Introduction
Appendiceal mucinous tumours are uncommon and are
detected in only 0.7-1.7% of individuals who undergo an
appendectomy. The preoperative diagnosis of appendiceal myxoma is
difficult and the results of imaging can only be used as a
reference. The diagnosis of the disease in the vast majority of
patients relies on the pathological examination (1). Appendiceal mucinous tumours are
low-grade malignant tumours with a high likelihood of metastatic
dissemination, and the most common type is low-grade appendiceal
mucinous neoplasm, which presents as an adenomatous change in the
appendix (2). The complications of
appendiceal mucinous tumours vary, but to the best of our
knowledge, cases resulting in autoamputation of the appendix have
not been reported. Appendiceal autoamputation is defined as the
separation of part of the appendix in the absence of surgical or
other invasive interventions and was first documented by Judd et
al in 1915(3). The clinical
manifestations of appendiceal mucinous tumours are typically
non-specific and their imaging features exhibit marked
heterogeneity, rendering preoperative diagnosis an arduous task. In
most cases, a conclusive diagnosis can only be achieved through
postoperative pathological examination. Over the past few decades,
extensive research has been conducted on the management of
appendiceal mucinous tumours, leading to the development of
well-established and standardized treatment protocols (4). Ruptured appendiceal mucinous tumours
are typically treated with cytoreductive surgery (CRS) combined
with hyperthermic intraperitoneal chemotherapy (HIPEC) (5); CRS is defined as the surgical removal
of all tumour tissue that is visible to the naked eye, as well as
the exploration of potential sites of involvement, and HIPEC is a
procedure whereby chemotherapeutic agents are dissolved in saline
at temperatures between 42.5-43.5˚C and then injected into the
abdominal cavity at a rate of 400 ml/min. This is achieved through
the use of an automated thermotherapy chemotherapy perfusion
device, with the aim of removing both free tumour cells and mucus
components. This comprehensive approach minimizes the risks of
residual tumour cells and local recurrence, thereby markedly
boosting the long-term survival rate of these patients and
remarkably enhancing their prognosis (6).
The present case report presents a particularly
unique case of appendiceal mucinous tumour. It highlights the
diagnostic challenges associated with this condition, the crucial
role of imaging and pathological examinations in its accurate
diagnosis and the standardized treatment protocol. By delving into
the rarity and complexity of the present case, the aim was to
enhance the clinical understanding of appendiceal mucinous tumours,
improving the ability of general surgeons to recognize, diagnose
and manage such cases in clinical practice.
Case report
A 68-year-old male patient (body mass index, 26.1
kg/m²) presented to the Emergency Department of the Second
Affiliated Hospital of Dalian Medical University (Dalian, China) in
March 2023 with a chief complaint of vague abdominal discomfort in
the right lower quadrant, which had been present for 10 days and
had recently worsened for 1 day. Although the patient took
anti-inflammatory medication prior to admission, the symptoms were
not notably relieved and the patient developed a fever reaching
37.5˚C. The postadmission temperature was 36.7˚C, and the white
blood cell (WBC) count was 11.90x109/l (normal range,
4.0-10.0x109/l). Physical examination revealed pressure
points limited to the right lower abdomen with mild rebound pain,
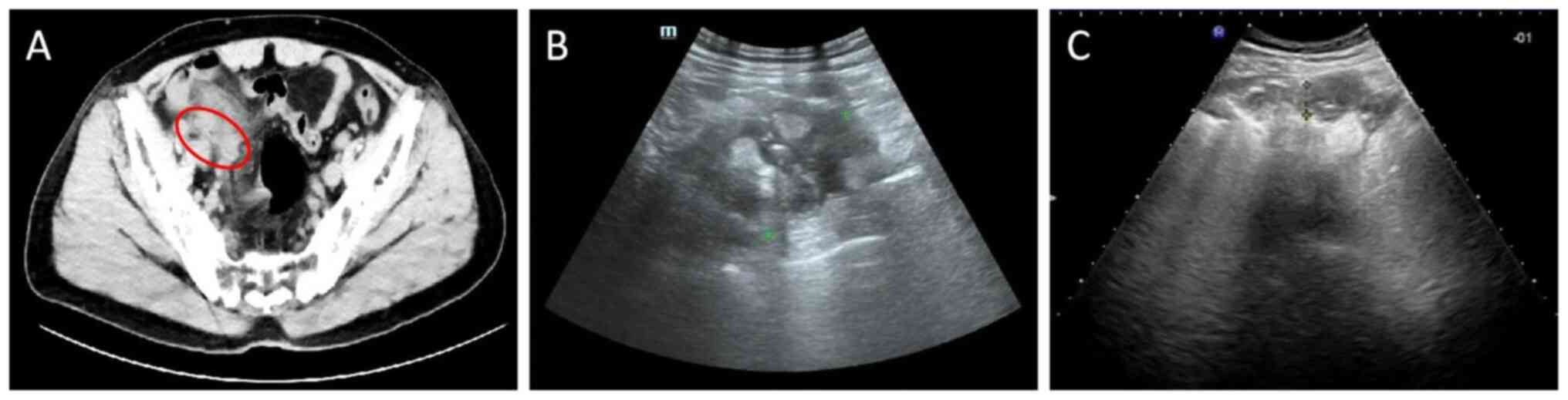
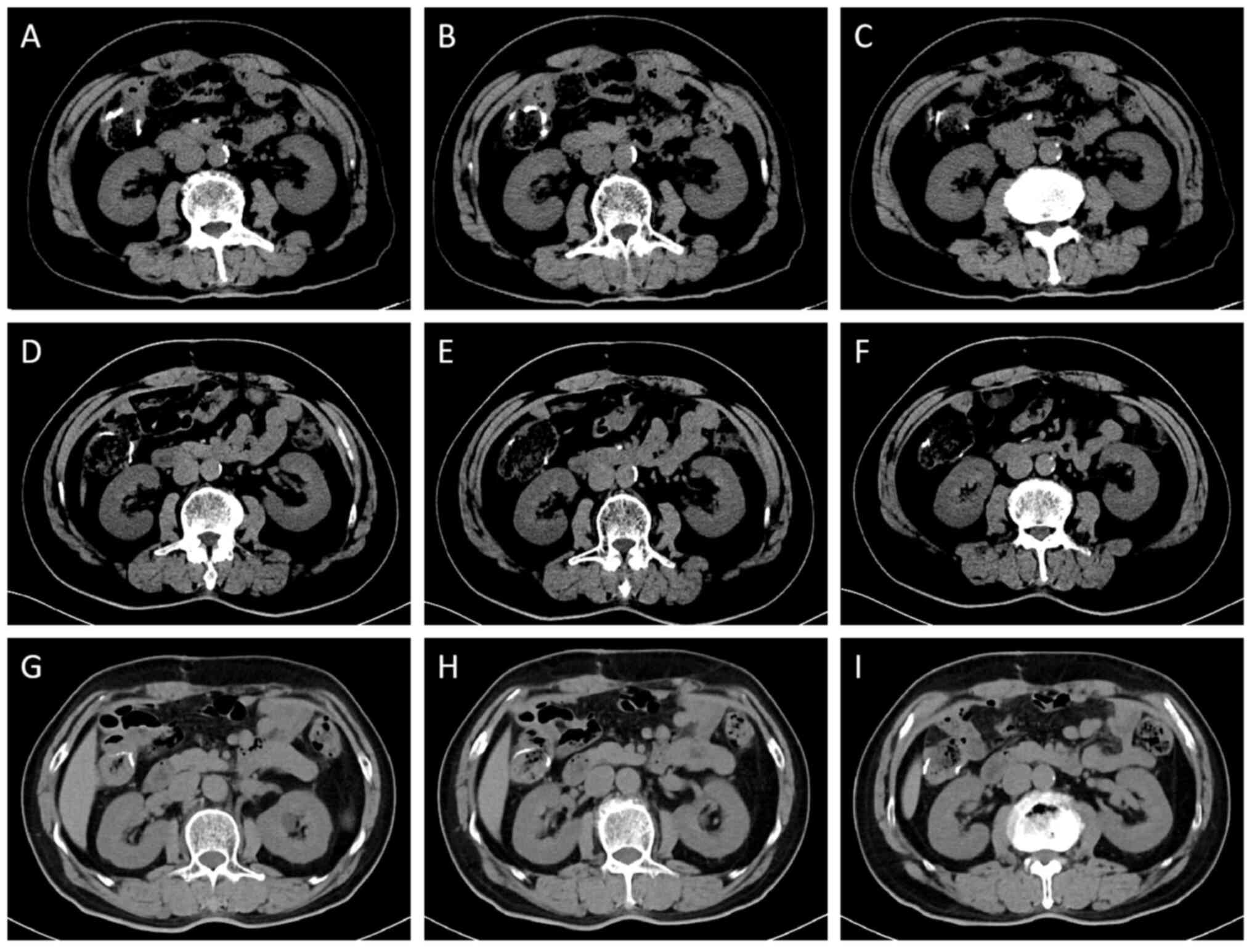
and CT examination revealed exudative effusive changes in the
ileocecal and right iliac fossa regions with adhesions in the
adjacent bowel (Fig. 1A);
additionally, ultrasound revealed the presence of a periappendiceal
inflammatory wrap (Fig. 1B). Based
on the patient's medical history and imaging findings, the appendix
was deemed to have formed complex adhesions with the surrounding
tissues. Following a discussion with the patient and their family,
it was determined that a complete removal of the appendix and
abscess in a single surgical procedure was not feasible;
consequently, a conservative treatment approach was implemented.
The conservative treatment regimen formulated for the patient
comprised the following measures: i) Nil per os (fasting); ii)
intravenous fluid replacement; and iii) anti-inflammatory therapy,
including a meloxicillin sulbactam injection, 3.75 g administered
intravenously every 12 h, with a planned course of 4 days (total
amount, 30 g). Ultrasound was repeated 3 days later, which revealed
that the abscess had decreased in size (Fig. 1C). Following a 6-day course of
treatment, the patient's body temperature returned to normal, the
abdominal pain abated and no compression or rebound pain was
observed upon examination. The patient was discharged from the
hospital in March 2023 in stable condition (WBC count:
3.56x109/l).
The patient was asked to undergo follow-up
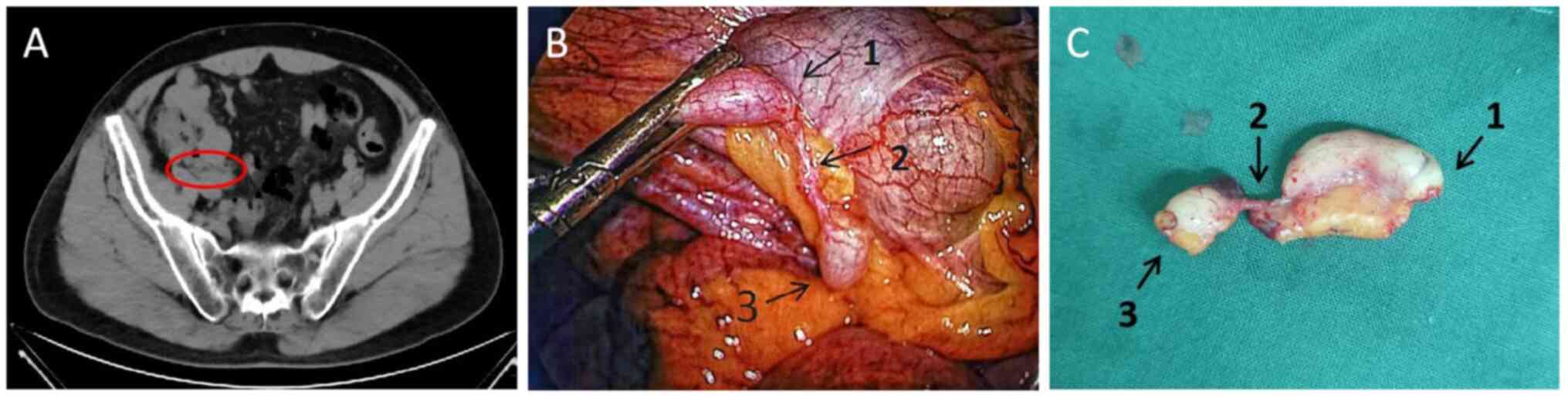
examinations, and 3 months after discharge, CT of the abdomen
revealed slight thickening of the appendix and that the surrounding
abscess had disappeared (Fig. 2A).
The patient was readmitted to the hospital in June 2023 with the
expectation of surgical treatment.
Exploration of the operative area revealed a
relatively fixed and locally oozing ileocecal bowel and its
mesentery, and adhesions due to a previous peripheral abscess were
considered. The adherent tissues were not forcibly separated and
thus the appendiceal autoamputation was discovered (Fig. 2B). The appendix was ~5 mm in
diameter, the entire layer was completely dissected, the proximal
end was ~3 cm long and the distal end was ~1 cm long. The two ends
were connected only by the mesentery, and the distance between the
dissected points was ~1.5 cm.
The appendicular artery was visible on the medial
side of the mesentery; additionally, the appendix at both ends of
the dissected point exhibited robust haemato-vascular activity,
with the capillaries discernible on the surface. The lumen
demonstrated slight distension, and neither the proximal nor distal
segments of the appendix presented any evidence of infarction, the
dissected surfaces were entirely sealed. As there was no marked
mucus component observed in the operative area, it was considered
to be a site of appendicitis and therefore the appendix was removed
(Fig. 2C).
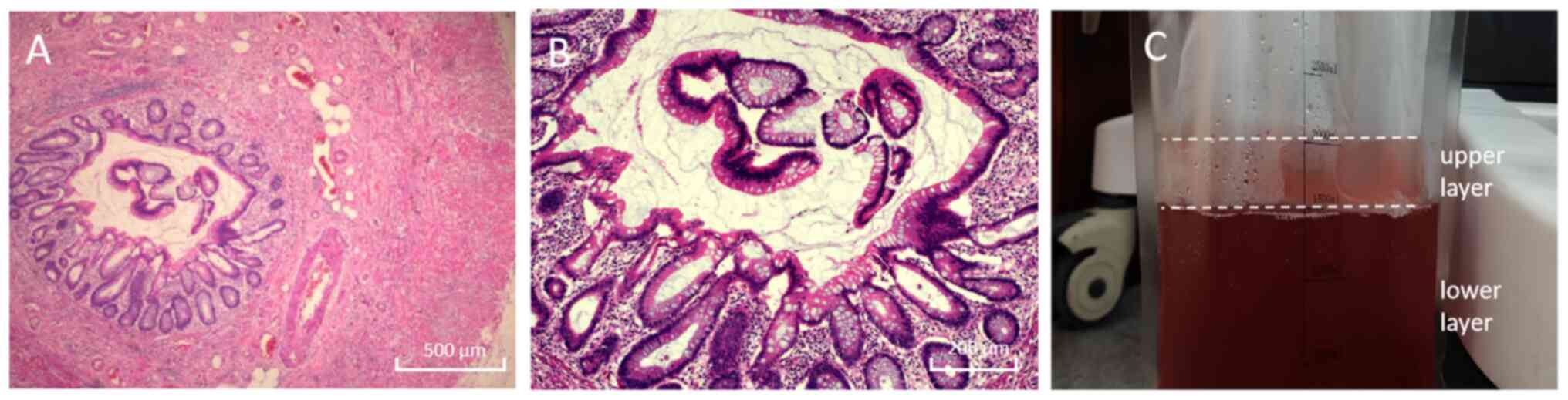
The results of the pathological examination (neutral
buffered formalin; 4% formaldehyde aqueous solution; 20-25˚C; 6-24
h) were returned 3 days later, and indicated a low-grade mucinous
tumour of the appendix (Fig. 3A
and B). The mucosal layer of the
appendix was thickened, with a smooth surface and no notable ulcers
or cauliflower-like protrusions. The appendiceal goblet cells and
mucus-secreting columnar cells were arranged in a columnar or
cuboidal shape, with nuclei located at the basal region of the
cells; the chromatin was uniformly distributed, nucleoli were
inconspicuous, mitotic figures were rare (≤1 per 10 high-power
fields) and no notable cytological atypia was observed. The
appendiceal epithelium and lamina propria were compressed, showing
thinning or even focal absence. Mucus had penetrated the glandular
basement membrane and infiltrated into the muscular layer of the
appendix, and a small number of lymphocytes and plasma cells were
seen infiltrating the area around the serosal layer. The
aforementioned pathological manifestations are consistent with the
pathological characteristics of low-grade appendiceal myxoma
(7).
Following confirmation of the diagnosis, right
hemicolectomy, resection of the greater omentum and partial
peritoneal resection were performed in July 2023 in accordance with
established treatment guidelines (8). Additionally, HIPEC (cisplatin 75
mg/m² for 60 min) was conducted; when HIPEC was performed, the
abdominal lavage fluid was stratified upon standing, with the upper
layer containing clear light red turbid mucus and the lower layer
containing dark red lavage fluid (Fig.
3C).
At 10 days post-surgery, the patient gradually
recovered intestinal function, as evidenced by normal defecation.
The patient was subsequently discharged without any new discomfort
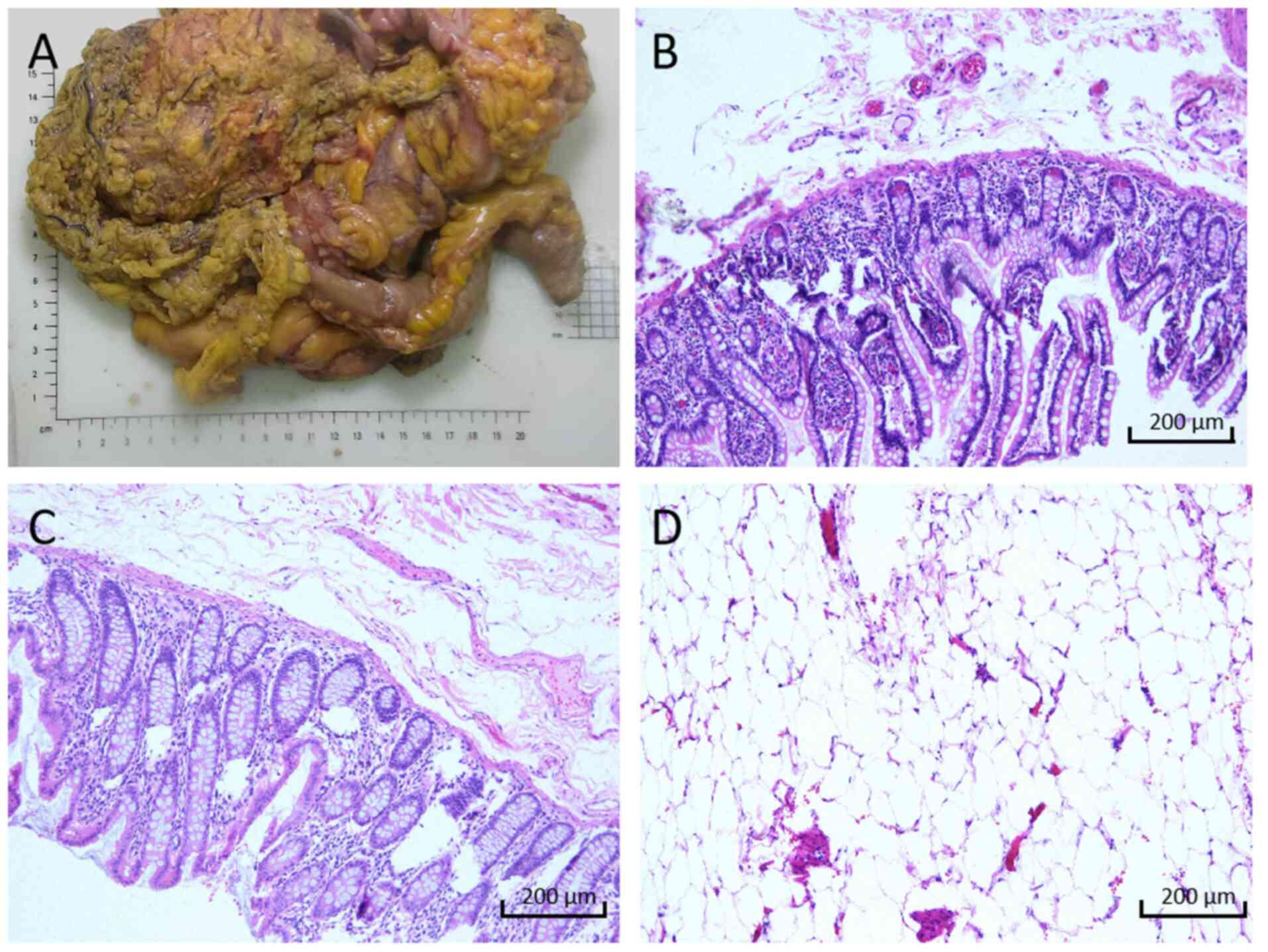
after eating, 10 days after the surgery. Pathological examination
of the appendiceal mucinous tumour revealed the absence of lesional
tissue on the ileocecal side, colonic side of the dissection and
peripheral circumferential margins. Additionally, no lesional
tissue was observed in the peri-intestinal lymph nodes (0/16)
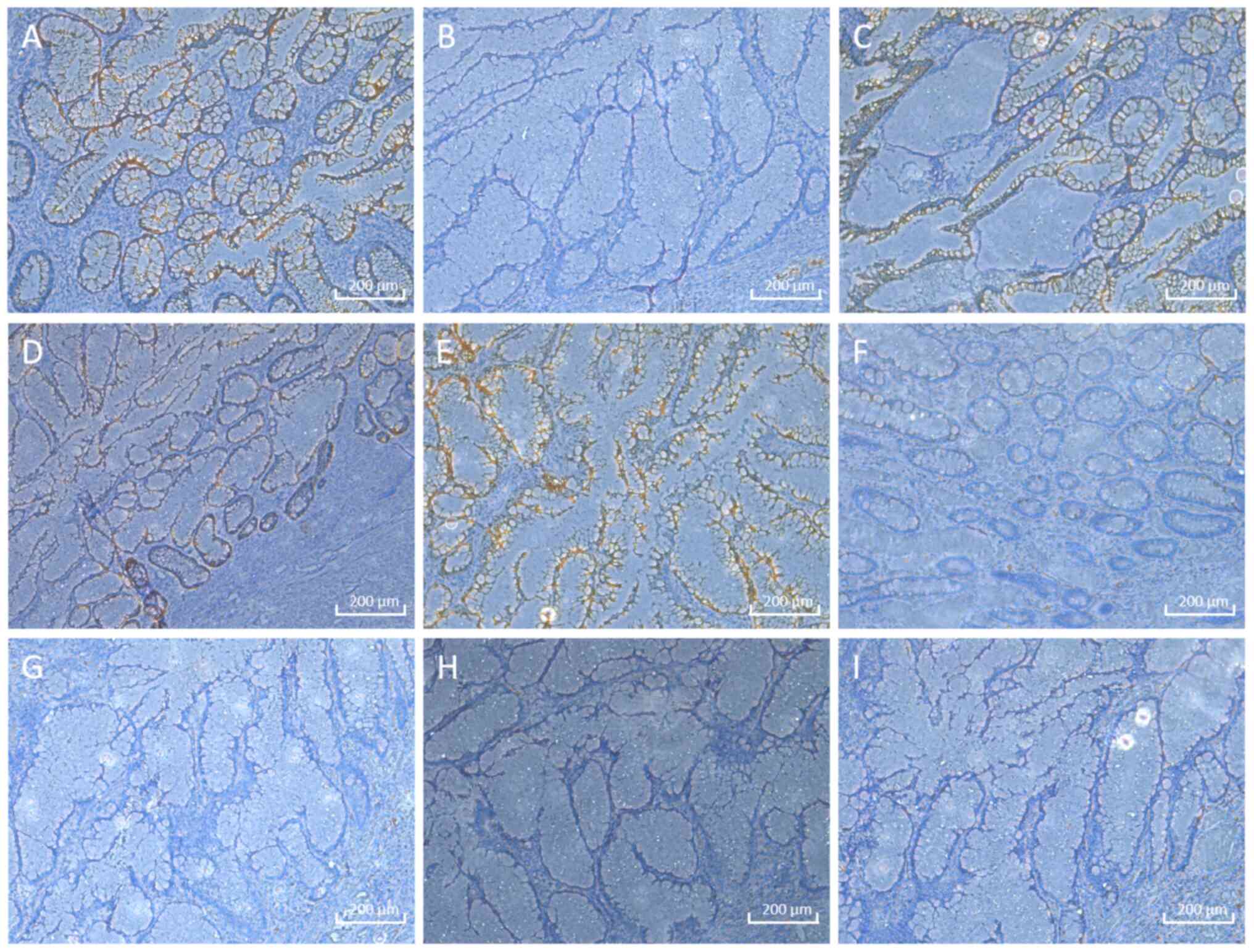
(Fig. 4). Immunohistochemistry was
performed (4% neutral buffered formalin fixative; 20-25˚C for 6-24
h; paraffin-embedded 4- to 5-µm sections), indicating cytokeratin
(CK)-pan+, CK7-, CK20+, caudal
type homeobox 2+, mucin (MUC2)+,
MUC5AC-, paired box (PAX)-2-,
PAX-8- and Ki-67- results (Fig. 5). Primary antibody details were as
follows: CK-pan mouse monoclonal antibody (7H8C4; 1:200; cat. no.
EM1712-42; HUABIO), CK7 recombinant monoclonal antibody (1:2,000;
cat. no. 86153-7-RR; Proteintech Group, Inc.), CK20 recombinant
monoclonal antibody (1:4,000; cat. no. 82428-1-RR; Proteintech
Group, Inc.), CDX2 recombinant monoclonal antibody (1:1,000; cat.
no. 82659-1-RR; Proteintech Group, Inc.), MUC2 polyclonal antibody
(1:2,000; cat. no. 27675-1-AP; Proteintech Group, Inc.), MUC5AC
polyclonal antibody (1:500; cat. no. 20725-1-AP; Proteintech Group,
Inc.), PAX2 polyclonal antibody (1:1,000; cat. no. 29307-1-AP;
Proteintech Group, Inc.), PAX8 polyclonal antibody (1:800; cat. no.
10336-1-AP; Proteintech Group, Inc.) and Ki-67 polyclonal antibody
(1:4,000; cat. no. 27309-1-AP; Proteintech Group, Inc.). Primary
antibodies were incubated at 4˚C for 12 h. Secondary antibody
details were as follows: Multi-rAb® Polymer HRP-Goat
Anti-Rabbit Recombinant Secondary Antibody (H+L) (ready-to-use;
cat. no. RGAR011; Proteintech Group, Inc.), Multi-rAb™ Polymer
HRP-Goat Anti-Mouse Recombinant Secondary Antibody (H+L)
(ready-to-use; cat. no. RGAM011; Proteintech Group, Inc.). The
secondary antibodies were incubated at room temperature for 30
min.
The patient underwent a total of three follow-up
reexaminations from July 2023 to June 2024, mainly including blood
biochemical and CT examinations. The 1-year follow-up after the
right hemicolectomy indicated that the patient had achieved
satisfactory postoperative recovery. Follow-up evaluation
demonstrated the absence of clinical symptoms (such as abdominal
discomfort and gastrointestinal dysfunction); physical examination
revealed normal abdominal signs, including negative tenderness, no
palpable masses, absent rebound tenderness and normal bowel sounds;
laboratory tests showed no abnormal findings: CEA, 0.97 ng/ml
(normal range, <5.0 ng/ml); CA125, 14.53 U/ml (normal range
<25 U/ml); CA19-9: consistently <1.20 U/ml (normal range,
<37 U/ml); and serial CT imaging revealed no evidence of disease
progression or recurrence (Fig.
6). A regular follow-up examination will be performed every
year in the future.
Discussion
In the present case report, a rare case of
appendiceal autoamputation due to an appendiceal mucinous tumour
was reported; the appendix was dissected into two parts, with good
haematology at both ends. An appendiceal mucocele is challenging to
diagnose preoperatively, and initially, it was assumed that the
appendix had autoamputated as a result of inflammation, so an
appendectomy was performed; however, the results of the
postoperative pathological examination revealed a diagnosis of
appendiceal mucocele. The most common presentation of an
appendiceal mucinous tumour is acute appendicitis combined with
perforation. This may present with numerous symptoms, including
abdominal pain (which may be located in the periumbilical area or
extensive abdomen and may progressively migrate to the right lower
abdomen), abdominal distension, an abdominal mass and bloody stool
(4). Most patients present with
atypical clinical manifestations; some individuals present with
lower back pain and ileocecal intestinal obstruction (9-11).
Appendiceal mucinous tumours are diagnosed on the basis of imaging
data and pathological findings. CT allows for straightforward,
rapid assessment of abdominal organs, but diagnosing appendiceal
mucinous tumours on the basis of CT findings alone is challenging
owing to the variable, ambiguous and nonspecific presentation of
these tumours on CT scans. Appendiceal mucinous tumour should be
considered when encountering a focal well-defined cystic mass with
slightly higher than water attenuation, thickened cystic wall with
ring mural enhancement and a characteristic progressive contrast
enhancement on CT imaging (7).
Consequently, a definitive diagnosis is often made on the basis of
incidental intraoperative findings and postoperative pathology.
Intraoperative visualisation of mucus draining from the appendix or
intra-abdominal findings of a distinct mucus component aids in the
pathological diagnosis of an appendiceal mucocele, as do
microscopic observation of villous or flat mucinous epithelial
hyperplasia with low-grade isoforms and a mucin component (1,7). The
immunohistochemical results were as follows: CK20-, MUC2- and
MUC5AC-positive, and PAX-2- and PAX-8-negative, mostly suggestive
of a diagnosis of an appendiceal mucinous tumour (12,13).
The immunohistochemical results were largely consistent with the
typical immunophenotypic characteristics of appendiceal myxoma,
which provides objective evidence for confirming the pathological
diagnosis. In addition, the immunohistochemical results further
provide a basis for the differential diagnosis of this disease, as
follows: i) CK-pan positivity excludes non-epithelial neoplasms,
such as carcinoid tumours, lymphomas and sarcomas (14); ii) CDX2 positivity rules out the
possibility of primary pancreatic myxoma (15); iii) Ki-67 negativity is consistent
with the biological behaviours of low-grade tumours, supporting the
exclusion of high-grade malignant tumours (16); and iv) PAX-2 and PAX-8 negativity
eliminated the possibility of metastatic tumours originating from
the genitourinary system or thyroid (17).
Rupture of an appendiceal mucinous tumour can result
in a variety of complications, but to the best of our knowledge,
cases leading to appendiceal autoamputation have not been reported.
Autoamputation of the appendix has been documented in cases of
appendiceal mucous cysts, gangrenous appendicitis, chronic pelvic
abscesses and blunt abdominal injury (18-22).
The aforementioned cases were identified as intraoperative
autoamputation of the appendix, and the underlying causes and
clinical manifestations of these occurrences exhibited notable
differences (including abdominal pain, calcified masses, fever and
free gas in the abdominal cavity).
The underlying aetiological mechanism of appendiceal
autoamputation resulting from the rupture of an appendiceal
mucinous tumour is the transformation of appendiceal cup cells into
tumour cells, which maintain the expression of mucin throughout the
process of proliferation, leading to the accumulation of mucus in
the appendiceal lumen (23). As
allometric tension increased, the likelihood of appendiceal rupture
and perforation increased concomitantly. Owing to the absence of
cell surface adhesion factors, appendiceal mucus tumour cells are
capable of traversing the abdominal cavity and continuing to
produce mucus (24). A clinical
study showed that ~20% of patients with appendiceal mucinous
tumours experienced appendiceal rupture or perforation, resulting
in the formation of a peritoneal pseudomucinous tumour (PMP)
(25). The 3-, 5- and 10-year
survival rates for patients who develop PMPs are 100, 86 and 45%,
respectively (26).
A review of the literature and relevant case
material revealed that an appendiceal mucocele can be caused by
chronic inflammation over a long period, which was discussed in the
context of the past medical history of the patient in the present
case. The current patient initially presented with a
periappendiceal abscess secondary to appendicitis, and following
anti-inflammatory and other symptomatic treatments, the
inflammation subsided and the abscess was absorbed; however, the
appendiceal inflammation was not resolved. After a prolonged period
of chronic inflammatory stimulation, the appendiceal cup cells
undergo tumour transformation, resulting in the formation of an
appendiceal mucocele, and the mucus produced by the tumour cells
accumulates within the appendiceal lumen. In the presence of
bacterial growth and infection, the appendix becomes gangrenous and
the entire layer of the appendix undergoes necrosis. Autoamputation
of the appendix occurs, and the fractured surface of the lumen
closes after the expulsion of necrotic material and mucus (27).
Treatment options for appendiceal mucinous tumours
have been progressively developed over the years, leading to more
standardized systemic treatment protocols currently in place. For
low-grade appendiceal mucinous tumours confined to the appendix,
the recommended treatment is right hemicolectomy, but for patients
whose appendix has ruptured secondary to PMP, CRS combined with
HIPEC (28,29), a protocol first proposed by Spratt
et al (30) in 1980, is
recommended. According to the guidelines, in the present study, a
right hemicolectomy, resection of the greater omentum and partial
peritoneal resection were performed and the surrounding tissues and
organs were cleared to minimise the risk of missing small lesions
(31,32). Surgery allows extensive removal of
solid tumour tissue but is limited in terms of addressing free
tumour cells and their mucus secretion; therefore, in the present
study, HIPEC was used to compensate for the lack of free tumour
cells and the mucus clearance ability of CRS (33). HIPEC kills tumour cells through the
following processes (34-36):
i) Increasing the concentration of local chemotherapy and enhancing
the direct cytotoxic effect of chemotherapy drugs; ii) causing
microvascular embolism in the tumour tissue, resulting in tissue
ischaemia and necrosis; iii) activating lysosomes to destroy the
cell structure to directly kill tumour cells in the S and M phases
of the cell cycle; and iv) destroying the membrane proteins of
tumour cells at the molecular level, interfering with the synthesis
process of DNA, RNA and proteins.
The CRS + HIPEC regimen combines the advantages of
surgical resection, local chemotherapy, hyperthermia and abdominal
lavage (37-40).
After years of investigation and research, CRS combined with HIPEC
has been identified as the optimal treatment option for patients
with appendiceal mucinous tumours combined with PMP (41). A follow-up analysis of 512 patients
with appendiceal mucinous tumours secondary to PMP who underwent
CRS combined with HIPEC was performed. Among these patients, ~25%
experienced recurrence, 375 were recurrence-free, 35 underwent
reoperation and 102 experienced recurrence but did not undergo
surgery. The 5-year survival rates were 90.9, 79.0 and 64.5%,
respectively (42).
In conclusion, the present case report describes a
rare case of an appendiceal mucinous tumour leading to
autoamputation of the appendix. The present report presents a
detailed analysis of the mechanism underlying the development of
appendiceal mucinous tumours, accompanied by a concise overview of
the literature on this subject. In addition, diagnosing appendiceal
mucinous tumours preoperatively is challenging, with the
specificity of the most commonly used imaging tests being
suboptimal. It is therefore recommended that full exploration of
the abdominal cavity and intraoperative frozen pathology is
conducted when the abdominal cavity is clear and the risk of
collateral injuries is low. This will facilitate an early
definitive diagnosis and standardise treatment, thus reducing
recurrence rates and prolonging survival.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
The present study was conceptualised by all authors.
CW and YuL wrote the manuscript and collected the data. CW, YuL, ZC
and SN designed and performed the analysis. YaL, BLi, Blu and XH
contributed to the acquisition and interpretation of data. All
authors have read and approved the final version of the manuscript.
CW and YuL confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines of the Declaration of Helsinki, and ethical review and
approval was waived for the single case report (Ethics Office, the
Second Affiliated Hospital of Dalian Medical University, Dalian,
China).
Patient consent for publication
Patient characteristics have been anonymized in
compliance with ethical standards. Written informed consent was
obtained from the next of kin for the publication of any associated
images or patient data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kawecka W, Adamiak-Godlewska A, Lewkowicz
D, Urbańska K and Semczuk A: Diagnostic difficulties in the
differentiation between an ovarian metastatic low-grade appendiceal
mucinous neoplasm and primary ovarian mucinous cancer: A case
report and literature review. Oncol Lett. 28(500)2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kabbani W, Houlihan PS, Luthra R, Hamilton
SR and Rashid A: Mucinous and nonmucinous appendiceal
adenocarcinomas: Different clinicopathological features but similar
genetic alterations. Mod Pathol. 15:599–605. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Judd JR: A specimen of ‘auto-amputation’
of the appendix. JAMA. 65(1179)1915.
|
|
4
|
Shaib WL, Assi R, Shamseddine A, Alese OB,
Staley C III, Memis B, Adsay V, Bekaii-Saab T and El-Rayes BF:
Appendiceal mucinous neoplasms: Diagnosis and management.
Oncologist. 22:1107–1116. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chow FC, Yip J, Foo DC, Wei R, Choi HK, Ng
KK and Lo OS: Cytoreductive surgery and hyperthermic
intraperitoneal chemotherapy (HIPEC) for colorectal and appendiceal
peritoneal metastases-The Hong Kong experience and literature
review. Asian J Surg. 44:221–228. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Auer RC, Sivajohanathan D, Biagi J, Conner
J, Kennedy E and May T: Indications for hyperthermic
intraperitoneal chemotherapy with cytoreductive surgery: A
systematic review. Eur J Cancer. 127:76–95. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yu XR, Mao J, Tang W, Meng XY, Tian Y and
Du ZL: Low-grade appendiceal mucinous neoplasms confined to the
appendix: Clinical manifestations and CT findings. J Investig Med.
68:75–81. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Govaerts K, Lurvink RJ, De Hingh IHJT, Van
der Speeten K, Villeneuve L, Kusamura S, Kepenekian V, Deraco M,
Glehen O and Moran BJ: PSOGI. Appendiceal tumours and pseudomyxoma
peritonei: Literature review with PSOGI/EURACAN clinical practice
guidelines for diagnosis and treatment. Eur J Surg Oncol. 47:11–35.
2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mannarini M, Maselli F, Giannotta G,
Cioeta M and Giovannico G: Low back pain as main symptom in
Low-grade Appendiceal Mucinous Neoplasm (LAMN): A case report.
Physiother Theory Pract. 41:230–238. 2025.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liapis SC, Perivoliotis K, Psarianos K,
Chatzinikolaou C, Moula AI, Skoufogiannis P, Balogiannis I and
Lytras D: A giant low-grade appendiceal mucinous neoplasm (LAMN)
presenting as ileocecal intussusception: A case report. J Surg Case
Rep. 2023(rjad273)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Komo T, Kohashi T, Hihara J, Oishi K,
Yoshimitsu M, Kanou M, Nakashima A, Aoki Y, Miguchi M, Kaneko M, et
al: Intestinal obstruction caused by Low-grade appendiceal mucinous
neoplasm: A case report and review of the literature. Int J Surg
Case Rep. 51:37–40. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ji H, Isacson C, Seidman JD, Kurman RJ and
Ronnett BM: Cytokeratins 7and 20, Dpc4, and MUC5AC in the
distinction of metastatic mucinous carcinomas in the ovary
fromprimary ovarian mucinous tumors: Dpc4 assists in identifying
metastatic pancreatic carcinomas. Int J Gynecol Pathol. 21:391–400.
2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dundr P, Singh N, Nozickova B, Nemejcova
K, Bartu M and Struzinska I: Primary mucinous ovarian tumors vs.
ovarian metastases from gastrointestinal tract, pancreas and
biliary tree: A review of current problematics. Diagn Pathol.
16(20)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Son EM, Kim JY, An S, Song KB, Kim SC, Yu
E and Hong SM: Clinical and prognostic significances of cytokeratin
19 and KIT expression in surgically resectable pancreatic
neuroendocrine tumors. J Pathol Transl Med. 49:30–36.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chu PG, Schwarz RE, Lau SK, Yen Y and
Weiss LM: Immunohistochemical staining in the diagnosis of
pancreatobiliary and ampulla of Vater adenocarcinoma: Application
of CDX2, CK17, MUC1, and MUC2. Am J Surg Pathol. 29:359–367.
2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kalvala J, Parks RM, Green AR and Cheung
KL: Concordance between core needle biopsy and surgical excision
specimens for Ki-67 in breast cancer-a systematic review of the
literature. Histopathology. 80:468–484. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ozcan A, de la Roza G, Ro JY, Shen SS and
Truong LD: PAX2 and PAX8 expression in primary and metastatic renal
tumors: A comprehensive comparison. Arch Pathol Lab Med.
136:1541–1551. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sachs L and Hoffman E: Autoamputation in
mucocele of the appendix. AMA Arch Surg. 63:712–714.
1951.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lovrenski J, Jokić R and Varga I:
Sonographically detected free appendicolith as a sign of retrocecal
perforated appendicitis in a 2-year-old child. J Clin Ultrasound.
44:395–398. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Weil BR, Al-Ibraheemi A, Vargas SO and
Rangel SJ: Autoamputation of the appendix presenting as a calcified
abdominal mass following necrotizing enterocolitis. Pediatr Dev
Pathol. 20:335–339. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Markey CM and Vestal LE: Autoamputation of
the appendix in a chronic adnexal abscess. Case Rep Obstet Gynecol.
2018(6010568)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sharma K, Tomar S, Sharma S and Bajpai M:
Floating appendix: Post-traumatic amputation of the appendix as
sequela or complication?: A case report. J Med Case Rep.
15(192)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Legué LM, Creemers GJ, de Hingh IHJT,
Lemmens VEPP and Huysentruyt CJ: Review: Pathology and its clinical
relevance of mucinous appendiceal neoplasms and pseudomyxoma
peritonei. Clin Colorectal Cancer. 18:1–7. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hinson FL and Ambrose NS: Pseudomyxoma
peritonei. Br J Surg. 85:1332–1339. 1998.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Smeenk RM, van Velthuysen ML, Verwaal VJ
and Zoetmulder FAN: Appendiceal neoplasms and pseudomyxoma
peritonei: A population based study. Eur J Surg Oncol. 34:196–201.
2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Misdraji J, Yantiss RK, Graeme-Cook FM,
Balis UJ and Young RH: Appendiceal mucinous neoplasms: A
clinicopathologic analysis of 107 cases. Am J Surg Pathol.
27:1089–1103. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zerem E, Salkic N, Imamovic G and Terzić
I: Comparison of therapeutic effectiveness of percutaneous drainage
with antibiotics versus antibiotics alone in the treatment of
periappendiceal abscess: Is appendectomy always necessary after
perforation of appendix? Surg Endosc. 21:461–466. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
McDonald JR, O'Dwyer ST, Rout S,
Chakrabarty B, Sikand K, Fulford PE, Wilson MS and Renehan AG:
Classification of and cytoreductive surgery for Low-grade
appendiceal mucinous neoplasms. Br J Surg. 99:987–92.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Huang Y, Alzahrani NA, Chua TC and Morris
DL: Histological subtype remains a significant prognostic factor
for survival outcomes in patients with appendiceal mucinous
neoplasm with peritoneal dissemination. Dis Colon Rectum.
60:360–367. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Spratt JS, Adcock RA, Muskovin M, Sherrill
W and McKeown J: Clinical delivery system for intraperitoneal
hyperthermic chemotherapy. Cancer Res. 40:256–260. 1980.PubMed/NCBI
|
|
31
|
Sugarbaker PH: Pont hepatique (hepatic
bridge), an important anatomic structure in cytoreductive surgery.
J Surg Oncol. 101:251–252. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Somashekhar SP, Ashwin KR, Yethadka R,
Zaveri SS, Ahuja VK, Rauthan A and Rohit KC: Impact of extent of
parietal peritonectomy on oncological outcome after cytoreductive
surgery and HIPEC. Pleura Peritoneum. 4(20190015)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li Y, Zhou YF, Liang H, Wang HQ, Hao JH,
Zhu ZG, Wan DS, Qin LX, Cui SZ, Ji JF, et al: Chinese expert
consensus on cytoreductive surgery and hyperthermic intraperitoneal
chemotherapy for peritoneal malignancies. World J Gastroenterol.
22:6906–6916. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gonzalez-Moreno S, Gonzalez-Bayon LA and
Ortega-Perez G: Hyperthermic intraperitoneal chemotherapy:
Rationale and technique. World J Gastrointest Oncol. 2:68–75.
2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Glehen O, Osinsky D, Cotte E, Kwiatkowski
F, Freyer G, Isaac S, Trillet-Lenoir V, Sayag-Beaujard AC, François
Y, Vignal J and Gilly FN: Intraperitoneal chemohyperthermia using a
closed abdominal procedure and cytoreductive surgery for the
treatment of peritoneal carcinomatosis: Morbidity and mortality
analysis of 216 consecutive procedures. Ann Surg Oncol. 10:863–869.
2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Elias D, Bonnay M, Puizillou JM, Antoun S,
Demirdjian S, El OA, Pignon JP, Drouard-Troalen L, Ouellet JF and
Ducreux M: Heated intraoperative intraperitoneal oxaliplatin after
complete resection of peritoneal carcinomatosis: Pharmacokinetics
and tissue distribution. Ann Oncol. 13:267–272. 2002.PubMed/NCBI View Article : Google Scholar
|
|
37
|
El-Kareh AW and Secomb TW: A theoretical
model for intraperitoneal delivery of cisplatin and the effect of
hyperthermia on drug penetration distance. Neoplasia. 6:117–127.
2004.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sinukumar S, Mehta S, As R, Damodaran D,
Ray M, Zaveri S, Kammar P and Bhatt A: . Analysis of clinical
outcomes of pseudomyxoma peritonei from appendicular origin
following cytoreductive surgery and hyperthermic intraperitoneal
Chemotherapy-A Retrospective study from INDEPSO. Indian J Surg
Oncol. 10 (Suppl 1):S65–S70. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wahba R, Schmidt T, Buchner D, Wagner T
and Bruns CJ: Surgical treatment of pseudomyxoma
peritonei-Cytoreductive surgery and hyperthermic intraperitoneal
chemotherapy. Chirurgie (Heidelb). 94:840–844. 2023.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
40
|
Baratti D, Kusamura S, Guaglio M, Milione
M, Pietrantonio F, Cavalleri T, Morano F and Deraco M: Relapse of
pseudomyxoma peritonei after cytoreductive surgery and hyperthermic
intraperitoneal chemotherapy: Pattern of failure, clinical
management and outcomes. Ann Surg Oncol. 30:404–414.
2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kusamura S, Barretta F, Yonemura Y,
Sugarbaker PH, Moran BJ, Levine EA, Goere D, Baratti D, Nizri E,
Morris DL, et al: The role of hyperthermic intraperitoneal
chemotherapy in pseudomyxoma peritonei after cytoreductive surgery.
JAMA Surg. 156(e206363)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lord AC, Shihab O, Chandrakumaran K,
Mohamed F, Cecil TD and Moran BJ: Recurrence and outcome after
complete tumour removal and hyperthermic intraperitoneal
chemotherapy in 512 patients with pseudomyxoma peritonei from
perforated appendiceal mucinous tumours. Eur J Surg Oncol.
41:396–399. 2015.PubMed/NCBI View Article : Google Scholar
|