Introduction
Heart failure (HF) occurs when the heart loses its
ability to pump sufficient amounts of blood to the body. HF
represents a pervasive global public health challenge, affecting
>64 million individuals worldwide, and exhibits a growing
prevalence due to the aging population (1,2). The
prevalence of HF exhibited a notable increase from 641.14/100,000
individuals in 1990 to 676.68/100,000 individuals in 2021, with men
(760.78/100,000) showing an increased incidence rate compared with
women (604/100,000) (3). HF may be
caused by numerous different conditions, including right
ventricular dysfunction, left ventricular (LV) dysfunction,
pericardial disease, valvular heart disease and obstructive lesions
in the great vessels or heart (4).
Despite notable improvements in pharmacological (nesiritide,
ularitide, inotropic agents, serelaxin, angiotensin II type 1
receptor, rolofylline) and non-pharmacological (ventilator support,
ultrafiltration) treatment strategies (5), HF continues to be associated with
high morbidity, frequent hospitalizations, impaired quality of life
and substantial mortality rates [32% deaths in patients who had HF
with LV ejection fraction (LVEF) <40%] (6). This burden is magnified in the
context of type 2 diabetes mellitus (T2DM), a frequent comorbidity
that not only accelerates HF progression but also complicates
management strategies (7).
Importantly, the pathophysiological association between
hyperglycemia, oxidative stress, insulin resistance, myocardial
fibrosis and inflammation, contributes to both functional and
structural deterioration of the heart (8). Thus, targeting the overlapping
mechanisms of diabetes and HF has been of great interest. Among
recent and emerging therapies, sodium-glucose cotransporter 2
inhibitors (SGLT2i), which were initially developed for glycemic
control, demonstrate promise as potent agents conferring
cardiovascular (CV) and renal protection, beyond the
glucose-lowering effect they exhibit (9,10).
Notable CV outcome trials in patients with T2DM first demonstrated
a reduction in hospitalizations related to HF and CV mortality,
establishing the foundation for dedicated HF trials that excluded
diabetes status as an inclusion criterion (11,12).
Subsequently, high-quality randomized controlled
trials (RCTs) across the spectrum of HF phenotypes, ranging from
reduced to preserved ejection fraction and including non-diabetic
individuals, have consistently demonstrated that SGLT2i reduce HF
hospitalizations, improve functional capacity and enhance
health-related quality of life (13). RCTs, such as the ‘Dapagliflozin and
Prevention of Adverse Outcomes in HF’ trial (DAPA-HF) and the
empagliflozin outcome trial in patients with preserved ejection
fraction (EMPEROR-preserved), have highlighted the efficacy of
SGLT2i in reducing HF-related hospitalizations and CV mortality,
extending their use to non-diabetic populations (14,15).
These patient-centered benefits, as evidenced by the Kansas City
Cardiomyopathy Questionnaire (KCCQ) and New York Heart Association
functional classification scores, underscore the broader importance
of SGLT2i in HF management (16).
Beyond their glucose-lowering effects, SGLT2i
exhibit pleiotropic benefits, including natriuresis, blood pressure
reduction, improved ventricular loading conditions and potential
direct myocardial effects (17).
Previous meta-analyses have supported the favorable effect of
SGLT2i on HF outcomes; however, they tended to include a limited
number of studies with heterogeneous populations (18,19).
Therefore, questions remain regarding the consistency and magnitude
of SGLT2i benefits across diabetic and non-diabetic patients, and
whether the observed outcomes are uniformly applicable across a
number of patient populations and HF phenotypes. Moreover, as
evidence continues to accumulate from both CV outcome trials and
HF-specific RCTs, an updated and more focused analysis with
additional variables is warranted. Therefore, the present
systematic review and meta-analysis aimed to collect evidence from
RCTs to evaluate the effect of SGLT2i on HF outcomes in adults with
and without T2DM. The primary objective was to determine whether
SGLT2i reduces the risk of hospitalization for HF across this
population. Secondary objectives included assessing the impact of
SGLT2i on CV mortality, all-cause mortality and adverse events.
Materials and methods
Data sources and search
Comprehensive literature searches were performed in
PubMed, Scopus, Web of Science and Cochrane Central, from database
inception to May 2025, applying combinations of vocabulary and key
words related to SGLT2i, empagliflozin, canagliflozin,
dapagliflozin and HF. These search terms were combined using
Boolean operators and the detailed search strategy is described in
Table SI. The present systematic
review and meta-analysis was designed following the Preferred
Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)
2020 guidelines (20).
Eligibility criteria and outcomes
Eligible studies included phase II-IV RCTs enrolling
adult patients (aged ≥18 years) diagnosed with HF, irrespective of
ejection fraction category or diabetes status. Studies must have
compared any SGLT2i with a placebo or standard-of-care treatment
and reported at least one of the following outcomes: i)
Hospitalization for HF (primary outcome); ii) CV mortality; iii)
all-cause mortality; iv) ejection fraction; v) myocardial
infraction; or vi) health-related quality of life assessed
according to the KCCQ. Studies that exclusively enrolled pediatric
populations, animal models or healthy volunteers were excluded.
Additionally, trials lacking extractable outcome data or those
without stratification by diabetes status were also excluded.
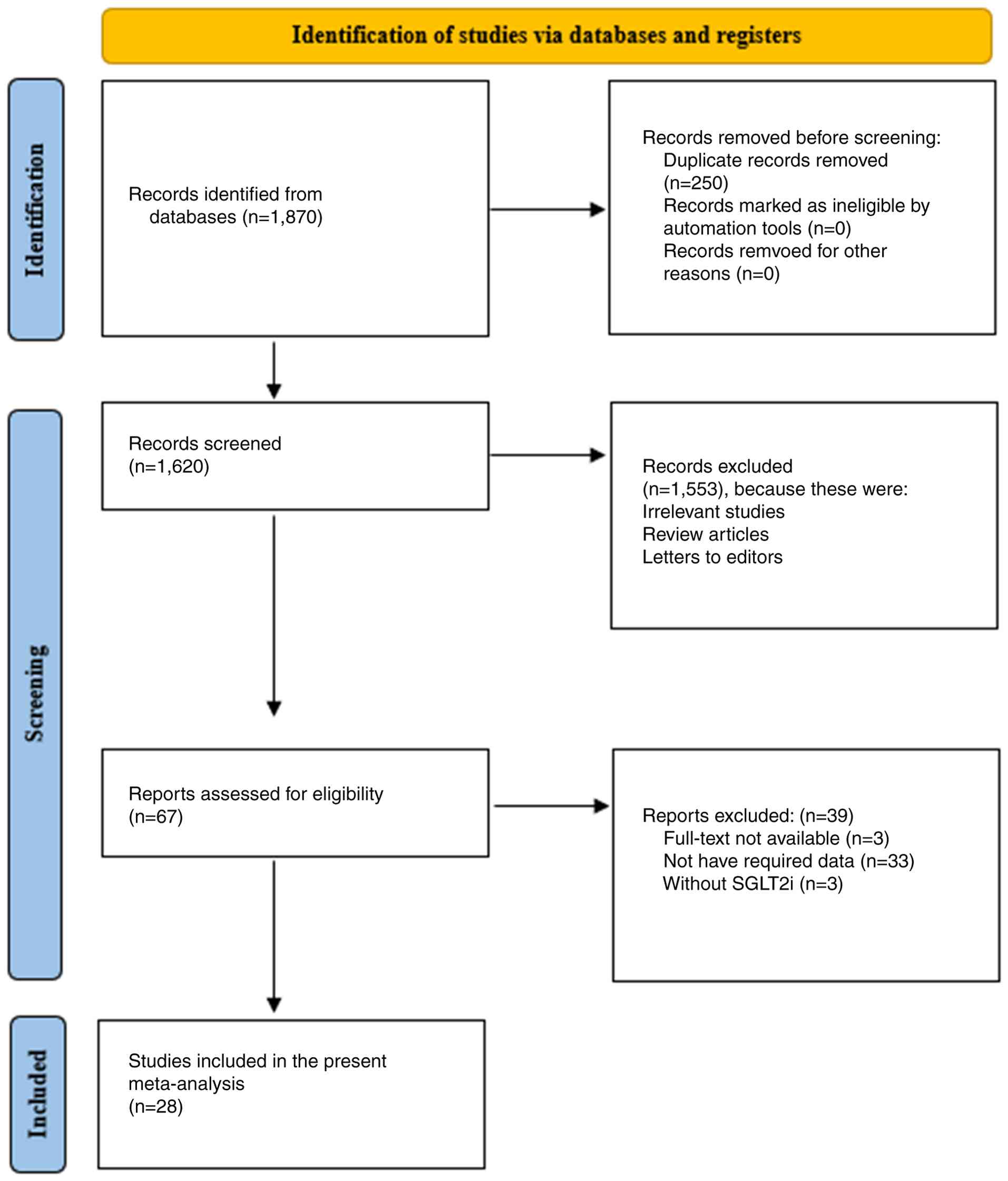
Study selection process
Independent screening processes were carried out by
two authors using the PRISMA flow chart. In the first phase, 1,870
studies were retrieved through a search in different electronic
databases and 250 duplicate studies were removed using the EndNote
X9 referencing software (Clarivate; www.endnote.com). In the second phase, 1,620 studies
were screened by checking their titles and abstracts, and 1,553
studies were excluded due to being irrelevant, or being reviews or
letters to editors, leaving 67 studies eligible for full-text
assessment. During the full-text assessment, 39 studies were
excluded due to non-availability of the full text, not reporting
the required outcomes or not having used SGLT2i. The study
selection process is illustrated in Fig. 1. Finally, 28 studies were selected
for qualitative and quantitative analysis. Discrepancies were
resolved through consensus or consultation with a third author.
Data extraction
Data were extracted from each eligible study by two
independent authors, using a predefined data collection form.
Extracted information included study characteristics (author, year
of publication, country, trial phase and sample size), participant
demographics (age, sex, diabetes status and baseline LVEF),
intervention and comparator details (agent, dosage, administration
route and treatment duration), follow-up periods and reported
effect estimates [hazard ratios (HRs), risk ratios and mean
differences with 95% CIs].
Methodological quality and risk of
bias assessment
Risk of bias was assessed at the study level using
the Cochrane Risk of Bias 2.0 tool (https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials),
with judgments made in the domains of randomization, deviations
from intended interventions, missing outcome data, measurement of
outcomes and selective reporting. Outcomes were reported in the
form of visualization judgments associated with each risk of bias
item and presented as percentages using the web-based application
Risk of Bias VISualization (21).
This entire process was performed by two independent authors and
any discrepancies were resolved with the consultation of a third
senior author.
Meta-analysis
For qualitative data, narrative synthesis was
performed, with key characteristics of studies and patients
presented as tables. Quantitative data were analyzed using RevMan
5.4 (https://www.cochrane.org/learn/courses-and-resources/software)
for the construction of forest plots using a random-effects model.
The association between HF clinical outcomes and SGLT2i was
measured using a χ2 test and P<0.05 was considered to
indicate a statistically significant difference. Heterogeneity
among studies was calculated using the I2 statistic,
with a heterogeneity of <25, 26-75 and >75% being considered
as low, moderate and high, respectively. Funnel plots were
constructed for publication bias. If the distribution of studies
was symmetrical and a clear funnel shape was observed, low
publication bias was found among the studies, while an asymmetrical
distribution of studies without a clear funnel shape indicated a
higher publication bias. The funnel plot asymmetry was evaluated
using Egger's linear regression test through RStudio software
(version 4.0.2; Posit Software, PBC) for Windows.
Certainty of evidence
Certainty of evidence was evaluated using the
Grading of Recommendations Assessment, Development and Evaluation
framework (22), a reproducible
and structured framework used for the assessment of outcomes
derived from the included studies. The level of certainty ranges
from low to high after considering specific criteria, such as the
risk of bias, result inconsistency and publication bias.
Results
General characteristics of
studies
Numerous included studies were performed in multiple
countries from Latin America, North America, South America, Europe
and Asia. However, a limited number of studies were also performed
in a single country, such as the USA (23-25),
the UK (26,27), Australia (28), Canada (29) and the Netherlands (30). The majority of the studies were
part of trials or programs, as described in Table I. Sample sizes varied notably and
the majority of the studies used large sample sizes ranging from
8,582 to 8,578 individuals in the intervention and placebo groups
(31), with the lowest sample size
of 162 individuals in both the intervention and placebo groups
(23). Overall, studies included
middle-aged and elderly individuals, between 61 and 73 years of
age, with predominant male representation (Table I). Furthermore, the majority of the
studies included patients with diabetes only (25,28,29,31-41),
while a reasonable number of studies analyzed both diabetic and
non-diabetic patients (23,24,26,27,30,42-50).
BMI also markedly ranged from average (<25 kg/m2) to
obese (35.1 kg/m2) (23,39).
Among the comorbidities, hypertension and kidney-associated
diseases were the most prevalent (Table I). In addition, a number of studies
focused on patients with an LVEF of <45 or <40% (23,27,32,36,42-44,47,48,50),
while one study focused on the preserved or reduced range of
ejection fraction and one study gave chronic kidney disease (CKD)
stage information (49), as
described in Table I.
 | Table ISummary of general characteristics of
the included studies. |
Table I
Summary of general characteristics of
the included studies.
| Study
characteristics | Participant
characteristics |
|---|
| First author,
year | Country | Trial phase | Sample size, N | Age, years | Sex, M:F | Diabetic
status | Comorbidities | BMI,
kg/m2 | Baseline LVEF | (Refs.) |
|---|
| Zinman et
al, 2015 | Multinational (42
countries from North America, Australia, New Zealand, Latin
America, Europe, Africa and Asia) | NA | Intervention group,
468,7; control group, 233,3 | Intervention group,
63.2; control group, 63.1 | Intervention group,
333,6:135,1; control group, 168,0:653 | Diabetic
patients | NA | Intervention group,
30.6; control group, 30.7 | NA | (41) |
| Fitchett et
al, 2016 | Multinational (42
countries) | EMPA-REG OUTCOME
trial | Intervention group,
468,7; control group, 233,3 | Intervention group,
63.1; control group, 63.1 | 505,4:196,6 | Diabetic
patients | NA | 30.6 | NA | (34) |
| Neal et al,
2017 | Multinational (30
countries) | NA | Intervention group,
579,5; control group, 434,7 | Intervention group,
63.2; control group, 63.4 | Intervention group,
375,9:203,6; control group, 275,0:159,7 | Diabetes
(chronic) | Hypertension | Intervention group,
31.9; control group, 32.0 | NA | (38) |
| Wanner et
al, 2018 | Multinational
(countries from Europe, North America, Asia, Africa, Latin America,
Australia and New Zealand) | NA | Intervention group,
464,7; control group, 222,7 | 61.0 | Intervention group,
330,2:134,5; control group, 167,1:556 | Diabetic
patients | Kidney
diseases | 30.8 | NA | (40) |
| Mahaffey et
al, 2018 | Multinational
(North America, South/Central America, Europe and the rest of the
world) | CANVAS program | 101,42 |
62.7-63.8a | 650,9:363,3 | Diabetic
patients | Hypertension |
31.7-32.5b | NA | (37) |
| Kato et al,
2019 | Multinational
(countries from North America, Latin America, Europe and the Asia
Pacific) | DECLARE-TIMI 58
trial | 171,60 | 63-65a | 108,11:634,9 | Diabetic
patients | Hypertension |
31.1-31.6b | <45% (n=671),
>45% (n=808) | (36) |
| McMurray et
al, 2019 | Multinational
(countries from North and South America, Europe and the Asia
Pacific) | Phase 3 | Intervention group,
237,3; control group, 237,1 | Intervention group,
66.2; control group, 66.5 | Intervention group,
180,9:564; control group, 1,826:545 | With or without
diabetes | NA | Intervention group,
28.2; control group, 28.1 | <40% | (48) |
| Wiviott et
al, 2019 | Multinational
(North America, Latin America, Europe and Asia Pacific) | DECLARE-TIMI 58
trial/phase 3 | Intervention group,
858,2; control group, 857,8 | Intervention group,
63.9; control group, 64.0 | Intervention group,
541,1:317,1; control group, 5,327:3,251 | Diabetic
patients | NA | Intervention group,
32.1; control group, 32.0 | NA | (31) |
| Perkovic et
al, 2019 | Australia | NA | Intervention group,
220,2; control group, 219,9 | Intervention group,
62.9; control group, 63.2 | Intervention group,
144,0:762; control group, 146,7:732 | Diabetic
patients | Hypertension | Intervention group,
31.4; control group, 31.3 | NA | (28) |
| Cannon et
al, 2020 | Multinational
(countries from North and South America, Asia, Europe, South
Africa, New Zealand and Australia) | NA | Intervention group,
549,9; control group, 274,7 | 64.4 | Intervention group,
386,6:163,3; control group, 1,903:844 | Diabetic
patients | Coronary artery
disease, cerebrovascular disease, peripheral arterial disease and
MI | Intervention group,
31.9; control group, 32.0 | NA | (33) |
| Heerspink et
al, 2020 | The
Netherlands | NA | Intervention group,
215,2; control group, 215,2 | Intervention group,
61.8; control group, 61.9 | Intervention group,
144,3:709; control group, 143,6:716 | With or without
diabetes | Kidney
diseases | Intervention group,
29.4; control group, 29.6 | NA | (30) |
| Kosiborod et
al, 2020 | Multinational
(countries from the Asia Pacific, Europe, North and South
America) | DAPA-HF trial | 444,3 | 66.3 | 345,2:991 | With or without
diabetes | Hypertension | NA | 6.8% | (46) |
| Inzucchi et
al, 2020 | Multinational
(countries from Europe, Asia, Latin America, North America and
Africa) | EMPA-REG OUTCOME
trial | Intervention group,
468,7; control group, 233,3 |
62.5-63.4a | 495,1:198,4 | Diabetic
patients | NA | NA | NA | (35) |
| Verma et al,
2020 | Canada | EMPA-REG OUTCOME
trial | 702,0 |
61.4-65.5a | NA | Diabetic
patients | Hypertension |
30.0-31.0b | NA | (29) |
| Ohkuma et
al, 2020 | Multinational | CANVAS program | 101,28 |
62.8-64.0a | 650,1:362,7 | Diabetic
patients | Hypertension | <25.0 or
>30.0 | NA | (39) |
| Bhatt et al,
2021 | Multinational
(countries from North America, Latin America, western Europe,
eastern Europe and the rest of the world) | NA | Intervention group,
608; control group, 614 | Intervention group,
69.0; control group, 70.0 | Intervention group,
410:198; control group, 400:214 | Diabetic
patients | NA | NA | <50%:
Intervention group (n=481) and control group (n=485) | (32) |
| McMurray et
al, 2021 | Multinational
(Europe, Asia Pacific, South and North America) | NA | 430,4 |
61.4-65.3a | 287,9:142,5 | With or without
diabetes | CKD, hypertension,
angina, MI and stroke | NA | NA | (49) |
| Nassif et
al, 2021 | USA | NA | Intervention group,
162; control group, 162 | Intervention group,
69.0; control group, 71.0 | Intervention group,
70:92; control group, 70:92 | With or without
diabetes | NA | Intervention group,
35.1; control group, 34.6 | Intervention group,
60%; control group, 60% | (23) |
| Anker et al,
2021 | Multinational
(countries from Latin and North America, Europe and Asia) | EMPEROR-preserved
trial | Intervention group,
299,7; control group, 299,1 | Intervention group,
71.8; control group, 71.9 | Intervention group;
165,9:133,8; control group, 165,3:133,8 | With or without
diabetes | NA | Intervention group,
29.77; control group, 29.90 | Intervention group:
≤50% (n=995), ≤60% (n=1,028), >60% (n=974); control group: ≤50%
(n=988), ≤60% (n=1,030) and >60% (n=973) | (42) |
| Anker et al,
2021 | Multinational
(countries from Latin and North America, Asia and Europe) | EMPEROR-reduced
trial | Intervention group,
299,7; control group, 299,1 |
66.3-67.6a | Intervention group,
165,9:133,8; control group, 165,3:133,8 | With or without
diabetes | Hypertension |
27.0-28.8b | ≤40% | (43) |
| Spertus et
al, 2022 | USA | CHIEF-HF | Intervention group,
222; control group, 226 | Intervention group,
62.9; control group, 64.0 | Intervention group,
118:104; control group, 129:97 | With or without
diabetes | NA | NA | NA | (24) |
| Kosiborod et
al, 2022 | Multinational
(countries from Asia, North America and Europe) | EMPULSE trial | Intervention group,
265; control group, 265 |
66.5-69.5a | 349:177 | With or without
diabetes | Hypertension |
27.4-32.6b | NA | (45) |
| Solomon et
al, 2022 | Multinational
(countries from Asia, Europe, Saudi Arabia, Latin and North
America) | NA | Intervention group,
313,1; control group, 313,2 | Intervention group,
71.8; control group, 71.5 | Intervention group,
176,7:136,4; control group, 174,9:138,3 | With or without
diabetes | Hypertension | NA | Intervention group,
54%; control group, 54.3% | (50) |
| Herrington et
al, 2023 | UK | NA | Intervention group,
330,4; control group, 330,5 | Intervention group,
63.9; control group, 63.8 | Intervention group,
220,7:109,7; control group, 221,0:109,5 | With or without
diabetes | NA | Intervention group,
29.7; control group, 29.8 | NA | (26) |
| Hernandez et
al, 2024 | Multinational (22
countries from North and Latin America, Europe and Asia) | NA | Intervention group,
326,0; control group, 326,2 | Intervention group,
63.6; control group, 63.7 | Intervention group,
244,8:812; control group, 244,9:813 | With or without
diabetes | Hypertension and
peripheral arterial disease | Intervention group,
28.1; control group, 28.1 | <45% in 78.4% of
patients | (44) |
| McMurray et
al, 2024 | Western Europe,
North America and the rest of the world | The DETERMINE
randomized clinical trials | DETERMINE-reduced
intervention group, 156; control group, 157. DETERMINE-preserved
intervention group, 253; control group, 251 | DETERMINE-reduced
intervention group, 69.0; control group, 69.0. DETERMINE-preserved
intervention group, 73.0; control group, 73.0 | DETERMINE-reduced
intervention group, 111:45; control group, 122:35.
DETERMINE-preserved intervention group, 162:91; control group,
158:93 | With or without
diabetes | NA | DETERMINE-reduced
intervention group, 28.0; control group, 29.0. DETERMINE-preserved
intervention group, 29.0; control group, 28.0 | DETERMINE-reduced
intervention group, 30%; control group, 29%. DETERMINE-preserved
intervention group, 50%; control group, 53% | (47) |
| Vaduganathan et
al, 2024 | USA | CANVAS program and
CREDENCE | 145,43 |
62-65.5a | 941,8:512,5 | Diabetic
patients | Kidney diseases and
hypertension | NA | NA | (25) |
| Petrie et
al, 2025 | UK | EMPACT-MI
trial | 652,2 |
63.0-64.0a | 489,2:163,0 | With or without
diabetes | Hypertension and
COPD |
27.0-29.0b | <45% | (27) |
Characteristics of intervention and
control groups
Among SGLT2i, the most commonly used agents were
empagliflozin (26,27,29,34,35,40-45),
dapagliflozin (23,30,31,36,46-50)
and canagliflozin (24,25,28,37-39),
while other less frequently used agents included ertugliflozin and
sotagliflozin (32,33). Most agents were administered orally
once daily; however, a wide range was observed in the dosages
administered, depending on the agent. Empagliflozin was
administered at a dose of 10 or 25 mg, dapagliflozin at 10 mg and
canagliflozin at either 100 or 300 mg, as described in Table II. Variation was also observed in
the treatment durations, ranging from 14 days (33,38,40,41,44)
to 2.6 years (34). Across all
included studies, a standard-of-care was used as a comparison
group, ensuring consistency in comparative evaluation (Table II).
 | Table IISummary of intervention and control
characteristics. |
Table II
Summary of intervention and control
characteristics.
| | Intervention and
control characteristics | |
|---|
| First author,
year | Agent | Dosage | Administration
route | Treatment
duration | Control | (Refs.) |
|---|
| Zinman et
al, 2015 | Empagliflozin | 10 or 25 mg
daily | Oral | 14 days | Placebo | (41) |
| Fitchett et
al, 2016 | Empagliflozin | 10 or 25 mg
daily | Oral | 2.6 years | Placebo | (34) |
| Neal et al,
2017 | Canagliflozin | 100 or 300 mg
daily | Oral | 14 days | Placebo | (38) |
| Wanner et
al, 2018 | Empagliflozin | 10 or 25 mg
daily | Oral | 14 days | Placebo | (40) |
| Mahaffey et
al, 2018 | Canagliflozin | NA | NA | NA | Placebo | (37) |
| Kato et al,
2019 | Dapagliflozin | NA | NA | NA | Placebo | (36) |
| McMurray et
al, 2019 | Dapagliflozin | 10 mg daily | Oral | NA | Placebo | (48) |
| Wiviott et
al, 2019 | Dapagliflozin | 10 mg daily | Oral | NA | Placebo | (31) |
| Perkovic et
al, 2019 | Canagliflozin | 100 mg daily | Oral | NA | Placebo | (28) |
| Cannon et
al, 2020 | Ertugliflozin | 5 or 15 mg
daily | Oral | 14 days | Placebo | (33) |
| Heerspink et
al, 2020 | Dapagliflozin | 10 mg daily | Oral | 2.4 years | Placebo | (30) |
| Kosiborod et
al, 2020 | Dapagliflozin | 10 mg daily | Oral | NA | Placebo | (46) |
| Inzucchi et
al, 2020 | Empagliflozin | 10 or 25 mg
daily | Oral | 12 weeks | Placebo | (35) |
| Verma et al,
2020 | Empagliflozin | 10 or 25 mg
daily | Oral | NA | Placebo | (29) |
| Ohkuma et
al, 2020 | Canagliflozin | 100 or 300 mg
daily | Oral | NA | Placebo | (39) |
| Bhatt et al,
2021 | Sotagliflozin | 200 mg daily | Oral | NA | Placebo | (32) |
| McMurray et
al, 2021 | Dapagliflozin | 10 mg daily | Oral | NA | Placebo | (49) |
| Nassif et
al, 2021 | Dapagliflozin | NA | NA | 12 weeks | Placebo | (23) |
| Anker et al,
2021 | Empagliflozin | 10 mg daily | Oral | NA | Placebo | (42) |
| Anker et al,
2021 | Empagliflozin | 10 mg daily | Oral | NA | Placebo | (43) |
| Spertus et
al, 2022 | Canagliflozin | 100 mg | Oral | 12 weeks | Placebo | (24) |
| Kosiborod et
al, 2022 | Empagliflozin | 10 mg daily | Oral | 90 days | Placebo | (45) |
| Solomon et
al, 2022 | Dapagliflozin | 10 mg daily | Oral | NA | Placebo | (50) |
| Herrington et
al, 2023 | Empagliflozin | 10 mg daily | Oral | NA | Placebo | (26) |
| Hernandez et
al, 2024 | Empagliflozin | 10 mg daily | Oral | 14 days | Placebo | (44) |
| McMurray et
al, 2024 | Dapagliflozin | NA | NA | NA | Placebo | (47) |
| Vaduganathan et
al, 2024 | Canagliflozin | 100 or 300 mg
daily | Oral | NA | Placebo | (25) |
| Petrie et
al, 2025 | Empagliflozin | 10 mg daily | Oral | NA | Placebo | (27) |
Outcomes
Overall, the majority of the studies reported a
significant reduction in hospitalization for HF and CV-associated
mortality with the use of SGLT2i, with HR values ranging from 0.51
to 2.64, indicating consistent benefits. Similarly, these agents
also demonstrated favorable outcomes in reducing all-cause
mortality, although a number of studies reported a neutral impact
in reducing these CV-mortality outcomes (33,50).
In addition, myocardial infarction rates (in studies that reported
them) also demonstrated a non-significant difference (31,33,38,41)
between the SGLT2i and placebo groups. However, quality of life was
improved after the administration of SGLT2i agents, as observed
through improved scores based on the KCCQ (Table III). A wide range was observed in
the follow-up duration of these trials, with most studies having
reported >1 year of follow-up (26-28,30,33,34,36,38,41,42,44,47,48)
and one study following up for <1 year (32). The most commonly occurring adverse
events associated with SGLT2i were genital mycotic infections,
urinary tract infections, volume depletion/hypotension, acute
kidney injury (AKI), lower limb amputation, severe hypoglycemia,
ketoacidosis and hypovolemia (Table
III). Overall, these results consistently supported the
efficacy and safety of SGLT2i in improving HF-associated outcomes
in patients with or without diabetes.
 | Table IIISummary of outcomes associated with
the application of sodium-glucose cotransporter 2 inhibitors. |
Table III
Summary of outcomes associated with
the application of sodium-glucose cotransporter 2 inhibitors.
| | Outcomes | |
|---|
| First author,
year | Hospitalization for
total HF | CV mortality | All-cause
mortality | Myocardia
infraction | QoL (KCCQ) | Follow-up | Adverse events | Conclusion | (Refs.) |
|---|
| Zinman et
al, 2015 | 0.65 (95% CI:
0.50-0.85) | 0.62 (95% CI:
0.49-0.77) | 0.68 (95% CI:
0.57-0.82) | 0.87 (95% CI:
0.70-1.09) | NA | 206 weeks | UTI | Empagliflozin was
found effective in reducing primary CV outcomes | (41) |
| Fitchett et
al, 2016 | 0.65 (95% CI:
0.50-0.85) | 0.66 (95% CI:
0.55-0.79) | 0.68 (95% CI:
0.57-0.82) | NA | NA | 3.1 years | Adverse events
occurred in both groups, like hypoglycemia | Empagliflozin
effectively reduced HF hospitalization and CV mortality | (34) |
| Neal et al,
2017 | NA | 0.87 (95% CI:
0.72-1.06) | 0.87 (95% CI:
0.74-1.01) | 0.86 (95% CI:
0.75-0.97) | NA | 188.2 weeks | Volume depletion,
kidney problems, diuresis | Canagliflozin
successfully lowered the risk of CV events | (38) |
| Wanner et
al, 2018 | 0.61 (95% CI:
0.42-0.87) | 0.71 (95% CI:
0.52-0.98) | 0.76 (95% CI:
0.59-0.99) | NA | NA | NA | Acute renal
failure, bone fracture, lower limb amputation and hyperkalemia | Empagliflozin
improved clinical outcomes | (40) |
| Mahaffey et
al, 2018 | 2.64 (95% CI:
1.90-3.65) | 2.51 (95% CI:
1.99-3.16) | 1.86 (95% CI:
1.57-2.22) | NA | NA | NA | Amputations,
genital infections, fractures, volume depletion and renal adverse
events | Canagliflozin
reduced CV outcomes | (37) |
| Kato et al,
2019 | 0.64 (95% CI:
0.43-0.95) | 0.55 (95% CI:
0.34-0.90) | 0.59 (95% CI:
0.40-0.88) | NA | NA | 4.2 years | Major hypoglycemia,
amputation, diabetic ketoacidosis, fracture, acute renal failure,
genital infection, urinary tract infection | Dapagliflozin
reduced CV outcomes | (36) |
| McMurray et
al, 2019 | 0.70 (95% CI:
0.59-0.83) | 0.82 (95% CI:
0.69-0.98) | 0.83 (95% CI:
0.71-0.97) | NA | NA | 18.2 months | Renal dysfunction,
hypoglycemia and volume depletion | Dapagliflozin
reduced HF in patients with and without diabetes | (48) |
| Wiviott et
al, 2019 | 0.73 (95% CI:
0.61-0.88) | 0.83 (95% CI:
0.73-0.95) | 0.93 (95% CI:
0.82-1.04) | 0.89 (95% CI:
0.77-1.01) | NA | 4.2 years | Renal failure and
ketoacidosis | Dapagliflozin did
not result in a higher or lower rate of MACE than placebo but did
result in a lower rate of CV mortality or hospitalization for
HF | (31) |
| Perkovic et
al, 2019 | 0.61 (95% CI:
0.47-0.80) | 0.78 (95% CI:
0.61-1.00) | 0.83 (95% CI:
0.68-1.02) | NA | NA | 2.62 years | Amputation,
fracture, renal cell carcinoma, bladder and breast cancer, acute
pancreatitis | Canagliflozin was
found to be effective in reducing cardiovascular events and kidney
failure events | (28) |
| Cannon et
al, 2020 | 0.70 (95% CI:
0.54-0.90) | 0.92 (95.8% CI:
0.77-1.11) | 0.93 (95% CI:
0.80-1.08) | 1.04 (95% CI:
0.86-1.27) | NA | 3.5 years | Amputation, UTI,
genital mycotic infection, hypovolemia, AKI and diabetic
ketoacidosis | Ertugliflozin was
non-inferior to placebo with respect to MACE | (33) |
| Heerspink et
al, 2020 | NA | 0.71 (95% CI:
0.55-0.92 | NA | NA | NA | 2.4 years | Amputation,
fracture, renal failure and volume depletion | Dapagliflozin
markedly reduced CV outcomes | (30) |
| Kosiborod et
al, 2020 | NA | 0.70 (95% CI:
0.57-0.86) | NA | NA | 2.8-point
improvement (intervention) | 12 months | NA | Dapagliflozin
reduced CV mortality and worsening HF across the range of baseline
KCCQ | (46) |
| Inzucchi et
al, 2020 | 1.91 (95% CI:
0.96-3.79) | 4.00 (95% CI:
2.26-7.11) | NA | NA | NA | NA | Non-fatal
myocardial infarction, non-fatal stroke | Empagliflozin
effectively improved CV outcomes | (35) |
| Verma et al,
2020 | 0.53 (95% CI:
0.28-1.01) | 0.75 (95% CI:
0.48-1.18) | 0.68 (95% CI:
0.48-0.97) | NA | NA | NA | UTI, volume
depletion and acute renal failure | Empagliflozin
reduced CV outcomes | (29) |
| Ohkuma et
al, 2020 | 0.67 (95% CI:
0.52-0.87) | 0.87 (95% CI:
0.72-1.06) | NA | NA | NA | NA | Amputation,
fractures, infection, serious hyperkalemia, diabetic
ketoacidosis | Canagliflozin
improved CV outcomes | (39) |
| Bhatt et al,
2021 | 0.64 (95% CI:
0.49-0.83) | 0.84 (95% CI:
0.58-1.22) | 0.82 (95% CI:
0.59-1.14) | NA | 4.10 (95% CI:
1.3-7.00) | 9 months | Diarrhea, severe
hypoglycemia, hypotension and AKI | Sotagliflozin
therapy markedly lowered CV mortality and hospitalizations | (32) |
| McMurray et
al, 2021 | 0.51 (95% CI:
0.34-0.76) | 0.68 (95% CI:
0.44-1.05) | 0.56 (95% CI:
0.34-0.93) | NA | NA | 2.4 years | Amputation,
fracture, renal adverse events, major hypoglycemia, volume
depletion | Dapagliflozin
reduced the risk of CV mortality and HF hospitalization | (49) |
| Nassif et
al, 2021 | NA | NA | Intervention group
(0.6%) and control group (1.2%) | NA | 5.8 points (95% CI:
2.3-9.2) | 12 weeks | AKI, volume
depletion and hypoglycemic events, lower limb amputation | Dapagliflozin
markedly improved patient-reported symptoms | (23) |
| Anker et al,
2021 | 0.73 (95% CI:
0.61-0.88) | 0.91 (95% CI:
0.76-1.09) | 1.00 (95% CI:
0.87-1.15) | NA | HR=1.32 (95% CI:
0.45-2.19) | 26.2 months | Uncomplicated
genital infections, UTI and hypotension were reported more
frequently in the intervention group | Empagliflozin
reduced the combined risk of CV mortality or hospitalization for
HF | (42) |
| Anker et al,
2021 | 0.70 (95% CI:
0.58-0.85) | 0.75 (95% CI:
0.65-0.86) | NA | NA | Difference in
change: 1.75 (95% CI: 0.5-3.0) | NA | Hypotension, volume
depletion, hypoglycemic events, ketoacidosis and lower limp
amputation | Empagliflozin
markedly improved CV and renal outcomes | (43) |
| Spertus et
al, 2022 | NA | NA | NA | NA | KCCQ TSS change:
4.3 points (95% CI: 0.8-7.8) higher in the intervention group | 12 weeks | Infections,
hypotension, increased urination | Canagliflozin
markedly improves symptom burden in HF, regardless of ejection
fraction or diabetes status | (24) |
| Kosiborod et
al, 2022 | NA | NA | NA | NA | Corrected
difference: 1.36 win ratio (95% CI: 1.09-1.68) | 90 days | NA | Empagliflozin
markedly improved symptoms, physical limitations and QoL | (45) |
| Solomon et
al, 2022 | 0.82 (95% CI:
0.73-0.92) | 0.88 (95% CI:
0.74-1.05) | 0.94 (95% CI:
0.83-1.07) | NA | Corrected
difference: 2.4 points (95% CI: 1.5-3.4) | 2.3 years | Amputation,
hypergly cemic events, ketoacidosis and volume depletion | Dapagliflozin
reduced the risk of worsening HF or CV mortality | (50) |
| Herrington et
al, 2023 | NA | 0.84 (95% CI:
0.60-1.19) | 0.87 (95% CI:
0.70-1.08) | NA | NA | 2 years | UTI, genital
infection, hyperkalemia, AKI, liver injury, lower limb amputation,
fractures and severe hypoglycemia | Empagliflozin was
found to be effective for reducing risk of progression of kidney
diseases and cardiovascular deaths | (26) |
| Hernandez et
al, 2024 | First intervention
group (118/3,260) and control group (153/3, 262); total inter
vention group (148/3, 260) and control group (207/3,262) | Intervention group
(280/3,260) and control group (338/3,262) | Intervention group
(20/3,260) and control group (30/3,262) | NA | NA | 17.9 months | Intervention group,
cardiac failure and cardiogenic shock; control group, cardiac
failure, cardiogenic shock and acute pulmonary edema | Empagliflozin
reduced the risk of HF | (44) |
| McMurray et
al, 2024 | NA | NA | NA | NA | KCCQ-TSS corrected
difference after 16 weeks: 4.2 (95% CI:1.0-8.2) favoring
dapagliflozin; KCCQ-PLS: 4.2 (95% CI: 0.0-8.3) | 16 weeks | Adverse events
occurred in both groups | Dapagliflozin
improved the KCCQ-TSS in patients with HF with reduced ejection
fraction, but did not improve KCCQ-PLS | (47) |
| Vaduganathan et
al, 2024 | Intervention group
(304) and control group (368) | NA | NA | NA | NA | 2.5 years | NA | Canagliflozin
reduced the total burden of HF hospitalizations | (25) |
| Petrie et
al, 2025 | First HR=0.77 (95%
CI: 0.60-0.98); total HR=0.67 (95% CI: 0.50-0.89) | NA | HR=0.96 (95% CI:
0.78-1.19) | NA | NA | 17.9 months | AKI, hypotension,
volume depletion, hypoglycemia, hepatic injury and
ketoacidosis | Empagliflozin
reduced first and total HF hospitalizations | (27) |
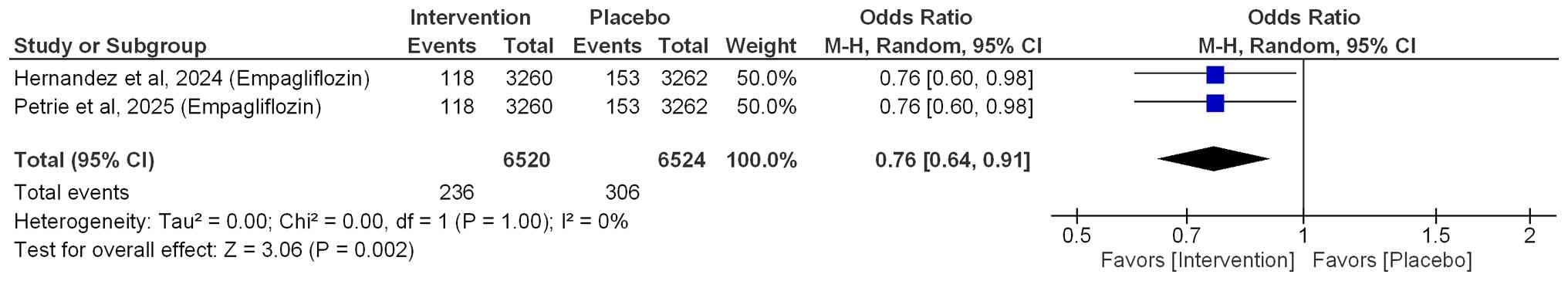
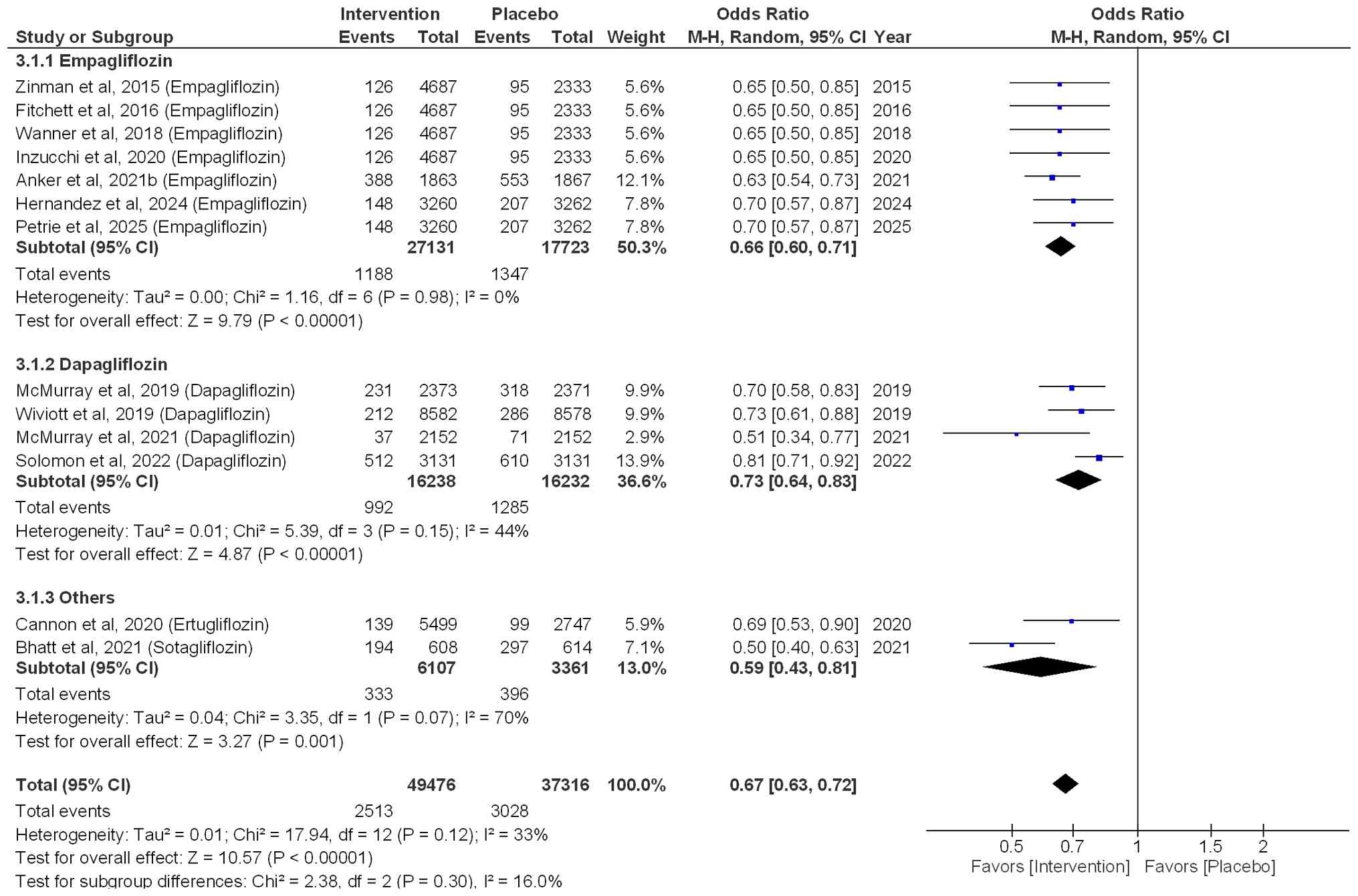
Hospitalization for HF
Among patients with HF who had diabetes and were
treated with empagliflozin, the risk of first hospitalization for
HF was significantly reduced by 24% (OR=0.76; 95% CI: 0.64-0.91;
P=0.002; I²=0%; Fig. 2). In
addition, the risk of hospitalization for total HF was reduced by
34% (OR=0.66; 95% CI: 0.60-0.71; P<0.00001; I²=0%), when
patients were treated with empagliflozin. Similarly, a significant
reduction of 27% (OR=0.73; 95% CI: 0.64-0.83; P<0.00001) was
observed when patients were treated with dapagliflozin, with
moderate heterogeneity (I2=44%). In addition, other
SGLT2i, such as ertugliflozin and sotagliflozin, also resulted in a
significant reduction of 41% in the risk of hospitalization for HF
(OR=0.59; 95% CI: 0.43-0.81; P=0.001), with a notable heterogeneity
(I2=70%). Overall, the pooled effect size (OR=0.67; 95%
CI: 0.63-0.72; P<0.00001; I²=33%) indicated that SGT2i
effectively reduced the risk of hospitalization for HF by 33%, as
shown in Fig. 3.
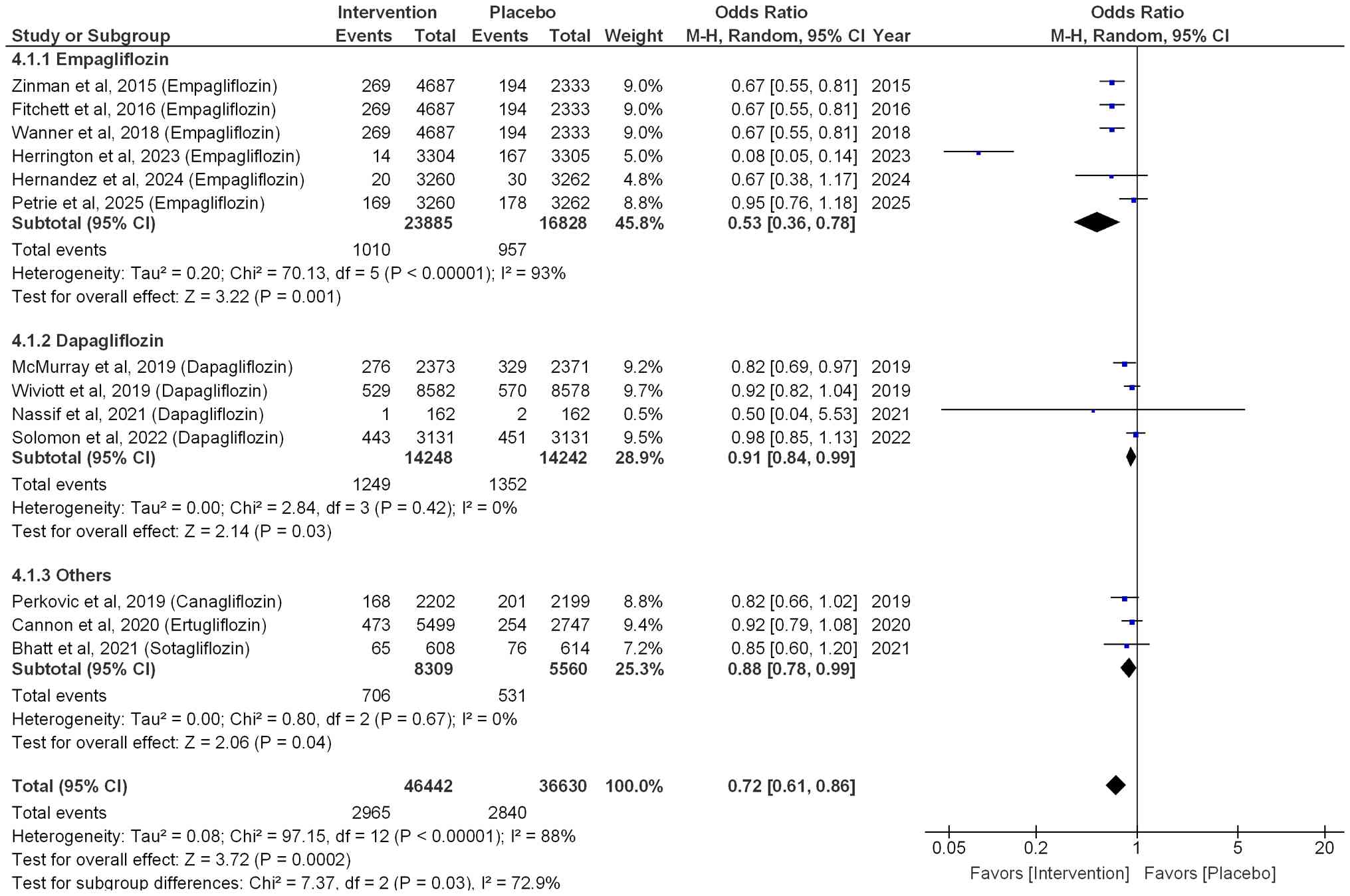
All-cause mortality and CV
mortality
Among patients with HF who had diabetes,
empagliflozin significantly reduced the risk of all-cause mortality
by 47% (OR=0.53; 95% CI: 0.36-0.78; P=0.001; I²=93%; Fig. 4). Dapagliflozin also resulted in a
significant reduction in all-cause mortality by 9% (OR=0.91; 95%
CI: 0.84-0.99; P=0.03; I2=0%). Other SGLT2i, such as
sotagliflozin, ertugliflozin and canagliflozin, also led to a
significant reduction in all-cause mortality by 12% (OR=0.88; 95%
CI: 0.78-0.99; P=0.04, I2=0%). Overall, a 28% reduction
was observed in all-cause mortality when patients were treated with
SGLT2i (OR=0.72; 95% CI: 0.61-0.86; P=0.0002; I2=88%),
as illustrated in Fig. 4.
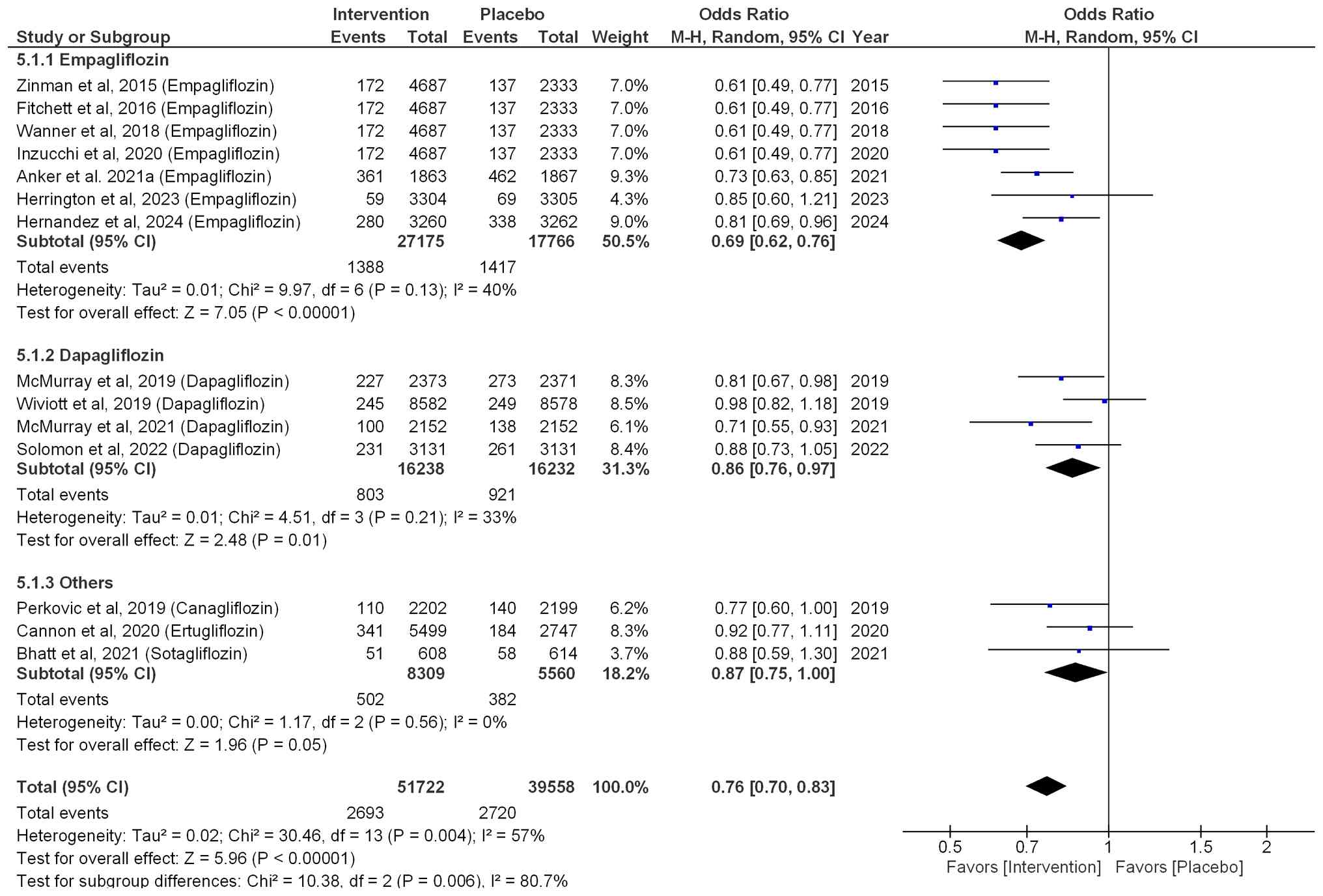
Similarly, regarding CV mortality, the risk was
reduced by 31% (OR=0.69; 95% CI: 0.62-0.76; P<0.00001; I²=40%)
when patients were treated with empagliflozin. Patients treated
with dapagliflozin exhibited a significant 14% reduction in
CV-associated mortality (OR=0.86; 95% CI: 0.76-0.97; P=0.01;
I2=33%). Similarly, patients treated with other SGLT2i
(sotagliflozin, ertugliflozin and canagliflozin) exhibited a
non-significant 13% reduction (OR=0.87; 95% CI: 0.75-1.00; P=0.05;
I2=0%). Overall, the pooled effect size (OR=0.76; 95%
CI: 0.70-0.83; P<0.00001; I²=57%) indicated that SGLT2i
effectively reduced the risk of CV-associated mortality by 24%, as
shown in Fig. 5.
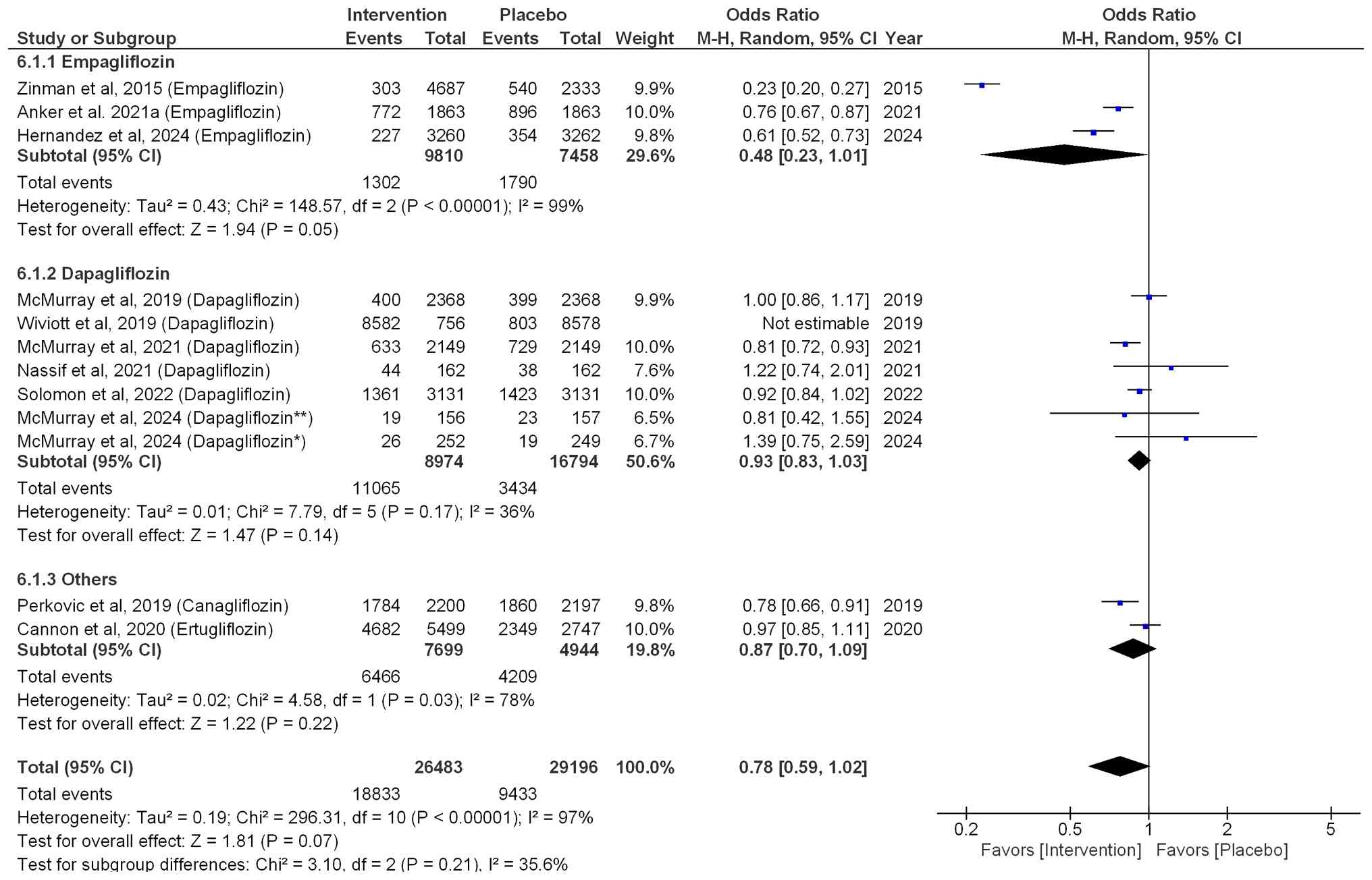
Adverse events
Within the empagliflozin treatment group, a
non-significant 52% reduction in the risk of adverse events was
demonstrated (OR=0.48; 95% CI: 0.23-1.01; P=0.05;
I2=99%). Dapagliflozin (OR=0.93; 95% CI: 0.83-1.03;
P=0.14; I2=36%) and other SGLT2i, such as ertugliflozin
and ertugliflozin (OR=0.87; 95% CI: 0.70-1.09; P=0.22;
I2=78%), did not significantly reduce the risk of
adverse events compared with that in the placebo group. Overall,
the pooled effect size for adverse events (OR=0.78; 95% CI:
0.59-1.02; P=0.07; I2=97%), indicated that SGLT2i
non-significantly reduced the risk of adverse events by 22%
compared with that in the placebo group (Fig. 6).
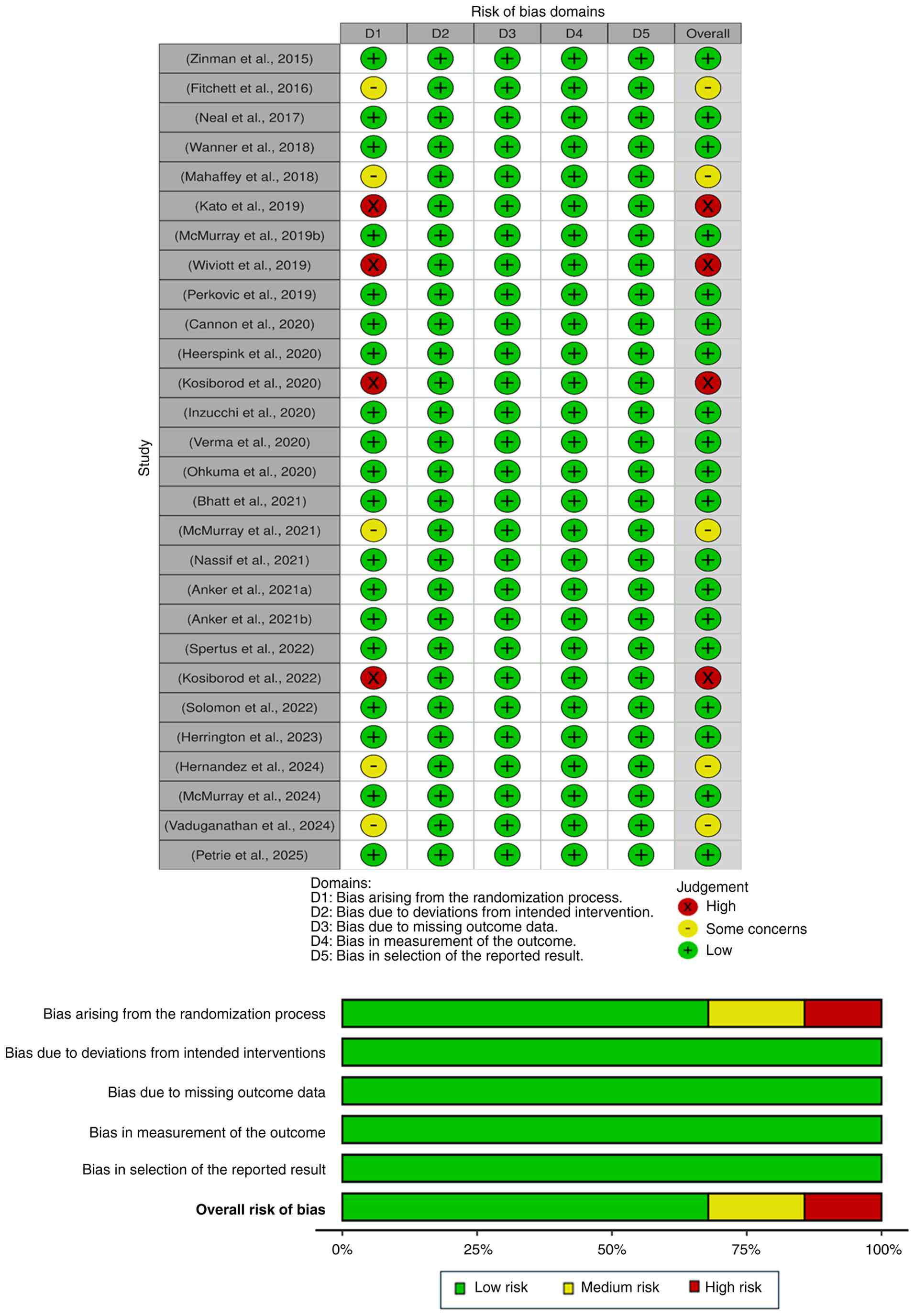
Methodological quality assessment
Overall, the majority of included studies exhibited
a low risk of bias in the randomization process, deviations from
intended interventions, missing outcome data, measurement of the
outcome and reporting results domains. However, a total of 4
studies exhibited a high risk of bias in the randomization domain
(31,36,45,46),
while 5 studies exhibited some concerns in the randomization domain
(25,34,37,44,49),
as illustrated in Fig. 7.
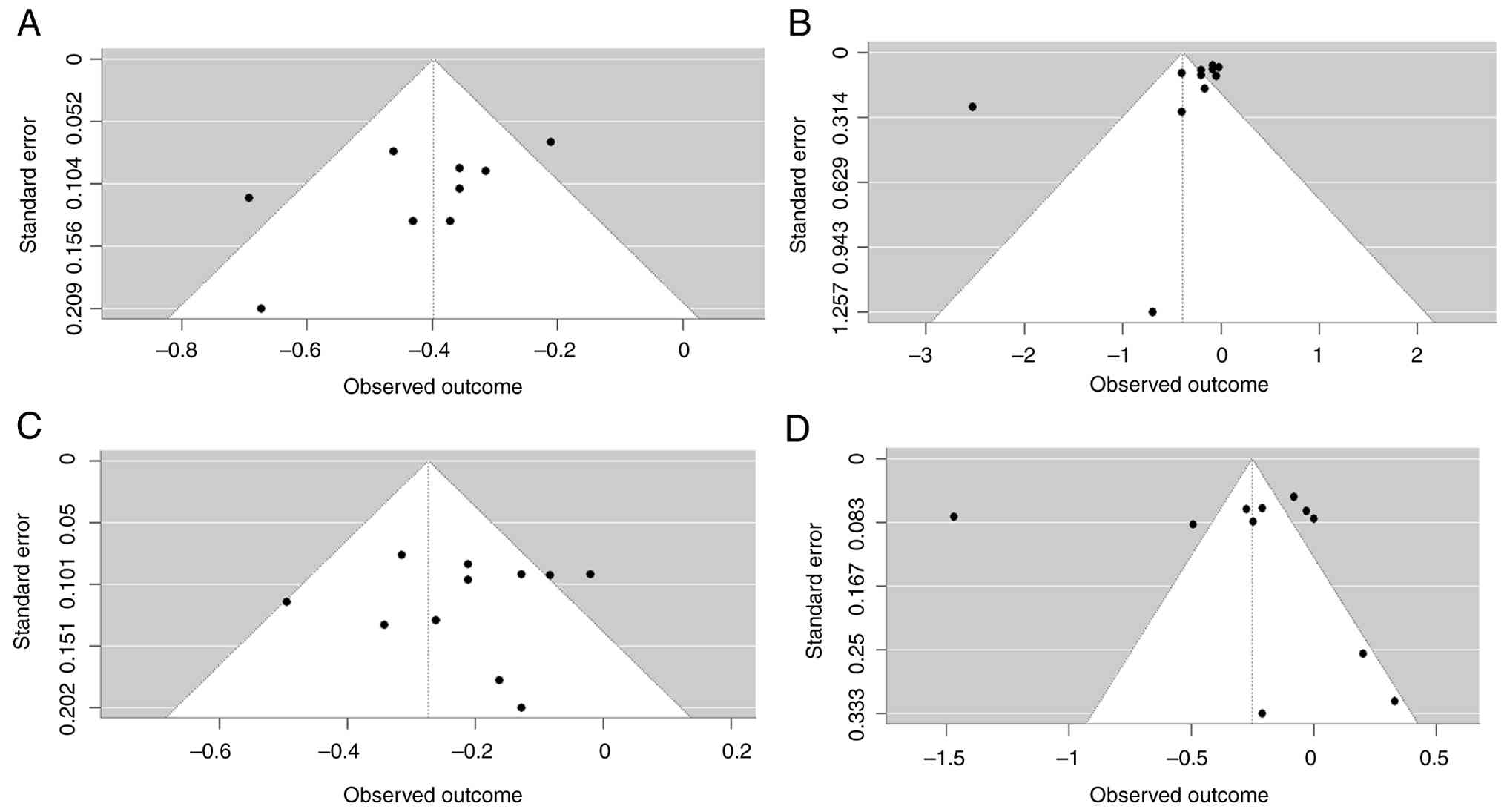
Publication bias
The Egger's regression test for publication bias
regarding the first hospitalization for HF was not performed due to
the presence of <10 studies. However, for total hospitalization
for HF (t=-2.21; P=0.048), the distribution of studies appeared
relatively asymmetrical around the effect size line, suggesting a
high publication bias (Fig. 8A).
On the other hand, the distribution of studies for all-cause
mortality (t=-2.10; P=0.058), CV-associated mortality (t=-0.72;
P=0.479) and adverse events (t=-0.01; P=0.988) appeared relatively
symmetrical around the effect size line, suggesting a low
publication bias (Fig. 8B-D).
Source of heterogeneity
However, despite efforts to further investigate
potential sources of heterogeneity, the available data did not
allow more conclusive results to be drawn, as the included studies
did not provide sufficient association data to evaluate the sources
of heterogeneity (such as the impact of age and sex of patients,
drug type, comorbidities, diabetes status and follow-up durations
on the outcomes). As a result, the sources of heterogeneity could
not be examined further despite a number of studies providing basic
demographic information, including age and sex.
Certainty of evidence
Outcomes, including the impact of SGLT2i on
hospitalization for HF (first and total), mortality (all-cause and
CV-associated) and adverse events in patients with or without
diabetes, were assessed in the domain of risk of bias,
inconsistency, indirectness, imprecision and publication bias.
Total hospitalization for HF, all-cause mortality and adverse
events were assessed in these domains and rated with low certainty
due to the presence of high heterogeneity and publication bias,
while, CV-associated mortality had moderate certainty due to
moderate heterogeneity. Furthermore, a high certainty was present
for the first hospitalization for HF, which may be due to the
limited number of studies, as publication bias test could not be
assessed (Table IV).
 | Table IVSummary of the certainty of
evidence. |
Table IV
Summary of the certainty of
evidence.
| Outcomes | Number of
studies | RoB | Inconsistency | Indirectness | Imprecision | Publication
bias | Effect size
(OR) | Certainty of
evidence |
|---|
| First
hospitalization for HF | Empagliflozin
(n=2) | Low to some
concerns | Not serious
(I2=0%) | Not serious | Not serious | Not assessed | 0.76 (95% CI:
0.64-0.91) | High ƟƟƟƟ |
| Total
hospitalization for HF | Empagliflozin
(n=7), dapagliflozin (n=4) and others (n=2) | Low to high | Partially serious
(I2=33%) | Not serious | Not serious | Present | 0.67 (95% CI:
0.63-0.72) | Low Ɵ |
| All-cause
mortality | Empagliflozin
(n=6), dapagliflozin (n=4) and others (n=3) | Low to high | Serious
(I2=88%) | Not serious | Not serious | Absent | 0.72 (95% CI:
0.61-0.86) | Low Ɵ |
| CV-associated
mortality | Empagliflozin
(n=7), dapagliflozin (n=4) and others (n=3) | Low to high | Serious
(I2=57%) | Not serious | Not serious | Absent | 0.76 (95% CI:
0.70-0.83) | Moderate ƟƟƟ |
| Adverse events | Empagliflozin
(n=3), dapagliflozin (n=7) and others (n=2) | Low to high | Serious
(I2=97%) | Not serious | Not serious | Absent | 0.78 (95% CI:
0.59-1.02) | Low Ɵ |
Discussion
Encompassing data from 28 studies, the present
comprehensive meta-analysis provided robust evidence regarding the
clinical benefits of SGLT2i across diabetic and non-diabetic
populations with HF. The outcomes of the present meta-analysis
indicated that SGLT2i were significantly associated with a relative
risk reduction in the composite endpoint of first and total
hospitalizations for HF in patients with and without diabetes by 24
and 33%, respectively. Similarly, pooled analysis for all-cause
mortality and CV-associated mortality also demonstrated a
significantly reduced risk in patients treated with SGLT2i by 28
and 24%, respectively. In addition, the risk of adverse events was
reduced by 22% in the SGLT2i-treated intervention group compared
with that in the placebo group; however, this result was not
significant. Furthermore, the majority of studies exhibited a low
risk of bias, except for a small number of studies, which exhibited
a high risk of bias or some concerns in the randomization process.
This was due to these studies not mentioning or explaining the
process used for randomization. For example, these studies
(31,36,45,46)
did not use a computer-generated randomization process or any other
standard procedure, like random number generators, flip coin
method, or block randomization. This may have influenced the
reliability of the pooled estimates, thereby underestimating the
true effect of treatment. However, most overall outcomes were found
without any publication bias, except for total hospitalizations,
which likely reflects an over-representation of studies reporting
significant reductions in total hospitalizations, while studies
with non-significant outcomes may remain unpublished or less
accessible. Due to this reason, positive findings may skew the
pooled effect estimate, indicating treatment to be more effective
than the true effect estimates if non-significant outcomes were
included. Therefore, the total hospitalization outcome in
particular should be interpreted with caution.
The present findings align with those of a previous
meta-analysis, which included 15 studies and observed a 29%
(HR=0.71; 95% CI: 0.67-0.77) reduction in the risk of first
hospitalization for HF in patients with diabetes treated with
SGLT2i. Similarly, CV-associated mortality was also reduced by 14%
(HR=0.86; 95% CI: 0.79-0.93) (51). The main difference between the
present study and this previous meta-analysis was the larger number
of studies being included in the present analysis, making it more
reliable. In addition, another difference was the inclusion of both
diabetic and non-diabetic patients in the present study. Similarly,
another previous meta-analysis included 8 studies, observing a
reduced relative risk in CV-associated mortality and HF
hospitalizations by 20% (52). The
main difference between this study and the present meta-analysis is
both the number of studies included in the analysis and the
parameters examined, such as adverse events, and all-cause
mortality. Greene et al (53) demonstrated that diabetic and
non-diabetic patients with HF exhibit an annual mortality rate of
8-10%, even in the presence of sTable symptoms. In addition, nearly
one in four patients either succumb to the disease or are
re-hospitalized within 30 days of a previous hospitalization for
HF. These findings provide support for the use of SGLT2i in
reducing both hospitalization for HF and CV mortality in this
population. Although absolute event rates varied across the trials,
from ~35 per 1,000 patient-years in the EMPA-REG OUTCOME (41), CANVAS (38) and EMPEROR-preserved trials to
>120 per 1,000 patient-years in the SOLOIST-WHF trial, low
heterogeneity in the treatment effects suggests the applicability
of these findings across a wide risk spectrum (32).
The benefits of SGLT2i are likely multifactorial and
may include favorable effects on cardiac remodeling. In addition,
in patients with diabetes, SGLT2i were found to be effective in
reducing both first and total hospitalizations for HF and in
reducing all-cause and CV-associated mortality. Diabetes has been
shown to significantly elevate the HF risk by ~2-fold in men and
5-fold in women (54). The
observed benefits in these patients appear to be largely
independent of glycemic control, as the outcomes did associate with
the changes in HbA1c across the trial (55). Instead, these effects may be
attributable to cardiorenal (55)
and systemic hemodynamic mechanisms (56). Within this bidirectional
pathophysiologic relationship, use of SGLT2i emerges as a safe and
effective therapeutic strategy.
Furthermore, a 22% reduction in adverse events was
observed in the SGLT2i-treated groups in the present study, which
is consistent with another recent meta-analysis showing that SGLT2i
markedly reduced the risk of kidney disease progression and AKI
across a broad spectrum of renal functions (diabetic kidney disease
or nephropathy, ischemic and hypertensive kidney disease),
irrespective of T2DM status (57).
SGLT2i can improve CV-associated outcomes by blocking glucose and
sodium reabsorption in the proximal renal tubules, causing
increased urinary excretion of both glucose and sodium, which
reduces preload and afterload through natriuresis and diuresis,
lowering interstitial fluid volume without notable electrolyte loss
or disturbance (58). This
reduction in sodium reabsorption also reduces renal oxygen demand
and improves tubuloglomerular feedback by reabsorption in the
proximal tubule, increasing sodium delivery to the macula densa and
contributing to improved heart function (59). In addition, SGLT2i also enhances
myocardial energy efficiency, and reduces fibrosis by
downregulating pro-inflammatory pathways (IL-6, TNF-alpha), cardiac
inflammation and oxidative stress whilst improving endothelial
function, collectively leading to reduced hospitalization and
mortality rates in both populations exhibiting HF with reduced
ejection fraction and HF with preserved ejection fraction (60). SGLT2i improve glycemic control and
reduce glucotoxicity in diabetic patients, while in non-diabetic
patients, the mechanisms are primarily hemodynamic and metabolic,
independent of glucose lowering (61,62).
Low-to-high heterogeneity was observed in the
present meta-analysis, which can be attributed to a number of
reasons. For example, baseline LVEF and presence of comorbidities,
such as CKD, are critical modifiers of the treatment response. In
the present study, these factors were not considered due to the
unavailability of uniform data for performing subgroup analysis.
These factors suggest that the response of patients with reduced
and preserved ejection fraction towards treatment may be different,
with studies indicating greater relative benefits in HF with
reduced ejection fraction (51,63).
Similarly, CKD severity can also markedly affect both the efficacy
and safety of SGLT2i, as renal function impacts drug
pharmacodynamics and the associated metabolic responses (64).
The findings of the present meta-analysis may have
important clinical implications for the management of HF in
patients with or without diabetes, as a significant reduction in
the risk of hospitalizations for HF, mortality and adverse events
was observed in the SGLT2i-treated groups. These outcomes strongly
suggest the routine incorporation of SGLT2i into HF treatment
protocols for both diabetic and non-diabetic patients.
The present study exhibits a number of strengths,
such as the inclusion of a large number of RCTs with diverse
populations from across the globe, enhancing the generalizability
of the present findings across both diabetic and non-diabetic
patients with HF. In addition, the present study also focused on
key outcomes, such as first and total hospitalizations for HF,
all-cause mortality, CV mortality and adverse events associated
with the administration of SGLT2i. Another strength may lie in the
inclusion of studies with a low risk of bias. However, certain
limitations should be considered during interpretation of the
results. Firstly, high heterogeneity was observed among the
studies, which can affect the generalizability of the outcomes.
This may have been due to the included population, as in certain
studies only diabetic patients were considered, while in other
studies, both diabetic and non-diabetic patients were included. The
heterogeneity may have also been impacted by the exclusion of
non-English studies. Another limitation may be the administration
of varying SGLT2i with different dosages and follow-up durations,
which were found to be non-consistent across all studies. Secondly,
a meta-analysis could not be performed regarding the impact of LVEF
and comorbidities such as CKD, due to the unavailability of
consistent data, which may have influenced the outcomes of the
present study. Thirdly, subgroup analysis between diabetic and
non-diabetic patients could not be performed, as HR values were
presented as composite measures that combined different clinical
outcomes (such as HF hospitalization and mortality), making them
unsuitable for pooling in a meaningful or methodologically sound
subgroup analysis. In addition, the reporting across studies lacked
sufficient consistency in outcome definitions (assessment tools
used for assessment of clinical outcomes, like hospitalization) and
stratification, further limiting the feasibility of combining these
data. Therefore, incorporating composite HRs that merge numerous
endpoints would affect or compromise the clarity and validity of
the present findings. Future studies are required to elucidate the
general understanding of the cardioprotective mechanisms and
long-term safety of SGLT2i.
In conclusion, the findings of the present
meta-analysis provided marked evidence that SGLT2i significantly
reduce the risk of first and total hospitalizations for HF,
all-cause mortality and CV mortality in both diabetic and
non-diabetic patients with HF. However, a non-significant reduction
in adverse events was observed. The consistent benefits
demonstrated across a wide range of clinical outcomes and
populations highlight the therapeutic importance of SGLT2i as a
treatment in HF management, supporting their clinical relevance and
routine use in improving outcomes. Future meta-analyses should aim
to focus on broader HF populations, including those with preserved
ejection fraction and numerous comorbidities. In addition, the
mechanism behind the cardioprotective impact of SGLT2i in
non-diabetic patients should be examined.
Supplementary Material
Literature search strategy from
different databases.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DAB and NHA conceptualized and designed the present
study, contributed to data analysis, interpretation and curation,
created graphs, contributed to the interpretation of results and
served a key role in writing the manuscript. DAB and NHA confirm
the authenticity of all the raw data. Both authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Savarese G, Becher PM, Lund LH, Seferovic
P, Rosano GMC and Coats AJS: Global burden of heart failure: A
comprehensive and updated review of epidemiology. Cardiovasc Res.
118:3272–3287. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sheraliev A: Pathophysiology of heart
failure, hemodynamic changes and their consequences. Mod Sci Res.
4:965–969. 2025.
|
|
3
|
Ran J, Zhou P, Wang J, Zhao X, Huang Y,
Zhou Q, Zhai M and Zhang Y: Global, regional, and national burden
of heart failure and its underlying causes, 1990-2021: Results from
the global burden of disease study 2021. Biomark Res.
13(16)2025.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dini FL, Pugliese NR, Ameri P, Attanasio
U, Badagliacca R, Correale M, Mercurio V, Tocchetti CG, Agostoni P
and Palazzuoli A: Heart Failure Study Group of the Italian Society
of Cardiology. Right ventricular failure in left heart disease:
From pathophysiology to clinical manifestations and prognosis.
Heart Fail Rev. 28:757–766. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shah P, Pellicori P, Cuthbert J and Clark
AL: Pharmacological and non-pharmacological treatment for
decompensated heart failure: What is new? Curr Heart Fail Rep.
14:147–157. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shahim B, Kapelios CJ, Savarese G and Lund
LH: Global public health burden of heart failure: An updated
review. Card Fail Rev. 9(e11)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Talha KM, Anker SD and Butler J: SGLT-2
inhibitors in heart failure: A review of current evidence. Int J
Heart Fail. 5(82)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Salvatore T, Pafundi PC, Galiero R,
Albanese G, Di Martino A, Caturano A, Vetrano E, Rinaldi L and
Sasso FC: The diabetic cardiomyopathy: The contributing
pathophysiological mechanisms. Front Med (Lausanne).
8(695792)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen J, Jiang C, Guo M, Zeng Y, Jiang Z,
Zhang D, Tu M, Tan X, Yan P, Xu X, et al: Effects of SGLT2
inhibitors on cardiac function and health status in chronic heart
failure: A systematic review and meta-analysis. Cardiovasc
Diabetol. 23(2)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vasquez-Rios G and Nadkarni GN: SGLT2
inhibitors: Emerging roles in the protection against cardiovascular
and kidney disease among diabetic patients. Int J Nephrol Renovasc
Dis. 13:281–296. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Singh J: A Comprehensive Analysis of
SGLT2-inhibition in Type 2 Diabetes and Heart Failure. University
of Dundee, 2019.
|
|
12
|
Fernandes GC, Fernandes A, Cardoso R,
Penalver J, Knijnik L, Mitrani RD, Myerburg RJ and Goldberger JJ:
Association of SGLT2 inhibitors with arrhythmias and sudden cardiac
death in patients with type 2 diabetes or heart failure: A
meta-analysis of 34 randomized controlled trials. Heart rhythm.
18:1098–1105. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Treewaree S, Kulthamrongsri N,
Owattanapanich W and Krittayaphong R: Is it time for class I
recommendation for sodium-glucose cotransporter-2 inhibitors in
heart failure with mildly reduced or preserved ejection fraction?:
An updated systematic review and meta-analysis. Front Cardiovasc
Med. 10(1046194)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
McMurray JJV, DeMets DL, Inzucchi SE,
Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O,
Ponikowski P, Sabatine MS, et al: The dapagliflozin and prevention
of Adverse-outcomes in heart failure (DAPA-HF) trial: Baseline
characteristics. Eur J Heart Fail. 21:1402–1411. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Packer M, Butler J, Zannad F, Filippatos
G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M,
et al: Effect of empagliflozin on worsening heart failure events in
patients with heart failure and preserved ejection fraction:
EMPEROR-preserved trial. Circulation. 144:1284–1294.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gao M, Bhatia K, Kapoor A, Badimon J,
Pinney SP, Mancini DM, Santos-Gallego CG and Lala A: SGLT2
inhibitors, functional capacity, and quality of life in patients
with heart failure: A systematic review and meta-analysis. JAMA
Netw Open. 7(e245135)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Savarese G, Butler J, Lund LH, Bhatt DL
and Anker SD: Cardiovascular effects of non-insulin
glucose-lowering agents: A comprehensive review of trial evidence
and potential cardioprotective mechanisms. Cardiovasc Res.
118:2231–2252. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Banerjee M, Pal R, Nair K and Mukhopadhyay
S: SGLT2 inhibitors and cardiovascular outcomes in heart failure
with mildly reduced and preserved ejection fraction: A systematic
review and meta-analysis. Indian Heart J. 75:122–127.
2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Minisy MM and Abdelaziz A: The role of
SGLT 2 inhibitors in heart failure with preserved ejection fraction
(HFpEF): A systematic review and Meta-analysis of randomized
controlled trials. BMC Cardiovasc Disord. 25(765)2025.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Parums DV: Review articles, systematic
reviews, meta-analysis, and the updated preferred reporting items
for systematic reviews and meta-analyses (PRISMA) 2020 guidelines.
Med Sci Monit. 27(e934475)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
McGuinness LA and Higgins JP: Risk-of-bias
VISualization (robvis): An R package and Shiny web app for
visualizing risk-of-bias assessments. Res Syn Methods. 12:55–61.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
González-Padilla DA and Dahm P: Evaluating
the certainty of evidence in Evidence-based medicine. Euro Urol
Focus. 9:708–710. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nassif ME, Windsor SL, Borlaug BA, Kitzman
DW, Shah SJ, Tang F, Khariton Y, Malik AO, Khumri T, Umpierrez G,
et al: The SGLT2 inhibitor dapagliflozin in heart failure with
preserved ejection fraction: A multicenter randomized trial. Nat
Med. 27:1954–1960. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Spertus JA, Birmingham MC, Nassif M,
Damaraju CV, Abbate A, Butler J, Lanfear DE, Lingvay I, Kosiborod
MN and Januzzi JL: The SGLT2 inhibitor canagliflozin in heart
failure: The CHIEF-HF remote, patient-centered randomized trial.
Nat Med. 28:809–813. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vaduganathan M, Cannon CP, Jardine MJ,
Heerspink HJL, Arnott C, Neuen BL, Sarraju A, Gogate J, Seufert J,
Neal B, et al: Effects of canagliflozin on total heart failure
events across the kidney function spectrum: Participant-level
pooled analysis from the CANVAS Program and CREDENCE trial. Eur J
Heart Fail. 26:1967–1975. 2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
The EMPA-KIDNEY Collaborative Group.
Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson
JR, Preiss D, Judge P, Mayne KJ, et al: Empagliflozin in patients
with chronic kidney disease. N Engl J Med. 388:117–127.
2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Petrie MC, Udell JA, Anker SD, Harrington
J, Jones WS, Mattheus M, Gasior T, van der Meer P, Amir O, Bahit
MC, et al: Empagliflozin in acute myocardial infarction in patients
with and without type 2 diabetes: A pre-specified analysis of the
EMPACT-MI trial. Eur J Heart Fail. 27:577–588. 2025.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Perkovic V, Jardine MJ, Neal B, Bompoint
S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull
S, et al: Canagliflozin and renal outcomes in type 2 diabetes and
nephropathy. N Engl J Med. 380:2295–2306. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Verma S, Sharma A, Zinman B, Ofstad AP,
Fitchett D, Brueckmann M, Wanner C, Zwiener I, George JT, Inzucchi
SE, et al: Empagliflozin reduces the risk of mortality and
hospitalization for heart failure across Thrombolysis In Myocardial
Infarction Risk Score for Heart Failure in Diabetes categories:
Post hoc analysis of the EMPA-REG OUTCOME trial. Diabetes Obes
Metab. 22:1141–1150. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Heerspink HJL, Stefánsson BV,
Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray
JJV, Lindberg M, Rossing P, et al: Dapagliflozin in patients with
chronic kidney disease. N Engl J Med. 383:1436–1446.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wiviott SD, Raz I, Bonaca MP, Mosenzon O,
Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF and Murphy SA:
Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N
Engl J Med. 380:347–357. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bhatt DL, Szarek M, Steg PG, Cannon CP,
Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, et
al: Sotagliflozin in patients with diabetes and recent worsening
heart failure. N Engl J Med. 384:117–128. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cannon CP, Pratley R, Dagogo-Jack S,
Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R,
Gallo S, Cosentino F, et al: Cardiovascular outcomes with
ertugliflozin in type 2 diabetes. N Engl J Med. 383:1425–1435.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fitchett D, Zinman B, Wanner C, Lachin JM,
Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC and Inzucchi
SE: EMPA-REG OUTCOME® trial investigators. Heart failure
outcomes with empagliflozin in patients with type 2 diabetes at
high cardiovascular risk: Results of the EMPA-REG
OUTCOME® trial. Eur Heart J. 37:1526–1534.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Inzucchi SE, Khunti K, Fitchett DH, Wanner
C, Mattheus M, George JT, Ofstad AP and Zinman B: Cardiovascular
benefit of empagliflozin across the spectrum of cardiovascular risk
factor control in the EMPA-REG OUTCOME trial. J Clin Endocrinol
Metab. 105:3025–3035. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kato ET, Silverman MG, Mosenzon O,
Zelniker TA, Cahn A, Furtado RHM, Kuder J, Murphy SA, Bhatt DL,
Leiter LA, et al: Effect of dapagliflozin on heart failure and
mortality in type 2 diabetes mellitus. Circulation. 139:2528–2536.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mahaffey KW, Neal B, Perkovic V, de Zeeuw
D, Fulcher G, Erondu N, Shaw W, Fabbrini E, Sun T, Li Q, et al:
Canagliflozin for primary and secondary prevention of
cardiovascular events: Results from the CANVAS Program
(Canagliflozin Cardiovascular Assessment Study). Circulation.
137:323–334. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Neal B, Perkovic V, Mahaffey KW, De Zeeuw
D, Fulcher G, Erondu N, Shaw W, Law G, Desai M and Matthews DR:
CANVAS Program Collaborative Group. Canagliflozin and
cardiovascular and renal events in type 2 diabetes. N Engl J Med.
377:644–657. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ohkuma T, Van Gaal L, Shaw W, Mahaffey KW,
de Zeeuw D, Matthews DR, Perkovic V and Neal B: Clinical outcomes
with canagliflozin according to baseline body mass index: Results
from post hoc analyses of the CANVAS Program. Diabetes Obes Metab.
22:530–539. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wanner C, Lachin JM, Inzucchi SE, Fitchett
D, Mattheus M, George J, Woerle HJ, Broedl UC, von Eynatten M and
Zinman B: EMPA-REG OUTCOME Investigators. Empagliflozin and
clinical outcomes in patients with type 2 diabetes mellitus,
established cardiovascular disease, and chronic kidney disease.
Circulation. 137:119–129. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zinman B, Wanner C, Lachin JM, Fitchett D,
Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ,
et al: Empagliflozin, cardiovascular outcomes, and mortality in
type 2 diabetes. N Engl J Med. 373:2117–2128. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Anker SD, Butler J, Filippatos G, Ferreira
JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V,
Chuquiure-Valenzuela E, et al: Empagliflozin in heart failure with
a preserved ejection fraction. N Engl J Med. 385:1451–161.
2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Anker SD, Butler J, Filippatos G, Khan MS,
Marx N, Lam CSP, Schnaidt S, Ofstad AP, Brueckmann M, Jamal W, et
al: Effect of empagliflozin on cardiovascular and renal outcomes in
patients with heart failure by baseline diabetes status: Results
from the EMPEROR-Reduced trial. Circulation. 143:337–349.
2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hernandez AF, Udell JA, Jones WS, Anker
SD, Petrie MC, Harrington J, Mattheus M, Seide S, Zwiener I, Amir
O, et al: Effect of empagliflozin on heart failure outcomes after
acute myocardial infarction: Insights from the EMPACT-MI trial.
Circulation. 149:1627–1638. 2024.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kosiborod MN, Angermann CE, Collins SP,
Teerlink JR, Ponikowski P, Biegus J, Comin-Colet J, Ferreira JP,
Mentz RJ, Nassif ME, et al: Effects of empagliflozin on symptoms,
physical limitations, and quality of life in patients hospitalized
for acute heart failure: Results From the EMPULSE trial.
Circulation. 146:279–288. 2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kosiborod MN, Jhund PS, Docherty KF, Diez
M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets
DL, et al: Effects of dapagliflozin on symptoms, function, and
quality of life in patients with heart failure and reduced ejection
fraction: Results from the DAPA-HF trial. Circulation. 141:90–99.
2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
McMurray JJV, Docherty KF, de Boer RA,
Hammarstedt A, Kitzman DW, Kosiborod MN, Maria Langkilde A, Reicher
B, Senni M, Shah SJ, et al: Effect of dapagliflozin versus placebo
on symptoms and 6-Minute walk distance in patients with heart
failure: The DETERMINE randomized clinical trials. Circulation.
149:825–838. 2024.PubMed/NCBI View Article : Google Scholar
|
|
48
|
McMurray JJV, Solomon SD, Inzucchi SE,
Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS,
Anand IS, Bělohlávek J, et al: Dapagliflozin in patients with heart
failure and reduced ejection fraction. Eur Heart J. 381:1995–2008.
2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
McMurray JJV, Wheeler DC, Stefánsson BV,
Jongs N, Postmus D, Correa-Rotter R, Chertow GM, Hou FF, Rossing P,
Sjöström CD, et al: Effects of dapagliflozin in patients with
kidney disease, with and without heart failure. JACC Heart failure.
9:807–820. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Solomon SD, McMurray JJV, Claggett B, de
Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam
CSP, Martinez F, et al: Dapagliflozin in heart failure with mildly
reduced or preserved ejection fraction. N Engl J Med.
387:1089–1098. 2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Usman MS, Bhatt DL, Hameed I, Anker SD,
Cheng AYY, Hernandez AF, Jones WS, Khan MS, Petrie MC, Udell JA, et
al: Effect of SGLT2 inhibitors on heart failure outcomes and
cardiovascular death across the cardiometabolic disease spectrum: A
systematic review and meta-analysis. Lancet Diabetes Endocrinol.
12:447–461. 2024.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Teo YH, Teo YN, Syn NL, Kow CS, Yoong CSY,
Tan BYQ, Yeo TC, Lee CH, Lin W and Sia CH: Effects of
Sodium/Glucose Cotransporter 2 (SGLT2) Inhibitors on cardiovascular
and metabolic outcomes in patients without diabetes mellitus: A
systematic review and meta-analysis of randomized-controlled
trials. J Am Heart Assoc. 10(e019463)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Greene SJ, Butler J and Fonarow GC:
Contextualizing risk among patients with heart failure. JAMA.
326:2261–2262. 2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kenny HC and Abel ED: Heart failure in
type 2 diabetes mellitus: Impact of glucose-lowering agents, heart
failure therapies, and novel therapeutic strategies. Circ Res.
124:121–141. 2019.
|
|
55
|
Kluger AY, Tecson KM, Lee AY, Lerma EV,
Rangaswami J, Lepor NE, Cobble ME and McCullough PA: Class effects
of SGLT2 inhibitors on cardiorenal outcomes. Cardiovasc Diabetol:
Aug 5, 2019 (Epub ahead of print).
|
|
56
|
Tromp J, Lim SL, Tay WT, Teng THK,
Chandramouli C, Ouwerkerk W, Wander GS, Sawhney JPS, Yap J,
MacDonald MR, et al: Microvascular disease in patients with
diabetes with heart failure and reduced ejection versus preserved
ejection fraction. Diabetes Care. 42:1792–1799. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Nuffield Department of Population Health
Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal
Trialists' Consortium. Impact of diabetes on the effects of sodium
glucose co-transporter-2 inhibitors on kidney outcomes:
Collaborative meta-analysis of large placebo-controlled trials.
Lancet. 400:1788–1801. 2022.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Abdelgadir E, Rashid F, Bashier A and Ali
R: SGLT-2 inhibitors and cardiovascular protection: Lessons and
gaps in understanding the current outcome trials and possible
benefits of combining SGLT-2 Inhibitors With GLP-1 agonists. J Clin
Med Res. 10:615–625. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Packer M, Wilcox CS and Testani JM:
Critical analysis of the effects of SGLT2 inhibitors on renal
tubular sodium, water and chloride homeostasis and their role in
influencing heart failure outcomes. Circulation. 148:354–372.
2023.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Dyck JRB, Sossalla S, Hamdani N, Coronel
R, Weber NC, Light PE and Zuurbier CJ: Cardiac mechanisms of the
beneficial effects of SGLT2 inhibitors in heart failure: Evidence
for potential off-target effects. J Mol Cell Cardiol. 167:17–31.
2022.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Hanke J, Romejko K and Niemczyk S:
Sodium-Glucose Cotransporter-2 inhibitors in diabetes and beyond:
Mechanisms, pleiotropic benefits, and clinical Use-Reviewing
protective effects exceeding glycemic control. Molecul.
30(4125)2025.PubMed/NCBI View Article : Google Scholar
|
|
62
|
McLean P and Bennett J: ‘Trey’ Woods E.
Chandrasekhar S, Newman N, Mohammad Y, Khawaja M, Rizwan A,
Siddiqui R, Birnbaum Y, et al: SGLT2 inhibitors across various
patient populations in the era of precision medicine: The
multidisciplinary team approach. npj Metab Health Dis.
3(29)2025.
|
|
63
|
Rastogi T and Girerd N: SGLT2 inhibitors
in heart failure with reduced ejection fraction: A paradigm shift
toward dual Cardio-renal protection. Heart Fail Clin. 18:561–577.
2022.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Siddiqui R, Obi Y, Dossabhoy NR and Shafi
T: Is there a role for SGLT2 inhibitors in patients with end-stage
kidney disease? Curr Hypertens Rep. 26:463–474. 2024.PubMed/NCBI View Article : Google Scholar
|