Introduction
Coronavirus disease 2019 (COVID-19) is known to
cause a variety of systemic and ocular manifestations, including
conjunctivitis, retinal vein occlusion, acute macular
neuroretinopathy and neovascular glaucoma (1-3).
Following adjustment of the national dynamic zero-COVID strategy in
China, a rapid surge in cases of COVID-19 occurred after November
14, 2022, particularly in Beijing (4). In December 2022 during an outbreak of
the Omicron variant of COVID-19, the number of individuals with
acute primary angle closure (APAC) following COVID-19 was observed
to increase sharply. Due to the Omicron variant s combination
of high transmissibility, altered tropism for ACE2-rich tissues and
ability to provoke a significant systemic inflammatory
response-even in mild cases - are the key characteristics that may
explain its observed association with unmasking or triggering APAC
in a broader, and often younger, population with susceptible ocular
anatomy (1). Several
ophthalmologists expressed concern regarding the potential impact
of COVID-19 on patients with APAC (5-7).
Zhou et al (5) reported
that during the 2020 lockdown in Wuhan, the incidence of blindness
secondary to APAC was higher than that during in 2021, when the
lockdown policy was not implemented. Barosco et al (6) reported a bilateral case of APAC that
may have been caused by COVID-19 treatment. In addition, Au
(7) described a case of monocular
APAC following COVID-19 during the Omicron outbreak in Hong Kong,
China.
Previous studies have mainly focused on the impact
of the COVID-19 pandemic on the self-management, telemedicine,
prognosis and mental state of patients with glaucoma, and research
on the clinical characteristics and pathogenesis of APAC following
COVID-19 is limited (8-10).
The present study reports on the clinical characteristics and
possible pathogenesis of APAC following COVID-19 infection and
their differences from those of APAC without COVID-19.
Patients and methods
Participants and perioperative
assessments
The present retrospective study of patients with
APAC following COVID-19 was performed at the Xiamen Eye Center of
Xiamen University (Xiamen, China). The study was approved by the
Institutional Review Board of Xiamen Eye Center (approval no.
XMYKZX-KY-2023-003) and was conducted in accordance with the tenets
of the Declaration of Helsinki. All analyzed data were anonymized
and deidentified.
All enrolled participants underwent a series of
ocular examinations and received appropriate treatments.
Demographic data, including age, sex and laterality,
epidemiological data, including the time interval between fever and
APAC onset (IFO), ocular parameters such as the anterior chamber
depth (ACD), lens thickness and lens nucleus hardness graded using
the Lens Opacities Classification System III (11), and treatment procedures were
collected from the electronic outpatient medical record system.
Ocular biometry was performed at presentation using a Lenstar
LS-900 instrument (Haag-Streit Diagnostics) and ultrasound
biomicroscopy (UBM) was also conducted. Pupil diameter measurements
were obtained without the administration of any antiglaucoma
treatments or drugs for miosis or intraocular pressure (IOP)
reduction. A single examiner (YX) assessed the anterior chamber
angle structure, including the quadrants of angle closure, and
evaluated ciliary body morphology for the presence or absence of
ciliary body detachment under darkroom conditions using UBM.
Grouping and perioperative
managements
The study included a post-COVID-19 group and a
pre-pandemic group. The inclusion criteria for the post-COVID-19
group were as follows: All patients diagnosed with APAC who had a
positive result for a severe acute respiratory syndrome coronavirus
2 (SARS-CoV-2) RNA test within 15 days prior to APAC onset, and who
first presented at the eye center between December 1, 2022 and
January 31, 2023. The exclusion criteria for the post-COVID-19
group were: i) Patients with APAC who had not undergone SARS-CoV-2
RNA testing; ii) patients with APAC who had previously undergone
anti-glaucoma surgery or cataract phacoemulsification (PH) with or
without intraocular lens implantation; and iii) patients with APAC
lacking complete epidemiological or ophthalmic examination data.
The pre-pandemic group comprised patients with APAC and complete
essential data who presented between December 1, 2018 and January
31, 2019, when COVID-19 was not prevalent. All patients, regardless
of their group assignment, were recruited from the Outpatient or
Emergency Department of Xiamen Eye Center. All patients were of
Chinese Han ethnicity, and all eyes affected by APAC in each
enrolled patient were included in the analysis.
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was performed to detect the open reading frame
1ab (ORF1ab) and nucleocapsid (N) genes of SARS-CoV-2 using a
TaqMan probe-based RT-qPCR method. A novel Coronavirus (2019-nCoV)
Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) (Sansure
Biotech Inc.) was used according to the manufacturer s
instructions, with human ribonuclease P as the internal control
gene. If both the ORF1ab and N genes exhibited a clear exponential
amplification phase with a cycle threshold value of ≤36, the result
was considered positive. The specific protocol encompassed three
main steps: First, RNA was extracted from nasopharyngeal swab
samples using the Sansure kit s proprietary lysis/binding
buffer, followed by reverse transcription at 50˚C for 30 min to
synthesize cDNA. Subsequent qPCR detection, employing undisclosed
primer and probe sequences, was then performed. The thermal cycling
conditions included cDNA pre denaturation at 95˚C for 1 min,
followed by 45 amplification cycles (denaturation at 95˚C for 15
sec, and annealing, extension and fluorescence collection at 60˚C
for 30 sec), yielding final cycle threshold values.
The diagnostic criteria for APAC were as follows
(5,12): i) Presence of at least two of the
following symptoms: Ocular or periocular pain, nausea and/or
vomiting, or a history of intermittent blurring of vision with
halos; ii) a IOP of >21 mmHg at presentation; and iii) presence
of at least one of the following signs: Conjunctival hyperemia,
cornea edema, a mid-dilated unreactive pupil or a shallow anterior
chamber. APAC was diagnosed by ophthalmologists (both YX and LZ).
In cases of disagreement, another two expert ophthalmologist (both
JZ and YW) were consulted to make the final decision.
All patients with a definite diagnosis of APAC,
regardless of group assignment, were managed according to the
following procedures. First, all patients were treated with a
miotic agent (2% pilocarpine, one drop every 10 min, 6 times in
total). The accompanying IOP-lowering eyedrops were selected from a
range of standard classes (beta-blocker, 2% carteolol, one drop,
twice daily; alpha-2 adrenergic agonist, 0.1% brimonidine, one
drop, 3 times daily; carbonic anhydrase inhibitor, 1% brinzolamide,
one drop, twice daily). The specific combination (1, 2 or 3 agents)
was personalized based on the patient s initial IOP (e.g.,
starting with 2 or 3 agents for high IOP >35 mmHg),
contraindications (e.g., beta-blockers for patients without
asthma), and, most importantly, their real-time response to the
initial treatment (e.g., potentially adding or removing agents
based on efficacy and tolerance), as outlined in the stepwise
management protocol. In summary, while the available menu of drugs
was standardized (carteolol, brimonidine, brinzolamide), the final
regimen for each patient was tailored based on a clinical decision
that integrated the initial presentation, patient-specific factors
and the observed therapeutic response. The use of glucocorticoid
eyedrops (typically 1% prednisolone acetate, one drop 4-8 times
daily) was guided by the severity of anterior chamber inflammation
(usually 1% prednisolone acetate, one drop, 4-8 times daily) and
tailored at the physician s discretion until surgery, rather
than adhering to a fixed, standardized protocol for all
patients.
After 1 h, if the IOP had not decreased, 250 ml
intravenous mannitol drip and oral ocular hypotensive medication
were administered, provided no contraindications existed (e.g.,
severe renal impairment, progressive heart failure, electrolyte
imbalances preclude mannitol, sulfa allergy, electrolyte imbalances
preclude acetazolamide). If these measures failed to reduce the IOP
to the normal range, argon laser peripheral iridoplasty (ALPI) with
or without yttrium aluminum garnet laser peripheral iridotomy (LPI)
was performed. Once the IOP was adequately reduced, appropriate
anti-glaucoma surgery was carried out in patients who meet the
surgical indications (10) to
prevent recurrent elevated IOP. All patients with APAC ultimately
underwent anti-glaucoma surgery, which including one of the
following three procedures: i) PH combined with goniosynechialysis
(GSL), ii) PH combined with GSL plus gonioscopy-assisted
transluminal trabeculotomy (GATT) or endoscopic
cyclophotocoagulation (ECP) and iii) trabeculectomy with or without
PH.
All surgical procedures were performed by one of two
glaucoma specialists (JZ or YW). All included patients, regardless
of group, were examined and operated on using the same equipment
and by the same technicians or glaucoma specialists.
Statistical analysis
All statistical analyses were performed using SPSS
Statistics for Windows software (version 25.0; IBM Corp.).
Continuous variables are expressed as the mean ± standard
deviation, median (interquartile range) or median (range),
depending on the data distribution. Categorical variables are
presented as counts and percentages. The Shapiro-Wilk test was used
to assess data normality.
Best corrected visual acuity (BCVA) values were
converted to logarithm of the minimum angle of resolution (logMAR)
units for analysis. For BCVA of counting fingers or worse, the
following conversions were applied: Counting fingers, 2.0 logMAR;
hand movements, 2.3 logMAR; light perception, 2.6 logMAR; and no
light perception, 2.9 logMAR (13).
Students t-test was used to compare continuous
variables, such as demographic data, ocular characteristics,
treatments and outcomes between two groups, where appropriate.
Mann-Whitney U test was used to compare two groups of hierarchical
variables, such as the number of quadrants of anterior chamber
angle closure; and Chi-square or Fisher s exact tests were
used to compare categorical variables.
To account for inter-eye correlation, a generalized
estimating equation (GEE) was used to further validate the key
variables, namely ACD and lens thickness, that exhibited
significant differences.
All patients with APAC following COVID-19 were
further divided into two groups according to sex, age (≤65 and
>65 years) or laterality, and the differences in demographic
features, the characteristics of affected eyes, and treatment
options between the two groups were compared. Two-sided P<0.05
was considered to indicate a statistically significant
difference.
Results
Demographic and epidemiological
findings of all recruited patients with APAC
A total of 86 eyes (43 right and 43 left) from 78
patients with APAC (21 males and 57 females) were enrolled in the
post-COVID-19 group and another 50 eyes (28 right and 22 left) from
48 patients with APAC (16 males and 32 females) were enrolled in
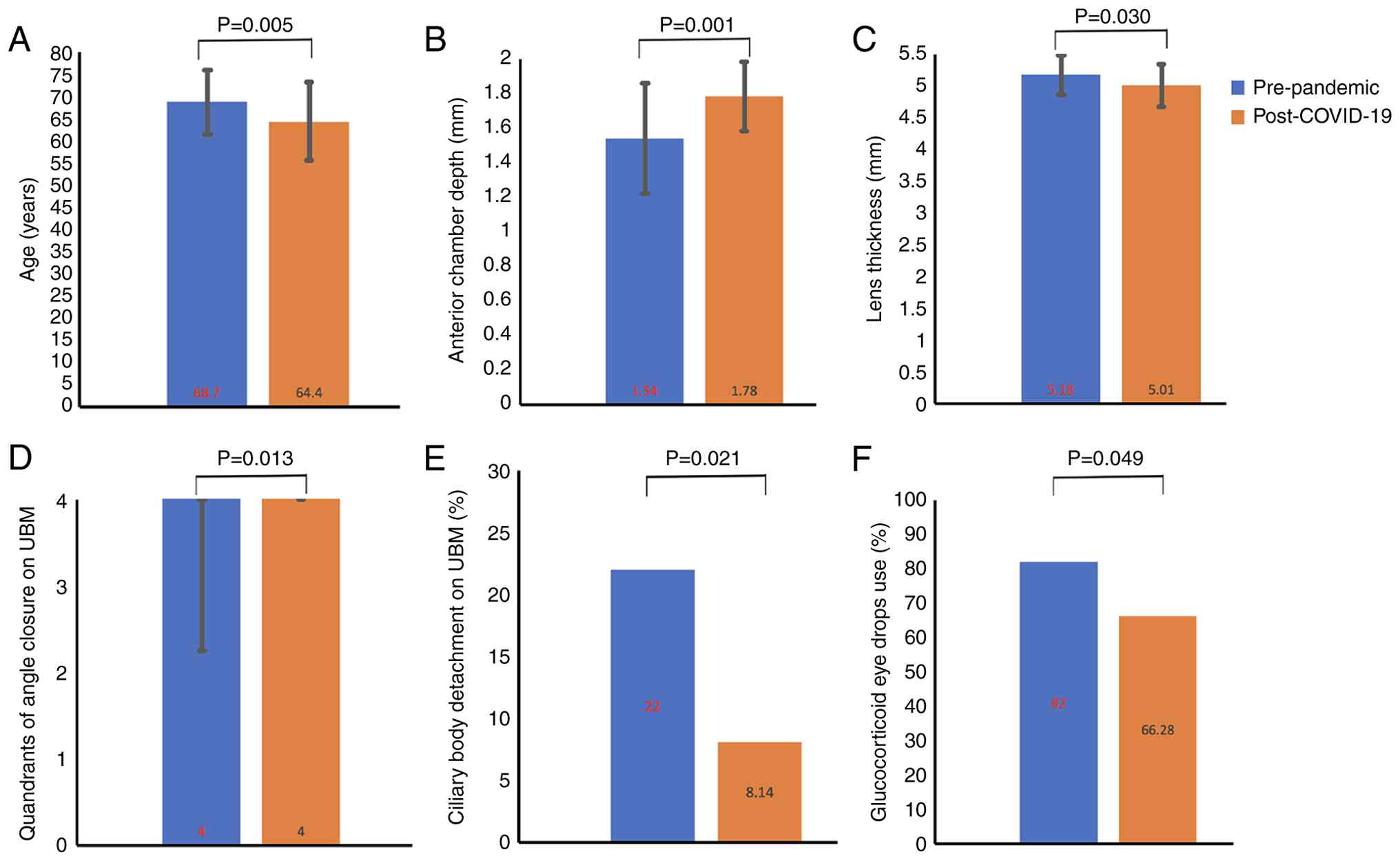
the pre-pandemic group. There was a significant difference in age
(64.4±8.8 vs. 68.7±7.2 years; P=0.005; Fig. 1A), but no statistically significant
difference in male percentage (26.92 vs. 33.33%; P=0.443) or
laterality distribution (right, 44.87%; left, 44.87%; both, 10.26%
vs. right, 54.17%; left, 41.67%; both 4.17%; P=0.370) between the
post-COVID-19 and pre-pandemic groups, respectively.
In the epidemiological analysis, 74.36% (58/78) of
the patients in the post-COVID-19 group experienced fever before
APAC onset. The median IFO was 1 day (range, 0-15 days) and the
median fever duration was 2 days (range, 0-4 days). Among patients
with fever, the maximum recorded body temperature was 39˚C and the
mean maximum body temperature was 38.26±0.56˚C. All 78 patients
manifested with either mild COVID-19 symptoms or were
asymptomatic.
When clinical manifestations and ocular structural
parameters were compared between the two groups, the post-COVID-19
group had a significantly greater ACD (1.78±0.20 vs. 1.54±0.32 mm;
P=0.001; Fig. 1B), thinner lens
(5.01±0.33 vs. 5.18±0.31 mm; P=0.030; Fig. 1C), more quadrants of angle closure
on UBM [4.00 (4.00-4.00) vs. 4.00 (2.25-4.00); P=0.013; Fig. 1D] and a lower prevalence of ciliary
body detachment (8.14 vs. 22.00%; P=0.021; Fig. 1E) compared with the pre-pandemic
group. By contrast, the post-COVID-19 and pre-pandemic groups had
similar median lens nucleus hardness gradings [2 (2-3) vs. 3 (2-3);
P=0.437], BCVA at first visit (1.28±0.77 vs. 1.49±0.80 logMAR;
P=0.336), axial length (AL; 22.35±0.73 vs. 22.37±0.59 mm; P=0.964),
maximum IOP (48.74±9.11 vs. 51.29±9.65 mmHg; P=0.319), prevalence
of glaucomatous subcapsular flecks (24.42 vs. 16.00%; P=0.248),
previous history of APAC (6.98 vs. 0.00%; P=0.140), pupil diameter
without any treatment (5.73±1.34 vs. 5.87±1.30 mm; P=0.715) and
corneal thickness (611.14±93.08 vs. 683.31±162.73 µm; P=0.106). The
demographic and clinical characteristics of all enrolled
participants with APAC and their affected eyes are summarized in
Table I.
 | Table IDemographic, epidemiological and
clinical characteristics of all patients with APAC. |
Table I
Demographic, epidemiological and
clinical characteristics of all patients with APAC.
| | Group | |
|---|
| Baseline
characteristics | Pre-pandemic (n=48)
unfavorable group group 1 group 2 | Post-COVID-19
(n=78) | P-value |
|---|
| Males | 16 (33.33) | 21 (26.92) | 0.443 |
| Age, years | 68.7±7.2 | 64.4±8.8 | 0.005 |
| Laterality | | | 0.370 |
|
Right | 26 (54.17) | 35 (44.87) | |
|
Left | 20 (41.67) | 35 (44.87) | |
|
Both | 2 (4.17) | 8 (10.26) | |
| Fever | - | 58 (74.36) | |
| Maximum body
temperature,˚C | - | 38.26±0.56 | |
| IFO,
daysa | - | 1 (0-15) | |
| Fever duration,
daysa | - | 2 (0-4) | |
| No. of affected
eyes | 50 | 86 | |
| ACD, mm | 1.54±0.32 | 1.78±0.20 | 0.001 |
| Lens thickness,
mm | 5.18±0.31 | 5.01±0.33 | 0.030 |
| Lens nucleus
hardness, gradeb | 3 (2-3) | 2 (2-3) | 0.437 |
| BCVA at first
visit, logMAR | 1.49±0.80 | 1.28±0.77 | 0.336 |
| AL, mm | 22.37±0.59 | 22.35±0.73 | 0.964 |
| Maximum IOP,
mmHg | 51.29±9.65 | 48.74±9.11 | 0.319 |
| Glaucomatic
subcapsular flecks | 8 (16.00) | 21 (24.42) | 0.248 |
| Previous history of
APAC | 0 (0) | 6 (6.98) | 0.140 |
| PD without any
treatments, mm | 5.87±1.30 | 5.73±1.34 | 0.715 |
| Corneal thickness,
µm | 683.31±162.73 | 611.14±93.08 | 0.106 |
| Ciliary body
detachment on UBM | 11 (22.00) | 7 (8.14) | 0.021 |
| Quadrants of angle
closure on UBMb | 4.00
(2.25-4.00) | 4.00
(4.00-4.00) | 0.013 |
| Treatments | | | |
|
Glucocorticoid
eye drops | 41 (82.00) | 57 (66.28) | 0.049 |
|
ALPI | 4 (8.00) | 3 (3.49) | 0.456 |
|
LPI | 0 (0) | 3 (3.49) | 0.465 |
|
ALPI +
LPI | 0 (0) | 3 (3.49) | 0.465 |
|
PH +
GSL | 19 (38.00) | 33 (38.37) | 0.966 |
|
PH + GSL +
ECP/GATT | 18 (36.00) | 28 (32.56) | 0.682 |
|
Trabeculectomy | 13 (26.00) | 25 (29.07) | 0.700 |
In the comparison of ACD and lens thickness between
the two groups, Students t-test showed a significant
difference. However, since some patients contributed both eyes to
the analysis, GEEs were used to account for inter-eye correlation.
For ACD, the GEE analysis confirmed a significant group effect
(Wald Chi-square, 13.647; P<0.001), with no significant effect
of laterality (Wald Chi-square, 0.005; P=0.943) or group x
laterality interaction (Wald Chi-square, 0.313; P=0.576). After
removing the non-significant group x laterality interaction term,
GEE analysis revealed that the group effect remained significant
(Wald Chi-square, 13.208; P<0.001), while laterality remained
non-significant (Wald Chi-square, 0.128; P=0.720). In the
comparison of lens thickness between the two groups, GEE analysis
also showed a significant group effect (Wald Chi-square, 6.257;
P=0.012), with non-significant laterality (Wald Chi-square, 1.277;
P=0.258) and group x laterality interaction (Wald Chi-square,
3.040; P=0.081). After removing the group x laterality interaction,
further GEE revealed that the group effect remained significant
(Wald Chi-square, 5.421; P=0.020) and laterality remained
non-significant (Wald Chi-square, 0.172; P=0.678).
Comparison of drug, laser and surgical
treatment methods between the post-COVID-19 and pre-pandemic
groups
In the comparison of treatment methods, the
proportion of cases treated in with glucocorticoid eyedrops in the
post-COVID-19 group was lower than that in the pre-pandemic group
(66.28 vs. 82.00%; P=0.049; Fig.
1F). However, there were no significant differences in the
proportions of patients treated with ALPI (3.49 vs. 8.00%;
P=0.456), LPI (3.49 vs. 0.00%; P=0.465), ALPI combined with LPI
(3.49 vs. 0.00%; P=0.465), PH combined with GSL (38.37 vs. 38.00%;
P=0.966), PH combined with GSL and either ECP or GATT (32.56 vs.
36.00%; P=0.682) and trabeculectomy with or without PH (29.07 vs.
26.00%; P=0.700) between the post-COVID-19 and pre-pandemic groups,
respectively. The detailed treatment methods of all participants
and affected eyes are summarized in Table I.
Comparison of demographic and clinical
characteristics between the sexes
All patients with APAC following COVID-19 were
divided into male (22 eyes from 21 men) and female (64 eyes from 57
women) groups according to sex. Analysis revealed that the female
group had a shallower ACD (1.74±0.18 vs. 1.86±0.24 mm; P=0.048),
higher BCVA at first visit (1.36±0.76 vs. 0.94±0.74 logMAR;
P=0.031) and shorter AL (22.22±0.69 vs. 22.92±0.59 mm; P=0.001).
However, no significant differences between the male and female
groups were detected for any other clinical features or treatments.
A comparison of the demographic and clinical characteristics
between male and female patients following COVID-19 is presented in
Table II.
 | Table IIComparison of demographic and
clinical characteristics between males and females with APAC
following COVID-19. |
Table II
Comparison of demographic and
clinical characteristics between males and females with APAC
following COVID-19.
| | Group | |
|---|
| Baseline
characteristics | Male (n=21) | Female (n=57) | P-value |
|---|
| Age, years | 62.5±10.2 | 64.9±8.6 | 0.328 |
| Laterality | | | 0.349 |
|
Right | 8 (38.10) | 27 (47.37) | |
|
Left | 12 (57.14) | 23 (40.35) | |
|
Both | 1 (4.76) | 7 (12.28) | |
| Fever | 16 (76.19) | 42 (73.68) | 0.822 |
| Maximum body
temperature,˚C | 38.36±0.47 | 38.22±0.59 | 0.548 |
| IFO,
daysa | 1 (0-10) | 1 (0-15) | 0.581 |
| Fever duration,
daysa | 1 (0-3) | 2 (0-4) | 0.160 |
| No. of affected
eyes | 22 | 64 | |
| ACD, mm | 1.86±0.24 | 1.74±0.18 | 0.048 |
| Lens thickness,
mm | 5.14±0.27 | 4.97±0.36 | 0.117 |
| Lens nucleus
hardness, gradeb | 2 (2-3) | 2 (2-3) | 0.293 |
| BCVA at first
visit, log MAR | 0.94±0.74 | 1.36±0.76 | 0.031 |
| AL, mm | 22.92±0.59 | 22.22±0.69 | 0.001 |
| Maximum IOP,
mmHg | 48.39±7.95 | 48.89±9.76 | 0.851 |
| Glaucomatic
subcapsular flecks | 3 (13.64) | 18 (28.13) | 0.172 |
| Previous history of
APAC | 2 (9.09) | 4 (6.25) | 1.000 |
| PD without any
treatments, mm | 5.82±1.30 | 5.71±1.34 | 0.781 |
| Corneal thickness,
µm | 617.07±73.99 | 615.00±104.72 | 0.946 |
| Ciliary body
detachment on UBM | 0 (0) | 7 (10.94) | 0.243 |
| Quadrants of angle
closure on UBMb | 4.00
(3.75-4.00) | 4.00
(4.00-4.00) | 0.685 |
| Treatments | | | |
|
Glucocorticoid
eye drops | 17 (77.27) | 40 (62.50) | 0.206 |
|
PH +
GSL | 8 (36.36) | 25 (39.06) | 0.822 |
|
PH + GSL +
ECP/GATT | 7 (36.00) | 21 (32.56) | 0.932 |
|
Trabeculectomy | 7 (31.82) | 18 (28.13) | 0.742 |
Comparison of demographic and clinical
features by age and laterality
Analysis by age group showed that patients aged
>65 years (42 eyes from 39 cases) developed APAC at a median
time of 1 day (range, 0-15 days) after COVID-19-induced fever,
while patients aged ≤65 years (44 eyes from 39 cases) had a
significantly longer median APAC development time of 2 days (range,
0-15 days) (P=0.023). In addition, the older patients had a
shallower ACD (1.76±0.18 vs. 1.79±0.22 mm; P=0.030), thicker lens
(5.17±0.27 vs. 4.87±0.34 mm; P=0.001), harder lens nucleus [3 (3-3)
vs. 2 (2-2); P<0.001] and a higher incidence of ciliary body
detachment on UBM (16.67 vs. 0.00%; P=0.015) than younger patients.
They also had a higher frequency of treatment with glucocorticoid
eyedrops (80.95 vs. 52.27%; P=0.005), or PH combined with GSL plus
either ECP or GATT (45.24 vs. 20.45%; P=0.014), and fewer
trabeculectomies with or without PH (19.05 vs. 38.64%; P=0.014). A
detailed comparison of the demographic and clinical features by age
grouping is shown in Table
III.
 | Table IIIComparison of demographic and
clinical characteristics between two age groups of patients with
APAC following COVID-19. |
Table III
Comparison of demographic and
clinical characteristics between two age groups of patients with
APAC following COVID-19.
| | Group | |
|---|
| Baseline
characteristics | ≤65 years
(n=39) | 65 years
(n=39) | P-value |
|---|
| Laterality | | | 0.479 |
|
Right | 19 (48.72) | 16 (41.03) | |
|
Left | 15 (38.46) | 20 (51.28) | |
|
Both | 5 (12.82) | 3 (7.69) | |
| Fever | 30 (76.92) | 28 (71.79) | 0.604 |
| Maximum body
temperature,˚C | 38.34±0.63 | 38.16±0.47 | 0.350 |
| IFO,
daysa | 2 (0-15) | 1 (0-15) | 0.023 |
| Fever duration,
daysa | 1 (0-3) | 1 (0-4) | 0.787 |
| No. of affected
eyes | 44 | 42 | |
| ACD, mm | 1.79±0.22 | 1.76±0.18 | 0.030 |
| Lens thickness,
mm | 4.87±0.34 | 5.17±0.27 | 0.001 |
| Lens nucleus
hardness, gradeb | 2 (2-2) | 3 (3-3) | <0.001 |
| BCVA at first
visit, logMAR | 1.31±0.79 | 1.26±0.76 | 0.847 |
| AL, mm | 22.34±0.71 | 22.46±0.75 | 0.519 |
| Maximum IOP,
mmHg | 50.90±8.12 | 48.17±10.04 | 0.272 |
| Glaucomatic
subcapsular flecks | 13 (29.55) | 8 (19.05) | 0.257 |
| Previous history of
APAC | 1 (2.27) | 5 (11.90) | 0.184 |
| PD without any
treatments, mm | 5.78±1.25 | 5.81±1.48 | 0.921 |
| Corneal thickness,
µm | 621.74±0.39 | 608.85±105.57 | 0.625 |
| Ciliary body
detachment on UBM | 0 (0) | 7 (16.67) | 0.015 |
| Quadrants of angle
closure on UBMb | 4.00
(4.00-4.00) | 4.00
(3.00-4.00) | 0.067 |
| Treatments | | | |
|
Glucocorticoid
eyedrops | 23 (52.27) | 34 (80.95) | 0.005 |
|
PH +
GSL | 18 (40.91) | 15 (35.71) | 0.620 |
|
PH + GSL +
ECP/GATT | 9 (20.45) | 19 (45.24) | 0.014 |
|
Trabeculectomy | 17 (38.64) | 8 (19.05) | 0.046 |
Finally, a comparison was performed according to
laterality (43 eyes in both the from right and left groups). The
results revealed no significant differences between these groups
for all eye structure parameters and treatment options. A detailed
comparison of demographic and clinical features by laterality
grouping is presented in Table
IV.
 | Table IVComparison of clinical
characteristics between eyes affected with APAC following
COVID-19. |
Table IV
Comparison of clinical
characteristics between eyes affected with APAC following
COVID-19.
| | Group by
laterality | |
|---|
| Baseline
characteristics | Right | Left | P-value |
|---|
| No. of affected
eyes | 43 | 43 | |
| ACD, mm | 1.76±0.18 | 1.80±0.23 | 0.427 |
| Lens thickness,
mm | 5.03±0.36 | 4.98±0.30 | 0.612 |
| Lens nucleus
hardness, gradea | 2 (2-3) | 2 (2-3) | 0.842 |
| BCVA at first
visit, logMAR | 1.30±0.74 | 1.28±0.81 | 0.931 |
| AL, mm | 22.38±0.66 | 22.33±0.83 | 0.821 |
| Maximum IOP,
mmHg | 48.29±10.39 | 49.28±7.46 | 0.633 |
| Glaucomatic
subcapsular flecks | 11 (25.58) | 10 (23.26) | 0.802 |
| Previous history of
APAC | 2 (4.65) | 4 (9.30) | 0.672 |
| PD without any
treatments, mm | 5.77±1.46 | 5.69±1.14 | 0.8022 |
| Corneal thickness,
µm | 617.60±94.78 | 603.07±91.99 | 0.542 |
| Ciliary body
detachment on UBM | 3 (6.98) | 4 (9.30) | 1.000 |
| Quadrants of angle
closure on UBMb | 4.00
(1.00-4.00) | 4.00
(0.00-4.00) | 0.767 |
| Treatments | | | |
|
Glucocorticoid
eye drops | 30 (69.77) | 27 (62.79) | 0.494 |
|
PH +
GSL | 16 (37.21) | 17 (39.53) | 0.825 |
|
PH + GSL +
ECP/GATT | 12 (27.91) | 16 (37.21) | 0.357 |
|
Trabeculectomy | 15 (34.88) | 10 (23.26) | 0.235 |
Discussion
In clinical practice, it was observed that the
two-month period from December 1, 2022 to January 31, 2023, when
the Omicron variant of COVID-19 was most prevalent in China, the
number of emergency cases of APAC increased markedly compared with
that between December 1, 2018 and January 31, 2019. Similarly,
during the COVID-19 epidemic in India, the number of emergency
glaucoma cases increased by 62.4% (14), further suggesting a possible
association between the prevalence of COVID-19 and the increased
presentation of acute ocular emergencies, including APAC.
Among patients with COVID-19, conjunctivitis is the
most common ocular manifestation, with blurred vision occurring in
4.8-12.8% of cases (15). By
contrast, APAC following SARS-CoV-2 infection not only presents
with conjunctival congestion resembling conjunctivitis, but is also
accompanied by eyelid swelling, vision loss and increased IOP.
Therefore, it poses a serious threat to visual health and warrants
greater attention from ophthalmologists. However, the exact
association between COVID-19 and APAC remains unclear.
The present study revealed that following COVID-19
exposure, APAC occurred in younger individuals than during the
pre-pandemic period (64.4±8.8 vs. 68.7±7.2 years; P=0.005), and the
patients had mild or asymptomatic infections. Similar age-related
trends have been reported for other diseases following COVID-19.
For example, Ashkenazy et al (16) described 12 cases of retinal venous
obstruction without hypertension, diabetes, glaucoma, underlying
hypercoagulable states or other common risk factors, all of whom
were under 50 years of age, and Fonollosa et al (17) reported 15 cases of retinal vein
occlusion following COVID-19 with a median age of onset of 39
years. However, Wu et al (18) observed that conjunctivitis
following COVID-19 was more common in patients with more severe
COVID-19 symptoms, and Romero-Castro et al (19) reported that among 117 cases of
severe COVID-19 there were 43 cases with abnormal ocular
manifestations. These reports suggest that while APAC following
COVID-19 is more common in patients with milder infections, the
influence of COVID-19 on the presentation of various ocular
conditions differs depending on disease type and infection
severity.
The time intervals between COVID-19 diagnosis and
the onset of various COVID-19 related diseases are known to differ.
For example, a study revealed that in patients who eventually died,
elevations in blood urea nitrogen and serum creatinine typically
occurred within 28 days following COVID-19 diagnosis (20). The peak incidence of
COVID-19-associated retinal venous occlusion has been reported to
be 6-8 weeks after infection onset (21). In addition, a case of panuveitis
and optic neuritis occurred ~2 weeks after SARS-CoV-2 infection in
60-year-old woman (22), while a
30-year-old man developed acute conjunctivitis in both eyes 13 days
after infection, with SARS-CoV-2 RNA detected in the conjunctival
secretions (23). It may be
inferred from these reports that acute inflammatory reactions
following COVID-19 usually occur within 1 month, particularly
within 2 weeks of infection. Similarly, in the present study, APAC
following COVID-19 developed within 15 days after the onset of
fever, with a median IFO of 1 day. This suggests that APAC onset
following COVID-19 may be associated with the acute inflammatory
reaction caused by SARS-CoV-2.
COVID-19 has been shown to cause extensive vascular
abnormalities in the conjunctiva, choroid and retina. Abdelmassih
et al (24) reported a
group of 14 patients with severe COVID-19, all of whom exhibited
abnormal choroidal angiography. Other studies have reported a
higher susceptibility of the retinal vein to occlusion and reduced
retinal thickness following COVID-19 (15,21,25).
In the present study, it was observed that cases with APAC
following COVID-19 had a greater ACD (1.78±0.20 vs. 1.54±0.32 mm;
P=0.001), thinner lens (5.01±0.33 vs. 5.18±0.31; P=0.030) and more
quadrants of angle closure [4.00 (4.00-4.00) vs. 4.00 (2.25-4.00);
P=0.013] on UBM than those in the pre-pandemic time period. These
findings suggest that APAC following COVID-19 may involve distinct
ocular characteristics and different risk factors than those
observed in pre-pandemic APAC cases (26).
While the post-COVID-19 group had a significantly
deeper average ACD than the pre-pandemic group, the values still
fell within the category of shallow ACD (defined as ACD <2.8 mm)
(27). This suggests that COVID-19
may act as a precipitating factor in eyes with pre-existing shallow
ACD, rather than serving as an independent risk factor for APAC;
specifically, COVID-19 infection may unmask or accelerate APAC in
susceptible individuals. We hypothesize that extended indoor
sedentary activities and reduced sunlight exposure during the
Omicron outbreak may have contributed to the risk of APAC in
SARS-CoV-2-positive patients. There is extensive literature on the
association between APAC onset and environmental factors (28-31).
Teikari et al (28,29) reported that fewer hours of sunshine
were associated with a higher incidence of APAC, and several
studies have reported a higher risk of APAC onset in December than
at other times of the year (29-31).
Coincidentally, the Omicron outbreak in China occurred between
December 1, 2022 and January 31, 2023, when there was little
sunshine and patients spent a larger proportion of time indoors,
thereby increasing the risk of APAC.
Apart from two cases that used 0.2 g ibuprofen for
self-management of myalgia, the vast majority of patients in the
present study with APAC following COVID-19 infection did not
receive any treatment for COVID-19 prior to enrollment, effectively
ruling out the possibility that COVID-19 treatment measures induced
APAC. In addition, the patients with APAC following COVID-19
exhibited a greater ACD and larger number of quadrants with angle
closure compared with those in pre-pandemic cases. This suggests
that the inflammatory response caused by SARS-CoV-2 infection may
serve as an additional induction factor for APAC. The possible
mechanisms underlying this may involve a combined effect involving
both anterior chamber angle blockage and inflammation, rather than
being solely attributable to pupillary block.
Another difference between the two groups of
patients with APAC was that patients with COVID-19-associated APAC
were less prone to ciliary body detachment on UBM (8.14 vs. 22.00%;
P=0.021) and less likely to use glucocorticoid eyedrops (66.28 vs.
82.00%; P=0.049). The reduced requirement for glucocorticoid
eyedrops in the post-COVID-19 group may indicate less disruption of
the blood-aqueous barrier, milder anterior chamber inflammation or
earlier intervention. These findings suggest a distinct
inflammatory profile in COVID-19-associated APAC that could
influence clinical management.
In the subgroup analysis of COVID-19-associated APAC
according to sex, female patients comprised a larger proportion of
the cohort (73.08%) than male patients (26.92%). The female
patients had a shallower ACD (1.74±0.18 vs. 1.86±0.24 mm; P=0.048),
poorer BCVA at first visit (1.36±0.76 vs. 0.94±0.74 log MAR;
P=0.031) and shorter AL (22.22±0.69 vs. 22.92±0.59; P=0.001). These
findings are consistent with previously reported pre-pandemic risk
factors for APAC (26,32-34),
which indicates that the presence of COVID-19 did not alter the
difference in clinical characteristics of APAC and treatment
methods between the sexes.
In the subgroup analysis by age, elderly patients
(>65 years) exhibited a significantly shallower ACD, thicker
lens and harder lens nucleus compared with those in younger
patients. APAC developed in elderly patients at a median IFO of 1
day after fever compared with 2 days in younger patients. These
findings suggest that older patients with pre-existing risk factors
may develop APAC more rapidly following COVID-19. Therefore, it is
necessary to be aware of the risk of APAC following COVID-19 in
elderly patients with APAC-related ocular characteristics. Another
difference observed between two age groups was the choice of
treatment methods. The older patients more frequently received
glucocorticoid eyedrops (80.95 vs. 52.27%; P=0.005), which may
reflect more severe damage of the blood-aqueous barrier. They also
were more frequently treated with PH combined with GSL and either
ECP or GATT (45.24 vs. 20.45%; P=0.014), reflecting more severe
lens opacity. By contrast, younger patients more frequently
underwent trabeculectomy with or without PH (38.64 vs. 19.05%;
P=0.014), likely due to having more quadrants of angle closure on
UBM [4.00 (4.00-4.00) vs. 4.00 (3.00-4.00); P=0.067] but milder
lens changes.
The present study is the first to describe in detail
the clinical characteristics of APAC following COVID-19 during the
Omicron outbreak in China, serving as a reference for the diagnosis
and treatment of patients with APAC during an epidemic outbreak.
However, the study has some limitations. These include its
retrospective, non-randomized design, and the lack of measurement
of systemic inflammatory markers, such as CRP or IL-6, or vitamin D
levels. This limits its ability to support a causal relationship
between COVID-19 and APAC through these pathways or to identify
predictive indicators. In addition, comparing patients from
different time periods may introduce selection bias due to
seasonal, environmental or healthcare variations. To minimize these
effects on COVID-19 as the primary factor under investigation, data
from the same months in the preceding year were used for
comparison. Although this may have introduced some bias, potential
confounding effects were mitigated by maintaining consistency in
equipment, surgical personnel and examiners in both groups.
However, variations in the duration of winter sunshine exposure
between years cannot be excluded. The analysis of patients from
different time periods was necessary as the availability of control
patients over the pandemic period was limited, resulting in an
insufficient sample size of patients without COVID-19 due to the
high infectivity of SARS-CoV-2. Furthermore, patients with APAC
following COVID-19 were younger than those without COVID-19. As no
data on the total number of patients with COVID-19 are available,
it was not possible to determine whether this age difference
reflects an increased susceptibility of younger individuals to
SARS-CoV-2 or a larger population base of younger COVID-19
patients. Moreover, all participants were of Chinese Han ethnicity,
so it is not clear whether the conclusions of the study can be
generalized to other races or nationalities. Finally, due to
limited detection methods, it was not possible to obtain evidence
of the presence of SARS-CoV-2 in the intraocular fluid. Therefore,
the possibility of concurrent intraocular infections in patients
with APAC following SARS-CoV-2 infection cannot be excluded.
In conclusion, patients with APAC following COVID-19
during the Omicron wave presented with a greater ACD compared with
that observed in patients with APAC prior to the pandemic, although
both were within the clinically shallow range. This suggests a
potential pathophysiological mechanism involving both anterior
chamber angle obstruction and inflammatory processes, possibly
triggered by SARS-CoV-2 infection, rather than pure pupillary
block. Ophthalmologists should maintain a high level of vigilance
for APAC in patients post-COVID-19 infection, even in those with
only moderately shallow ACD, and pay particular attention to
individuals with pre-existing anatomic risk factors. However, these
recommendations should be interpreted with caution given the
retrospective design of the study, as well as the single-center
origin and unmeasured potential confounding factors of the
patients. Further large-scale, prospective, multi-center studies
are warranted to validate these observations. These limitations
will be addressed in future research.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors contributions
SS, YX, and JZ conceived and planned the study and
wrote the manuscript. YX, YW and LZ acquired the data. SS, YW and
JZ analyzed the data, and participated in manuscript discussion and
revision. SS conducted statistical analysis of the data. YX and LZ
checked and confirmed the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Review
Board of Xiamen Eye Center (Xiamen, China; approval no.
XMYKZX-KY-2023-003) and was conducted in accordance with the tenets
of the Declaration of Helsinki. All patients provided written
informed consent to participate.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors information
Dr Shancheng Si: ORCID:0009-0003-9159-7972.
References
|
1
|
Pan Y, Wang L, Feng Z, Xu H, Li F, Shen Y,
Zhang D, Liu WJ, Gao GF and Wang Q: Characterisation of SARS-CoV-2
variants in Beijing during 2022: An epidemiological and
phylogenetic analysis. Lancet. 401:664–672. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Soman M, Indurkar A, George T, Sheth JU
and Nair U: Rapid onset neovascular glaucoma due to
COVID-19-related retinopathy. J Curr Glaucoma Pract. 16:136–140.
2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen L, Deng C, Chen X, Zhang X, Chen B,
Yu H, Qin Y, Xiao K, Zhang H and Sun X: Ocular manifestations and
clinical characteristics of 535 cases of COVID-19 in Wuhan, China:
A cross-sectional study. Acta Ophthalmol. 98:e951–e959.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang Y and Stewart JM: Retinal and
choroidal manifestations of COVID-19. Curr Opin Ophthalmol.
32:536–540. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhou L, Wu S, Wang Y, Bao X, Peng T, Luo W
and Ortega-Usobiaga J: Clinical presentation of acute primary angle
closure during the COVID-19 epidemic lockdown. Front Med
(Lausanne). 9(1078237)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Barosco G, Morbio R, Chemello F, Tosi R
and Marchini G: Bilateral angle-closure during hospitalization for
coronavirus disease-19 (COVID-19): A case report. Eur J Ophthalmol.
32:NP75–NP82. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Au SCL: From acute angle-closure to
COVID-19 during Omicron outbreak. Vis J Emerg Med.
29(101514)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tejwani S, Angmo D, Nayak BK, Sharma N,
Sachdev MS, Dada T and Sinha R: Prepared in Association with the
AIOS and GSI Expert Group; Composition of the All India
Ophthalmological Society (AIOS) and Glaucoma Society of India (GSI)
Expert Group includes the Writing Committee (as listed) and the
following members. Devindra Sood, et al: Preferred practice
guidelines for glaucoma management during COVID-19 pandemic. Indian
J Ophthalmol. 68:1277–1280. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pujari R, Chan G and Tapply I:
Addenbrookes Glaucoma COVID response consortium and Bourne RR. The
impacts of COVID-19 on glaucoma patient outcomes as assessed by
POEM. Eye (Lond). 36:653–655. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhou W, Lin H, Ren Y, Lin H, Liang Y, Chen
Y and Zhang S: Mental health and self-management in glaucoma
patients during the COVID-19 pandemic: A cross-sectional study in
China. BMC Ophthalmol. 22(474)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chylack LT Jr, Wolfe JK, Singer DM, Leske
MC, Bullimore MA, Bailey IL, Friend J, McCarthy D and Wu SY: The
lens opacities classification system III. The longitudinal study of
cataract study group. Arch Ophthalmol. 111:831–836. 1993.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gao X, Lv A, Lin F, Lu P, Zhang Y, Song W,
Zhu X, Zhang H, Liao M, Song Y, et al: Efficacy and safety of
trabeculectomy versus peripheral iridectomy plus goniotomy in
advanced primary angle-closure glaucoma: Study protocol for a
multicentre, non-inferiority, randomised controlled trial (the TVG
study). BMJ Open. 12(e 062441)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Niederer RL, Sharief L, Tomkins-Netzer O
and Lightman SL: Uveitis in sarcoidosis-clinical features and
comparison with other non-infectious uveitis. Ocul Immunol Inflamm.
31:367–373. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rajendrababu S, Durai I, Mani I, Ramasamy
KS, Shukla AG and Robin AL: Urgent and emergent glaucoma care
during the COVID-19 pandemic: An analysis at a tertiary care
hospital in South India. Indian J Ophthalmol. 69:2215–2221.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sen M, Honavar SG, Sharma N and Sachdev
MS: COVID-19 and eye: A review of ophthalmic manifestations of
COVID-19. Indian J Ophthalmol. 69:488–509. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ashkenazy N, Patel NA, Sridhar J, Yannuzzi
NA, Belin PJ, Kaplan R, Kothari N, Benitez Bajandas GA, Kohly RP,
Roizenblatt R, et al: Hemi- and central retinal vein occlusion
associated with COVID-19 infection in young patients without known
risk factors. Ophthalmol Retina. 6:520–530. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fonollosa A, Hernández-Rodríguez J,
Cuadros C, Giralt L, Sacristán C, Artaraz J, Pelegrín L,
Olate-Pérez Á, Romero R, Pastor-Idoate S, et al: Characterizing
COVID-19-related retinal vascular occlusions: A case series and
review of the literature. Retina. 42:465–475. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L
and Wu K: Characteristics of ocular findings of patients with
coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA
Ophthalmol. 138:575–578. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Romero-Castro RM, Ruiz-Cruz M, Alvarado-de
la Barrera C, González-Cannata MG, Luna-Villalobos YA,
García-Morales AK, Ablanedo-Terrazas Y, González-Navarro M and
Ávila-Ríos S: Posterior segment ocular findings in critically ill
patients with COVID-19. Retina. 42:628–633. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu YM, Xie J, Chen MM, Zhang X, Cheng X,
Li H, Zhou F, Qin JJ, Lei F, Chen Z, et al: Kidney function
indicators predict adverse outcomes of COVID-19. Med. 2:38–48.e2.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Modjtahedi BS, Do D, Luong TQ and Shaw J:
Changes in the incidence of retinal vascular occlusions after
COVID-19 Diagnosis. JAMA Ophthalmol. 140:523–527. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Benito-Pascual B, Gegúndez JA, Díaz-Valle
D, Arriola-Villalobos P, Carreño E, Culebras E, Rodríguez-Avial I
and Benitez-Del-Castillo JM: Panuveitis and optic neuritis as a
possible initial presentation of the novel coronavirus disease 2019
(COVID-19). Ocul Immunol Inflamm. 28:922–925. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen L, Liu M, Zhang Z, Qiao K, Huang T,
Chen M, Xin N, Huang Z, Liu L, Zhang G and Wang J: Ocular
manifestations of a hospitalised patient with confirmed 2019 novel
coronavirus disease. Br J Ophthalmol. 104:748–751. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Abdelmassih Y, Azar G, Bonnin S, Scemama
Timsit C, Vasseur V, Spaide RF, Behar-Cohen F and Mauget-Faysse M:
COVID-19 associated choroidopathy. J Clin Med.
10(4686)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kanra AY, Altınel MG and Alparslan F:
Evaluation of retinal and choroidal parameters as neurodegeneration
biomarkers in patients with post-covid-19 syndrome. Photodiagnosis
Photodyn Ther. 40(103108)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang L, Huang W, Huang S, Zhang J, Guo X,
Friedman DS, Foster PJ and He M: Ten-year incidence of primary
angle closure in elderly Chinese: The Liwan Eye Study. Br J
Ophthalmol. 103:355–360. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Qian T, Du J, Ren R, Zhou H, Li H, Zhang Z
and Xu X: Vault-Correlated efficacy and safety of implantable
collamer lens V4c implantation for myopia in patients with shallow
anterior chamber depth. Ophthalmic Res. 66:445–456. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Teikari JM, O Donnell J, Nurminen M
and Raivio I: Acute closed angle glaucoma and sunshine. J Epidemiol
Community Health. 45:291–293. 1991.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Teikari J, Raivio I and Nurminen M:
Incidence of acute glaucoma in Finland from 1973 to 1982. Graefes
Arch Clin Exp Ophthalmol. 225:357–360. 1987.PubMed/NCBI View Article : Google Scholar
|
|
30
|
David R, Tessler Z and Yassur Y:
Epidemiology of acute angle-closure glaucoma: Incidence and
seasonal variations. Ophthalmologica. 191:4–7. 1985.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mehta SK, Mir T, Freedman IG, Sheth AH,
Sarrafpour S, Liu J and Teng CC: Emergency department presentations
of acute primary angle closure in the United States from 2008 to
2017. Clin Ophthalmol. 16:2341–2351. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang X, Liu Y, Wang W, Chen S, Li F,
Huang W, Aung T and Wang N: Why does acute primary angle closure
happen? Potential risk factors for acute primary angle closure.
Surv Ophthalmol. 62:635–647. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li M, Chen Y, Jiang Z, Chen X, Chen J and
Sun X: What are the characteristics of primary angle closure with
longer axial length? Invest Ophthalmol Vis Sci. 59:1354–1359.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Choudhari NS, Khanna RC, Marmamula S,
Mettla AL, Giridhar P, Banerjee S, Shekhar K, Chakrabarti S, Murthy
GVS, Gilbert C and Rao GN: Andhra Pradesh eye disease study group.
Fifteen-Year incidence rate of primary angle closure disease in the
Andhra Pradesh eye disease study. Am J Ophthalmol. 229:34–44.
2021.PubMed/NCBI View Article : Google Scholar
|