Introduction
Paragangliomas (PGLs) are rare neuroendocrine tumors
originating from paraganglionic cells adjacent to blood vessels and
nerves. The tumors are classifiable as parasympathetic or
sympathetic in type. Parasympathetic PGLs primarily involve the
head and neck region, whereas sympathetic variants typically arise
in the retroperitoneum, thoracic cavity and pelvis (1,2).
Such tumors are seldom encountered, having an estimated annual
incidence of 2-8 cases per million individuals (1,3).
Overall, 6-19% are known to exhibit malignant behavior, developing
distant metastases that often affect the regional lymph nodes,
lungs and bones (1,4). According to World Health Organization
guidelines, all PGLs should be viewed as potentially malignant and
thus require vigilant, long-term follow-up, even after surgery
(3,5).
Head and neck paragangliomas (HNPGLs) account for
65-70% of all PGLs (1). Although
generally slow-growing, HNPGLs must be regarded as potentially
malignant subsets of PGL, warranting long-term follow-up after
treatment (4). Common sites of
origin include the carotid body, temporal bone, jugular foramen,
mastoid foramen and areas surrounding the vagus nerve (2,6).
The estimated incidence of HNPGL ranges from 0.3-1
case per 100,000 individuals every year in the general population,
underscoring its rarity (1). Our
limited understanding of their biological attributes and a lack of
standardized treatment strategies complicates interventional
decisions, whether surgery, radiotherapy or observation, which
remain challenging in each instance (1,6).
Importantly, those tumors that recur near critical structures (such
as the brainstem) are in need of safe and effective therapeutic
strategies.
The present study details the course of a patient
undergoing two surgical procedures for PGL. The tumor arose and
then recurred at the jugular foramen, invading the medulla
oblongata. Use of hypofractionated intensity-modulated proton
therapy (IMPT) ultimately allowed successful tumor control. A
discussion of pertinent resources in the literature is also
provided.
Case report
Patient history: The patient was a
52-year-old woman who first experienced tinnitus in the left ear in
November 2013 and presented to Yanshan Phoenix Hospital (Beijing,
China). No clear cause was determined, but the patient was
diagnosed with sensorineural hearing loss at the same hospital
several months later. The patient received oral neurotrophic drugs
and hyperbaric oxygen therapy. Subsequently, vasodilators,
Traditional Chinese Medicine (herbal medicine), and acupuncture
were administered at the Departments of Internal Medicine and
Otolaryngology of Yanshan Phoenix Hospital, but there was no major
improvement. The left-sided hearing loss continued to progress.
In August 2014, the patient also developed
hoarseness. Left vocal cord paralysis was observed using a
laryngeal fiberscope, so standard oral neurotrophic therapy (drug
name and dose unknown) was initiated at Fangshan Chinese Medicine
Hospital (Beijing, China), again without improvement. Another month
after this, the patient presented with left-sided tongue deviation
and dysphagia during liquid intake. According to the patient's
medical history records from Zhuozhou Chinese Medicine Hospital
(Zhuozhou, China), a space-occupying lesion was visible on magnetic
resonance imaging (MRI). This lesion involved the left
cerebellopontine angle, which raised suspicion of a primary tumor
at the jugular foramen. The patient underwent a left paraganglioma
resection via the retroauricular transmastoid and labyrinthine
approach, combined with abdominal fat filling for cavity
obliteration at the Chinese People's Liberation Army General
Hospital (Beijing, China).
The pathology report issued by the Chinese People's
Liberation Army General Hospital documented that postoperative
tissue analysis confirmed the diagnosis of paraganglioma.
Immunohistochemical staining was performed, demonstrating focal
positivity for chromogranin A (CgA), as well as synaptophysin
(Syn), CD56, S-100 protein and vimentin positivity. The Ki-67 index
was low (3%) (data not shown).
Approximately 6 and one-half years after the initial
resection, diplopia became an issue. Preoperative MRI at that time
confirmed a recurrent lesion (data not shown). A second tumor
resection took place 3 months later, at Beijing Tiantan Hospital
(Beijing, China). Following analysis using standard procedures
(fixation in 10% neutral buffered formalin, paraffin embedding and
sectioning to 4 µm thickness, followed by hematoxylin and eosin
staining), histological findings confirmed recurrent PGL. As shown
in Fig. S1A, the tumor cells
exhibited a nested arrangement (Zellballen pattern).
Immunohistochemical staining performed using standard procedures
(fixation in 10% neutral buffered formalin, paraffin embedding and
sectioning to 4 µm thickness, followed by staining using DAB as the
chromogen) showed strong positivity for CD56 (Fig. S1B) and CgA (Fig. S1C). The Ki-67 proliferation index
was low (3-7%) (Fig. S1D). S-100
protein staining was positive in the sustentacular cells
surrounding the tumor nests (Fig.
S1E). Furthermore, the pathology report recorded positive
results for other markers, including Syn, somatostatin receptor
type 2 and CD34 (in vascular endothelium) (data not shown). There
was partial positivity for transcription factor SOX10, and staining
for STAT6, cytokeratin, thyroid transcription factor-1 and
progesterone receptor was negative. Postoperatively, left facial
nerve paralysis was apparent.
At 18 months after the second surgery, tumor
progression was evident on MRI performed at Beijing Tiantan
Hospital, and had extended into the medulla oblongata.
Current presentation
The patient underwent concurrent IMPT at Hebei
Yizhou Cancer Hospital (Zhuozhou, China) in May 2023.
Planning and delivery of
hypofractionated proton beam therapy (PBT)
PBT was performed as a standalone procedure, using
intensity-modulated proton therapy (IMPT) with pencil-beam scanning
(PBS) and limiting treatment to local irradiation. The gross tumor
volume (GTV) was configured by fusing contrast-enhanced MRI and
planning computed tomography (CT) views, adding a 5-mm margin to
GTV to ascertain clinical target volume (CTV). The enhancing lesion
on MRI signified the GTV. For treatment planning, the patient's
head was immobilized in a custom-made thermoplastic mask, and daily
cone-beam CT (CBCT) imaging input offered guidance. Planning CT was
performed in the treatment position at a 1-mm slice thickness, with
CTV representing a 3-mm expansion of GTV. A further 3- to 5-mm
margin was added to the CTV to arrive at planning target volume
(PTV). IMPT served for dose delivery, adjusting prescribed doses
based on tumor location. The treatment plan was designed to ensure
that ≥95% of the PTV received prescribed dosing at the isocenter. A
summary of the doses prescribed and constraints for organs at risk
(OARs) is provided as Fig. 1. The
equivalent dose in 2 Gy fractions (EQD2) was calculated to evaluate
dosing, setting α/β ratios of 2 for the brainstem, optic nerves and
optic chiasm, and 10 for the tumor itself. The presumed relative
biological effectiveness of protons was 1.1, and the prescribed
dose for the GTV was 40 Gy in 15 fractions (EQD2, 42.09 Gy,
assuming α/β=10).
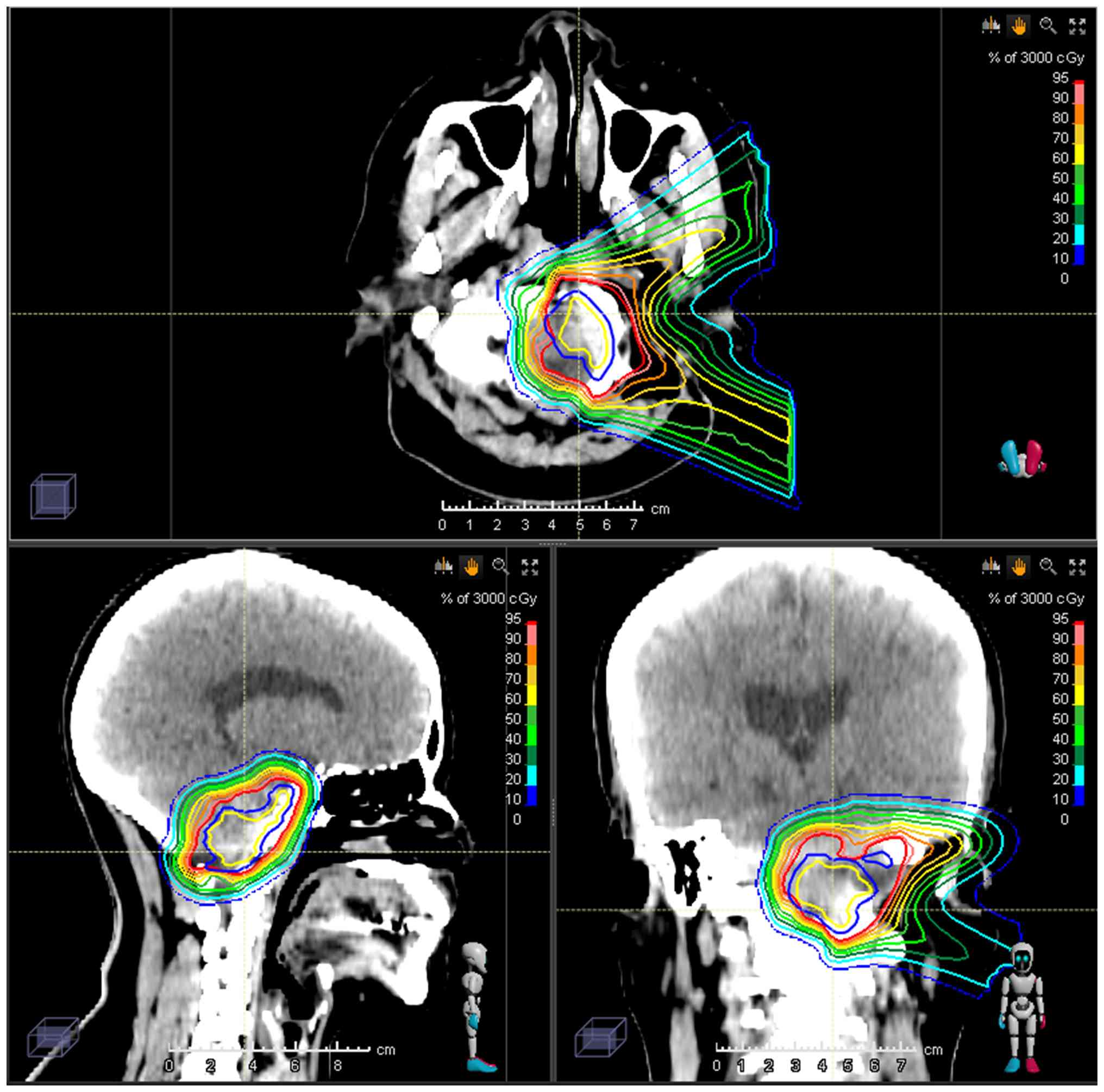
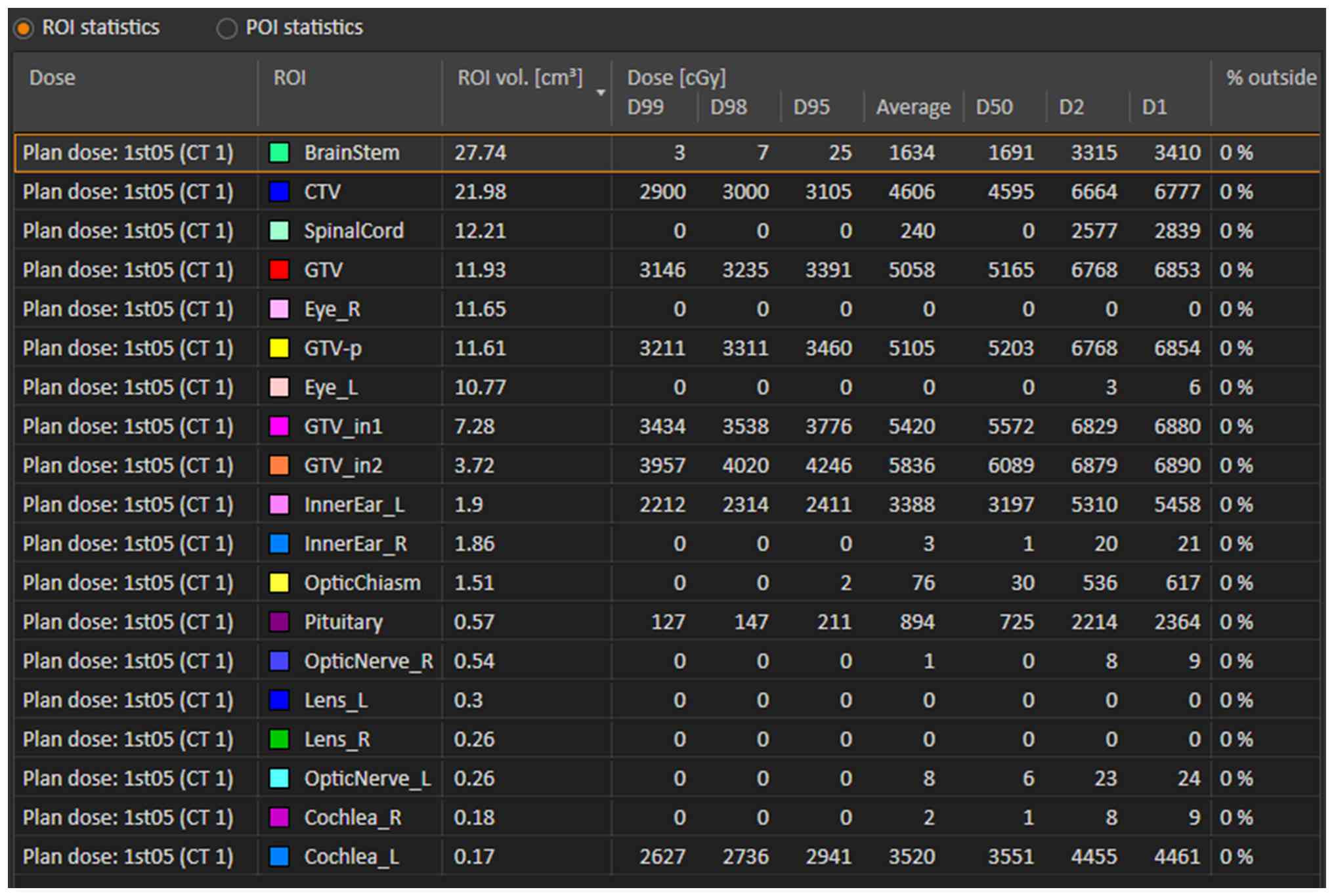 | Figure 1Summary of prescribed doses and
constraints for organs at risk. CTV, clinical target volume; GTV,
gross target volume; R, right; L, left; GTV_in1, GTV reduced by
2-mm margin; GTV_in2, GTV reduced by 3-mm margin; ROI, region of
interest; GTV-p, gross tumor volume-primary; CT, computed
tomography (planning image); D99, dose received by 99% of the
target volume. |
Two interior subvolumes of GTV were specified as GTV
reduced by 2-mm margin and GTV reduced by 3-mm margin. Prescribed
doses were 50 Gy/15 fractions (EQD2, 5.49 Gy) and 60 Gy/15
fractions (EQD2, 70.00 Gy), respectively.
The CTV determined as the contrast-enhancing lesion
on MRI plus a 3-mm margin, received 30 Gy in 15 fractions (EQD2,
30.00 Gy).
As OARs, the cochlea received 44.61 Gy in 15
fractions (EQD2, 55.48 Gy, assuming α/β=2) and the brainstem
received 34.1 Gy in 15 fractions (EQD2, 36.42 Gy, assuming α/β=2)
(Fig. 2).
Pretreatment physical examination. At the
start of PBT, the patient presented with left facial nerve
paralysis and oral commissure deviation to the right. Both pupils
were equal in size (~3 mm) and reactive to light. Right-eye
esotropia and left-eye diplopia were both noted, without visual
field defects. Complete hearing loss was also documented on the
left, whereas the right ear retained normal function. The patient
reported intermittent dysphagia, with choking during fluid intake
and difficulty consuming solid foods.
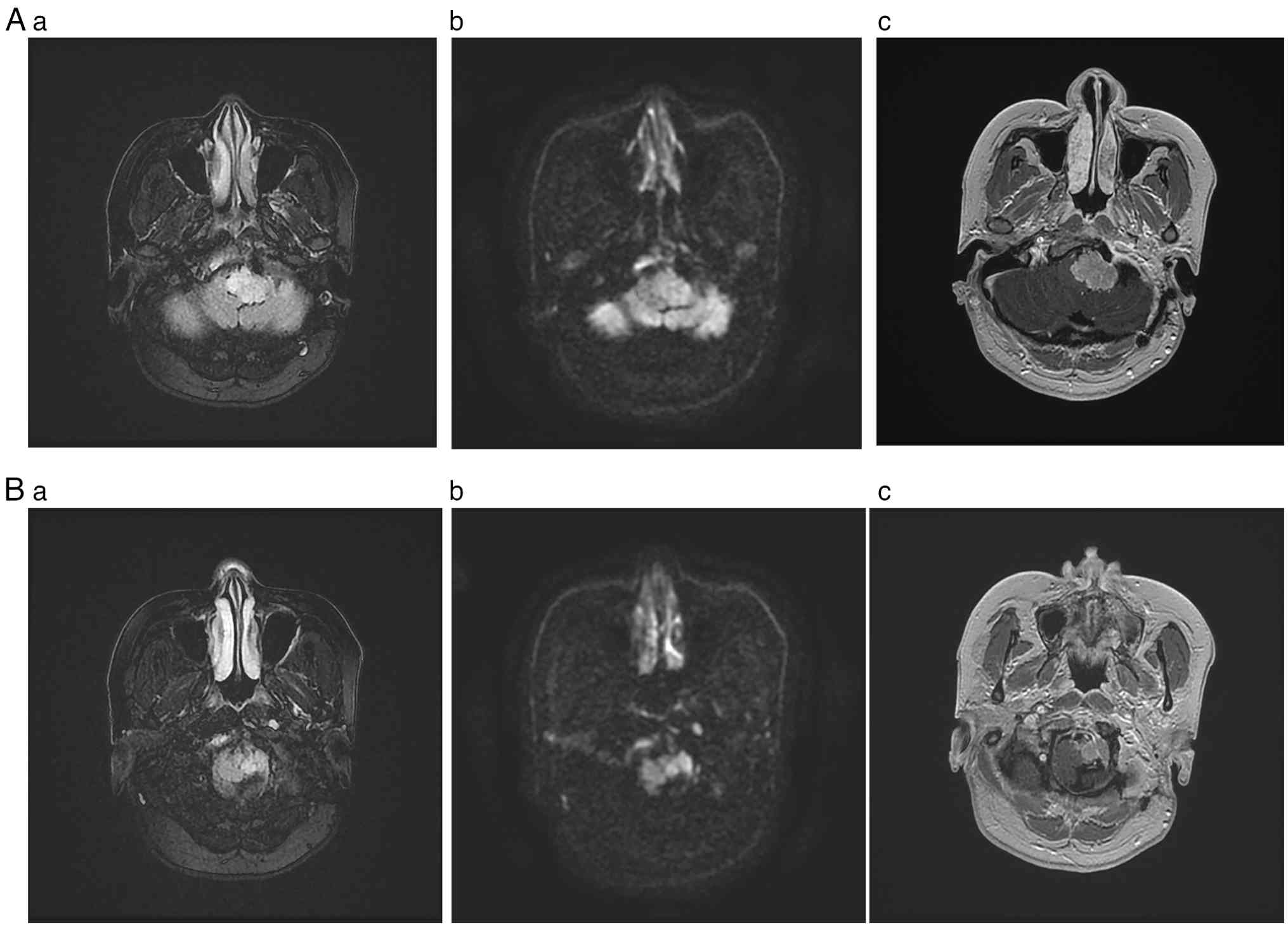
Imaging findings at baseline. Pretreatment
MRI revealed a mass lesion of the left anterior medulla (jugular
bulb region), showing somewhat increased signal intensity on both
T1- and T2-weighted images. This lesion was heterogeneous but
proved strongly enhanced by contrast, displaying an irregular shape
and measuring ~2.5x2.1x1.7 cm. The medulla was compressed, deformed
and displaced to the right (Fig.
3).
Follow-up imaging evaluation at 11 months
post-surgery. MRI showed no evidence of significant tumor
growth or recurrence 11 months after treatment, indicating stable
post-treatment changes. Follow-up imaging conducted 18 months after
treatment also demonstrated stable conditions, supporting the
long-term efficacy of PBT in suppressing tumor progression and
preventing recurrence (Fig.
4).
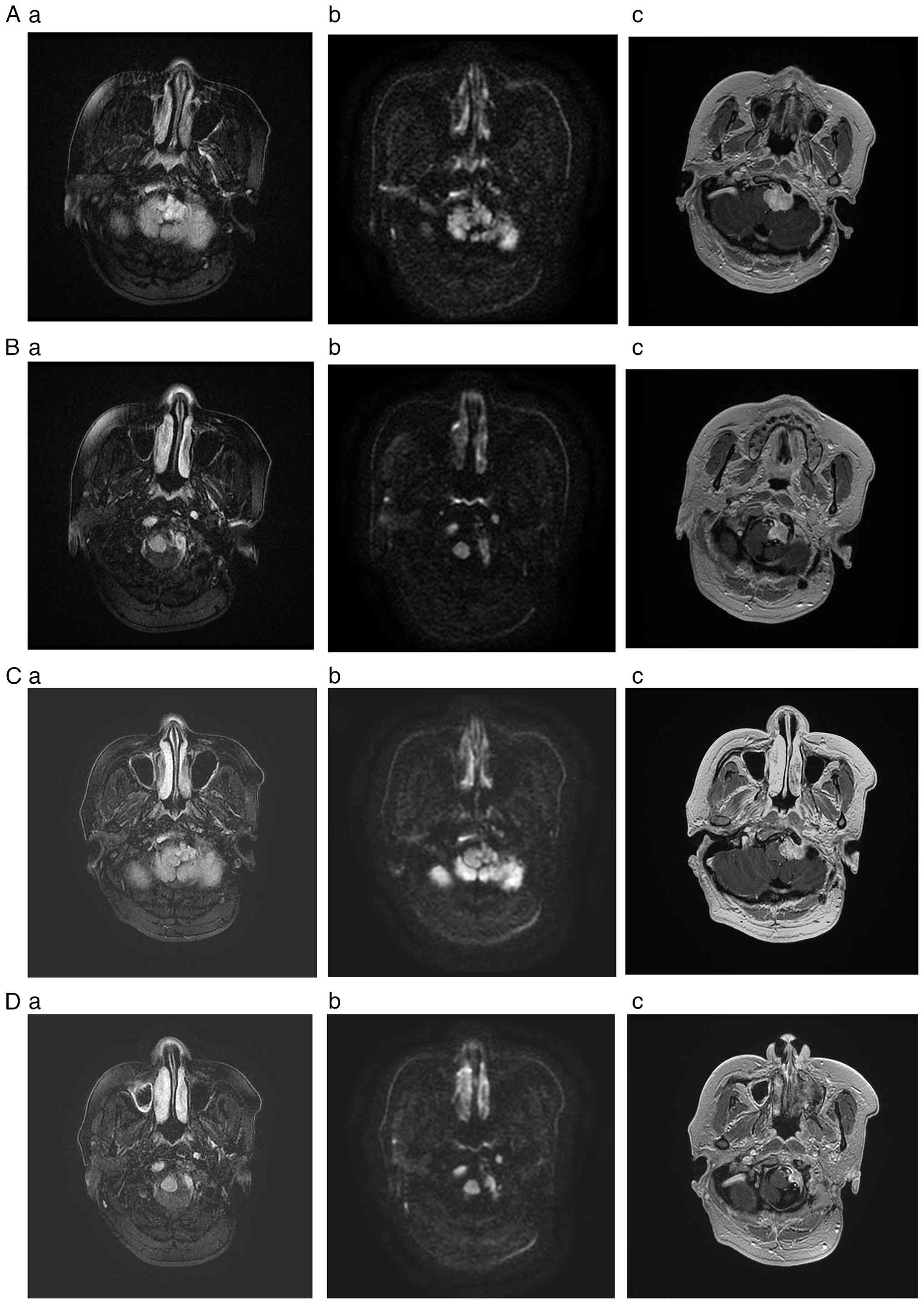 | Figure 4Magnetic resonance imaging study
during follow-up. (A and B) Images obtained at 11 months
post-treatment (April 2024). Images (A) and (B) represent different
axial section levels. (C and D) Images obtained at 18 months
post-treatment (December 2024). Images (C) and (D) represent
different axial section levels. (A-a, B-a, C-a and D-a) T2-weighted
fluid-attenuated inversion recovery sequences marked by a nodular,
somewhat hyperintense signal involving the left jugular bulb region
and the left lateral medulla oblongata. (A-b, B-b, C-b and D-b) DWI
demonstrating isointense or now vaguely hypointense signaling in
these areas. (A-c, B-c, C-c and D-c) T1-weighted imaging, again
disclosing moderate contrast enhancement in a heterogeneous pattern
at the aforementioned sites. Relative to pretreatment status in
Fig. 3 (May 2023), follow-up views
(A and B) at 11 months and (C and D) 18 months confirm significant
tumor volume reduction and diminished DWI signal intensity,
suggesting pronounced decline in tumor activity. DWI,
diffusion-weighted imaging. |
Clinical course 1 year after PBT. The
patient's neurological function had markedly improved at the 1-year
follow-up, showing substantial recovery of the facial nerve palsy.
The strabismus and diplopia had largely resolved, and there were
major strides in the abatement of choking frequency and swallowing
difficulty. The patient's hearing had also partially returned on
the left, although vertigo persisted.
Overall, the patient had experienced acute adverse
effects, such as vertigo, headache, nausea, vomiting and reflux,
during the first year after treatment. These were effectively
addressed through symptomatic management. By the 1-year mark, most
symptoms had considerably lessened, indicating good overall
tolerability of PBT. Late adverse effects further improved over
time, gradually diminishing between 6- and 18-months
post-treatment. This aided the recovery process and enhanced the
patient's quality of life. The patient is currently followed up
with clinical assessments and MRI scans every 3 months. Given the
high local control rates typically associated with high-dose PBT
for paragangliomas, the likelihood of local recurrence is
considered low, and the long-term prognosis for tumor control is
expected to be favorable. The patient has remained recurrence-free
for 24 months since PBT, showing marked symptom improvement
Discussion
As seen in the present patient, PGL recurrence in
close proximity to vital neurovascular structures has serious
clinical ramifications. Both anatomical considerations and
difficulties entailed in separating tumor from nerves and vessels
create inordinate opportunities for surgically related damage and
complications. The majority of PGLs arise within the head and neck
regions, and commonly originate from paraganglionic tissue near the
carotid body, vagus nerve, middle ear and jugular foramen (2,6).
Those abutting the jugular foramen are spawned by adventitial
paraganglionic cells of the jugular bulb, set within the temporal
bone; they are slow-growing and yet intensely vascular. Although
typically benign, such tumors may enlarge, infiltrate or destroy
surrounding bony structures and inflict complications due to mass
effects (1,2,7).
Traditionally, their clinical management (especially those at the
jugular foramen) has centered on complete surgical resection
(8,9). However, advances in neuroimaging and
radiotherapy technologies, along with mounting demand for less
invasive strategies in this rather benign setting, have prompted a
shift toward more conservative efforts fostering retention of
cranial nerve function (10,11).
For PGLs at the jugular foramen, the reported rates
of total resection vary (83-90%), depending on published sources
(7-9,12,13).
Conventional surgical procedures are more disruptive in general,
calling for external auditory canal closure and facial nerve
manipulation. Risks of cranial nerve palsy, dysphagia and
cerebrospinal fluid leakage are thereby increased. To minimize such
hazards (especially in large tumors) and preserve cranial nerve
function, a subtotal resection is often preferred (10,14-16).
A number of studies have confirmed that a subtotal resection not
only provides tumor stability or volume reduction, but also helps
safeguard critical neurovascular structures (10,14-18).
Huy et al (18) analyzed
patients after treatment with a combined subtotal resection and
adjuvant radiotherapy. Although some (27%) experienced regrowth,
which was curtailed through additional therapy, the majority (73%)
showed residual tumor stability (18). This combination approach maintains
a balance between tumor removal and functional preservation.
The decision to combine a subtotal resection with
radiotherapy should be based on the size and location of the tumor,
and the presence or absence of neurological symptoms. In a study of
47 patients with tumors classified as Fisch C or D (19), a subtotal resection was the more
frequent choice in patients who were elderly or had no preoperative
neurological deficits (20).
Another study involving 56 patients found that a subtotal resection
was often performed in the context of advanced tumors classified as
Fisch C3 or D (21).
Radiotherapy is particularly suitable for elderly
patients or for those with comorbidities, as it is less invasive
and carries fewer complications (11,22-25).
The patient in the present study showed complete tumor-related
brainstem compression, so postoperative complications seemed
unavoidable. Radiotherapy alone was therefore chosen as the primary
treatment method.
The principal dosimetric advantage of PBT lies in
the physical properties of the Bragg peak. This allows for the
delivery of high-dose, conformal radiation to the tumor while
largely sparing adjacent OARs from the broader low-dose ‘bath’
associated with intensity-modulated radiation therapy (IMRT), which
may intensify long-term toxicity risk (26,27).
This capability is vital for sensitive structures such as the
brainstem, cochlea and salivary glands. Several dosimetric studies
have confirmed that PBT can significantly lower radiation doses to
these critical structures, thereby reducing risks of hearing
impairment, xerostomia and hypothyroidism, with some evidence
suggesting improved long-term survival (26,27).
Furthermore, the IMPT with PBS utilized in the
present case represents the most advanced form of PBT. Unlike older
passive scattering techniques, PBS employs a fine proton pencil
beam that is magnetically scanned across the tumor layer-by-layer
(11). This technology creates
exceptionally steep dose gradients, providing superior dose
conformality and the ability to pre-set tolerable doses for OARs,
ensuring an optimal dose distribution that further minimizes
exposure compared with traditional broad-beam PBT. This level of
precision is particularly advantageous when treating tumors in
complex anatomical regions, such as the skull base (27,28).
However, these dosimetric advantages must be weighed
against practical limitations. The primary disadvantages of PBT are
its substantially higher cost and limited availability. Moreover,
its precision makes it highly sensitive to positional uncertainties
arising from patient setup or physiological motion. Such shifts can
alter the position of the Bragg peak, potentially undermining
target coverage and the OAR-sparing benefit (26). Therefore, rigorous quality
assurance, such as the daily CBCT used in this case, is essential
to mitigate these risks (27,28).
In contrast to PBT, while IMRT is dosimetrically inferior in terms
of OAR sparing, it is more widely accessible, less costly and
offers greater robustness against tumor motion, making it a more
tolerant option for such uncertainties.
Stereotactic radiosurgery (SRS) and IMRT my offer
high local control rates but often confer more risk in terms of
acute and late toxicities, owing to limited dose modulation in
surrounding tissues (22,29,30).
Despite the reported efficacy of SRS in controlling PGLs, acute
side effects, such as headache and fatigue, and late toxicities,
including dysphagia and otalgia, have resulted (30,31).
PBT is broadly categorized as broad-beam methodology
and IMPT. Various studies have reported the efficacy of PBT
treatment in patients with PGLs (27,28).
During one single-center trial, a median dose of 50.4 GyE over
25-34 fractions reduced tumor volumes by ≥20% in 40% of patients
with HNPGLs (28). A second study
similarly documented 100% local control in 10 patients receiving
45-67 Gy [median, 50.4 gray equivalents (GyE)] who were followed up
for a median of 24.6 months (26).
Yet another study (median follow-up time, 50.1 months) showed 100%
local disease control after irradiation at 50.4 GyE (32). The aforementioned findings imply
that local tumor control is attainable at doses of ~50 GyE.
However, in treating malignant PGLs of high grade or with recurrent
features, doses of 54-60 Gy may be warranted (22,33-35).
For greater precision in dose delivery, IMPT by PBS
was used in the present case, rather than engaging conventional
broad-beam techniques. Earlier efforts at PBT use in patients with
PGL have deployed broad-beam methods alone, without this advanced
PBS technology. As in IMRT, PBS allows for pre-defined dose
constraints on nearby normal tissues and augmented dose conformity
to the CTV, minimizing adjacent critical organ exposure (26-28).
Moreno et al (36) reported respective differences in
dose distribution characteristics of broad-beam and PBS methods,
emphasizing that PBS allows for more flexible adjustment of the
spread-out Bragg peak (SOBP) along the beam axis (36). By contrast, broad-beam techniques
utilize ridge filters to generate a uniform SOBP, which may result
in excessive radiation to surrounding tissues if the shape of the
CTV is suboptimal (37,38). PBS instead may deliver individually
modulated spots tailored to tumor shape, forming an SOBP that
follows tumor contours more precisely. The combination of IMPT and
PBS permits focal dose escalation to the tumor center and spares
normal tissues at the perimeter (39,40).
In the present patient, tumor shrinkage was observed
relatively early after PBS irradiation, coupled with symptomatic
improvement. Given the tumor's proximity to the brainstem and the
overt prognostic devastation dealt by cranial nerve dysfunction,
these extraordinary results may underscore the utility of PBS-based
proton therapeutics in treating similar cases.
Beyond dosimetric considerations, practical factors,
such as treatment cost and duration, also influence modality
selection. Within China, PBT (vs. IMRT) is associated with
substantially higher costs (US $40,000 vs. US $4,000-14,000) and
prolonged treatment sessions (30-45 min vs. 15-20 min) (41). Patient selection is therefore
paramount. PBT is preferentially recommended for specific patient
populations, including children and young adults in whom long-term
growth, development and radiation-induced secondary malignancy
risks are major concerns. PBT is also an ideal option in instances
where tumors border critical OARs or where cost is not an issue.
Conversely, IMRT may be more appropriate for patients with limited
life expectancies, certain tumor profiles (large, superficial or
highly mobile), or dire economic prospects (42).
To minimize the risk of radiation-induced toxicity,
strict dose constraints were applied during PBT in the present
study. The fractionation scheme was designed to achieve tumor
shrinkage and impart clinical improvement, both deemed essential to
therapeutic optimization. Fig. 1
delineates the patient's treatment regimen, including the
prescribed doses and aforementioned constraints.
In reviewing the current literature, a trend towards
less invasive, function-preserving strategies for recurrent jugular
foramen PGLs is clear, especially when surgical options are
exhausted (20-24).
The present report contributes to the limited but growing body of
evidence supporting the use of advanced radiation therapies for
this rare and complex condition.
However, the limitations of the present study must
be acknowledged. As a single case report, strong conclusions cannot
be drawn, and the favorable outcomes observed in the patient cannot
be generalized to the entire population of patients with this
disease. This highlights a significant potential research gap:
there is a lack of large-scale, prospective studies to define the
optimal radiation dose, fractionation and long-term outcomes for
anaplastic PGLs treated with PBT.
Additionally, it should be noted that the specific
immunohistochemical findings for the recurrent tumor described
within the present case were extracted from the official clinical
pathology reports, as the original slide image files from the
external institution were no longer retrievable due to the passage
of time.
Therefore, our future recommendations include the
establishment of multi-center registries to collect more data on
these rare tumors. Further studies with extended follow-up are
essential to fully assess the long-term efficacy and safety of PBT,
to confirm its benefits in preserving neurocognitive function and
to evaluate the risk of late-onset toxicities or secondary
malignancies. Such research will be crucial to solidifying the role
of PBT in the treatment algorithm for similar cases.
In conclusion, the present case highlights the
clinical challenges imposed by the recurrence of PGL at the jugular
foramen. Surgery remains a cornerstone of treatment but is clearly
quite limited under these circumstances. Proton therapy,
particularly if delivered via PBS and hypofractionated regimens,
offers an effective and precise alternative modality that may
protect critical structures while ensuring meaningful tumor
control. Long-term follow-up is essential to monitor disease
progression and evaluate the durability of outcomes post-treatment.
However, the merit of advanced proton therapy techniques in such
complex scenarios stems from compelling evidence and is hard to
deny.
Supplementary Material
Pathological findings from the second
surgery (recurrence). (A) Hematoxylin and eosin (H&E) staining
(original magnification, x100) showing tumor cells arranged in a
characteristic nested pattern (‘Zellballen’) separated by a rich
capillary network. (B) Immunohistochemical staining showing tumor
cells positive for CD56 (original magnification, x200). (C) Tumor
cells showing strong positivity for chromogranin A (original
magnification, x200). (D) The Ki-67 proliferation index is low at
3-7% (original magnification, x200). (E) S-100 protein staining
highlighting the sustentacular cells surrounding the tumor nests
(original magnification, x200).
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by institutional funds from
Hebei Yizhou Cancer Hospital (Zhuozhou, China).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
SS was responsible for conceptualization,
supervision, methodology, validation, project administration and
manuscript writing (reviewing/editing). WW was responsible for
conceptualization, data curation, investigation and manuscript
writing (original draft). SS and WW confirm the authenticity of all
the raw data. YY performed the formal data analysis, investigation
and manuscript writing (review/editing). JB was responsible for
methodology and data analysis. YZ assisted with resources and data
collection. DZ assisted in investigation and data curation. JZ
assisted with resources and follow-up data collection. SZ performed
data integration and visualization. ZW and JW were responsible for
radiotherapy dose data acquisition and software use. JK performed
data organization and visualization. LY was responsible for formal
analysis and manuscript writing (reviewing/editing). MM assisted in
investigation and data validation. HS was responsible for the
methodology and conceptualization. All authors took part in the
direct clinical care of the patient, and each author contributed
significantly to the data collection, dose acquisition or formal
analysis processes. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures involving human participants complied
with ethical standards of applicable institutional and national
research committees, and with the principles of the 1964
Declaration of Helsinki, its later amendments or comparable ethical
standards. This case report was approved by the Institutional
Review Board of Hebei Yizhou Cancer Hospital (Zhuozhou, China).
Written informed consent was obtained from the patient. Although
some journals may not require formal ethics approval for a single
case report, it is the policy of Hebei Yizhou Cancer Hospital to
obtain such approval for any publication involving patient data to
ensure the highest standards of patient privacy protection and
ethical conduct.
Patient consent for publication
The patient depicted herein granted written informed
consent for publication of this case report and its accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Palade DO, Hainarosie R, Zamfir A,
Vrinceanu D, Pertea M, Tusaliu M, Mocanu F and Voiosu C:
Paragangliomas of the head and neck: A review of the latest
diagnostic and treatment methods. Medicina (Kaunas).
60(914)2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Strasser V and Steinbichler T:
Paragangliomas of the head and neck. Radiologie (Heidelb).
64:960–970. 2024.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
3
|
Mete O, Asa SL, Gill AJ, Kimura N, de
Krijger RR and Tischler A: Overview of the 2022 WHO classification
of paragangliomas and pheochromocytomas. Endocr Pathol. 33:90–114.
2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sethi RV, Sethi RK, Herr MW and Deschler
DG: Malignant head and neck paragangliomas: Treatment efficacy and
prognostic indicators. Am J Otolaryngol. 34:431–438.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Brewczyński A, Kolasińska-Ćwikła A,
Jabłońska B and Wyrwicz L: Pheochromocytomas and
paragangliomas-current management. Cancers (Basel).
17(1029)2025.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Strasser V and Steinbichler T:
Paragangliome im Kopf-Hals-Bereich Paragangliomas of the head and
neck. HNO. 72:598–608. 2024.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
7
|
Moe KS, Li D, Linder TE, Schmid S and
Fisch U: An update on the surgical treatment of temporal bone
paraganglioma. Skull Base Surg. 9:185–194. 1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sanna M, Jain Y, De Donato G, Rohit Lauda
L and Taibah A: Management of jugular paragangliomas: The Gruppo
Otologico experience. Otol Neurotol. 25:797–804. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jackson CG, McGrew BM, Forest JA,
Netterville JL, Hampf CF and Glasscock ME III: Lateral skull base
surgery for glomus tumors: Long-term control. Otol Neurotol.
22:377–382. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Manzoor NF, Yancey KL, Aulino JM, Sherry
AD, Khattab MH, Cmelak A, Morrel WG, Haynes DS, Bennett ML,
O'Malley MR, et al: Contemporary management of jugular
paragangliomas with neural preservation. Otolaryngol Head Neck
Surg. 164:391–398. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chuong M, Badiyan SN, Yam M, Li Z, Langen
K, Regine W, Morris C, Snider J III, Mehta M, Huh S, et al: Pencil
beam scanning versus passively scattered proton therapy for
unresectable pancreatic cancer. J Gastrointest Oncol. 9:687–693.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Manolidis S, Jackson CG, Von Doersten PG,
Pappas D and Glasscock ME: Lateral skull base surgery: The otology
group experience. Skull Base Surg. 7:129–137. 1997.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Borba LA, Araújo JC, de Oliveira JG, Filho
MG, Moro MS, Tirapelli LF and Colli BO: Surgical management of
glomus jugulare tumors: A proposal for approach selection based on
tumor relationships with the facial nerve. J Neurosurg. 112:88–98.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
de Brito R, Cisneros Lesser JC, Lopes PT
and Bento RF: Preservation of the facial and lower cranial nerves
in glomus jugulare tumor surgery: Modifying our surgical technique
for improved outcomes. Eur Arch Otorhinolaryngol. 275:1963–1969.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wanna GB, Sweeney AD, Carlson ML, Latuska
RF, Rivas A, Bennett ML, Netterville JL and Haynes DS: Subtotal
resection for management of large jugular paragangliomas with
functional lower cranial nerves. Otolaryngol Head Neck Surg.
151:991–995. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li D, Zeng XJ, Hao SY, Wang L, Tang J,
Xiao XR, Meng GL, Jia GJ, Zhang LW, Wu Z and Zhang JT:
Less-aggressive surgical management and long-term outcomes of
jugular foramen paragangliomas: A neurosurgical perspective. J
Neurosurg. 125:1143–1154. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Anderson JL, Khattab MH, Anderson C,
Sherry AD, Luo G, Manzoor N, Attia A, Netterville J and Cmelak AJ:
Long-term outcomes for the treatment of paragangliomas in the
upfront, adjuvant, and salvage settings with stereotactic
radiosurgery and intensity-modulated radiotherapy. Otol Neurotol.
41:133–140. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Huy PT, Kania R, Duet M, Dessard-Diana B,
Mazeron JJ and Benhamed R: Evolving concepts in the management of
jugular paraganglioma: A comparison of radiotherapy and surgery in
88 cases. Skull Base. 19:83–91. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fisch U and Mattox D: Microsurgery of the
Skull Base. Thieme, Stuttgart, 1988.
|
|
20
|
Tran Ba Huy P, Chao PZ, Benmansour F and
George B: Long-term oncological results in 47 cases of jugular
paraganglioma surgery with special emphasis on the facial nerve
issue. J Laryngol Otol. 115:981–987. 2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhao P, Zhang Y, Lin F, Kong D, Feng Y and
Dai C: Comparison of surgical outcomes between early and advanced
class of jugular paragangliomas following application of our
modified surgical techniques. Sci Rep. 13(885)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lassen-Ramshad Y, Ozyar E, Alanyali S,
Poortmans P, van Houtte P, Sohawon S, Esassolak M, Krengli M, Villa
S, Miller R, et al: Paraganglioma of the head and neck region,
treated with radiation therapy, a Rare Cancer Network study. Head
Neck. 41:1770–1776. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sharma M, Meola A, Bellamkonda S, Jia X,
Montgomery J, Chao ST, Suh JH, Angelov L and Barnett GH: Long-term
outcome following stereotactic radiosurgery for glomus jugulare
tumors: A single institution experience of 20 years. Neurosurgery.
83:1007–1014. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jansen TTG, Kaanders JHAM, Beute GN,
Timmers HJLM, Marres HAM and Kunst HPM: Surgery, radiotherapy or a
combined modality for jugulotympanic paraganglioma of Fisch class C
and D. Clin Otolaryngol. 43:1566–1572. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Suárez C, Rodrigo JP, Bödeker CC, Llorente
JL, Silver CE, Jansen JC, Takes RP, Strojan P, Pellitteri PK,
Rinaldo A, et al: Jugular and vagal paragangliomas: Systematic
study of management with surgery and radiotherapy. Head Neck.
35:1195–1204. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cao KI, Feuvret L, Herman P, Bolle S,
Jouffroy T, Goudjil F, Amessis M, Rodriguez J, Dendale R and
Calugaru V: Proton therapy of head and neck paragangliomas: A
monocentric study. Cancer Radiother. 22:31–37. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chartier J, Beddok A, Cao KI, Feuvret L,
Herman P, Bolle S, Goudjil F, Sauvaget E, Choussy O, Dendale R and
Calugaru V: Protontherapy to maintain local control of head and
neck paragangliomas. Acta Oncol. 62:400–403. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kang KH, Lebow ES, Niemierko A, Bussière
MR, Dewyer NA, Daly J, McKenna MJ, Lee DJ, Loeffler JS, Busse PM
and Shih HA: Proton therapy for head and neck paragangliomas: A
single institutional experience. Head Neck. 42:670–677.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gottfried ON, Liu JK and Couldwell WT:
Comparison of radiosurgery and conventional surgery for the
treatment of glomus jugulare tumors. Neurosurg Focus.
17(E4)2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ehret F, Ebner DK, McComas KN, Gogineni E,
Andraos T, Kim M, Lo S, Schulder M, Redmond KJ, Muacevic A, et al:
The Radiosurgery Society case-based discussion of the management of
head and neck or skull base paragangliomas with stereotactic
radiosurgery and radiotherapy. Pract Radiat Oncol. 14:225–233.
2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fatima N, Pollom E, Soltys S, Chang SD and
Meola A: Stereotactic radiosurgery for head and neck
paragangliomas: A systematic review and meta-analysis. Neurosurg
Rev. 44:741–752. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Henzel M, Hamm K, Gross MW, Surber G,
Kleinert G, Failing T, Sitter H, Strassmann G and
Engenhart-Cabillic R: Fractionated stereotactic radiotherapy of
glomus jugulare tumors. Local control, toxicity, symptomatology,
and quality of life. Strahlenther Onkol. 183:557–562.
2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bianchi LC, Marchetti M, Brait L,
Bergantin A, Milanesi I, Broggi G and Fariselli L: Paragangliomas
of head and neck: A treatment option with CyberKnife radiosurgery.
Neurol Sci. 30:479–485. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gilbo P, Morris CG, Amdur RJ, Werning JW,
Dziegielewski PT, Kirwan J and Mendenhall WM: Radiotherapy for
benign head and neck paragangliomas: A 45-year experience. Cancer.
120:3738–3743. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Galland-Girodet S, Maire JP, De-Mones E,
Benech J, Bouhoreira K, Protat B, Demeaux H, Darrouzet V and Huchet
A: The role of radiation therapy in the management of head and neck
paragangliomas: Impact of quality of life versus treatment
response. Radiother Oncol. 111:463–467. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Moreno AC, Frank SJ, Garden AS, Rosenthal
DI, Fuller CD, Gunn GB, Reddy JP, Morrison WH, Williamson TD,
Holliday EB, et al: Intensity modulated proton therapy (IMPT) - The
future of IMRT for head and neck cancer. Oral Oncol. 88:66–74.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Paganetti H: Range uncertainties in proton
therapy and the role of Monte Carlo simulations. Phys Med Biol.
57:R99–R117. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Akagi T, Kanematsu N, Takatani Y, Sakamoto
H, Hishikawa Y and Abe M: Scatter factors in proton therapy with a
broad beam. Phys Med Biol. 51:1919–1928. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Reese AS, Das SK, Kirkpatrick JP and Marks
LB: Quantifying the dosimetric trade-offs when using
intensity-modulated radiotherapy to treat concave targets
containing normal tissues. Int J Radiat Oncol Biol Phys.
73:585–593. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kaulfers T, Lattery G, Cheng C, Zhao X,
Selvaraj B, Wu H, Chhabra AM, Choi JI, Lin H, Simone CB II, et al:
Pencil beam scanning proton bragg peak conformal FLASH in prostate
cancer stereotactic body radiotherapy. Cancers (Basel).
16(798)2024.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li G, Qiu B, Huang YX, Doyen J, Bondiau
PY, Benezery K, Xia YF and Qian CN: Cost-effectiveness analysis of
proton beam therapy for treatment decision making in paranasal
sinus and nasal cavity cancers in China. BMC Cancer.
20(599)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Paganetti H, Athar BS, Moteabbed M, Adams
JY, Schneider U and Yock TI: Assessment of radiation-induced second
cancer risks in proton therapy and IMRT for organs inside the
primary radiation field. Phys Med Biol. 57:6047–6061.
2012.PubMed/NCBI View Article : Google Scholar
|