Mental health, recognized as a fundamental human
right, holds critical importance globally. However, mental illness
is escalating worldwide (1). Major
mental disorders, such as depressive disorders, anxiety disorders,
bipolar disorder, schizophrenia and autism spectrum disorders,
contribute significantly to this burden. The Global Burden of
Diseases, Injuries and Risk Factors Study (GBD) 2019 highlights a
substantial increase in the burden of non-communicable diseases,
including mental health conditions, over the past three decades
(1990-2019) (2,3). While diagnostic rates of mental
disorders may appear higher in high-income countries due to better
healthcare infrastructure and reporting, access to effective
treatment and mental health services remains insufficient across
both high- and low-income regions (4).
Schizophrenia, a severe mental disorder with a
global lifetime prevalence of ~0.4%, stands as one of the leading
causes of disability worldwide (5-7).
Numerous affected individuals experience long-term functional
impairment, with significant life-altering consequences such as
social isolation, self-stigmatization and increased difficulty in
forming relationships (8).
Schizophrenia is not characterized by pathognomonic symptoms, but
rather by a constellation of features typically categorized into
positive, negative and cognitive domains (9). Positive symptoms reflect distortions
of normal perception and behavior, including delusions,
hallucinations and disorganized behavior. Negative symptoms involve
diminished emotional expression and motivation, manifesting as
avolition, asociality and affective flattening. Cognitive deficits,
such as impairments in attention, working memory and executive
functioning, are increasingly recognized as core features of the
disorder (10,11). Some researchers argue that
cognitive impairment is the primary determinant of long-term
functional disability in schizophrenia (12). Diagnosing schizophrenia and
evaluating treatment effectiveness remain challenging, primarily
due to the absence of reliable, objective biomarkers (13). This review describes the potential
pathogenesis of schizophrenia from environmental and genetic
factors. Further summarising model studies of schizophrenia, this
work integrates genetic, environmental and modelling perspectives
to advance the understanding of the disorder from both holistic and
cellular-molecular viewpoints. This approach yields significant
benefits for research into characteristic biomarkers,
pathophysiology and aetiological mechanisms.
This is a narrative review aiming to summarise
current research on the influence of genetic and environmental
factors on schizophrenia, alongside studies concerning research
models for schizophrenia. The primary themes are the pathogenesis
of schizophrenia (genetic and environmental factors) and model
studies (animal and cellular models). References were retrieved
from the PubMed database {using the following search string:
{[(schizophrenia) OR (psychosis)] AND [(pathogenesis) OR
(mechanism) OR (gene) OR (environment); (schizophrenia) OR
(psychosis)] AND [(model) OR (modeling) OR (animal model) OR (cell
model)]}, encompassing reviews, randomised controlled trials,
meta-analyses and experimental research data from the past decade.
Based on this review, we have screened articles exploring the
pathogenesis of schizophrenia from both genetic and environmental
perspectives, as well as disease research methodologies utilising
animal and cellular models. Studies incorporating schizophrenia
diagnoses will be included. Research primarily consisting of review
reports will be excluded. Model studies failing to explicitly state
that relevant ethical approvals were obtained will be excluded.
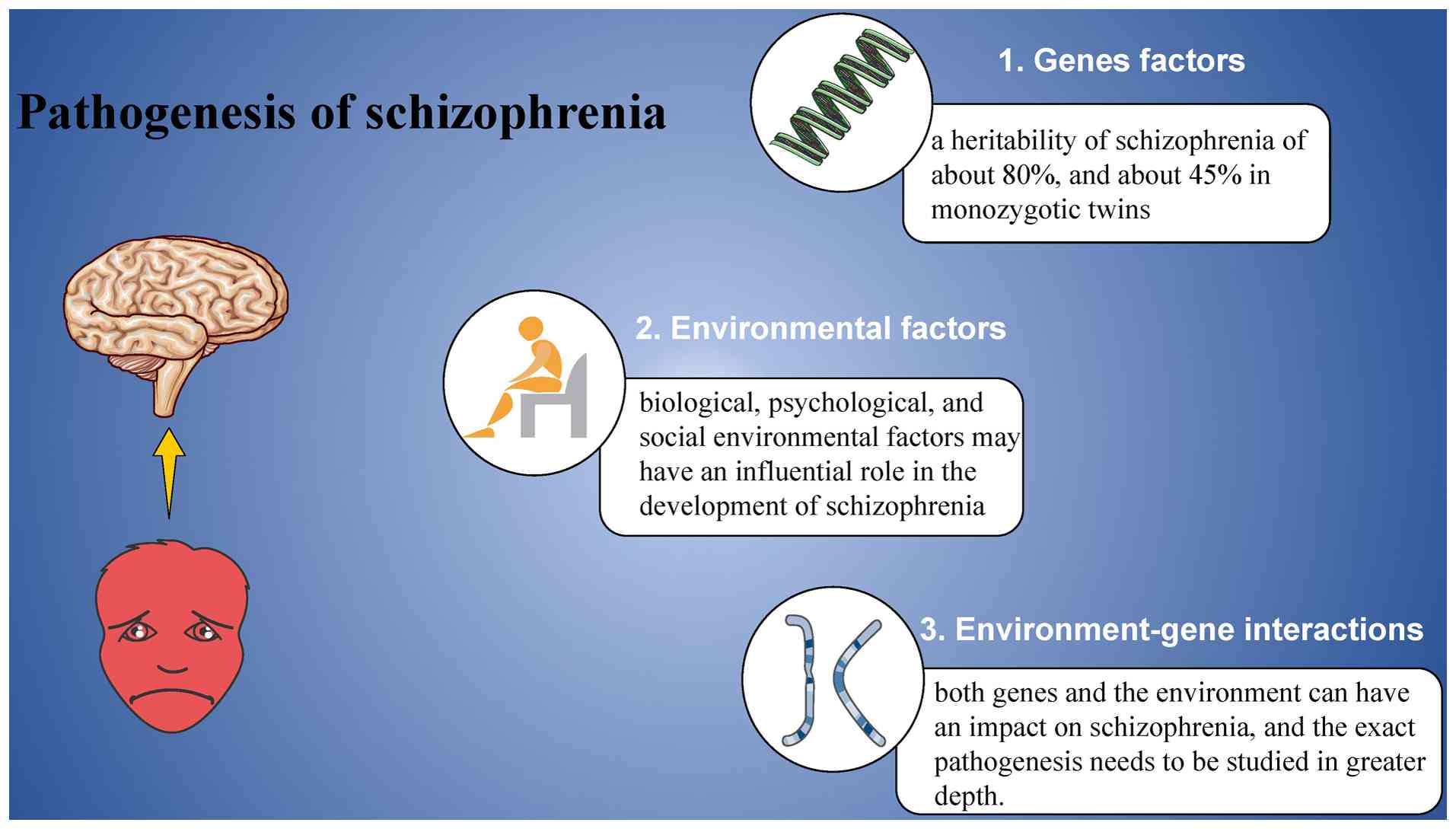
The precise pathogenesis of schizophrenia remains to
be fully elucidated. However, it is generally accepted that
genetic, environmental and immunological factors are key
contributors to the disorder (14,15).
The causes of schizophrenia are multifactorial, typically arising
from the complex interplay of these factors (Fig. 1) (16).
Genetic factors play a significant role in
schizophrenia's etiology, with heritability estimates of ~80 and
~45% for monozygotic twins (17).
Following the completion of the human genome sequencing in 2003,
genetic research has expanded considerably (18). Approximately 70-80% of human genes
are expressed in the brain, and numerous genes involved in synaptic
transmission have been linked to psychiatric disorders (19). Schizophrenia exhibits familial
aggregation, and studies involving adoption and twins suggest that
genetic factors outweigh environmental influences (20,21).
The 22q11.2 gene deletion was one of the first variants identified
in relation to schizophrenia, but no single genetic mutation has
been conclusively tied to the disorder (22,23).
Individuals with a deletion in the glutathione S-transferase theta
1 gene within the 22q11.2 region exhibit an increased risk of
developing schizophrenia (24). A
genetic study encompassing 36,989 patients with schizophrenia and
113,075 controls revealed 108 independent loci strongly associated
with the disorder. These include the gene encoding dopamine D2
receptor, the primary target of nearly all antipsychotic drugs, as
well as genes involved in glutamatergic signaling and synaptic
plasticity, such as glutamate ionotropic receptor
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid type subunit
1, glutamate ionotropic receptor noncompetitive malondialdehyde
type subunit 2A and serine racemase, providing genetic support for
existing hypotheses that implicate both dopaminergic and
glutamatergic systems in schizophrenia (25-27).
A separate study of 5,220 patients with schizophrenia and 18,823
controls identified 145 independent gene loci linked to the
disorder (28). These risk
variants primarily cluster in neuronal and synaptic gene sets, and
ongoing research with larger sample sizes continues to enhance the
understanding of schizophrenia's genetic underpinnings (29). Certain researchers proposed that
common genetic variants account for 30-50% of schizophrenia's
genetic liability, but the risk posed by any individual variant is
limited (30).
Notably, several studies have identified new
mutations clustered in loss-of-function genes in individuals with
childhood-onset schizophrenia (31). These studies highlight that many of
the mutations affect genes linked to neurodevelopmental disorders,
including ATPase Na+/K+ transporting subunit alpha 3, Up-frameshift
suppressor 3B, snf2 related CREBBP activator protein and
polynucleotide kinase 3'-phosphatase (32-34).
Further research has underscored the role of
epigenetic modifications in schizophrenia (35). Epigenetic processes, including
histone modification, DNA methylation, chromatin remodeling and
non-coding RNA expression, contribute to the regulation of gene
expression. Guidotti et al (36) suggested that targeting the link
between reduced Reelin expression in patients with schizophrenia
and epigenetic dysregulation may offer a more promising therapeutic
approach. A large-scale study by Jaffe et al (37) identified 2,104 methylated CpG sites
in the forebrain cortex of schizophrenia cases, which differed
significantly from controls. Additionally, histone deacetylase
(HDAC) inhibitors have shown potential for improving clinical
symptoms of schizophrenia (38).
Evidence from brain samples of patients with schizophrenia
indicates altered epigenetic markers, including elevated H3R17
methylation levels (39),
increased cortical HDAC1 expression (40) and reduced cortical HDAC2 levels
(41).
Environmental factors, once overlooked, are now
widely acknowledged as significant contributors to the development
of schizophrenia. It is increasingly recognized that a combination
of biological, psychological and social environmental influences
may play a critical role in the onset and progression of the
disorder, as summarized in Table I
(42-53).
Environmental factors (such as prenatal infections,
childhood trauma and adolescent cannabis use) can shape
schizophrenia risk across critical developmental windows, from
early gestation to young adulthood, impacting both individuals and
populations. These factors may play a more significant role in the
onset of schizophrenia than common genetic variants identified in
global genomic studies (54).
Schizophrenia's origins may be rooted in
neurodevelopmental disruptions during the fetal stage. Research has
shown that maternal exposure to infections or stress during
pregnancy can increase the likelihood of schizophrenia in offspring
(55-57).
Early investigations into psychiatric disorders following in-utero
infections primarily focused on schizophrenia (58). A significant amount of research has
examined the effects of Toxoplasma gondii infection, though
the exact mechanism by which it contributes to schizophrenia
remains elusive. Toxoplasma gondii, a unicellular eukaryote
primarily hosted by felines but capable of infecting any
warm-blooded animal, has been associated with behavioral
alterations in humans (59). In
healthy individuals, Toxoplasma gondii infection typically
shows no overt clinical symptoms, but during pregnancy, it can be
transmitted to the fetus via the placenta (60). Serological studies have revealed
significantly higher serum levels of anti-Toxoplasma IgG and
IgM in patients with schizophrenia compared to healthy controls
(61-63).
Current research indicates that Toxoplasma infection may
disrupt central nervous system neurotransmitter regulation, the
blood-brain barrier, immune stress responses and inflammatory
reactions, factors closely associated with schizophrenia
pathogenesis (64,65).
Childhood adversity is also known as childhood
trauma, such as abuse, domestic violence and neglect (66). Individuals with schizophrenia who
have experienced such adversity may exhibit earlier cognitive
deficits, possibly due to neurodevelopmental impairments (67). There is substantial evidence
linking childhood maltreatment to more severe psychotic symptoms,
with those affected being more than four times as likely to develop
a psychotic disorder following trauma (68,69).
Additionally, such adversity is strongly associated with positive
symptoms of schizophrenia, such as hallucinations and delusions
(70).
Several studies have also highlighted the influence
of the social environment on the onset of schizophrenia. Factors
such as social adversity, discrimination, poverty and substance
use-especially cannabis-have all been identified as potential
contributors to schizophrenia development (71-75).
As research has advanced, it has become increasingly
evident that schizophrenia is a multifactorial disorder, prompting
more studies focused on the interaction between genetic and
environmental factors in its development. Epidemiological studies
have established a clear link between infection and schizophrenia
(76). Among the infectious
agents, Toxoplasma gondii has been the most extensively
studied, with substantial evidence supporting its association with
schizophrenia. A meta-analysis by Wang et al (77) revealed that
schizophrenia-associated genes were notably enriched in the
Toxoplasma gondii IgG seropositive population, suggesting
that Toxoplasma infection may influence the expression of
genes related to schizophrenia. However, a more recent study by
Lori et al (78) indicated
that signs of Toxoplasma infection do not serve as reliable
predictors of schizophrenia. As previously noted, Toxoplasma
gondii infection exerts a significant influence on
schizophrenia by affecting associated genetic loci. Additional
studies have indicated that inflammatory environments and stress
responses within the central nervous system may contribute to
psychiatric disorders, including schizophrenia, by affecting the
hypothalamic-pituitary-adrenal axis (79), with anti-inflammatory medications
showing promise in alleviating certain schizophrenia symptoms
(80). In the context of
environment-gene interactions in schizophrenia, neuroinflammation
driven by infectious agents has been recognized as a potential
mechanism. Elevated peripheral inflammatory markers, such as
C-reactive protein, have been observed in patients with
schizophrenia (81). Furthermore,
high levels of immune antibodies in infected mothers have been
suggested as potential causes of neuropathological changes
(82). Neuropathological
alterations in offspring resulting from maternal infection with
infectious viruses such as influenza and measles may increase the
incidence rate by 1-20% (83).
Cannabis use interacts with genetic factors in the
pathogenesis of schizophrenia (84). Further studies suggest that
individuals with a genetic predisposition may be more susceptible
to developing schizophrenia as a result of cannabis use (85). Cannabis use may influence the
development of schizophrenia by affecting specific genetic loci,
potentially alleviating symptoms of central nervous system
disorders (86). A study on the
catechol-o-methyltransferase (COMT) Val158Met polymorphism revealed
that the COMT Val158Met Val allele is associated with an increased
risk of psychosis following cannabis use (87). In a further study, Met allele
homozygotes for the COMT Val158Met polymorphism were more sensitive
to stress and exhibited a neurotic tendency in response to daily
stressors (88). Additionally,
research on specific alleles of the Protein Kinase B and fatty acid
amide hydrolase genes has demonstrated that cannabis users with
certain genetic variations are more susceptible to showing
psychotic symptoms (89). However,
the relationship between cannabis use and schizophrenia is complex
and somewhat controversial. Cannabis is known to alleviate stress
symptoms and individuals who have experienced high levels of stress
or depression may be more inclined to use it (90). This highlights the intricate nature
of the link between cannabis use and schizophrenia, further
reinforcing the understanding that schizophrenia is a
multifactorial mental illness.
Extensive evidence indicates that both genetic and
environmental factors contribute to schizophrenia, although the
exact pathogenesis remains elusive. More comprehensive studies
exploring the interaction between genes and the environment could
offer valuable insights into the causes, pathology and potential
treatment options for schizophrenia.
Constructing an appropriate model is a powerful
approach to understanding how a disease affects an organism. The
schizophrenia model, described here from both an animal and
cellular perspective, provides a key example.
The unique complexity of the human brain in both
function and structure poses challenges for schizophrenia research.
Studies of the living patient's brain primarily rely on
neuroimaging and cognitive testing, while direct analysis of brain
tissue is restricted to post-mortem investigations. Factors such as
ongoing environmental stress, genetic influences and drug effects
in patients complicate research, necessitating the development of
reliable animal models. An effective model should encompass three
core characteristics: Face validity, construct validity and
predictive validity (91). Face
validity requires that the model replicates the symptoms of
schizophrenia, while construct validity ensures the model mimics
the structural and functional abnormalities observed in the
disease. Predictive validity ensures that therapeutic interventions
can reverse the disease-like phenotype. Animal models, including
both invertebrates and vertebrates, such as non-human primates,
offer valuable tools for investigating the mechanisms underlying
schizophrenia risk. The MATRICS program, developed by the National
Institute of Mental Health, identified seven domains relevant to
schizophrenia and proposed corresponding rodent-based assays
(92).
Established high-risk factors, such as maternal
infections and early neurodevelopmental disorders, play a critical
role in the pathophysiology of schizophrenia. Animal models
addressing these factors often involve perinatal and/or early
postnatal environmental manipulation, or the administration of
medications, followed by longitudinal studies of offspring
development. One of the earliest approaches to modeling
schizophrenia in animals involved inducing ventral hippocampal
lesions in rats through localized toxin injections on postnatal day
7. Another widely used model is the administration of
methylazomethanol (MAM) to pregnant rats (93), which induces neuroanatomical,
electrophysiological and behavioral alterations in offspring, with
the timing of MAM exposure-particularly on gestational day 17-being
a critical determinant of model fidelity (94).
Inducing maternal immune activation (MIA) in
pregnant rodents is another widely used strategy for constructing
schizophrenia models. Strong evidence suggests that rodents and
primates subjected to MIA during gestation produce offspring
exhibiting anatomical, neurochemical, electrophysiological and
behavioral alterations consistent with schizophrenia (95). Using this model, Steullet et
al (96) demonstrated that
changes in parasympathetic interneurons and their associated
perineuronal networks (extracellular matrix structures critical for
structural and synaptic plasticity) in the prefrontal cortex and
hippocampal formation were linked to cognitive deficits, findings
aligning with autopsy reports from patients with schizophrenia
(97).
The most commonly utilized pharmacological agents in
animal models of schizophrenia are dopamine enhancers and
noncompetitive malondialdehyde (NMDA) receptor antagonists.
Amphetamine, a dopamine-enhancing agent known to induce psychosis
in humans, does not cause social impairment in rodents with
continuous administration, although it does affect certain
forebrain-associated cognitive functions (98). Studies have shown that amphetamine
impairs cholinergic basal neurons in rat cerebral cortical
projections, suggesting that dysregulation of this activity is
related to attentional dysfunction (99-101).
Wearne et al (102)
identified 96 differentially expressed proteins in the prefrontal
cortex of methamphetamine-treated rats, with 20% linked to
schizophrenia pathogenesis. Additionally, certain dopamine
enhancers are known to elevate tumor necrosis factor α (TNFα) and
MDA, signaling increased inflammation and oxidative stress
(103). NMDA receptor antagonists
are often used in rodents to simulate the hypofunction of NMDA
glutamate receptors observed in patients with schizophrenia
(104). The NMDA receptor
antagonist pentachlorophenol (PCP) induces hallucinations,
delusions, speech impoverishment and cognitive deficits in humans,
while in rodents, PCP causes social withdrawal and cognitive
impairments.
Advances in human genome-wide studies have recently
revealed a growing list of schizophrenia-associated and candidate
genes (105), emphasizing the
importance of genetic factors in the development of animal models.
The identification of these candidate genes has significantly
contributed to refining and enhancing schizophrenia model systems
(106,107). Certainly, animal models used in
modern research must obtain approval from the Animal Research
Ethics Committee and comply with the animal experiment guidelines
or directives formulated by relevant institutions.
In the post-genome-wide era of schizophrenia, the
development of cellular models incorporating
schizophrenia-associated variants has become a crucial tool for
understanding the disease. Both autopsy and in vivo
neuroimaging studies have highlighted neuronal and glial cell
abnormalities as prominent features of schizophrenia.
Artificially manipulating SH-SY5Y cells to construct
cellular models greatly benefits research into schizophrenia at the
cellular and molecular levels. SH-SY5Y cells treated with the NMDA
antagonist MK-801 are commonly used to model neuronal damage seen
in patients with schizophrenia. MK-801 downregulates the sirtuin
1/microRNA (miRNA)-134 signaling pathway and Ca2+
influx, replicating dysfunction in the prefrontal cortex (112). Additionally, SH-SY5Y cells are
utilized to investigate the molecular mechanisms underlying reduced
neurite spines, a known susceptibility factor for schizophrenia
that is also observed in postmortem brain samples from patients
(113). Lentiviral infection of
SH-SY5Y cells with short hairpin RNA targeting the schizophrenia
susceptibility gene DISC1 results in mitochondrial fragmentation
and respiratory defects. Studies on DISC1 in SH-SY5Y cells suggest
that DISC1-depleted cells fail to maintain proper mitochondrial
oxidative phosphorylation complex function, and mitochondrial
abnormalities may have a role in the onset of schizophrenia
(114). SHANK2, a gene encoding a
protein essential for the formation and development of
glutamatergic synapses, has also been implicated in schizophrenia
pathogenesis (115). Mutations in
SHANK2 in SH-SY5Y cells impair early neuronal differentiation,
alter cell growth properties and reduce both pre- and post-synaptic
protein expression (116).
Collectively, these studies strongly support the use of SH-SY5Y
cells as a reliable model for investigating schizophrenia.
Additionally, SH-SY5Y cells have proven valuable as a screening
platform for potential antipsychotic drugs (117).
Induced pluripotent stem cells (iPSCs) were
initially utilized to model diseases linked to highly penetrant
genetic variants with significant phenotypic effects, and more
recently, iPSC-derived neurons have been employed to construct
models of psychiatric disorders. Although iPSCs are not commonly
used to model schizophrenia-related processes directly, genetic
variants associated with schizophrenia can substantially affect
iPSCs. For instance, the 22q11.2 deletion has been shown to reduce
the proliferation rate of iPSCs in culture (118). Furthermore, the study of
patient-derived iPSCs enables the screening of genetic variants
that may not be detectable through human genome-wide studies, while
also shedding light on the roles of relevant candidate variants.
Although the reproducibility of iPSC models remains a subject of
debate, careful selection of iPSC lines for reprogramming, as well
as thoughtful consideration of subjects and controls, can mitigate
many of their limitations (119).
iPSC-derived neurons mainly include neural
progenitor cells (NPCs), glutamatergic neurons, dopaminergic
(DAergic) neurons and γ-aminobutyric acid-secreting (GABAergic)
neurons. Studies involving NPCs have broadened the understanding of
schizophrenia, highlighting its complexity as a neurodevelopmental
disorder. The glutamatergic neurons model is based on the
glutamatergic hypothesis, where SNP-4bp mutations in DISC1 in
glutamatergic neurons result in reduced presynaptic labeling and
defective neurotransmitter release. Notably, the mutant phenotype
can be reversed by restoring the DISC1 sequence, thereby
demonstrating how synaptic defects contribute to schizophrenia
(120). The DAergic neurons model
is linked to the dopaminergic dysfunction hypothesis, which remains
central to schizophrenia research, supported by both animal models
and human studies (121). The
iPSC-derived DAergic neurons model helps elucidate the molecular
mechanisms underlying the relationship between genetic variants and
dopaminergic dysfunction in patients with schizophrenia. While most
studies on iPSC-derived GABAergic neurons have focused on
Huntington's disease, research has indicated the downregulation of
key GABA pathway genes in GABAergic neurons derived from patients
with schizophrenia, suggesting there is still significant room for
further exploration of GABAergic neurons in schizophrenia (122).
Non-neuronal cells can be reprogrammed into neuronal
cells or used directly as cell models. It has been demonstrated
that fibroblasts can be directly converted into patient-specific
inducible neurons, such as glutamatergic and GABAergic neurons,
through transcription factor overexpression and the addition of
miRNAs (123). Non-neuronal
cells, like HEK293 cells, can also serve as models for studying
genes and proteins associated with schizophrenia. The
primate-specific mutant isoform Kv11.1-3.1 of the brain
voltage-gated potassium channel exhibits aberrant folding and
impaired migration to the plasma membrane, which correlates with
prolonged neuronal action potentials observed in patients with
schizophrenia. Researchers have developed a platform for screening
drugs that restore Kv11.1-3.1 function using HEK293 cells (124). Studies on the DPYSL2 isoform in
HEK293 cells have shown that alterations in risk alleles can lead
to changes in cellular morphology, affecting synaptic transmission
and correlating with schizophrenia susceptibility (125,126).
However, current research on schizophrenia largely
focuses on relatively narrow perspectives and the symptoms of
schizophrenia exhibit significant individual variation. Such
individual variations pose significant challenges to the
establishment of disease models and the conduct of more in-depth
research. Furthermore, differing hypotheses exist regarding the
pathogenesis of schizophrenia, and genetic outcomes also vary
across different populations. This review primarily employed broad
keywords in its literature search (such as schizophrenia, mental
illness, model studies, animal models, cellular models,
environment, genes and epigenetics), and consequently may have
overlooked certain publications. Advancing the translation of
findings from animal/cell model research into human pathology
facilitates researchers' deeper understanding of schizophrenia and
aids in drug screening and validation.
The causes of schizophrenia are unknown and include
complex genetic inheritance, the environment and the interaction of
the two. Current findings suggest that schizophrenia involves a
number of different neurotransmitters, including dopamine,
glutamate and serotonin. More research related to schizophrenia
remains to be done, and the construction of more accurate models
will facilitate further exploration of the pathogenesis of
schizophrenia as well as its treatment.
At present, mental disorders pose considerable
challenges both in diagnosis and treatment. Schizophrenia lacks
precise biomarkers or pathological criteria for diagnosis, and
psychiatric disorders frequently co-occur with it, presenting
symptoms that vary significantly among individuals. The current
mainstream medications for treating schizophrenia carry significant
side effects, resulting in a markedly reduced quality of life for
patients. Whether through human genomics research or more complex
and precise biological modelling, the aim is to gain deeper
insights into schizophrenia, uncover its precise mechanisms, and
identify therapeutic drugs with enhanced efficacy and reduced side
effects. In the current state of mental health, research into
mechanisms and therapeutic drug development is of considerable
value and significance.
Not applicable.
Funding: No funding was received.
Not applicable.
SL contributed to the writing and editing of this
review. CL collected the data. Data authentication is not
applicable. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Patel V, Saxena S, Lund C, Thornicroft G,
Baingana F, Bolton P, Chisholm D, Collins PY, Cooper JL, Eaton J,
et al: The lancet commission on global mental health and
sustainable development. Lancet. 392:1553–1598. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
GBD 2019 Demographics Collaborators.
Global age-sex-specific fertility, mortality, healthy life
expectancy (HALE), and population estimates in 204 countries and
territories, 1950-2019: A comprehensive demographic analysis for
the Global Burden of Disease Study 2019. Lancet. 396:1160–1203.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
GBD 2019 Diseases and Injuries
Collaborators. Global burden of 369 diseases and injuries in 204
countries and territories, 1990-2019: A systematic analysis for the
Global Burden of Disease Study 2019. Lancet. 396:1204–1222.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
GBD 2019 Mental Disorders Collaborators.
Global, regional, and national burden of 12 mental disorders in 204
countries and territories, 1990-2019: A systematic analysis for the
Global Burden of Disease Study 2019. Lancet Psychiatry. 9:137–150.
2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Khavari B and Cairns MJ: Epigenomic
dysregulation in schizophrenia: In search of disease etiology and
biomarkers. Cells. 9(1837)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
McCutcheon RA, Reis Marques T and Howes
OD: Schizophrenia-an overview. JAMA Psychiatry. 77:201–210.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Saha S, Chant D, Welham J and McGrath J: A
systematic review of the prevalence of schizophrenia. PLoS Med.
2(e141)2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jauhar S, Johnstone M and McKenna PJ:
Schizophrenia. Lancet. 399:473–486. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bueno-Antequera J and Munguía-Izquierdo D:
Exercise and Schizophrenia. Adv Exp Med Biol. 1228:317–332.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Correll CU and Schooler NR: Negative
symptoms in schizophrenia: A review and clinical guide for
recognition, assessment, and treatment. Neuropsychiatr Dis Treat.
16:519–534. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Faden J and Citrome L: Schizophrenia: One
name, many different manifestations. Med Clin North Am. 107:61–72.
2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Javitt DC: Cognitive impairment associated
with schizophrenia: From pathophysiology to treatment. Annu Rev
Pharmacol Toxicol. 63:119–141. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ma H, Cheng N and Zhang C: Schizophrenia
and alarmins. Medicina (Kaunas). 58(694)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zamanpoor M: Schizophrenia in a genomic
era: A review from the pathogenesis, genetic and environmental
etiology to diagnosis and treatment insights. Psychiatr Genet.
30:1–9. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chauhan P, Kaur G, Prasad R and Singh H:
Pharmacotherapy of schizophrenia: Immunological aspects and
potential role of immunotherapy. Expert Rev Neurother.
21:1441–1453. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Helaly AMN and Ghorab DSED: Schizophrenia
as metabolic disease. What are the causes? Metab Brain Dis.
38:795–804. 2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Stilo SA and Murray RM: Non-genetic
factors in schizophrenia. Curr Psychiatry Rep.
21(100)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Collins FS, Morgan M and Patrinos A: The
human genome project: Lessons from large-scale biology. Science.
300:286–290. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Peedicayil J: Genome-environment
interactions and psychiatric disorders. Biomedicines.
11(1209)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tsuang M: Schizophrenia: Genes and
environment. Biol Psychiatry. 47:210–220. 2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kendler KS and Diehl SR: The genetics of
schizophrenia: A current, genetic-epidemiologic perspective.
Schizophr Bull. 19:261–285. 1993.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wahbeh MH and Avramopoulos D:
Gene-environment interactions in schizophrenia: A literature
review. Genes (Basel). 12(1850)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Takata A, Xu B, Ionita-Laza I, Roos JL,
Gogos JA and Karayiorgou M: Loss-of-function variants in
schizophrenia risk and SETD1A as a candidate susceptibility gene.
Neuron. 82:773–780. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ansari-Lari M, Zendehboodi Z, Masoudian M
and Mohammadi F: Additive effect of glutathione S-transferase T1
active genotype and infection with Toxoplasma gondii for increasing
the risk of schizophrenia. Nord J Psychiatry. 75:275–280.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Coyle JT: Passing the torch: The
ascendance of the glutamatergic synapse in the pathophysiology of
schizophrenia. Biochem Pharmacol. 228(116376)2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Harrison PJ and Bannerman DM: GRIN2A
(NR2A): A gene contributing to glutamatergic involvement in
schizophrenia. Mol Psychiatry. 28:3568–3572. 2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang CY, Xiao X, Zhang Z, Hu Z and Li M:
An alternative splicing hypothesis for neuropathology of
schizophrenia: evidence from studies on historical candidate genes
and multi-omics data. Mol Psychiatry. 27:95–112. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pardiñas AF, Holmans P, Pocklington AJ,
Escott-Price V, Ripke S, Carrera N, Legge SE, Bishop S, Cameron D,
Hamshere ML, et al: Common schizophrenia alleles are enriched in
mutation-intolerant genes and in regions under strong background
selection. Nat Genet. 50:381–389. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Forsyth JK and Asarnow RF: Genetics of
childhood-onset schizophrenia 2019 update. Child Adolesc Psychiatr
Clin N Am. 29:157–170. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Schizophrenia Working Group of the
Psychiatric Genomics Consortium. Biological insights from 108
schizophrenia-associated genetic loci. Nature. 511:421–427.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ambalavanan A, Girard SL, Ahn K, Zhou S,
Dionne-Laporte A, Spiegelman D, Bourassa CV, Gauthier J, Hamdan FF,
Xiong L, et al: De novo variants in sporadic cases of childhood
onset schizophrenia. Eur J Hum Genet. 24:944–948. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chaumette B, Ferrafiat V, Ambalavanan A,
Goldenberg A, Dionne-Laporte A, Spiegelman D, Dion PA, Gerardin P,
Laurent C, Cohen D, et al: Missense variants in ATP1A3 and FXYD
gene family are associated with childhood-onset schizophrenia. Mol
Psychiatry. 25:821–830. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Smedemark-Margulies N, Brownstein CA,
Vargas S, Tembulkar SK, Towne MC, Shi J, Gonzalez-Cuevas E, Liu KX,
Bilguvar K, Kleiman RJ, et al: A novel de novo mutation in ATP1A3
and childhood-onset schizophrenia. Cold Spring Harb Mol Case Stud.
2(a001008)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jolly LA, Homan CC, Jacob R, Barry S and
Gecz J: The UPF3B gene, implicated in intellectual disability,
autism, ADHD and childhood onset schizophrenia regulates neural
progenitor cell behaviour and neuronal outgrowth. Hum Mol Genet.
22:4673–4687. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Richetto J and Meyer U: Epigenetic
modifications in schizophrenia and related disorders: Molecular
scars of environmental exposures and source of phenotypic
variability. Biol Psychiatry. 89:215–226. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Guidotti A, Grayson DR and Caruncho HJ:
Epigenetic RELN dysfunction in schizophrenia and related
neuropsychiatric disorders. Front Cell Neurosci.
10(89)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jaffe AE, Gao Y, Deep-Soboslay A, Tao R,
Hyde TM, Weinberger DR and Kleinman JE: Mapping DNA methylation
across development, genotype and schizophrenia in the human frontal
cortex. Nat Neurosci. 19:40–47. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sharma RP, Rosen C, Kartan S, Guidotti A,
Costa E, Grayson DR and Chase K: Valproic acid and chromatin
remodeling in schizophrenia and bipolar disorder: Preliminary
results from a clinical population. Schizophr Res. 88:227–231.
2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Akbarian S, Ruehl MG, Bliven E, Luiz LA,
Peranelli AC, Baker SP, Roberts RC, Bunney WE Jr, Conley RC, Jones
EG, et al: Chromatin alterations associated with down-regulated
metabolic gene expression in the prefrontal cortex of subjects with
schizophrenia. Arch Gen Psychiatry. 62:829–840. 2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bahari-Javan S, Varbanov H, Halder R,
Benito E, Kaurani L, Burkhardt S, Anderson-Schmidt H, Anghelescu I,
Budde M, Stilling RM, et al: HDAC1 links early life stress to
schizophrenia-like phenotypes. Proc Natl Acad Sci USA.
114:E4686–E4694. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Schroeder FA, Gilbert TM, Feng N, Taillon
BD, Volkow ND, Innis RB, Hooker JM and Lipska BK: Expression of
HDAC2 but Not HDAC1 transcript is reduced in dorsolateral
prefrontal cortex of patients with schizophrenia. ACS Chem
Neurosci. 8:662–668. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Dickerson F, Schroeder JR, Nimgaonkar V,
Gold J and Yolken R: The association between exposure to herpes
simplex virus type 1 (HSV-1) and cognitive functioning in
schizophrenia: A meta-analysis. Psychiatry Res.
291(113157)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tripathy S, Singh N, Singh A and Kar SK:
COVID-19 and psychotic symptoms: The view from psychiatric
immunology. Curr Behav Neurosci Rep. 8:172–178. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Banerjee D and Viswanath B:
Neuropsychiatric manifestations of COVID-19 and possible pathogenic
mechanisms: Insights from other coronaviruses. Asian J Psychiatr.
54(102350)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Klein HC, Guest PC, Dobrowolny H and
Steiner J: Inflammation and viral infection as disease modifiers in
schizophrenia. Front Psychiatry. 14(1231750)2023.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Pandey JP, Namboodiri AM and Elston RC:
Immunoglobulin G genotypes and the risk of schizophrenia. Hum
Genet. 135:1175–1179. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Inyang B, Gondal FJ, Abah GA, Minnal
Dhandapani M, Manne M, Khanna M, Challa S, Kabeil AS and Mohammed
L: The role of childhood trauma in psychosis and schizophrenia: A
systematic review. Cureus. 14(e21466)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Thomson P and Jaque SV: Childhood
adversity and the creative experience in adult professional
performing artists. Front Psychol. 9(111)2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Buscemi V, Chang WJ, Liston MB, McAuley JH
and Schabrun S: The role of psychosocial stress in the development
of chronic musculoskeletal pain disorders: Protocol for a
systematic review and meta-analysis. Syst Rev.
6(224)2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Li KJ, Chen A and DeLisi LE: Opioid use
and schizophrenia. Curr Opin Psychiatry. 33:219–224.
2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Guloksuz S, Rutten BPF, Pries LK, Ten Have
M, de Graaf R, van Dorsselaer S, Klingenberg B, van Os J and
Ioannidis JPA: European Network of National Schizophrenia Networks
Studying Gene-Environment Interactions Work Package 6 (EU-GEI WP6)
Group. The complexities of evaluating the exposome in psychiatry: A
data-driven illustration of challenges and some propositions for
amendments. Schizophr Bull. 44:1175–1179. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Rodriguez-Gonzalez A and Orio L:
Microbiota and alcohol use disorder: Are psychobiotics a novel
therapeutic strategy? Curr Pharm Des. 26:2426–2437. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Clausing ES and Non AL: Epigenetics as a
mechanism of developmental embodiment of stress, resilience, and
cardiometabolic risk across generations of latinx immigrant
families. Front Psychiatry. 12(696827)2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Jaaro-Peled H and Sawa A:
Neurodevelopmental factors in schizophrenia. Psychiatr Clin North
Am. 43:263–274. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Karlsson H and Dalman C: Epidemiological
studies of prenatal and childhood infection and schizophrenia. Curr
Top Behav Neurosci. 44:35–47. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ju S, Shin Y, Han S, Kwon J, Choi TG, Kang
I and Kim SS: The gut-brain axis in schizophrenia: The implications
of the gut microbiome and SCFA production. Nutrients.
15(4391)2023.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Tandon R, Nasrallah H, Akbarian S,
Carpenter WT Jr, DeLisi LE, Gaebel W, Green MF, Gur RE, Heckers S,
Kane JM, et al: The schizophrenia syndrome, circa 2024: What we
know and how that informs its nature. Schizophr Res. 264:1–28.
2024.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Al-Haddad BJS, Oler E, Armistead B,
Elsayed NA, Weinberger DR, Bernier R, Burd I, Kapur R, Jacobsson B,
Wang C, et al: The fetal origins of mental illness. Am J Obstet
Gynecol. 221:549–562. 2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Desmettre T: Toxoplasmosis and behavioural
changes. J Fr Ophtalmol. 43:e89–e93. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Maldonado YA and Read JS: COMMITTEE ON
INFECTIOUS DISEASES. Diagnosis, treatment, and prevention of
congenital toxoplasmosis in the United States. Pediatrics.
139(e20163860)2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Chen X, Chen B, Hou X, Zheng C, Yang X, Ke
J, Hu X and Tan F: Association between Toxoplasma gondii infection
and psychiatric disorders in Zhejiang, Southeastern China. Acta
Trop. 192:82–86. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Liu T, Gao P, Bu D and Liu D: Association
between Toxoplasma gondii infection and psychiatric disorders: A
cross-sectional study in China. Sci Rep. 12(15092)2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Veleva I, Stoychev K, Stoimenova-Popova M,
Stoyanov L, Mineva-Dimitrova E and Angelov I: Toxoplasma gondii
seropositivity and cognitive function in adults with schizophrenia.
Schizophr Res Cogn. 30(100269)2022.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Acquarone M, Poleto A, Perozzo AF,
Gonçalves PFR, Panizzutti R, Menezes JRL, Neves GA and Barbosa HS:
Social preference is maintained in mice with impaired startle
reflex and glutamate/D-serine imbalance induced by chronic cerebral
toxoplasmosis. Sci Rep. 11(14029)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Lucchese G: From toxoplasmosis to
schizophrenia via NMDA dysfunction: Peptide overlap between
toxoplasma gondii and N-Methyl-d-Aspartate receptors as a potential
mechanistic link. Front Psychiatry. 8(37)2017.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Bhavsar V, Boydell J, McGuire P, Harris V,
Hotopf M, Hatch SL, MacCabe JH and Morgan C: Childhood abuse and
psychotic experiences-evidence for mediation by adulthood adverse
life events. Epidemiol Psychiatr Sci. 28:300–309. 2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Sheffield JM, Karcher NR and Barch DM:
Cognitive deficits in psychotic disorders: A lifespan perspective.
Neuropsychol Rev. 28:509–533. 2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Kaufman J and Torbey S: Child maltreatment
and psychosis. Neurobiol Dis. 131(104378)2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Powers A, Fani N, Cross D, Ressler KJ and
Bradley B: Childhood trauma, PTSD, and psychosis: Findings from a
highly traumatized, minority sample. Child Abuse Negl. 58:111–118.
2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Vila-Badia R, Butjosa A, Del Cacho N,
Serra-Arumí C, Esteban-Sanjusto M, Ochoa S and Usall J: Types,
prevalence and gender differences of childhood trauma in
first-episode psychosis. What is the evidence that childhood trauma
is related to symptoms and functional outcomes in first episode
psychosis? A systematic review. Schizophr Res. 228:159–179.
2021.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Abdel-Baki A, Ouellet-Plamondon C, Salvat
É, Grar K and Potvin S: Symptomatic and functional outcomes of
substance use disorder persistence 2 years after admission to a
first-episode psychosis program. Psychiatry Res. 247:113–119.
2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Di Forti M, Quattrone D, Freeman TP,
Tripoli G, Gayer-Anderson C, Quigley H, Rodriguez V, Jongsma HE,
Ferraro L, La Cascia C, et al: The contribution of cannabis use to
variation in the incidence of psychotic disorder across Europe
(EU-GEI): A multicentre case-control study. Lancet Psychiatry.
6:427–436. 2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Morgan C, Charalambides M, Hutchinson G
and Murray RM: Migration, ethnicity, and psychosis: Toward a
sociodevelopmental model. Schizophr Bull. 36:655–664.
2010.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Porter M and Haslam N: Predisplacement and
postdisplacement factors associated with mental health of refugees
and internally displaced persons: A meta-analysis. JAMA.
294:602–612. 2005.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Veling W, Selten JP, Susser E, Laan W,
Mackenbach JP and Hoek HW: Discrimination and the incidence of
psychotic disorders among ethnic minorities in The Netherlands. Int
J Epidemiol. 36:761–768. 2007.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Benros ME and Mortensen PB: Role of
infection, autoimmunity, atopic disorders, and the immune system in
schizophrenia: Evidence from epidemiological and genetic studies.
Curr Top Behav Neurosci. 44:141–159. 2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Wang AW, Avramopoulos D, Lori A, Mulle J,
Conneely K, Powers A, Duncan E, Almli L, Massa N, McGrath J, et al:
Genome-wide association study in two populations to determine
genetic variants associated with Toxoplasma gondii infection and
relationship to schizophrenia risk. Prog Neuropsychopharmacol Biol
Psychiatry. 92:133–147. 2019.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Lori A, Avramopoulos D, Wang AW, Mulle J,
Massa N, Duncan EJ, Powers A, Conneely K, Gillespie CF, Jovanovic
T, et al: Polygenic risk scores differentiate schizophrenia
patients with toxoplasma gondii compared to toxoplasma seronegative
patients. Compr Psychiatry. 107(152236)2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Xiao J, Prandovszky E, Kannan G, Pletnikov
MV, Dickerson F, Severance EG and Yolken RH: Toxoplasma gondii:
Biological parameters of the connection to schizophrenia. Schizophr
Bull. 44:983–992. 2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Hong J and Bang M: Anti-inflammatory
strategies for schizophrenia: A review of evidence for therapeutic
applications and drug repurposing. Clin Psychopharmacol Neurosci.
18:10–24. 2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Avramopoulos D, Pearce BD, McGrath J,
Wolyniec P, Wang R, Eckart N, Hatzimanolis A, Goes FS, Nestadt G,
Mulle J, et al: Infection and inflammation in schizophrenia and
bipolar disorder: A genome wide study for interactions with genetic
variation. PLoS One. 10(e0116696)2015.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Estes ML and McAllister AK: Maternal
immune activation: Implications for neuropsychiatric disorders.
Science. 353:772–777. 2016.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Reisinger S, Khan D, Kong E, Berger A,
Pollak A and Pollak DD: The poly(I:C)-induced maternal immune
activation model in preclinical neuropsychiatric drug discovery.
Pharmacol Ther. 149:213–226. 2015.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Caspi A, Moffitt TE, Cannon M, McClay J,
Murray R, Harrington H, Taylor A, Arseneault L, Williams B,
Braithwaite A, et al: Moderation of the effect of adolescent-onset
cannabis use on adult psychosis by a functional polymorphism in the
catechol-O-methyltransferase gene: longitudinal evidence of a gene
X environment interaction. Biol Psychiatry. 57:1117–1127.
2005.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Zammit S, Spurlock G, Williams H, Norton
N, Williams N, O'Donovan MC and Owen MJ: Genotype effects of
CHRNA7, CNR1 and COMT in schizophrenia: Interactions with tobacco
and cannabis use. Br J Psychiatry. 191:402–407. 2007.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Akhtar MJ, Yar MS, Grover G and Nath R:
Neurological and psychiatric management using COMT inhibitors: A
review. Bioorg Chem. 94(103418)2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Henquet C, Rosa A, Krabbendam L, Papiol S,
Fananás L, Drukker M, Ramaekers JG and van Os J: An experimental
study of catechol-o-methyltransferase Val158Met moderation of
delta-9-tetrahydrocannabinol-induced effects on psychosis and
cognition. Neuropsychopharmacology. 31:2748–2757. 2006.PubMed/NCBI View Article : Google Scholar
|
|
88
|
van Winkel R, Stefanis NC and Myin-Germeys
I: Psychosocial stress and psychosis. A review of the
neurobiological mechanisms and the evidence for gene-stress
interaction. Schizophr Bull. 34:1095–1105. 2008.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Hindocha C, Quattrone D, Freeman TP,
Murray RM, Mondelli V, Breen G, Curtis C, Morgan CJA, Valerie
Curran H and Di Forti M: Do AKT1, COMT and FAAH influence reports
of acute cannabis intoxication experiences in patients with first
episode psychosis, controls and young adult cannabis users? Transl
Psychiatry. 10(143)2020.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Hiemstra M, Nelemans SA, Branje S, van
Eijk KR, Hottenga JJ, Vinkers CH, van Lier P, Meeus W and Boks MP:
Genetic vulnerability to schizophrenia is associated with cannabis
use patterns during adolescence. Drug Alcohol Depend. 190:143–150.
2018.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Dzirasa K and Covington HE 3rd: Increasing
the validity of experimental models for depression. Ann N Y Acad
Sci. 1265:36–45. 2012.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Hvoslef-Eide M, Mar AC, Nilsson SR, Alsiö
J, Heath CJ, Saksida LM, Robbins TW and Bussey TJ: The NEWMEDS
rodent touchscreen test battery for cognition relevant to
schizophrenia. Psychopharmacology (Berl). 232:3853–3872.
2015.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Modinos G, Allen P, Grace AA and McGuire
P: Translating the MAM model of psychosis to humans. Trends
Neurosci. 38:129–138. 2015.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Talamini LM, Ellenbroek B, Koch T and Korf
J: Impaired sensory gating and attention in rats with developmental
abnormalities of the mesocortex. Implications for schizophrenia.
Ann N Y Acad Sci. 911:486–494. 2000.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Paylor JW, Lins BR, Greba Q, Moen N, de
Moraes RS, Howland JG and Winship IR: Developmental disruption of
perineuronal nets in the medial prefrontal cortex after maternal
immune activation. Sci Rep. 6(37580)2016.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Steullet P, Cabungcal JH, Coyle J,
Didriksen M, Gill K, Grace AA, Hensch TK, LaMantia AS, Lindemann L,
Maynard TM, et al: Oxidative stress-driven parvalbumin interneuron
impairment as a common mechanism in models of schizophrenia. Mol
Psychiatry. 22:936–943. 2017.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Careaga M, Murai T and Bauman MD: Maternal
immune activation and autism spectrum disorder: From rodents to
nonhuman and human primates. Biol Psychiatry. 81:391–401.
2017.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Sams-Dodd F: A test of the predictive
validity of animal models of schizophrenia based on phencyclidine
and D-amphetamine. Neuropsychopharmacology. 18:293–304.
1998.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Kittirattanapaiboon P, Mahatnirunkul S,
Booncharoen H, Thummawomg P, Dumrongchai U and Chutha W: Long-term
outcomes in methamphetamine psychosis patients after first
hospitalisation. Drug Alcohol Rev. 29:456–461. 2010.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Lecomte T, Dumais A, Dugré JR and Potvin
S: The prevalence of substance-induced psychotic disorder in
methamphetamine misusers: A meta-analysis. Psychiatry Res.
268:189–192. 2018.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Medhus S, Rognli EB, Gossop M, Holm B,
Mørland J and Bramness JG: Amphetamine-induced psychosis:
Transition to schizophrenia and mortality in a small prospective
sample. Am J Addict. 24:586–589. 2015.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Wearne TA, Mirzaei M, Franklin JL,
Goodchild AK, Haynes PA and Cornish JL: Methamphetamine-induced
sensitization is associated with alterations to the proteome of the
prefrontal cortex: Implications for the maintenance of psychotic
disorders. J Proteome Res. 14:397–410. 2015.PubMed/NCBI View Article : Google Scholar
|
|
103
|
El-Sayed El-Sisi A, Sokkar SS, El-Sayed
El-Sayad M, Sayed Ramadan E and Osman EY: Celecoxib and omega-3
fatty acids alone and in combination with risperidone affect the
behavior and brain biochemistry in amphetamine-induced model of
schizophrenia. Biomed Pharmacother. 82:425–431. 2016.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Kalinichev M, Robbins MJ, Hartfield EM,
Maycox PR, Moore SH, Savage KM, Austin NE and Jones DN: Comparison
between intraperitoneal and subcutaneous phencyclidine
administration in Sprague-Dawley rats: A locomotor activity and
gene induction study. Prog Neuropsychopharmacol Biol Psychiatry.
32:414–422. 2008.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Dennison CA, Legge SE, Pardiñas AF and
Walters JTR: Genome-wide association studies in schizophrenia:
Recent advances, challenges and future perspective. Schizophr Res.
217:4–12. 2020.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Keller MC: Evolutionary perspectives on
genetic and environmental risk factors for psychiatric disorders.
Annu Rev Clin Psychol. 14:471–493. 2018.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Power RA, Kyaga S, Uher R, MacCabe JH,
Långström N, Landen M, McGuffin P, Lewis CM, Lichtenstein P and
Svensson AC: Fecundity of patients with schizophrenia, autism,
bipolar disorder, depression, anorexia nervosa, or substance abuse
vs their unaffected siblings. JAMA Psychiatry. 70:22–30.
2013.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Bray NJ, Kapur S and Price J:
Investigating schizophrenia in a ‘dish’: Possibilities, potential
and limitations. World Psychiatry. 11:153–155. 2012.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Kovalevich J and Langford D:
Considerations for the use of SH-SY5Y neuroblastoma cells in
neurobiology. Methods Mol Biol. 1078:9–21. 2013.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Bell M, Bachmann S, Klimek J,
Langerscheidt F and Zempel H: Axonal TAU sorting requires the
C-terminus of TAU but is independent of ANKG and TRIM46 Enrichment
at the AIS. Neuroscience. 461:155–171. 2021.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Paik S, Somvanshi RK and Kumar U:
Somatostatin-mediated changes in microtubule-associated proteins
and retinoic acid-induced neurite outgrowth in SH-SY5Y cells. J Mol
Neurosci. 68:120–134. 2019.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Farber NB, Wozniak DF, Price MT, Labruyere
J, Huss J, St Peter H and Olney JW: Age-specific neurotoxicity in
the rat associated with NMDA receptor blockade: Potential relevance
to schizophrenia? Biol Psychiatry. 38:788–796. 1995.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Berdenis van Berlekom A, Muflihah CH,
Snijders GJLJ, MacGillavry HD, Middeldorp J, Hol EM, Kahn RS and de
Witte LD: Synapse pathology in schizophrenia: A meta-analysis of
postsynaptic elements in postmortem brain studies. Schizophr Bull.
46:374–386. 2020.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Piñero-Martos E, Ortega-Vila B, Pol-Fuster
J, Cisneros-Barroso E, Ruiz-Guerra L, Medina-Dols A, Heine-Suñer D,
Lladó J, Olmos G and Vives-Bauzà C: Disrupted in schizophrenia 1
(DISC1) is a constituent of the mammalian mitochondrial contact
site and cristae organizing system (MICOS) complex, and is
essential for oxidative phosphorylation. Hum Mol Genet.
25:4157–4169. 2016.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Peykov S, Berkel S, Schoen M, Weiss K,
Degenhardt F, Strohmaier J, Weiss B, Proepper C, Schratt G, Nöthen
MM, et al: Identification and functional characterization of rare
SHANK2 variants in schizophrenia. Mol Psychiatry. 20:1489–1498.
2015.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Unsicker C, Cristian FB, von Hahn M,
Eckstein V, Rappold GA and Berkel S: SHANK2 mutations impair
apoptosis, proliferation and neurite outgrowth during early
neuronal differentiation in SH-SY5Y cells. Sci Rep.
11(2128)2021.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Wu S, Wang P, Tao R, Yang P, Yu X, Li Y,
Shao Q, Nie F, Ha J, Zhang R, et al: Schizophrenia-associated
microRNA-148b-3p regulates COMT and PRSS16 expression by targeting
the ZNF804A gene in human neuroblastoma cells. Mol Med Rep.
22:1429–1439. 2020.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Toyoshima M, Akamatsu W, Okada Y, Ohnishi
T, Balan S, Hisano Y, Iwayama Y, Toyota T, Matsumoto T, Itasaka N,
et al: Analysis of induced pluripotent stem cells carrying 22q11.2
deletion. Transl Psychiatry. 6(e934)2016.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Lee IS, Carvalho CM, Douvaras P, Ho SM,
Hartley BJ, Zuccherato LW, Ladran IG, Siegel AJ, McCarthy S,
Malhotra D, et al: Characterization of molecular and cellular
phenotypes associated with a heterozygous CNTNAP2 deletion using
patient-derived hiPSC neural cells. NPJ Schizophr. 1:15019-.
2015.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Wen Z, Nguyen HN, Guo Z, Lalli MA, Wang X,
Su Y, Kim NS, Yoon KJ, Shin J, Zhang C, et al: Synaptic
dysregulation in a human iPS cell model of mental disorders.
Nature. 515:414–418. 2014.PubMed/NCBI View Article : Google Scholar
|
|
121
|
McCutcheon RA, Krystal JH and Howes OD:
Dopamine and glutamate in schizophrenia: Biology, symptoms and
treatment. World Psychiatry. 19:15–33. 2020.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Cohen SM, Tsien RW, Goff DC and Halassa
MM: The impact of NMDA receptor hypofunction on GABAergic neurons
in the pathophysiology of schizophrenia. Schizophr Res. 167:98–107.
2015.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Raabe FJ, Slapakova L, Rossner MJ,
Cantuti-Castelvetri L, Simons M, Falkai PG and Schmitt A:
Oligodendrocytes as a new therapeutic target in schizophrenia: From
histopathological findings to neuron-oligodendrocyte interaction.
Cells. 8(1496)2019.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Tran NN, Ladran IG and Brennand KJ:
Modeling schizophrenia using induced pluripotent stem cell-derived
and fibroblast-induced neurons. Schizophr Bull. 39:4–10.
2013.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Liu Y, Pham X, Zhang L, Chen PL, Burzynski
G, McGaughey DM, He S, McGrath JA, Wolyniec P, Fallin MD, et al:
Functional variants in DPYSL2 sequence increase risk of
schizophrenia and suggest a link to mTOR signaling. G3 (Bethesda).
5:61–72. 2014.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Stratton H, Boinon L, Moutal A and Khanna
R: Coordinating synaptic signaling with CRMP2. Int J Biochem Cell
Biol. 124(105759)2020.PubMed/NCBI View Article : Google Scholar
|