Introduction
Among urological cancers, the incidence of bladder
cancer is the highest, and that in men is approximately 2.5 times
higher than that in women. Approximately 95% of bladder cancers are
urothelial carcinoma. The peak of incidence occurs in the 60–70
year age group, while there are few young patients with this
disease under 50 years of age. The response rate of treatment for
advanced metastatic transitional cell carcinoma is approximately
40%, in spite of M-VAC chemotherapy (methotrexate, vinblastine,
doxorubicin and cisplatin), the gold standard treatment (1,2).
Particularly in the case of metastasis to lymph nodes and other
organs, the survival rate is low, 20%. There are many reasons for
the low response and survival rates. Since elderly patients cannot
tolerate anti-tumor drugs, the dose intensity must be decreased
(3). Continued administration of
anti-cancer drugs becomes difficult due to the physical and mental
distress caused by their severe side effects (4).
The efficacy of hyperthermia in combination with
chemotherapy has been established experimentally and clinically
(5,6). Although chemotherapy is used at all
medical institutions, very few institutions employ it in
combination with hyperthermia.
The use of hyperthermia is not widespread at many
medical facilities, due to the difficulty of heating and
maintaining tissues at a temperature of 43°C or higher.
Additionally, the costs of the devices used for applying clinical
hyperthermia along with the associated personnel costs are
high.
Thus, the effects of mild hyperthermic heating at a
lower temperature of 41°C were examined in contrast to hyperthermia
at temperatures of 43°C or higher. The temperature used for mild
hyperthermia is not sufficiently high enough to kill tumor cells,
but it is relatively easy to heat and maintain cells and tissue at
41°C. In addition, mild hyperthermia is a less severe therapy for
cancer patients than standard hyperthermia.
We previously reported that the cytotoxic effects of
anti-tumor drugs were increased by combining anti-tumor drugs
(adriamycin, vincristine) with mild hyperthermia using the NALM-6
leukemia cell line, and confirmed the effectiveness of mild
hyperthermia (7).
In this experiment, using a human bladder cancer
cell line, the combined effect of mild hyperthermia with cisplatin
and adriamycin was examined. These drugs are the main anti-tumor
drugs in the M-VAC chemotherapeutic regimen, which is the typical
standard therapy for progressive urothelial cancer.
Materials and methods
Cell line
T24 cells, a human bladder cell line, were cultured
in RPMI-1640 culture medium including 10% FCS and were used in
experiments during the log phase of growth. T24 cells were sown on
96-well plates at 5×104/0.2 ml culture medium/well.
These plates were used for experiments after one day in a
CO2 incubator at 37°C.
Cell survival rate
The cell survival rate was measured by the
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT)
method (Cell Proliferation Kit; Roche Diagnostics, Mannheim,
Germany) (8). After cell
treatment, MTT reagent was added (1/10) to the cell reaction
medium. Following incubation at 37°C for 2 h, the same volume of
10% SDS was added to the reaction medium. The absorbance at 550 nm
was measured.
Thermal sensitivity of the human bladder
cancer T24 cell line
T24 cells in 96-well plates were incubated for 0–5 h
at 37, 39, 41, 43, 45 and 47°C, respectively, with a device capable
of maintaining very precise temperatures (temperature accuracy
±0.01°C; Nihon Kouseikagaku Res. Co., Osaka, Japan). The survival
rate of cells was measured by the MTT method.
Response of human bladder cancer T24
cells to anti-tumor drugs
Cisplatin (CDDP) and adriamycin (ADR) are typical
carcinostatics for the treatment of bladder cancer with M-VAC
chemotherapy. These two anti-tumor drugs were used in combination
with mild hyperthermia, and the anti-tumor effect on human bladder
cancer T24 cells was examined. In the combination experiment with
carcinostatics and mild hyperthermia, human bladder cancer T24
cells were treated with final CDDP concentrations of 20 and 200
μg/ml or ADR concentrations of 4 and 40 μg/ml at 41°C for 0–5 h.
Total incubation time (at both 41 and 37°C) of all samples was 12
h. After 12 h, the MTT reagent was added to the incubation medium,
and the cell survival rate was measured. For example, when samples
were treated at 41°C for 3 h, they were incubated at 37°C in a
CO2 incubator for 9 h.
Results
Thermal sensitivity of human bladder
cancer cells
As shown in Fig. 1,
almost all (1.0) the human bladder cancer T24 cells survived at 37,
39 and 41°C after 5 h of incubation. With treatment at 43°C, the
survival rate of T24 cells was 0.94 after 2 h of incubation, 0.8
after 3 h and 0.06–0.09 after 4–5 h. At 45°C, the survival rate of
T24 cells was 0.69 after 2 h of incubation, 0.17 after 3 h and 0.05
after 4–5 h. At 47°C, the survival rate of T24 cells decreased
linearly to 0.05 after 3 h of incubation.
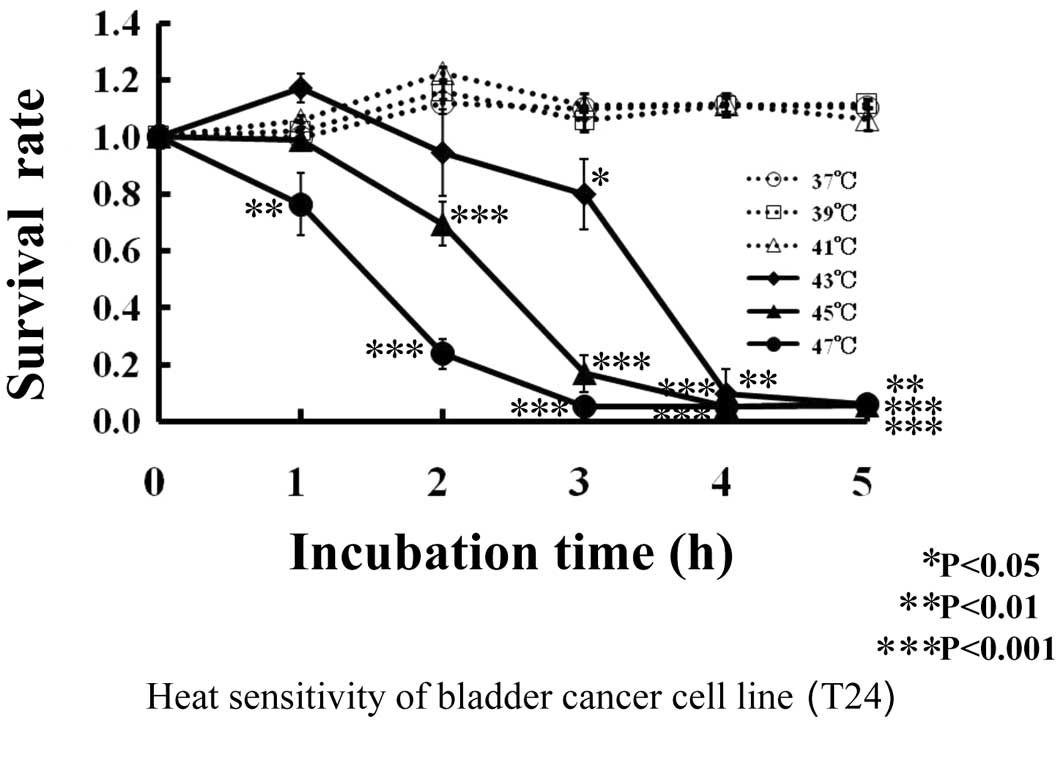 | Figure 1.Thermal sensitivity of human bladder
cancer cell line, T24s. T24 cells were incubated at 37°C (--○--),
39°C (--□--), 41°C (--▵--), 43°C (♦), 45°C (▴) or 47°C (•) for 0,
1, 2, 3, 4 and 5 h, respectively. Viability of T24 cells was
measured by the MTT method after incubation. *P<0.05,
**P<0.01 and ***P<0.001 compared to 0 h of incubation at
37°C. |
Human bladder cancer T24 cells were not influenced
by the thermal effect of 41°C, and cell death was noted from 43°C,
just as with cells in general.
Sensitivity of human bladder cancer T24
cells to anti-tumor drugs
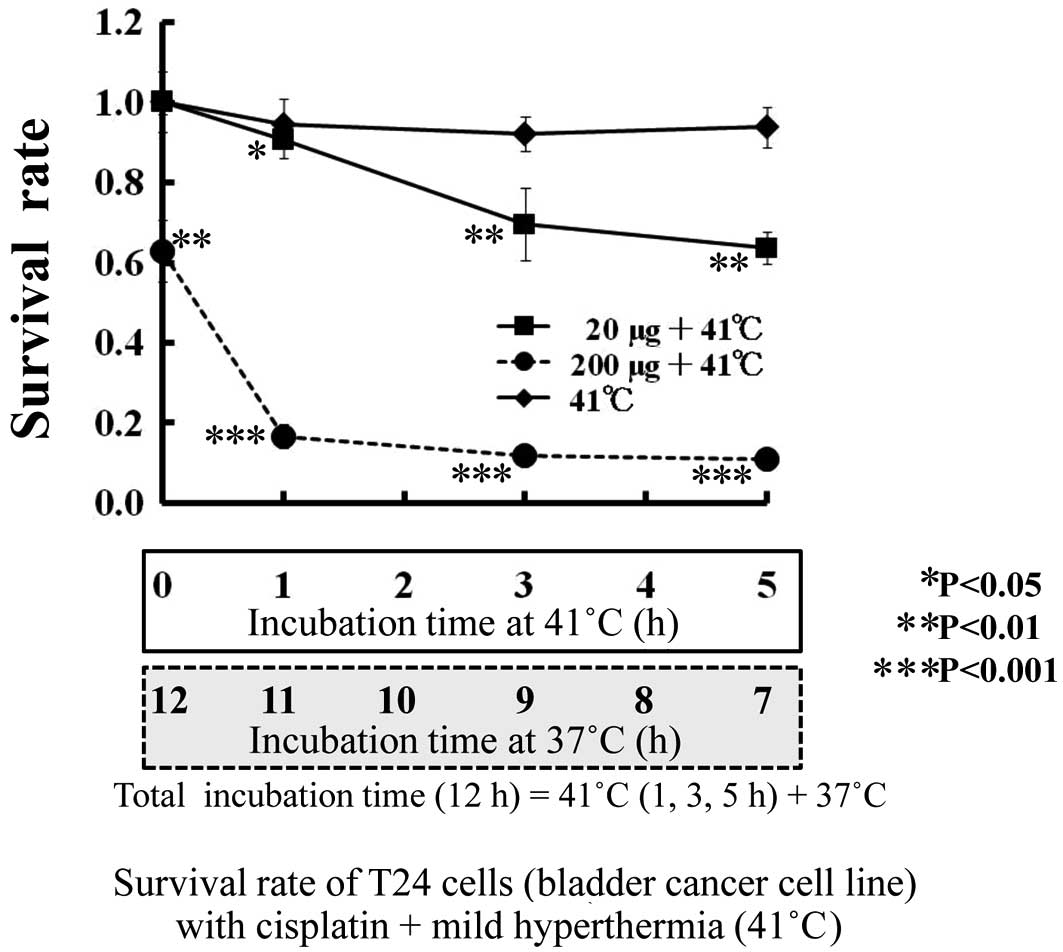
The sensitivity of T24 cells to CDDP (0, 2 and 20
μg/ml) is shown by the survival rate (y-axis) at 0 h of incubation
at 41°C (12-h incubation at 37°C) (Fig. 2). The survival rate of T24 cells in
CDDP (0 μg/ml) after 12 h of incubation at 37°C (without mild
hyperthermia) was 1.0. Additionally, the survival rate of T24 cells
at a low concentration (20 μg/ml) of CDDP was 1.0, and that at a
high concentration (200 μg/ml) of CDDP was 0.62 (P<0.01).
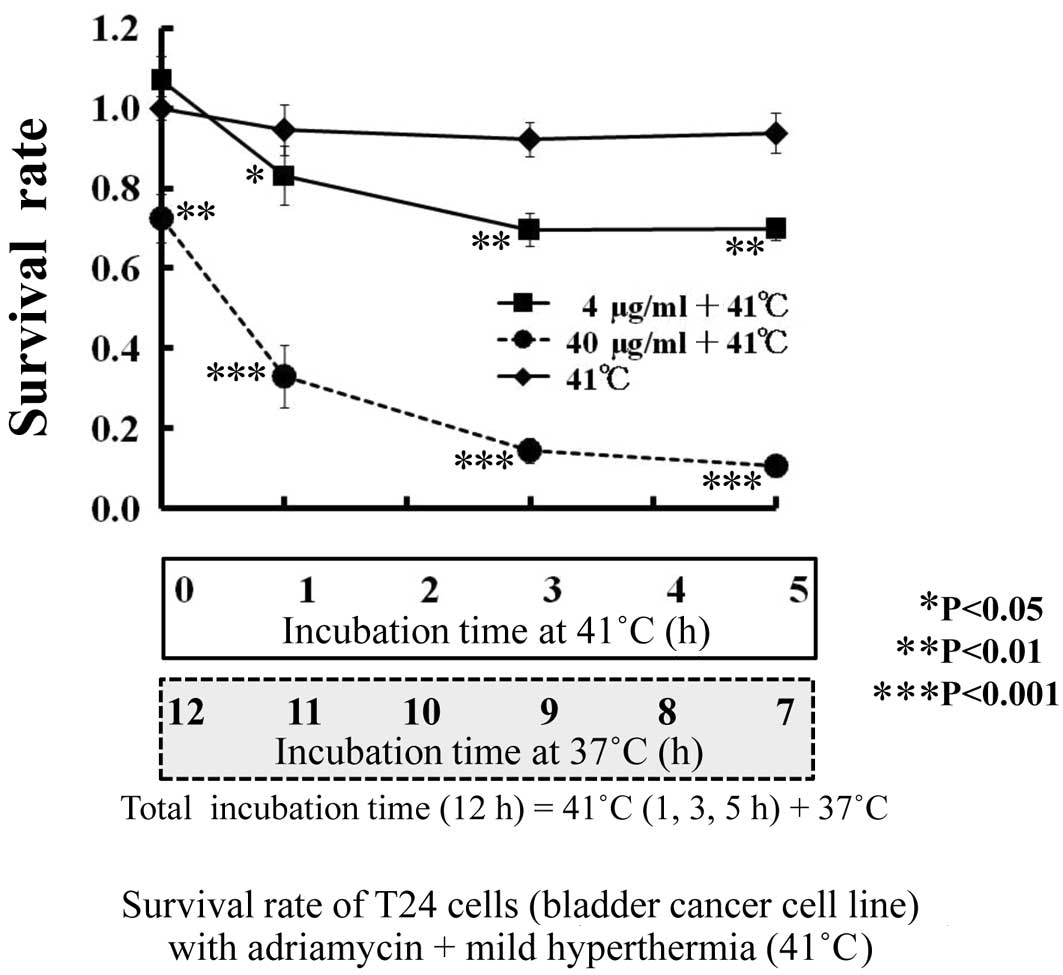
The sensitivity of T24 cells to ADR is shown by the
survival rate (y-axis) at 0 h of incubation at 41°C (Fig. 3). Similar to CDDP, the survival
rate at a low concentration (4 μg/ml) of ADR was 1.0 and that at a
high concentration (40 μg/ml) of ADR decreased the survival to 0.72
(P<0.01).
Combination of anti-tumor drugs and mild
hyperthermia
The survival rate of T24 cells with a combination of
anti-tumor drugs and mild hyperthermia is shown in Figs. 2 and 3. As shown in Fig. 1, regarding thermal sensitivity, the
survival rate of T24 cells was not altered by mild hyperthermia at
41°C for 5 h; nor was it altered by anti-tumor drugs, such as a low
CDDP concentration (2 μg/ml) for 12 h (Fig. 2). However, the survival rate
decreased with a combination of mild hyperthermia at 41°C and a low
concentration of anti-tumor drugs (CDDP 20 μg/ml or ADR 4
μg/ml).
Mild hyperthermia at 41°C or a low concentration of
anti-tumor drugs CDDP and ADR had no independent influence on the
survival of T24 cells. The viability of T24 cells was decreased
from 1.0 to 0.90 (P<0.05), 0.70 (P<0.01) and 0.63 (P<0.01)
by the combination of mild hyperthermia at 41°C and 20 μg/ml CDDP
for 0, 1, 3 and 5 h, respectively. The viability of T24 cells was
0.62 at a CDDP concentration of 200 μg/ml without mild
hyperthermia. Therefore, the viability of T24 cells at a CDDP
concentration of 20 μg/ml with mild hyperthermia at 41°C for 5 h
had the same anti-tumor effect as a 10-fold higher ADR
concentration (200 μg/ml).
The viability of T24 cells with 200 μg/ml CDDP was
decreased from 0.62 to 0.16 (P<0.001) in combination with mild
hyperthermia at 41°C for 1 h, and to 0.11 (P<0.001) at 41°C for
3 h. The addition of mild hyperthermia at 41°C for 1 h quadrupled
the anti-tumor activity of CDDP (200 μg/ml).
As with CDDP, the viability of T24 cells with 4
μg/ml ADR was decreased from 1.0 to 0.83 (P<0.05), 0.70
(P<0.01) and 0.69 (P<0.01) in combination with mild
hyperthermia at 41°C for 0, 1, 3 and 5 h, respectively. Viability
of T24 cells with 40 μg/ml ADR was 0.72. Therefore, the cell
viability when 4 μg/ml ADR was combined with mild hyperthermia at
41°C for 3 h showed the same anti-tumor effect as a 10-fold higher
ADR concentration (40 μg/ml).
The viability of T24 cells with 40 μg/ml ADR
decreased from 0.72 to 0.33 (P<0.001) when combined with mild
hyperthermia at 41°C after 1 h, and to 0.10 (P<0.001) at 41°C
after 5 h. The addition of mild hyperthermia at 41°C for 1 h caused
a 2.2-fold increase in the anti-tumor activity of 40 μg/ml ADR.
Discussion
Hyperthermia as a cancer therapy requires heating to
43°C or higher in order to kill tumor cells. However, it is
technically difficult to heat and maintain some organs and tissues
at that temperature. Mild hyperthermia is easy, safe and
cost-effective, and can be practically performed at any medical
facility.
We previously reported that mild hyperthermia at
41°C was effective in combination with chemotherapy, instead of
hyperthermia at 43°C, in an in vitro experiment using a
leukemia cell line NALM-6 (7).
Mild hyperthermia markedly enhanced the anti-tumor effects of
anti-tumor drugs.
M-VAC chemotherapy (methotrexate, vinblastine,
doxorubicin and cisplatin) is the gold standard therapy for
transitional cell carcinoma of the urothelium (1,2).
However, M-VAC chemotherapy is associated with severe adverse drug
reactions, such as renal damage, bone marrow depression and
gastrointestinal toxicity (nausea and vomiting) (3,4). In
addition, its response rate is not always high, at about 40%. We
examined whether the anti-tumor effect in the leukemic cell line
could be extended to transitional cell carcinoma of the urothelium
by combining mild hyperthermia with M-VAC chemotherapy in an in
vitro experiment. In this experiment, the anti-tumor effects of
CDDP and ADR, which have the main target action in M-VAC
chemotherapy, were examined using the human bladder cancer cell
line T24.
The thermal sensitivity of T24 cells was the same as
that of cells in general. The survival rate of T24 cells was not
altered by mild hyperthermia at 41°C, and was decreased by heating
at over 43°C.
CDDP and ADR are well-known anti-tumor drugs in
M-VAC chemotherapy for transitional cell carcinoma of the
urothelium (1–4). The survival rate of T24 cells was not
affected by a low CDDP concentration (20 μg/ml) or mild
hyperthermia at 41°C, independently. However, the survival rate was
significantly decreased by a low CDDP concentration (20 μg/ml) in
combination with mild hyperthermia at 41°C according to the
incubation time. The anti-tumor effect of a low CDDP concentration
(20 μg/ml) and 41°C for 5 h resulted in the same survival rate as
that of a 10-fold higher concentration (200 μg/ml) of CDDP.
As with CDDP, another anti-tumor drug, ADR, showed
the same effects. The anti-tumor effect of 4 μg/ml ADR at 41°C for
3 h achieved the same survival rate as a 10-fold higher ADR
concentration (40 μg/ml).
Based on these results, a low concentration of
anti-tumor drugs that have no tumor cell killing effect would be
expected to have a 10 times greater anti-tumor effect in
combination with mild hyperthermia at 41°C. Given that, at the same
concentration of anti-tumor drugs, the anti-tumor effect of 200
μg/ml CDDP increased 4-fold (P<0.001) and the effect of 40 μg/ml
ADR increased 2.2-fold (P<0.001) in combination with mild
hyperthermia at 41°C for 1 h, the increase in tumor cell killing
activity of anti-tumor drugs by combination with mild hyperthermia
was confirmed.
It is reported that CDDP combines with the DNA
strand in cancer cells and inhibits the DNA synthesis and cell
division of cancer cells (9). The
mechanism of the anti-tumor effect of ADR is the inhibition of both
DNA and RNA synthesis after ADR forms a complex with DNA in tumor
cells and inhibits both DNA polymerase and RNA polymerase reactions
(10,11).
It seems that combination with mild hyperthermia
potentiated the cytotoxic effects of these anti-tumor drugs by
inducing apoptosis. Kameda et al reported that apoptosis was
significantly enhanced when mild hyperthermia was combined with an
anti-tumor drug (12).
Hyperthermia was found to inhibit the repair of DNA
damage by anti-tumor drugs and radiation and increased the
anti-tumor activity of chemotherapy and radiotherapy (13). Shioura et al (14) and Kano et al (15) reported an increase in the cytotoxic
effects of combined treatment with low hyperthermia (40°C) and
bleomycin in vitro. Ono et al also reported that mild
hyperthermia increased blood flow and enhanced the uptake of
anti-tumor drugs into tumor tissue (16). Furthermore, mild hyperthermia was
more effective in promoting heat-mediated suicide-gene (HSP 70)
expression than high temperature therapy (17). Mild temperatures below 41°C showed
significantly smaller energies in Arrhenius plots for some
anti-tumor drugs than those observed with temperatures above 41°C
(18). Recently, Ahmed et
al reported mild hyperthermia- and hyperthermia-induced
enhancement of drug cytotoxicity in apoptosis (19).
From these reports, it was suggested that mild
hyperthermia enhances apoptosis and the anti-tumor effects of
chemotherapy through an increase in the uptake of carcinostatics
into tumor cells, and inhibits the repair of tumor cell killing by
anti-tumor drugs.
These experimental results indicate the possibility
that the dose of anti-tumor drugs can be decreased by combination
with mild hyperthermia, thus reducing the side effects of the
drugs. In poorly effective chemotherapy, a higher response rate is
expected by combination with mild hyperthermia.
We found clinically mild hyperthermia to be safe,
cost-effective and easy to perform using a far-infrared apparatus
(20,21). With the approval of the ethics
committee of our University, combination therapy with mild
hyperthermia and chemotherapy for progressive bladder cancer was
performed with exceptional results (22,23).
We previously reported that combination therapy with mild
hyperthermia and M-VAC chemotherapy (methotrexate, vinblastine,
doxorubicin and cisplatin) reduces gastrointestinal side effects
and potentiates the anti-tumor effect, with an excellent response
rate of 83% for advanced or metastatic transitional cell carcinoma
of the urothelium (24). This
basic in vitro research strongly supports our clinical
results.
References
|
1.
|
Sternberg CN, Yagoda A, Scher HI, et al:
Preliminary results of M-VAC (methotrexate, vinblastine,
doxorubicin and cisplatin) for transitional cell carcinoma of the
urothelium. J Urol. 133:403–407. 1985.PubMed/NCBI
|
|
2.
|
Masse HM, Hansen SW, Robert JT, et al:
Gemcitabine and cisplatin versus methotrexate, vinblastine,
doxorubicin and cisplatin in advanced or metastatic bladder cancer;
results of a large, randomized, multinational, multicancer, phase
III study. J Clin Oncol. 18:3068–3077. 2000.
|
|
3.
|
Saxman SB, Propert KJ, Einborn LH, et al:
Long-term follow-up of a phase III intergroup study of cisplatin
alone or in combination with methotrexate, vinblastine and
doxorubicin in patients with metastatic urothelial carcinoma; a
cooperative group study. J Clin Oncol. 15:2564–2569.
1997.PubMed/NCBI
|
|
4.
|
Sternberg CN, Yagoda A, Scher HI, et al:
Methotrexate, vinblastine, doxorubicin and cisplatin for advanced
transitional cell carcinoma of the urothelium. Efficacy and
patterns of response and relapse. Cancer. 64:2448–2458. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Mizuno S: Biological and medical grounds
of combination with hyperthermia and radiotherapy. Hyperthermia
Manual (in Japanese). Matsuda T: Magupuros Publication Co.; pp.
1–5. 1991
|
|
6.
|
Mitsuhashi N: Positioning in combination
therapy and future subjects. Hyperthermia – Guide Book of
Hyperthermia for Cancer Therapy (in Japanese). Japanese Society for
Thermal Medicine, Mainichi-kenkousaron Publication Co.; pp. 6–7.
2008
|
|
7.
|
Itoh Y, Kazaoka Y, Nitta M, et al:
Combining anti-tumor drugs with mild hyperthermia increases the
cytotoxic effects of drugs on human leukemia cells in vitro.
Mol Med Rep. 2:411–415. 2009.PubMed/NCBI
|
|
8.
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Zwelling LA and Kohn KW: Mechanism of
action of cis-dichlorodiammineplatinum (II). Cancer Treat Rep.
63:1439–1444. 1979.PubMed/NCBI
|
|
10.
|
Di M: Adriamycin (NSC-123127): mode and
mechanism of action. Cancer Chemother Rep. 6:91–106. 1975.
|
|
11.
|
Nemoto T and Takahira H: Uptake of
adriamycin into the cells and the interaction with DNA.
Yakugakuzasshi (in Japanese). 93:1498–1508. 1973.
|
|
12.
|
Kameda K, Kondo T, Tanabe K, et al: The
role of intracellular Ca2+ in apoptosis induced by
hyperthermia and its enhancement by verapamil in U937 cells. Int J
Radiat Oncol. 49:1369–1379. 2001.PubMed/NCBI
|
|
13.
|
Hall EJ and Roizin T: Biological effects
of heat. Cancer Res. 44:S4708–S4713. 1984.
|
|
14.
|
Shioura H, Hayashi S, Matsumoto H, et al:
The effects of combined treatments with low hyperthermia and
bleomycin on survival of murine L cells. Clin Cancer Res.
16:147–152. 1997.PubMed/NCBI
|
|
15.
|
Kano E, Furukawa-Furuya M and Nitta K:
Sensitivity of bleomycin-resistant variant cells enhanced by 40°C
hyperthermia in vitro. Int J Hyperthermia. 4:5547–5553.
1988.PubMed/NCBI
|
|
16.
|
Ono H, Ando S and Suzuki T: The drug
uptake in the tumor with the mild-hyperthermia treatment in
combination with the chemotherapy in vivo. Jpn J Hyperthermic
Oncol. 22:23–33. 2006. View Article : Google Scholar
|
|
17.
|
Huang Q, Hu JK and Lohr F: Heat-induced
gene expression as a novel targeted cancer gene therapy strategy.
Cancer Res. 60:3435–3439. 2000.PubMed/NCBI
|
|
18.
|
Urano M, Kuroda M and Nishimura Y: For the
clinical application of thermal chemotherapy given at mild
temperatures. Int J Hyperthermia. 15:79–107. 1999. View Article : Google Scholar
|
|
19.
|
Ahmed K, Hori T and Yu DA: Hyperthermia
chemo-sensitization, chemical thermo-sensitization and apoptosis.
Thermal Med. 24:1–12. 2008. View Article : Google Scholar
|
|
20.
|
Itoh Y, Tazawa K, Wada K, et al: Induction
of HSP 70 in lymphocytes by whole body far-infrared hyperthermia.
Jpn J Hyperthermic Oncol. 21:209–220. 2005.
|
|
21.
|
Itoh Y, Ogawa K and Tazawa K: Improvement
of athletic performances by heat shock protein 70 induced with mild
hyperthermia. Jpn J Clin Physiol. 38:13–21. 2008.
|
|
22.
|
Itoh Y and Yamada Y: Enhancement of the
anti-tumor effects and improvement of QOL by the combination with
mild hyperthermia. In: 67th Annual Meeting of the Japanese Cancer
Association (JCA 2008) – Proceedings; pp. 3612008
|
|
23.
|
Yamada Y, Itoh Y and Honda Y: Increase of
anti-tumor effect by combination therapy with mild hyperthermia and
chemotherapy for bladder cancer. Thermal Med (in Japanese).
24:S652008.
|
|
24.
|
Yamada Y, Itoh Y, Aoki S, et al:
Preliminary results of M-VAC chemotherapy combined with mild
hyperthermia, a new therapeutic strategy for advanced or metastatic
transitional cell carcinoma of the urothelium. Cancer Chemother
Pharmacol. 64:1079–1083. 2009. View Article : Google Scholar : PubMed/NCBI
|