Introduction
Myopia, also known as nearsightedness, is a
refractive condition in which distant objects are seen in low
definition due to light focusing anterior to the retina (1). The prevalence of myopia has risen
substantially worldwide, particularly among children and
adolescents (2). It has been
estimated that ~50% of the global population will experience myopia
by 2050 (2,3). In addition, it is estimated that as
many as 10% of cases might be classified as being high myopia,
characterized by major ocular complications, including retinal
detachment, glaucoma, myopic maculopathy and choroidal
neovascularization. These complications are associated with a high
risk of irreversible vision loss, highlighting the need for
aggressive management to control the progression of myopia in
childhood, when ocular growth and development are most active
(4).
Several factors may explain why the prevalence of
pediatric myopia is increasing. Among environmental factors,
reduced exposure to natural light and increased engagement in
near-work activities, such as screen use and reading, have been
cited, and genetic predisposition also plays a key role (5). Children with myopic parents are at an
elevated risk of developing myopia, with a study demonstrating a
compounded risk when both parents are myopic (2). Axial elongation, the main structural
correlate of myopia severity, is driven by an interplay between
genetic and environmental factors. In addition to promoting myopia
progression, axial elongation increases the risk of
sight-threatening complications, making it an important target for
myopia control interventions (6,7).
Orthokeratology (OK) is a corneal reshaping therapy
in which the patient wears specially designed rigid gas-permeable
lenses overnight, and has emerged as a promising approach for
myopia control (8). These lenses
temporarily reshape the corneal epithelium, allowing for clear
unaided vision during the day. More importantly, OK lenses are
postulated to reduce the extent of axial elongation by peripheral
retinal defocus remodeling (9).
The underlying mechanism is the establishment of a peripheral
myopic defocus that counteracts hyperopic defocus-induced axial
elongation. Numerous clinical studies and meta-analyses have shown
that OK lenses significantly slow axial elongation growth compared
with that achieved using conventional corrective treatments such as
single-vision glasses or contact lenses (1-10).
Other ocular parameters, such as refractive error,
contrast sensitivity and higher-order aberrations, are also altered
when OK lenses are used. It is hypothesized that the peripheral
myopic defocus caused by OK lenses is the main mechanism by which
retinal growth signals are modulated and the progression of myopia
is reduced. However, the underlying mechanism remains incompletely
understood. Considerable variability in treatment outcomes among
patients has been reported (11,12).
This may be attributed to factors including differences in lens
design, patient age, the initial refractive error of the eye,
biomechanical properties of the cornea, and compliance with the
wear schedule and protocols. In addition, some concerns remain
regarding the long-term safety and efficacy of OK lens wear,
particularly concerning corneal health and infection risk (12).
Despite considerable research, substantial knowledge
gaps persist regarding the comprehensive effects of OK on visual
and structural ocular parameters. Most existing studies focus on
short-term refractive outcomes, while few long-term studies have
evaluated the impact of OK on axial elongation and other important
metrics. Furthermore, heterogeneous study designs, variable
follow-up times and inconsistencies in the reporting of outcomes
limit the comparability of the findings. A previous study has
contextualized OK as a pivotal modality within the expanding
spectrum of noninvasive, lens-mediated ocular therapies, aligning
with broader innovations in nanoparticle-based drug delivery
systems (13).
The present prospective cohort study aimed to assess
the effect of OK lens wear on visual parameters, refractive error
and axial length in children with myopia over an extended follow-up
period. Changes in these parameters were evaluated in detail to
provide evidence-based insights into the role of OK as a myopia
control intervention and to contribute to the optimization of
pediatric myopia management strategies.
Materials and methods
Study design and setting
The single-arm prospective cohort study was
conducted over 3 years, from February 1, 2020 to February 28, 2023.
The study took place at The First Affiliated Hospital of Anhui
Medical University (Hefei, China) and Anhui Women and Children's
Medical Center (Hefei, China), both of which specialize in
pediatric ophthalmology.
Study population
The study enrolled myopic children <18 years of
age who were seeking vision correction and met the inclusion
criteria. Eligible participants had a spherical equivalent
refractive error between -1.00 and -6.00 diopters (D) and
astigmatism ≤1.50 D. Exclusion criteria were any history of ocular
surgery, active ocular disease, systemic conditions affecting
vision or poor compliance with lens wear. Participants with
contraindications to OK, such as irregular corneas or severe dry
eye, were also excluded. Recruitment was carried out using
convenience sampling from patients presenting to the clinic for
refractive correction.
Sample size estimation
The sample size was calculated to ensure adequate
statistical power for detecting a significant difference in axial
length changes between baseline and follow-up. Based on previous
studies, the expected effect size for axial elongation reduction
was set at 0.25 mm per year, with a standard deviation (SD) of 0.40
mm. Using a two-tailed a of 0.0 and a power of 80, the required
sample size was calculated using the following formula: n=2 x
(Zα/2 + Zβ)2
x σ2/Δ2 in which:
Zα/2 is 1.96, corresponding to a
95% confidence interval, Zβ is 0.84, providing 80%
power; σ is 0.40 mm as the assumed SD; and Δ is 0.25 mm,
representing the minimum detectable difference. The calculated
sample size was 156. Based on an anticipated dropout rate of 20%, a
total of 188 participants were enrolled in the study.
Intervention
Participants were fitted with OK lenses specifically
designed for myopia management. Lens fitting was performed by
experienced practitioners following a standardized protocol to
ensure optimal centration and alignment. Lenses were worn overnight
for ≥8 h and removed upon waking. Participants were provided with
detailed instructions on lens handling, cleaning and storage.
Follow-up visits were scheduled at 1 week, 1 month and every 3
months thereafter to monitor compliance, assess lens fit and
address any adverse events.
The OK lenses used in the present study featured a
reverse geometry design with four-zone architecture, including a
central optical zone, reverse curve, alignment zone and peripheral
curve, optimized for overnight wear and corneal reshaping. All
lenses were made of high-Dk materials to ensure adequate oxygen
permeability during closed-eye conditions. Lens parameters,
including base curve, diameter and sagittal depth, were customized
based on corneal topography and refractive error. To minimize the
influence of lens-induced corneal flattening on axial length
measurements, all AL assessments were performed after a minimum
washout period of 24-48 h without lens wear, as recommended in
previous studies (1,2,3,5,7,10).
This protocol ensured that transient corneal changes did not
confound biometric outcomes (2).
Outcome measures
The primary outcome measure was change in axial
length, assessed using low-coherence optical biometry, for example
using an IOLMaster device (IOLMaster 500; Carl Zeiss Meditec AG).
Secondary outcomes included changes in spherical equivalent
refractive error, measured via cycloplegic autorefraction, and
visual parameters such as uncorrected distance visual acuity (UDVA)
and best-corrected visual acuity (BCVA). Corneal topography was
performed at each visit to monitor changes in corneal shape and
ensure lens stability.
Data collection
Baseline data included demographic information,
ocular history and comprehensive ocular examination results.
Measurements of axial length, corneal curvature and refractive
error were performed at baseline and each follow-up visit using
standardized equipment and procedures. Adverse events, including
corneal staining, conjunctival redness and lens intolerance, were
recorded and managed according to established OK safety guidelines
(2). Outcomes were evaluated by
clinicians blinded to the study. The level of compliance with OK
lens wear in children was evaluated based on a combination of
behavioral, clinical and follow-up adherence parameters: High
compliance was defined as ≥90% adherence to wear time, full
attendance at follow-ups, proper hygiene, and no missed logs or
adverse event reporting; moderate compliance was defined as 60-89%
adherence, and occasional missed visits or minor lapses in
hygiene/logging; and low compliance was defined as <60%
adherence, frequent missed visits, poor hygiene or unreported
adverse events (2,9).
Statistical analysis
Data were analyzed using SPSS software, version 27.0
(IBM Corp.). Data are presented at the mean ± SD. Descriptive
statistics were used to summarize demographic and baseline
characteristics. Paired t-tests were employed to compare changes
from baseline to 36-month follow-up (14). Bartlett's test was performed to
check the uniformity of SDs for normally distributed continuous
variables. Repeated measures analysis of variance (ANOVA) with
Bonferroni correction was used to analyze axial length changes over
multiple time points. One-way ANOVA with post hoc Tukey's test was
used to analyze axial length changes among compliance groups.
Mixed-effects regression models were used to evaluate the
longitudinal effects of OK on axial length while adjusting for
potential confounders such as age, baseline refractive error and
compliance. P<0.05 was considered to indicate a statistically
significant result. Forest plots were drawn using metaanalysisonline.com (ELIXIR Hungary) (15) using the setting ‘random effect
model’, method ‘inverse’, summary measures ‘standard mean
difference’ and between study heterogeneity estimator
‘DerSimonian-Laird’. I2>50% was considered
moderate heterogeneity.
Results
Study participants
The study window was from February 1, 2020 to
February 28, 2023, a total of 37 months. All 188 children completed
a full 36-month follow-up and none dropped out.
Baseline visual parameters
The study included 188 participants, 100 (53.2%)
males and 88 (46.8%) females. The mean age of the participants was
12.5±2.1 years. At baseline (Table
I), the mean spherical equivalent refractive error was
-3.25±1.05 D, the mean axial length was 24.12±0.63 mm, and the UDVA
was 0.48±0.22 logarithm of the minimum angle of resolution
(logMAR). These parameters confirmed the enrollment of moderately
myopic participants suitable for OK intervention.
 | Table IVisual parameters as baseline and
36-month follow-up. |
Table I
Visual parameters as baseline and
36-month follow-up.
| | Baseline | 36-month
follow-up | |
|---|
| Visual parameter | Mean | SD | Mean | SD | Mean change | t-value | P-value |
|---|
| Spherical equivalent
refractive error, D | -3.25 | 1.05 | -2.82 | 0.18 | 0.43 | 10.28 | <0.01 |
| Axial length, mm | 24.12 | 0.63 | 24.70 | 0.12 | 0.58 | -66 | <0.01 |
| Uncorrected
distance visual acuity, logMAR | 0.48 | 0.22 | 0.12 | 0.12 | -0.36 | 20 | <0.01 |
| Best corrected
visual acuity, logMAR | 0.08 | 0.25 | 0.05 | 0.01 | -0.03 | 41 | 0.03 |
Compliance and axial length
changes
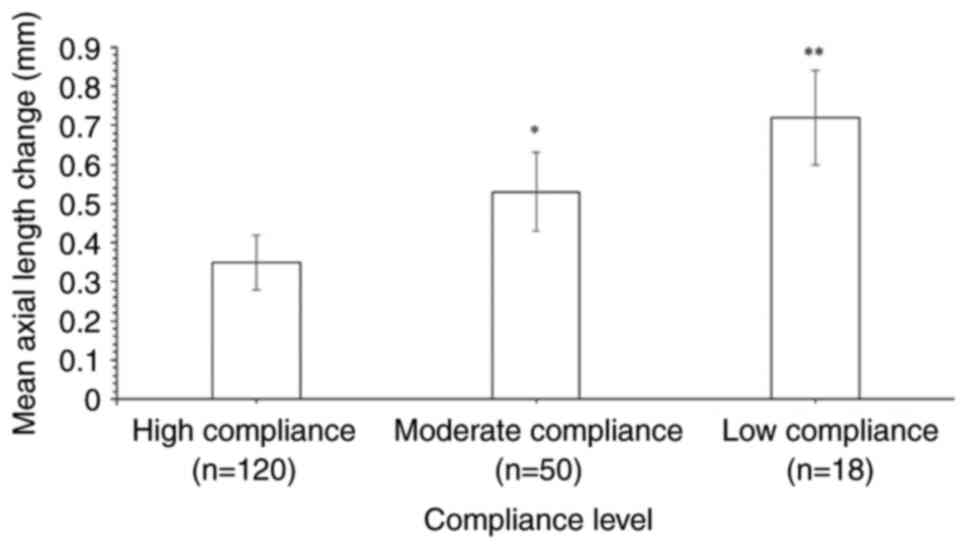
As shown in Fig. 1,
120 (63.6%), 50 (26/6%) and 18 (9.6%) children had high, moderate
and low levels of compliance. Participants with high compliance
exhibited the lowest mean change in axial length (0.35±0.07 mm),
while participants with low compliance exhibited the highest mean
change (0.72±0.12 mm). Participants with medium compliance
exhibited a mean change of 0.53±0.1 mm. Differences between the
high and moderate compliance groups and between the high and low
compliance groups were statistically significant (P<0.05),
emphasizing the importance of adhering to lens wear protocols.
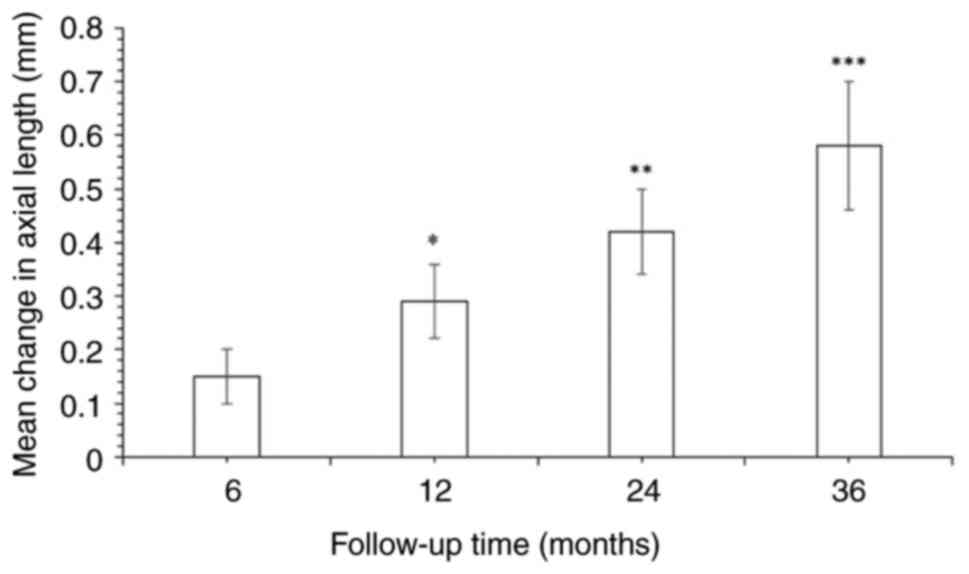
Changes in axial length over time
The mean increase in axial length was 0.15±0.05 mm
at 6 months and reached 0.58±0.12 mm at 36 months. Statistically
significant changes in mean axial length were observed over time,
highlighting a progressive increase in axial length over time
during OK lens wear (P<0.05; Fig.
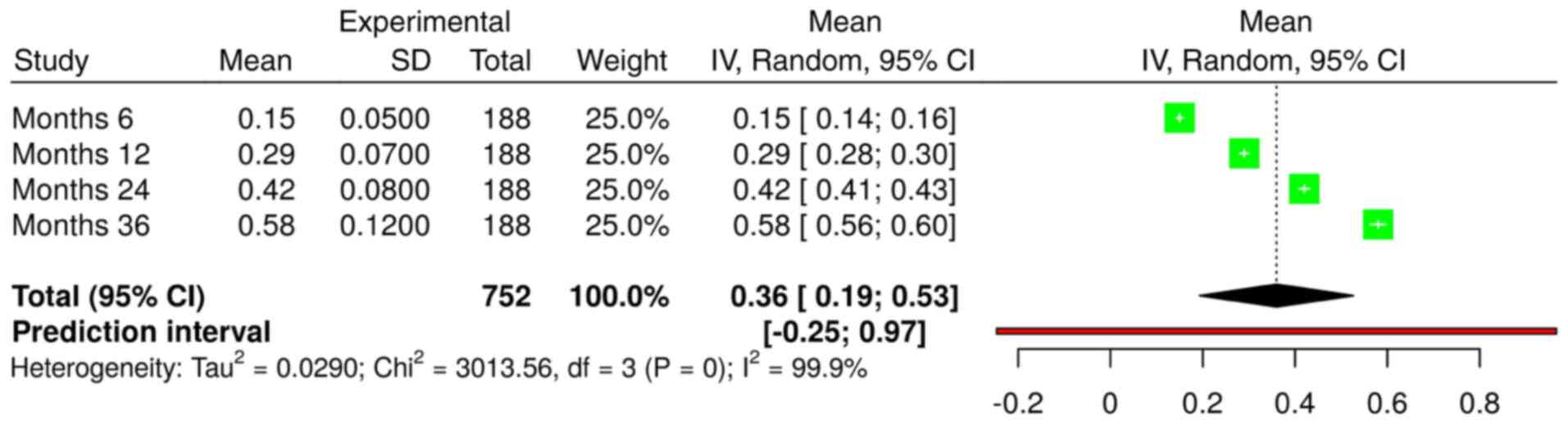
2). Evaluations of axial length changes were performed at 6,
12, 24 and 36 months for all 188 patients using a random effects
model with the inverse variance method (Fig. 3). The summarized mean raw (MRAW)
was 0.36 with a 95% confidence interval of 0.19-0.53. Significant
heterogeneity was detected (P<0.01), suggesting variable effects
in magnitude and/or direction. The I2 value indicates
that 99.9% of the variability among the four time points of
evaluation arises from heterogeneity over the time of OK use,
rather than random chance. The analysis confirmed that the axial
length increased over time.
Visual parameter changes
UDVA improved significantly from 0.48±0.22 to
0.12±0.12 logMAR by month 36, with a mean change of -0.36 logMAR
(P<0.01). The refractive error also improved by +0.43 D
(P<0.01) over this period. BCVA improved significantly from
0.08±0.25 to 0.05±0.01 during month 36. These results demonstrate
that OK lenses improved vision and effectively managed refractive
error (Table I).
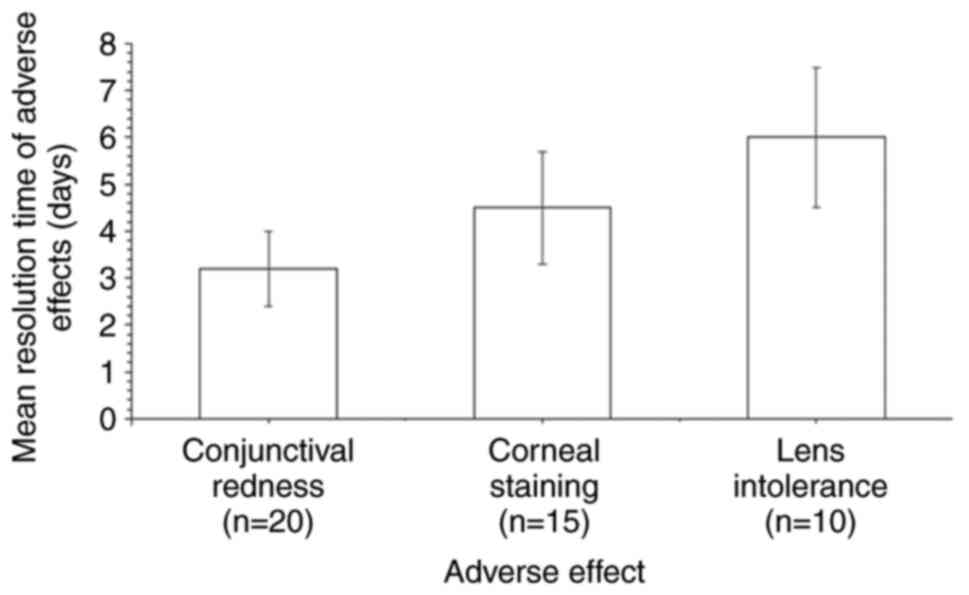
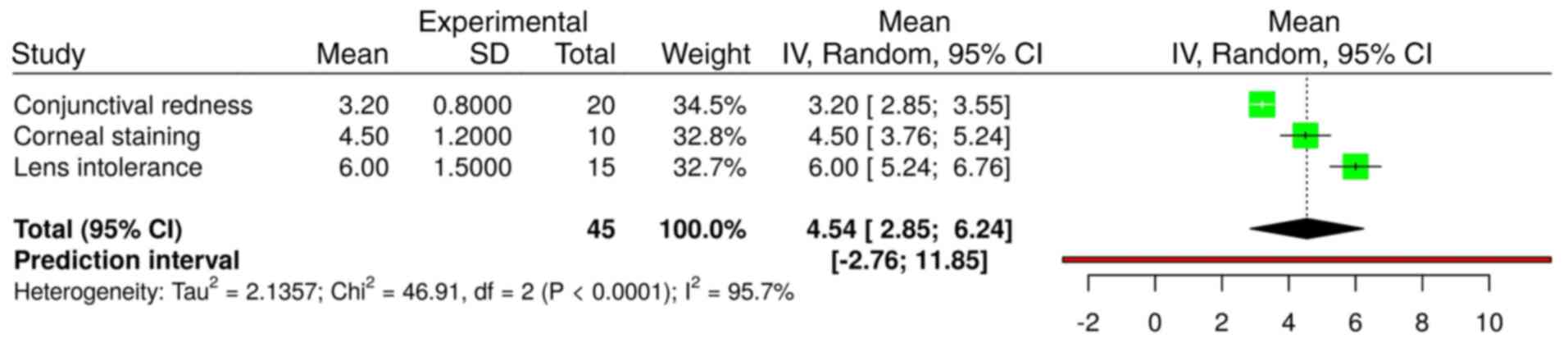
Adverse events during follow-up
Adverse events included corneal staining 15 (8.0%),
lens intolerance 10 (5.3%) and conjunctival redness 20 (10.6%)
(Fig. 4). The mean resolution time
of adverse effects ranged from 3.2 to 6.0 days. Although these
events were relatively infrequent, careful monitoring was necessary
to ensure their timely resolution. The mean resolution times of the
three adverse events were analyzed in a total of 45 patients
(Fig. 5) using a random effects
model with the inverse variance method. The summarized MRAW was
4.54 with a 95% confidence interval of 2.85-6.24. Significant
heterogeneity was detected (P<0.01), indicating inconsistency in
the magnitude and/or direction of resolution times. The
I2 value indicates that 95.7% of the variability in the
mean resolution time of the three adverse events was attributable
to heterogeneity rather than random chance.
Changes in axial length and refractive
error over the follow-up period
Paired t-test analysis revealed significant changes
in both axial length and refractive error over the 36-month
follow-up period (Table I). The
mean axial length increased from 24.12±0.63 mm at baseline to
24.70±0.12 mm at the 36-month follow-up, with a mean change of 0.58
mm (P<0.01). Similarly, refractive error improved from
-3.25±1.05 D at baseline to -2.82±0.18 D at 36 months, showing a
mean improvement of +0.43 D (P<0.01). These findings indicate
that OK lenses are associated with changes in structural and
refractive outcomes over the study period.
Independent factors influencing axial
length changes
A mixed-effects regression analysis provided
additional insights into the factors influencing changes in axial
length (Table II). Age was
inversely associated with axial elongation, with a coefficient (β)
of -0.02±0.01 (P<0.01), suggesting that younger participants
experienced greater increases in axial length. Baseline refractive
error was positively associated with axial elongation, with a
β-coefficient of 0.15±0.04 (P<0.01), indicating that children
with higher baseline myopia experienced greater axial growth.
Compliance level was a strong predictor of changes in axial length,
with high compliance associated with reduced elongation, with a
β-coefficient of -0.18±0.05 (P<0.01). These results highlight
the importance of baseline characteristics and adherence to OK lens
protocols on axial length progression.
 | Table IIIndependent factors influencing axial
length changes. |
Table II
Independent factors influencing axial
length changes.
| Predictor
variable | Coefficient β | Standard error | t-value | P-value |
|---|
| Age, years | -0.02 | 0.01 | -2.67 | <0.01 |
| Baseline refractive
error | 0.15 | 0.04 | 3.75 | <0.01 |
| Compliance
level | -0.18 | 0.05 | -3.6 | <0.01 |
Discussion
The findings of the present study demonstrated that
OK lenses effectively slowed axial elongation and enhanced
refractive outcomes in children with myopia over 36 months, with
the greatest benefits observed among those who consistently adhered
to the prescribed lens-wearing schedule. These results suggest that
OK lenses may be a valuable tool for mitigating myopia progression
and reducing the potential risk of long-term complications, such as
retinal detachment and glaucoma. Mixed-effects regression
highlighted the importance of considering individual factors, such
as age and baseline refractive error, when tailoring treatment
strategies. Future research should focus on the optimization of
lens designs, enhancement of adherence strategies, and
investigation of the long-term ocular health implications of
prolonged OK lens use.
Although numerous studies have demonstrated the
efficacy of OK in the management of myopia progression (16-19),
few have extensively studied its safety in pediatric populations
(17-21).
Previous studies suggest that the annual incidence of ocular
adverse events associated with OK lens use may be ~20% in children
(20-23).
However, a major limitation of these studies is that no
standardized definition exists for what constitutes an adverse
event, and safety assessments are often narrow in scope, being
confined to a limited range of complications. Given the frequent
use of OK lenses for myopia management (1), and their long-term application, which
often spans several years throughout childhood and adolescence
(4,21) and may extend into adulthood
(6), robust long-term safety
assessments are important.
Regarding adverse events and safety, the present
study revealed that 8.0% of participants experienced corneal
staining, 5.3% reported lens intolerance and 10.6% exhibited
conjunctival redness, with all adverse events resolving within a
mean of 3.2-6.0 days. These findings indicate a relatively low
incidence of adverse events, similar to that reported by
Santodomingo-Rubido et al (18), who reported an overall annual
adverse event rate of 13%, with corneal staining being the most
common issue, although the frequency of adverse events was higher
than that observed in the present study. Similarly, Hu et al
(24) reported a 22.7% incidence
of corneal adverse events over 1 year, which is also higher than
that in the present study. A major limitation of the present study
is that, unlike that of Hu et al (24), the present study did not evaluate
associations between adverse events and individual risk factors
such as age, spherical equivalent refraction or history of allergic
conjunctivitis. Future studies could stratify adverse events by
age, lens type and other relevant factors when identifying
potential risk factors.
The present study found a mean axial elongation of
0.58 mm over 36 months, with a slower progression among
participants with high compliance (0.35 mm) than in participants
with low compliance (0.72 mm). This is consistent with the findings
of Zhu et al (25), who
reported significantly slower axial elongation in patients treated
with OK (0.22 mm) compared with that in patients wearing
single-vision spectacles (0.35 mm) over 12 months. However, the
current study has a longer follow-up duration, and directly
evaluated compliance as a factor influencing axial length changes,
which Zhu et al (25) did
not examine.
The significant improvement in mean UDVA from 0.48
to 0.12 logMAR in the present study is consistent with the findings
of Hahn et al (26), who
reported significant improvements in UDVA and reductions in
spherical equivalent over 6 months of OK lens wear. However, Hahn
et al (26) also observed
higher-order aberrations (HOAs), particularly spherical
aberrations, which were not assessed in the present study.
Compliance emerged as a key determinant in the
present study, with high compliance associated with a significant
reduction in axial elongation. This is consistent with the findings
of Chang et al (27), who
emphasized the importance of parental knowledge and adherence to OK
care protocols for achieving favorable outcomes. While Chang et
al (27) focused on parental
compliance behaviors, the present study assessed compliance
directly among pediatric participants and its impact on clinical
outcomes.
Notably, the present study did not assess treatment
zone decentration (TZD) as a variable, unlike Chu et al
(28), who reported that
decentered OK lenses slowed axial elongation more effectively in
children with higher spherical equivalent refraction. This
represents a gap in the findings of the study, as TZD may influence
the efficacy of OK treatment.
The present study has an adequate sample size and
duration of follow-up, with a clear focus on safety outcomes in
patients based on 3 years of data. In addition, the topic is
important and relatively underexplored in the ophthalmological
literature, and the large study group and longitudinal design are
key strengths. However, the reliance on a single cohort may
restrict the generalizability of the findings. Although it was
designed as a prospective study, no parallel, crossover or matched
historical control group was included, which precludes the ability
to make direct comparisons or infer treatment-specific effects.
Also, no details of previous myopia progression were available,
which could have allowed within-subject comparisons to assess the
extent of the slowing achieved by treatment. Compliance and
adherence levels were self-reported, introducing potential
reporting bias. In addition, the analysis of adverse events may be
underpowered for the detection of rare complications such as
microbial keratitis. The study population comprised myopic children
presenting for vision correction and may not represent progressive
childhood myopes. Lastly, corneal biomechanical parameters, which
may have contributed to observed outcomes, were not assessed.
Based on these findings, it is recommended that OK
should be considered a valuable option for myopia management in
pediatric patients, particularly those with moderate myopia. To
maximize efficacy, healthcare providers should emphasize the
importance of lens wear compliance through regular monitoring and
patient education. In addition, future studies should aim to
include larger, more diverse populations and explore combinations
of OK with other myopia control strategies, such as atropine
therapy, to optimize outcomes. Long-term safety considerations
should also remain a priority in research and clinical
practice.
In conclusion, the present study demonstrated that
OK lenses are a highly effective intervention for the management of
pediatric myopia, significantly improving refractive error and
visual acuity over 3 years. The findings highlight the benefits of
OK in addressing structural and functional aspects of myopia.
Compliance emerged as a crucial factor influencing treatment
outcomes, with higher adherence to lens wear protocols associated
with significantly lower axial elongation rates. These results
underscore the importance of regular follow-up and robust patient
education to ensure the proper use of OK lenses.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL served as project administrator and contributed
to supervision, resources, methodology, validation and literature
review. RZ contributed to the investigation, resources,
visualization, methodology and literature review. YS contributed to
resources, conceptualization, methodology, visualization and
literature review. RL contributed to resources, methodology,
software, data curation, formal analyses, literature review and
supervision. All authors contributed to the drafting and editing of
the manuscript. YL and RL confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study adhered to the ethical principles of the
Declaration of Helsinki, and was approved by the Ethics Committee
of The First Affiliated Hospital of Anhui Medical University and
Anhui Women and Children's Medical Center (approval no. AMUfah1514;
January 15, 2020). Written informed consent was obtained from all
participants and their legal guardians after detailed explanation
of the study protocol, including the risks and benefits of OK lens
wear. Participants were regularly followed up to ensure the early
detection and management of any adverse events, and participants
retained the right to withdraw from the study at any time without
affecting their standard of care.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
The ORCIDs of the authors are as follows: YL,
0009-0008-0286-3762; RZ, 0000-0003-3472-3576; YS,
0009-0002-2720-9018; and RL, 0009-0005-6352-5642.
References
|
1
|
Jonas JB, Ang M, Cho P, Guggenheim JA, He
MG, Jong M, Logan NS, Liu M, Morgan I, Ohno-Matsui K, et al: IMI
prevention of myopia and its progression. Invest Ophthalmol Vis
Sci. 62(6)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bullimore MA and Johnson LA: Overnight
orthokeratology. Cont Lens Anterior Eye. 43:322–332.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nti AN and Berntsen DA: Optical changes
and visual performance with orthokeratology. Clin Exp Optom.
103:44–54. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang J, Lu X, Cheng Z, Zou D, Shi W and
Wang T: Alterations of conjunctival microbiota associated with
orthokeratology lens wearing in myopic children. BMC Microbiol.
23(397)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wan K, Yau HT, Cheung SW and Cho P:
Corneal thickness changes in myopic children during and after
short-term orthokeratology lens wear. Ophthalmic Physiol Opt.
41:757–767. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lee SS, Lingham G, Sanfilippo PG, Hammond
CJ, Saw SM, Guggenheim JA, Yazar S and Mackey DA: Incidence and
progression of myopia in early adulthood. JAMA Ophthalmol.
140:162–169. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sankaridurg P, Berntsen DA, Bullimore MA,
Cho P, Flitcroft I, Gawne TJ, Gifford KL, Jong M, Kang P, Ostrin
LA, et al: IMI 2023 digest. Invest Ophthalmol Vis Sci.
64(7)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang Y, Wu Q, Pan W, Wen L, Luo Z, Wu H,
Ran G, Yang Z and Li X: Characteristics of the ocular surface in
myopic child candidates of orthokeratology lens wear. Ophthalmol
Ther. 12:3067–3079. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hung LL, Liao LL, Chen HJ, Lin HL and
Chang LC: Factors associated with follow-up visits in parents with
myopic children wearing orthokeratology lens. J Nurs Res.
30(e244)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Qin J, Qing H, Ji N, Lyu T, Ma H, Shi M,
Yu S, Ma C and Fu A: Changes in axial length in anisometropic
children wearing orthokeratology lenses. Front Med (Lausanne).
10(1266354)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen Y, Liu M, Lu H, Zhang Y, Luo D, Pan
H, Wan C, Szentmáry N and Shi L: Impact of overnight wear of
orthokeratology lens on thickness of tear film lipid layer in
children with myopia. Klin Monbl Augenheilkd. 240:1151–1157.
2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhu Q and Zhao Q: Short-term effect of
orthokeratology lens wear on choroidal blood flow in children with
low and moderate myopia. Sci Rep. 12(17653)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li D, Ye Q and Li C: Trends in noninvasive
ocular nanoparticle drug delivery: A bibliometric analysis
(2004-2023). Biomol Biomed. 25:1949–1960. 2025.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ashara KC and Shah KV: The study of
chloramphenicol for ophthalmic formulation. IJSRR. 7 (Suppl
1):S173–S178. 2018.
|
|
15
|
Fekete JT and Gyorffy B:
MetaAnalysisOnline.com: An online tool for the rapid meta-analysis
of clinical and epidemiological studies. J Med Internet Res.
27(e64016)2025.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Zhang Y, Sun X and Chen Y: Controlling
anisomyopia in children by orthokeratology: A one-year randomised
clinical trial. Cont Lens Anterior Eye. 46(101537)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wu H, Peng T, Zhou W, Huang Z, Li H, Wang
T, Zhang J, Zhang K, Li H, Zhao Y, et al: Choroidal vasculature act
as predictive biomarkers of Long-term ocular elongation in myopic
children treated with orthokeratology: A prospective cohort study.
Eye Vis. 10(27)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Santodomingo-Rubido J, Cheung SW and
Villa-Collar C: ROMIO/MCOS/TO-SEE Groups. The safety of
orthokeratology contact lens wear in slowing the axial elongation
of the eye in children. Cont Lens Anterior Eye.
48(102258)2025.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhong Y, Ke L, Qiong W and Liu F:
Orthokeratology lens for management of myopia in anisometropic
children: A contralateral study. Cont Lens Anterior Eye. 43:40–43.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang J, Li Z, Cheng Z, Wang T and Shi W:
Comparison of the clinical efficacy of orthokeratology and 0.01%
atropine for retardation of myopia progression in myopic children.
Cont Lens Anterior Eye. 47(102094)2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li X, Huang Y, Zhang J, Ding C, Chen Y,
Chen H and Bao J: Treatment zone decentration promotes retinal
reshaping in Chinese myopic children wearing orthokeratology
lenses. Ophthalmic Physiol. 42:1124–1132. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhu MJ, Ding L, Du LL, Chen J, He XG, Li
SS and Zou HD: Photopic pupil size change in myopic orthokeratology
and its influence on axial length elongation. Int J Ophthalmol.
15:1322–1330. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhu S, Song Y, Yang B, Wang X, Ma W, Dong
G and Liu L: The relationship between accommodative and binocular
function with myopia progression in myopic children undergoing
orthokeratology. Cont Lens Anterior Eye. 47(102171)2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hu P, Zhao Y, Chen D and Ni H: The safety
of orthokeratology in myopic children and analysis of related
factors. Cont Lens Anterior Eye. 44:89–93. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhu Q, Yin J, Li X, Hu M, Xue L, Zhang J,
Zhou Y, Zhang X, Zhu Y and Zhong H: Effects of long-term wear and
discontinuation of orthokeratology lenses on the eyeball parameters
in children with myopia. Int J Med Sci. 20:50–56. 2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hahn IK, Lee D, Lee DH, Lee H, Tchah H and
Kim JY: Serially checked spherical aberration can evaluate the
anti-myopia effect of orthokeratology lens in children. J Pers Med.
12(1686)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chang LC, Sun C and Liao LL: Compliance
with orthokeratology care among parents of young children in
Taiwan. Cont Lens Anterior Eye. 44(101427)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chu M, Zhao Y, Hu P, Chen D, Yu Y and Ni
H: Is orthokeratology treatment zone decentration effective and
safe in controlling myopic progression? Eye Contact Lens.
49:147–151. 2023.PubMed/NCBI View Article : Google Scholar
|