Introduction
Myopia is a refractive error frequently attributed
to elongation of the ocular axis (1). Severe myopia can lead to various
complications, including myopic macular degeneration and amblyopia,
resulting in vision impairment and marked health consequences,
including retinal detachment and early-onset cataracts (2,3). In
addition, myopia has been classified among the five serious ocular
conditions that may lead to blindness (4). In recent years, the prevalence of
myopia has increased rapidly, and the age of onset has gradually
decreased to 15.6±4.2 years, making it a global health concern
(5), with the global prevalence of
myopia projected to reach 50% of the population by 2050(4). The choroid plays a crucial role in
the development of myopia (6).
Myopic visual signals can lead to decreased choroidal blood flow,
leading to a reduced supply of oxygen and nutrients to the sclera.
These conditions result in reduced scleral strength and thickness,
axial elongation and the focus of light rays in front of the
retina, thereby causing myopia (7-9).
Following the application of orthokeratology lenses
to treat myopia, the centre of the cornea flattens, the peripheral
retinal refractive state shifts from relative hyperopic defocus to
myopic defocus (10-12),
the axial length (AL) of the eye shows decelerated growth and
choroidal thickness (CT) markedly increases (13-15).
Small incision lenticule extraction (SMILE) and femtosecond
laser-assisted in situ keratomileusis (FS-LASIK) are
currently the predominant corneal refractive surgical procedures.
SMILE and FS-LASIK correct myopia by ablating the central stroma of
the cornea, thereby reducing corneal curvature and enabling light
to focus on the retina. Both these procedures yield comparable
outcomes in terms of safety, efficacy, predictability and stability
for correcting myopia and myopic astigmatism (16,17).
Similarly, SMILE and FS-LASIK also reduces the central curvature of
the cornea and alters the defocus state of the retina (18-20).
Thus, whether the choroid undergoes the same changes after SMILE
and FS-LASIK as observed after orthokeratology lens use remains
unclear. To the best of our knowledge, studies on the choroid after
corneal refractive surgery are relatively limited. Optical
coherence tomography (OCT) represents a breakthrough in ophthalmic
imaging, allowing for the scanning of multiple intraocular tissue
structures and the evaluation of choroidal vasculature (21). The present study aimed to apply OCT
to measure central and peripheral CT and blood flow density in
patients with myopia before and after SMILE and FS-LASIK surgeries,
to observe the choroidal changes induced by these two
procedures.
Materials and methods
Patients
The present study enrolled patients who underwent
SMILE or FS-LASIK surgery for myopia correction at Jinan Mingshui
Eye Hospital (Jinan, China) between November 2020 and October 2022.
All patients provided written informed consent for refractive
surgery and the use of their data in research. The inclusion
criteria were as follows: i) Age 18-30 years; ii) maximum annual
increase in myopic spherical equivalent (SE) of -0.5 diopters (D)
within 2 years; iii) SE ranging from -3.00 to -10.00 D; and iv)
normal fundus oculi. Exclusion criteria included: i) Keratoconus or
corneal ectasia; ii) corneal dystrophy; iii) scar constitution; iv)
conjunctivitis; v) keratitis; vi) compromised immune function; and
vii) mental instability. A total of 56 patients were included, with
35 patients in the SMILE group and 21 patients in the FS-LASIK
group. The sex distribution and age range of the patients are
presented in the results section. The study protocol adhered to the
principles of The Declaration of Helsinki and received approval
from the Human Ethics Committee of Jinan Mingshui Eye Hospital
(approval no. 2020-020).
Ophthalmological examination
SE, AL, ablation depth (AD), CT and blood flow
density were measured before surgery and at 1 week, 1 month and 3
months postoperatively. AL was obtained using the IOLMaster 500
(Zeiss AG; Oberkochen; Germany) to measure the distance from the
anterior corneal surface to the retinal pigment epithelium.
CT was measured using ‘Radial Dia 6.0 mm overlap
radial scanning mode’ of OCT for the macula (DRI-OCT Triton; Topcon
Corporation). A circular area with a 6-mm diameter centred on the
macular fovea was scanned. CT was defined as the vertical distance
from Bruch's membrane to the choroid-sclera junction and was
measured in micrometres. The scanning area was divided into the
following regions for CT measurements: The centre of the macula
(CT-C), 0.5 mm nasal (CT-N1), 1.5 mm nasal (CT-N2), 0.5 mm temporal
(CT-T1), 1.5 mm temporal (CT-T2), 0.5 mm superior (CT-S1), 1.5 mm
superior (CT-S2), 0.5 mm inferior (CT-I1) and 1.5 mm inferior
(CT-I2) to the centre, as shown in Fig. 1.
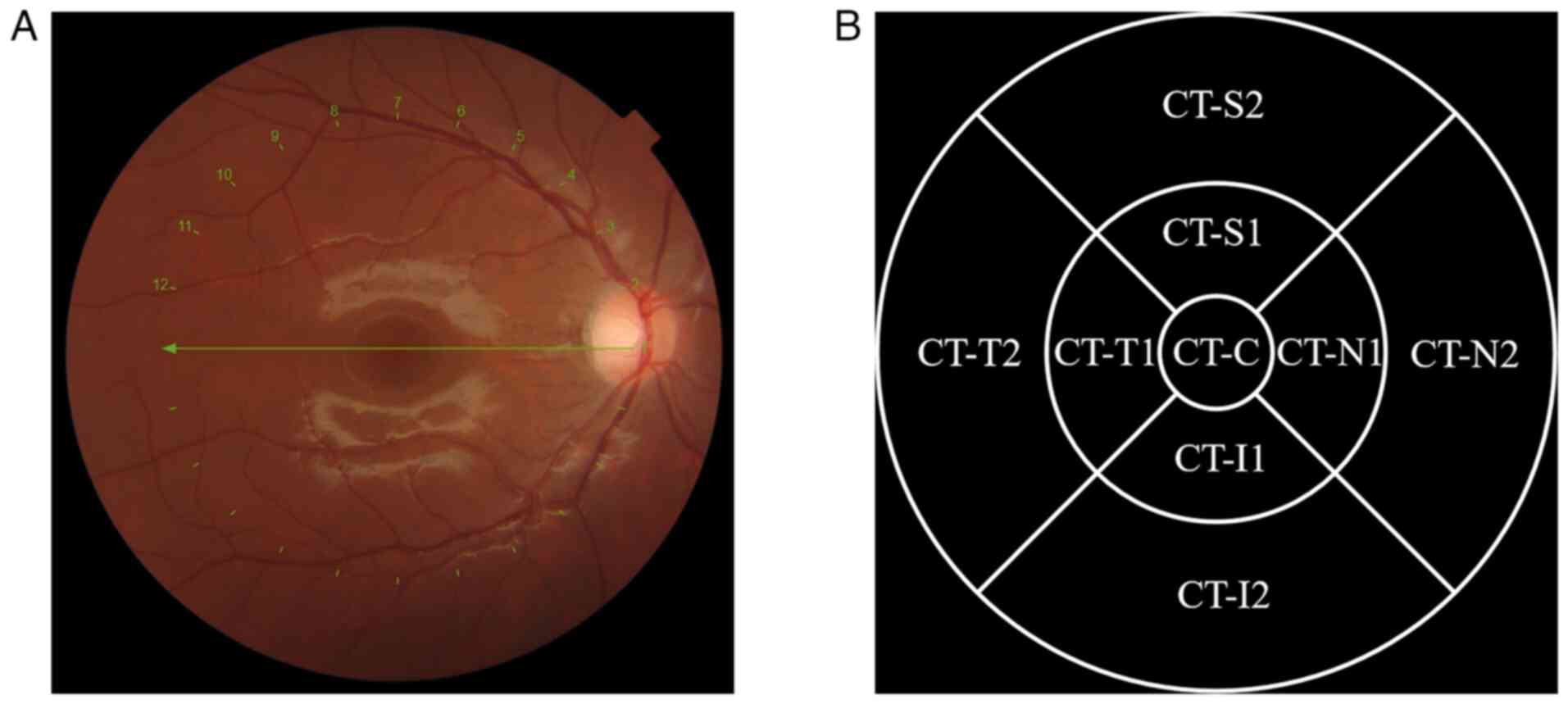 | Figure 1Measurement of CT. (A) Imaging of CT
during optical coherence tomography scanning. (B) The scanned area
was divided into different regions in the form of quadrants,
including the following regions: CT-C, CT-N1, CT-N2, CT-T1, CT-T2,
CT-S1, CT-S2, CT-I1 and CT-I2. Numbers 1 to 12 indicate the
positions of the captured images. CT, choroidal thickness; CT-C, CT
at the centre of the macula; CT-N1, CT at 0.5 mm nasal; CT-N2, CT
at 1.5 mm nasal; CT-T1, CT at 0.5 mm temporal; CT-T2, CT at 1.5 mm
temporal; CT-S1, CT at 0.5 mm superior; CT-S2, CT at 1.5 mm
superior; CT-I1, CT at 0.5 mm inferior; CT-I2, CT at 1.5 mm
inferior. |
To measure blood flow density, the ‘OCT Angiography
(OCTA) 4.5x4.5’ scanning mode was selected (DRI-OCT Triton; Topcon
Corporation). The macular centre was examined in a 4.5x4.5 mm area.
Scattering of the retina generates artifacts anterior to the
choroid, and the sensitivity of OCTA decreases sharply with
increasing scanning depth, both of which affect the imaging quality
of the blood flow (22,23). Therefore, to minimize measurement
errors, the depth range of 10.4-31.2 µm below Bruch's membrane was
selected to obtain the choriocapillaris blood flow density (CD)
(22,24). The scanning area was divided into
the following regions for CD measurements: Centre of the macula,
0.5 mm nasal, 0.5 mm temporal (CD-T), 0.5 mm superior and 0.5 mm
inferior to the centre, as shown in Fig. 2.
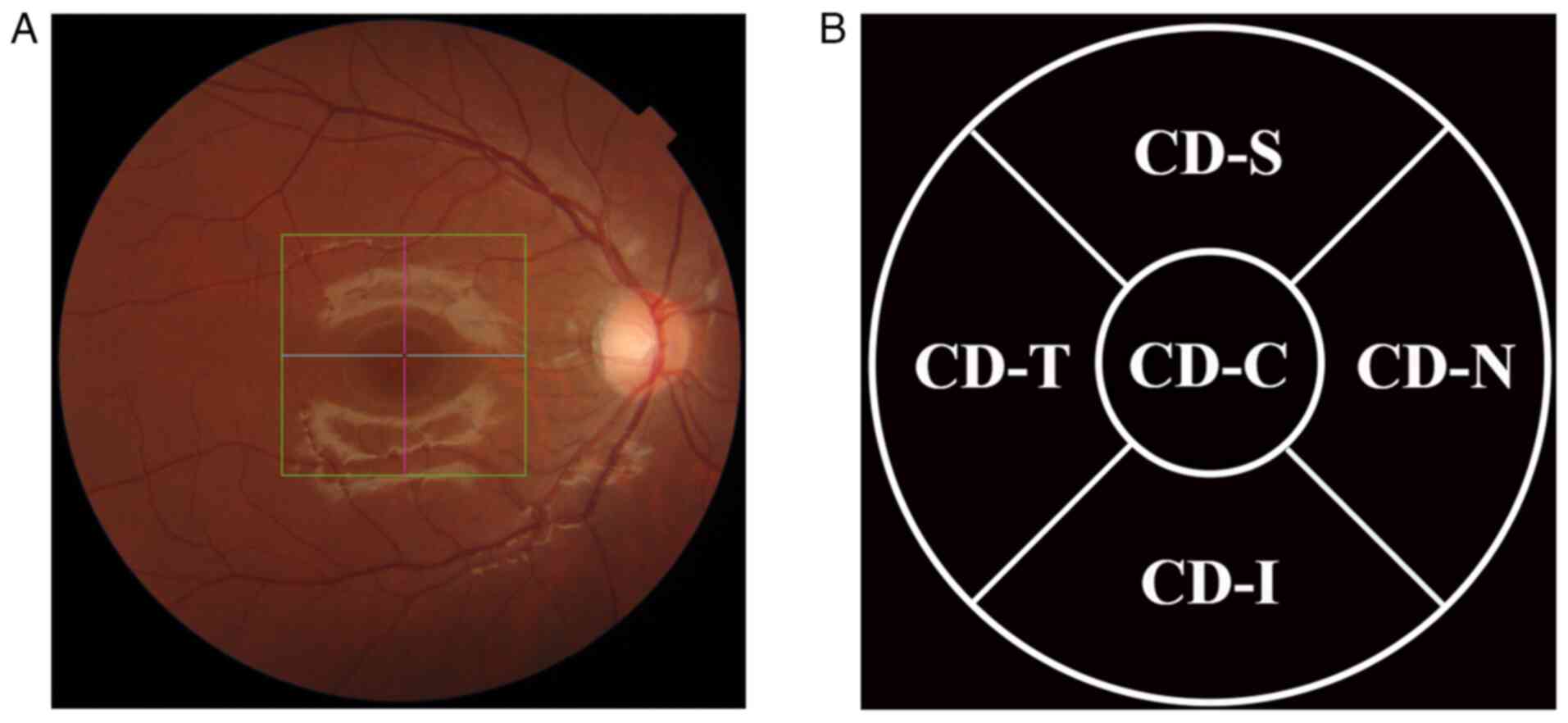 | Figure 2Measurement of CD. (A) Imaging of the
choriocapillaris vessels during optical coherence tomography
scanning. The square in the picture represents the scanning area
used by the instrument to measure blood flow density, and the cross
is the center of the scan. (B) The scanned area was divided into
different regions in the form of quadrants, including the following
regions: CD-C, CD-N, CD-T, CD-S and CD-I. CD, choriocapillaris
blood flow density; CD-C, CD at the centre of the macula; CD-N, CD
at 0.5 mm nasal; CD-T, CD at 0.5 mm temporal; CD-S, CD at 0.5 mm
superior; CD-I, CD at 0.5 mm inferior. |
All examinations were conducted between 8:00 and
11:00 a.m. to account for the circadian rhythm of the choroid
(25). In addition, all procedures
were performed by the same experienced optometrist to avoid
systematic errors.
Surgical procedure
SMILE was performed using the VisuMax femtosecond
laser (VisuMax-500; Zeiss AG) to create corneal lenticules and
caps. The surgical plan was developed based on preoperative
refractive diopter and entered into the device. Laser scanning was
then initiated, followed by lenticule removal using forceps through
the upper aperture.
FS-LASIK was performed as a two-step procedure.
First, a corneal flap was created using the VisuMax femtosecond
laser. Next, the patient was transferred to the excimer laser
system (AMARIS-500E; SCHWIND eye-tech-solution GmbH), where the
corneal flap was opened and the corneal stroma was ablated. Normal
saline was used to irrigate the stromal bed and conjunctival sac,
resulting in a smooth corneal flap. All surgeries were performed by
the same qualified and experienced ophthalmologist.
Statistical analysis
SPSS 25.0 statistical software (IBM Corp.) was used
for data analysis. Normally distributed data are presented as the
mean ± standard deviation, while non-normally distributed data are
presented as the median (25th percentile, 75th percentile). When
comparing the baseline characteristics between the SMILE and
FS-LASIK groups, independent sample Student's t-test was used for
the analysis of normally distributed data, while the non-parametric
Mann-Whitney U test was used for data not conforming to a normal
distribution. When conducting overall intergroup and intragroup
analyses of various parameters, a two-way mixed ANOVA was used for
normally distributed data, while non-normally distributed data were
analysed using the non-parametric K-related sample Friedman test
for within-group comparisons and the Mann-Whitney U test for
between-group comparisons, all followed by a Bonferroni post hoc
test or correction as necessary. Correlations between datasets were
performed using Spearman correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Baseline characteristics
The present study included 56 patients (112 eyes;
all surgeries were performed on both eyes). The patients had a mean
age of 23.07±3.61 years and a mean SE of -5.75±1.77 D. The SMILE
group had a mean age of 22.89±3.01 years and a mean SE of
-5.76±1.67 D; this group included 35 patients (15 male patients and
20 female patients). The FS-LASIK group had a mean age of
23.38±4.51 years and a mean SE of -5.74±1.79 D; this group included
21 patients (7 male patients and 14 female patients). No
significant differences were found between the groups in age,
preoperative SE, AL, AD of the corneal stroma, CT or CD
(P>0.05). Table I presents the
baseline characteristic comparisons between the two groups.
 | Table IComparison of baseline
characteristics between the SMILE and FS-LASIK groups. |
Table I
Comparison of baseline
characteristics between the SMILE and FS-LASIK groups.
| Variable | SMILE | FS-LASIK | t/Z-value | P-value |
|---|
| Age, years | 22.89±3.01 | 23.38±4.51 | -0.447 | 0.658 |
| SE, D | -5.76±1.67 | -5.74±1.79 | 1.580 | 0.343 |
| AL, mm | 25.45 (24.70,
26.08) | 25.62 (24.99,
26.34) | -0.884 | 0.377 |
| AD, µm | 98.37±25.27 | 92.74±22.35 | 1.192 | 0.236 |
| CT-C, µm | 227.69±82.07 | 211.83±63.11 | 1.323 | 0.189 |
| CT-N1, µm | 200.00 (151.25,
248.00) | 177.00 (144.00,
226.75) | -1.328 | 0.184 |
| CT-N2, µm | 159.00 (123.00,
216.75) | 141.50 (112.75,
202.00) | -1.614 | 0.107 |
| CT-T1, µm | 241.24±80.51 | 225.65±65.97 | 1.205 | 0.231 |
| CT-T2, µm | 252.27±76.23 | 230.42±63.50 | 1.678 | 0.096 |
| CT-S1, µm | 235.87±76.60 | 208.32±64.40 | 2.030 | 0.055 |
| CT-S2, µm | 245.74±71.49 | 216.88±58.12 | 2.202 | 0.060 |
| CT-I1, µm | 236.70±83.19 | 216.43±62.74 | 1.693 | 0.094 |
| CT-I2, µm | 234.50 (178.75,
297.75) | 217.50 (170.50,
245.50) | -1.668 | 0.095 |
| CD-C, % | 55.52±4.91 | 55.23±4.66 | 0.534 | 0.595 |
| CD-N, % | 55.94±3.83 | 55.48±2.44 | 1.015 | 0.312 |
| CD-T, % | 57.22 (55.10,
58.90) | 55.89 (53.72,
56.88) | -2.515 | 0.067 |
| CD-S, % | 51.01±3.91 | 50.90±3.35 | 0.398 | 0.691 |
| CD-I, % | 53.19 (51.06,
56.15) | 53.74 (51.09,
55.60) | -0.237 | 0.621 |
Changes in SE and AL. Changes in
SE
The SE at all postoperative time points was
significantly increased compared with that before the surgery in
each group (P<0.001). Statistically significant differences
between the two groups were observed at 1 week and 1 month
postoperatively (P<0.05) but not at the remaining time points
(P>0.05) (Table II).
 | Table IIChanges in SE and AL before and after
the operation in the SMILE and FS-LASIK groups. |
Table II
Changes in SE and AL before and after
the operation in the SMILE and FS-LASIK groups.
| A, SE, D |
|---|
| Variable | Pre-operation | 1 week | 1 month | 3 months |
F/χ2-value | P-value |
|---|
| SMILE | -5.74±1.79 | 0.00±0.30 | -0.03±0.27 | 0.01±0.31 | 612.340 | <0.001 |
| FS-LASIK | -5.76±1.67 | 0.33±0.28 | 0.29±0.28 | -0.06±0.05 | 598.430 | <0.001 |
| P-value | 0.343 | <0.001 | 0.010 | 0.610 | | |
| B, AL, mm |
| Variable | Pre-operation | 1 week | 1 month | 3 months |
F/χ2-value | P-value |
| SMILE | 25.45 (24.70,
26.08) | 25.29 (24.61,
25.94) | 25.32 (24.59,
25.93) | 25.29 (24.59,
25.93) | 137.586 | <0.001 |
| FS-LASIK | 25.62 (24.99,
26.34) | 25.48 (24.88,
26.14) | 25.48 (24.85,
26.14) | 25.50 (24.85,
26.16) | 84.274 | <0.001 |
| P-value | 0.377 | 0.465 | 0.447 | 0.220 | | |
Changes in AL. The AL at each time point
postoperatively was significantly shorter than preoperative AL in
both groups (P<0.001). No significant differences were found
between the two groups at each time point before and after surgery
(P>0.05) (Table II).
Correlation between the shortening of AL and
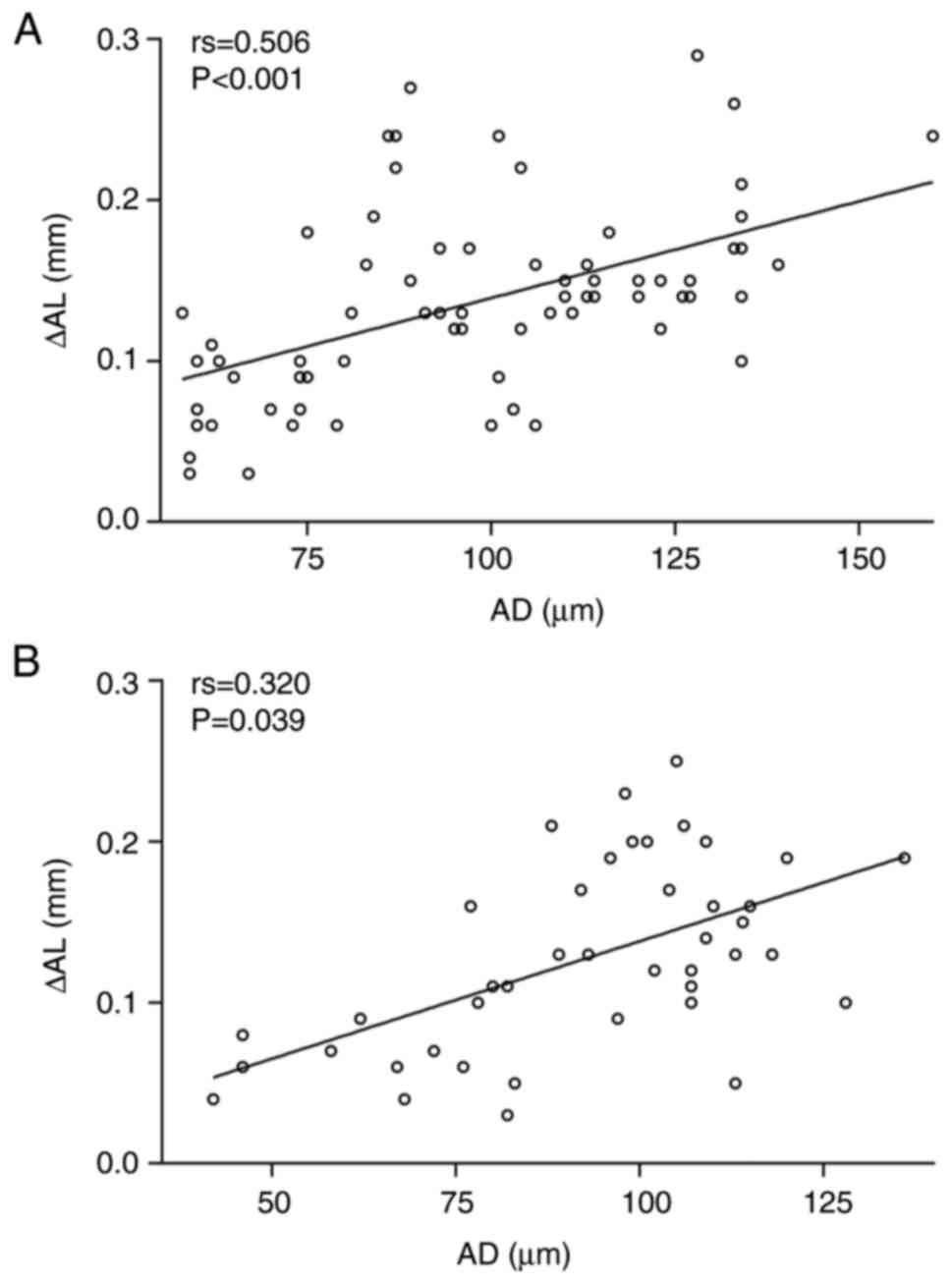
AD. A significant correlation was found between the shortening
of AL and AD at 3 months postoperatively in the SMILE group
compared with the preoperative values (P<0.001; Fig. 3A). In addition, a significant
correlation was also found between the shortening of AL and AD at 3
months postoperatively in the FS-LASIK group (P=0.039; Fig. 3B).
Changes in CT. Changes in CT in the
SMILE group
CT-C, CT-N1, CT-N2, CT-S1, CT-S2, CT-I1 and CT-I2
were significantly increased at 1 week postoperatively compared
with their preoperative levels (P<0.001), followed by a
decreasing trend and the return to preoperative thickness by 3
months after surgery (P>0.05). CT-T1 and CT-T2 remained
significantly increased compared with preoperative values at all
postoperative time points (P<0.001); however, these two
measurement indicators showed a slow growth trend at 1 month and 3
months postoperatively. Details on the levels of the CT parameters
are presented in Table III and
Fig. 4.
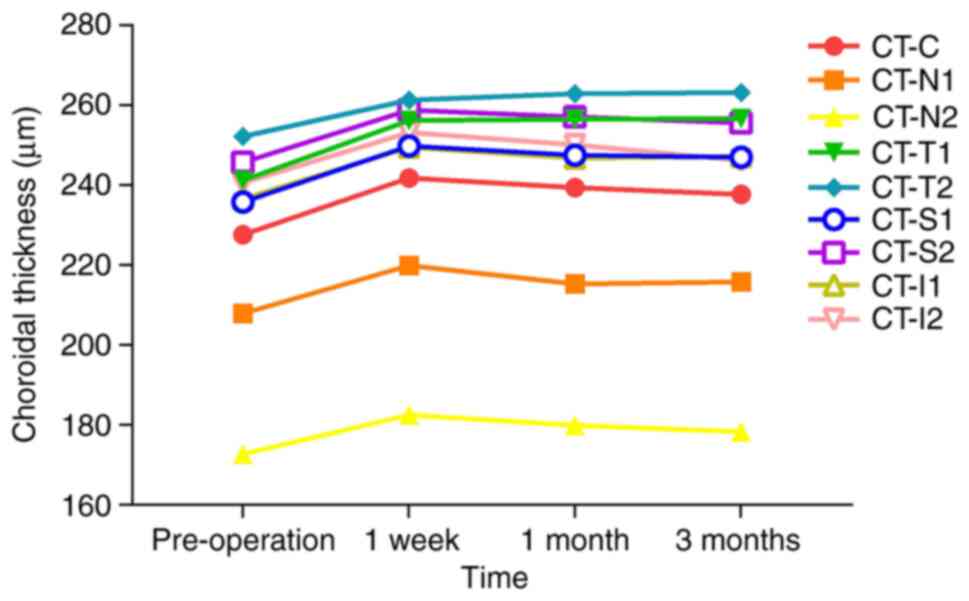 | Figure 4Change in the trend of CT before and
after the operation in the small incision lenticule extraction
group measured across different regions. Pre, pre-operation; CT,
choroidal thickness; CT-C, CT at the centre of the macula; CT-N1,
CT at 0.5 mm nasal; CT-N2, CT at 1.5 mm nasal; CT-T1, CT at 0.5 mm
temporal; CT-T2, CT at 1.5 mm temporal; CT-S1, CT at 0.5 mm
superior; CT-S2, CT at 1.5 mm superior; CT-I1, CT at 0.5 mm
inferior; CT-I2, CT at 1.5 mm inferior. |
 | Table IIIChanges in CT before and after the
operation in the SMILE and FS-LASIK groups. |
Table III
Changes in CT before and after the
operation in the SMILE and FS-LASIK groups.
| A, CT-C, µm |
|---|
| Variable | Pre-operation | 1 week | 1 month | 3 months |
F/χ2-value | P-value |
|---|
| SMILE | 227.69±82.07 | 241.83±84.35 | 239.46±83.41 | 237.77±81.29 | 14.024 | <0.001 |
| FS-LASIK | 211.83±63.11 | 216.48±64.39 | 218.20±64.83 | 218.15±65.76 | 1.889 | 0.035 |
| P-value | 0.189 | 0.050 | 0.108 | 0.128 | | |
| B, CT-N1, µm |
| Variable | Pre-operation | 1 week | 1 month | 3 months |
F/χ2-value | P-value |
| SMILE | 200.00 (151.25,
248.00) | 207.50 (155.00,
279.25) | 205.00 (153.50,
265.00) | 206.00 (152.50,
271.00) | 31.108 | <0.001 |
| FS-LASIK | 177.00 (144.00,
226.75) | 187.50 (151.25,
228.75) | 191.50 (144.25,
248.50) | 192.00 (134.50,
239.75) | 8.632 | 0.031 |
| P-value | 0.184 | 0.168 | 0.234 | 0.129 | | |
| C, CT-N2, µm |
| Variable | Pre-operation | 1 week | 1 month | 3 months |
F/χ2-value | P-value |
| SMILE | 159.00 (123.00,
216.75) | 163.00 (12825,
230.25) | 155.00 (122.00,
217.00) | 154.00 (12.002,
220.25) | 25.536 | <0.001 |
| FS-LASIK | 141.50 (112.75,
202.00) | 150.50 (115.75,
202.25) | 145.00 (113.50,
193.00) | 149.50 (105.00,
189.75) | 10.832 | 0.013 |
| P-value | 0.107 | 0.188 | 0.220 | 0.222 | | |
| D, CT-T1, µm |
| Variable | Pre-operation | 1 week | 1 month | 3 months |
F/χ2-value | P-value |
| SMILE | 241.24±80.51 | 256.24±80.22 | 256.57±82.46 | 256.76±81.77 | 17.534 | <0.001 |
| FS-LASIK | 225.65±65.97 | 234.62±70.26 | 233.00±69.26 | 230.70±70.09 | 2.581 | 0.057 |
| P-value | 0.231 | 0.103 | 0.085 | 0.060 | | |
| E, CT-T2, µm |
| Variable | Pre-operation | 1 week | 1 month | 3 months |
F/χ2-value | P-value |
| SMILE | 252.27±76.23 | 261.31±73.37 | 262.96±75.08 | 263.26±75.09 | 6.238 | <0.001 |
| FS-LASIK | 230.42±63.50 | 237.25±66.84 | 237.50±67.50 | 237.68±65.17 | 3.113 | 0.039 |
| P-value | 0.096 | 0.063 | 0.045 | 0.058 | | |
| F, CT-S1, µm |
| Variable | Pre-operation | 1 week | 1 month | 3 months |
F/χ2-value | P-value |
| SMILE | 235.87±76.60 | 249.80±79.13 | 247.50±79.54 | 247.00±79.30 | 18.432 | <0.001 |
| FS-LASIK | 208.32±64.40 | 216.58±62.16 | 219.52±61.59 | 219.68±61.93 | 3.097 | 0.030 |
| P-value | 0.055 | 0.016 | 0.061 | 0.056 | | |
| G, CT-S2, µm |
| Variable | Pre-operation | 1 week | 1 month | 3 months |
F/χ2-value | P-value |
| SMILE | 245.74±71.49 | 258.83±75.15 | 257.16±76.20 | 255.63±73.09 | 12.220 | <0.001 |
| FS-LASIK | 216.88±58.12 | 223.22±62.13 | 225.58±64.18 | 226.83±61.10 | 3.683 | 0.030 |
| P-value | 0.060 | 0.010 | 0.022 | 0.064 | | |
| H, CT-I1, µm |
| Variable | Pre-operation | 1 week | 1 month | 3 months |
F/χ2-value | P-value |
| SMILE | 236.70±83.19 | 249.56±86.29 | 246.83±87.99 | 247.23±84.41 | 13.852 | <0.001 |
| FS-LASIK | 216.43±62.74 | 223.30±65.26 | 222.45±64.91 | 221.47±68.42 | 1.966 | 0.140 |
| P-value | 0.094 | 0.058 | 0.077 | 0.060 | | |
| I, CT-I2, µm |
| Variable | Pre-operation | 1 week | 1 month | 3 months |
F/χ2-value | P-value |
| SMILE | 234.50 (178.75,
297.75) | 237.00 (201.25,
327.00) | 229.50 (199.00,
304.25) | 230.00 (193.25,
284.00) | 27.965 | <0.001 |
| FS-LASIK | 217.50 (170.50,
245.50) | 225.00 (171.75,
257.00) | 218.50 (170.50,
261.00) | 222.00 (161.75,
271.50) | 11.310 | 0.010 |
| P-value | 0.095 | 0.072 | 0.100 | 0.092 | | |
Changes in CT in the FS-LASIK group. CT-N1,
CT-N2 and CT-I2 significantly increased at 1 week postoperatively
compared with their preoperative levels (all P<0.05),
followed by gradual decrease by 1 month. No significant difference
was found at 3 months postoperatively compared with the baseline
(P>0.05). CT-T1 and CT-I1 showed no significant changes but
followed a pattern of initial increase followed by reduction at 1
month postoperatively. All other measurement sites showed
significantly greater thickness than their preoperative levels at
all postoperative time points (P<0.05) but T2, S1 and S2
exhibited a slow increase, and CT-C exhibited a decrease at 3
months postoperatively. Details on the levels of the CT parameters
are presented in Table III and
Fig. 5.
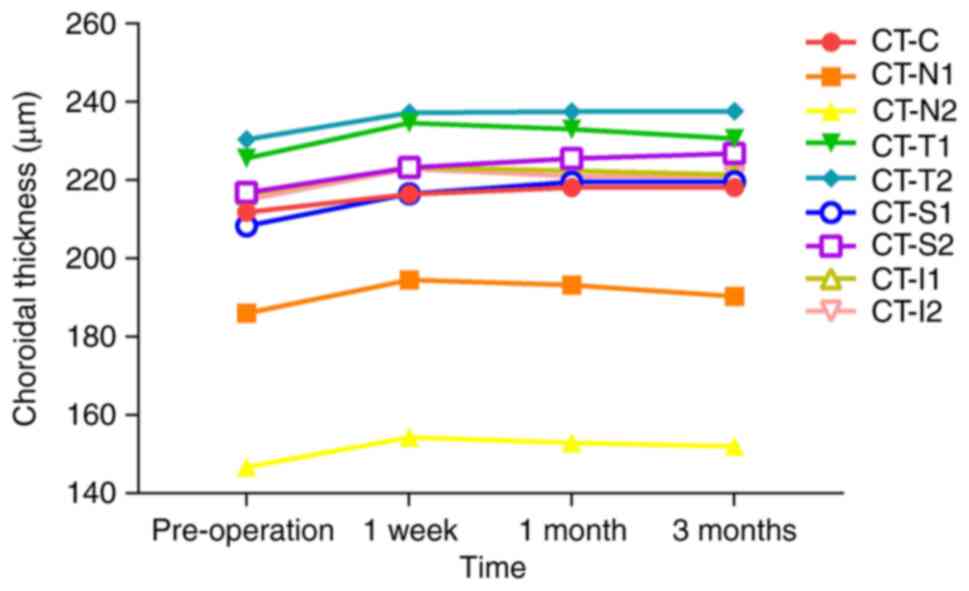 | Figure 5Change in the trend of CT before and
after the operation in the femtosecond laser-assisted in
situ keratomileusis group measured across different regions.
Pre, pre-operation; CT, choroidal thickness; CT-C, CT at the centre
of the macula; CT-N1, CT at 0.5 mm nasal; CT-N2, CT at 1.5 mm
nasal; CT-T1, CT at 0.5 mm temporal; CT-T2, CT at 1.5 mm temporal;
CT-S1, CT at 0.5 mm superior; CT-S2, CT at 1.5 mm superior; CT-I1,
CT at 0.5 mm inferior; CT-I2, CT at 1.5 mm inferior. |
Comparison of CT between the two groups. The
CT-T2 in the SMILE group was significantly thicker than that in the
FS-LASIK group at 1 month postoperatively (P=0.045). CT-S1 in the
SMILE group was significantly thicker than that in the FS-LASIK
group at 1 week (P=0.016), as was CT-S2 at both 1 week and 1 month
postoperatively (P<0.05). No significant differences were
observed between groups at other measurement sites across the
postoperative timepoints (P>0.05) (Table III).
Changes in CD. Changes in CD in the
SMILE group
A significant decrease in CD-T was observed at 1
week postoperatively (P=0.010), but the values returned to
preoperative levels by 1 month. No significant changes were found
at other observation sites (Table
IV).
 | Table IVChanges in CD before and after the
operation in the SMILE and FS-LASIK groups. |
Table IV
Changes in CD before and after the
operation in the SMILE and FS-LASIK groups.
| A, CD-C, % |
|---|
| Variable | Pre-operation | 1 week | 1 month | 3 months |
F/χ2-value | P-value |
|---|
| SMILE | 55.52±4.91 | 55.19±6.02 | 55.54±5.48 | 55.18±5.87 | 0.249 | 0.843 |
| FS-LASIK | 55.23±4.66 | 57.15±5.14 | 55.10±4.84 | 55.40±5.42 | 2.694 | 0.050 |
| P-value | 0.595 | 0.052 | 0.634 | 0.893 | | |
| B, CD-N, % |
| Variable | Pre-operation | 1 week | 1 month | 3 months |
F/χ2-value | P-value |
| SMILE | 55.94±3.83 | 55.35±3.58 | 55.36±4.01 | 55.69±4.13 | 0.852 | 0.467 |
| FS-LASIK | 55.48±2.44 | 55.31±3.91 | 54.81±3.69 | 56.12±4.32 | 1.832 | 0.160 |
| P-value | 0.312 | 0.756 | 0.810 | 0.681 | | |
| C, CD-T, % |
| Variable | Pre-operation | 1 week | 1 month | 3 months |
F/χ2-value | P-value |
| SMILE | 57.22 (55.10,
58.90) | 56.43 (54.28,
57.86) | 56.30 (54.36,
58.45) | 56.97 (54.52,
59.06) | 10.898 | 0.012 |
| FS-LASIK | 55.89 (53.72,
56.88) | 55.25(53.73,
57.49) | 56.10 (53.15,
57.80) | 56.58 (53.69,
58.33) | 0.756 | 0.521 |
| P-value | 0.067 | 0.261 | 0.140 | 0.489 | | |
| D, CD-S, % |
| Variable | Pre-operation | 1 week | 1 month | 3 months |
F/χ2-value | P-value |
| SMILE | 51.01±3.91 | 51.58±3.86 | 51.94±3.84 | 52.06±4.74 | 2.029 | 0.111 |
| FS-LASIK | 50.90±3.35 | 52.08±3.50 | 51.86±3.53 | 50.89±4.07 | 2.492 | 0.085 |
| P-value | 0.691 | 0.794 | 0.730 | 0.189 | | |
| E, CD-I, % |
| Variable | Pre-operation | 1 week | 1 month | 3 months |
F/χ2-value | P-value |
| SMILE | 53.19 (51.06,
56.15) | 53.83 (50.34,
56.46) | 53.76 (51.66,
57.07) | 54.45 (50.32,
57.56) | 2.584 | 0.460 |
| FS-LASIK | 53.74 (51.09,
55.60) | 54.02 (51.28,
56.01) | 53.62 (50.62,
56.57) | 53.74 (50.10,
58.13) | 0.368 | 0.699 |
| P-value | 0.621 | 0.995 | 0.757 | 0.843 | | |
Changes in CD in the FS-LASIK group. No
significant changes in CD parameters were observed among
preoperative and postoperative groups (Table IV).
Comparison of CD between the two groups. No
statistically significant differences were found between the
surgical groups at any time point before or after surgery (Table IV).
Discussion
The choroid, an essential ocular tissue, responds to
changes in internal visual signal transmission, and visual signals
of peripheral myopic defocus on the retina induce choroidal
thickening (26-28).
Recent studies have shown that after correcting myopia with
orthokeratology lenses, peripheral retinal myopic defocus results
in increased CT (13-15).
Although corneal refractive surgery also alters the retinal defocus
state during myopia correction (19,20),
the present study, in conjunction with existing research (29,30),
revealed that choroidal responses may not entirely mirror those
observed with orthokeratology.
In the present study, after SMILE and FS-LASIK, CT
increased initially and then gradually returned to baseline at the
majority of measurement sites. Among the two groups, only a very
small number of measurement sites showed significant differences in
choroidal thickness at very few time points. These findings are
consistent with those of previous research. Xu et al
(29) reported that CT increased
after FS-LASIK but returned to baseline levels 3 months
postoperatively. Zhang et al (30) observed a temporary increase in CT 2
h after FS-LASIK, which returned to preoperative levels at 1 week;
however, the average thickness at 3 months postoperatively exceeded
that at 2 h after the operation, which differs from the present
study results. The authors attributed the transient increase to
elevated intraocular pressure during surgery, which may stimulate
the choroid, causing thickening. As intraocular pressure
stabilizes, CT initially recovers, followed by an increase possibly
related to myopic defocus of the peripheral retina, similar to
orthokeratology mechanisms (30).
Another study demonstrated that myopic defocus promotes the
recovery of CT and slows axial elongation (31), further supporting the association
between choroidal changes and retinal defocus state. In the present
study, choroidal thickening occurred during the early postoperative
period. In addition to the aforementioned mechanisms, we
hypothesize that changes in signal molecule transmission along the
retina-choroid pathway may contribute to these findings. Previous
studies indicated that alterations in visual signals can regulate
the release of dopamine in the dopamine pathway (32,33);
an increase in dopamine secretion can lead to the thickening of the
choroid and inhibit the progression of myopia, whereas a decrease
in secretion has the opposite effect (7,34).
Corneal refractive surgery reduces central corneal curvature,
shifting the peripheral retina defocus from hyperopic to myopic
(19,20); consequently, this emmetropization
of the visual signal increases the secretion of dopamine from the
retina to the choroid, increasing CT. This hypothesis aligns with
the perspectives shared in the study by Cheng et al
(35), where it was also
considered that the release of dopamine is what caused the change
in the thickness of the choroid. This mechanism may occur during
the early postoperative period, as retinal areas adapt to the
altered visual environment, after which dopamine secretion
decreases (7) and CT gradually
returns to baseline. In the present study, in both the SMILE and
FS-LASIK groups, some measurement points, primarily at the macular
centre (CT-C), temporal side (CT-T) or superior region (CT-S),
remained increased compared with their preoperative levels
throughout the study. These regions are known to have relatively
greater CT (36,37), and thicker choroid exhibits a
delayed response to visual signalling (7). Although CT in these regions stayed
above baseline, these variables demonstrated a slow increase or
decline. However, the clinical relevance of CT changes remains
unclear; the present study only tracked patients until 3 months
post-surgery, and more long-term observations are required to
clarify the outcomes.
Xu et al (29) reported a notable decrease in
choroidal blood flow density 1 day after surgery, which returned to
baseline near the macular centre by 1 month. Similarly, Chen et
al (38) observed reduced
vascular density in and around the optic disc 1 day after SMILE,
with recovery by 1 week postoperatively. Another study revealed an
initial decline in vascular density after FS-LASIK, followed by a
gradual increase and return to preoperative levels within 1 month
(39). In the present study, CD
decreased at a single site in the SMILE group but returned to
baseline. No significant changes were observed in other areas.
These findings are consistent with those of Chen et al
(40), who reported no significant
changes in superficial or deep retinal vascular density
post-surgery. In the present study, CD was specifically measured to
minimize artifacts from the anterior retina and reduce potential
errors from scanning deeper layers of the choroid (22-24).
We hypothesize that postoperative changes in choroidal blood flow
density may not be entirely absent but could occur in the deeper
choroidal vasculature, which remains underexplored. Currently,
although OCTA can be used to image the choriocapillaris blood flow,
this technique has limitations (22,41),
including reduced detection sensitivity in deeper tissues and
artifacts during scanning, which limits the conclusions on the
overall choroidal blood flow in the present study. Based on the
results of the current study, it is proposed that temporary changes
in intraocular pressure do not markedly affect choroidal blood
flow. A previous study supports this view, showing that choroidal
blood flow remains relatively stable during the early stages of
intraocular pressure elevation after surgery (42). In the present study, although CT
changed during the observation period, blood flow density remained
largely stable, suggesting no strong association between the two
parameters. Some studies have also found no clear association
between CT and blood flow (29,43).
Moreover, another study indicated that CT is more closely related
to choroidal vascularity than to choriocapillaris perfusion
(44). Given the structural
complexity of the choroid, evaluating its vascular characteristics
remains challenging, and further research is required to better
understand choroidal vascular responses following corneal
refractive surgery.
The present study also observed postoperative
reductions in AL in both groups. AL elongation and choroidal
thinning are known to correlate closely with myopia progression
(45,46). Peripheral hyperopic defocus in
myopia can further accelerate AL elongation (47), whereas correcting myopia can delay
this progression (48). Chen et
al (49) reported that in
adolescents using orthokeratology lenses, AL growth slowed and CT
increased markedly. Peripheral retinal myopic defocus induced by
orthokeratology has been shown to substantially alter ocular
development and slow myopia progression (10-12).
In the current study, postoperative AL was significantly shorter
than baseline, consistent with the effects observed with
orthokeratology. The IOLMaster 500 (Zeiss AG) used for AL
measurement in the present study calculates the distance from the
anterior corneal surface to the retinal pigment epithelium
(50); corneal refractive surgery
reduces corneal thickness through stromal ablation, which may lead
to shorter AL. The present data also indicated that AL shortening
was correlated with stromal AD. However, Xu et al (29) reported no significant association
between reduced AL and choroidal parameters. Therefore, it cannot
be definitively concluded that AL reduction is related to increased
CT or to changes in retinal myopic defocus.
The present study has several limitations, including
a small sample size and a relatively short follow-up period, which
limited the ability to assess long-term postoperative choroidal
changes. Owing to limitations in imaging sensitivity, blood flow
density in deeper choroidal layers was not assessed. Future studies
should address these aspects for a more comprehensive understanding
of the choroid.
In conclusion, an increase in CT following SMILE and
FS-LASIK surgeries was observed in the present study. However, by
the end of the observation period, CT at most locations had
returned to preoperative levels, indicating that the change was not
sustained. In addition, no significant changes in CD were observed.
The present findings suggest that SMILE and FS-LASIK exert minimal
influence on the choroid.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
The present study was conceived and designed by YL
and SY. The experiments were performed by SY, MT and JZ. The data
were analyzed by SY, TQ, JH and YD. YL contributed materials. SY,
YL and TQ wrote the manuscript. All authors read and approved the
final manuscript. JZ and YL confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The study protocol adhered to the principles of The
Declaration of Helsinki and received approval from the Human Ethics
Committee of Jinan Mingshui Eye Hospital (approval no. 2020-020).
All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jonas JB, Panda-Jonas S, Dong L and Jonas
RA: Clinical and anatomical features of myopia. Asia Pac J
Ophthalmol (Phila). 13(100114)2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Du Y, Meng J, He W, Qi J, Lu Y and Zhu X:
Complications of high myopia: An update from clinical
manifestations to underlying mechanisms. Adv Ophthalmol Pract Res.
4:156–163. 2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Landreneau JR, Hesemann NP and Cardonell
MA: Review on the myopia pandemic: Epidemiology, risk factors, and
prevention. Mo Med. 118:156–163. 2021.PubMed/NCBI
|
|
4
|
Arnoldi K: Growing pains: The incidence
and prevalence of myopia from 1950 to 2050. J Binocul Vis Ocul
Motil. 74:118–121. 2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liang J, Pu Y, Chen J, Liu M, Ouyang B,
Jin Z, Ge W, Wu Z, Yang X, Qin C, et al: Global prevalence, trend
and projection of myopia in children and adolescents from 1990 to
2050: A comprehensive systematic review and meta-analysis. Br J
Ophthalmol. 109:362–371. 2025.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu Y, Wang L, Xu Y, Pang Z and Mu G: The
influence of the choroid on the onset and development of myopia:
From perspectives of choroidal thickness and blood flow. Acta
Ophthalmol. 99:730–738. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Brown DM, Mazade R, Clarkson-Townsend D,
Hogan K, Datta Roy PM and Pardue MT: Candidate pathways for retina
to scleral signaling in refractive eye growth. Exp Eye Res.
219(109071)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ostrin LA, Harb E, Nickla DL, Read SA,
Alonso-Caneiro D, Schroedl F, Kaser-Eichberger A, Zhou X and
Wildsoet CF: IMI-the dynamic choroid: New insights, challenges, and
potential significance for human myopia. Invest Ophthalmol Vis Sci.
64(4)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jonas JB, Jonas RA, Bikbov MM, Wang YX and
Panda-Jonas S: Myopia: Histology, clinical features, and potential
implications for the etiology of axial elongation. Prog Retin Eye
Res. 96(101156)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tang T, Lu Y, Li X, Zhao H, Wang K, Li Y
and Zhao M: Comparison of the long-term effects of atropine in
combination with orthokeratology and defocus incorporated multiple
segment lenses for myopia control in Chinese children and
adolescents. Eye (Lond). 38:1660–1667. 2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Erdinest N, London N, Lavy I, Berkow D,
Landau D, Morad Y and Levinger N: Peripheral defocus and myopia
management: A mini-review. Korean J Ophthalmol. 37:70–81.
2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vincent SJ, Cho P, Chan KY, Fadel D,
Ghorbani-Mojarrad N, González-Méijome JM, Johnson L, Kang P,
Michaud L, Simard P and Jones L: CLEAR-orthokeratology. Cont Lens
Anterior Eye. 44:240–269. 2021.
|
|
13
|
Zhang R, Zhuang S, Zhou Y, Chin MP, Sun L,
Jhanji V and Zhang M: Associations between choroidal thickness and
rate of axial elongation in orthokeratology lens users.
Photodiagnosis Photodyn Ther. 51(104450)2025.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu M, Huang J, Xie Z, Wang Y, Wang P, Xia
R, Liu X, Su B, Qu J, Zhou X, et al: Dynamic changes of choroidal
vasculature and its association with myopia control efficacy in
children during 1-year orthokeratology treatment. Cont Lens
Anterior Eye. 48(102314)2025.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang XQ, Chen M, Zeng LZ and Liu LQ:
Investigation of retinal microvasculature and choriocapillaris in
adolescent myopic patients with astigmatism undergoing
orthokeratology. BMC Ophthalmol. 22(382)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lin MY, Tan HY and Chang CK: Myopic
regression after FS-LASIK and SMILE. Cornea. 43:1560–1566.
2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ramirez-Miranda A, Mota ADL, Rosa GGDL,
Serna-Ojeda JC, Valdez-García JE, Fábregas-Sánchez-Woodworth D,
Navas A, Jiménez-Corona A and Graue-Hernandez EO: Visual and
refractive outcomes after SMILE versus FS-LASIK: A paired-eye
study. Cir Cir. 92:758–768. 2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kim BK and Chung YT: Comparison of changes
in corneal thickness and curvature after myopia correction between
SMILE and FS-LASIK. J Refract Surg. 39:15–22. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Khanjian AT, Khodzhabekyan NV, Tarutta EP,
Harutyunyan SG and Milash SV: Changes in the wavefront and
peripheral defocus profile after excimer laser and orthokeratology
corneal reshaping in myopia. Vestn Oftalmol. 139:87–92.
2023.PubMed/NCBI View Article : Google Scholar : (In Russian).
|
|
20
|
Du Y, Zhou Y, Ding M, Zhang M and Guo Y:
Changes in relative peripheral refraction and optical quality in
Chinese myopic patients after small incision lenticule extraction
surgery. PLoS One. 18(e0291681)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zeppieri M, Marsili S, Enaholo ES, Shuaibu
AO, Uwagboe N, Salati C, Spadea L and Musa M: Optical coherence
tomography (OCT): A brief look at the uses and technological
evolution of ophthalmology. Medicina (Kaunas).
59(2114)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tan B, Chua J, Wong D, Liu X, Ismail M and
Schmetterer L: Techniques for imaging the choroid and choroidal
blood flow in vivo. Exp Eye Res. 247(110045)2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Spaide RF: Choriocapillaris flow features
follow a power law distribution: Implications for characterization
and mechanisms of disease progression. Am J Ophthalmol. 170:58–67.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Singh RB, Perepelkina T, Testi I, Young
BK, Mirza T, Invernizzi A, Biswas J and Agarwal A: Imaging-based
assessment of choriocapillaris: A comprehensive review. Semin
Ophthalmol. 38:405–426. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Stone RA, Tobias JW, Wei W, Carlstedt X,
Zhang L, Iuvone PM and Nickla DL: Diurnal gene expression patterns
in retina and choroid distinguish myopia progression from myopia
onset. PLoS One. 19(e0307091)2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
She Z and Gawne TJ: The parameters
governing the anti-myopia efficacy of chromatically simulated
myopic defocus in tree shrews. Transl Vis Sci Technol.
13(6)2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ma JX, Tian SW and Liu QP: Effectiveness
of peripheral defocus spectacle lenses in myopia control: A
meta-analysis and systematic review. Int J Ophthalmol.
15:1699–1706. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Troilo D, Nickla DL and Wildsoet CF:
Choroidal thickness changes during altered eye growth and
refractive state in a primate. Invest Ophthalmol Vis Sci.
41:1249–1258. 2000.PubMed/NCBI
|
|
29
|
Xu Z, Gui S, Huang J, Li Y, Lu F and Hu L:
Effect of femtosecond laser in situ keratomileusis on the
choriocapillaris perfusion and choroidal thickness in myopic
patients. Curr Eye Res. 46:878–884. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang J, He FL, Liu Y and Fan XQ:
Comparison of choroidal thickness in high myopic eyes after
FS-LASIK versus implantable collamer lens implantation with
swept-source optical coherence tomography. Int J Ophthalmol.
13:773–781. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhu X: Temporal integration of visual
signals in lens compensation (a review). Exp Eye Res. 114:69–76.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang Y and Wildsoet CF: RPE and choroid
mechanisms underlying ocular growth and myopia. Prog Mol Biol
Transl Sci. 134:221–240. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Roy S and Field GD: Dopaminergic
modulation of retinal processing from starlight to sunlight. J
Pharmacol Sci. 140:86–93. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhou X, Pardue MT, Iuvone PM and Qu J:
Dopamine signaling and myopia development: What are the key
challenges. Prog Retin Eye Res. 61:60–71. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cheng D, Qiao YL, Zhu XY, Ruan KM, Lian
HL, Shen MX, Wang SL, Shen LJ and Ye YF: Change in choroid
thickness and vascularity index associated with accommodation and
aberration after small-incision lenticule extraction. Int J
Ophthalmol. 18:672–682. 2025.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gupta P, Jing T, Marziliano P, Cheung CY,
Baskaran M, Lamoureux EL, Wong TY, Cheung CM and Cheng CY:
Distribution and determinants of choroidal thickness and volume
using automated segmentation software in a population-based study.
Am J Ophthalmol. 159:293–301. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mori Y, Miyake M, Hosoda Y, Uji A, Nakano
E, Takahashi A, Muraoka Y, Miyata M, Tamura H, Ooto S, et al:
Distribution of choroidal thickness and choroidal vessel dilation
in healthy Japanese individuals: The Nagahama study. Ophthalmol
Sci. 1(100033)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen Y, Liao H, Sun Y and Shen X:
Short-term changes in the anterior segment and retina after small
incision lenticule extraction. BMC Ophthalmol.
20(397)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang P, Hu X, Zhu C, Liu M, Yuan Y and Ke
B: Transient alteration of retinal microvasculature after
refractive surgery. Ophthalmic Res. 64:128–138. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chen M, Dai J and Gong L: Changes in
retinal vasculature and thickness after small incision lenticule
extraction with optical coherence tomography angiography. J
Ophthalmol. 2019(3693140)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gandhi S, Pattathil N and Choudhry N:
OCTA: Essential or gimmick? Ophthalmol Ther. 13:2293–2302.
2024.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kiyota N, Shiga Y, Ichinohasama K, Yasuda
M, Aizawa N, Omodaka K, Honda N, Kunikata H and Nakazawa T: The
impact of intraocular pressure elevation on optic nerve head and
choroidal blood flow. Invest Ophthalmol Vis Sci. 59:3488–3496.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sogawa K, Nagaoka T, Takahashi A, Tanano
I, Tani T, Ishibazawa A and Yoshida A: Relationship between
choroidal thickness and choroidal circulation in healthy young
subjects. Am J Ophthalmol. 153:1129–1132.e1. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang Y, Liu M, Xie Z, Wang P, Li X, Yao X,
Tian J, Han Y, Chen X, Xu Z, et al: Choroidal circulation in 8- to
30-year-old chinese, measured by SS-OCT/OCTA: Relations to age,
axial length, and choroidal thickness. Invest Ophthalmol Vis Sci.
64(7)2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Tian F, Zheng D, Zhang J, Liu L, Duan J,
Guo Y, Wang Y, Wang S, Sang Y, Zhang X, et al: Choroidal and
retinal thickness and axial eye elongation in Chinese junior
students. Invest Ophthalmol Vis Sci. 62(26)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Xie J, Ye L, Chen Q, Shi Y, Hu G, Yin Y,
Zou H, Zhu J, Fan Y, He J and Xu X: Choroidal thickness and its
association with age, axial length, and refractive error in Chinese
adults. Invest Ophthalmol Vis Sci. 63(34)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chakraborty R, Read SA and Collins MJ:
Hyperopic defocus and diurnal changes in human choroid and axial
length. Optom Vis Sci. 90:1187–1198. 2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Read SA, Collins MJ and Sander BP: Human
optical axial length and defocus. Invest Ophthalmol Vis Sci.
51:6262–6269. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chen Z, Xue F, Zhou J, Qu X and Zhou X:
Effects of orthokeratology on choroidal thickness and axial length.
Optom Vis Sci. 93:1064–1071. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Shi Q, Wang GY, Cheng YH and Pei C:
Comparison of IOL-Master 700 and IOL-Master 500 biometers in ocular
biological parameters of adolescents. Int J Ophthalmol.
14:1013–1017. 2021.PubMed/NCBI View Article : Google Scholar
|