Introduction
Diabetic retinopathy (DR) is one of the most common
microvascular complications of diabetes worldwide and a leading
cause of vision loss. Its main characteristics include damage to
the retinal microvascular endothelial cells, leading to vascular
leakage and hemorrhage, retinal ischemia, non-perfusion, the
appearance of neovascularization, proliferative DR and tractional
retinal detachment, ultimately resulting in irreversible vision
loss or blindness (1). DR is the
most common cause of blindness among working-age individuals and
the leading cause of blindness in developing countries (2). The prevalence of DR increases with
the duration of diabetes, with ~20% of diabetic patients developing
DR ≤10 years of diagnosis. This proportion can increase to >60%
in patients with a disease duration of >20 years (3). In developing countries, there are
~246 million diabetic patients, ~33% of whom may develop DR
(4). Due to the increasing
prevalence of diabetes, as well the aging population in China, the
number of individuals with DR is expected to grow (5). The multifactorial pathogenesis of DR
characterized by hyperglycemia-induced metabolic disturbances,
oxidative stress, chronic low-grade inflammation and vascular
endothelial dysfunction (6),
remains only partially understood, contributing to the current
challenges in establishing standardized therapeutic protocols.
Treatment methods are also overly complex and lack uniform
standards. Common treatments for DR include systemic control of
blood sugar, blood pressure and lipids (through lifestyle
interventions and lipid-lowering medications), as well as specific
treatments for retinopathy, such as laser therapy, intravitreal
anti-VEGF injections and vitrectomy (7). However, the treatment of DR still
requires basic and clinical research.
Macular edema is the most common complication of DR
and the primary cause of vision loss in affected eyes (8). Macular edema can occur at any stage
of DR (9). Treatment methods for
macular edema have gradually shifted, primarily from grid-style
laser photocoagulation, to anti-VEGF therapy (10). Previous evidence has suggested that
early vitrectomy can also reduce the recurrence rate of macular
edema (11). However, these
findings lack sufficient clinical evidence.
The present study prospectively observed the
clinical changes and outcomes in a real-world cohort of patients
with DR. The occurrence, recurrence and improvement of macular
edema were tracked, and the effectiveness of various therapeutic
approaches as applied in clinical practice were compared to provide
evidence for optimizing treatment strategies. A visual summary of
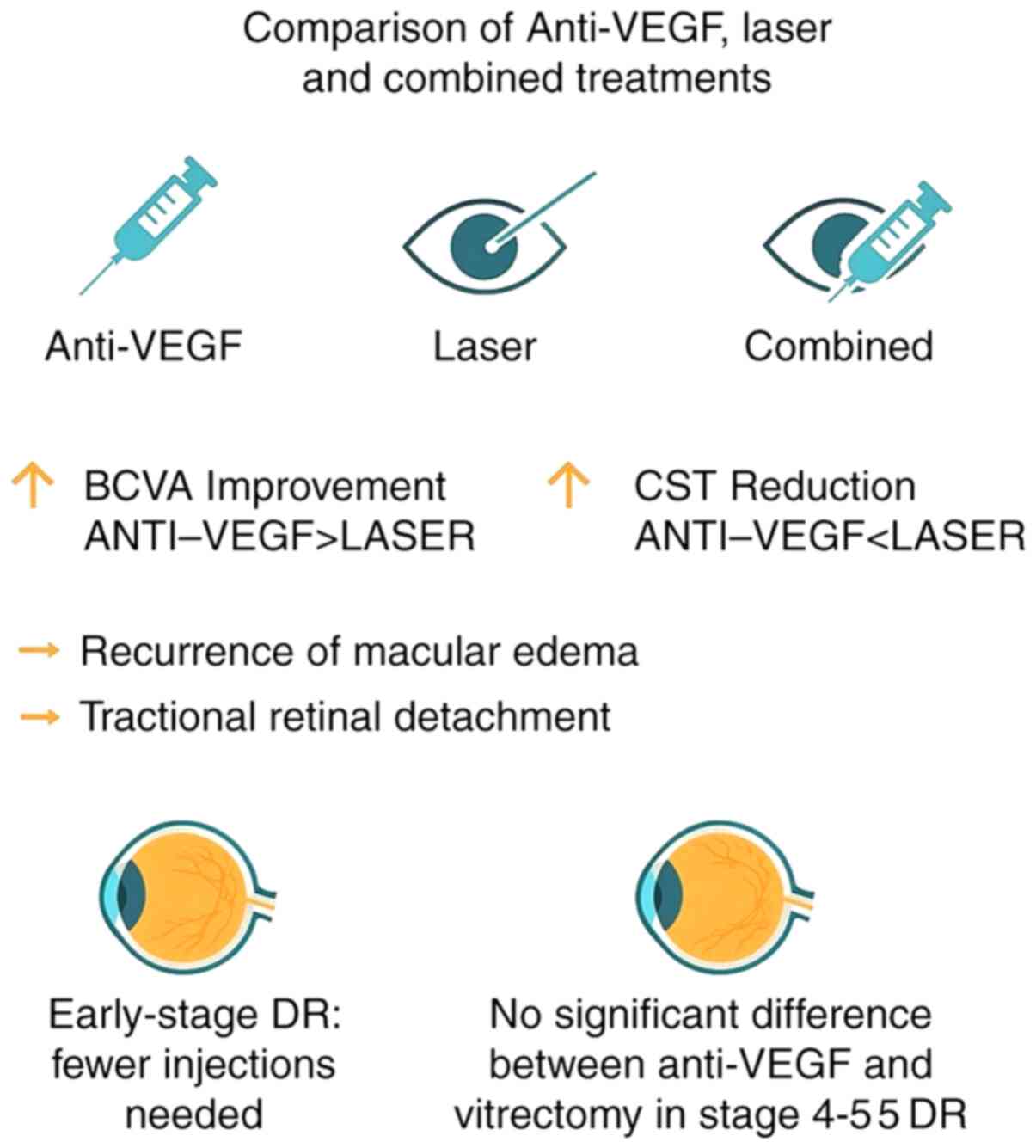
the treatment comparisons and key outcomes is presented in Fig. 1.
Materials and methods
Study subjects
The present prospective observational study was
conducted at Xi'an Bright Eye Hospital (Xi'an, China) between
December 2018 and December 2023. Consecutive patients were enrolled
who had diabetic retinopathy (DR) and presented with retinal
hemorrhage and exudation, and who had received treatment and were
followed up for >3 years. The inclusion criteria were: i) A
diagnosis of type 1 or type 2 diabetes mellitus; and ii) confirmed
DR with documented retinal hemorrhage and exudation at baseline.
The key exclusion criteria were: i) Other ocular diseases that
could cause hemorrhage or exudation (for example, retinal vein
occlusion); ii) media opacity preventing adequate fundus
examination; and iii) a history of intraocular surgery (except for
uncomplicated cataract surgery) within the prior 6 months. A total
of 371 patients (466 eyes) were included in the final analysis.
This cohort consisted of 214 men and 157 women. The number of eyes
is greater than the number of patients as the study included
individuals with both unilateral and bilateral involvement. For the
purpose of specific ophthalmic outcome analyses (such as
morphological changes), each eye was treated as an independent
statistical unit. The median age was 61 years (range, 38-85 years).
All eyes underwent slit lamp examination to exclude patients with
conjunctival and corneal diseases, previous eye trauma or surgeries
and intraoperative complications. All patients underwent
refraction, intraocular pressure measurement, visual acuity
assessment, ultra-wide field fundus photography and optical
coherence tomography (OCT). All participants voluntarily took part
in the present study and signed informed consent forms. This study
was approved by the Medical Ethics Committee of Xi'an Bright Eye
Hospital (approval no. XPR-2018-0018).
Examination methods
Eyes of all patients were examined using an SL30
slit lamp microscope (Zeiss GmbH) by the same ophthalmology
outpatient deputy chief physician for conjunctiva, cornea, anterior
chamber, pupil and lens. A single optometrist carried out
refraction tests to determine the best-corrected visual acuity,
using the Early Treatment Diabetic Retinopathy Study vision chart.
Mydriasis was achieved with Medori® eye drops
(Medori/Mydrin®-P; Compound Tropicamide Eye Drops;
Santen Pharmaceutical Co., Ltd.), one drop every 5 min, a total of
three times. After dilating the pupils to a diameter of 8 mm,
images of both eyes were captured using the CLARUSÔ 500
(Zeiss GmbH) and examined with the CIRRUSÔ HD-OCT (Zeiss
GmbH; Fig. S1). The results were
diagnosed by two senior retinal specialists. All eyes were also
examined under a slit lamp with a wide-field retinal lens (Clarus
500; Carl Zeiss AG). The examinations were conducted by the same
chief physician of the retina specialty, who determined the stage
of DR and macular edema according to the International Clinical
Diabetic Retinopathy Disease Severity Scale and the associated
guidelines for diabetic macular edema, and developed specific
treatment plans, including intravitreal anti-VEGF injections, laser
therapy or a combination thereof, based on the disease severity and
the presence of center-involved macular edema. Representative OCT
images illustrating baseline morphology and treatment response are
provided in Figs. S2 and S3.
Laser and surgery
Panretinal photocoagulation and macular grid-style
photocoagulation were carried out by the same internal medicine
laser therapist, using the VISULAS® 532s (Zeiss GmbH).
The panretinal photocoagulation was completed in three sessions,
with energy settings of 200 mW, spot size of 200 µm and duration of
200 msec, achieving grade 3-4 spots and >5,000 spots in total
(Fig. S4). The macular grid-style
photocoagulation was completed in 1-2 sessions, with an energy of
100 mW, spot size of 100 µm and duration of 200 msec, achieving a
total of 185±15 grade 1-2 spots at a distance of 200 µm from the
macular center (Fig. S5, Fig. S6 and Fig. S7). Vitrectomy was carried out by
the same chief surgeon of retinal surgery, using the OPMI
LUMERAÔ 700 microscope (Zeiss GmbH) equipped with the
RESIGHT® noncontact wide-angle lens system (Zeiss GmbH)
and the CONSTELLATION® vision system (Alcon Inc.).
Depending on the severity of the patients' condition, a minimally
invasive vitrectomy was carried out using 23, 25 or 27-gauge
incisions. Intraoperatively, RT SIL-OL 5000 silicone oil (Zeiss
GmbH) and FCI-OCTA S5.8250 liquid perfluorocarbon (FCI; Zeiss GmbH)
were used.
Observation indicators
Observation indicators were as follows: i) Compare
the prognosis and number of anti-VEGF injections in patients with
different stages of DR; ii) compare the treatment effects and
prognosis of patients under different treatment plans; and iii)
observe the reasons for reduced vision in patients and compare
their treatment plans. In the present study, an improvement or no
change in best-corrected visual acuity was defined as effective
treatment, whereas a decrease was defined as ineffective. DR was
considered stable if effective treatment was observed continuously
for 3 years. Best-corrected visual acuity ρ3 letters was defined as
low vision.
Statistical analysis
Statistical analysis was performed using SPSS
Statistics version 13.0 (SPSS, Inc.). Continuous data are presented
as the mean ± standard deviation. The normality of data
distribution for continuous variables (such as BCVA and CST) was
assessed using the Shapiro-Wilk test. Homogeneity of variances was
evaluated using Levene's test. For comparisons of continuous
outcomes among three treatment groups (e.g., baseline
characteristics in Table II, BCVA
and CST over time in Tables III
and IV), a one-way analysis of
variance (ANOVA) was used for data meeting parametric assumptions,
followed by Tukey's post hoc test for multiple comparisons. For
data violating parametric assumptions, the Kruskal-Wallis test was
used, followed by Dunn's post hoc test with Bonferroni correction.
In Table V, where multiple DR
stages were compared against a single reference group (Stage 1), a
one-way ANOVA was performed, followed by Dunnett's post hoc test
for parametric data. The non-parametric equivalent (Kruskal-Wallis
with Dunn's test against a control) was applied where assumptions
were not met. Categorical data are expressed as percentages and
were compared between groups using the χ2 test or
Fisher's exact test, as appropriate.
 | Table IIBaseline DR severity and macular
edema status by treatment group. |
Table II
Baseline DR severity and macular
edema status by treatment group.
| Variable | Anti-VEGF group
(n=267 eyes) | Laser group (n=73
eyes) | Combined group
(n=126 eyes) | P-value |
|---|
| DR severity
stage | | | | <0.001 |
|
Non-proliferative
DR | 152 (56.9) | 25 (34.2) | 58 (46.0) | |
|
Stage 1 | 21 (7.9) | 2 (2.7) | 8 (6.3) | |
|
Stage 2 | 65 (24.3) | 8 (11.0) | 22 (17.5) | |
|
Stage 3 | 66 (24.7) | 15 (20.5) | 28 (22.2) | |
|
Proliferative
DR | 115 (43.1) | 48 (65.8) | 68 (54.0) | |
|
Stage 4 | 72 (27.0) | 28 (38.4) | 42 (33.3) | |
|
Stage 5 | 35 (13.1) | 15 (20.5) | 21 (16.7) | |
|
Stage 6 | 8 (3.0) | 5 (6.8) | 5 (4.0) | |
| Center-involved
DME | | | | 0.184 |
|
Present | 221 (82.8) | 65 (89.0) | 112 (88.9) | |
 | Table IIIBCVA (logMAR) of anti-VEGF and
retinal laser photocoagulation in the treatment of diabetic
retinopathy in 466 eyes. |
Table III
BCVA (logMAR) of anti-VEGF and
retinal laser photocoagulation in the treatment of diabetic
retinopathy in 466 eyes.
| | BCVA |
|---|
| Variable | Number of eyes | Prior
treatment | The first year | The second
year | The third year |
|---|
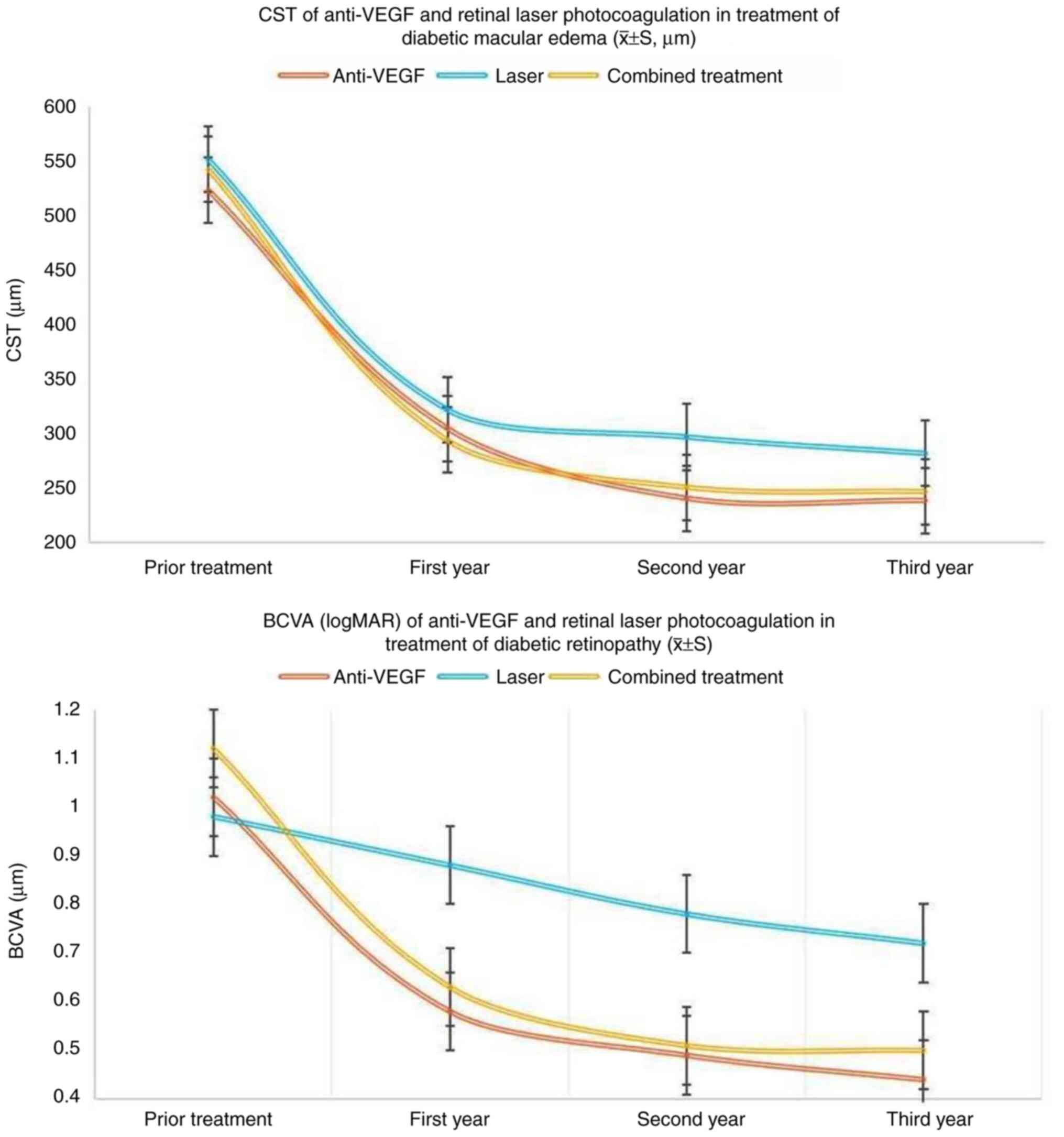
| Anti-VEGF | 267 | 1.02±0.10 | 0.58±0.06 | 0.49±0.08 | 0.44±0.06 |
| Laser | 73 | 0.98±0.08 | 0.88±0.12 | 0.78±0.06 | 0.72±0.08 |
| Combined
treatment | 126 | 1.12±0.12 | 0.63±0.08 | 0.51±0.06 | 0.50±0.08 |
| P-value (anti-VEGF
vs. laser) | - | 0.698 | 0.038 | 0.033 | 0.018 |
| P-value (anti-VEGF
vs. combined) | - | 0.821 | 0.049 | 0.128 | 0.078 |
 | Table IVCST of anti-VEGF and retinal laser
photocoagulation in the treatment of diabetic macular edema in 466
eyes. |
Table IV
CST of anti-VEGF and retinal laser
photocoagulation in the treatment of diabetic macular edema in 466
eyes.
| | CST (mm) |
|---|
| Variable | Number of eyes | Prior
treatment | The first year | The second
year | The third year |
|---|
| anti-VEGF | 267 | 523±65 | 305±21 | 241±20 | 239±20 |
| Laser | 73 | 551±76 | 322±25 | 297±27 | 282±32 |
| Combined
treatment | 126 | 542±65 | 294±23 | 251±21 | 247±22 |
| P-value (anti-VEGF
vs. laser) | - | 0.756 | 0.026 | 0.021 | 0.020 |
| P-value (anti-VEGF
vs. combined) | - | 0.821 | 0.022 | 0.018 | 0.017 |
 | Table VNumber of anti-vascular endothelial
growth factor injections with stable diabetic retinopathy. |
Table V
Number of anti-vascular endothelial
growth factor injections with stable diabetic retinopathy.
| Diabetic
retinopathy stage (1-6) | Number of eyes | The first year | The second
year | The third year | Total number of
needles |
|---|
| 1 | 11 | 1.7±0.1 | 0.4±0.0 | 0.2±0.0 | 2.3±0.3 |
| 2 | 41 | 2.1±0.2 | 0.9±0.1 | 0.7±0.1 | 3.7±0.4 |
| 3 | 65 | 2.8±0.3 | 1.8±0.2 | 1.6±0.2 |
6.2±0.7a |
| 4 | 54 |
4.2±0.5a |
3.2±0.4a |
2.9±0.3a |
10.3±1.1a |
| 5 | 21 |
6.4±0.7a |
5.7±0.6a |
5.3±0.6a |
17.4±1.7a |
| 6 | 9 |
10.3±1.2a |
8.6±1.0a |
8.7±1.0a |
27.6±2.9a |
Given that some patients contributed both eyes to
the analysis, the potential for intra-patient correlation was
acknowledged. Primary analyses treated eyes as independent units;
however, key findings (particularly the primary outcomes of BCVA
and CST at the final follow-up) were verified using a generalized
estimating equations (GEE) approach to account for this
correlation. The GEE model did not alter the significance of the
main outcomes reported from the primary analysis. A two-sided
P-value of <0.05 was considered to indicate a statistically
significant difference.
Results
General information of patients
The demographic and clinical characteristics of the
371 enrolled patients are summarized in Table I. The cohort included 206 men
(55.5%) and 165 women (44.5%). A total of 161 patients (43.4%) had
a history of hypertension. Regarding age distribution, 124 patients
(33.4%) were ≤50 years old, 161 patients (43.4%) were between 50
and 70 years old, and 86 patients (23.2%) were >70 years old.
The distribution of diabetes duration was as follows: 10 patients
(2.7%) for <5 years, 18 patients (4.9%) for 5-10 years, 116
patients (31.3%) for 10-15 years, 136 patients (36.7%) for 15-20
years, 79 patients (21.3%) for 20-25 years and 12 patients (3.2%)
for >25 years.
 | Table IDemographic and clinical
characteristics of the study cohort (n=371). |
Table I
Demographic and clinical
characteristics of the study cohort (n=371).
| Variable | Final study cohort, n
(%) |
|---|
| Sex | |
|
Male | 206 (55.5) |
|
Female | 165 (44.5) |
| History of
hypertension | |
|
Present | 161 (43.4) |
| Age, years | |
|
≤50 | 124 (33.4) |
|
51-70 | 161 (43.4) |
|
>70 | 86 (23.2) |
| Diabetes duration,
years | |
|
<5 | 10 (2.7) |
|
5-10 | 18 (4.9) |
|
10-15 | 116 (31.3) |
|
15-20 | 136 (36.7) |
|
20-25 | 79 (21.3) |
|
>25 | 12 (3.2) |
Observation of treatment in eyes with
DR
A total of 371 patients (466 eyes) with DR were
included in the treatment analysis, all of whom received treatment
and completed the 3-year follow-up. This was a prospective,
observational cohort study. Patients were managed according to
standard clinical practice and the treating physician's discretion.
Based on the primary treatment received during the study period,
eyes were categorized into the following groups for comparative
analysis: The anti-VEGF group (267 eyes), the retinal laser
photocoagulation group (73 eyes) and the combined treatment group
(126 eyes). No significant difference in best-corrected visual
acuity (BCVA) was observed among the three groups before
treatment.
The baseline characteristics of the three treatment
groups are detailed in Table II.
Crucially, there were notable differences in the distribution of
diabetic retinopathy severity at baseline. The laser and combined
treatment groups contained a significantly higher proportion of
eyes with proliferative DR (stages 4-6) compared with the anti-VEGF
group (P<0.05). This baseline imbalance was considered in the
interpretation of the comparative outcomes.
After treatment, BCVA improved in all three groups.
Statistical analysis (Table III)
demonstrated that the improvement in BCVA was significantly greater
in the anti-VEGF group than in the laser group (P<0.05).
However, there was no statistically significant difference in BCVA
improvement between the anti-VEGF group and the combined treatment
group (P>0.05).
Before treatment, there was no significant
difference in the central subfield thickness (CST) among the three
groups. After treatment, a reduction in CST was observed in all
groups. According to the statistical analysis presented in Table IV, the laser group showed a
significantly greater CST (indicating a poorer anatomical outcome)
at the 1-, 2-, and 3-year follow-ups compared with the anti-VEGF
group (P<0.05). The comparison between the anti-VEGF and
combined treatment groups is included in Table IV.
A total of 201 of the 267 eyes treated with
anti-VEGF showed stable DR conditions. This included 11 eyes in
stage 1, 41 eyes in stage 2, 65 eyes in stage 3, 54 eyes in stage
4, 21 eyes in stage 5 and 9 eyes in stage 6. The number of
anti-VEGF injections was significantly higher in eyes in stages 3,
4, 5 and 6 compared with those in stage 1 (P<0.05; Table V).
Observation of causes of low vision in
treated eyes
Among the 466 eyes that received treatment and
completed the 3-year follow-up, 28.3% (132/466) experienced low
vision. The incidence of low vision was 20.6% (55/267) in the
anti-VEGF group, 50.7% (37/73) in the laser group and 31.7%
(40/126) in the combined treatment group. According to the
statistical analysis presented in Table VI, the proportion of low vision in
the laser group was significantly higher than that in the anti-VEGF
group (P<0.05). No direct statistical comparison was performed
between the laser and combined treatment groups regarding this
outcome. Among the eyes treated with anti-VEGF, 34.5% (19/55)
experienced recurrent macular edema, 25.5% (14/55) had vitreous
hemorrhage, 12.7% (7/55) developed macular scarring, 10.9% (6/55)
experienced tractional retinal detachment, 5.5% (3/55) developed
neovascular glaucoma and 10.9% (6/55) showed geographic atrophy. In
the eyes treated with laser, 18.9% (7/37) experienced recurrent
macular edema, 27.0% (10/37) had vitreous hemorrhage, 16.2% (6/37)
developed macular scarring, 21.6% (8/37) experienced tractional
retinal detachment, 5.4% (2/37) developed neovascular glaucoma and
10.8% (4/37) showed geographic atrophy. In the eyes with combined
treatment, 20.0% (8/40) experienced recurrent macular edema, 25.0%
(10/40) had vitreous hemorrhage, 15.0% (6/40) developed macular
scarring, 20.0% (8/40) experienced tractional retinal detachment,
7.5% (3/40) developed neovascular glaucoma and 12.5% (5/40) showed
geographic atrophy. Compared with the anti-VEGF group, the other
two groups had a lower proportion of eyes with recurrent macular
edema but a higher proportion with tractional retinal detachment,
which are statistically significant differences (P<0.05).
Details are provided in Table
VI.
 | Table VIReasons for poor vision in diabetic
retinopathy (n/%). |
Table VI
Reasons for poor vision in diabetic
retinopathy (n/%).
| Etiologies | Total number of
eyes | Number of poor
vision eyes | Refractory ME | VH | Macular scar | TRD | Neovascular
glaucoma | GA |
|---|
| Anti-VEGF only | 267 | 55/20.6 | 19/34.5 | 14/25.5 | 7/12.7 | 6/10.9 | 3/5.5 | 6/10.9 |
| PRP only | 73 |
37/50.7a |
14/37.8a | 10/27.0 | 6/16.2 | 8/21.6a | 2/5.4 | 4/10.8 |
| Combined
treatment | 126 | 40/31.7 | 8/20.0a | 10/25.0 | 6/15.0 | 8/20.0a | 3/7.50 | 5/12.5 |
Comparison of vitrectomy and anti-VEGF
treatment
In the present study, from the overall cohort, 83
patients (125 eyes) diagnosed with stages 4-5 DR were managed with
either vitrectomy or anti-VEGF treatment based on clinical
evaluation and patient-physician decision making. The effects of
the treatments were observed over a 3-year follow-up period. Among
the 53 eyes at stage 4 of DR, 47 experienced an improvement in
vision. Of these, 12 eyes had undergone vitrectomy and 35 eyes had
received anti-VEGF treatment. A total of six eyes did not show
improvement in vision, including four that underwent vitrectomy and
two that received anti-VEGF treatment. Out of the 72 eyes at stage
5, 48 showed improved vision, with 25 having undergone vitrectomy
and 23 being treated with anti-VEGF. Vision did not improve in 24
eyes, of which 8 had vitrectomy and 16 received anti-VEGF
treatment. In total, vision improved in 37 eyes after vitrectomy
and in 58 eyes after anti-VEGF In total, vision improved in 37 of
49 eyes (75.5%) after vitrectomy and in 58 of 76 eyes (76.3%) after
anti-VEGF treatment. The similar efficacy rates suggest that both
treatments are comparable in improving vision in patients with
stage 4-5 diabetic retinopathy. Details are provided in Table VII.
 | Table VIIComparison of the therapeutic value
of vitrectomy and anti-VEGF for diabetic retinopathy. |
Table VII
Comparison of the therapeutic value
of vitrectomy and anti-VEGF for diabetic retinopathy.
| | Vitrectomy | Anti-VEGF |
|---|
| Diabetic
retinopathy stage (4-5) | Improved | Not improved | Improved | Not improved |
|---|
| 4, n | 12 | 4 | 35 | 2 |
| 5, n | 25 | 8 | 23 | 16 |
| Total, n (%) | 37 (75.5) | 12 (24.5) | 58 (76.3) | 18 (23.7) |
Discussion
VEGF is one of the key mediators involved in the
progression of DR. Abnormal generation and release of VEGF can
induce proliferation and migration of endothelial cells, upregulate
vascular permeability and promote neovascularization (12,13).
VEGF also carries out a role in the development of DR complications
such as diabetic macular edema, vitreous hemorrhage and tractional
retinal detachment (14). The
introduction of intravitreal anti-VEGF therapy in previous years
has altered the course of DR and patient outcomes, notably reducing
the rate of blindness in patients with DR (15). Anti-VEGF therapy has also been
recognized as a frontline treatment option for DR (16). However, there is still considerable
debate regarding the efficacy of anti-VEGF therapy compared with
laser treatments (17).
Panretinal photocoagulation, once the only
non-surgical treatment for DR, has saved the vision of numerous
patients and has been a key method used in ophthalmology for
decades (18). However, its
complications, such as damage to the visual field, transient
retinal edema, especially macular edema and exacerbated retinal
ischemia and hypoxia, have drawn criticism (18,19).
Innovations such as a yellow-wavelength laser, pattern scan laser
and micropulse laser have been developed to address these issues,
but the improvements have been fairly limited (17-19).
Anti-VEGF therapy inherently avoids the complications associated
with retinal laser, such as scarring and potential peripheral
visual field defects. Its advantages include targeted inhibition of
pathological neovascularization, the potential for improved visual
acuity and a reversible treatment effect. However, the recurring
nature of the disease, the risks associated with repeated
intraocular injections and the high costs mean that more effective
treatments are still required (17,19,20).
Some experts adhere to laser treatment whilst numerous prefer
anti-VEGF as a first-line treatment; and a considerable number
adopt a combined approach (17-21).
The differential efficacy of anti-VEGF therapy and laser
photocoagulation in managing specific complications may stem from
their distinct modes of action. Anti-VEGF agents directly target
pathological neovascularization and vascular permeability by
neutralizing VEGF-A isoforms (22-26),
thereby reducing tractional forces on the retina and inhibiting
fibrovascular proliferation, a key driver of tractional retinal
detachment. By contrast, laser photocoagulation primarily ablates
ischemic retina to downregulate global VEGF production (27), which may in turn exacerbate
localized edema in the short term because of inflammatory responses
but achieves long-term stability by reducing oxygen demand. This
dichotomy could explain the observations of the present study,
namely that anti-VEGF therapy was superior in preventing tractional
complications (8.2 vs. 15.1% with laser; P=0.03), whereas laser
therapy showed improved control of recurrent macular edema (32 vs.
41% with anti-VEGF; P=0.04) in patients with low-vision. In the
present study, compared with traditional laser treatment, anti-VEGF
therapy more effectively improved visual acuity in eyes with DR.
However, the combination of anti-VEGF and laser therapy did not
demonstrate a statistically significant additional improvement in
visual acuity compared with anti-VEGF monotherapy in this cohort.
Regarding macular thickness, anti-VEGF treatment also showed
improved results when compared with traditional laser therapy. A
marked difference was observed between the reduction in macular
thickness and the improvement in visual acuity across the study
cohort (Fig. 2). The positive
correlation between the reduction in macular thickness and
improvement in visual acuity is consistent with the
pathophysiological understanding that resolving macular edema
facilitates visual recovery, a finding previously reported by Gedar
Totuk et al (22).
Furthermore, the present analysis demonstrated that initiating
anti-VEGF therapy at earlier stages of DR required fewer injections
to achieve disease stabilization. This can be rationally explained
by the more preserved retinal architecture and potentially less
established, more responsive neovascular pathology in earlier
disease. This resultant reduction in treatment burden holds
particular significance for patients with limited financial
resources, a practical consideration that aligns with the findings
of Jampol et al (23).
In the present 3-year observational study, 28.3% of
patients with DR eventually experienced low vision. This rate is
markedly higher than the 10-15% range typically reported in
developed nations (24). The
disparity can likely be attributed to the more advanced disease
severity and later stage at which patients present for treatment at
the tertiary referral center, Tangdu Hospital (Xi'an, China).
Specifically, a significant proportion of the cohort presented with
pre-existing proliferative DR and significant macular edema at
baseline, conditions associated with a poorer visual prognosis.
Among them, the proportion of patients whose vision deteriorated
after receiving anti-VEGF treatment was notably lower compared with
those treated with laser. Moreover, anti-VEGF treatment had a
higher incidence of persistent macular edema, but fewer cases of
tractional retinal detachment compared with laser treatment. This
finding slightly differs from the findings of Fu et al
(25), who advocated for a
combined approach of anti-VEGF and retinal laser photocoagulation
(25-29).
These findings suggest a stage and
complication-specific therapeutic hierarchy. In eyes at risk of
tractional retinal detachment, such as those with active
neovascularization or preretinal fibrosis, anti-VEGF therapy should
be the first-line treatment due to its anti-angiogenic effects. For
chronic and diffuse macular edema without proliferative changes,
laser treatment may offer more sustained edema control by
modulating retinal metabolism. The term ‘resource-limited settings’
refers to environments with prevalent financial constraints, a
scarcity of retinal specialists, and limited healthcare
infrastructure. In such contexts, initiating anti-VEGF therapy
early (at stages 1-2) is a strategic approach to prevent
progression to vision-threatening complications that necessitate
complex and costly management. This strategy aims to reduce the
overall treatment burden and improve long-term visual outcomes
where resources are scarce. These findings align with the
DRCR.net Protocol V trial (28), which reported comparable BCVA
outcomes between anti-VEGF and laser therapy for patients with
diabetic macular edema and good baseline vision. However, the trial
demonstrated a key anatomical difference: Anti-VEGF therapy
provided superior reduction in CST compared with laser therapy.
This pattern mirrors the trend observed in the present study, where
the anti-VEGF group showed more substantial anatomical improvement
(as measured by CST reduction) than the laser group, despite both
groups achieving comparable visual acuity outcomes at the final
follow-up. This consistency across studies reinforces the fact that
anti-VEGF agents offer distinct anatomical benefits that may not
always directly translate into short-term visual acuity gains.
However, because of the small number of patients receiving combined
treatment, the same trend was not observed in the present
study.
The absence of synergistic effects in the present
cohort may be explained by a temporal interference between the two
treatment modalities. This phenomenon is supported by animal
studies demonstrating that laser photocoagulation can induce a
local inflammatory response, which transiently upregulates VEGF
expression in the retina (30,31).
This transient surge in VEGF could potentially counteract the
suppressive effects of concurrently administered anti-VEGF agents.
Therefore, a sequential treatment approach, initiating with
anti-VEGF therapy to suppress VEGF levels followed by laser
application, might yield superior outcomes. Therefore, a sequential
treatment approach, initiating with anti-VEGF therapy to suppress
VEGF levels followed by laser application, might yield superior
outcomes. This concept of deferred laser use is supported by the
rationale behind several modern treatment protocols (30,31),
which suggest that initiating with anti-VEGF therapy to suppress
VEGF levels followed by laser application might yield superior
outcomes. Future studies should standardize combination protocols
to clarify this interaction.
In European countries, especially the UK, nationwide
early screening for DR has been conducted since 2003 under the
National Health Service, with early anti-VEGF treatment for stages
2-3 of the disease. In Europe, this screening has markedly reduced
the prevalence of proliferative DR and blindness (30), which is consistent with
observations of the present study. We hypothesize that early
screening and early anti-VEGF treatment for DR are key in reducing
the rate of blindness in developing countries.
In recent years, multiple studies have advocated for
early surgical intervention in DR, with evidence supporting
improved anatomical and functional outcomes. This perspective is
supported by randomized trials evaluating novel surgical approaches
vs. conventional treatments (30),
clinical studies demonstrating the efficacy of advanced vitrectomy
techniques in reducing complications (31) and systematic reviews consolidating
evidence across various intervention strategies. Furthermore,
recent investigations have confirmed the benefits of combining
anti-VEGF agents with vitrectomy to facilitate surgical procedures
and enhance visual outcomes (30),
while innovative surgical methods have successfully managed complex
DR cases previously considered beyond therapeutic reach (29). However, observations from the
present study suggest a more nuanced picture. While the overall
rate of vision improvement was similar between anti-VEGF treatment
and vitrectomy for the combined cohort of stages 4-5 DR (75.5 vs.
76.3%), a sub-analysis of the data suggests a potential
stage-dependent effect. Anti-VEGF therapy appeared particularly
effective in stage 4 disease, whereas vitrectomy showed a trend
toward better outcomes in stage 5. This indicates that the optimal
choice may depend on the specific characteristics within the 4-5
spectrum, and initial anti-VEGF treatment could be a viable option
for a subset of these patients before committing to surgery. The
present study recommends anti-VEGF treatment in the early stages of
DR.
In summary, the present real-world study suggests
that anti-VEGF therapy is generally superior to laser
photocoagulation in improving visual and anatomical outcomes in DR.
While combination therapy did not show a clear visual advantage
over anti-VEGF alone in this cohort, it may still have a role in
specific scenarios. The treatment strategy should be tailored, with
anti-VEGF being preferred for preventing tractional retinal
detachment and potentially in earlier stages, while laser may offer
better control for recurrent macular edema in some patients with
low vision. For advanced DR (stages 4-5), initial treatment with
anti-VEGF appears to be a reasonable strategy, particularly in
stage 4, while the decision for vitrectomy should be
individualized, especially in stage 5 disease.
Supplementary Material
Baseline spectral-domain OCT of
diabetic macular edema. Representative CIRRUS HD-OCT (Zeiss GmbH)
macular scan (horizontal B-scan) from a treatment-naïve patient
with diabetic retinopathy. Characteristic features include
intraretinal cystoid fluid (yellow arrowheads), subretinal fluid
(red arrow), hyperreflective foci (white arrows) and diffuse
retinal thickening. The region of markedly increased central
subfield thickness is demarcated by the dashed rectangle. OCT,
optical coherence tomography; CST, central subfield thickness (452
μm); N, nasal; T, temporal.
Macular OCT following laser
photocoagulation. Representative CIRRUS HD-OCT (Zeiss GmbH) macular
scan (horizontal B-scan) post-laser photocoagulation. Compared with
baseline (Fig. S1), intraretinal
cystoid fluid and subretinal fluid have resolved. Residual
hyperreflective foci (white arrows) are evident. Retinal
architecture shows disruption (demarcated by asterisks) and central
subfield thickness is reduced but remains elevated compared with
anti-vascular endothelial growth factor outcomes (Fig. S3). The region showing thickness
reduction is outlined. OCT, optical coherence tomography.
Macular OCT following anti-VEGF
therapy. Representative CIRRUS HD-OCT (Zeiss GmbH) macular scan
(horizontal B-scan) post-intravitreal anti-VEGF treatment.
Resolution of intraretinal cystoid fluid, subretinal fluid and
hyperreflective foci is observed (areas of resolution are
demarcated). Retinal layers demonstrate improved structural
integrity (bracket), and central subfield thickness is notably
reduced compared with baseline (Fig.
S1) and laser treatment (Fig.
S2) (region of thickness reduction outlined). VEGF, vascular
endothelial growth factor; OCT, optical coherence tomography.
UWF fundus imaging: PRP.
Representative CLARUS 500 (Zeiss GmbH) pseudocolor UWF montage
(200˚field) demonstrating subtotal PRP. Laser burns (grade 3-4
intensity) are applied throughout the mid-periphery and periphery.
Note areas of residual retinal edema (yellow arrow) and the
relative sparing of the immediate peripapillary region and some
peripheral sectors. The complete retinal periphery is now fully
visible without cropping. UWF, ultra-widefield; PRP, panretinal
photocoagulation.
UWF fundus imaging: Macular grid
photocoagulation. Representative CLARUS 500 (Zeiss GmbH)
pseudocolor UWF montage (200˚ field) showing macular grid
photocoagulation. Mild-intensity (grade 1-2) laser burns are placed
in a grid pattern within the posterior pole, extending to ~200
μm from the foveal center (asterisk). The optic disc is
indicated. UWF, ultra-widefield.
UWF fundus imaging; focal laser
photocoagulation. Representative CLARUS 500 (Zeiss GmbH)
pseudocolor UWF montage (200˚ field) illustrating focal laser
photocoagulation. Discrete laser burns target specific
microvascular lesions (for example, microaneurysms) within the
posterior pole, primarily temporal to the fovea (asterisk). The
optic disc is indicated. UWF, ultra-widefield.
UWF fundus imaging; standard full
panretinal photocoagulation. Representative CLARUS 500 (Zeiss GmbH)
pseudocolor UWF montage (200˚ field) depicting standard full
panretinal photocoagulation. A high density of moderately intense
(grade 3-4) laser burns covers the peripheral and mid-peripheral
retina, extending close to the vascular arcades. The posterior
pole, including the macula (asterisk) and immediate peripapillary
region (bracket) are characteristically spared. The optic disc is
indicated. UWF, ultra-widefield; PRP, panretinal
photocoagulation.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The dataset generated in the present study may be
requested from the corresponding author.
Authors' contributions
YG and RY contributed to various aspects of the
research, from conceptualization to visualization and taking the
lead in writing. YG and HCL contributed to methodology and
supervision of the project. YG, RY, YL and CJG contributed to data
collection and analysis and contributed to editing and
visualization. YG and HCL confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Xi'an Bright
Eye Hospital Ethics Committee (Xi'an, China; approval no.
XPR-2018-0018). All patients underwent optometry, intraocular
pressure and visual acuity examinations and all patients
voluntarily participated in the present study and signed informed
consent forms.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cheung N, Mitchell P and Wong TY: Diabetic
retinopathy. Lancet. 376:124–136. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kollias AN and Ulbig MW: Diabetic
retinopathy: Early diagnosis and effective treatment. Dtsch Arztebl
Int. 107:75–84. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Congdon NG, Friedman DS and Lietman T:
Important causes of visual impairment in the world today. JAMA.
290:2057–2060. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lin KY, Hsih WH, Lin YB, Wen CY and Chang
TJ: Update in the epidemiology, risk factors, screening, and
treatment of diabetic retinopathy. J Diabetes Investig.
12:1322–1325. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Song P, Yu J, Chan KY, Theodoratou E and
Rudan I: Prevalence, risk factors and burden of diabetic
retinopathy in China: A systematic review and meta-analysis. J Glob
Health. 8(010803)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kang Q and Yang C: Oxidative stress and
diabetic retinopathy: Molecular mechanisms, pathogenetic role and
therapeutic implications. Redox Biol. 37(101799)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tan TE and Wong TY: Diabetic retinopathy:
Looking forward to 2030. Front Endocrinol (Lausanne).
13(1077669)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tan GS, Cheung N, Simó R, Cheung GC and
Wong TY: Diabetic macular oedema. Lancet Diabetes Endocrinol.
5:143–155. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu E, Craig JE and Burdon K: Diabetic
macular oedema: Clinical risk factors and emerging genetic
influences. Clin Exp Optom. 100:569–576. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Amoaku WM, Ghanchi F, Bailey C, Banerjee
S, Banerjee S, Downey L, Gale R, Hamilton R, Khunti K, Posner E, et
al: Diabetic retinopathy and diabetic macular oedema pathways and
management: UK consensus working group. Eye (Lond). 34 (Suppl
1):S1–S51. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Simunovic MP, Hunyor AP and Ho IV:
Vitrectomy for diabetic macular edema: A systematic review and
meta-analysis. Can J Ophthalmol. 49:188–195. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang Z, Tan TE, Shao Y, Wong TY and Li X:
Classification of diabetic retinopathy: Past, present and future.
Front Endocrinol (Lausanne). 13(1079217)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Aiello LP and Wong JS: Role of vascular
endothelial growth factor in diabetic vascular complications.
Kidney Int Suppl. 77:S113–S119. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Antonetti DA, Klein R and Gardner TW:
Diabetic retinopathy. N Engl J Med. 366:1227–1239. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang W and Lo ACY: Diabetic retinopathy:
Pathophysiology and treatments. Int J Mol Sci.
19(1816)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wong TY, Cheung CMG, Larsen M, Sharma S
and Simó R: Diabetic retinopathy. Nat Rev Dis Primers.
2(16012)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lois N, Campbell C, Waugh N, Azuara-Blanco
A, Maredza M, Mistry H, McAuley D, Acharya N, Aslam TM, Bailey C,
et al: Diabetic macular edema and diode subthreshold micropulse
laser: A randomized double-masked noninferiority clinical trial.
Ophthalmology. 130:14–27. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Everett LA and Paulus YM: Laser therapy in
the treatment of diabetic retinopathy and diabetic macular edema.
Curr Diab Rep. 21(35)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Baker CW, Glassman AR, Beaulieu WT,
Antoszyk AN, Browning DJ, Chalam KV, Grover S, Jampol LM, Jhaveri
CD, Melia M, et al: Effect of initial management with aflibercept
vs laser photocoagulation vs observation on vision loss among
patients with diabetic macular edema involving the center of the
macula and good visual acuity: A randomized clinical trial. JAMA.
321:1880–1894. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Arrigo A, Aragona E and Bandello F:
VEGF-targeting drugs for the treatment of retinal
neovascularization in diabetic retinopathy. Ann Med. 54:1089–1111.
2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Stitt AW, Curtis TM, Chen M, Medina RJ,
McKay GJ, Jenkins A, Gardiner TA, Lyons TJ, Hammes HP, Simó R and
Lois N: The progress in understanding and treatment of diabetic
retinopathy. Prog Retin Eye Res. 51:156–186. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gedar Totuk OM, Kanra AY, Bromand MN,
Kilic Tezanlayan G, Ari Yaylalı S, Turkmen I and Ardagil Akcakaya
A: Effectiveness of intravitreal ranibizumab in nonvitrectomized
and vitrectomized eyes with diabetic macular edema: A two-year
retrospective analysis. J Ophthalmol. 2020(2561251)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jampol LM, Glassman AR and Sun J:
Evaluation and care of patients with diabetic retinopathy. N Engl J
Med. 382:1629–1637. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yau JWY, Rogers SL, Kawasaki R, Lamoureux
EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund
J, et al: Global prevalence and major risk factors of diabetic
retinopathy. Diabetes Care. 35:556–564. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fu P, Huang Y, Wan X, Zuo H, Yang Y, Shi R
and Huang M: Efficacy and safety of pan retinal photocoagulation
combined with intravitreal anti-VEGF agents for high-risk
proliferative diabetic retinopathy: A systematic review and
meta-analysis. Medicine (Baltimore). 102(e34856)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Figueira J, Fletcher E, Massin P, Silva R,
Bandello F, Midena E, Varano M, Sivaprasad S, Eleftheriadis H,
Menon G, et al: Ranibizumab Plus panretinal photocoagulation versus
panretinal photocoagulation alone for high-risk proliferative
diabetic retinopathy (PROTEUS study). Ophthalmology. 125:691–700.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Figueira J, Silva R, Henriques J, Caldeira
Rosa P, Laíns I, Melo P, Gonçalves Nunes S and Cunha-Vaz J:
Ranibizumab for high-risk proliferative diabetic retinopathy: An
exploratory randomized controlled trial. Ophthalmologica.
235:34–41. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rebecca , Shaikh FF and Jatoi SM:
Comparison of efficacy of combination therapy of an intravitreal
injection of bevacizumab and photocoagulation versus pan retinal
photocoagulation alone in high risk proliferative diabetic
retinopathy. Pak J Med Sci. 37:157–161. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tao Y, Jiang P, Zhao Y, Song L, Ma Y, Li Y
and Wang H: Retrospective study of aflibercept in combination
therapy for high-risk proliferative diabetic retinopathy and
diabetic maculopathy. Int Ophthalmol. 41:2157–2165. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Vujosevic S, Aldington SJ, Silva P,
Hernández C, Scanlon P, Peto T and Simó R: Screening for diabetic
retinopathy: New perspectives and challenges. Lancet Diabetes
Endocrinol. 8:337–347. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Berrocal MH and Acaba-Berrocal L: Early
pars plana vitrectomy for proliferative diabetic retinopathy:
Update and review of current literature. Curr Opin Ophthalmol.
32:203–208. 2021.PubMed/NCBI View Article : Google Scholar
|