Introduction
Bronchopulmonary dysplasia (BPD) is a chronic lung
disease that remains a notable cause of morbidity in preterm
infants, particularly those born at low gestational ages (<28
weeks) or with very low birth weight (<1,000 g). Globally, among
infants born at <28 weeks' gestation, reported incidence of BPD
ranges from 10 to 89% depending on region and definition, with
population-based rates in recent meta-analyses of very low birth
weight (<1,500 g) or very low gestational age (<32 weeks)
neonates estimated at approximately 21% (95% CI 19-24%) (1). Despite advances in neonatal intensive
care, the incidence of BPD has not markedly declined, largely due
to the increased survival of extremely preterm infants who remain
at the highest risk (2-4).
BPD is characterized by abnormal alveolar and vascular development,
resulting from a complex interplay of antenatal, perinatal and
postnatal factors, including inflammation, oxygen toxicity,
mechanical ventilation and sepsis (5).
Early identification of infants at high risk for BPD
is crucial for optimizing management strategies and minimizing
long-term complications such as recurrent wheezing, reduced
pulmonary function, asthma-like symptoms, feeding difficulties,
growth failure, and neurodevelopmental impairments including
cognitive delays, motor dysfunction, and increased risk of cerebral
palsy (6,7). However, the current diagnostic
criteria for BPD, as updated by the National Institute of Child
Health and Human Development (NICHD) in 2018, rely on the need for
respiratory support or supplemental oxygen at 36 weeks
postmenstrual age, which is defined as gestational age at birth
plus the infant's postnatal age. Because this assessment point
occurs after birth, it limits the usefulness of the criteria for
early risk stratification (8,9).
This highlights the need for early-stage predictive tools to
identify high-risk infants before BPD diagnosis is clinically
confirmed.
Previous studies have explored risk factors for BPD,
such as gestational age, birth weight and exposure to invasive
ventilation. However, to the best of our knowledge, few studies
have integrated these variables into a comprehensive, clinically
applicable prediction model (10-12).
Furthermore, the utility of advanced statistical tools such as
nomograms in quantifying individualized risk has been underexplored
in neonatal populations. Nomograms provide a graphical
representation of complex statistical models, and are particularly
valuable in clinical practice for their simplicity and
interpretability (13).
The present study aimed to develop and validate a
robust risk prediction model for BPD in preterm infants,
incorporating readily available clinical and demographic variables.
Using a retrospective cohort of 120 preterm infants, a nomogram was
constructed to estimate individualized risk, and its performance
was evaluated in terms of discrimination, calibration and internal
validation. The present model aimed to provide an early and
accurate assessment of BPD risk, thereby supporting clinical
decision-making and improving outcomes for preterm infants.
Patients and methods
Study design and population
The present retrospective cohort study was conducted
to develop and validate a prediction model for BPD in preterm
infants. Data were collected from 120 preterm infants admitted to
the neonatal intensive care unit (NICU) of The Affiliated Hospital
of Southwest Medical University, Luzhou, Sichuan, China, between
January 2020 and December 2022. Infants were stratified into two
groups based on their BPD status, defined according to the 2018
diagnostic criteria published by the NICHD: A BPD group (n=34) and
a non-BPD group (n=86).
Inclusion criteria included preterm infants with
gestational ages <32 weeks and birth weights ≤2,500 g who were
admitted to the NICU within the first week of life. Exclusion
criteria included i) major congenital anomalies or chromosomal
abnormalities; ii) severe congenital heart defects; iii) genetic or
metabolic disorders; iv) mortality or discharge before 14 days of
life; and v) transfer to another hospital within 7 days of birth.
During the study period (January 2020-December 2022), a total of
156 preterm infants with gestational age <32 weeks were admitted
to the NICU of The Affiliated Hospital, Southwest Medical
University (Luzhou, China). Of these, 36 patients were excluded for
the following reasons: Major congenital anomalies or chromosomal
abnormalities (n=5), severe congenital heart defects (n=4), genetic
or metabolic disorder (n=2), early mortality or discharge before 14
days of life (n=15) and transfer to another hospital within 7 days
of birth (n=10). The final analysis therefore included 120 infants,
representing an inclusion rate of 76.9%. Ethical approval for the
study was obtained from the Institutional Review Board of The
Affiliated Hospital, Southwest Medical University (approval no.
KY2025055), and informed consent was waived due to the
retrospective nature of the study.
Data collection
Clinical and demographic data were collected from
electronic medical records. Maternal factors included maternal age
(years), parity, pre-pregnancy body mass index (BMI,
kg/m2) and the presence of chronic medical conditions
such as hypertension, diabetes or thyroid disease. Antenatal
corticosteroid administration was recorded in detail, including
whether any course was received, the specific agent used
(betamethasone or dexamethasone) and whether the course was
complete or partial according to the standardized institutional
protocol. Chorioamnionitis was diagnosed through clinical or
histopathological findings. The mode of delivery (vaginal or
cesarean section) was also documented. Neonatal characteristics
encompassed gestational age measured in completed weeks, birth
weight in grams, sex, and Apgar scores at 1 and 5 min, which were
documented to assess immediate postnatal adaptation (14). Detailed respiratory support data
were obtained, including the duration (in days) and type of support
provided, such as continuous positive airway pressure (CPAP),
non-invasive positive pressure ventilation (NIPPV) and
high-frequency oscillatory ventilation (HFOV). Additionally,
neonatal comorbidities were recorded, including the occurrence of
sepsis confirmed by positive blood cultures or clinical diagnosis,
patent ductus arteriosus (PDA) diagnosed via echocardiography,
intraventricular hemorrhage (IVH, graded using Papile's
classification) and necrotizing enterocolitis (NEC, identified
based on modified Bell's criteria) (15). For the purposes of the prediction
model, these comorbidities were defined as events occurring within
the first 14 days of life, ensuring that only early-onset cases
were included to maintain the model's utility for early risk
stratification of BPD. Although the formal definitions for sepsis,
PDA, IVH and NEC in the present dataset were restricted to events
occurring within the first 14 days of life, in clinical practice,
the majority of these conditions in the present cohort were either
diagnosed or strongly suspected within the first 72 h after birth,
based on early clinical signs, laboratory findings, and
echocardiographic or cranial ultrasound assessments. Therefore,
these variables retain practical utility for early postnatal risk
stratification. Finally, key outcome measures included the length
of hospital stay (in days) and the presence or absence of BPD,
defined according to the 2018 NICHD criteria. This comprehensive
dataset provided a robust foundation for analyzing factors
contributing to BPD in preterm infants.
During the study period, respiratory management in
the NICU followed a standardized protocol based on gestational age,
respiratory distress severity and oxygen requirements. Initial
support typically involved CPAP for infants with mild-to-moderate
respiratory distress. NIPPV was applied when CPAP was insufficient,
or in cases of apnea or moderate respiratory acidosis. HFOV was
typically initiated in cases of severe respiratory failure or
persistent hypercapnia despite conventional support. Intubation and
mechanical ventilation were considered in cases of refractory
hypoxemia, recurrent apnea unresponsive to non-invasive support or
clinical deterioration. Decisions regarding escalation or
de-escalation of support were made collaboratively by the attending
neonatologist and the respiratory team based on ongoing clinical
assessments.
Statistical analysis
Statistical analyses were conducted to summarize
baseline characteristics, develop a predictive model and evaluate
its performance. Baseline characteristics were described using the
mean ± standard deviation for normally distributed continuous
variables, median (interquartile range) for non-normally
distributed continuous variables and frequencies (percentages) for
categorical variables. Comparisons between groups were performed
using independent Student's t-tests for normally distributed
continuous variables, the Mann-Whitney U test for non-normally
distributed variables, and χ2 or Fisher's exact tests
for categorical variables. P<0.05 was considered to indicate a
statistically significant difference. Univariate logistic
regression analyses were used to assess the association between
each predictor variable and the risk of BPD. Prior to multivariate
modeling, collinearity among candidate predictors (particularly
gestational age and birth weight) was assessed using variance
inflation factors (VIFs). All VIF values were <2, indicating no
significant multicollinearity. Variables with P<0.1 in
univariate analysis were included in a multivariate logistic
regression model, where a stepwise backward elimination approach
was applied to identify independent predictors. The results were
expressed as odds ratios (ORs) with 95% confidence intervals (CIs).
To evaluate model performance, discrimination was assessed using a
receiver operating characteristic (ROC) curve, with calculation of
the area under the curve (AUC), while calibration was evaluated
using a calibration curve comparing predicted and observed
probabilities. The Hosmer-Lemeshow test was used to assess
goodness-of-fit. Based on the multivariate logistic regression
model, a nomogram was constructed to visually represent the BPD
risk prediction tool. Points were assigned to each predictor based
on the magnitude of regression coefficients in the multivariable
logistic regression model, with the predictor contributing the
largest coefficient scaled to 100 points and other predictors
allocated proportionally. The total points were then mapped onto
the corresponding predicted probability of BPD. Internal validation
of the nomogram was performed using bootstrapping with 1,000
resamples to ensure robustness. All analyses were conducted using R
software (version 4.2.2; Posit Software, PBC), with the ‘rms’ and
‘pROC’ packages utilized for nomogram construction and ROC curve
analysis (16,17). There were no missing data for the
variables included in the analysis; therefore, no imputation or
exclusion due to missingness was required.
Results
Baseline characteristics
A total of 120 preterm infants were included in the
analysis, of whom 34 (28.3%) developed BPD. The baseline
characteristics of the study population stratified by BPD status
are summarized in Table I. There
were no significant differences between the two groups in terms of
maternal characteristics, including age, primiparity, pre-pregnancy
BMI or prevalence of chronic medical conditions such as
hypertension or diabetes. Antenatal corticosteroid exposure was
also comparable between groups, with similar proportions receiving
any corticosteroid, similar distributions of specific agents
(betamethasone or dexamethasone) and no difference in the
proportion of patients completing the full treatment course.
Infants in the BPD group had significantly lower gestational age
(28.02±1.05 vs. 29.29±1.70 weeks; P<0.001) and lower median
birth weight (1,036.3 vs. 1,244.7 g; P<0.001) compared with
those in the non-BPD group. CPAP use was significantly less
frequent in the BPD group (35.3 vs. 59.3%; P=0.018) than in the
non-BPD group, whereas HFOV use was more common (35.3 vs. 17.4%;
P=0.035). Comorbidities, including sepsis (58.8 vs. 8.1%;
P<0.001), PDA (55.9 vs. 29.1%; P=0.006), IVH (29.4 vs. 11.6%;
P=0.018) and NEC (23.5 vs. 5.8%; P=0.013), were significantly more
prevalent in the BPD group than in the non-BPD group. The median
length of hospital stay was significantly longer among infants with
BPD (69.5 vs. 34 days; P<0.001).
 | Table IBaseline characteristics of the study
population stratified by BPD status. |
Table I
Baseline characteristics of the study
population stratified by BPD status.
| Characteristic | Non-BPD (n=86) | BPD (n=34) | P-value |
|---|
| Maternal
characteristics | | | |
|
Maternal
age, years (mean ± SD) | 29.7±4.8 | 30.1±5.1 | 0.642 |
|
Primiparity
(%) | 49 (57.0) | 18 (52.9) | 0.683 |
|
Chronic
hypertension, n (%) | 6 (7.0) | 4 (11.8) | 0.462 |
|
Diabetes
mellitus, n (%) | 8 (9.3) | 3 (8.8) | >0.999 |
|
Pre-pregnancy
BMI, kg/m² (mean ± SD) | 22.9±3.4 | 23.4±3.6 | 0.514 |
|
Antenatal
corticosteroids use | | | |
|
Any,
n (%) | 64 (81.7) | 27 (79.4) | 0.565 |
|
Betamethasone,
n (%) | 39 (45.3) | 16 (47.1) | 0.859 |
|
Dexamethasone,
n (%) | 25 (29.1) | 11 (32.4) | 0.815 |
|
Complete
course, n (%) | 58 (67.4) | 24 (70.6) | 0.753 |
| Neonatal
characteristics | | | |
|
Gestational
age, weeks (mean ± SD) | 29.29±1.70 | 28.02±1.05 | <0.001 |
|
Birth
weight, g [median (IQR)] | 1,244.7
(989.9-1,354.0) | 1,036.3
(890.2-1,123.2) | <0.001 |
|
Male sex, n
(%) | 45 (52.3) | 22 (64.7) | 0.218 |
| Respiratory support
use | | | |
|
CPAP, n
(%) | 51 (59.3) | 12 (35.3) | 0.018 |
|
NIPPV, n
(%) | 37 (43.0) | 21 (61.8) | 0.064 |
|
HFOV, n
(%) | 15 (17.4) | 12 (35.3) | 0.035 |
| Comorbidities | | | |
|
Sepsis, n
(%) | 7 (8.1) | 20 (58.8) | <0.001 |
|
PDA, n
(%) | 25 (29.1) | 19 (55.9) | 0.006 |
|
IVH, n
(%) | 10 (11.6) | 10 (29.4) | 0.018 |
|
NEC, n
(%) | 5 (5.8) | 8 (23.5) | 0.013 |
| Length of stay,
days [median (IQR)] | 34.0
(31.0-37.0) | 69.5
(67.0-72.8) | <0.001 |
Univariate and multivariate
analyses
The results of univariate and multivariate logistic
regression analyses for predictors of BPD are presented in Table II. In the univariate analysis,
lower gestational age, lower birth weight, absence of CPAP, use of
HFOV, and presence of sepsis, PDA, IVH and NEC were significantly
associated with an increased risk of BPD (P<0.05 for all). In
the multivariate model, lower gestational age (OR=0.625; 95% CI,
0.420-0.932; P=0.021), sepsis (OR=12.847; 95% CI, 3.187-51.789;
P<0.001), PDA (OR=4.514; 95% CI, 1.324-15.389; P=0.016) and IVH
(OR=5.926; 95% CI, 1.217-28.845; P=0.028) remained independent
predictors of BPD. Although birth weight did not reach statistical
significance in the multivariate model (OR=0.997; P=0.059), it was
retained due to its established clinical importance in neonatal
outcome prediction (18).
 | Table IIUnivariate and multivariate logistic
regression analyses of risk factors for bronchopulmonary
dysplasia. |
Table II
Univariate and multivariate logistic
regression analyses of risk factors for bronchopulmonary
dysplasia.
| Characteristic | Univariate OR (95%
CI) | P-value | Multivariate OR
(95% CI) | P-value |
|---|
| Gestational age,
weeks | 0.570
(0.419-0.777) | <0.001 | 0.625
(0.420-0.932) | 0.021 |
| Birth weight,
g | 0.997
(0.994-0.999) | <0.001 | 0.997
(0.994-1.000) | 0.059 |
| Male sex | 1.670
(0.735-3.796) | 0.221 | - | - |
| Antenatal
steroids | 1.326
(0.507-3.470) | 0.566 | - | - |
| CPAP use | 2.671
(1.171-6.093) | 0.020 | 2.158
(0.656-7.098) | 0.205 |
| NIPPV use | 2.139
(0.949-4.822) | 0.067 | 2.458
(0.731-8.271) | 0.146 |
| HFOV use | 2.582
(1.053-6.332) | 0.038 | 1.725
(0.440-6.758) | 0.434 |
| Sepsis | 16.122
(5.748-45.225) | <0.001 | 12.847
(3.187-51.789) | <0.001 |
| PDA | 3.091
(1.359-7.028) | 0.007 | 4.514
(1.324-15.389) | 0.016 |
| IVH | 3.167
(1.177-8.517) | 0.022 | 5.926
(1.217-28.845) | 0.028 |
| NEC | 4.985
(1.499-16.575) | 0.009 | 3.663
(0.718-18.698) | 0.119 |
Model performance and validation
The predictive performance of the multivariate model
was evaluated through discrimination and calibration analyses. The
nomogram demonstrated excellent discrimination, with an apparent
AUC of 0.918 (95% CI, 0.866-0.971) in the derivation dataset
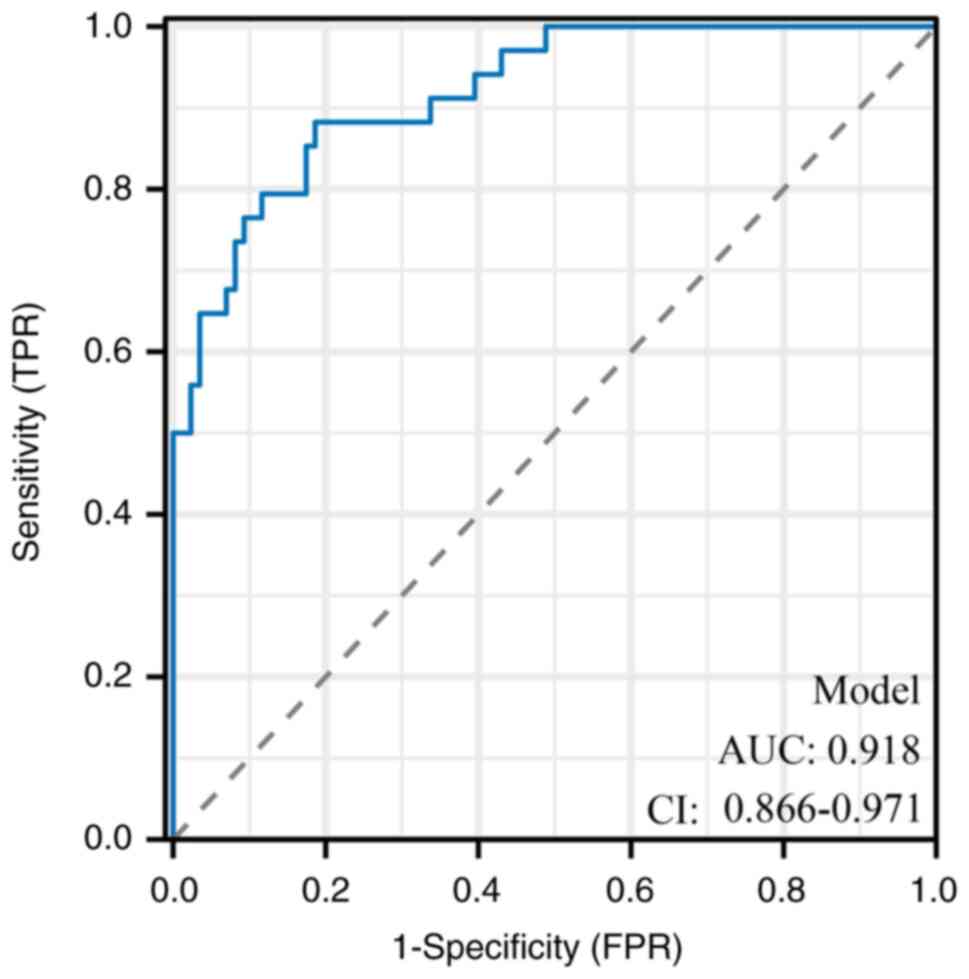
(Fig. 1). This result indicates
indicating only minimal optimism and confirms the robustness of the
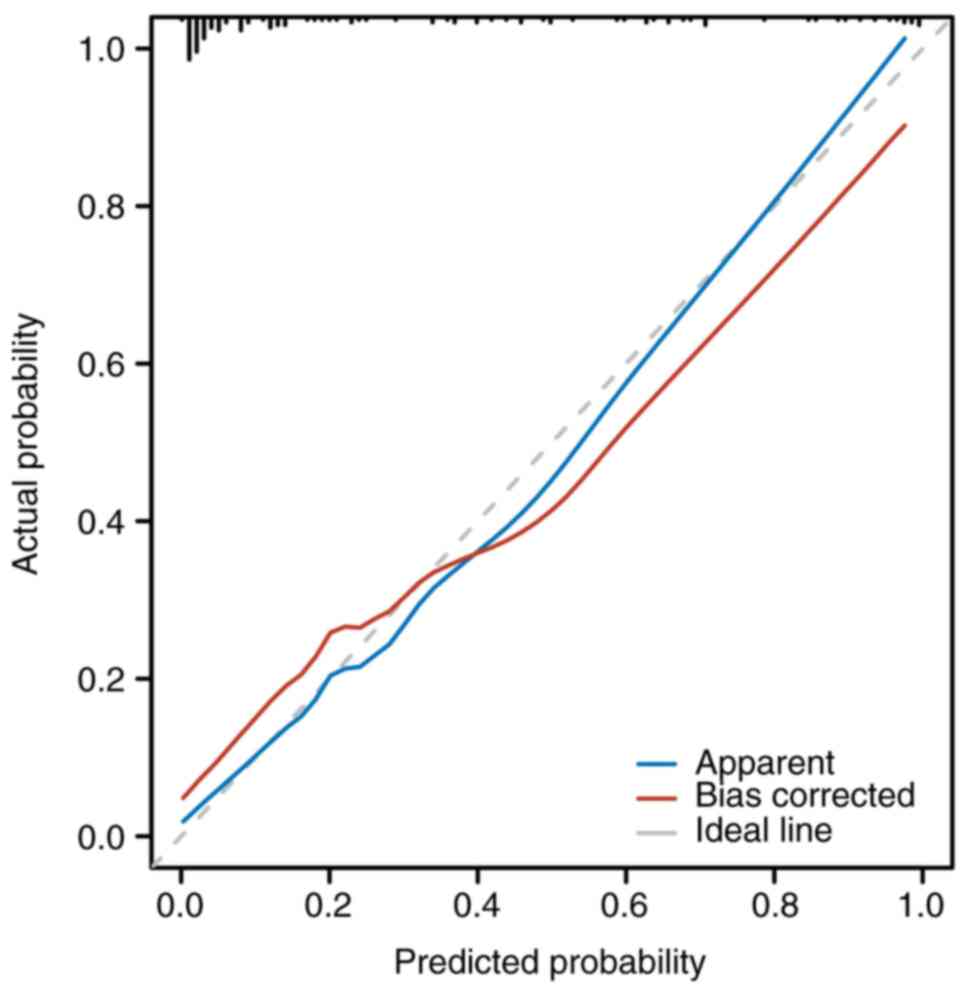
model after adjustment for potential overfitting. The calibration
performance of the internally validated model is shown in Fig. 2, which demonstrates strong
agreement between observed and predicted probabilities. The
Hosmer-Lemeshow test yielded a non-significant result (P>0.05),
indicating a good overall model fit.
Nomogram construction
Based on the final multivariate model, a clinical
nomogram was constructed to estimate the individualized risk of BPD
in preterm infants (Fig. 3). The
predictors included in the nomogram were gestational age, birth
weight, sex, antenatal steroid use, respiratory support modalities
(CPAP, NIPPV and HFOV), and comorbidities (sepsis, PDA, IVH and
NEC). Although certain predictors did not reach statistical
significance in the multivariate model, they were retained in the
nomogram because of their established clinical relevance and
routine use in neonatal risk assessment, consistent with accepted
nomogram-building practices. Each factor contributed a weighted
score to the total, which corresponded to the predicted probability
of BPD. This nomogram offered a practical and interpretable tool
for bedside application in neonatal intensive care settings.
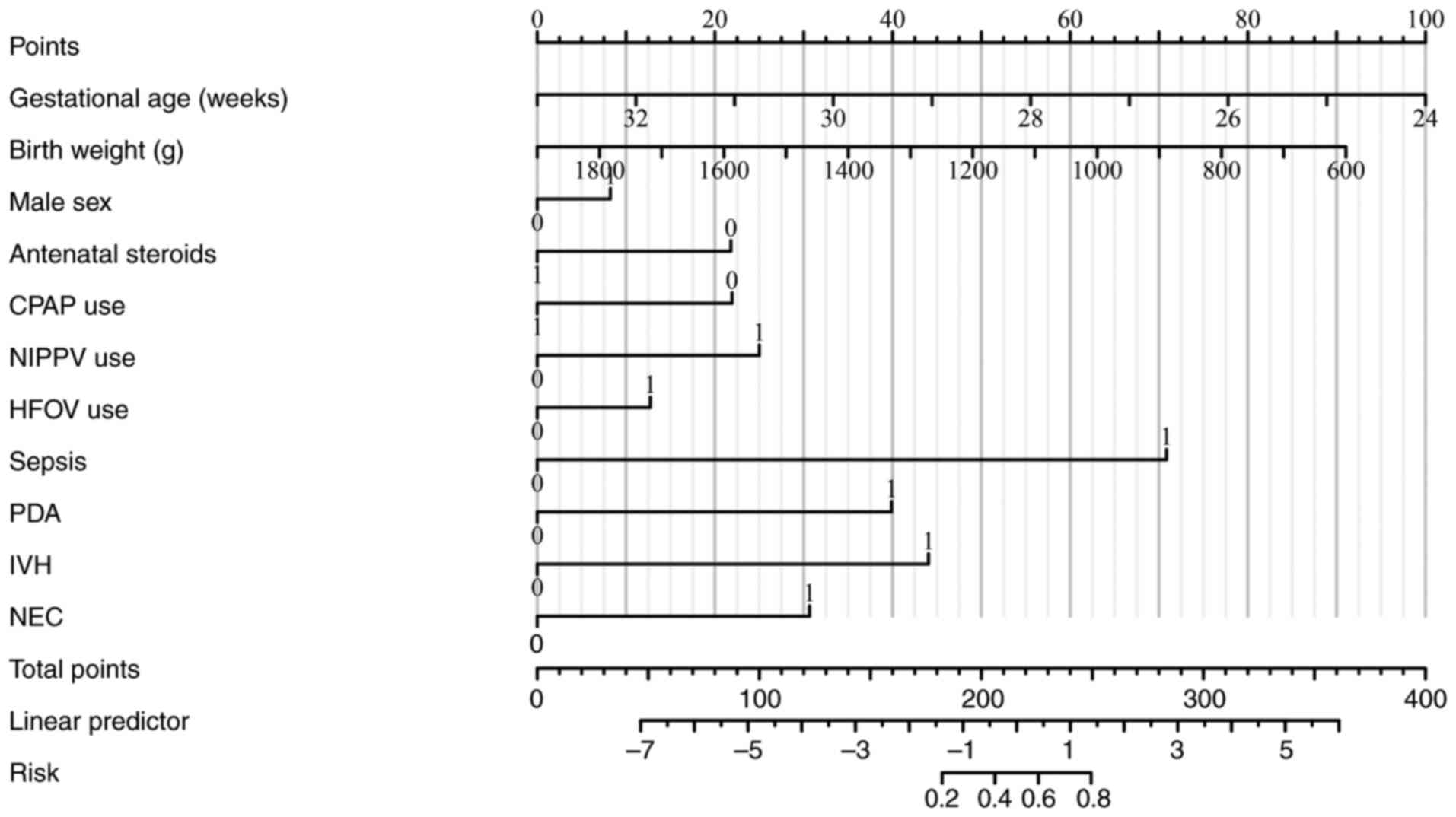 | Figure 3Nomogram for BPD risk prediction. The
nomogram visualizes the contribution of multiple predictors to the
risk of BPD. Predictors include gestational age, birth weight, sex,
antenatal steroids, respiratory support modes, sepsis, PDA, IVH and
NEC. The total points correspond to the predicted probability of
BPD. BPD, bronchopulmonary dysplasia CPAP, continuous positive
airway pressure; NIPPV; non-invasive positive pressure ventilation;
HFOV, high-frequency oscillatory ventilation; PDA, patent ductus
arteriosus; IVH, intraventricular hemorrhage; NEC, necrotizing
enterocolitis. |
Discussion
The present study provides a comprehensive analysis
of risk factors for BPD and presents a validated predictive model
for early identification of at-risk preterm infants. The present
model demonstrated high discriminatory power, with an area under
the ROC curve of 0.918, and robust calibration, highlighting its
potential clinical utility.
Here, gestational age and birth weight emerged as
critical predictors of BPD, which is consistent with prior research
highlighting the heightened vulnerability of extremely preterm
infants and those with impaired early growth (19-21).
Lower gestational age (OR=0.625; P=0.021) reflects the immaturity
of the lung parenchyma, surfactant deficiency, and susceptibility
to barotrauma and oxygen toxicity (5). Similarly, reduced birth weight
(P=0.059 in multivariate analysis) underscores the importance of
intrauterine growth and its association with postnatal outcomes
(22). These findings reinforce
the need for strategies targeting antenatal interventions, such as
corticosteroids and optimal timing of delivery, to reduce
prematurity-related risks.
In the present study, sepsis showed the strongest
independent association with BPD in the multivariate model
(OR=12.847; P<0.001), with an effect size notably greater than
that of the other significant predictors. This increased risk
aligns with prior studies linking systemic inflammation to
disrupted alveolarization and pulmonary vascular remodeling
(23,24). Pro-inflammatory cytokines and
reactive oxygen species released during sepsis amplify lung injury,
perpetuating the cycle of inflammation and impaired repair
mechanisms (25,26). This underscores the importance of
stringent infection control measures in the NICU, including early
recognition and management of sepsis.
Notably, the OR value for sepsis in the multivariate
analysis conducted in the present study was markedly high
(OR=12.847). This magnitude likely reflects both the strong
biological plausibility of the association and the relatively
concentrated distribution of severe early-onset sepsis in the
present BPD cohort. All cases of sepsis included in the present
analysis were diagnosed within the first 14 days of life, a
critical period for lung development, during which systemic
inflammation can exert disproportionate and irreversible effects on
alveolarization and microvascular maturation (27). Early-onset sepsis in preterm
infants has been shown to trigger release of cytokines (including
IL-6, TNF-α and IL-1β), oxidative stress and endothelial
dysfunction, which collectively disrupt pulmonary angiogenesis and
promote interstitial fibrosis (23,24,26).
Furthermore, severe infection frequently necessitates prolonged
invasive ventilation and higher oxygen exposure, compounding the
risk of ventilator-induced lung injury and oxygen toxicity
(25).
Clinically, this finding underscores the imperative
for early sepsis prevention and prompt antimicrobial treatment in
the NICU, particularly during the first 2 postnatal weeks.
Stringent infection control protocols, rapid pathogen
identification and optimized empiric antibiotic strategies may
therefore represent critical interventions to mitigate BPD risk in
high-risk preterm populations.
PDA (OR=4.514; P=0.016) was a significant predictor
identified in the present study, in agreement with previous studies
suggesting that prolonged ductal patency exacerbates pulmonary
overcirculation, increasing the risk of pulmonary edema and
ventilator dependency (28,29).
The role of PDA treatment in mitigating BPD remains controversial;
however, the present findings suggest that early closure, whether
pharmacological or surgical, may reduce the burden of
ventilator-associated lung injury in high-risk infants.
IVH (OR=5.926; P=0.028) was strongly associated with
BPD, reflecting shared mechanisms of systemic inflammation,
oxidative stress and vascular fragility (30,31).
The interplay between brain and lung injury highlights the
importance of a multidisciplinary approach to managing extremely
preterm infants, focusing on neuroprotection and minimizing
invasive procedures that increase hemodynamic instability.
While CPAP and high-frequency oscillatory
ventilation (HFOV) were significant in univariate analysis, their
effects did not remain significant in multivariate models. This may
indicate that respiratory support modalities reflect underlying
disease severity, rather than acting as direct predictors of BPD.
Nonetheless, the judicious use of non-invasive ventilation and
minimizing oxygen toxicity remain cornerstones of BPD prevention
(32,33). CPAP is generally regarded as a
protective modality that reduces ventilator-associated lung injure
(34) In the present cohort, CPAP
use was lower among infants who ultimately developed BPD, whereas
escalation to HFOV was more frequent. This pattern most likely
reflects baseline illness severity rather than a detrimental effect
of CPAP: Infants with more severe initial respiratory compromise
were less able to tolerate CPAP and required earlier escalation to
invasive ventilation or HFOV. Thus, the observed group difference
in respiratory support should be interpreted as a marker of
underlying disease severity, consistent with prior reports showing
that the choice of respiratory support modality typically reflects
the infant's clinical acuity rather than functioning as an
independent predictor of BPD (35,36).
NEC, a systemic inflammatory condition, was
significant in the present univariate analysis, but lost
significance in multivariate modeling. This result may reflect
collinearity with sepsis and other variables. Nevertheless, the
present findings align with previous research highlighting NEC as a
driver of systemic inflammation and impaired lung development
(15). Preventative measures, such
as human milk feeding and probiotics, could have dual benefits for
gut and lung health in preterm infants (37).
The nomogram constructed in the present study
provides a user-friendly tool for bedside application. While some
predictors in the final model, such as sepsis or HFOV use, were
formally coded as occurring within the first 14 days, in the
majority of cases, these events were clinically apparent or could
be anticipated within the first 72 h after birth. For example,
early-onset sepsis in preterm infants often manifests within the
first 48 h, and the need for HFOV is typically determined during
initial respiratory stabilization (38). As such, the model maintains its
intended role for early risk stratification and timely intervention
in clinical practice. By integrating gestational age, birth weight,
sepsis, PDA and IVH, the model facilitates individualized risk
stratification and enables timely interventions. Compared with
earlier models that primarily relied on gestational age and birth
weight (39,40), the present approach incorporates
comorbidities, thus enhancing predictive accuracy. Importantly, the
present study introduces several novel aspects: Unlike prior models
that typically focused on gestational age and birth weight alone,
the present nomogram integrates comorbidities such as sepsis, PDA
and IVH, variables with high clinical relevance yet often excluded
from earlier predictive tools. The development of a visually
intuitive nomogram enhances practical usability, making the model
more accessible for bedside application by clinicians. Moreover, to
the best of our knowledge, this is one of the few studies conducted
in Southwest China that has systematically developed and internally
validated a BPD risk prediction model, thereby addressing a
regional gap in neonatal research and contributing valuable
insights to the global literature. Although birth weight and sex
were not statistically significant in the present multivariate
model, they were retained in the nomogram due to their strong
clinical relevance and prior validation as BPD risk factors
(41). Including such variables
ensures clinical interpretability and aligns with accepted
practices in predictive model development (42).
The present model's high AUC (0.918) surpasses
benchmarks reported in similar studies (11), underscoring its robustness.
Moreover, the calibration curve in the present study demonstrated
excellent agreement between predicted and observed probabilities,
thus ensuring reliability across various clinical settings. These
strengths make the model particularly valuable for guiding early
interventions, such as caffeine therapy, optimization of
ventilation strategies and nutritional support (43).
The inclusion of these parameters significantly
enhances predictive accuracy while maintaining applicability within
the first 72 h of life in most cases. The comprehensive collection
of maternal, neonatal and comorbid factors allowed for a thorough
exploration of BPD risk determinants, while multivariate logistic
regression ensured the identification of independent predictors.
Furthermore, the application of bootstrapping for internal
validation enhanced the reliability of the model and reduced the
risk of overfitting. The visually intuitive nomogram bridges the
gap between complex statistical models and bedside decision-making,
facilitating rapid individualized risk estimation by
clinicians.
However, certain limitations exhibited by the
current study should be acknowledged. First, the model underwent
only internal validation using bootstrapping without any external
validation, which restricts its generalizability and positions it
as a preliminary exploratory tool; external validation in diverse
cohorts is necessary to confirm its applicability across different
clinical environments. Furthermore, the ratio of BPD events (n=34)
to the 11 candidate predictors initially entered into the
multivariate model resulted in an events-per-variable ratio of
~3.1:1, which is below the commonly recommended threshold of
10:1(44). To minimize this risk,
a stepwise backward elimination process was applied to retain only
the most stable predictors and performed bootstrap internal
validation to assess and adjust for optimism. This yielded a
bias-corrected AUC of 0.902, which was only slightly lower than the
apparent AUC, supporting the relative stability of the model.
Nevertheless, external validation in larger, multi-center cohorts
is essential to confirm the generalizability of the present
findings. The retrospective design of the present study, despite
meticulous data collection, is inherently prone to selection and
information biases. Additionally, the current study was conducted
in a single center with a relatively small sample size (n=120; BPD
cases=34), which may limit statistical power and precluded more
granular subgroup analyses by gestational age and birth weight
categories. The relatively small number of events also means that
some clinically important variables, such as birth weight and
infant sex, did not reach statistical significance in multivariable
analysis.
Future research should focus on addressing the
limitations of the present study and further advancing the field of
BPD risk prediction. Conducting multicenter, prospective studies
with larger and more diverse populations will be essential to
validate the current model externally and ensure its
generalizability. Incorporating biomarkers such as peripheral blood
cytokines (e.g., IL-6, IL-8) or molecular signatures identified
through transcriptomic approaches may enhance the precision of the
model and provide insights into the underlying mechanisms of BPD
(45). Additionally, integrating
advanced imaging techniques, such as lung ultrasound or magnetic
resonance imaging, may offer novel perspectives on disease
progression and early risk stratification. Long-term follow-up
studies are crucial to evaluate the predictive impact of the model
on neurodevelopmental and respiratory outcomes, ensuring that early
identification translates into improved quality of life for preterm
infants. Finally, assessing the real-world clinical utility of the
model, including its integration into electronic health records and
decision-support systems, will determine its potential to optimize
neonatal care and resource allocation.
Since only internal validation was performed, the
present study should be regarded as a preliminary exploratory
effort requiring future external validation to confirm its broader
applicability. In the current study, a clinically meaningful
preliminary risk prediction model for BPD in preterm infants was
constructed. The inclusion criteria were intentionally broad
(gestational age <32 weeks and birth weight ≤2,500 g) to ensure
the applicability of the model to a wider range of preterm infants
in routine clinical practice. Although this approach may include
infants at relatively lower risk for BPD, it enhances the
generalizability and relevance of the model in diverse neonatal
care settings. While the model demonstrates strong discrimination
and calibration, the relatively small sample size (n=120) and
single-center design aspect of the current study may limit the
generalizability of the present findings. Nevertheless, the use of
readily accessible clinical predictors and the application of 1,000
bootstrap resamples for internal validation provide a reliable
foundation for further model development. This exploratory analysis
offers an important first step toward establishing a practical risk
stratification tool. Although internal validation using 1,000
bootstrap resamples indicates strong model stability and reduces
overfitting risk, the absence of external validation remains a key
limitation of the present study. Without testing it on an
independent cohort, the generalizability of the model across
different populations and clinical practices cannot be fully
ensured. Future research should therefore prioritize external
validation through large-scale, multicenter, prospective studies to
confirm the performance of the nomogram in broader settings.
Establishing external credibility through future validation is
essential for integrating this model into routine clinical practice
and reinforcing its predictive value.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YSP conceived and designed the study, and
participated in data collection, statistical analysis and
manuscript drafting. LX participated in data acquisition,
interpretation of results and critical revision of the manuscript.
WBD was responsible for data management, literature review and
assisting in statistical analysis. JWD conceived and designed the
study and critically revised the manuscript. All authors have read
and approved the final manuscript. YSP and LX confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was conducted in accordance with
The Declaration of Helsinki and was approved by the Institutional
Review Board of The Affiliated Hospital, Southwest Medical
University (approval no. KY2025055). Due to the retrospective
nature of the present study, the requirement for informed consent
was waived by the ethics committee. All patient data were fully
anonymized and utilized exclusively for research purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siffel C, Kistler KD, Lewis JFM and Sarda
SP: Global incidence of bronchopulmonary dysplasia among extremely
preterm infants: A systematic literature review. J Matern Fetal
Neonatal Med. 34:1721–1731. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Perveen S, Chen CM, Sobajima H, Zhou X and
Chen JY: Editorial: Bronchopulmonary dysplasia: Latest advances.
Front Pediatr. 11(1303761)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Algarni SS, Ali K, Alsaif S, Aljuaid N,
Alzahrani R, Albassam M, Alanazi R, Alqueflie D, Almutairi M,
Alfrijan H, et al: Changes in the patterns of respiratory support
and incidence of bronchopulmonary dysplasia; a single center
experience. BMC Pediatr. 23(357)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Moreira A, Noronha M, Joy J, Bierwirth N,
Tarriela A, Naqvi A, Zoretic S, Jones M, Marotta A, Valadie T, et
al: Rates of bronchopulmonary dysplasia in very low birth weight
neonates: A systematic review and meta-analysis. Respir Res.
25(219)2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dankhara N, Holla I, Ramarao S and
Kalikkot Thekkeveedu R: Bronchopulmonary dysplasia: Pathogenesis
and pathophysiology. J Clin Med. 12(4207)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sikdar O, Harris C and Greenough A:
Improving early diagnosis of bronchopulmonary dysplasia. Expert Rev
Respir Med. 18:283–294. 2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Abdelrazek AA, Kamel SM, Elbakry AAE and
Elmazzahy EA: Lung ultrasound in early prediction of
bronchopulmonary dysplasia in pre-term babies. J Ultrasound.
27:653–662. 2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pérez-Tarazona S, Marset G, Part M, López
C and Pérez-Lara L: Definitions of bronchopulmonary dysplasia:
Which one should we use? J Pediatr. 251:67–73.e2. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Higgins RD, Jobe AH, Koso-Thomas M,
Bancalari E, Viscardi RM, Hartert TV, Ryan RM, Kallapur SG,
Steinhorn RH, Konduri GG, et al: Bronchopulmonary dysplasia:
Executive summary of a workshop. J Pediatr. 197:300–308.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sucasas Alonso A, Pértega Diaz S, Sáez
Soto R and Avila-Alvarez A: Epidemiology and risk factors for
bronchopulmonary dysplasia in preterm infants born at or less than
32 weeks of gestation. An Pediatr (Engl Ed). 96:242–251.
2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
He W, Zhang L, Feng R, Fang WH, Cao Y, Sun
SQ, Shi P, Zhou JG, Tang LF, Zhang XB and Qi YY: Risk factors and
machine learning prediction models for bronchopulmonary dysplasia
severity in the Chinese population. World J Pediatr. 19:568–576.
2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang H, Fang J, Su Q, et al: Development
and validation of a nomogram for predicting bronchopulmonary
dysplasia in very-low-birth-weight infants. Front Pediatr.
9(648828)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sun X, Li L, He L, Wang S, Pan Z and Li D:
Preoperative malnutrition predicts poor early immune recovery
following gynecologic cancer surgery: A retrospective cohort study
and risk nomogram development. Front Immunol.
16(1681762)2025.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Papile LA: The Apgar score in the 21st
century. N Engl J Med. 344:519–520. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hu X, Liang H, Li F, Zhang R, Zhu Y, Zhu X
and Xu Y: Necrotizing enterocolitis: Current understanding of the
prevention and management. Pediatr Surg Int. 40(32)2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Robin X, Turck N, Hainard A, Tiberti N,
Lisacek F, Sanchez JC and Müller M: pROC: An open-source package
for R and S+ to analyze and compare ROC curves. BMC Bioinformatics.
12(77)2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang Z and Kattan MW: Drawing nomograms
with R: Applications to categorical outcome and survival data. Ann
Transl Med. 5(211)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Basnet K, Yadav BK, Khan SA, Bhattarai CD,
Adhikari K, Bajgain A, Karki M and Yadav R: Birth weight status of
newborns and its relationship with other anthropometric parameters.
Medicine (Baltimore). 104(e45374)2025.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Maytasari GM, Haksari EL and
Prawirohartono EP: Predictors of bronchopulmonary dysplasia in
infants with birth weight less than 1500 g. Glob Pediatr Health.
10(2333794X231152199)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang L, Lin XZ, Shen W, Wu F, Mao J, Liu
L, Chang YM, Zhang R, Ye XZ, Qiu YP, et al: Risk factors of
extrauterine growth restriction in very preterm infants with
bronchopulmonary dysplasia: A multi-center study in China. BMC
Pediatr. 22(363)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yoneda K, Seki T, Kawazoe Y, Ohe K and
Takahashi N: Neonatal Research Network of Japan. Immediate
postnatal prediction of death or bronchopulmonary dysplasia among
very preterm and very low birth weight infants based on gradient
boosting decision trees algorithm: A nationwide database study in
Japan. PLoS One. 19(e0300817)2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li L, Guo J, Wang Y, Yuan Y, Feng X, Gu X,
Jiang S, Chen C, Cao Y, Sun J, et al: Association of neonatal
outcome with birth weight for gestational age in Chinese very
preterm infants: A retrospective cohort study. Ital J Pediatr.
50(203)2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Qiao X, Yin J, Zheng Z, Li L and Feng X:
Endothelial cell dynamics in sepsis-induced acute lung injury and
acute respiratory distress syndrome: Pathogenesis and therapeutic
implications. Cell Commun Signal. 22(241)2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gong T, Wang QD, Loughran PA, Li YH, Scott
MJ, Billiar TR, Liu YT and Fan J: Mechanism of lactic
acidemia-promoted pulmonary endothelial cells death in sepsis: role
for CIRP-ZBP1-PANoptosis pathway. Mil Med Res.
11(71)2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Deng L, Xie W, Lin M, Xiong D, Huang L,
Zhang X, Qian R, Huang X, Tang S and Liu W: Taraxerone inhibits M1
polarization and alleviates sepsis-induced acute lung injury by
activating SIRT1. Chin Med. 19(159)2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xu Y, Xin J, Sun Y, Wang X, Sun L, Zhao F,
Niu C and Liu S: Mechanisms of sepsis-induced acute lung injury and
advancements of natural small molecules in its treatment.
Pharmaceuticals (Basel). 17(472)2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mižíková I and Thébaud B: Perinatal
origins of bronchopulmonary dysplasia-deciphering normal and
impaired lung development cell by cell. Mol Cell Pediatr.
10(4)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
El-Khuffash A, Mullaly R and McNamara PJ:
Patent ductus arteriosus, bronchopulmonary dysplasia and pulmonary
hypertension-a complex conundrum with many phenotypes? Pediatr Res.
94:416–417. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Villamor E, van Westering-Kroon E,
Gonzalez-Luis GE, Bartoš F, Abman SH and Huizing MJ: Patent ductus
arteriosus and bronchopulmonary dysplasia-associated pulmonary
hypertension: A bayesian meta-analysis. JAMA Netw Open.
6(e2345299)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Faro DC, Di Pino FL and Monte IP:
Inflammation, oxidative stress, and endothelial dysfunction in the
pathogenesis of vascular damage: Unraveling novel cardiovascular
risk factors in fabry disease. Int J Mol Sci.
25(8273)2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Panda P, Verma HK, Lakkakula S, Merchant
N, Kadir F, Rahman S, Jeffree MS, Lakkakula BVKS and Rao PV:
Biomarkers of oxidative stress tethered to cardiovascular diseases.
Oxid Med Cell Longev. 2022(9154295)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shahzad T, Dong Y, Behnke NK, Brandner J,
Hilgendorff A, Chao CM, Behnke J, Bellusci S and Ehrhardt H:
Anti-CCL2 therapy reduces oxygen toxicity to the immature lung.
Cell Death Discov. 10(311)2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Behnke J, Dippel CM, Choi Y, Rekers L,
Schmidt A, Lauer T, Dong Y, Behnke J, Zimmer KP, Bellusci S and
Ehrhardt H: Oxygen toxicity to the immature lung-part II: The unmet
clinical need for causal therapy. Int J Mol Sci.
22(10694)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kaltsogianni O, Dassios T and Greenough A:
Neonatal respiratory support strategies-short and long-term
respiratory outcomes. Front Pediatr. 11(1212074)2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ramaswamy VV, Devi R and Kumar G:
Non-invasive ventilation in neonates: A review of current
literature. Front Pediatr. 11(1248836)2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dou C, Yu YH, Zhuo QC, Qi JH, Huang L,
Ding YJ, Yang DJ, Li L, Li D, Wang XK, et al: Longer duration of
initial invasive mechanical ventilation is still a crucial risk
factor for moderate-to-severe bronchopulmonary dysplasia in very
preterm infants: A multicentrer prospective study. World J Pediatr.
19:577–585. 2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sjödin KS, Sjödin A, Ruszczyński M,
Kristensen MB, Hernell O, Szajewska H and West CE: Targeting the
gut-lung axis by synbiotic feeding to infants in a randomized
controlled trial. BMC Biol. 21(38)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tana M, Tirone C, Aurilia C, Lio A,
Paladini A, Fattore S, Esposito A, De Tomaso D and Vento G:
Respiratory management of the preterm infant: Supporting
evidence-based practice at the bedside. Children (Basel).
10(535)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jassem-Bobowicz JM, Klasa-Mazurkiewicz D,
Żawrocki A, Stefańska K, Domżalska-Popadiuk I, Kwiatkowski S and
Preis K: Prediction Model for bronchopulmonary dysplasia in preterm
newborns. Children (Basel). 8(886)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yin J, Liu L, Li H, Hou X, Chen J, Han S
and Chen X: Mechanical ventilation characteristics and their
prediction performance for the risk of moderate and severe
bronchopulmonary dysplasia in infants with gestational age <30
weeks and birth weight <1,500 g. Front Pediatr.
10(993167)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Huang LY, Lin TI, Lin CH, Yang SN, Chen
WJ, Wu CY, Liu HK, Wu PL, Suen JL, Chen JS and Yang YN:
Comprehensive analysis of risk factors for bronchopulmonary
dysplasia in preterm infants in Taiwan: A four-year study. Children
(Basel). 10(1822)2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Efthimiou O, Seo M, Chalkou K, Debray T,
Egger M and Salanti G: Developing clinical prediction models: A
step-by-step guide. BMJ. 386(e078276)2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Oliphant EA, Hanning SM, McKinlay CJD and
Alsweiler JM: Caffeine for apnea and prevention of
neurodevelopmental impairment in preterm infants: Systematic review
and meta-analysis. J Perinatol. 44:785–801. 2024.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Peduzzi P, Concato J, Kemper E, Holford TR
and Feinstein AR: A simulation study of the number of events per
variable in logistic regression analysis. J Clin Epidemiol.
49:1373–1379. 1996.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Luo L, Luo F, Wu C, Zhang H, Jiang Q, He
S, Li W, Zhang W, Cheng Y, Yang P, et al: Identification of
potential biomarkers in the peripheral blood of neonates with
bronchopulmonary dysplasia using WGCNA and machine learning
algorithms. Medicine (Baltimore). 103(e37083)2024.PubMed/NCBI View Article : Google Scholar
|