Introduction
Presacral tumors, also known as tumors of the
presacral or retrorectal space, are rare and heterogeneous lesions
arising in the anatomically complex area between the rectum and
sacrum. The incidence of these tumors is projected to be ~1 in
every 40,000 patients, with a higher prevalence observed in females
and an average age of onset of 30 years (1). The classification of presacral tumors
is intricate, with the tumors being traditionally categorized into
the following five groups based on histological origin: Congenital
tumors (such as epidermoid cysts and teratomas), neurogenic tumors
(including schwannomas and ganglioneuromas), osseous tumors (such
as chordomas and chondrosarcomas), soft-tissue tumors (such as
liposarcomas) and other inflammatory or infectious lesions
(2-4).
Among these, malignant variants, such as chordomas, are
characterized by rapid growth, invasive characteristics and
potential for distant metastasis, and occur more frequently in
males. By contrast, benign lesions, including teratomas and cysts,
typically manifest as slow-growing masses with variable cystic or
solid appearances on imaging studies, and predominate in females.
Presacral tumors are often asymptomatic in the early stages but may
present with non-specific symptoms, including pelvic discomfort,
bowel or urinary disturbances, and sciatic pain as the tumor grows
(5,6). The present study reports the rare
case of a young woman with bilateral sacral anterior cysts who
exhibited abdominal pain as the only symptom. To the best of our
knowledge, this has not been reported in previous literature. A
discussion of the literature based around this rare tumor is also
presented in the current study.
Case report
A 32-year-old previously healthy female presented to
the First People's Hospital of Xiaoshan District (Hangzhou, China)
in September 2024 with unexplained abdominal pain, which was the
first occurrence of such symptoms within the preceding 2 months.
The patient exhibited no signs of intestinal dysfunction or
symptoms indicative of urinary tract irritation. Notably, a digital
rectal examination revealed a solid, fixed mass located on the
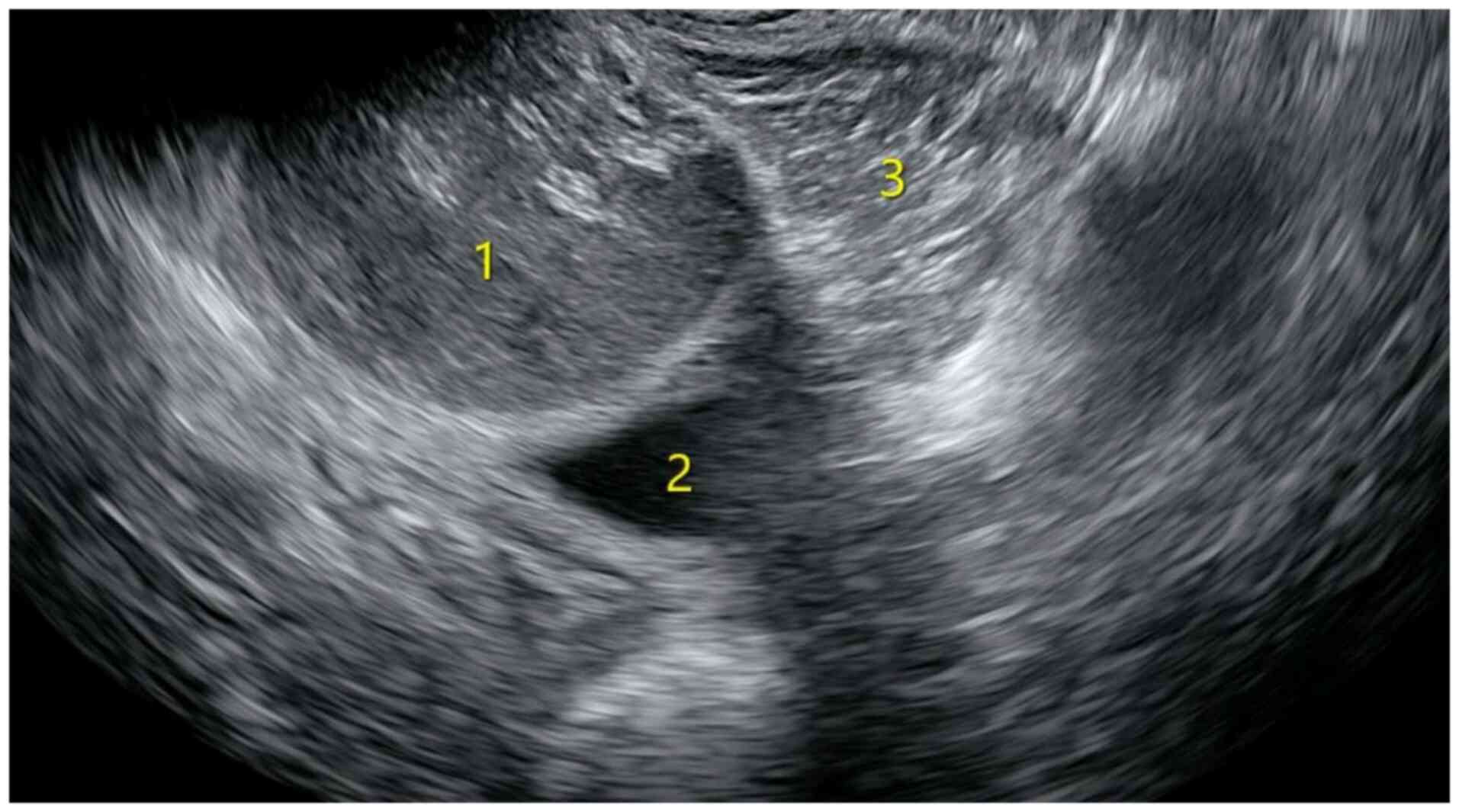
posterolateral wall of the rectum. Gynecological ultrasound prior
to surgery revealed two unevenly strong echo masses in the pelvic
cavity that were located posterior to the cervix and lateral to the
rectum (Fig. 1). The larger mass
measured ~8.0x6.5x6.1 cm, whereas the second largest measured
~6.4x6.0x4.8 cm. Both masses had clear boundaries and regular
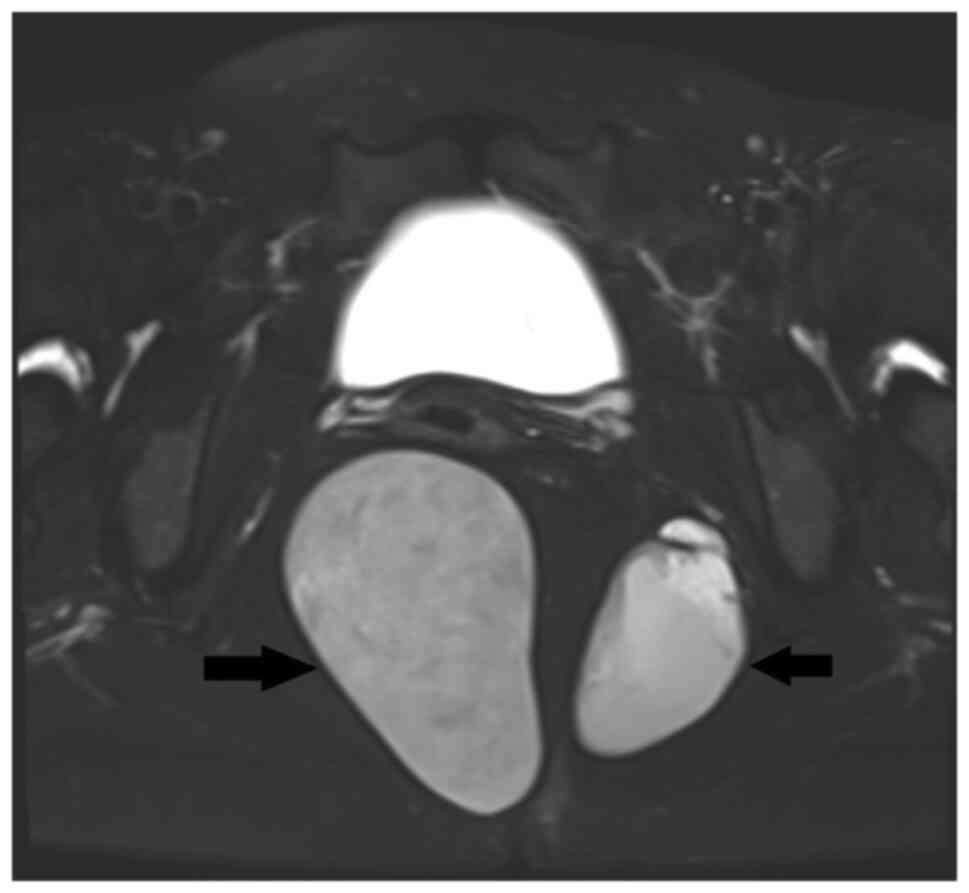
shapes, with no obvious blood flow signals detected. MRI revealed
two abnormal signal shadows in the perirectal and bilateral rectal
fossa. The larger shadow measured ~53x81 mm, with a clear boundary
and smooth edges. The cyst fluid exhibited low signal intensity on
T1-weighted imaging (T1WI), and high signal intensity on T2WI
(Fig. 2). The internal signal was
slightly mixed, with pronounced high signal intensity on
diffusion-weighted imaging (DWI) and low signal intensity on
apparent diffusion coefficient (ADC) mapping. No enhancement within
the cyst was observed after contrast administration. A circular low
signal intensity was visible on T2WI in the cyst wall, which was
moderately enhanced after contrast administration.
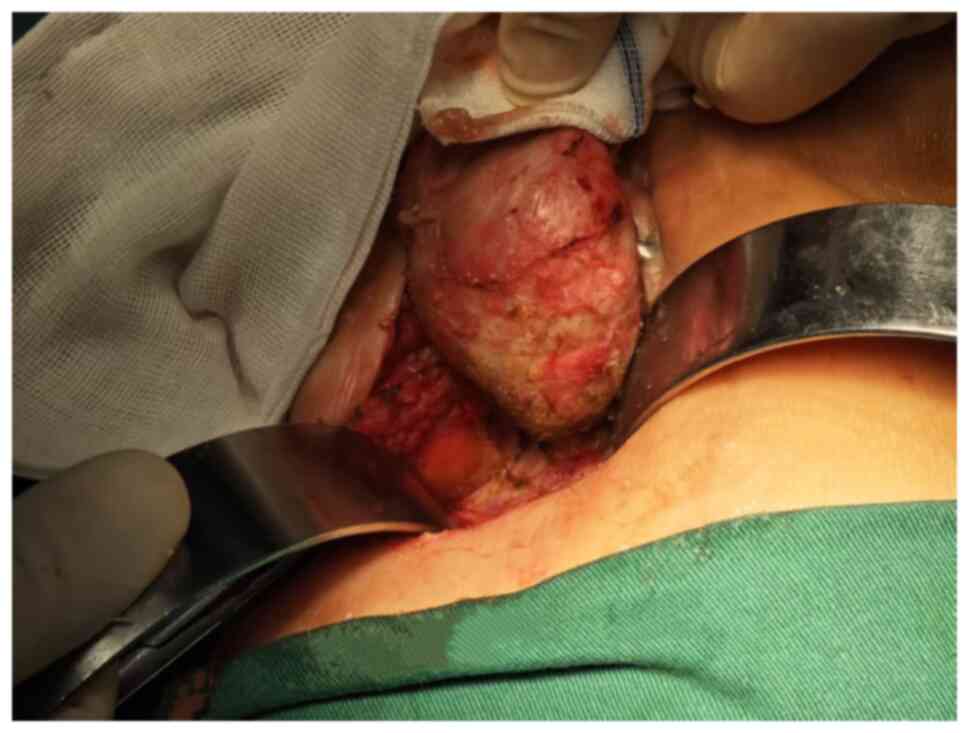
The patient underwent a mass resection via the
sacral approach under general anesthesia. A circular incision was
made at the anal margin, positioned 3 cm from the lower edge of the
coccyx, measuring ~8 cm in length. Subsequently, the subcutaneous
tissue was meticulously dissected layer by layer. Initially, the
subcutaneous tissue was separated along the perimeter of the right
pelvic mass until its base was reached. The mass exhibited well
defined boundaries, a discernible capsule and a firm consistency,
allowing for complete excision under direct visualization (Fig. 3). The left pelvic mass was excised
using an identical technique. Following thorough hemostasis, a
negative pressure drainage device was placed in the surgical sites
of both masses, and the wound was closed with intermittent sutures.
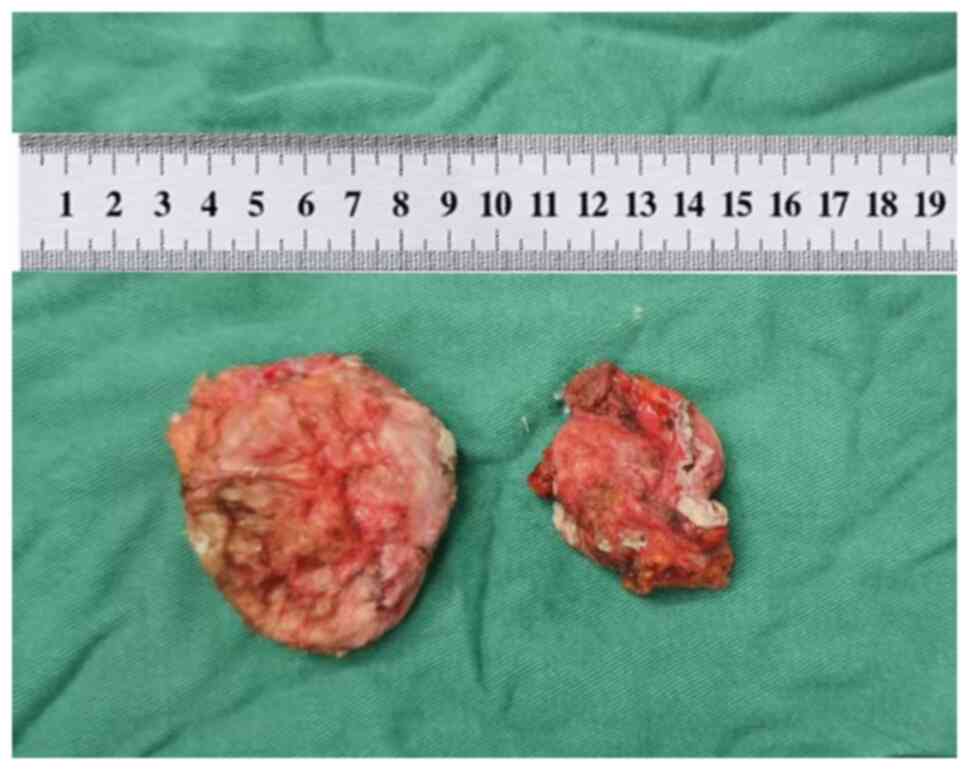
Postoperatively, the excised mass was examined and identified as a
bean curd-like material, encased in a distinct envelope (Fig. 4). The excised tissues were
submitted for pathological examination. The tissue specimens were
fixed in 10% neutral buffered formalin for 24 h at room temperature
and routinely embedded in paraffin. The tissues were sliced to a
3-µm thickness and baked at 60˚C for 30 min. Using the conventional
hematoxylin and eosin staining method, hematoxylin staining was
performed for 5 min and eosin staining for 2 min at room
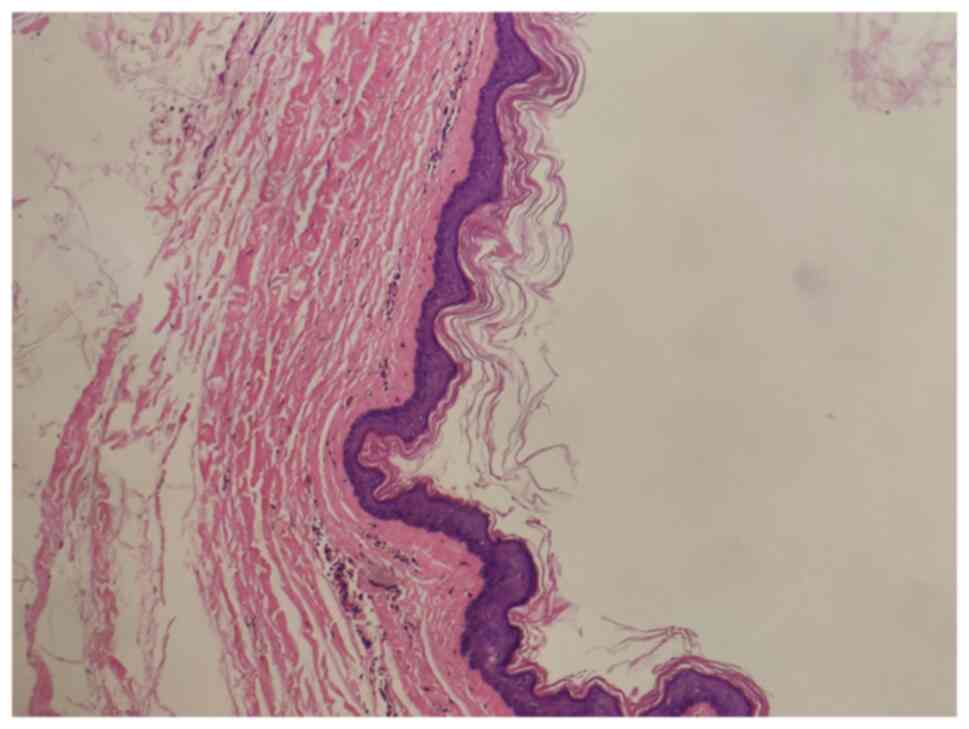
temperature. Light microscopic examination revealed a cyst lined by
epidermis-like keratinized stratified squamous epithelium,
confirming that both masses were epidermoid cysts (Fig. 5). The wound exhibited satisfactory
healing, and the patient was discharged on the seventh
postoperative day. Regular follow-up visits were conducted
approximately every 3 months, beginning 1 month postoperatively
when complete wound healing. The 12-month assessment, performed in
September 2025, revealed no evidence of presacral tumor recurrence.
Given the current disease-free status and favorable postoperative
course, the risk of subsequent recurrence appears to be low.
Discussion
The precise etiology of presacral tumors remains
incompletely elucidated, with a strong association with embryonic
developmental abnormalities. This region represents an area of
caudal bud embryo remnants, where abnormal tissue differentiation
may lead to the formation of congenital lesions (7). For example, a previous study
indicated a potential association between the occurrence of
sacrococcygeal teratoma and abnormalities in isochromosome 12p
(8). However, molecular and
biological research on presacral tumors remains in its early stages
due to the rarity of these neoplasms and their pathological
diversity. Furthermore, the anatomical configuration of this region
is remarkably complex, bounded anteriorly by the mesorectum,
posteriorly by the presacral fascia, and laterally by the iliac
vessels and ureters, presenting significant surgical challenges
(9). The therapeutic difficulty in
the present case stemmed not only from the intricate presacral
anatomy but also from the discontinuous bilateral distribution of
the lesions, which substantially increased the difficulty of
achieving an en bloc resection. After comprehensive preoperative
preparation and imaging assessment, a posterior approach was used
and complete tumor excision was achieved. The successful outcome
depended not only on a meticulous intraoperative technique but also
on thorough preoperative imaging evaluation and careful
intraoperative route planning.
MRI has emerged as the cornerstone imaging modality
for assessing presacral tumors due to its superior soft-tissue
resolution. MRI provides detailed visualization of tumor
characteristics and extent, and their association with adjacent
structures, which is critical for preoperative planning (10). Multisequence MRI techniques have
demonstrated considerable value in tumor characterization: T1WI can
identify fatty components, assisting in the diagnosis of teratomas,
whereas T2WI helps in differentiating cystic components from their
solid counterparts (11).
Additionally, DWI and ADC mapping have demonstrated robust
diagnostic performance, and have been widely used both for
preoperative evaluation and postoperative follow-up of presacral
tumors. For example, in preoperative staging, changes in ADC values
can indicate the invasiveness and risk of metastasis of tumors,
thereby guiding the selection of surgical plans (12). In the present case, MRI effectively
delineated the patient's dual discontinuous cysts and their
anatomical positioning relative to surrounding tissues and organs.
MRI serves as an indispensable reference for facilitating
comprehensive and precise surgical resection. During the surgical
procedure, the discontinuity between the two masses initially
hindered the ability to locate the smaller mass; however, with the
assistance of MRI, the mass situated in the deeper region could
ultimately be identified and excised. Consequently, we recommend
performing an MRI examination prior to surgical intervention for
presacral tumors, in order to aid in diagnosis and treatment.
Computed tomography (CT) both offers comprehensive
anatomical insights and facilitates the differentiation between
cystic and solid lesions (13).
Moreover, it effectively delineates bone invasion and the
calcification features of tumors, with particular emphasis on
chordomas and giant cell tumors affecting the sacrum (14,15).
A previous study demonstrated that 78% of malignant sacral tumors
exhibit bone disruption on CT, contrasting with the compressive
bone remodeling typically observed in benign tumors (16). CT also affords valuable insights
into tumor vascularization, which aids in preoperative risk
assessment (17).
Ultrasound remains a cost-effective, non-radiative
and real-time imaging tool for presacral tumor screening,
especially for cystic lesions such as teratomas (18). Transabdominal and transrectal
ultrasound scans are able to delineate tumor size, location and
internal echo patterns. Furthermore, Doppler ultrasound evaluates
tumor vascularity, assisting in surgical risk stratification, and
ultrasound-guided percutaneous biopsy provides precise tissue
sampling, thereby minimizing complications. A study of
ultrasound-guided biopsy of thoracic lesions in 146 patients
reported a diagnostic accuracy of 90.5%, with a complication rate
of 2.7% (19,20). In the present case study, since
both the ultrasound and MRI scans of the patient's cyst indicated a
benign cystic mass with well-defined boundaries and smooth edges,
and to avoid unnecessary complications, a puncture biopsy was not
performed.
Multimodal imaging has a pivotal role in diagnosing
presacral tumors. MRI remains the gold standard due to its superior
soft-tissue characterization (20). In the present case, on the basis of
the preoperative imaging assessment, especially MRI, the lesion in
the presacral region was initially suspected to be a benign
epidermoid cyst. The differential diagnosis for presacral tumors
primarily encompasses teratoma (18), chordoma (16), schwannoma (21), neurofibroma (22), giant cell tumor of bone (23) and osteosarcoma (24), among others.
Surgical resection remains the cornerstone of
treatment for presacral tumors, especially for benign lesions and
resectable malignancies (11,25,26).
The selection of the type of surgical approach is primarily
determined by tumor location, size, association with adjacent
structures and surgeon experience. Currently employed approaches
include anterior (transabdominal), posterior (transsacral) and
combined approaches (27).
The anterior approach is advantageous for tumors
located predominantly anterior to the sacrum, providing excellent
exposure, especially for lesions above the S3 level. However,
sacral resection through an anterior approach may be limited, and
protection of the lower sacral nerves can be challenging. One
documented case reported a presacral epidermoid cyst situated as
high as the S3 vertebra, measuring 65x50 cm, which was excised via
abdominal surgery (28). Another
case involved a sacral anterior cyst measuring 45x40 cm located at
the S5 level; due to its distance from the sacrum, a
laparoscopic-assisted transabdominal resection was performed
(29). By contrast, the mass in
the present patient was notably larger, exceeding 8 cm, and it was
bilateral in nature, with no similar cases reported to date. Given
the tumor's size and location, and the characteristics of both
lesions, it was determined that a posterior surgical approach was
more appropriate, ultimately achieving a complete tumor excision.
The posterior approach is deemed appropriate for tumors that are
located inferior to the S3 level. However, this approach presents
significant limitations, including inadequate control over the
pelvic vasculature and the potential risk of injury to the lateral
pelvic nerves (27,30). Preoperative MRI providing precise
anatomical visualization, combined with meticulously executed
surgical techniques, effectively prevented the anticipated
iatrogenic injuries considered in the preoperative assessment.
The combined approach merges anterior and posterior
exposures to enhance visualization of complex presacral tumors,
especially large or multi-structure tumors (31). The approach provides extensive
exposure for curative treatment and allows control of the internal
iliac and sacral vessels to reduce the bleeding risk. However, one
disadvantage is that it often requires longer surgery times and
position changes, which can increase the risk of complications,
such as pelvic instability and neurological deficits in the
perineal or lower limb areas.
Radiation therapy has a pivotal role in the
management of presacral tumors, especially malignant lesions such
as chordomas and soft-tissue sarcomas, and in cases with incomplete
resection (5). Although
conventional three-dimensional conformal radiation therapy provides
a degree of local control, its application in the presacral region
is often limited by the proximity of critical organs (for example,
the rectum, bladder and small intestine), resulting in either
inadequate dose delivery or excessive normal tissue irradiation.
Technological advancements have facilitated the widespread
implementation of precision radiotherapy techniques, including
intensity-modulated radiation therapy (32), stereotactic radiosurgery (33) and image-guided radiation therapy
(34), for sacral tumors.
Conventional chemotherapy demonstrates limited
efficacy for most presacral tumors, and is primarily utilized for
specific pathological subtypes, such as neuroendocrine neoplasms,
malignant teratoid neoplasm, osteosarcoma, chondrosarcoma and
chordoma (24,35,36).
Regarding epidermoid cysts, it was reported that a 66-year-old
female patient with malignant transformation of presacral
epidermoid cysts and parapharyngeal metastasis received FOLFOX
chemotherapy (comprising the drugs folinic acid, fluorouracil and
oxaliplatin) after the operation and achieved good results,
although the effectiveness of this scheme for other presacral
tumors still requires further study (37).
The advent of immune checkpoint inhibitors has
revolutionized treatment paradigms for various malignancies, with
emerging applications in presacral malignant tumors. A previous
study has demonstrated that certain sacral tumors, including
chondrosarcoma and chordomas, exhibit the expression of programmed
death-ligand 1 and immune cell infiltration within the tumor
microenvironment, suggesting potential immunotherapy responsiveness
(38).
In conclusion, in the current case report, a
challenging case of bilateral presacral tumors is presented, where
preoperative MRI was shown to serve a pivotal diagnostic and
surgical planning role. The imaging studies comprehensively
characterized the tumors' benign morphology, while also revealing
significant anatomical complexities that potentially complicated
surgical intervention. Recognizing the intrinsic surgical
challenges posed by the tumors' location and interconnected spatial
configuration, a posterior surgical approach was designed. This
strategic decision was predicated on minimizing potential operative
risks and maximizing surgical precision. Employing intraoperative
MRI guidance, the smaller, potentially challenging tumor was
successfully identified and precisely localized, thereby preventing
potential surgical omission and guaranteeing complete oncological
clearance. This surgical strategy exemplified the synergistic
application of imaging technologies and refined surgical
techniques, successfully mitigating potential operative risks and
facilitating a comprehensive resection with minimal surrounding
tissue trauma.
Several limitations exist in the present study.
First, this is a single-case report with a limited sample size,
which restricts the generalizability of the findings. Second,
standardized quantitative imaging metrics or a reproducible scoring
system to guide surgical approach selection were not established,
which limits methodological reproducibility and clinical
applicability. To address these limitations, future studies should
assemble multicenter, larger cohorts to validate the present
observations and improve external validity, and should develop
standardized quantitative imaging scores and objective preoperative
planning protocols to guide approach selection. Through these
efforts, the diagnostic workflows can be optimized and the
therapeutic strategies refined for presacral lesions.
Acknowledgements
The authors extend their sincere gratitude to
Professor Jingyong Luo and Professor Lijiang Chen from The First
People's Hospital of Xiaoshan District (Hangzhou, China) for
providing invaluable suggestions and guidance in the composition of
this article.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LZ conceived the study. YH gathered medical images,
conducted the surgical procedure and wrote the original draft
manuscript. GL collected, analyzed and interpreted the patient
data. The manuscript was critically reviewed by GL and LZ. All
authors have read and approved the final version of the manuscript.
YH, GL and LZ confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The study was approved by institutional Review Board
of The First People's Hospital of Xiaoshan District (Hangzhou,
China; approval no. 2025-14).
Patient consent for publication
The patient provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baek SK, Hwang GS, Vinci A, Jafari MD,
Jafari F, Moghadamyeghaneh Z and Pigazzi A: Retrorectal tumors: A
comprehensive literature review. World J Surg. 40:2001–2015.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Balci B, Yildiz A, Leventoğlu S and Mentes
B: Retrorectal tumors: A challenge for the surgeons. World J Gastro
Surg. 13:1327–1337. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Brown IS, Sokolova A, Rosty C and Graham
RP: Cystic lesions of the retrorectal space. Histopathology.
82:232–241. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
A E, Prakash A, Ashta A, Garg A, Verma A
and Padaliya P: Pediatric presacral tumors with intraspinal
extension: A rare entity with diagnostic challenges. Acta Radiol.
64:3056–3073. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Noël G, Habrand JL, Jauffret E, de
Crevoisier R, Dederke S, Mammar H, Haie-Méder C, Pontvert D,
Hasboun D, Ferrand R, et al: Radiation therapy for chordoma and
chondrosarcoma of the skull base and the cervical spine. Prognostic
factors and patterns of failure. Strahlenther Onkol. 179:241–248.
2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Barraqué M, Filippello A, Brek A, Baccot
S, Porcheron J and Barabino G: Surgical management of retro-rectal
tumors in the adult. J Visc Surg. 156:229–237. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cearns MD, Hettige S, De Coppi P and
Thompson DNP: Currarino syndrome: Repair of the dysraphic anomalies
and resection of the presacral mass in a combined neurosurgical and
general surgical approach. J Neurosurg Pediatr. 22:584–590.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Emerson RE, Kao CS, Eble JN, Grignon DJ,
Wang M, Zhang S, Wang X, Fan R, Masterson TA, Roth LM and Cheng L:
Evidence of a dual histogenetic pathway of sacrococcygeal
teratomas. Histopathology. 70:290–300. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Otote J, Butnari V, Ravichandran PS,
Mansuri A, Ahmed M, Pestrin O, Rajendran N and Kaul S: Presacral
tumors: A systematic review of literature. J Clin Imag Sci.
14(17)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yu ML, Lee KH, Fang B and Lau V: Sacral
tumours and their mimics: Pictorial review and diagnostic strategy.
Clin Radiol. 76:153.e9–153.e16. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fuchs B, Dickey ID, Yaszemski MJ, Inwards
CY and Sim FH: Operative management of sacral chordoma. J Bone
Joint Surg Am. 87:2211–2216. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yin P, Chen W, Fan Q, Yu R, Liu X, Liu T,
Wang D and Hong N: Development and evaluation of a deep learning
framework for pelvic and sacral tumor segmentation from
multi-sequence MRI: A retrospective study. Cancer Imaging.
25(34)2025.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Malutan AM, Suciu VE, Ignat FL, Diculescu
D, Ciortea R, Boțan EC, Bucuri CE, Roman MP, Nati I, Ormindean C
and Mihu D: Tailgut cyst-gynecologist's pitfall: Literature review
and case report. Diagnostics (Basel). 15(108)2025.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang YK, Chan CM, Zhang Q, Xu HR and Niu
XH: Computer navigation-aided resection of sacral chordomas. Chin
Med J (Engl). 129:162–168. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Huang W, Peng Y, Zhang Y, Chao F, Li L,
Qiu Y, Gao J and Kang L: Multimodality imaging of an unusual giant
cell tumor of thoracic spine with mediastinal invasion: A case
report. Am J Nucl Med Mol Imaging. 13:289–294. 2023.PubMed/NCBI
|
|
16
|
Jin CJ, Berry-Candelario J, Reiner AS,
Laufer I, Higginson DS, Schmitt AM, Lis E, Barzilai O, Boland P,
Yamada Y and Bilsky MH: Long-term outcomes of high-dose
single-fraction radiosurgery for chordomas of the spine and sacrum.
J Neurosurg Spine. 32:79–88. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Weidlich A, Schaser KD, Weitz J, Kirchberg
J, Fritzmann J, Reeps C, Schwabe P, Melcher I, Disch A, Dragu A, et
al: Surgical and oncologic outcome following sacrectomy for primary
malignant bone tumors and locally recurrent rectal cancer. Cancers
(Basel). 16(2334)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Batukan C, Ozgun MT and Basbug M: First
trimester diagnosis of sacrococcygeal teratoma using two- and
three-dimensional ultrasound. J Clin Ultrasound. 39:160–163.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kim JW and Shin SS: Ultrasound-guided
percutaneous core needle biopsy of abdominal viscera: Tips to
ensure safe and effective biopsy. Korean J Radiol. 18:309–322.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Patel N, Maturen KE, Kaza RK, Gandikota G,
Al-Hawary MM and Wasnik AP: Imaging of presacral masses-a
multidisciplinary approach. Brit J Radiol.
89(20150698)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Makni A, Fetirich F, Mbarek M and Ben
Safta Z: Presacral schwannoma. J Visc Surg. 149:426–427.
2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hebert-Blouin M, Sullivan PS, Merchea A,
Leonard D, Spinner RJ and Dozois EJ: Neurological outcome following
resection of benign presacral neurogenic tumors using a
nerve-sparing technique. Dis Colon Rectum. 56:1185–1193.
2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Raskin KA, Schwab JH, Mankin HJ,
Springfield DS and Hornicek FJ: Giant cell tumor of bone. J Am Acad
Orthop Surg. 21:118–126. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Whelan JS and Davis LE: Osteosarcoma,
chondrosarcoma, and chordoma. J Clin Oncol. 36:188–193.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Burke JR, Shetty K, Thomas O, Kowal M,
Quyn A and Sagar P: The management of retrorectal tumours: Tertiary
centre retrospective study. BJS Open. 6(zrac044)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Galán C, Hernández MP, Martínez MC,
Sánchez A, Bollo J and Targarona EM: Surgical treatment of
retrorectal tumors: A plea for a laparoscopic approach. Surg
Endosc. 37:9080–9088. 2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Naseri A, Behboudi B, Faryabi A, Tafti
SMA, Sharifi A, Keramati MR, Fazeli MS, Keshvari A, Zeinalizadeh M,
Asbagh RA, et al: Surgical management of retrorectal tumors: A
single-center 12 years' experience. Ann Coloproctol: Oct 11, 2022
(Epub ahead of print).
|
|
28
|
Riojas CM, Hahn CD and Johnson EK:
Presacral epidermoid cyst in a male: A case report and literature
review. J Surg Educ. 67:227–232. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yoshida N, Nakamura K, Shigaki T,
Fujiyoshi K, Koushi K, Yoshida T, Isobe T, Mori N, Sudo T, Sakai H,
et al: Laparoscopic transabdominal approach for resection of
presacral epidermoid cyst in an obese man: A case report. Surg Case
Rep. 10(120)2024.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Aranda-Narváez JM, González-Sánchez AJ,
Montiel-Casado C, Sánchez-Pérez B, Jiménez-Mazure C, Valle-Carbajo
M and Santoyo-Santoyo J: Posterior approach (Kraske procedure) for
surgical treatment of presacral tumors. World J Gastrointest Surg.
4:126–130. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Böhm B, Milsom JW, Fazio VW, Lavery IC,
Church JM and Oakley JR: Our approach to the management of
congenital presacral tumors in adults. Int J Colorectal Dis.
8:134–138. 1993.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Osborn VW, Lee A and Yamada Y:
Stereotactic body radiation therapy for spinal malignancies.
Technol Cancer Res Treat. 17(1533033818802304)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhuang H, Zhuang H, Lang N and Liu J:
Precision stereotactic radiotherapy for spinal tumors: Mechanism,
efficacy, and issues. Front Oncol. 10(826)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sterzing F, Engenhart-Cabillic R, Flentje
M and Debus J: Image-guided radiotherapy: A new dimension in
radiation oncology. Dtsch Arztebl Int. 108:274–280. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Violante T, Murphy B, Ferrari D, Graham
RP, Navin P, Merchea A, Larson DW, Dozois EJ, Halfdanarson TR and
Perry WR: Presacral Neuroendocrine neoplasms: A multi-site review
of surgical outcomes. Ann Surg Oncol. 31:4551–4557. 2024.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nakano Y, Hasegawa D, Stewart DR, Schultz
KAP, Harris AK, Hirato J, Uemura S, Tamura A, Saito A, Kawamura A,
et al: Presacral malignant teratoid neoplasm in association with
pathogenic DICER1 variation. Mod Pathol. 32:1744–1750.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kitano D, Komatsu Y and Omori M: A case of
oropharyngeal carcinoma accompanying a presacral malignant
epidermoid cyst. Cureus. 16(e69841)2024.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Traylor JI, Pernik MN, Plitt AR, Lim M and
Garzon-Muvdi T: Immunotherapy for chordoma and chondrosarcoma:
Current evidence. Cancers (Basel). 13(2408)2021.PubMed/NCBI View Article : Google Scholar
|