Adequate sequence
generation was reported in 1/8 studies, while baseline
characteristics were comparable in 7/8 (37-41,43,44).
Allocation concealment was not described in any report and
therefore was rated as high risk across all the studies. Random
housing and outcome assessments were not reported in any study,
resulting in universally unclear ratings. A total of three studies
(37,38,44)
described the blinding of experimenters and the blinding of outcome
assessors. By contrast, incomplete outcome data were adequately
addressed in all studies and selective outcome reporting was judged
as low risk in 7/8 (37-41,43,44).
Other sources of bias were consistently rated as low risk.
Collectively, these findings indicated that although the internal
validity of the included studies was limited by insufficient
reporting of randomization, housing and blinding, the risk of
attrition and reporting bias was generally low.
Level of evidence. Level of evidence for
SOST-positive cells, ABL and related subgroups was determined to be
moderate. However, the levels of evidence for TMD, BVF, OCN, P1NP
and TRAP5b were lower (Table
SIV). The decrease in the level of evidence was primarily due
to inconsistency and imprecision or less overlap in CI, small
P-values for the heterogeneity test, large I2 value and
failure to perform sensitivity analyses. None of the included
studies reported osteonecrosis or other adverse events associated
with sclerostin inhibition
Discussion
The present analysis highlighted the potential of
Scl inhibition in mitigating ABL and promoting osteogenesis in
experimental periodontitis models (37,38,41-43).
The mechanisms underlying these effects are complex but may involve
key pathways including Wnt/β-catenin signaling, which regulates
osteoblast differentiation and bone formation (13-15,41,43).
In addition, Scl inhibition may act via the RANKL/OPG pathway,
thereby balancing osteoclast activity and limiting excessive bone
resorption (16,37,40,42).
The decrease in the number of SOST-positive cells further suggested
that Scl inhibition effectively targets its intended biological
pathway at the histological level (39,41,44).
Scl inhibition not only attenuated alveolar bone
loss (ABL) but also promoted regeneration through three primary
mechanisms. First, activation of the canonical Wnt pathway restores
β-catenin translocation to the nucleus, rescuing osteogenic
differentiation in periodontal ligament stem cells (13-15,41,43,45).
Second, modulation of the RANKL/OPG pathway limits
osteoclastogenesis by suppressing macrophage colony-stimulating
factor secretion from platelet-derived growth factor receptor-β
(PDGFRβ)-positive osteoprogenitors (16,37,40,42,46,47).
Third, disruption of the TNF-α/NF-κB/SOST feedback loop reduces
inflammation-induced osteocyte apoptosis and RANKL production,
thereby supporting bone preservation and regeneration (39,48,49).
Together, these multi-pathway interactions highlight the key role
of Scl in periodontitis, namely bone microenvironment
crosstalk.
Low-dose regimens (5 mg/kg) (38) showed greater efficacy compared with
higher doses (25 mg/kg), particularly in maintaining bone formation
and decreasing ABL (37,41,42,44).
This paradox suggests that treatment duration and timing may be
more influential compared with dose alone. In periodontal models,
longer treatment (22 vs. 4-6 weeks) promoted cumulative Wnt
activation, underscoring the importance of optimizing both dose and
duration for clinical translation (38). Administration route also influenced
outcomes, with subcutaneous delivery (37,40,42)
outperforming intraperitoneal injection, potentially due to
decreased hepatic first-pass metabolism (38,41,45).
However, the current evidence is limited and the superiority of
lower-dose regimens should be interpreted cautiously. More data are
needed to confirm the optimal dose-time association and future
studies should systematically examine dosing schedules and
treatment durations to validate these observations.
The present study primarily reflects therapeutic
models, as preventive evidence was scarce. A number of studies
applied therapeutic interventions following ligature removal
(37,41-43).
Taut et al (42) tested
both preventive and therapeutic arms, but only the therapeutic arm
reported quantitative data, hindering pooled analysis of preventive
effects. Hadaya et al (38)
retained ligatures during treatment to model persistent
inflammation. Compared with inflammation-resolved therapeutic
models, this design produced a larger effect size for ABL but less
pronounced regenerative benefits. These findings suggested that
persistent inflammation limits the anabolic effects of Scl
inhibition, even when short-term improvements in bone turnover
markers are observed. The coexistence of preventive and therapeutic
designs may therefore obscure biological interpretation and should
be considered when extrapolating to clinical contexts.
To assess the robustness of the present results, a
leave-one-out sensitivity analysis was performed, sequentially
excluding each study and recalculating the pooled effect size.
Results remained consistent across all iterations, indicating that
no single study disproportionately influenced the overall findings.
This supported the validity of the present conclusions despite
heterogeneity and small sample sizes.
Notably, transient increases in bone formation
markers such as P1NP have been reported, consistent with findings
from osteoporosis models (38,42,46).
None of the included studies reported osteonecrosis or similar
adverse events (38). This
suggested a favorable safety profile compared with antiresorptive
therapies such as bisphosphonates, which are associated with
medication-related osteonecrosis of the jaw. The absence of major
adverse outcomes supports the translational potential of Scl
inhibition.
A number of limitations should be acknowledged.
First, heterogeneity across studies was moderate-high, stemming
from differences in animal species, periodontitis induction method,
intervention protocol (dose, duration, route of administration,
preventive vs. therapeutic design and presence or absence of
residual inflammation). These variations complicate direct
comparisons and may explain the variability in effect sizes.
However, sensitivity analysis demonstrated that the overall
conclusions remained robust despite this heterogeneity. Second, the
overall risk of bias was high or unclear across a number of
domains. A number of studies did not adequately describe
randomization procedures and allocation concealment was rarely
reported, raising concerns regarding potential selection bias.
While a number of studies reported blinding of investigators or
outcome assessors, these practices were inconsistent, increasing
the risk of performance and detection bias. In addition, small
sample size and incomplete methodological reporting further
decreased the certainty of evidence and may limit reproducibility.
These shortcomings highlight the need for stricter adherence to
established guidelines such as the ARRIVE guidelines (50) or SYRCLE (35), which emphasize transparent
reporting of randomization, allocation concealment and blinding.
Strengthening these aspects in future animal studies may minimize
bias and improve reliability. Third, the use of different outcome
assessment methods created additional inconsistency. Techniques
such as micro-CT, histology, immunohistochemistry and serum
biomarkers vary in sensitivity, quantification accuracy and the
biological processes they capture. For example, micro-CT provides
precise three-dimensional measurements of alveolar bone, whereas
histology and immunohistochemistry yield localized and often
semi-quantitative information. Serum biomarkers, by contrast,
reflect systemic rather than site-specific changes in bone
turnover. Furthermore, different biomarkers (such as OCN, P1NP and
TRAP5b) and detection approaches (immunohistochemistry vs. serum
assays) were used across studies. These discrepancies complicate
comparisons, contribute to variability in effect sizes and may
explain why some results had low statistical significance. Such
inconsistencies may also affect the reliability of the results.
Consequently, the overall certainty of evidence was downgraded to
moderate to low according to GRADE. Future studies should use
standardized and validated detection protocols or coordinate
primary outcome measures and biomarker panels, to enhance
comparability, reproducibility and reliability. Finally, the
predominance of ligature-induced models (7/8 studies) limits the
generalizability of findings to bacterially driven periodontitis
(37-44).
These limitations underscore the need for more rigorous and
standardized protocols in future. Clear reporting of randomization,
allocation concealment and blinding, along with coordination of
biomarker detection methods and outcome assessments, are key in
reducing heterogeneity and improving evidence quality. In parallel,
systematic investigation of preventive vs. therapeutic settings,
optimized dose-time regimens, alternative delivery routes and
diverse disease models are necessary to delineate the translational
potential of Scl inhibition in periodontal regeneration (37-44).
In conclusion, the present analysis suggested Scl
inhibition notably decreased ABL and enhanced the expression of
bone formation markers in preclinical periodontitis models. The
improved efficacy of low-dose subcutaneous regimens (5 mg/kg)
suggested that sustained Wnt activation rather than transient
peak-dose stimulation may underlie the superior therapeutic
response. These findings warrant clinical trials evaluating Scl-Ab
as an adjunct to conventional periodontal therapy (for example,
scaling and root planing), prioritizing dose optimization and
standardized imaging protocols.
Supplementary Material
Mechanism of sclerostin-mediated
regulation of bone remodeling, integrating Wnt/β-catenin,
RANKL/OPG, BMP and inflammatory pathways. LRP, lipoprotein
receptor-related protein; RANKL, receptor activator of nuclear
factor κ-B ligand; OPG, osteoprotegerin; Runx2, RUNX family
transcription factor 2; SOST, sclerostin.
Flow chart of study selection
according to PRISMA guidelines. WOS, Web of Science.
Subgroup analysis comparing studies
with ligature removal vs. retention. Scl, sclerostin; df, degrees
of freedom; LDD, low-dose doxycycline; CAPE, caffeic acid phenethyl
ester; SMD, standardized mean difference; IV, inverse variance
model.
Leave-one-out sensitivity analysis
showing the pooled effect size following sequential exclusion of
each study. LDD, low-dose doxycycline; CAPE, caffeic acid phenethyl
ester.
Search strategy.
Details of outcomes variables.
SYRCLE (The Systematic Review Centre
for Laboratory Animal Experimentation) results.
Level of evidence.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MY contributed to data analysis using RevMan and
EndNote software, constructed figures, analyzed data, and wrote and
revised the manuscript. YM conceived the study, constructed
figures, analyzed data, and wrote and revised the manuscript. XQ
designed the study methodology, contributed to project
administration and resources, and provided supervision. ZQ
contributed to the study conception, critical revision of the
manuscript for important intellectual content, and overall
supervision of the project. JS contributed to study validation,
data interpretation and resource coordination. MY and YM confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Abdulkareem AA, Al-Taweel FB, Al-Sharqi
AJB, Gul SS, Sha A and Chapple ILC: Current concepts in the
pathogenesis of periodontitis: From symbiosis to dysbiosis. J Oral
Microbiol. 15(2197779)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Meyle J and Chapple I: Molecular aspects
of the pathogenesis of periodontitis. Periodontol 2000. 69:7–17.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hajishengallis G and Chavakis T: Local and
systemic mechanisms linking periodontal disease and inflammatory
comorbidities. Nat Rev Immunol. 21:426–440. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tonetti MS, Greenwell H and Kornman KS:
Staging and grading of periodontitis: Framework and proposal of a
new classification and case definition. J Clin Periodontol 45
Suppl. 20:S149–S161. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kwon T, Lamster IB and Levin L: Current
concepts in the management of periodontitis. Int Dent J.
71:462–476. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Graziani F, Karapetsa D, Alonso B and
Herrera D: Nonsurgical and surgical treatment of periodontitis: How
many options for one disease? Periodontol 2000. 75:152–188.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Slots J: Periodontitis: Facts, fallacies
and the future. Periodontol 2000. 75:7–23. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Haque MM, Yerex K, Kelekis-Cholakis A and
Duan K: Advances in novel therapeutic approaches for periodontal
diseases. BMC Oral Health. 22(492)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Preshaw PM and Bissett SM: Periodontitis
and diabetes. Br Dent J. 227:577–584. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhou N, Zou F, Cheng X, Huang Y, Zou H,
Niu Q, Qiu Y, Shan F, Luo A, Teng W and Sun J: Porphyromonas
gingivalis induces periodontitis, causes immune imbalance, and
promotes rheumatoid arthritis. J Leukoc Biol. 110:461–473.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cardoso EM, Reis C and Manzanares-Céspedes
MC: Chronic periodontitis, inflammatory cytokines, and
interrelationship with other chronic diseases. Postgrad Med.
130:98–104. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Persson GR: Periodontal complications with
age. Periodontol 2000. 78:185–194. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Napimoga MH, Nametala C, da Silva FL,
Miranda TS, Bossonaro JP, Demasi AP and Duarte PM: Involvement of
the Wnt-β-catenin signalling antagonists, sclerostin and
dickkopf-related protein 1, in chronic periodontitis. J Clin
Periodontol. 41:550–557. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mihara A, Yukata K, Seki T, Iwanaga R,
Nishida N, Fujii K, Nagao Y and Sakai T: Effects of sclerostin
antibody on bone healing. World J Orthop. 12:651–659.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jacobsen CM: Application of
anti-Sclerostin therapy in non-osteoporosis disease models. Bone.
96:18–23. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kitaura H, Marahleh A, Ohori F, Noguchi T,
Shen WR, Qi J, Nara Y, Pramusita A, Kinjo R and Mizoguchi I:
Osteocyte-related cytokines regulate osteoclast formation and bone
resorption. Int J Mol Sci. 21(5169)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liao C, Liang S, Wang Y, Zhong T and Liu
X: Sclerostin is a promising therapeutic target for oral
inflammation and regenerative dentistry. J Transl Med.
20(221)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chatzopoulos GS, Costalonga M, Mansky KC
and Wolff LF: WNT-5a and SOST levels in gingival crevicular fluid
depend on the inflammatory and osteoclastogenic activities of
periodontal tissues. Medicina (Kaunas). 57(788)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yakar N, Guncu GN, Akman AC, Pınar A,
Karabulut E and Nohutcu RM: Evaluation of gingival crevicular fluid
and peri-implant crevicular fluid levels of sclerostin, TWEAK,
RANKL and OPG. Cytokine. 113:433–439. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chatzopoulos GS, Koidou VP and Wolff LF:
Expression of Wnt signaling agonists and antagonists in
periodontitis and healthy subjects, before and after non-surgical
periodontal treatment: A systematic review. J Periodontal Res.
57:698–710. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chatzopoulos GS, Mansky KC, Lunos S,
Costalonga M and Wolff LF: Sclerostin and WNT-5a gingival protein
levels in chronic periodontitis and health. J Periodontal Res.
54:555–565. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ozden FO, Demir E, Lutfioglu M, Acarel EE,
Bilgici B and Atmaca A: Effects of periodontal and bisphosphonate
treatment on the gingival crevicular levels of sclerostin and
dickkopf-1 in postmenopausal osteoporosis with and without
periodontitis. J Periodontal Res. 57:849–858. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Delgado-Calle J, Sato AY and Bellido T:
Role and mechanism of action of sclerostin in bone. Bone. 96:29–37.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Iwamoto R, Koide M, Udagawa N and
Kobayashi Y: Positive and negative regulators of sclerostin
expression. Int J Mol Sci. 23(4895)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tanaka S and Matsumoto T: Sclerostin: From
bench to bedside. J Bone Miner Metab. 39:332–340. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cosman F, Crittenden DB, Adachi JD,
Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki
EM, Miyauchi A, et al: Romosozumab treatment in postmenopausal
women with osteoporosis. N Engl J Med. 375:1532–1543.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Martiniakova M, Babikova M and Omelka R:
Pharmacological agents and natural compounds: Available treatments
for osteoporosis. J Physiol Pharmacol. 71:2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tinsley BA, Dukas A, Pensak MJ, Adams DJ,
Tang AH, Ominsky MS, Ke HZ and Lieberman JR: Systemic
administration of sclerostin antibody enhances bone morphogenetic
protein-induced femoral defect repair in a rat model. J Bone Joint
Surg Am. 97:1852–1859. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Korn P, Kramer I, Schlottig F, Tödtman N,
Eckelt U, Bürki A, Ferguson SJ, Kautz A, Schnabelrauch M, Range U,
et al: Systemic sclerostin antibody treatment increases
osseointegration and biomechanical competence of
zoledronic-acid-coated dental implants in a rat osteoporosis model.
Eur Cell Mater. 37:333–346. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Virdi AS, Irish J, Sena K, Liu M, Ke HZ,
McNulty MA and Sumner DR: Sclerostin antibody treatment improves
implant fixation in a model of severe osteoporosis. J Bone Joint
Surg Am. 97:133–140. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li X, Ominsky MS, Villasenor KS, Niu QT,
Asuncion FJ, Xia X, Grisanti M, Wronski TJ, Simonet WS and Ke HZ:
Sclerostin antibody reverses bone loss by increasing bone formation
and decreasing bone resorption in a rat model of male osteoporosis.
Endocrinology. 159:260–271. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cumpston M, Li T, Page MJ, Chandler J,
Welch VA, Higgins JP and Thomas J: Updated guidance for trusted
systematic reviews: A new edition of the cochrane handbook for
systematic reviews of interventions. Cochrane Database Syst Rev.
10(ED000142)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Drevon D, Fursa SR and Malcolm AL:
Intercoder reliability and validity of WebPlotDigitizer in
extracting graphed data. Behav Modif. 41:323–339. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hooijmans CR, Rovers MM, de Vries RB,
Leenaars M, Ritskes-Hoitinga M and Langendam MW: SYRCLE's risk of
bias tool for animal studies. BMC Med Res Methodol.
14(43)2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Higgins JPT and Green S: Cochrane handbook
for systematic reviews of interventions. Version 5.1.0. The
Cochrane Collaboration, London, 2011. www.handbook.cochrane.org.
|
|
37
|
Chen H, Xu X, Liu M, Zhang W, Ke HZ, Qin
A, Tang T and Lu E: Sclerostin antibody treatment causes greater
alveolar crest height and bone mass in an ovariectomized rat model
of localized periodontitis. Bone. 76:141–148. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hadaya D, Gkouveris I, Soundia A,
Bezouglaia O, Boyce RW, Stolina M, Dwyer D, Dry SM, Pirih FQ,
Aghaloo TL and Tetradis S: Clinically relevant doses of sclerostin
antibody do not induce osteonecrosis of the jaw (ONJ) in rats with
experimental periodontitis. J Bone Miner Res. 34:171–181.
2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kim JH, Kim AR, Choi YH, Jang S, Woo GH,
Cha JH, Bak EJ and Yoo YJ: Tumor necrosis factor-α antagonist
diminishes osteocytic RANKL and sclerostin expression in diabetes
rats with periodontitis. PLoS One. 12(e0189702)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ozaki Y, Koide M, Furuya Y, Ninomiya T,
Yasuda H, Nakamura M, Kobayashi Y, Takahashi N, Yoshinari N and
Udagawa N: Treatment of OPG-deficient mice with WP9QY, a
RANKL-binding peptide, recovers alveolar bone loss by suppressing
osteoclastogenesis and enhancing osteoblastogenesis. PLoS One.
12(e0184904)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ren Y, Han X, Ho SP, Harris SE, Cao Z,
Economides AN, Qin C, Ke H, Liu M and Feng JQ: Removal of SOST or
blocking its product sclerostin rescues defects in the
periodontitis mouse model. FASEB J. 29:2702–2711. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Taut AD, Jin Q, Chung JH, Galindo-Moreno
P, Yi ES, Sugai JV, Ke HZ, Liu M and Giannobile WV: Sclerostin
antibody stimulates bone regeneration after experimental
periodontitis. J Bone Miner Res. 28:2347–2356. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yang X, Han X, Shu R, Jiang F, Xu L, Xue
C, Chen T and Bai D: Effect of sclerostin removal in vivo on
experimental periodontitis in mice. J Oral Sci. 58:271–276.
2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yiğit U, Kırzıoğlu FY and Özmen Ö: Effects
of low dose doxycycline and caffeic acid phenethyl ester on
sclerostin and bone morphogenic protein-2 expressions in
experimental periodontitis. Biotech Histochem. 97:567–575.
2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ominsky MS, Boyce RW, Li X and Ke HZ:
Effects of sclerostin antibodies in animal models of osteoporosis.
Bone. 96:63–75. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ashifa N, Viswanathan K, Sundaram R and
Srinivasan S: Sclerostin and its role as a bone modifying agent in
periodontal disease. J Oral Biosci. 63:104–110. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
de Vries TJ and Huesa C: The osteocyte as
a novel key player in understanding periodontitis through its
expression of RANKL and sclerostin: A review. Curr Osteoporos Rep.
17:116–121. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sapir-Koren R and Livshits G: Osteocyte
control of bone remodeling: Is sclerostin a key molecular
coordinator of the balanced bone resorption-formation cycles?
Osteoporos Int. 25:2685–2700. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Li X, Niu QT, Warmington KS, Asuncion FJ,
Dwyer D, Grisanti M, Han CY, Stolina M, Eschenberg MJ, Kostenuik
PJ, et al: Progressive increases in bone mass and bone strength in
an ovariectomized rat model of osteoporosis after 26 weeks of
treatment with a sclerostin antibody. Endocrinology. 155:4785–4797.
2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Percie du Sert N, Hurst V, Ahluwalia A,
Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl
U, et al: The ARRIVE guidelines 2.0: Updated guidelines for
reporting animal research. PLoS Biol. 18(e3000410)2020.PubMed/NCBI View Article : Google Scholar
|