Introduction
Hematological neoplasms lead to multiple
cardiovascular complications depending on their type and mechanism.
In patients with myeloproliferative malignancies, the outcome is
influenced not only by the disease itself, but also by severe
cardiovascular complications. The most frequent complications are
thrombotic events, pulmonary hypertension, accelerated
atherosclerosis leading to ischemic heart disease and heart failure
(1). Patients with
lymphoproliferative malignancies have a greater risk of
chemotherapy-associated cardiomyopathy, heart failure and
arrhythmia compared with the general population, effects that are
related either to the primary malignancy or to the specific
treatment (anthracyclines, tyrosine kinase inhibitors, Bruton's
tyrosine kinase inhibitors, stem cell transplantation and radiation
therapy) (2). Venous and arterial
thrombosis, as well as intracardiac thrombosis, are usually found
in myeloproliferative neoplasms, mainly in polycythemia vera (PV)
(3), with thrombosis being the
leading cause of morbidity and mortality globally in PV and
essential thrombocythemia (4),
while cardiac metastases are usually identified in hematological
tumors of the lymphoid line (that is lymphoproliferative neoplasms,
mainly lymphomas) (5).
Cardiac neoplasms can be either primary or
secondary, with the most common tumors identified being metastatic
(6). Among primary tumors, ~90%
are benign (including atrial myxomas and lipomas) (7). Secondary cardiac tumors are of
various histological types, with lung adenocarcinoma being the most
frequent cardiac metastasis identified in autopsy studies (14.6%),
followed by poorly differentiated lung carcinoma (12.4%), squamous
cell lung carcinoma (11.8%) and lymphomas/leukemias (10.1%)
(8).
Secondary cardiac involvement in lymphomas accounts
for ~10% of cardiac metastases (8), although the percentages vary (between
8.7 and 20%), as identified by previous autopsy studies (8,9).
Secondary cardiac lymphomas are more frequent in patients with
disseminated disease, rarely presenting as primary cardiac tumors
(10,11). Of these, the most frequent
lymphomas identified are B-cell lymphomas, with the diffuse large
B-cell lymphoma (DLBCL) subtype of non-Hodgkin lymphoma (NHL) being
the most frequent (11,12). Burkitt lymphoma (BL), another
subtype of NHL, is a rare and aggressive B-cell lymphoma,
characterized by rapid cell proliferation (12), with a relatively low prevalence in
the general population (~0.8% of all adult B-cell lymphomas)
(5). However, their prevalence
increases markedly in patients who are HIV-positive, with BL being
the second most common type of NHL after DLBCL in this subgroup of
patients (13).
Patients living with HIV infection (PLWHIV) are at
an increased cardiovascular risk compared with the general
population. A high prevalence of traditional and specific risk
factors (chronic inflammation, endothelial dysfunction, immune
dysregulation and antiretroviral therapy) can lead to accelerated
atherosclerosis and, consequently, to ischemic heart disease
(14). The symptomatology of acute
coronary syndromes in these patients can be atypical, creating
diagnostic issues. PLWHIV can develop other cardiac conditions,
such as pericardial disease, endocarditis, dilated cardiomyopathy
and pulmonary hypertension (15,16).
The increased incidence of cardiovascular disease in this
population outlines the need for a higher degree of suspicion and
cardiovascular screening in these patients, especially concerning
the early detection and treatment of conditions such as myocardial
infarction, heart failure, hypertension or arrhythmia, all highly
prevalent in this population group (16). Additionally, lifestyle optimization
and the primary prevention of cardiovascular disease in PLWHIV need
to be considered to prevent these secondary outcomes (16).
HIV infection, in addition to the aforementioned
particular risk factors and secondary cardiovascular effects,
affects the development of hematological and non-hematological
neoplasms, with >40% of HIV-positive individuals developing
acquired immunodeficiency syndrome (AIDS)-associated lymphomas
(13). Even though the incidence
of malignant tumors is higher in HIV-positive individuals, some
[such as Kaposi sarcoma, a multicentric tumor caused by infection
with the oncovirus Kaposi sarcoma-associated herpesvirus (KSHV)],
have a risk inversely related to the CD4+ count, while
others (such as BL), even though slightly more prevalent in the
AIDS stage of the infection, frequently appear in patients with a
normal CD4+ cell count and are not associated with
specific oncoviruses (13,17,18).
However, lymphoproliferative disorders, mainly lymphomas, are the
most common malignancies in PLWHIV, who exhibit either a high or
low T-helper cell count (17).
Hodgkin lymphomas (HLs) and NHL are the main types
of HIV-associated malignancies, with NHL being the most common and
the leading cause of HIV-associated mortality globally (13). In PLWHIV, 25-30% of NHL are BL or
Burkitt-like lymphomas (BLLs), being the second most common subtype
of NHL after DLBCL (13). BL and
BLL are also frequently identified and should be taken into
consideration in patients with a normal CD4+ cell count,
with the risk of developing BL in PLWHIV being more strongly
associated with cumulative HIV viremia (19,20),
outlining the need for a high degree of suspicion even in patients
who are not in the AIDS stage of HIV infection (13,18).
Oncoviruses, as aforementioned, also have an important role in the
development of HIV-associated lymphomas, with Epstein-Barr virus
(EBV) and KSHV being responsible for different subtypes of
lymphomas in PLWHIV (20). In
these patients, the uncontrolled proliferation of virus-infected
cells and expression of viral oncogenic proteins is induced by
HIV-triggered immunosuppression (20). While EBV latency type II
[expressing EBV nuclear antigen 1 (EBNA1) and latent membrane
proteins (LMP)-1 and -2] leads to an increased risk of development
of HL in HIV-infected individuals, other oncoviruses such as
KSHV/human herpesvirus 8 (HHV8) are linked to NHL, particularly
DLBCL or primary effusion lymphoma (PEL) (20). BLs are not linked with these and
often appear sporadically in PLWHIV, more likely secondary to
cumulative HIV viremia, as aforementioned or occasionally in
association with EBV latency type I (expressing only EBNA1, without
expressing LMP1 or LMP2) (20).
Despite being one of the most common lymphomas
diagnosed in PLWHIV, BL rarely represents a cause for intracardiac
masses, with limited cases presented in the literature and with the
disseminated form of the disease being more common than the primary
cardiac form (21-23).
While there is no clear or specific molecular mechanism identified
yet underlying cardiac metastasizing in BLs found in PLWHIV,
cardiac involvement potentially arises secondary to numerous
mechanisms. Firstly, early hematogenous spread appears secondary to
the high circulating tumor burden in BLs, caused by MYC
dysregulation, which drives rapid proliferation in tumor cells
(reflected by a markedly high Ki-67 index of >95%) and by the
loss of immune surveillance found especially in PLWHIV; and,
secondly, through transvenous extension from caval or mediastinal
disease, explaining the preference for the right atrium when it
comes to secondary cardiac masses in these patients (5,13,21,24).
The present report encompasses an illustrative case
of cardiac involvement in a patient with BL and HIV infection,
highlighting the diagnostic challenges, management and clinical
course of such cases.
Case report
The present study describes the case of a 57
year-old man, known to have immunological class B2 HIV infection
diagnosed 1.5 years prior to the current presentation, who has been
treated since diagnosis with antiretroviral therapy (ART)
consisting of a combination of doravirine, lamivudine and tenofovir
disoproxil, with a CD4+ count of 236
cells/mm3 (13.11%) (normal range, 500-1,500
cells/mm3), CD8+ count of 1,175
cells/mm3 (65.30%) (normal range, 170-1,000
cells/mm3) and a CD4+/CD8+
fraction of 0.20 (normal range, 1-3), 1 month prior to
presentation, suggestive of moderate-severe immunosuppression. The
HIV infection history of the patient was notable due to a
CD4+ count of 349 cells/mm3 and a
CD4+/CD8+ fraction of 0.20, with a HIV
viremia of 117 viruses/ml shortly after ART initiation, suggestive
of moderate immunosuppression. The patient presented in April 2025
to the emergency room of Coltea Clinical Hospital (Bucharest,
Romania) with rapidly growing (~3 months), painful, round, soft and
adherent left latero-thoracic and right latero-cervical
subcutaneous masses, measuring 12x7 cm (latero-thoracic) and 9x5 cm
(latero-cervical) in size. Clinical examination revealed
tachycardia with an irregular heart rate (HR) of 140 bpm, skin
pallor, nail clubbing, absent lung sounds in the left pulmonary
base, no crackles on pulmonary auscultation, normal blood pressure,
blood oxygen saturation of 96%, normal respiratory rate, light
exertional dyspnea and no palpitations, dizziness or other
complaints at the moment of presentation. The patient also
mentioned an unintentional weight loss of ~10% in the past 2 years
as well as night sweats.
Considering the clinical presentation and patient
history, laboratory tests were ordered, along with an
electrocardiogram (ECG) and a chest X-ray. The laboratory tests
revealed a normal white blood cell count [5,720 cells/µl
(4,000-11,000 cells/µl normal range), including 35% lymphocytes
(20-45% normal range) and 56% neutrophiles (43-65% normal range)],
mild microcytic anemia [hemoglobin, 10.3 g/dl (14-17 g/dl normal
range); mean corpuscular hemoglobin, 22.5 pg (27-33 pg normal
range); and mean corpuscular volume, 74.3 fl (80-96 fl normal
range)] with no iron deficit [decreased total iron binding capacity
of 219 µmol/dl (250-450 µmol/dl normal range) with increased iron
saturation of 40% (16-25% normal range) and increased ferritin],
mild thrombocytosis [platelet count, 454,000 cells/µl
(150,000-400,000 cells/µl normal value)], inflammatory syndrome
with an increase in all inflammatory biomarkers measured
[C-reactive protein (CRP), 9.4 mg/dl (normal value <0.3 mg/dl);
erythrocyte sedimentation rate, 86 mm/h (3-8 mm/h normal range);
fibrinogen, 596 mg/dl (150-400 mg/dl normal range); and ferritin,
870 ng/ml (24-260 ng/ml normal range)] and an elevated N-terminal
pro B-type natriuretic peptide (NT-proBNP) value, suggestive of
heart failure [2,260 pg/ml (0-300 pg/ml normal range)]. An ECG
revealed atrial fibrillation with a rapid ventricular response
[heart rate (HR), 140 bpm], with a QRS complex duration of <100
msec, diffuse QRS microvoltage and no notable repolarization
abnormalities nor pathological Q waves.
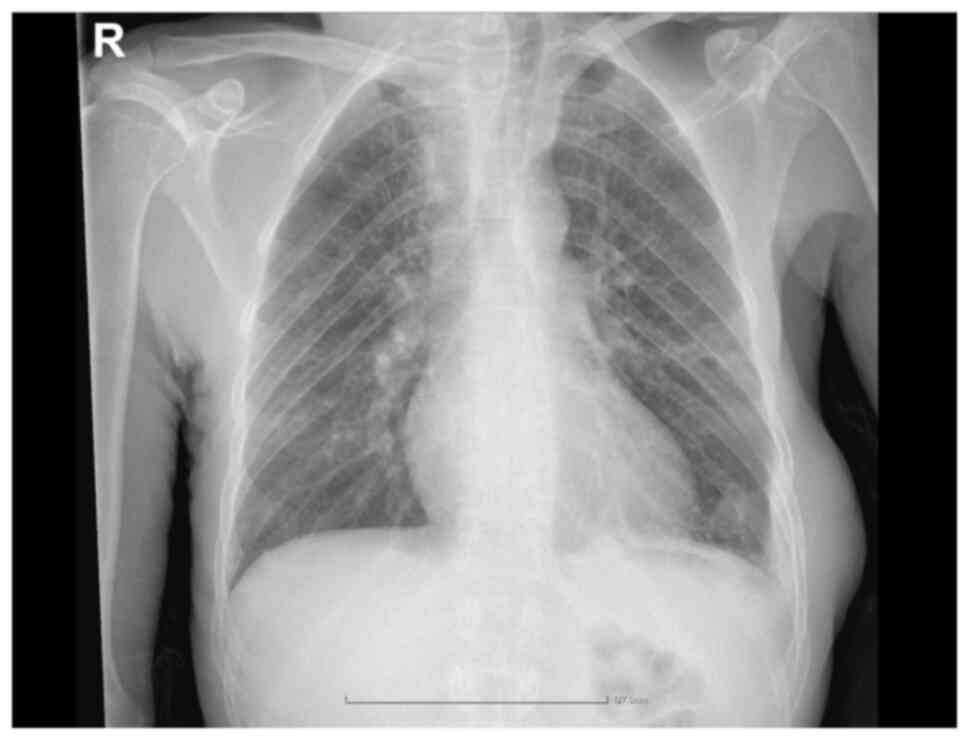
Furthermore, a chest X-ray revealed left basal
pleural effusion and right upper mediastinum enlargement with
secondary tracheal compression (Fig.
1).
Considering these findings (newly diagnosed atrial
fibrillation, diffuse QRS microvoltage on the ECG and an increased
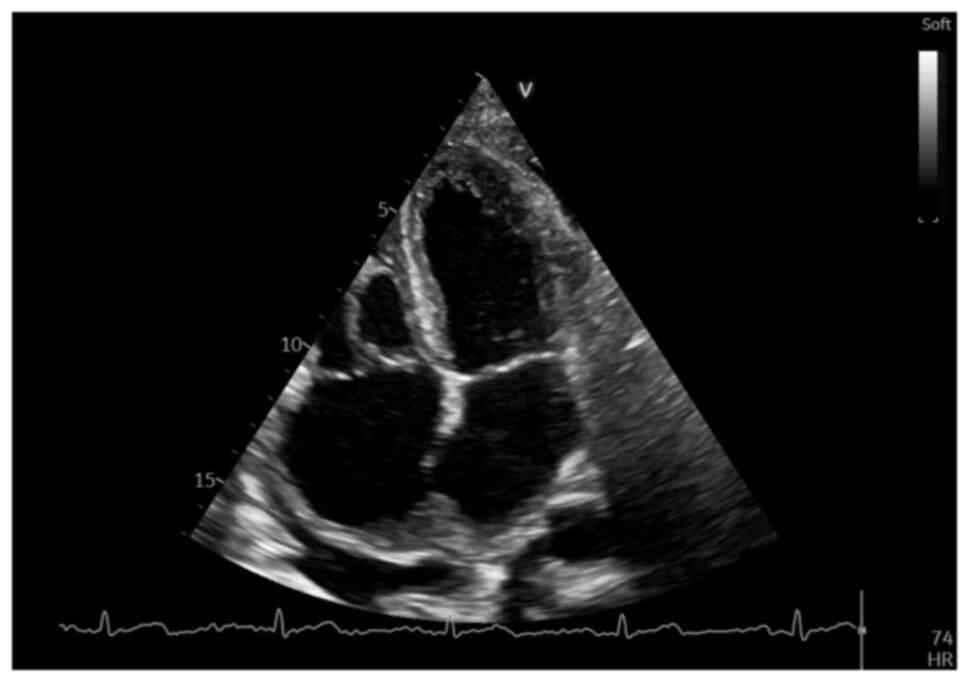
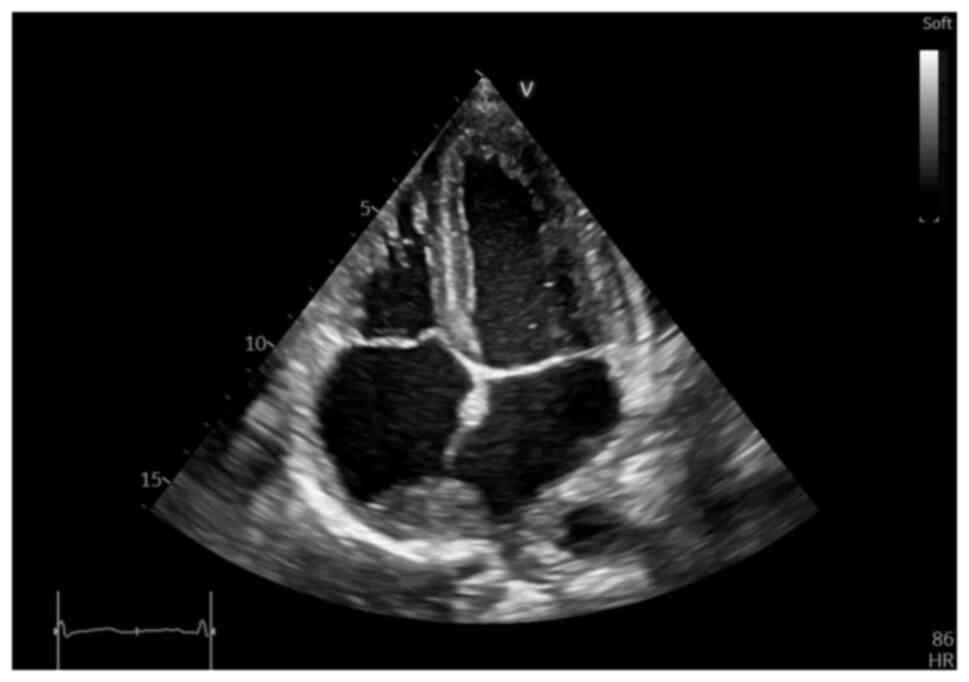
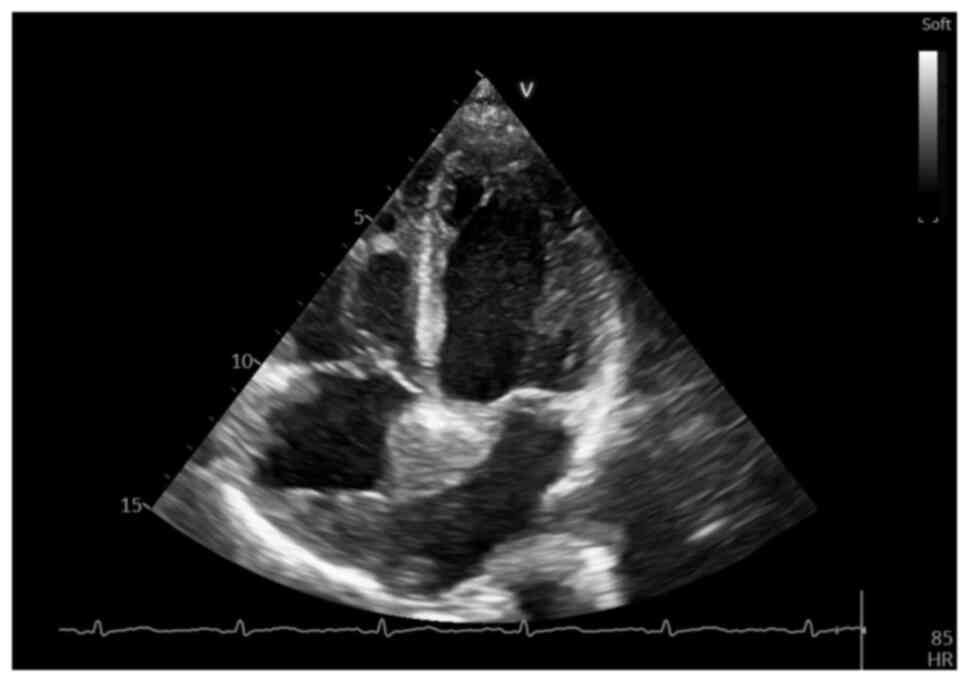
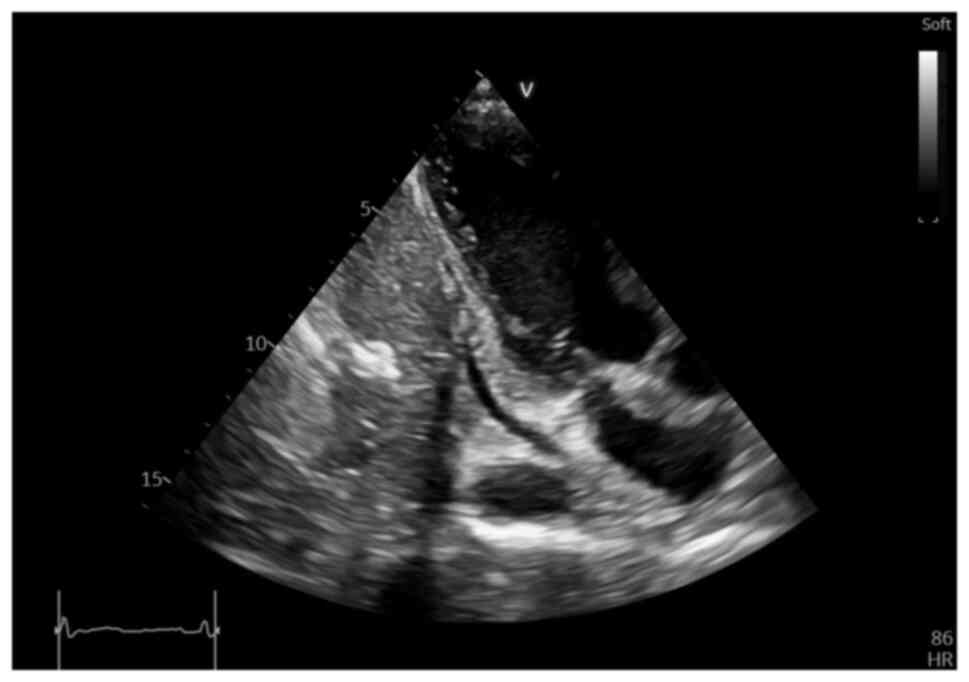
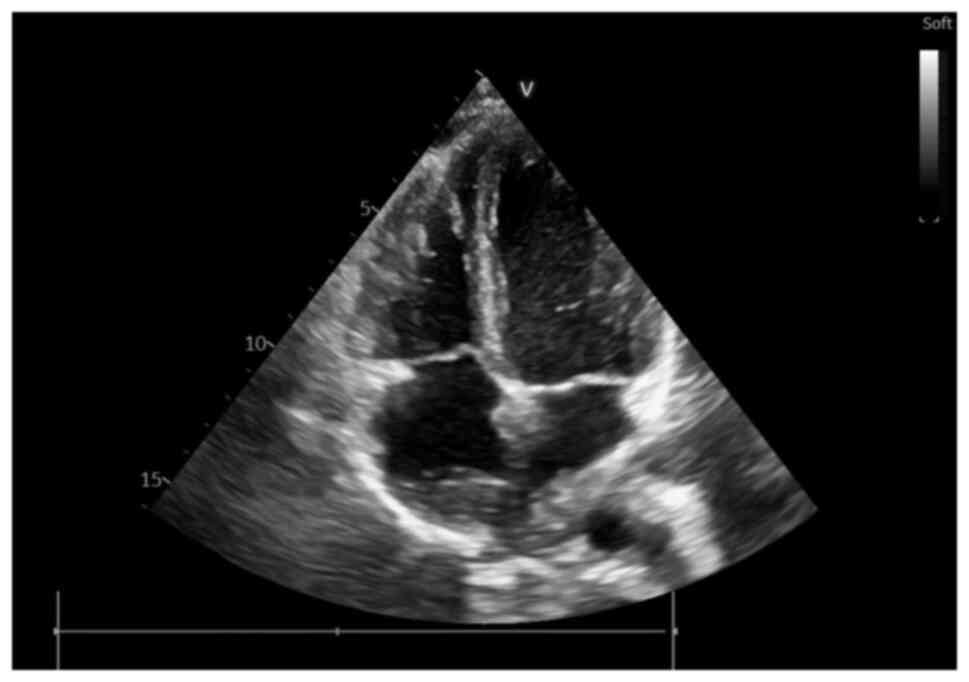
NT-proBNP value) echocardiography was performed, which revealed an
irregular, diffusely hyperechoic, immobile upper atrial mass, which
occupied ~1/3 of the right atrial (RA) area (4.2 cm2,
with an RA area of 16.9 cm2) and infiltrated the
interatrial septum, extending to the anterior wall and roof of the
left atrium (LA), also involving the right upper pulmonary vein
(Fig. 2, Fig. 3 and Fig. 4), a small pericardial effusion and
mild tricuspid regurgitation, with no apparent flow obstruction at
the level of the tricuspid valve.
Functionally, the left ventricle demonstrated normal
filling pressures, but a left ventricular global longitudinal
strain (GLS) of -19.7% with a slightly reduced ejection fraction
(EF) of 48% (Fig. 5). The slight
reduction in GLS and EF, without any specific segmentary
distribution, suggesting an ischemic etiology, was suspected to be
due to tumoral infiltration. As a consequence, a CT scan was
ordered to further understand the tumoral infiltration extent and
distribution.
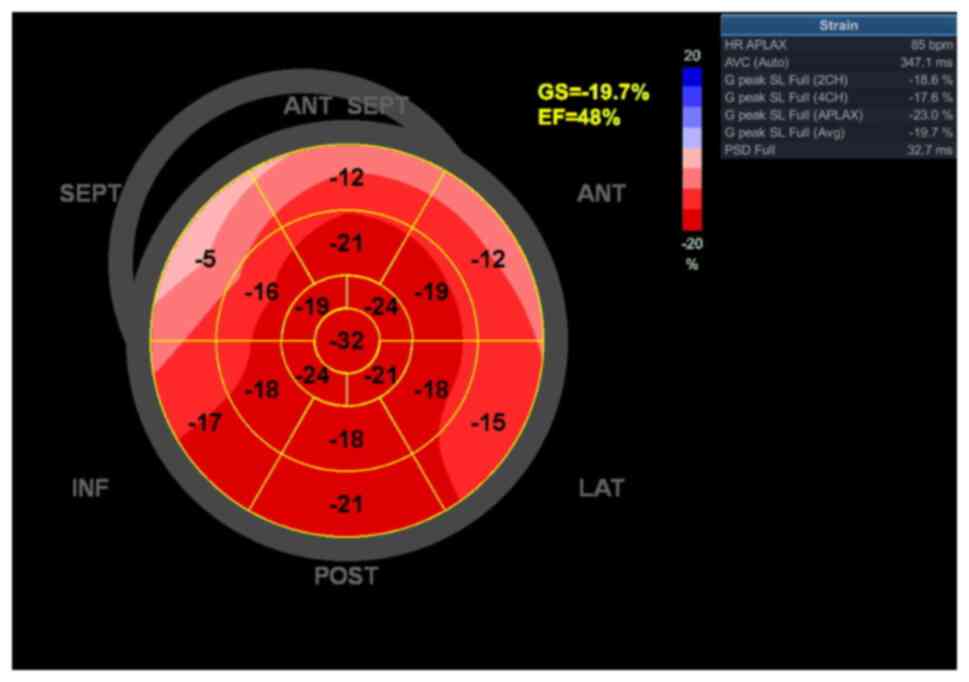 | Figure 5Echocardiography bull's-eye plot at
the initial evaluation showing a slightly reduced global strain and
ejection fraction, localized at the level of the basal segments of
the antero-lateral, antero-septal and septal walls and
interventricular septum. SEPT, septal wall; ANT, anterior; LAT,
lateral wall; POST, posterior; INF, inferior; GS, global
longitudinal strain; EF, ejection fraction; AVC, aortic valve
closure; HR, heart rate; APLAX, apical long-axis; PSD, peak strain
dispersion; G peak SL, global peak longitudinal strain. |
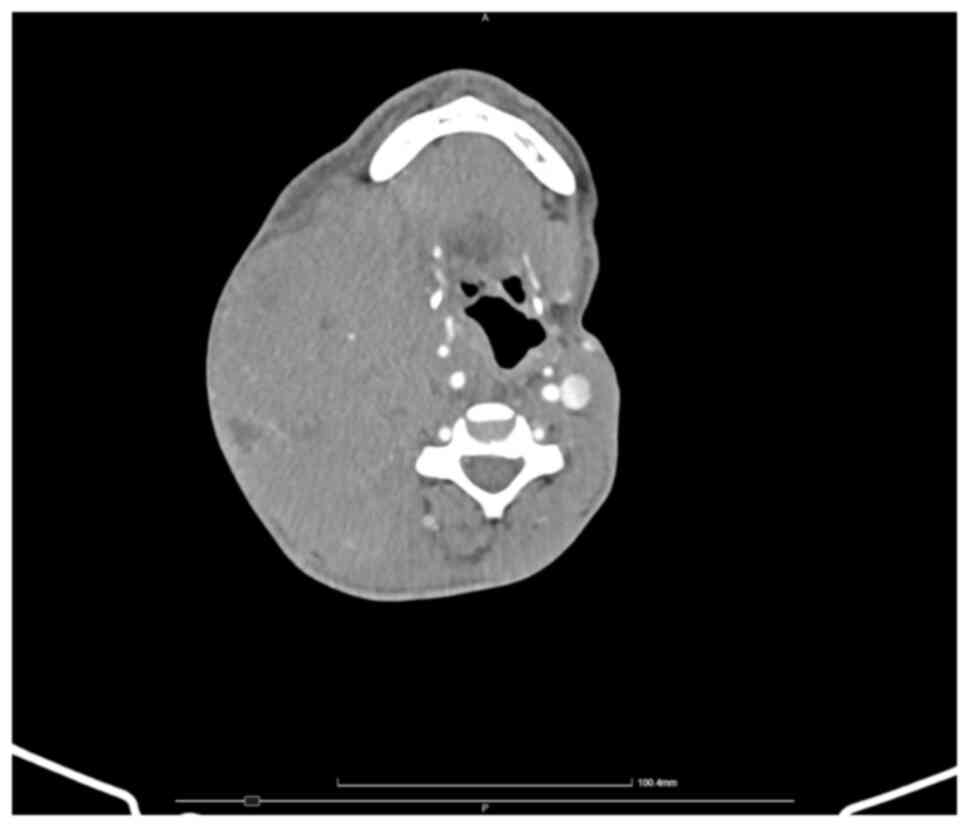
The whole-body CT showed multiple large masses,
including: i) A right lateral cervical mass of ~8.3x12x18 cm, with
a diffuse iodine uptake, necrosis areas and pharyngeal, laryngeal,
tracheal and thyroid compression, alongside compression and
stenosis of the right common, internal and external carotid
arteries and apparent extrinsic obstruction of the internal jugular
vein (Fig. 6); ii) a left
latero-thoracic mass of 8.4x4x11 cm with diffuse iodine uptake and
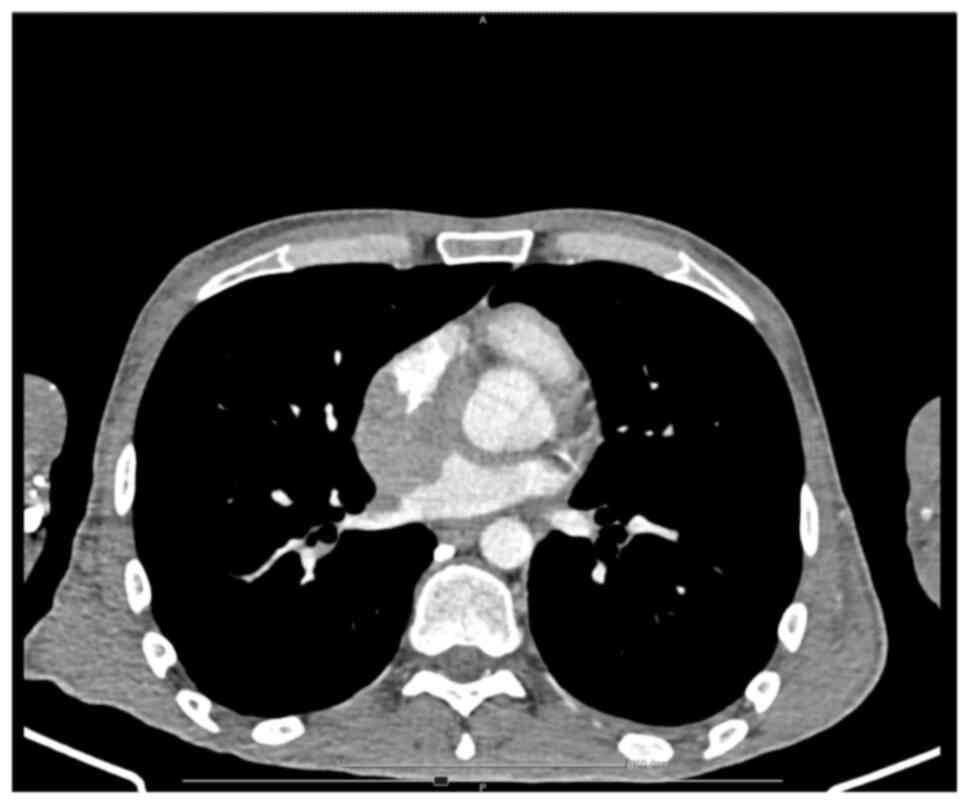
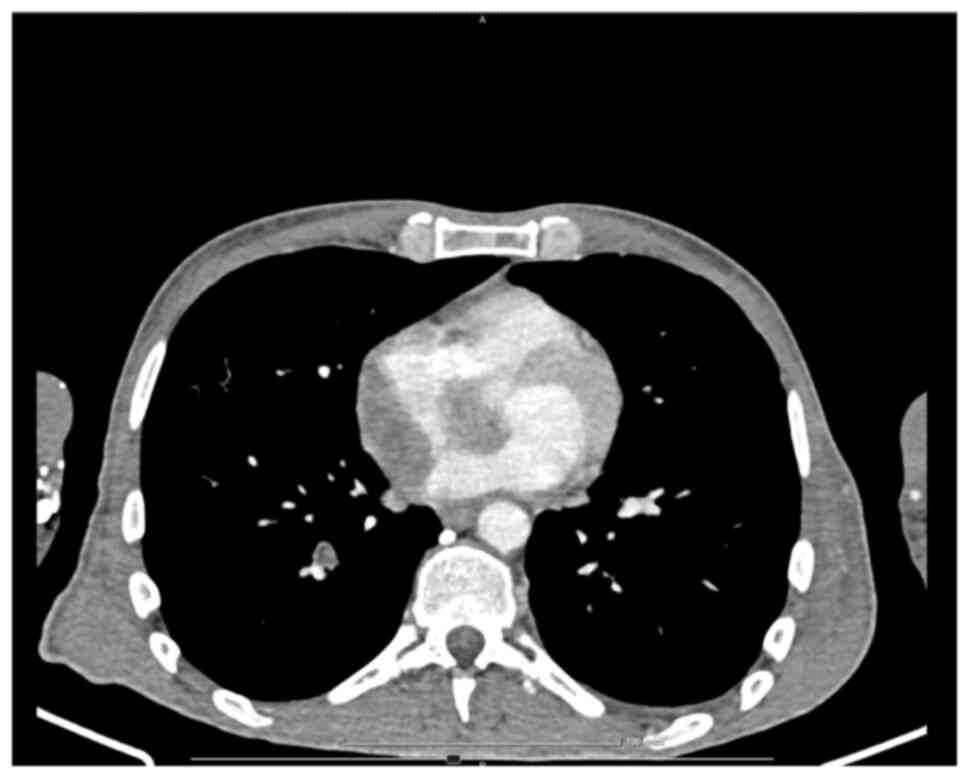
necrosis areas; and iii) an RA mass, which infiltrated the LA and
the right upper pulmonary vein, extending to the interatrial
septum, surrounding the aortic root and further extending to the
base of the interventricular septum and right ventricle (Figs. 7 and 8). No central nervous system (CNS)
involvement was identified, and neither any other notable findings
on the CT scan. Considering the cardiac infiltration observed on
the CT scan, which extended to the base of the left and right
ventricles, the slight reduction in EF and the reduced GLS observed
at the level of the basal segments of the antero-lateral,
antero-septal and septal walls as well as the interventricular
septum was interpreted in the context of tumoral infiltration.
Considering these findings, anticoagulant therapy
was initiated with a direct oral coagulant, due to the high
thromboembolic risk in the context of atrial fibrillation
(apixaban, 5 mg twice daily, with no interactions with ART) and a
β-blocker (metoprolol) for rate control. Additionally, a discussion
was held regarding the addition of a sodium-glucose cotransporter-2
[inhibitor to the treatment regimen (dapagliflozin or
empagliflozin, sodium-glucose cotransporters used in treating type
2 diabetes and heart failure)] according to the current guideline
recommendations and considering the presence of heart failure
(25); however, after careful
consideration of the potential benefits (a reduction in heart
failure mortality and hospitalization) and risks (increased risk of
urinary tract infections in the context of HIV co-infection with
moderate-severe immunosuppression), it was decided, in accordance
with the wishes of the patient, to temporarily withhold the
addition of dapagliflozin or empagliflozin to the treatment regimen
until an improved immune control was obtained with ART (changed
from doravirine, lamivudine and tenofovir disoproxil to
bictegravirum/emtricitabine/tenofovir alafenamide after
hematological diagnosis and chemotherapy initiation).
An incisional biopsy of the right latero-cervical
mass was made and the fragment was sent for histopathological and
immunohistochemical analyses. The patient was closely monitored
clinically and biologically awaiting the results, with
echocardiographic evaluations repeated weekly. After 1 week from
the initial evaluation, the patient exhibited an increase in size
and discomfort in the latero-thoracic and latero-cervical regions,
with notable growth of the cardiac mass observed during
echocardiographic re-evaluation, and no other morphological or
functional changes observed compared with the initial evaluation
(Fig. 9). The conversion to sinus
rhythm was obtained spontaneously, without the need for rhythm
control therapy, and sinus rhythm was maintained throughout
subsequent evaluations, although certain changes in repolarization
were observed on subsequent ECG evaluations, as the tumoral mass
expanded in size (negative T waves in V1-V3), with no other
repolarization abnormalities or pathological Q waves and a right
bundle branch block (RBBB) QRS morphology (rsR' aspect of the QRS
segment, suggestive of incomplete RBBB), with a QRS complex <100
msec and a slightly prolonged corrected QT (QTc) interval of 458
msec (corrected with the Fridericia formula:
QTc=QT/RR1/3). Additionally, a decrease in NT-proBNP
levels shortly after spontaneous restoration of the sinus rhythm
(786 pg/ml) was observed.
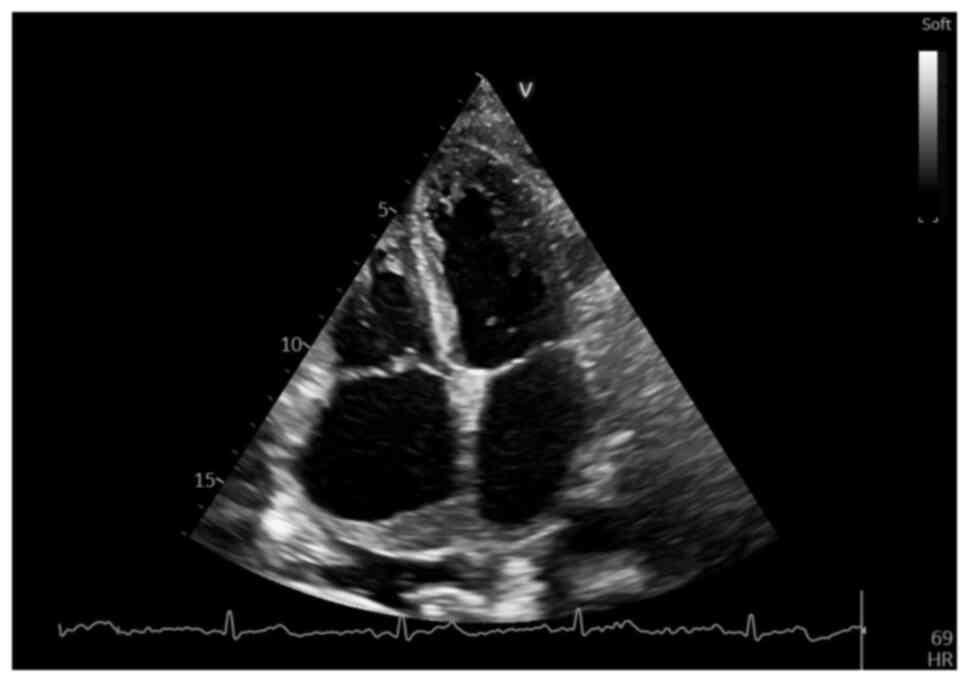
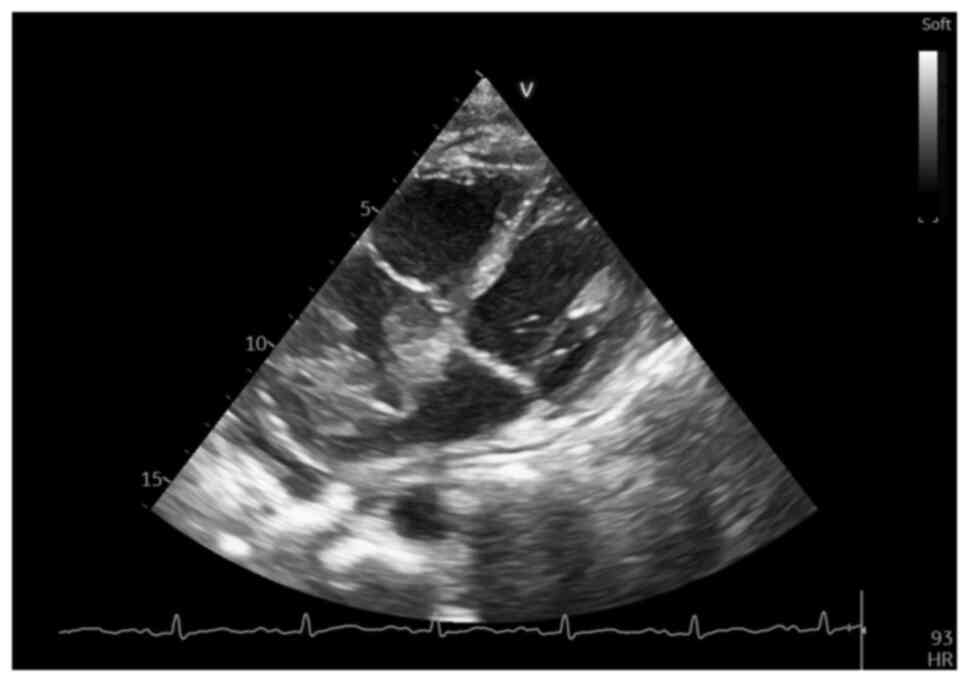
A subsequent echocardiographic follow-up evaluation
at 3 weeks after the initial one, while awaiting the tissue
immunohistochemical analysis results, revealed a marked increase in
size of the RA mass, which now occupied >1/2 of the RA area (9.4
cm2 with an RA area of 16.6 cm2),
infiltrating almost entirely the interatrial septum and more of the
anterior wall, as well as the roof of the LA and right upper
pulmonary vein (Fig. 10),
demonstrating a notably rapid increase in the size of the cardiac
tumor, but well tolerated at the time, with no apparent flow
obstruction or further changes in cardiac function. However, the
rapid growth of the latero-thoracic and latero-cervical masses made
echocardiographic assessment difficult, and the aggressive growth
of the cardiac mass highlighted the need for a quick, definitive
diagnosis and the initiation of therapy.
Tissue immunohistochemical analysis was performed,
using the following procedure: Sections with a thickness of 2.5 µm
obtained from formalin-fixed paraffin-embedded (FFPE) tissue
samples were cut and affixed to positively charged microscope
slides. Subsequently, these slides were subjected to a heating
process at a temperature of 60˚C for 1 h in a dry oven, thereby
enhancing the adhesion of the tissue and softening the paraffin.
The immunohistochemistry procedure was conducted as per established
protocol using a Ventana BenchMark ULTRA autostainer (Roche Tissue
Diagnostics). The sections went through steps of deparaffinisation,
rehydration and antigen retrieval with Ventana's CC1 (prediluted,
ph 8.0) solution (Roche Tissue Diagnostics). Primary antibodies
were applied according to the manufacturer's dilution guidelines
and permitted to interact with the sections. The primary antibodies
utilized in this study included the following: Anti-c-MYC (cat. no.
790-4628) Rabbit Monoclonal Primary Antibody (ready-to-use; Roche
Diagnostics GmbH), CONFIRM anti-bcl-2 (cat. no. 790-4464) Mouse
Monoclonal Primary Antibody (ready-to-use; Roche Diagnostics GmbH),
CONFIRM anti-Ki-67 (cat. no. 790-4286) Rabbit Monoclonal Primary
Antibody (ready-to-use; Roche Diagnostics GmbH), CONFIRM anti-CD20
(cat. no. 760-2531) Primary Antibody (ready-to-use; Roche
Diagnostics GmbH), CONFIRM anti-CD3 (cat. no. 790-4341) Rabbit
Monoclonal Primary Antibody (ready-to-use; Roche Diagnostics GmbH),
CD138/syndecan-1 (cat. no. 760-4248) Mouse Monoclonal Antibody
(ready-to-use; Roche Diagnostics GmbH), VENTANA anti-CD10 (cat. no.
790-4506) Rabbit Monoclonal Primary Antibody (ready-to-use; Roche
Diagnostics GmbH), bcl-6 (cat. no. 760-4241) Mouse Monoclonal
Antibody (ready-to-use; Roche Diagnostics GmbH), MUM1 (cat. no.
760-6082) Rabbit Monoclonal Primary Antibody (ready-to-use; Roche
Diagnostics GmbH), Epstein-Barr Virus (cat. no. 760-2640) Mouse
Monoclonal Antibody (ready-to-use; Roche Diagnostics GmbH), HHV-8
(cat. no. 760-4260) Mouse Monoclonal Antibody (ready-to-use; Roche
Diagnostics GmbH) and anti-CD30 (cat. no. 790-4858) Mouse
Monoclonal Primary Antibody (ready-to-use; Roche Diagnostics GmbH).
The incubation periods were established as 16 min for MYC and Ki67,
and 32 min for BCL2, BCL6, CD10, CD20, CD3, MUM1, CD138, EBV-LMP1,
HHV-8 and CD30. The visualization process was conducted using the
Ultraview universal DAB IHC detection kit (cat. no. 760-500; Roche
Diagnostics GmbH), followed by counterstaining utilizing
hematoxylin II for 8 min and a bluing solution for 8 min at room
temperature in the automated BenchMark ULTRA (cat. no. 750-600;
Roche Diagnostics GmbH). The slides were meticulously cleaned and
dehydrated through a graded series of ethanol and xylene. Finally,
the slides were affixed with mounting media onto microscope slides.
The subsequent analysis showed a CD20 diffusely positive, CD10
positive, B cell lymphoma 6 (BCL6) positive, multiple myeloma
oncogene 1 (MUM1) negative, CD30 and BCL2 negative, and c-Myc 70%
positive tissue (Fig. 11A and
B). Positivity for CD3 was
observed in frequent small intratumoral T cells, while CD138 was
positive in rare intratumoral plasmacytes and negative in tumoral
cells. The tissue was negative for EBV-LMP1 and HHV8 (thus
excluding other oncovirus-driven hematological malignancies
identified in PLWHIV, such as DLBCL, PEL or HL and supporting a
diagnosis of BL), with a very high proliferation index Ki-67 of
~98%, helping differentiate BL from DLBCL, which has a lower index
of proliferation (Fig. 11C)
(26).
The identified malignant cells also possessed strong
basophilia and multiple prominent basophilic nucleoli and multiple
mitoses observed under standard histological analysis, performed
according to the following procedure by the Pathology Department:
H&E staining was performed on 2.5-µm FFPE sections. Tissue was
fixed in 10% neutral-buffered formalin, processed through graded
ethanol and xylene and embedded in paraffin. Sections mounted on
charged slides were dewaxed in xylene, rehydrated through
descending alcohol concentrations and stained with hematoxylin
(0.1% solution) for 5 min, followed by bluing in water, and
counterstaining with eosin (0.5% solution) for 2 min, at room
temperature. Slides were then dehydrated in 70, 95 and 100%
ethanol, cleared in xylene and coverslipped. Histological
evaluation was performed using a standard light microscope at x20
and x40 magnification (Fig. 11D).
Thus, considering the presence of frequent basophilic monomorphic
cells with prominent basophilic nucleoli on histological analysis,
along with the diffusely positive CD20, positive BCL6 (Fig. S1A), intensely positive CD10
(Fig. S1B), negative MUM1
(Fig. S1C) and negative BCL2
(Fig. S1D), with a positive c-Myc
of 70% and the high index of proliferation, close to 100% (Ki67 of
98%), a diagnosis of non-Hodgkin BL as BL international prognostic
index 2(27), high-risk secondary
to HIV immunosuppression, was made (26). Chemotherapy with a
hyperfractionated cyclophosphamide, doxorubicin, vincristine,
methotrexate, cytarabine and dexamethasone (hyper CVAD) regimen was
initiated considering the markedly high hematological risk of the
patient according to the BL international prognostic index (namely,
>40 years old, with a high index of tumoral progression and
lactate dehydrogenase >3-fold the upper limit of the normal
range, with a value of 879 U/l). The regimen included the following
agents and dosing, and was administered according to the following
protocol: On cycles 1, 3, 5 and 7 (3-4 weeks between cycles), 600
mg/m2 cyclophosphamide was administered intravenously
(IV) over 2 h every 12 h for six doses starting on day 1. Mesna
(600 mg/m2/day) was administred by continuous IV
infusion on days 1-3, starting 1 h before, plus 2 mg vincristine IV
on days 4 and 11, plus 50 mg/m2 doxorubicin IV on day 4
and 40 mg dexamethasone orally on days 1-4 and 11-14. On cycles 2,
4, 6 and 8 (3-4 weeks between cycles), 200 mg/m methotrexate was
administered IV over 2 h followed by 800 mg/m2 IV over
22 h on day 1, plus 3 g/m2 cytarabine IV over 2 h every
12 h for four doses starting on day 2, plus 15 mg leucovorin IV
every 6 h for eight doses beginning 12 h after the completion of
methotrexate infusion, and increased to 50 mg IV every 6 h if
methotrexate levels were >20 µM/l at baseline, were >1.0 µM/l
at 24 h or were >0.1 µM/l at 48 h after the end of methotrexate
infusion, until levels were <0.1 µM/l. Methylprednisolone (50
mg) was administered IV every 12 h on days 1-3.
However, considering the markedly high
cardiovascular risk according to the score developed by the Heart
Failure Association in collaboration with the International
Cardio-Oncology Society (HFA-ICOS) (25), namely, elevated baseline NT-proBNP,
history of heart failure and a selected treatment plan with
anthracyclines, the patient was closely monitored with repeated
echocardiographic evaluations during chemotherapy initiation. The
patient responded well to the regimen, with a notable reduction in
all tumoral masses after chemotherapy initiation. In an
echocardiographic evaluation made 5 days after chemotherapy
initiation, the cardiac mass occupied <1/5 of the RA area (2.7
cm2 with an RA area of 20.6 cm2, right atrial
dilation compared with the initial measurement and a marked tumor
area reduction compared with the first and second evaluation),
measured ~2.3x1.2 cm and was restricted to the roof of the RA and
upper third of the interatrial septum, with minimal involvement of
the LA roof and right upper pulmonary vein compared with
pretherapeutic evaluation and no extension to the base of the right
or left ventricles (Figs. 12 and
13).
After 2 weeks post-therapy initiation, a notable
reduction in size of the latero-cervical and latero-thoracic
tumoral masses was observed and the compression of the carotid
arteries and the jugular vein subsequently remitted. The patient
also demonstrated a notable improvement in symptoms, with remission
of the pain that was present during initial and subsequent
evaluations. The laboratory results exhibited a marked reduction in
inflammatory syndrome biomarkers (CRP, 1.6 mg/dl and fibrinogen,
521 mg/dl) and a decrease in the platelet count (218,000 cells/µl),
with the persistence of microcytic anemia (hemoglobin, 9 g/dl) and
a reduction in white blood cell count (2,590 cells/µl, including
45% lymphocytes and 40.7% neutrophiles). The patient maintained the
sinus rhythm and no other cardiovascular complications associated
with treatment were observed during monitoring. The ECG showed a
notable improvement compared with previous evaluations (namely,
sinus rhythm with an HR of 90 bpm and no QRS morphology changes,
indicative of remission of RBBB morphology) and the remission of
the negative T waves in V2-V3, which became positive, with no other
repolarization abnormalities and correction of the slightly
prolonged QTc interval (now 435 msec with the Fridericia formula).
However, a diffuse QRS microvoltage was still observed (Fig. 14).
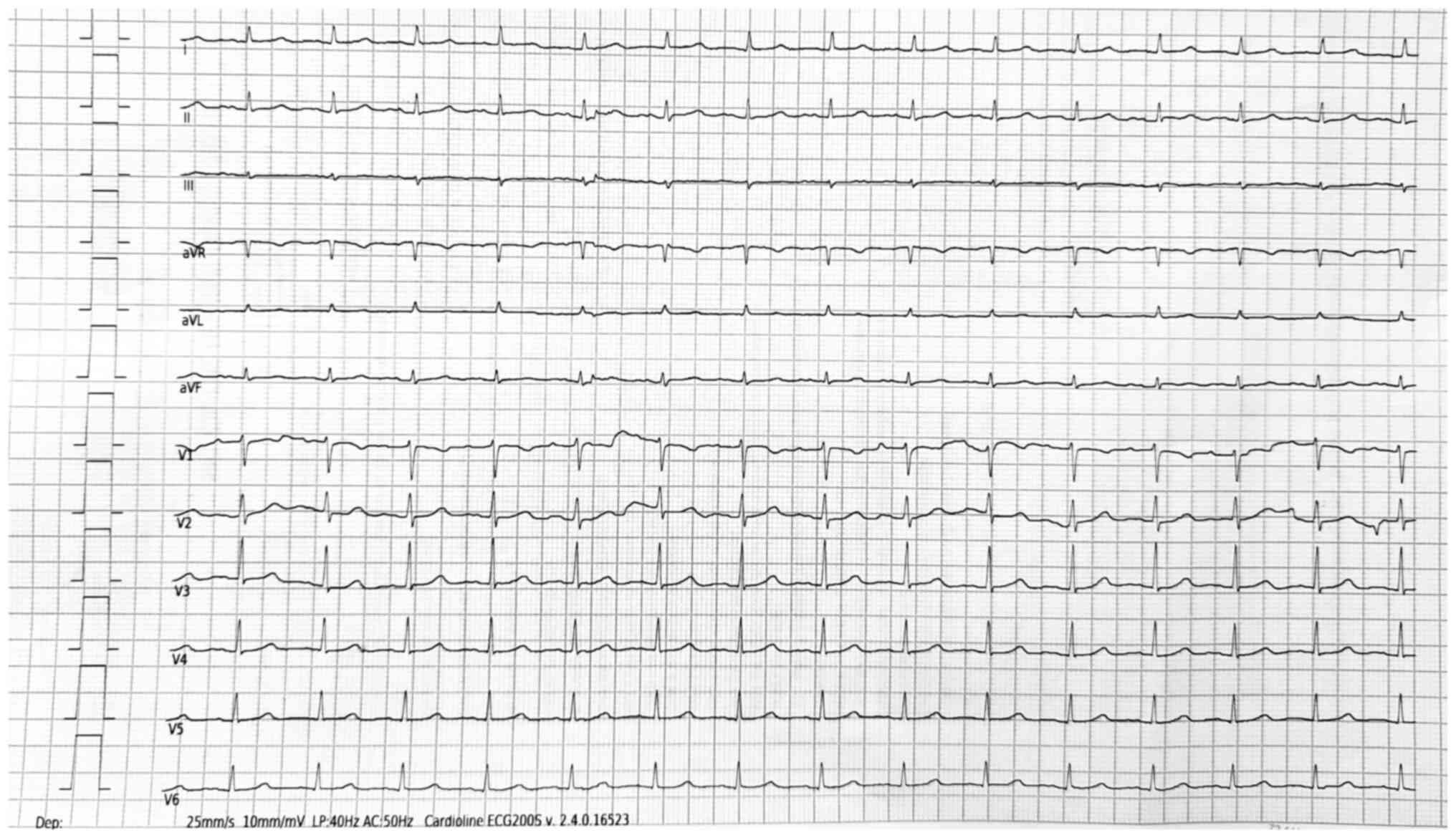 | Figure 14Post-chemotherapy electrocardiogram
showing sinus rhythm, a heart rate of 90 bpm, remission of the
right bundle branch block morphology, remission of the negative T
waves in V2-V3, diffuse QRS microvoltage (showing in this case loss
of electrical signal caused probably by tumoral infiltration)and
normalization of the corrected QT interval (435 msec, Fridericia
formula). V1-V6 refer to precordial leads. aVR, augmented vector
right; aVL, augmented vector left; aVF, augmented vector foot; LP,
low pass; AC, alternating current. |
There were no further changes in cardiac function or
morphology observed in subsequent echocardiographic evaluations
until discharge, which occurred 2 weeks after the initiation of the
first cycle of the hyper CVAD protocol. The patient continued to be
monitored after chemotherapy initiation for cardiovascular or
systemic side effects through regular clinical evaluations every 2
weeks, laboratory tests, ECG monitoring and echocardiographic
screenings. Furthermore, recommendations for the prevention of
cardiovascular disease, including lifestyle changes, as well as the
benefits and risks of the cardiovascular treatment prescribed, were
offered, in addition to information regarding possible adverse
cardiovascular effects of the chemotherapy regimen and the early
identification of warning signs and symptoms requiring further
evaluation (including sudden onset dyspnea or dyspnea aggravation,
palpitations, dizziness, chest pain or lower extremity edema).
After three cycles of chemotherapy, there were no
marked changes in infectious status (HIV immunological class C3 and
moderate-severe immunosuppression after the hematological diagnosis
and ART change) or cardiac tumor size compared with chemotherapy
initiation, with the persistence of the small tumoral mass at the
level of the RA roof, without any marked growth in size, but with
an improved left ventricular function on longitudinal strain
evaluation (including a GLS of -19.1%, with minimal anterior,
septal and lateral basal dyskinesia and with a calculated EF of
54%, which was an increase from the initial 48%; Fig. 15). This was a marked improvement
from the previous evaluation, perhaps secondary to tumor
infiltration reduction at the level of the left ventricular base.
The ECG objectified a slight reduction in the QRS microvoltage and
sinus rhythm, with no repolarization abnormalities or QTc
prolongation. However, a slightly increased NT-proBNP level was
observed at the end of the third cycle of chemotherapy (1,810
pg/ml), without the presence of any symptoms or signs of heart
failure, with no other cardiovascular complications to date.
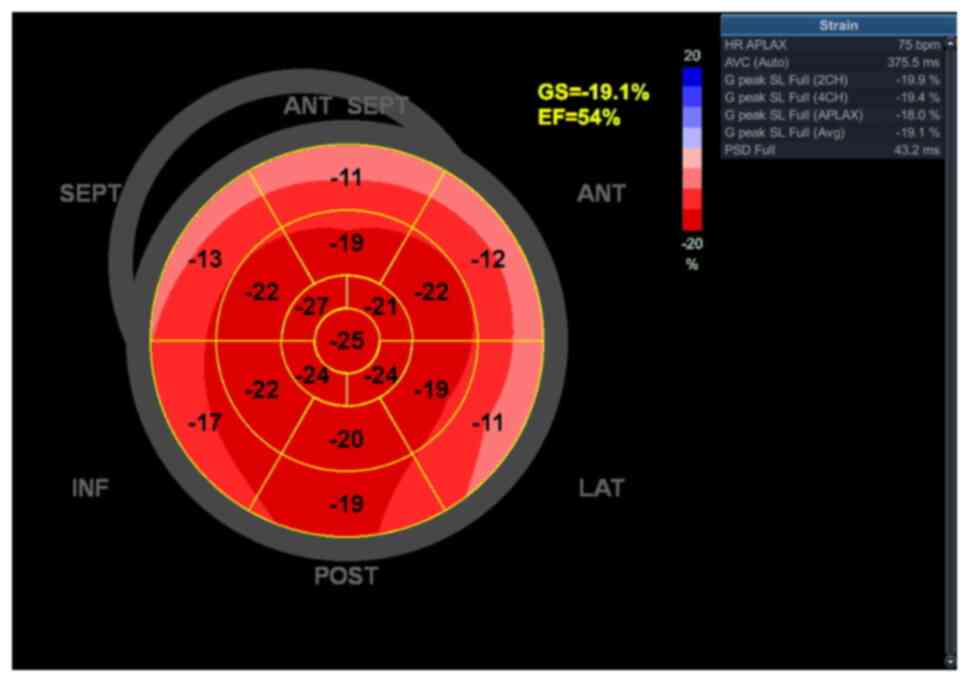 | Figure 15Echocardiography bull's-eye plot
recorded after 3 cycles of chemotherapy showing slightly reduced
GS, with minimal dyskinesia localized at the level of the basal
segments of the antero-lateral, antero-septal, septal and lateral
walls and preserved ejection fraction. SEPT, septal wall; ANT,
anterior; LAT, lateral wall; POST, posterior; INF, inferior; GS,
global longitudinal strain; EF, ejection fraction; AVC, aortic
valve closure; HR, heart rate; APLAX, apical long-axis; PSD, peak
strain dispersion; G peak SL, global peak longitudinal strain. |
Literature review and discussion
Hematological malignancies may have multiple
cardiovascular complications, usually related to thrombosis, heart
failure, cardiomyopathy or secondary cardiac metastases, which are
either associated with the primary malignancy or with chemotherapy
(1,2). These complications depend on the
malignancy subtype, with fluid malignancies such as
myeloproliferative neoplasms leading to an increased risk in
thrombosis and lymphoid line neoplasms such as lymphomas
occasionally leading to cardiac metastases (4,5).
Usually, cardiac tumors have a secondary origin,
with primary cardiac tumors being benign in nature and rarely
identified (6,7). Among cardiac tumors, lymphomas
exhibit an incidence in the general population of ~10%, although
the values vary between 8.7-20% according to previous autopsy
studies (8,9). The majority of cardiac lymphomas
appear secondary to disseminated disease and rarely present as
primary cardiac malignancies (10,11).
Even though lymphomas are not the most frequent cardiac tumors
identified, their prevalence increases markedly in PLWHIV, with
>40% of HIV-positive individuals developing AIDS-associated
lymphomas and thus having an increased risk of secondary cardiac
metastases compared with the general population (13).
ART has notably improved the prognosis of PLWHIV in
previous years. In the early ART era HIV infection had multiple
secondary cardiovascular effects documented, such as pericarditis,
endocarditis and dilated cardiomyopathy; nowadays, with improved
immunological control, atherosclerotic heart disease has become the
most important cardiovascular complication in these patients,
mainly due to a higher prevalence of traditional risk factors such
as chronic inflammation and endothelial dysfunction (16), leading to an increased risk in
heart failure, arrhythmias, myocardial infarction or hypertension
(15). However, the increased
incidence of lymphomas, mainly NHL (with DLBCL being the most
frequent subtype, followed by BL) (13), needs to be considered due to the
rapid tumor growth and aggressive nature of these neoplasms,
leading to the necessity of rapidly diagnosing and initiating
treatment in these cases (21).
BLs are a subtype of B-cell lymphomas, characterized by rapid
growth; however, they are rarely a cause for secondary cardiac
metastases, with limited cases documented to date and most of them
having been identified in HIV-positive individuals (12,21).
In this regard, a PubMed literature review was conducted to analyze
adult cardiac BL cases published during the last 15 years
(2010-2025). The search was performed using the keyword ‘cardiac
Burkitt lymphoma’. Inclusion criteria were study publication and
diagnosis between 2010 and 2025, and patients diagnosed with
primary or secondary cardiac BL with the intracardiac mass location
mentioned and with documented age, sex and response to treatment.
All cases of BLL or ambiguous line lymphomas were excluded, as well
as patients aged <18 years (since the prevalence, incidence and
etiology differ markedly compared with the adult population) and
cases without a comprehensive documentation of the diagnostic or
treatment response. Furthermore, 2 cases were excluded, as they
were published in Chinese and could not be translated accurately.
The 13 cases identified are summarized in Table I (21,22,28-38).
 | Table IDocumented cases of adult Burkitt
cardiac lymphomas based on a PubMed search of the last 15 years
(2010-2025). |
Table I
Documented cases of adult Burkitt
cardiac lymphomas based on a PubMed search of the last 15 years
(2010-2025).
| First author,
year | Age, years | Sex | Presenting
symptoms | HIV status | Intracardiac mass
location/s | Diagnostic
modality | Treatment
regimen | Outcome | (Refs.) |
|---|
| Tzachanis et
al, 2014 | 44 | F | Early satiety,
abdominal bloating and epigastric discomfort | Negative | Right atrium | CT scan, TTE,
pericardiocentesis and MRI | Cyclophosphamide,
vincristine, doxorubicin and high-dose methotrexate; ifosfamide,
etoposide and high-dose cytarabine; rituximab | Complete
remission | (28) |
| Lazkani et
al, 2015 | 38 | M | Dyspnea,
tachycardia and palpitations | Positive | Right atrium | CT scan, TTE and
MRI | Rituximab,
etoposide, prednisone, vincristine, cyclophosphamide and
doxorubicin | Remission (6
weeks) | (29) |
| Chan et al,
2016 | 27 | M | Palpitations and
dizziness | Positive | Right atrium, right
ventricular base and interatrial septum | CT scan, TTE and
TEE | Modified
cyclophosphamide, vincristine, doxorubicin, and
methotrexate/ifosfamide; etoposide and cytarabine | Partial remission
(12 weeks) | (21) |
| Vervloet et
al, 2017 | 49 | M | Dyspnea, chest pain
and lower limb edema | Positive | Right atrium, left
atrium and interatrial septum | X-ray, TTE and
MRI | Methotrexate and
fractionated high doses of ifosfamide; rituximab, cyclophosphamide,
doxorubicin, vincristine and prednisone | Complete
remission | (30) |
| Vilaça et
al, 2017 | 64 | M | Lipothymia, nausea
and palpitations | Positive | Right atrium, right
ventricle base and interventricular apical septum | CT scan, TTE and
MRI | Prednisolone,
vincristine, daunorubicin, L-asparaginase, rituximab and adaptive
radiation therapy | Partial
remission | (22) |
| Usry et al,
2020 | 80 | F | Dyspnea | N/A | Right atrium,
interatrial septum and inlet ventricular septum | TTE and CT
scan | Rituximab,
etoposide, prednisone, vincristine, cyclophosphamide and
doxorubicin | Partial
remission | (31) |
| Khalid et
al, 2020 | 49 | M | Dyspnea and
palpitations | Positive | Right atrium and
right ventricle base | TTE and CT
scan | Rituximab,
cyclophosphamide, vincristine, doxorubicin and methotrexate | Partial remission
(complete response N/A) | (32) |
| Chen et al,
2020 | 68 | M | Mild chest pain,
nausea and wheezing | N/A | Right atrium | TTE, CT scan, MRI
and PET-CT | Rituximab,
etoposide, prednisone, vincristine, cyclophosphamide and
doxorubicin | Complete
remission | (33) |
| Martinez et
al, 2021 | 77 | F | N/A | N/A | Right atrium, left
atrium and interatrial septum | PET-CT, EUS and CT
scan | Rituximab,
etoposide, prednisone, vincristine, cyclophosphamide and
doxorubicin | Complete
remission | (34) |
| Khaba et al,
2021 | 30 | F | Dyspnea,
non-productive cough and leg edema | Positive | Right atrium | X-ray and TTE | Modified
cyclophosphamide, vincristine, doxorubicin, and
methotrexate/ifosfamide; etoposide and cytarabine | Complete
remission | (35) |
| Hérnandez Jimenez
et al, 2021 | 65 | F | Cough, fatigue,
dyspnea and bilateral leg edema | Negative | Right atrium | TTE and MRI | None | Deceased (shortly
after biopsy) | (36) |
| Schmiester et
al, 2022 | 54 | M | Paranasal sinus
swelling, fatigue and dyspnea on exertion | Negative | Right ventricle and
right atrium | CT scan, TTE and
MRI | Cyclophosphamide,
doxorubicin and prednisone; rituximab, vincristine, cytarabine,
etoposide, methotrexate, ifosfamide and dexamethasone; intrathecal
prophylaxis with cytarabine, dexamethasone and methotrexate | Complete
remission | (37) |
| Rector et
al, 2022 | 70 | M | Constipation,
eating discomfort and unintentional weight loss | Negative | Right atrium and
right ventricle | CT scan, TTE and
MRI | Vincristine,
doxorubicin, dexamethasone and rituximab; etoposide, prednisone,
vincristine, cyclophosphamide and hydroxydaunorubicin; intrathecal
methotrexate without complication | Deceased | (38) |
Out of these cases, there were no observed
differences regarding sex (5 female patients and 8 male patients),
with a median age of 55 years and an age range of 27-80 years. The
main presenting symptom was dyspnea (7 cases), with palpitations
being reported by ~1/3 of the patients, who were eventually
diagnosed with cardiac BL (4 cases). There were 4 HIV-negative
patients, 6 HIV-positive patients and 3 patients with their HIV
status not available (no testing was mentioned), outlining the
increased prevalence of cardiac BL in PLWHIV. All the cases
featured RA involvement, with 5 cases having an isolated mass
limited to the RA, without any extension to the other cardiac walls
or chambers, confirming past reviews of literature that noted the
RA as the main and preferred localization of cardiac BL (21,29,33-36).
The majority of patients responded well to treatment, complete
remission documented for 6 cases and partial remission for 5,
although in 2 cases, the patients deceased shortly after therapy
initiation. The chemotherapeutic regimens varied, with multiple
regimens employed across 15 years of evolving oncological
therapies. The main diagnostic method used was transthoracic
echocardiography, which highlights the importance of
echocardiographic evaluation and screening, even when a small level
of suspicion is present. CT scans and MRI were also used as
diagnostic or screening tools (particularly CT scans) or as methods
of in-depth description of the nature and extension of the cardiac
involvement (particularly MRI).
Considering the low prevalence and the aggressive
nature of these cardiac tumors, as outlined in the literature
review and in the aforementioned case presented, a high threshold
of suspicion is necessary when diagnosing these types of cardiac
metastasis and echocardiographic screening is key in these cases
(24,39), particularly in PLWHIV, in whom the
prevalence of cardiac BL is increased compared with the general
population, irrespective of their CD4+ cell count or
immune status (13,21). Beyond standard transthoracic
echocardiography, which is readily available and non-invasive, thus
making it the method of choice when it comes to cardiac evaluation
when an index of suspicion is present, CT scans, cardiac MRI or
transesophageal echocardiography also have a diagnostic role when
it is unclear whether cardiac involvement exists or when a more
in-depth characterization is necessary (9,21).
Often, cardiac BLs are localized solely at the RA level and, in the
majority of cases, there is some RA involvement, even when other
chambers and walls are occupied or infiltrated by tumoral masses,
making the careful evaluation of right atrial morphology and
function key when diagnosing these types of cardiac tumor (21).
Treatment with a hyper CVAD regimen is highly
effective, particularly in BL with high-risk features, and is
associated with a low incidence of CNS relapse (40), although cardiovascular risk
stratification (clinical, biological, imaging modalities and risk
scores, including the aforementioned HFA-ICOS score) and monitoring
of the side effects related to anthracycline treatment (heart
failure, left ventricular dysfunction and cardiomyopathy) is
necessary in patients receiving this protocol (41).
In conclusion, hematological malignancies have
numerous complications, which are either associated with treatment
or with the disease, including, but not limited to, thrombosis,
heart failure, cardiomyopathy, rhythm and rate disturbances. One
notable complication is the potential of these malignancies to
determine cardiac metastases, especially when in tumors of the
lymphoid line. Out of these, NHL, particularly BL and DLBCL, even
though rare in the general population, are much more frequent in
PLWHIV and exhibit rapid and aggressive growth and subsequently, a
markedly poor prognosis. Thus, patients with HIV infection, even
those with a normal CD4+ cell count, need a high index
of suspicion for the diagnosis of these types of lymphoma
(particularly BL and BLL) and secondary cardiac metastases,
especially since rapid diagnosis and initiation of treatment
improves the otherwise poor prognosis of these patients. However,
patients still need to be monitored for adverse cardiovascular
effects, considering that the use of multiple treatment regimens
has demonstrated cardio-toxic effects and also considering the
important chronic and acute cardiovascular complications of HIV
infection, even though, to the best of our knowledge, at present,
there is no clear standardized protocol regarding the evaluation
and follow-up of this group of patients.
The literature review and the case presented in the
present report underline the importance of a high index of
suspicion, echocardiographic screening, rapid diagnosis and
therapeutic intervention in patients with BL with secondary cardiac
metastases and history of HIV infection, even in PLWHIV with a
normal CD4+ count, considering their low prevalence
(even though higher than the prevalence in the general population)
and aggressive nature, as outlined in the sequential
echocardiographic evaluations in the present case report (the rapid
growth of the tumor mass was observed in a short span, doubling in
size over the course of ~3 weeks) and otherwise poor prognosis of
this diagnosis in a occasionally neglected group of patients. The
present case presentation also outlines, in conjunction with the
literature review, the rapid and remarkable response to treatment
(in this case with a hyper CVAD regimen) when a quick diagnosis is
made and an adequate treatment regimen is employed. Additionally,
the patient also particularly presented with no cardiac adverse
effects to the treatment regimen administered, with an increase in
left ventricular function and left ventricular longitudinal strain
during treatment, even in the context of anthracycline treatment.
However, considering the potential cardiotoxicity of the regimen
and of the hematological diseases, periodic cardiologic follow-up
is necessary in these patients, especially during anti-neoplastic
treatment, to identify potential cardiovascular complications and
address them as early as possible.
Supplementary Material
Immunohistochemistry findings
supporting the diagnosis of Burkitt lymphoma. (A) Positive B cell
lymphoma 6 maker. (B) Strongly positive CD10 marker. (C) Negative
multiple myeloma oncogene 1. (D) Negative BCL2. [(A-D)
Magnification, x20; scale bar, 100 μm].
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AG participated in the design, drafting, reviewing
and rewriting of the article throughout its development. IM
participated in the drafting and reviewing of the manuscript, as
well as in the acquisition and interpretation of the review data.
AC contributed to the design and review of the article and provided
insight into the main cardiovascular complications of hematological
malignancies. MB further revised the article offering substantial
contributions to its design and aided in the comprehensive
description and presentation of HIV-associated cardiovascular
complications. AM and CS were involved in patient care, acquisition
of data and manuscript drafting, offering valuable insight into
patient symptoms, signs and effects to treatment throughout
monitoring. RR contributed by designing and reviewing the data and
describing the patient's HIV status in chronological and logical
order. ALG contributed to the conception of the article, revised
the draft and performed further literature and article reviews,
interpreting, adding and rearranging the data in a logical,
detailed and coherent manner. AG and ALG confirm the authenticity
of all the raw data. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Approval from the Medical Ethics Committee for
Clinical Studies of the Coltea Clinical Hospital (Bucharest,
Romania) was obtained prior to submission (approval no.
10/10.07.2025).
Patient consent for publication
Written informed consent for the publication of case
data and images was obtained from the patient prior to
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leiva O, Hobbs G, Ravid K and Libby P:
Cardiovascular disease in myeloproliferative neoplasms: JACC:
CardioOncology state-of-the-art review. JACC CardioOncol.
4:166–182. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Herrmann J, McCullough KB and Habermann
TM: How I treat cardiovascular complications in patients with
lymphoid malignancies. Blood. 139:1501–1516. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gangadharamurthy D and Shih H:
Intracardiac thrombosis in polycythemia vera. BMJ Case Rep.
2013(bcr2012008214)2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kelliher S and Falanga A: Thrombosis in
myeloproliferative neoplasms: A clinical and pathophysiological
perspective. Thromb Update. 5(100081)2021.
|
|
5
|
Campo E, Stein H, Harris N, et al: Burkitt
lymphoma. In: Swerdlow SH, Harris NL, Jaffe ES, Pileri SA, Stein H,
Thiele J, editors. WHO classification of tumours of haematopoietic
and lymphoid tissues. Revised 4th ed. Vol. 2. WHO Press, p. 586,
2018.
|
|
6
|
Bajdechi M, Onciul S, Costache V, Brici S
and Gurghean A: Right atrial lipoma: A case report and literature
review. Exp Ther Med. 24(697)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tyebally S, Chen D, Bhattacharyya S,
Mughrabi A, Hussain Z, Manisty C, Westwood M, Ghosh AK and Guha A:
Cardiac tumors: JACC CardioOncology state-of-the-art review.
JACC CardioOncol. 2:293–311. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bussani R, De-Giorgio F, Abbate A and
Silvestri F: Cardiac metastases. J Clin Pathol. 60:27–34.
2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
O'Mahony D, Peikarz RL, Bandettini WP,
Arai AE, Wilson WH and Bates SE: Cardiac involvement with lymphoma:
A review of the literature. Clin Lymphoma Myeloma. 8:249–252.
2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lichtenberger JP III, Dulberger AR,
Gonzales PE, Bueno J and Carter BW: MR imaging of cardiac masses.
Top Magn Reson Imaging. 27:103–111. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jeudy J, Burke AP and Frazier AA: Cardiac
lymphoma. Radiol Clin North Am. 54:689–710. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Al-Mehisen R, Al-Mohaissen M and Yousef H:
Cardiac involvement in disseminated diffuse large B-cell lymphoma,
successful management with chemotherapy dose reduction guided by
cardiac imaging: A case report and review of literature. World J
Clin Cases. 7:191–202. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Berhan A, Bayleyegn B and Getaneh Z:
HIV/AIDS associated lymphoma: Review. Blood Lymphat Cancer.
12:31–45. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bajdechi M, Gurghean A, Bataila V,
Scafa-Udriste A, Radoi R, Oprea AC, Marinescu A, Ion S, Chioncel V,
Nicula A, et al: Cardiovascular risk factors, angiographical
features and short-term prognosis of acute coronary syndrome in
people living with human immunodeficiency virus: Results of a
retrospective observational multicentric Romanian study.
Diagnostics (Basel). 13(1526)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bajdechi M, Gurghean A, Bataila V,
Scafa-Udriste A, Bajdechi GE, Radoi R, Oprea AC, Chioncel V,
Mateescu I, Zekra L, et al: Particular aspects related to CD4+
level in a group of HIV-infected patients and associated acute
coronary syndrome. Diagnostics (Basel). 13(2682)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Feinstein MJ: HIV and cardiovascular
disease: From insights to interventions. Top Antivir Med.
29:407–411. 2021.PubMed/NCBI
|
|
17
|
Yarchoan R and Uldrick TS: HIV-associated
cancers and related diseases. N Engl J Med.
378(2145)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bush LM, Urrutia JG, Rodriguez EA and
Perez MT: AIDS-associated cardiac lymphoma: A review: Apropos a
case report. J Int Assoc Provid AIDS Care. 14:482–490.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hernández-Ramírez RU, Shiels MS, Dubrow R
and Engels EA: Cancer risk in HIV-infected people in the USA from
1996 to 2012: A population-based, registry-linkage study. Lancet
HIV. 4:e495–e504. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dolcetti R, Vaccher E and Carbone A:
Lymphoproliferations in people living with HIV: Oncogenic pathways,
diagnostic challenges and new therapeutic opportunities. Cancers
(Basel). 17(2088)2025.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chan O, Igwe M, Breburda CS and Amar S:
Burkitt lymphoma presenting as an intracardiac mass: Case report
and review of literature. Am J Case Rep. 17:553–558.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vilaca A, Monteiro M, Pimentel T and
Marques H: Intracardiac mass from Burkitt's lymphoma in an
immunocompromised patient: A very rare form of presentation. BMJ
Case Rep. 2017(bcr2017221001)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Soldea S, Iovanescu M, Berceanu M, Mirea
O, Raicea V, Bezna MC, Rocsoreanu A and Donoiu I: Cardiovascular
disease in HIV patients: A comprehensive review of current
knowledge and clinical implications. Int J Mol Sci.
26(1837)2025.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bruce CJ: Cardiac tumours: Diagnosis and
management. Heart. 97:151–160. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lyon AR, Lopez-Fernandez T, Couch LS,
Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D,
Cordoba R, Cosyns B, et al: 2022 ESC guidelines on cardio-oncology
developed in collaboration with the European hematology
association, the European society for therapeutic radiology and
oncology and the international cardio-oncology society: Developed
by the task force on cardio-oncology of the European society of
cardiology. Eur Heart J. 43:4229–4361. 2022.
|
|
26
|
Harlendea N and Harlendo K: Ki-67 as a
Marker to differentiate Burkitt lymphoma and diffuse large B-cell
lymphoma: A literature review. Cureus. 16(e72190)2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Olszewski AJ, Jakobsen LH, Collins GP,
Cwynarski K, Bachanova V, Blum KA, Boughan KM, Bower M, Dalla Pria
A, Danilov A, et al: Burkitt lymphoma international prognostic
index. J Clin Oncol. 39:1129–1138. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tzachanis D, Dewar R, Luptakova K, Chang
JD and Joyce RM: Primary cardiac Burkitt lymphoma presenting with
abdominal pain. Case Rep Hematol. 2014(687598)2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lazkani M, Rivera-Bonilla T, Eddin M,
Girotra S and Pershad A: The evanescent right atrial mass. Indian
Heart J. 67:485–488. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Vervloet DM, De Pauw M, Demulier L,
Vercammen J, Terryn W, Steel E and Vandekerckhove L: Aggressive
extensive cardiac mass in an HIV-1-infected patient: Should we go
for comfort therapy? Acta Clin Belg. 72:198–200. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Usry CR, Wilson AS and Bush KNV: Primary
cardiac lymphoma manifesting as complete heart block. Case Rep
Cardiol. 2020(3825312)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Khalid K, Faza N, Lakkis NM and Tabbaa R:
Cardiac involvement by Burkitt lymphoma in a 49-year-old man. Tex
Heart Inst J. 47:210–212. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen X, Lin Y and Wang Z: Primary cardiac
lymphoma: A case report and review of literature. Int J Clin Exp
Pathol. 13:1745–1749. 2020.PubMed/NCBI
|
|
34
|
Martinez HA, Kuijvenhoven JC and Annema
JT: Intracardiac EUS-B-guided FNA for diagnosing cardiac tumors.
Respiration. 100:918–922. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Khaba MC, Kampetu MF, Rangaka MC, Karodia
M, Ramoroko SP and Madzhia EI: Primary cardiac lymphoma in HIV
infected patients: A clinicopathological report of two cases. Ann
Med Surg (Lond). 69(102757)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hernandez Jimenez CA, Schlie Villa W and
Ordinola Navarro A: Cardiac Burkitt's lymphoma presenting with
heart failure. QJM. 114:589–590. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Schmiester M, Tranter E, Lorusso A,
Blaschke F, Geisel D, Bullinger L, Damm F and Na IK: Acute left
ventricular insufficiency in a Burkitt lymphoma patient with
myocardial involvement and extensive local tumor cell lysis: A case
report. BMC Cardiovasc Disord. 22(31)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Rector G, Koh SJ and Tabbaa R: A case of
isolated cardiac Burkitt lymphoma causing right-sided heart
failure. Tex Heart Inst J. 49(e217575)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
De Filippo M, Chernyschova N, Maffei E,
Rovani C, De Blasi M, Beghi C and Zompatori M: Primary cardiac
Burkitt's type lymphoma: Transthoracic echocardiography,
multidetector computed tomography and magnetic resonance findings.
Acta Radiol. 47:167–171. 2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Samra B, Khoury JD, Morita K, Ravandi F,
Richard-Carpentier G, Short NJ, El Hussein S, Thompson P, Jain N,
Kantarjian H and Jabbour E: Long-term outcome of hyper-CVAD-R for
Burkitt leukemia/lymphoma and high-grade B-cell lymphoma: Focus on
CNS relapse. Blood Adv. 5:3913–3918. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cardinale D, Iacopo F and Cipolla CM:
Cardiotoxicity of anthracyclines. Front Cardiovasc Med.
7(26)2020.PubMed/NCBI View Article : Google Scholar
|
















![Representative histopathology and
immunophenotype supporting the diagnosis of Burkitt lymphoma. (A)
Ki-67 proliferation index of ~98%. (B) CD20 highlights diffuse
B-cell phenotype. (C) Nuclear c-Myc expression in ~70% of tumor
cells(D) Standard H&E staining showing diffuse B-cell
infiltrate with strong basophilia and multiple prominent basophilic
nucleoli, with multiple mitoses [(A-C) magnification, x20; scale
bar, 100 µm; (D) magnification, x40; scale bar, 50 µm].](/article_images/etm/31/2/etm-31-02-13046-g10.jpg)