Introduction
Pulmonary fibrosis (PF) is a chronic, fatal disease
characterized by progressive lung tissue scarring and functional
decline, ultimately leading to impaired gas exchange. The core
pathological mechanisms involve aberrant fibroblast activation,
excessive extracellular matrix deposition, persistent oxidative
stress and chronic inflammation (1,2).
Idiopathic PF, the most common form of PF, has a median survival of
only 2.5-3.5 years and to the best of our knowledge, there is no
curative treatment at present (3).
Present clinical therapies, such as pirfenidone and nintedanib, can
only slow disease progression but are associated with side effects,
including gastrointestinal disturbances and hepatic toxicity
(4). Given these limitations,
naturally derived compounds such as quercetin have garnered notable
translational interest due to their multi-target mechanisms and
favorable safety profiles. Therefore, exploring naturally derived
compounds with favorable safety profiles to intervene in the
fibrotic process has become a promising area of research.
Quercetin (3,5,7,3',4'-pentahydroxyflavone) is a
natural flavonoid compound found in plants such as apples, onions
and tea and in Traditional Chinese Medicine, is known for its
multifaceted biological activities (5,6). In
recent years, an increasing number of studies have demonstrated the
potential therapeutic effects of quercetin in PF. Research has
shown that quercetin can inhibit the onset and progression of PF
through numerous mechanisms, such as suppressing the production of
inflammatory cytokines, promoting apoptosis and inhibiting the
expression and activity of TGF-β1 (7-9).
Furthermore, quercetin exhibits a good safety profile, is low-cost
and readily accessible (10),
making it a notable area of research interest. Existing studies
(11,12) primarily focus on animal models and
in vitro mechanisms, while clinical translational evidence
remains insufficient. In addition, while in vitro and animal
studies provide valuable evidence, the translational potential of
quercetin may be affected by the fragmented nature of preclinical
data and methodological heterogeneity.
To address the aforementioned issues, the present
meta-analysis consolidates preclinical evidence to quantitatively
assess the efficacy of quercetin in improving outcomes related to
PF. By accumulating data on fibrotic markers, inflammatory
cytokines and oxidative stress parameters, the present review
systematically evaluates the therapeutic effects of quercetin in
PF. The findings aim to provide a theoretical basis for clinical
trials exploring quercetin as an adjunctive treatment for PF,
advancing its experimental potential toward clinical efficacy.
Materials and methods
Literature search strategy
A systematic search was performed across PubMed
(https://pubmed.ncbi.nlm.nih.gov/), Web
of Science (https://www.webofscience.com/), Embase (https://www.embase.com/), Ovid (https://ovidsp.ovid.com/) and Cochrane Library
(https://www.cochranelibrary.com/)
databases between inception and January 1, 2025. Following the
population, intervention, comparison, outcomes and study (PICOS)
framework (13), the search
strategy was designed using the keywords ‘quercetin’ and ‘pulmonary
fibrosis’, including both subject headings and free terms. The
detailed search strategy is provided in Tables SI, SII, SIII, SIV and SV. The search approach included
keywords, full-text searches and Medical Subject Headings, with no
restrictions on publication type, sample size, study design or
methods of exposure or outcome measurement. In addition, gray
literature was manually searched using Google Scholar (https://scholar.google.com/). The present study is a
secondary analysis and did not require ethical approval for animal
or human experiments.
Inclusion and exclusion criteria
Inclusion criteria were established based on the
PICOS framework and were as follows: i) Population [animal models
of PF (rodents)]; ii) intervention (monotherapy with quercetin or
its derivatives); iii) comparator (placebo or blank control); iv)
outcome; and v) study design (randomized or non-randomized
controlled trials with full text available).
Several outcome measures were considered suitable.
The outcome measures assessed were: i) Basic characteristics [body
weight and lung index (lung index=lung weight (mg)/body weight
(g)]; ii) fibrosis markers [hydroxyproline content, Ashcroft score
(14), α-smooth muscle actin
(α-SMA) and collagen I (Col I)]; iii) inflammatory cytokines
(TNF-α, IL-6, IL-1β, TGF-β1, total cell count, leukocyte count,
neutrophils, lymphocytes, eosinophils and macrophages); and iv)
oxidative stress indictors [superoxide dismutase (SOD) activity,
malondialdehyde (MDA) levels, glutathione (GSH), catalase (CAT),
nitric oxide (NO) and thiobarbituric acid-reactive substances
(TBARS)].
Exclusion criteria for article screening were as
follows: i) Exclusively in vitro studies, clinical trials,
reviews or conference abstracts; and ii) incomplete data (for
example, charts with unannotated values), duplicate publications or
studies where the full text was unavailable.
Literature screening and data
extraction
For screening, two independent researchers conducted
separate literature searches using the predefined search strategy.
The search results were imported into NoteExpress software (version
4.0.0.9855; Beijing Aegean Software Co., Ltd.) and checked for
duplicates. Next, the titles and abstracts were initially screened
according to the inclusion and exclusion criteria, after which the
full texts of the selected studies were read for further screening.
The basic information of the studies that were ultimately included
was extracted. Any disagreements were resolved through discussion
or consultation with a third-party expert to reach a consensus.
Data extraction followed the guidelines outlined in the Preferred
Reporting Items for Systematic Reviews and Meta-Analyses (15) statement. The following data were
extracted from the studies: i) Study characteristics, authors,
publication year, species/strain, sex, age, sample size and
modeling methods; ii) intervention details, quercetin dosage,
administration route and intervention duration; iii) outcome
measures, mean values, SD and sample size (n) for both experimental
and control groups; and iv) methodological quality, information on
randomization, blinding and allocation concealment, among others.
If different dosages were used in the studies, the highest dosage
results were extracted. When results were measured at multiple time
points, data from the longest duration were recorded. If the data
were presented solely in graphical form, the authors were contacted
to obtain the raw data. If no response was received, numerical
values were extracted using Engauge Digitizer software (version
12.1; Engauge Open Source Developers). If different values were
obtained by the two researchers, the mean of the value was
calculated to produce a single estimate for analysis, thereby
reducing measurement error.
Quality assessment/bias risk
analysis
The risk of bias in animal studies was assessed
using the SYRCLE risk of bias tool (16), which includes 10 categories: i)
Sequence generation; ii) baseline characteristics; iii) allocation
concealment; iv) random housing; v) blinding implementation; vi)
random outcome assessment; vii) blinded outcome assessment; viii)
incomplete outcome data; ix) selective outcome reporting; and x)
other sources of bias. Each category was rated as high, unclear or
low risk of bias. Quality assessment was performed by three
researchers and any discrepancies in the ratings were resolved
through discussion.
Statistical analysis
Data analysis was performed using RevMan (version
5.4; The Cochrane Collaboration) and Stata (version 17.0; StataCorp
LP) software. Continuous variables are expressed as standardized
mean differences (SMDs) with 95% CI. Heterogeneity was assessed
using the I2 statistic (with I2 >50%
indicating significant heterogeneity). A random-effects model was
applied when I2 >50%, while a fixed-effects model was
used when I2 ≤50%. To explore potential sources of
heterogeneity, an exploratory meta-regression analysis was
performed on all available data, incorporating study
characteristics as covariates. Publication bias was evaluated both
visually using funnel plots and statistically using Egger's linear
regression test and Begg's rank correlation test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Search results
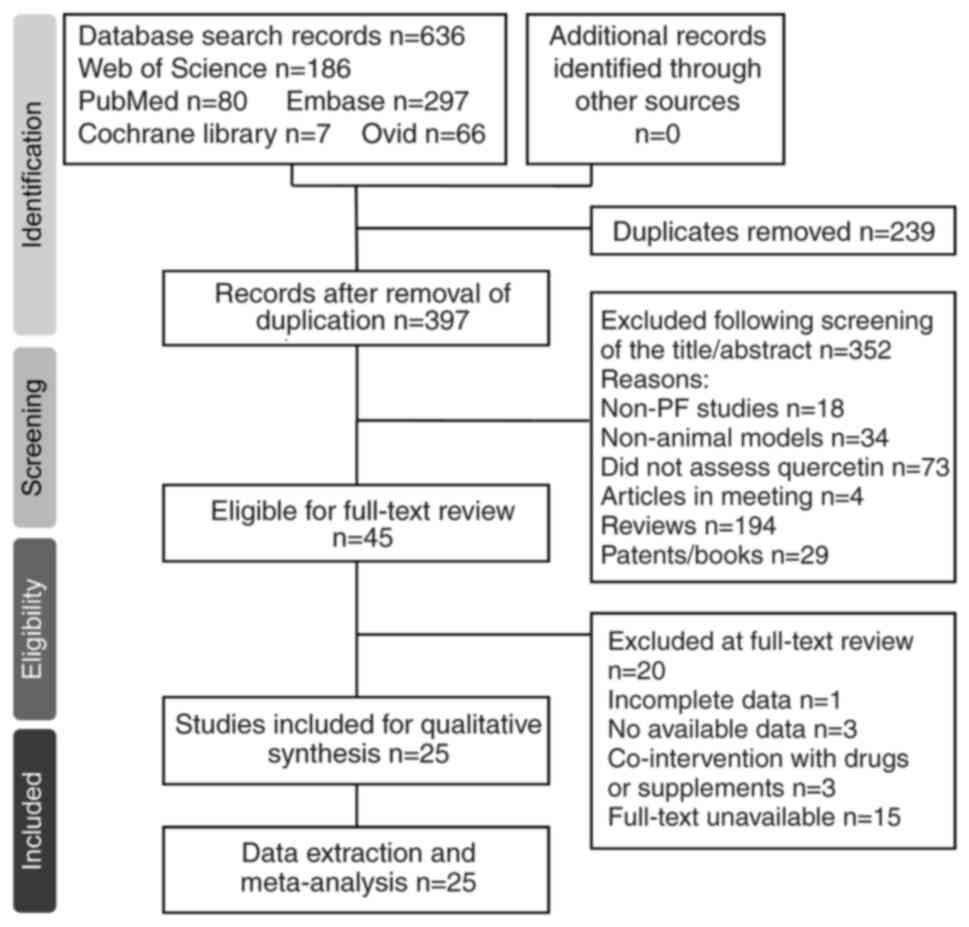
Literature searches yielded a total of 636 articles
(PubMed, 80; Web of Science, 186; Embase, 297; Cochrane Library, 7;
Ovid, 66). After removing 23 duplicated articles, 613 articles were
screened based on titles and abstracts, resulting in the exclusion
of 352 studies that did not meet the inclusion criteria. Full-text
evaluation was performed on 45 articles, leading to the exclusion
of 20 studies due to irrelevance or incomplete data. Manual
searching on Google Scholar did not reveal any additional
qualifying studies. Ultimately, 25 studies (17-41)
were included in the present meta-analysis (Fig. 1). The basic characteristics of the
included studies are shown in Table
I.
 | Table IBasic characteristics of the included
studies. |
Table I
Basic characteristics of the included
studies.
| First author,
year | Country | Age | Total, n | Species; model | PF induction
method | Quercetin dose
(route) | Duration | Outcome | (Refs.) |
|---|
| Baowen et
al, 2010 | China | N | 40 | Rats;
Sprague-Dawley | Bleomycin
(intratracheal) | 5 mg/kg
(intravenous) | 28 days | TNF-α, IL-1β, IL-6,
total cell numbers, lymphocytes, neutrophils, macrophages and
hydroxyproline | (17) |
| Mehrzadi et
al, 2021 | Iran | N | 20 | Male rats;
Wistar | Bleomycin
(intratracheal) | 75 mg/kg (oral
gavage) | 28 days | TNF-α, IL-6, GSH,
CAT, NO, TBARS, Ashcroft score, lung index and hydroxyproline | (18) |
| Martinez et
al, 2008 | Brazil | N | 28 | Male hamsters;
Mesocricetus auratus | Bleomycin
(intratracheal) | 30 mg/kg
(intraperitoneal) | 14 days | GSH, TBARS and
hydroxyproline | (19) |
| Verma et al,
2013 | India | 10-12 weeks | 20 | Male rats;
Wistar | Bleomycin
(intratracheal) | 100 mg/kg
(oral) | 20 days | TNF-α, CAT, SOD,
MDA, weight, hydroxyproline, total cell numbers, neutrophils,
macrophages, eosinophil and lymphocytes | (20) |
| Impellizzeri et
al, 2015 | Italy | N | 20 | Male mice;
CD1(ICR) | Bleomycin
(intratracheal) | 10 mg/kg
(oral) | 7 days | Ashcroft score,
total cell numbers, weight, neutrophils, lymphocytes, macrophages,
eosinophil and leucocytes | (21) |
| Park et al,
2010 | Korea | N | 8 | Male rats;
Sprague-Dawley | Paraquat
(intratracheal) | 50 mg/kg
(intraperitoneal) | 14 days | GSH, MDA and
NO | (22) |
| Taslidere et
al, 2014 | Turkey | 3-4 months | 14 | Albino female rats;
Wistar | CCl4
(intraperitoneal) | 25 mg/kg
(intraperitoneal) | 10 days | GSH, MDA and
CAT | (23) |
| Geng et al,
2022 | China | 6-8 weeks | 20 | Female mice;
C57BL/6 | SiO2
(intratracheal) | 100 mg/kg (oral
gavage) | 28 days | TNF-α, IL-1β, IL-6
and Col I | (24) |
| Geng et al,
2023 | China | 8 weeks | 10 | Male mice;
C57BL/6 | SiO2
(intratracheal) | 100 mg/kg
(intraperitoneal) | 28 days | α-SMA and Col
I | (25) |
| Hohmann et
al, 2019 | America | >12 months | 14 | Male and female
mice; C57BL/6 | Bleomycin
(intratracheal) | 30 mg/kg
(intraperitoneal) | 21 days | Weight and
hydroxyproline | (26) |
| Wu et al,
2024 | China | 8 weeks | 10 | Female rats;
Wistar | Bleomycin
(intratracheal) | 75 mg/kg
(oral) | 28 days | α-SMA, Col I,
hydroxyproline and weight | (27) |
| Liu et al,
2013 | China | 6-8 weeks | 46 | Female mice;
C57BL/6 | Cobalt-60γ
radiation (16 Gy; thoracic irradiation) | 5 mg/kg
(intraperitoneal) | 24 weeks | α-SMA, TNF-α,
TGF-β1, SOD, MDA, total cell numbers, hydroxy proline and Ashcroft
score | (28) |
| Verma et al,
2022 | India | 8-10 weeks | 12 | Female mice;
C57BL/6 | γ radiation (12 Gy)
(thoracic irradiation) | 10 mg/kg
(intramuscularly) | 16 weeks | α-SMA, TNF-α,
IL-1β, IL-6, TGF-β1, MDA, NO, lung index, macrophages, total cell
numbers, Ashcroft score and leucocytes | (29) |
| Boots et al,
2020 | Germany | 10-12 weeks | 23 | Male and female
mice; C57BL/6 | Bleomycin
(pharyngeal administration) | 200 mg/kg
(oral) | 21 days | TNF-α and
weight | (30) |
| Wei et al,
2016 | China | 18 weeks | 40 | Male rats;
Sprague-Dawley | Bleomycin
(intraperitoneal) | 3 mg/kg
(intraperitoneal) | 36 days | Ashcroft score,
hydroxyproline and MDA | (31) |
| Oka et al,
2019 | Nigeria | N | 12 | Female rats;
Wistar | Amiodarone
(intratracheal) | 20 mg/kg
(oral) | 21 days | GSH, CAT, total
cell numbers and macrophages | (32) |
| Qin et al,
2017 | China | 5-6 weeks | 24 | Male rats;
Wistar | X-ray (15Gy)
(pulmonary apex irradiation) | 100 mg/kg
(inhaled) | 4 months | Leucocytes | (33) |
| Ding et al,
2024 | China | 6-8 weeks | 12 | Male mice;
C57BL/6 | PM2:5
(intratracheal) | 50 mg/kg (oral
gavage) | 60 days | TNF-α, IL-1β, IL-6,
TGF-β1, Col I, Ashcroft score and lung index | (34) |
| Malayeri et
al, 2016 | Iran | N | 20 | Male rats;
Sprague-Dawley | Bleomycin
(intratracheal) | 50 mg/kg
(intraperitoneal) | 28 days | TNF-α | (35) |
| El-Sayed et
al, 2009 | Egypt | N | 14 | Albino male rats;
N | Paraquat
(intraperitoneal) | 50 mg/kg
(p.o.) | 21 days | GSH, SOD, NO and
TBARS | (36) |
| Fang et al,
2023 | China | 6 weeks | 20 | Male mice;
C57BL/6 | 10 mg/ml OVA and
alum adjuvant (intraperitoneal) OVA (inhaled) | 30 mg/kg
(gavage) | 21 days | TGF-β1, α-SMA,
total cell numbers, neutrophils, lymphocytes, eosinophil and
macrophages | (37) |
| Yao et al,
2023 | China | N | 20 | Rats;
Sprague-Dawley | Silica suspension
(intratracheal) | 2 mg/kg
(intratracheal) | 28 days | TNF-α, IL-1β,
α-SMA, SOD, weight and lung index | (38) |
| Yang et al,
2020 | China | 6-8 weeks | 24 | Male mice;
BALB/c | Cigarette smoke
(inhaled) | 50 mg/kg
(intraperitoneal) | 12 weeks | TNF-α, IL-1β, total
cell numbers, neutrophils and macrophages | (39) |
| Zhang et al,
2024 | China | 6-8 weeks | 12 | Male mice;
C57BL/6 | Bleomycin
(intratracheal) | 50 mg/kg
(intragastric administration) | 3 weeks | Ashcroft score and
hydroxyproline | (40) |
| Toker et al,
2024 | Turkey | N | 16 | Albino male rats;
Wistar | Bleomycin
(intratracheal) | 50 mg/kg
(intraperitoneal) | 21 days | α-SMA | (41) |
Basic characteristics of included
studies
A total of 25 studies were included in the analysis,
performed across 11 countries. Among these, 12 studies were from
China, 2 each from India, Iran and Turkey and 1 each from the
United States, Brazil, Egypt, Germany, Italy, South Korea and
Nigeria. All studies were preclinical controlled trials utilizing
rat models. Of these, 24 studies used quercetin as the sole
intervention, while one study employed a derivative of quercetin.
The experimental animals included male rats (60%), female rats
(24%), mixed sex (8%) and those with unspecified sex (8%). The age
of the animals varied notably, ranging from 6 weeks to >12
months, with 10 studies not reporting the age. The primary outcome
measures included body weight (5 studies), lung index (4 studies),
fibrosis markers (hydroxyproline content in 9 studies, α-SMA in 6
studies and COL I in 4 studies), histopathological scores (Ashcroft
score in 7 studies), inflammatory markers (TNF-α in 11 studies,
IL-1β in 6 studies and IL-6 in 5 studies), inflammatory cell counts
(total cell count in 7 studies, macrophage count in 7 studies,
neutrophil count in 5 studies, lymphocyte count in 4 studies,
eosinophil count in 3 studies and white blood cell count in 3
studies) and oxidative stress markers (MDA levels in 7 studies, GSH
levels in 6 studies, SOD and CAT enzyme activities in 4 studies
each and TBARS in 3 studies).
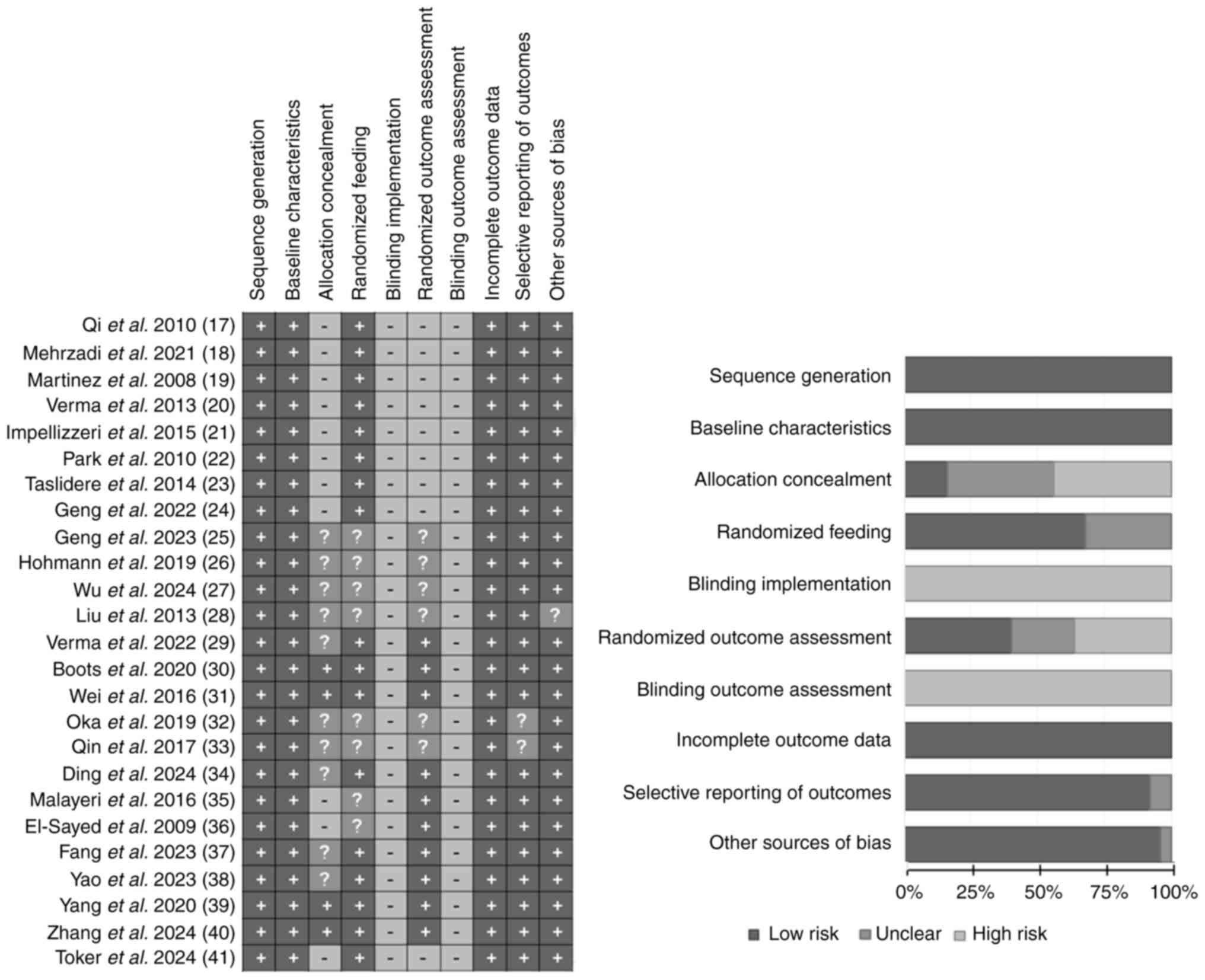
Quality assessment of the studies
Among the 25 studies, the assessment results for
sequence generation, baseline characteristics, incomplete outcome
data, selective outcome reporting and other sources of bias were
generally favorable, with 25, 25, 25, 23 and 24 studies rated as
‘low risk of bias’, respectively. No studies were scored as ‘high
risk of bias’ for random housing. However, the assessments for
blinding and blinding of outcome assessment were poor, with all 25
studies rated as ‘high risk of bias’. Overall, the studies
performed well regarding randomization and outcome data, but there
was notable bias in the implementation of blinding. The specific
findings are summarized in Fig. 2.
Due to the nature of study subjects and interventions, blinding of
both participants and researchers was difficult and thus most of
the studies did not report implementing double blinding.
Research results
The present meta-analysis demonstrated that
quercetin supplementation elicited notable changes across multiple
physiological domains. As summarized in Table II, a significant increase in body
weight was observed in the quercetin group compared with the
control group (n=5; SMD=1.78; 95% CI, 0.72 to 2.84;
I2=73%; P=0.0010). Similarly, lung index values were
significantly reduced following quercetin intervention (n=4;
SMD=-1.55; 95% CI, -3.04 to -0.05; I2=83%; P=0.04).
 | Table IIResults of the meta-analysis for each
outcome indicator. |
Table II
Results of the meta-analysis for each
outcome indicator.
| | Heterogeneity test
results | | Meta-analysis
results |
|---|
| Outcome
indicator | I2,
% | P-value | Effect models | SMD | 95% CI | P-value |
|---|
| Weight | 73 | 0.005 | Random | 1.78 | (0.72, 2.84) | 0.001 |
| Lung index | 83 | 0.0005 | Random | -1.55 | (-3.04, -0.05) | 0.040 |
| TBARS | 9 | 0.330 | Fixed | -1.15 | (-1.69, -0.61) | <0.0001 |
| Ashcroft score | 73 | 0.001 | Random | -2.20 | (-3.21, -1.18) | <0.0001 |
| Hydroxyproline | 75 | <0.0001 | Random | -2.05 | (-2.91, -1.18) | <0.00001 |
| Col I | 0 | 0.710 | Fixed | -1.77 | (-2.85, -0.69) | 0.001 |
| α-SMA | 53 | 0.060 | Random | -2.25 | (-3.17, -1.32) | <0.00001 |
| TNF-α | 80 | <0.0001 | Random | -1.73 | (-2.65, -0.82) | 0.0002 |
| IL-1β | 0 | 0.410 | Fixed | -2.77 | (-3.55, -2.00) | <0.00001 |
| IL-6 | 0 | 0.410 | Fixed | -1.45 | (-2.07, -0.83) | <0.00001 |
| TGF-β1 | 0 | 0.980 | Fixed | -2.68 | (-3.58, -1.78) | <0.00001 |
| GSH | 85 | <0.00001 | Random | 1.93 | (0.52, 3.34) | 0.007 |
| CAT | 41 | 0.170 | Fixed | 1.99 | (1.30, 2.68) | <0.00001 |
| SOD | 0 | 0.690 | Fixed | 2.36 | (1.60, 3.12) | <0.00001 |
| MDA | 58 | 0.030 | Random | -2.56 | (-3.46, -1.66) | <0.00001 |
| NO | 53 | 0.090 | Random | -2.42 | (-3.63, -1.21) | <0.0001 |
| Neutrophils | 89 | <0.00001 | Random | -3.73 | (-6.50, -0.95) | 0.009 |
| Lymphocytes | 80 | 0.002 | Random | -0.74 | (-2.20, 0.73) | 0.320 |
| Macrophages | 82 | <0.00001 | Random | -1.85 | (-3.36, -0.35) | 0.020 |
| Eosinophil | 79 | 0.009 | Random | -1.66 | (-3.25, -0.06) | 0.040 |
| Total cell
numbers | 37 | 0.150 | Fixed | -1.32 | (-1.87, -0.78) | <0.00001 |
| Leucocytes | 77 | 0.010 | Random | -2.33 | (-3.89, -0.77) | 0.003 |
Effect of quercetin on
fibrosis-related markers
Regarding fibrosis-related markers, quercetin
administration resulted in marked improvements. Notably,
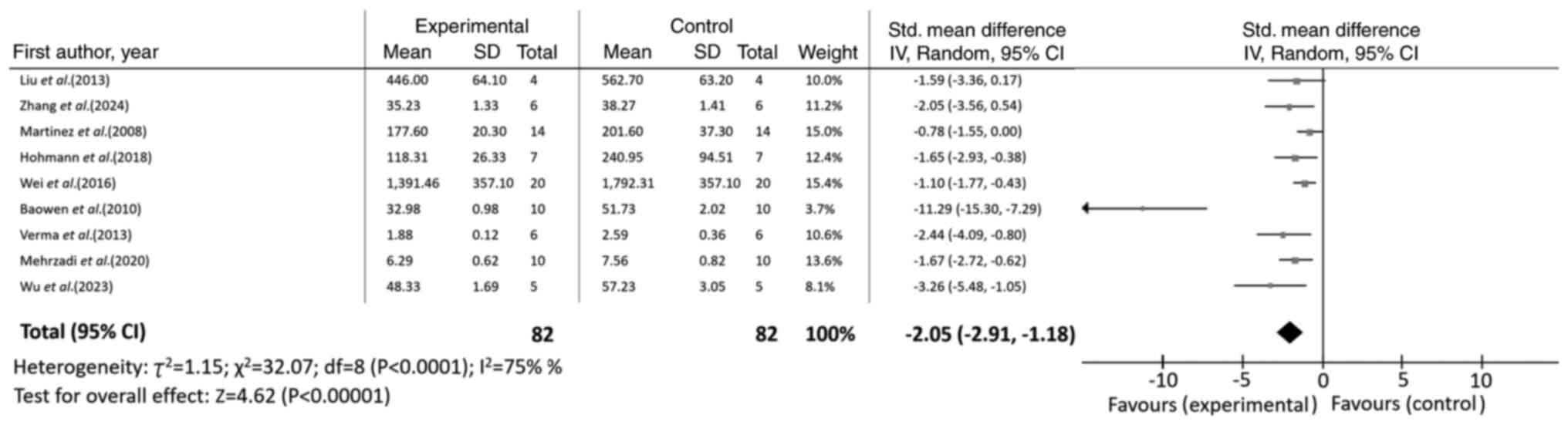
hydroxyproline levels were significantly decreased (n=9; SMD=-2.05;
95% CI, -2.91 to -1.18; I2=75%; P<0.00001), as shown
in Fig 3. Consistently, the
Ashcroft score was also significantly lower in the quercetin group
(n=7; SMD=-2.20; 95% CI, -3.21 to -1.18; I2=73%;
P<0.0001). Furthermore, expression levels of Col I (n=4;
SMD=-1.77; 95% CI, -2.85 to -0.69; I2=0%; P=0.001) and
α-SMA (n=6; SMD=-2.25; 95% CI, -3.17 to -1.32; I2=53%;
P<0.00001) were significantly suppressed, further supporting the
anti-fibrotic effect of quercetin (Table II).
Effect of quercetin on inflammatory
markers
Analysis of inflammatory parameters revealed notable
modulation by quercetin, which was evaluated through
pro-inflammatory cytokines and inflammatory cells. The quercetin
group exhibited significantly lower levels of key pro-inflammatory
cytokines, including TNF-α (n=11; SMD=-1.73; 95% CI, -2.65 to
-0.82; I2=80%; P=0.0002), IL-1β (n=6; SMD=-2.77; 95% CI,
-3.55 to -2.00; I2=0%; P<0.00001, IL-6 (n=5;
SMD=-1.45; 95% CI, -2.07 to -0.83; I2=0%; P<0.00001)
and TGF-β1 (n=4; SMD=-2.68; 95% CI, -3.58 to -1.78;
I2=0%; P<0.00001) (Table II).
With regards to inflammatory cell infiltration,
quercetin supplementation significantly reduced counts of
neutrophils (n=5; SMD=-3.73; 95% CI, -6.50 to -0.95;
I2=89%; P=0.009), macrophages (n=7; SMD=-1.85; 95% CI,
-3.36 to -0.35; I2=82%; P=0.02), eosinophils (n=3;
SMD=-1.66; 95% CI, -3.25 to -0.06; I2=79%; P=0.04),
leukocytes (n=3; SMD=-2.33; 95% CI, -3.89 to -0.77;
I2=77%; P=0.003) and total cells (n=7; SMD=-1.32; 95%
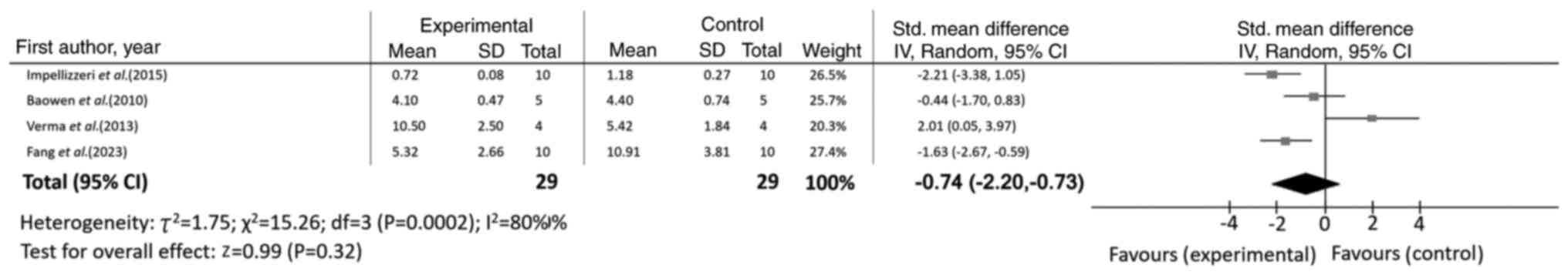
CI, -1.87 to -0.78; I2=37%; P<0.00001) (Table II). However, no significant effect
was observed on lymphocyte count (n=4; SMD=-0.74; 95% CI, -2.20 to
0.73; I2=80%; P=0.32), as illustrated in Fig. 4.
Effect of quercetin on oxidative
stress markers
Quercetin supplementation significantly alleviated
oxidative stress, as evidenced by the increased activities of
antioxidant enzymes. Specifically, SOD activity was significantly
enhanced (n=4; SMD=2.36; 95% CI, 1.60 to 3.12; I2=0%;
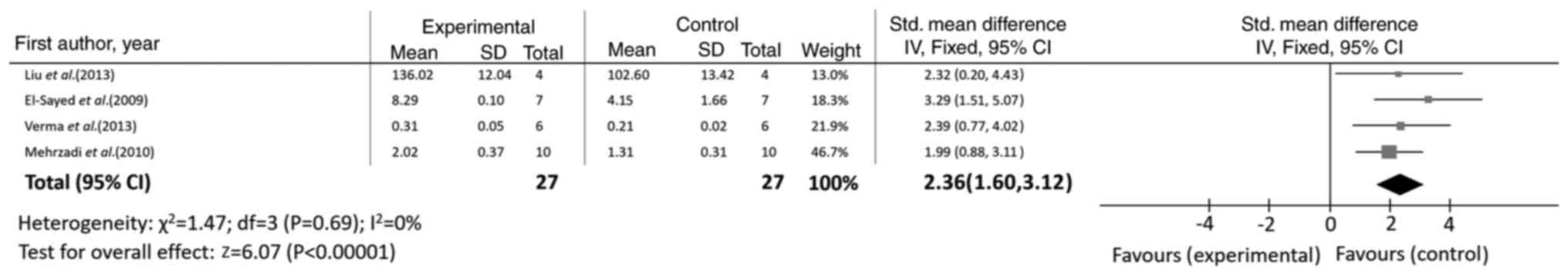
P<0.00001), as shown in Fig. 5.
CAT (n=4; SMD=1.99; 95% CI, 1.30 to 2.68; I2=41%;
P<0.00001) activity and GSH levels were also significantly
increased (n=6; SMD=1.93; 95% CI, 0.52 to 3.34; I2=85%;
P=0.007) (Table II).
Conversely, quercetin significantly reduced the
levels of biomarkers of oxidative damage, including MDA (n=7;
SMD=-2.56; 95% CI, -3.46 to -1.66; I2=58%;
P<0.00001), NO (n=4; SMD=-2.42; 95% CI, -3.63 to -1.21;
I2=53%; P<0.0001) and TBARS (n=3; SMD=-1.15; 95% CI,
-1.69 to -0.61; I2=9%; P<0.0001) (Table II).
Meta-regression analysis for sources
of heterogeneity
To explore potential sources of heterogeneity across
the included studies, a meta-regression analysis was performed
using four covariates: i) Animal model type (‘Model’); ii) fibrosis
induction method (‘Pfinductiod’); iii) quercetin dosage
(‘Quercetindose’); and i) intervention duration (‘Duration’).
The results indicated that quercetin dosage and
intervention duration were the most influential factors
contributing to heterogeneity across multiple outcome measures
(Table III). Specifically,
higher quercetin dosage was significantly associated with increased
levels of the antioxidant marker GSH [unstandardized regression
coefficients (coef.)=0.107; P=0.035] and lymphocyte count
(coef.=0.040; P=0.035), decreased total inflammatory cell count
(coef.=-0.025; P=0.027) and leukocyte cell count (coef.=-0.030;
P=0.002). A longer intervention duration was significantly
associated with increased CAT activity (coef.=0.101; P=0.044) and
elevated GSH levels (coef.=0.228; P=0.017).
 | Table IIIMeta-regression analysis of potential
moderators for various outcome measures. |
Table III
Meta-regression analysis of potential
moderators for various outcome measures.
| Outcome
measure | Moderator | Coefficient | SE | z-value | P-value | 95% CI |
|---|
| α-SMA | Model | -0.171 | 0.342 | -0.50 | 0.618 | -0.841 to
0.500 |
| | Pfinductiod | -0.293 | 0.273 | -1.07 | 0.284 | -0.829 to
0.243 |
| | Quercetindose | -0.009 | 0.020 | -0.46 | 0.642 | -0.048 to
0.030 |
| | Duration | 0.018 | 0.012 | 1.54 | 0.124 | -0.005 to
0.042 |
| CAT | Model | -1.389 | 0.799 | -1.74 | 0.082 | -2.956 to
0.178 |
| | Pfinductiod | -0.082 | 0.284 | -0.29 | 0.773 | -0.638 to
0.475 |
| | Quercetindose | -0.007 | 0.018 | -0.37 | 0.709 | -0.042 to
0.029 |
| | Duration | 0.101 | 0.050 | 2.02 | 0.044a | 0.003 to 0.198 |
| Col I | Model | -0.442 | 0.355 | -1.24 | 0.214 | -1.138 to
0.255 |
| | Pfinductiod | -0.302 | 0.366 | -0.83 | 0.409 | -1.020 to
0.415 |
| | Quercetindose | -0.011 | 0.034 | -0.33 | 0.739 | -0.077 to
0.055 |
| | Duration | -0.022 | 0.044 | -0.51 | 0.612 | -0.108 to
0.063 |
| GSH | Model | 0.479 | 0.455 | 1.05 | 0.292 | -0.413 to
1.371 |
| | Pfinductiod | -0.030 | 0.547 | -0.05 | 0.957 | -1.102 to
1.042 |
| | Quercetindose | 0.107 | 0.051 | 2.11 | 0.035a | 0.008 to 0.207 |
| | Duration | 0.228 | 0.096 | 2.38 | 0.017a | 0.040 to 0.416 |
| Hydroxyproline | Model | 0.475 | 0.507 | 0.94 | 0.349 | -0.519 to
1.468 |
| | Pfinductiod | 0.475 | 1.405 | 0.34 | 0.735 | -2.279 to
3.229 |
| | Quercetindose | 0.009 | 0.029 | 0.33 | 0.744 | -0.047 to
0.066 |
| | Duration | 0.005 | 0.019 | 0.26 | 0.798 | -0.033 to
0.043 |
| IL-1β | Model | -0.204 | 0.212 | -0.96 | 0.336 | -0.621 to
0.212 |
| | Pfinductiod | -0.119 | 0.232 | -0.51 | 0.610 | -0.574 to
0.336 |
| | Quercetindose | -0.006 | 0.016 | -0.39 | 0.695 | -0.039 to
0.026 |
| | Duration | -0.018 | 0.015 | -1.20 | 0.230 | -0.046 to
0.011 |
| IL-6 | Model | -0.153 | 0.160 | -0.96 | 0.338 | -0.466 to
0.160 |
| | Pfinductiod | -0.094 | 0.194 | -0.48 | 0.629 | -0.474 to
0.287 |
| | Quercetindose | -0.019 | 0.014 | -1.31 | 0.190 | -0.046 to
0.009 |
| | Duration | -0.006 | 0.010 | -0.58 | 0.561 | -0.025 to
0.013 |
| MDA | Model | 0.468 | 0.160 | 2.93 | 0.003a | 0.155 to 0.782 |
| | Pfinductiod | 0.164 | 0.354 | 0.46 | 0.643 | -0.529 to
0.858 |
| | Quercetindose | -0.007 | 0.015 | -0.45 | 0.653 | -0.037 to
0.023 |
| | Duration | 0.012 | 0.008 | 1.55 | 0.121 | -0.003 to
0.027 |
| NO | Model | -0.386 | 0.198 | -1.95 | 0.051 | -0.775 to
0.002 |
| | Pfinductiod | -0.586 | 0.546 | -1.07 | 0.284 | -1.656 to
0.485 |
| | Quercetindose | -0.016 | 0.053 | -0.31 | 0.760 | -0.119 to
0.087 |
| | Duration | 0.008 | 0.023 | 0.33 | 0.740 | -0.037 to
0.053 |
| Ashcroft score | Model | 0.759 | 0.507 | 1.50 | 0.135 | -0.236 to
1.753 |
| | Pfinductiod | 0.565 | 0.650 | 0.87 | 0.385 | -0.709 to
1.838 |
| | Quercetindose | -0.059 | 0.038 | -1.53 | 0.125 | -0.133 to
0.016 |
| | Duration | 0.015 | 0.017 | 0.88 | 0.379 | -0.019 to
0.049 |
| SOD | Model | 0.210 | 0.163 | 1.29 | 0.198 | -0.110 to
0.530 |
| | Pfinductiod | 0.378 | 0.311 | 1.22 | 0.224 | -0.232 to
0.989 |
| | Quercetindose | -0.0003 | 0.014 | -0.02 | 0.984 | -0.027 to
0.027 |
| | Duration | 0.0004 | 0.008 | 0.05 | 0.960 | -0.015 to
0.016 |
| TBARS | Model | -0.005 | 0.194 | -0.02 | 0.981 | -0.385 to
0.376 |
| | Pfinductiod | -0.129 | 0.314 | -0.41 | 0.682 | -0.745 to
0.487 |
| | Quercetindose | -0.044 | 0.027 | -1.59 | 0.112 | -0.097 to
0.010 |
| | Duration | -0.070 | 0.046 | -1.54 | 0.124 | -0.160 to
0.019 |
| TGF-β1 | Model | 0.451 | 0.921 | 0.49 | 0.624 | -1.353 to 2.25 |
| | Pfinductiod | 0.155 | 0.320 | 0.48 | 0.628 | -0.473 to
0.783 |
| | Quercetindose | 0.014 | 0.032 | 0.43 | 0.668 | -0.048 to
0.076 |
| | Duration | -0.004 | 0.008 | -0.48 | 0.631 | -0.020 to
0.012 |
| TNF-α | Model | 0.584 | 0.223 | 2.62 | 0.009 | 0.147 to 1.021 |
| | Pfinductiod | 0.136 | 0.309 | 0.44 | 0.660 | -0.469 to
0.741 |
| | Quercetindose | 0.015 | 0.008 | 1.83 | 0.067 | -0.001 to
0.031 |
| | Duration | 0.013 | 0.012 | 1.05 | 0.295 | -0.011 to
0.036 |
| Leukocyte | Model | 0.953 | 0.398 | 2.39 | 0.017 | 0.172 to 1.734 |
| | Pfinductiod | -0.653 | 1.114 | -0.59 | 0.558 | -2.837 to
1.531 |
| | Quercetindose | -0.030 | 0.010 | -3.07 | 0.002 | -0.049 to
-0.011 |
| | Duration | -0.013 | 0.020 | -0.67 | 0.501 | -0.052 to
0.025 |
| Lung index | Model | 0.615 | 0.444 | 1.38 | 0.166 | -0.256 to
1.485 |
| | Pfinductiod | 0.581 | 0.726 | 0.80 | 0.423 | -0.842 to
2.004 |
| | Quercetindose | 0.052 | 0.043 | 1.22 | 0.223 | -0.032 to
0.137 |
| | Duration | 0.027 | 0.031 | 0.89 | 0.375 | -0.033 to
0.088 |
| Macrophages | Model | -0.712 | 0.634 | -1.12 | 0.261 | -1.956 to
0.531 |
| | Pfinductiod | -0.947 | 0.312 | -3.03 | 0.002 | -1.559 to
-0.335 |
| | Quercetindose | 0.039 | 0.035 | 1.11 | 0.266 | -0.030 to
0.109 |
| | Duration | -0.009 | 0.033 | -0.26 | 0.796 | -0.074 to
0.057 |
| Lymphocytes | Model | -0.674 | 0.570 | -1.18 | 0.237 | -1.791 to
0.444 |
| | Pfinductiod | -0.290 | 0.511 | -0.57 | 0.571 | -1.291 to
0.712 |
| | Quercetindose | 0.040 | 0.019 | 2.11 | 0.035 | 0.003 to 0.077 |
| | Duration | 0.096 | 0.140 | 0.68 | 0.495 | -0.179 to
0.371 |
| Eosinophils | Model | -0.790 | 0.636 | -1.24 | 0.214 | -2.038 to
0.457 |
| | Pfinductiod | -0.102 | 0.525 | -0.19 | 0.846 | -1.132 to
0.927 |
| | Quercetindose | 0.033 | 0.010 | 3.17 | 0.002 | 0.013 to 0.053 |
| | Duration | 0.140 | 0.142 | 0.99 | 0.324 | -0.139 to
0.419 |
| Body weight | Model | -0.350 | 0.444 | -0.79 | 0.430 | -1.220 to
0.520 |
| | Pfinductiod | 2.915 | 1.076 | 2.71 | 0.007 | 0.806 to 5.024 |
| | Quercetindose | -0.023 | 0.012 | -2.01 | 0.044 | -0.046 to
-0.001 |
| | Duration | 0.018 | 0.092 | 0.20 | 0.843 | -0.162 to
0.198 |
| Total cell
count | Model | -0.032 | 0.269 | -0.12 | 0.905 | -0.560 to
0.496 |
| | Pfinductiod | -0.280 | 0.197 | -1.42 | 0.156 | -0.667 to
0.106 |
| | Quercetindose | -0.025 | 0.011 | -2.22 | 0.027a | -0.047 to
-0.003 |
| | Duration | 0.007 | 0.007 | 0.91 | 0.362 | -0.008 to
0.021 |
| Neutrophils | Model | -0.510 | 0.940 | -0.54 | 0.587 | -2.353 to
1.332 |
| | Pfinductiod | 0.234 | 0.680 | 0.34 | 0.731 | -1.098 to
1.566 |
| | Quercetindose | -0.032 | 0.053 | -0.61 | 0.543 | -0.136 to
0.072 |
| | Duration | 0.031 | 0.068 | 0.46 | 0.645 | -0.102 to
0.165 |
The choice of fibrosis induction method emerged as a
significant source of heterogeneity for macrophage infiltration
(coef.=-0.947; P=0.002) and changes in body weight (coef.=2.915;
P=0.007). Conversely, the type of animal model was significantly
associated with variations in MDA (coef.=0.468; P=0.003) and TNF-α
levels (coef.=0.584; P=0.009).
For numerous outcomes, including inflammatory
cytokines (IL-1β and IL-6) and fibrosis markers (COL I and α-SMA),
none of the examined covariates demonstrated a significant
moderating effect (all P>0.05), suggesting that other unmeasured
factors likely contributed to the observed heterogeneity.
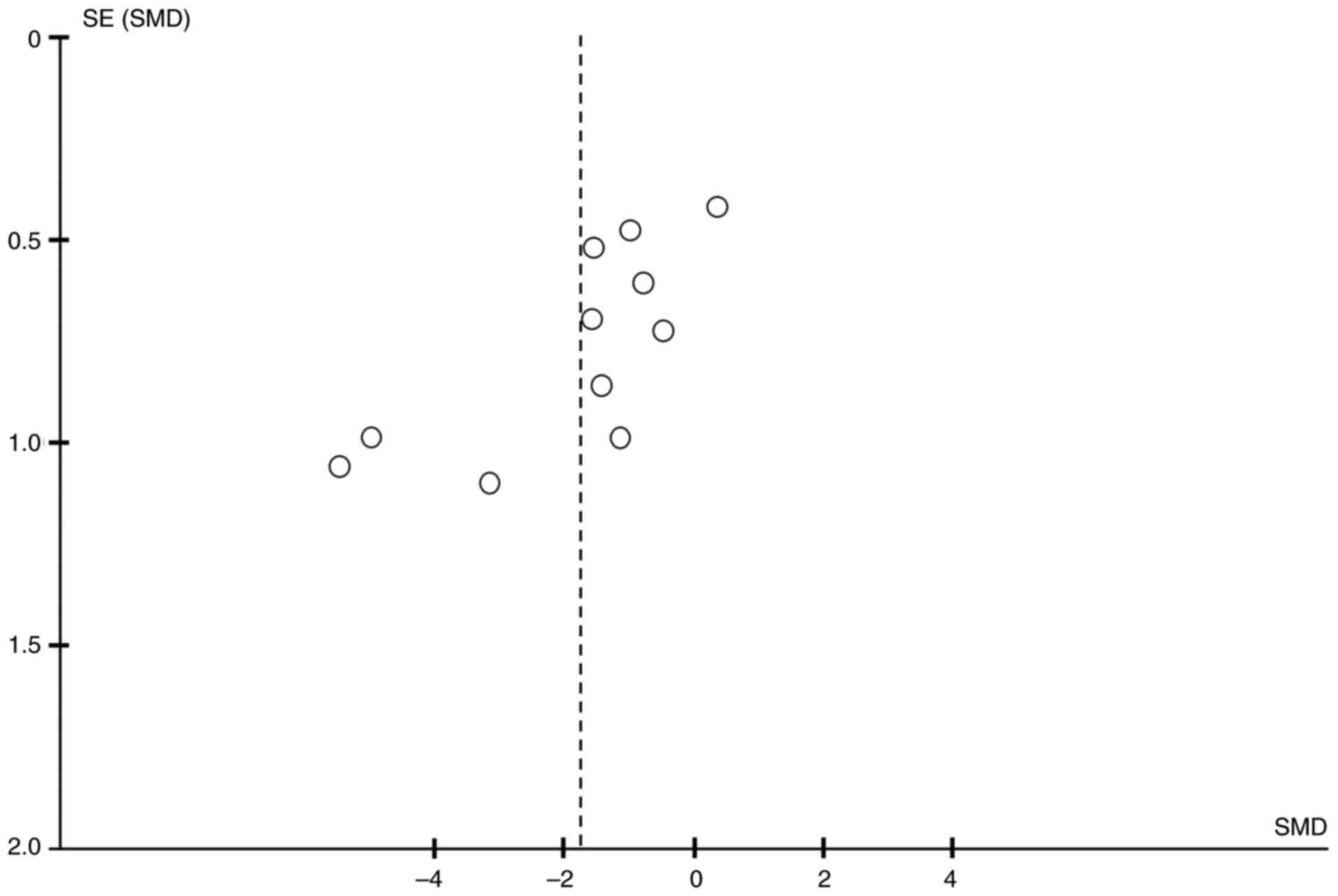
Publication bias
The potential for publication bias was
systematically evaluated for all outcomes using both Egger's linear
regression test and Begg's rank correlation test (Table IV). The visual inspection of
funnel plots indicated general symmetry for numerous outcomes (for
example, TNF-α; Fig. 6). In
accordance with methodological recommendations (including the
Cochrane Handbook), statistical tests for funnel plot asymmetry,
such as Egger's test, are only recommended when a meta-analysis
contains ≥10 studies. Among all outcomes in the present analysis,
TNF-α exhibited the largest number of studies (n=11), meeting this
minimum threshold. Therefore, funnel plots and statistical tests
for this outcome were selectively performed and reported to provide
a meaningful assessment. The statistical tests demonstrated that no
significant publication bias was detected for the majority of
outcomes (all P>0.05).
 | Table IVResults of the Begg's and Egger's
tests for assessment of potential publication bias. |
Table IV
Results of the Begg's and Egger's
tests for assessment of potential publication bias.
| | | Begg's test | Egger's test |
|---|
| Outcome
measure | n | z-value | P-value | t-value | P-value |
|---|
| Boby weight | 5 | 1.96 | 0.050 | 2.25 | 0.110 |
| Lung index | 4 | -0.68 | 0.497 | -1.84 | 0.207 |
| TBARS | 3 | -0.52 | 0.602 | -2.10 | 0.283 |
| Ashcroft score | 7 | -1.35 | 0.176 | -1.87 | 0.120 |
| Hydroxyproline
content | 9 | -2.50 | 0.012a | -4.60 | 0.002b |
| Col I | 4 | -2.04 | 0.042a | -5.14 | 0.036a |
| α-SMA | 6 | -1.69 | 0.091 | -1.04 | 0.357 |
| TNF-α | 11 | -1.95 | 0.052 | -3.45 | 0.007b |
| IL-1β | 6 | -0.94 | 0.348 | -0.81 | 0.463 |
| IL-6 | 5 | -1.47 | 0.142 | -1.78 | 0.173 |
| TGF-β1 | 4 | 0.68 | 0.497 | 0.20 | 0.860 |
| GSH | 6 | 1.69 | 0.091 | 4.32 | 0.012a |
| CAT | 4 | 1.36 | 0.174 | 1.22 | 0.347 |
| SOD | 4 | 0.68 | 0.497 | 1.18 | 0.359 |
| MDA | 7 | 0.45 | 0.652 | -0.27 | 0.801 |
| NO | 4 | -1.36 | 0.174 | -1.88 | 0.200 |
| Neutrophils | 5 | -1.47 | 0.142 | -1.90 | 0.154 |
| Lymphocytes | 4 | 1.36 | 0.174 | 2.94 | 0.099 |
| Macrophages | 7 | -0.15 | 0.881 | -0.58 | 0.586 |
| Eosinophils | 3 | 0.52 | 0.602 | 0.33 | 0.800 |
| Total cell
count | 7 | -1.35 | 0.176 | -2.20 | 0.079 |
| Leukocyte
count | 3 | -1.57 | 0.117 | -1.08 | 0.475 |
However, significant publication bias was identified
for four specific outcomes. Egger's test yielded statistically
significant results for hydroxyproline (t=-4.60; P=0.002), Col I
(t=-5.14; P=0.036), TNF-α (t=-3.45; P=0.007) and GSH (t=4.32;
P=0.012). The findings from Begg's test further supported the
presence of a significant bias for hydroxyproline (z=-2.50;
P=0.012) and Col I (z=-2.04; P=0.042), while the result for TNF-α
was of borderline statistical significance (P=0.052), and no
significant bias was detected for GSH (P=0.091) using this
method.
Discussion
The present meta-analysis evaluated the therapeutic
potential of quercetin in experimental models of PF to explore
potential sources of heterogeneity in its efficacy. The present
comprehensive analysis indicated that quercetin intervention may
exert regulatory effects across multiple physiological processes,
including body weight recovery, attenuation of fibrosis
progression, suppression of inflammatory responses and reduction of
oxidative stress. These findings are consistent with previous
experimental studies (42-44).
Previous studies have suggested that quercetin may
inhibit collagen synthesis through modulation of the TGF-β1/Smad
signaling pathway (45,46), while also promoting collagen
degradation by regulating the MMP/TIMP balance; thereby
demonstrating anti-fibrotic effects. This is reflected in reduced
levels of hydroxyproline, Col I, α-SMA and lower Ashcroft scores.
However, meta-regression analysis indicated that the type of animal
model may be a notable source of heterogeneity in fibrosis markers,
suggesting that the genetic backgrounds of different animal strains
may influence treatment responsiveness.
With regards to anti-inflammatory mechanisms, the
present study observed that quercetin may reduce the levels of
pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6 and
TGF-β1, as suggested in the literature through inhibition of the
NF-κB and MAPK signaling pathways (47-49).
Notably, the method of fibrosis induction markedly influenced the
degree of inflammatory cell infiltration, indicating that different
induction methods (for example, bleomycin vs. silica) may activate
distinct inflammatory pathways, thereby perhaps contributing to
variability in treatment outcomes.
In relation to its antioxidant effects, quercetin
may enhance the activities of SOD, CAT and GSH through activation
of the nuclear factor erythroid 2-related factor 2
(Nrf2)/Kelch-like ECH-associated protein 1/antioxidant response
element pathway (50,51). Meta-regression analysis revealed a
positive association between quercetin dosage and GSH levels and
intervention duration was also notably associated with CAT activity
and GSH, suggesting that its antioxidant effects may be dose- and
time-dependent.
Interactions among experimental design parameters
add complexity to the assessment of efficacy. The present study
found that both animal model selection and induction method jointly
influence treatment outcomes. For example, the present data
indicate that different induction methods may produce markedly
distinct pathological phenotypes across animal strains.
Furthermore, we hypothesize that there may be an interaction
between quercetin dosage and intervention duration, suggesting that
long-term high-dose treatment could yield synergistic effects,
although this hypothesis warrants further validation.
It should be noted that the high heterogeneity
observed in the present study may affect the interpretation of
results. Although several indicators, including hydroxyproline,
lung index, TNF-α and neutrophil count, exhibited high
heterogeneity (I2>75%), this variation largely
reflects methodological diversity across studies rather than
fundamental differences in treatment effects. Importantly,
meta-regression analysis demonstrated the beneficial therapeutic
effect of quercetin across all studies, with heterogeneity
primarily influencing the magnitude rather than the direction of
the effect.
Notably, quercetin did not demonstrate a notable
effect on lymphocyte count, contrasting with its pronounced effects
on innate immune cells. This may indicate differential regulatory
activity on innate compared with adaptive immune responses. For
other outcomes with high heterogeneity but statistical
significance, the results suggested context-dependent
variability.
Moreover, for certain indicators (for example,
IL-1β, IL-6, Col I or α-SMA), none of the covariates examined
showed notable influence, indicating that other unmeasured sources
of heterogeneity, such as animal age, sex differences or analytical
method variations, may be present.
The present meta-analysis provided a comprehensive
and quantitative summary of the current preclinical evidence
regarding the therapeutic effects of quercetin in experimental PF.
One of the primary strengths is the integration of data from 25
independent studies, which enhances the statistical power and
allows for a more robust estimation of treatment effects across
multiple outcome domains, including fibrotic, inflammatory and
oxidative stress parameters. The application of random-effects
meta-analysis and meta-regression analysis further strengthens the
present study by accounting for between-study heterogeneity and
exploring the influence of key experimental variables, such as
dosage, duration, animal model and induction method. This approach
not only increases the reliability of the findings but also helps
identify subtle and consistent treatment effects that may not be
apparent in individual studies. Moreover, the present study offers
novel insights into the context-dependent efficacy of quercetin and
highlights potential sources of heterogeneity, thereby contributing
to the optimization of future preclinical research design.
Several limitations should be considered when
interpreting the present results. First, the inclusion of studies
with varying methodological quality, particularly in areas such as
randomization and blinding, a common issue in animal studies, may
introduce bias and affect the validity of pooled effect estimates.
Second, the presence of notable heterogeneity, although partially
explained by meta-regression, remains a concern, as unmeasured
factors such as animal age, sex and specific analytical protocols
may contribute to variability. Third, the reliance on data
extracted from figures in certain studies, despite efforts to
obtain original datasets, may have introduced inaccuracies in
measurement. Finally, all included studies were conducted in animal
models, which inherently limits the direct translatability of the
findings to human patients. These limitations, however, are
reflective of broader challenges in preclinical meta-research
rather than specific flaws in the current methodology.
The present meta-analysis indicated that quercetin
administration was associated with notable improvements in
fibrotic, inflammatory and oxidative stress parameters, potentially
through the modulation of key pathways such as TGF-β1/Smad, NF-κB
and Nrf2 signaling.
However, these findings must be interpreted with
caution due to the inherent limitations of the included preclinical
studies. The notable methodological heterogeneity, variability in
experimental design and lack of clinical validation, all of which
are common in animal research, undermine the robustness of the
results and render their translational relevance to human disease
uncertain. Consequently, the implications of the present analysis
should be considered hypothesis-generating rather than
definitive.
To strengthen the evidence, future investigations
should prioritize: i) Standardizing experimental protocols to
minimize heterogeneity; ii) performing rigorous dose-response and
time-course studies; iii) validating these findings across a
broader range of PF models; and iv) enhancing data transparency and
reproducibility.
In summary, while the present meta-analysis
highlights the promising therapeutic potential of quercetin and
provides a rationale for further mechanistic investigation, its
ultimate clinical value can only be established through
well-designed future clinical trials.
Supplementary Material
Search strategy for PubMed.
Search strategy for Cochrane
Library.
Search strategy for Embase.
Search strategy for Ovid.
Search strategy for Web of
Science.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by funding from
Guangzhou Municipal Science and Technology Project on Traditional
Chinese Medicine and Integrated Traditional Chinese and Western
Medicine (grant no. 20252A010010).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LHC wrote the manuscript, conceived the present
study, collected the data and analyzed the data. LC conceived the
present study, analyzed the data and wrote the manuscript. CML
designed the present study and reviewed the manuscript. LHC and CML
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang Y, Lv M, Xu Q, Wang X and Fang Z:
Extracellular vesicles in idiopathic pulmonary fibrosis:
Pathogenesis, biomarkers and innovative therapeutic strategies. Int
J Nanomedicine. 19:12593–12614. 2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Richeldi L, Collard HR and Jones MG:
Idiopathic pulmonary fibrosis. Lancet. 389:1941–1952.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lederer DJ and Martinez FJ: Idiopathic
pulmonary fibrosis. New Engl J Med. 378:1811–1823. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kou M, Jiao Y, Li Z, Wei B, Li Y, Cai Y
and Wei W: Real-world safety and effectiveness of pirfenidone and
nintedanib in the treatment of idiopathic pulmonary fibrosis: A
systematic review and meta-analysis. Eur J Clin Pharmacol.
80:1445–1460. 2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chagas MDSS, Behrens MD, Moragas-Tellis
CJ, Penedo GXM, Silva AR and Goncalves-de-Albuquerque CF: Flavonols
and flavones as Potential anti-inflammatory, antioxidant, and
antibacterial compounds. Oxid Med Cell Longev.
2022(9966750)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rajesh RU and Sangeetha D: Therapeutic
potentials and targeting strategies of quercetin on cancer cells:
Challenges and future prospects. Phytomedicine.
133(155902)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Godoy MCX, Monteiro GA, Moraes BHD, Macedo
JA, Goncalves GMS and Gambero A: Addition of polyphenols to drugs:
The potential of controlling ‘Inflammaging’ and fibrosis in human
senescent lung fibroblasts in vitro. Int J Mol Sci.
25(7163)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Reyes-Jimenez E, Ramirez-Hernandez AA,
Santos-Alvarez JC, Velázquez-Enríquez JM, González-García K,
Carrasco-Torres G, Villa-Treviño S, Baltiérrez-Hoyos R and
Vásquez-Garzón VR: Coadministration of 3'5-dimaleamylbenzoic acid
and quercetin decrease pulmonary fibrosis in a systemic sclerosis
model. Int Immunopharmacol. 122(110664)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wiggins Z, Chioma OS, Drake WP and
Langford M: The effect of quercetin on human lung fibroblasts and
regulation of collagen production. Am J Resp Crit Care.
(207)2023.
|
|
10
|
Andres S, Pevny S, Ziegenhagen R, Bakhiya
N, Schäfer B, Hirsch-Ernst KI and Lampen A: Safety aspects of the
use of quercetin as a dietary supplement. Mol Nutr Food Res.
(62)2018.PubMed/NCBI View Article : Google Scholar : doi:
10.1002/mnfr.201700447.
|
|
11
|
Liu X, Liang Q, Qin Y, Chen Z and Yue R:
Advances and perspectives on the Anti-fibrotic mechanisms of the
quercetin. Am J Chin Med. 53:1411–1440. 2025.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sellares J and Rojas M: Quercetin in
idiopathic pulmonary fibrosis: Another brick in the senolytic wall.
Am J Respir Cell Mol Biol. 60:3–4. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Amir-Behghadami M and Janati A:
Population, Intervention, Comparison, Outcomes and Study (PICOS)
design as a framework to formulate eligibility criteria in
systematic reviews. Emerg Med J. 37(387)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ashcroft T, Simpson JM and Timbrell V:
Simple method of estimating severity of pulmonary fibrosis on a
numerical scale. J Clin Pathol. 41:467–470. 1988.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Hooijmans CR, Rovers MM, de Vries RBM,
Leenaars M, Ritskes-Hoitinga M and Langendam MW: SYRCLE's risk of
bias tool for animal studies. BMC Med Res Methodol.
14(43)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Baowen Q, Yulin Z, Xin W, Wenjing X, Hao
Z, Zhizhi C, Xingmei D, Xia Z, Yuquan W and Lijuan C: A further
investigation concerning correlation between anti-fibrotic effect
of liposomal quercetin and inflammatory cytokines in pulmonary
fibrosis. Eur J Pharmacol. 642:134–139. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mehrzadi S, Hosseini P, Mehrabani M,
Siahpoosh A, Goudarzi M, Khalili H and Malayeri A: Attenuation of
Bleomycin-induced pulmonary fibrosis in wistar rats by combination
treatment of two natural phenolic compounds: Quercetin and Gallic
acid. Nutr Cancer. 73:2039–2049. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Martinez JA, Ramos SG, Meirelles MS,
Verceze AV, Arantes MR and Vannucchi H: Effects of quercetin on
Bleomycin-induced lung injury: A preliminary study. J Bras Pneumol.
34:445–452. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kushwah L, Verma R, Gohel D, Patel M,
Marvania T and Balakrishnan S: Evaluating the ameliorative
potential of quercetin against the Bleomycin-induced pulmonary
fibrosis in wistar rats. Pulm Med. 2013:280–289. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Impellizzeri D, Talero E, Siracusa R,
Alcaide A, Cordaro M, Maria Zubelia J, Bruschetta G, Crupi R,
Esposito E, Cuzzocrea S and Motilva V: Protective effect of
polyphenols in an inflammatory process associated with experimental
pulmonary fibrosis in mice. Brit J Nutr. 114:853–865.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Park HK, Kim SJ, Kwon DY, Park JH and Kim
YC: Protective effect of quercetin against paraquat-induced lung
injury in rats. Life Sci. 87:181–186. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Taslidere E, Esrefoglu M, Elbe H, Cetin A
and Ates B: Protective effects of melatonin and quercetin on
experimental lung injury induced by carbon tetrachloride in rats.
Exp Lung Res. 40:59–65. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Geng F, Xu M, Zhao L, Zhang H, Li J, Jin
F, Li Y, Li T, Yang X, Li S, et al: Quercetin alleviates pulmonary
fibrosis in mice exposed to silica by inhibiting macrophage
senescence. Front Pharmacol. 13(912029)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Geng F, Zhao L, Cai Y, Zhao Y, Jin F, Li
Y, Li T, Yang X, Li S, Gao X, et al: Quercetin alleviates pulmonary
fibrosis in silicotic mice by inhibiting macrophage transition and
TGF-β-Smad2/3 pathway. Curr Issues Mol Biol. 45:3087–3101.
2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hohmann MS, Habiel DM, Coelho AL, Verri
JWA and Hogaboam CM: Quercetin enhances ligand-induced apoptosis in
senescent idiopathic pulmonary fibrosis fibroblasts and reduces
lung fibrosis in vivo. Am J Resp Cell Mol. 60:28–40.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wu W, Wu X, Qiu L, Wan R, Zhu X, Chen S,
Yang X, Liu X and Wu J: Quercetin influences intestinal
dysbacteriosis and delays alveolar epithelial cell senescence by
regulating PTEN/PI3K/AKT signaling in pulmonary fibrosis. Naunyn
Schmiedebergs Arch Pharmacol. 397:4809–4822. 2024.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu H, Xue J, Li X, Ao R and Lu Y:
Quercetin liposomes protect against Radiation-induced pulmonary
injury in a murine model. Oncol Lett. 6:453–459. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Verma S, Dutta A, Dahiya A and Kalra N:
Quercetin-3-Rutinoside alleviates radiation-induced lung
inflammation and fibrosis via regulation of NF-κB/TGF-β1 signaling.
Phytomedicine. 99(154004)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Boots AW, Veith C, Albrecht C, Bartholome
R, Drittij MJ, Claessen SMH, Bast A, Rosenbruch M, Jonkers L, van
Schooten FJ and Schins RPF: The dietary antioxidant quercetin
reduces hallmarks of bleomycin-induced lung fibrogenesis in mice.
BMC Pulm Med. 20:112–116. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wei QF, Wang XH, Zhang XY, Song LJ, Wang
YM, Wang Q, Lv F and Li XF: Therapeutic effects of quercetin on
bleomycin induced pulmonary fibrosis in rats. Int J Clin Exp Med.
9:5161–5167. 2016.
|
|
32
|
Oka VO, Okon UE and Osim EE: Pulmonary
responses following quercetin administration in rats after
intratracheal instillation of amiodarone. Niger J Physiol Sci.
34:63–68. 2019.PubMed/NCBI
|
|
33
|
Qin M, Chen W, Cui J, Li W, Liu D and
Zhang W: Protective efficacy of inhaled quercetin for radiation
pneumonitis. Exp Ther Med. 14:5773–5778. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ding S, Jiang J and Li Y: Quercetin
alleviates PM2.5-induced chronic lung injury in mice by targeting
ferroptosis. PeerJ. 12(e16703)2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Malayeri AR, Hemmati AA, Arzi A, Rezaie A,
Ghafurian-Boroojerdnia M and Khalili HR: A comparison of the
effects of quercetin hydrate with those of vitamin E on the levels
of IL-13, PDGF, TNF-α, and INF-γ in bleomycin-induced pulmonary
fibrosis in rats. Jundishapur J Nat Ph. 11(e27705)2016.
|
|
36
|
El-Sayed NS and Rizk SM: The protective
effect of quercetin, green tea or malt extracts against
experimentally-induced lung fibrosis in rats. African J Pharm
Pharmacol. 3:191–201. 2009.
|
|
37
|
Fang Y, Jin W, Guo Z and Hao J: Quercetin
alleviates Asthma-induced airway inflammation and remodeling
through downregulating periostin via blocking TGF-β1/smad pathway.
Pharmacology. 108:432–443. 2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yao J, Li Y, Meng F, Shen W and Wen H:
Enhancement of suppression oxidative stress and inflammation of
quercetin by Nano-decoration for ameliorating silica-induced
pulmonary fibrosis. Environ Toxicol. 38:1494–1508. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yang T, Wang H, Li Y, Zeng Z, Shen Y, Wan
C, Wu Y, Dong J, Chen L and Wen F: Serotonin receptors 5-HTR2A and
5-HTR2B are involved in cigarette smoke-induced airway
inflammation, mucus hypersecretion and airway remodeling in mice.
Int Immunopharmacol. 81(106036)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang H, Hua H, Liu J, Wang C, Zhu C, Xia
Q, Jiang W, Cheng X, Hu X and Zhang Y: Integrative analysis of the
efficacy and pharmacological mechanism of Xuefu Zhuyu decoction in
idiopathic pulmonary fibrosis via evidence-based medicine,
bioinformatics, and experimental verification. Heliyon.
10(e38122)2024.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Toker C, Kuyucu Y, Saker D, Kara S,
Guzelel B and Mete UO: Investigation of miR-26b and miR-27b
expressions and the effect of quercetin on fibrosis in experimental
pulmonary fibrosis. J Mol Histol. 55:25–35. 2024.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Geng Q, Yan L, Shi C, Zhang L, Li L, Lu P,
Cao Z, Li L, He X, Tan Y, et al: Therapeutic effects of flavonoids
on pulmonary fibrosis: A preclinical Meta-analysis. Phytomedicine.
132(155807)2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang X, Cai Y, Zhang W and Chen X:
Quercetin ameliorates pulmonary fibrosis by inhibiting SphK1/S1P
signaling. Biochem Cell Biol. 96:742–751. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xiao C, Tang Y, Ren T, Kong C, You H, Bai
XF, Huang Q, Chen Y, Li LG, Liu MY, et al: Treatment of silicosis
with quercetin depolarizing macrophages via inhibition of
mitochondrial damage-associated pyroptosis. Ecotoxicol Environ Saf.
286(117161)2024.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang H, Yang L, Han Q and Xu W: Original
antifibrotic effects of quercetin on TGF-β1-induced vocal fold
fibroblasts. Am J Transl Res. 14:8552–8561. 2022.PubMed/NCBI
|
|
46
|
Widiatmoko A, Fitri LE, Endharti AT and
Murlistyarini S: The effect of quercetin on phosphorylated p38,
Smad7, Smad2/3 nuclear translocation and collagen type I of keloid
fibroblast culture. J Biotech Res. 16:32–42. 2024.
|
|
47
|
Takano M, Deguchi J, Senoo S, Izumi M,
Kawami M and Yumoto R: Suppressive effect of quercetin against
bleomycin-induced Epithelial-mesenchymal transition in alveolar
epithelial cells. Drug Metab Pharmacokinet. 35:522–526.
2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lee GB, Kim Y, Lee KE, Vinayagam R, Singh
M and Kang SG: Anti-inflammatory effects of quercetin, rutin, and
troxerutin result from the inhibition of NO production and the
reduction of COX-2 levels in RAW 264.7 cells treated with LPS. Appl
Biochem Biotechnol. 196:8431–8452. 2024.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Boots AW, Haenen GRMM and Bast A: Health
effects of quercetin: From antioxidant to nutraceutical. Eur J
Pharmacol. 585:325–337. 2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kostyuk VA, Potapovich AI, Speransky SD
and Maslova GT: Protective effect of natural flavonoids on rat
peritoneal macrophages injury caused by asbestos fibers. Free Radic
Bio Med. 21:487–493. 1996.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Veith C, Drent M, Bast A, van Schooten FJ
and Boots AW: The disturbed redox-balance in pulmonary fibrosis is
modulated by the plant flavonoid quercetin. Toxicol Appl Pharmacol.
336:40–48. 2017.PubMed/NCBI View Article : Google Scholar
|