Introduction
In Japan, gastric cancer is the most common (22.2%)
and colorectal cancer is the second most common (18.1%) form of
cancer (1). The morbidity and
mortality rates for gastric cancer peaked in the 1960s, and both
rates have since decreased in Japan (2,3).
However, the morbidity and mortality rates for colorectal cancer
have markedly been increasing (2).
Chemotherapy plays an important role in the treatment of
gastrointestinal cancers.
5-Fluorouracil (5-FU), which is a frequently used
chemotherapeutic drug, was first synthesized by Dushinsky and
Heidelberger in 1956 (4). Later,
Heidelberger et al reported that 5-FU exhibited an antitumor
effect (5). The median survival
time of patients with unresectable advanced gastric cancer, which
is 3–4 months without any treatment, can be prolonged to 7 months
after treatment with 5-FU, 5-FU + doxorubicin, or 5-FU +
doxorubicin + mytomycin C (6).
Chemotherapy has thus improved the survival time and disease-free
period of patients with metastatic colorectal cancer (7,8).
Meanwhile, the median survival time of patients with advanced
colorectal cancer, which is 6 months without any treatment, can be
improved to 10–12 months after treatment with 5-FU or 5-FU +
leucovorin (LV) (9).
To select the most appropriate drug for use in
individual patients, the utility of anticancer drug sensitivity
tests, such as the succinic dehydrogenase inhibition test and the
histoculture drug response assay, has been examined. Both of these
methods directly judge chemotherapeutic sensitivity by removing
cancer cells from the cancer tissues and culturing them with
various anticancer drugs. Alternatively, gene expression analyses,
which do not require cell culturing with anticancer drugs, for
genes related to chemotherapeutic sensitivity have been attracting
attention, along with progress in the analysis of the molecular
mechanisms of anticancer drug action.
Thymidylate synthase (TS), which is a target enzyme
of 5-FU and is also a rate-limiting enzyme in DNA synthesis, and
dihydropyrimidine dehydrogenase (DPD), which is related to 5-FU
metabolism, have been studied as predictors of prognosis or
anticancer drug sensitivity (10–21).
These studies on 5-FU-related anticancer drug-sensitivity factors
in gastrointestinal cancers are of significance in improving the
efficiency of adjuvant chemotherapy for advanced and recurrent
cancers and in helping to identify optimal therapies for individual
gastrointestinal cancer patients (i.e., establishing customized
chemotherapy).
We studied the relationships between the outcomes of
gastric and colorectal cancer patients who received treatment with
orally active derivatives of fluoropyrimidine after surgery and the
expression of genes related to chemotherapeutic sensitivity. Here,
we present our findings and discuss the clinical significance of
genes related to chemotherapeutic sensitivity.
Materials and methods
The subjects included 45 patients who underwent
adjuvant chemotherapy with orally active derivatives of
fluoropyrimidine after undergoing curative surgery between January
2001 and September 2007 in the Department of General and
Gastrointestinal Surgery, 2nd Teaching Hospital of Fujita Health
University. Of the 45 patients, 21 had gastric cancer and 24 had
colorectal cancer. All of the patients received adjuvant
chemotherapy with UFT (uracil/tegafur) + oral LV, S-1 (tegafur
gimeracil oteracil potassium), or 5-FU. In the UFT + LV regimen,
300 mg/m2/day of UFT and 75 mg/m2/day of LV
were divided into three oral doses per day (both agents were
administered simultaneously). One course consisted of 4 weeks of
medication and 1 week of rest. In the S-1 regimen, 80
mg/m2/day of S-1 was divided into two oral doses per
day. One course consisted of 4 weeks of medication and 2 weeks of
rest. In the 5-FU regimen, 200–300 mg/body/day of 5-FU were divided
into three oral doses per day, administered each day.
We prepared formalin-fixed, paraffin-embedded
specimens of the resected tumors, and 5-μm thick sections were
stained with H&E to identify the cancer cells. Then, isolated
cancer cells were removed from 10-μm thick sections using the
laser-captured microdissection technique (22). Total RNA was extracted and the
expression of ten genes [TS, DPD, thymidine phosphorylase (TP),
folylpolyglutamate synthetase (FPGS), γ-glutamyl hydrolase (GGH),
dihydrofolate reductase (DHFR), excision repair cross-complementing
gene 1 (ERCC1), topoisomerase 1 (Topo1), epidermal growth factor
receptor (EGFR) and vascular endothelial growth factor (VEGF)] was
measured using the RT-PCR method with a TaqMan probe (Applied
Biosystems, Foster City, CA). The expression of β-actin was also
measured as an internal control. The sequence products shown in
Table I were used as the primers
for TS, DPD, TP, FPGS, GGH, DHFR, ERCC1, Topo1, EGFR, VEGF and
β-actin genes. The expression levels of each gene were normalized
using the expression level of β-actin. PCR was performed using an
ABI PRISM 7900 Sequence Detection System (Applied Biosystems). The
PCR conditions consisted of 42 cycles at 95°C for 15 sec and 60°C
for 1 min, followed by 50°C for 10 sec and 95°C for 10 min.
 | Table I.Sequences of the PCR primers. |
Table I.
Sequences of the PCR primers.
| Gene name | Primer sequence
(forward) | Primer sequence
(reverse) | Probe sequence |
|---|
| TS |
GCCTCGGTGTGCCTTTCA |
CCCGTGATGTGCGCAAT |
TCGCCAGCTACGCCCTGCTCA |
| DPD |
AGGACGCAAGGAGGGTTTG |
GTCCGCCGAGTCCTTACTGA |
CAGTGCCTACAGTCTCGAGTCTGCCAGTG |
| TP |
CCTTGGATAAGCTGGAGTCTATTCC |
CCTGGTCCAGCAGCACTTG |
TCAATGTCATCCAGAGCCCAGAGCAGAT |
| FPGS |
GGCTGGAGGAGACCAAGGAT |
CATGAGTGTCAGGAAGCGGA |
CAGCTGTGTCTCCATGCCCCCCTAC |
| GGH |
GTGGCAATGCCGCTGAA |
CAACTCAGTAGGAAAATTCTGGAACA |
TTCACTGGAGGTCAATTGCACAGCAGA |
| DHFR |
GTCCTCCCGCTGCTGTCA |
GCCGATGCCCATGTTCTG |
TTCGCTAAACTGCATCGTCGCTGTGTC |
| ERCC1 |
GGGAATTTGGCGACGTAATTC |
GCGGAGGCTGAGGAACAG |
CACAGGTGCTCTGGCCCAGCACATA |
| Topo1 |
TGTAGCAAAGATGCCAAGGT |
TGTTATCATGCCGGACTTCT |
CCTTCTCCTCCTCCAGGACATAAGTGGA |
| VEGF |
AGTGGTCCCAGGCTGCAC |
TCCATGAACTTCACCACTTCGT |
TGATTCTGCCCTCCTCCTTCTGCCAT |
| EGFR |
TGCGTCTCTTGCCGGAAT |
GGCTCACCCTCCAGAAGGTT |
ACGCATTCCCTGCCTCGGCTG |
| β-actin |
GAGCGCGGCTACAGCTT |
TCCTTAATGTCACGCACGATTT |
ACCACCACGGCCGAGCGG |
We then analyzed the relationships between the
clinicopathological factors, including age, gender, cancer
location, pathological type, depth of invasion, lymph node
metastasis, stage and site of cancer, and relapse rate, as well as
the relationships between the gene expression levels and the
relapse rate.
The risk of relapse was evaluated using the Cox
proportional hazards model. The cut-off value was determined using
the receiver operating characteristic (ROC) curve analysis. The
survival rate was calculated using the Kaplan-Meier method. The
survival rate was evaluated using the log-rank test, and a p-value
of <0.05 was considered statistically significant. The
statistical analysis software SPSS v.11 (SPSS Japan Inc., Japan)
was used for all analyses. This study was approved by the Fujita
Health University Ethics Review Board for Epidemiological and
Clinical Studies.
Results
The median observation period was 41 months. Twelve
of the 21 gastric cancer patients (57.1%) and 11 of the 24
colorectal cancer patients (45.8%) relapsed. Although the results
of a univariate analysis revealed that none of the examined gene
expression was related to relapse in the gastric cancer patients,
ERCC1 was related to relapse in the colorectal cancer patients
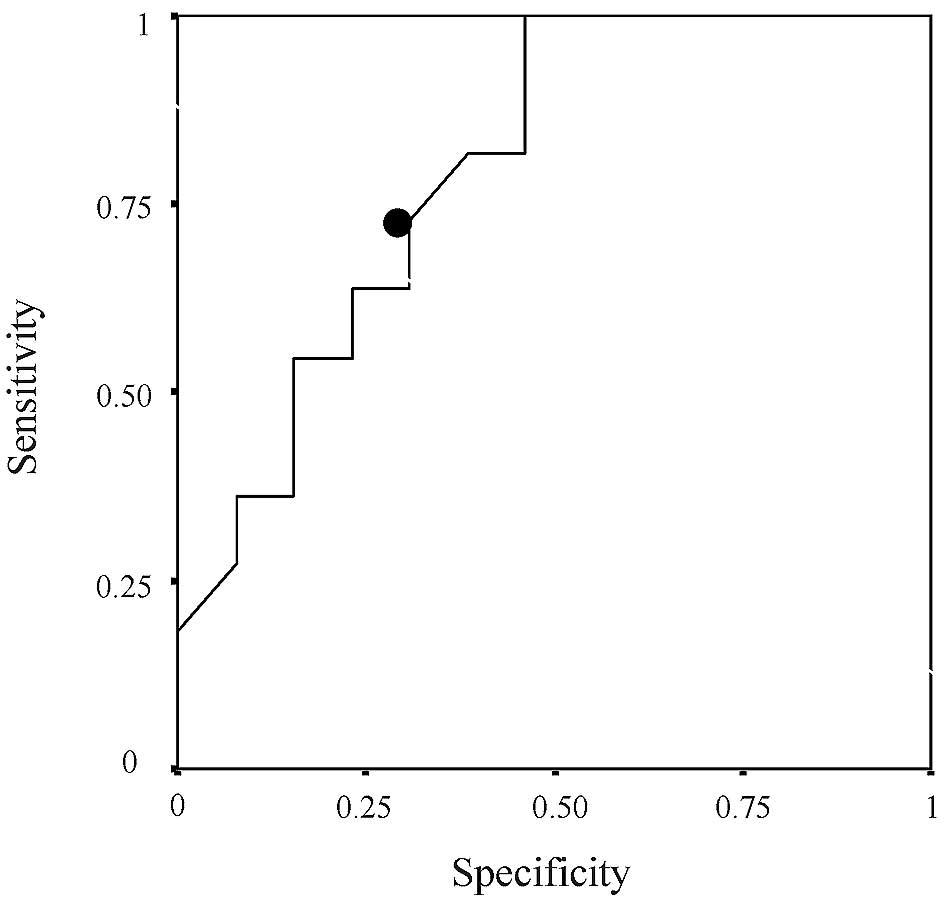
[hazard ratio (HR), 3.137; 95% CI, 1.167–8.431; P=0.023] (Tables II–VII). A value of 1.295 was set as the
cut-off value for ERCC1 expression based on the results of the ROC
curve (Fig. 1); values of ≥1.295
were defined as high expression, while values of <1.295 were
defined as low expression. Eight of the 12 patients in the
high-expression group (67%) and 3 of the 12 patients in the
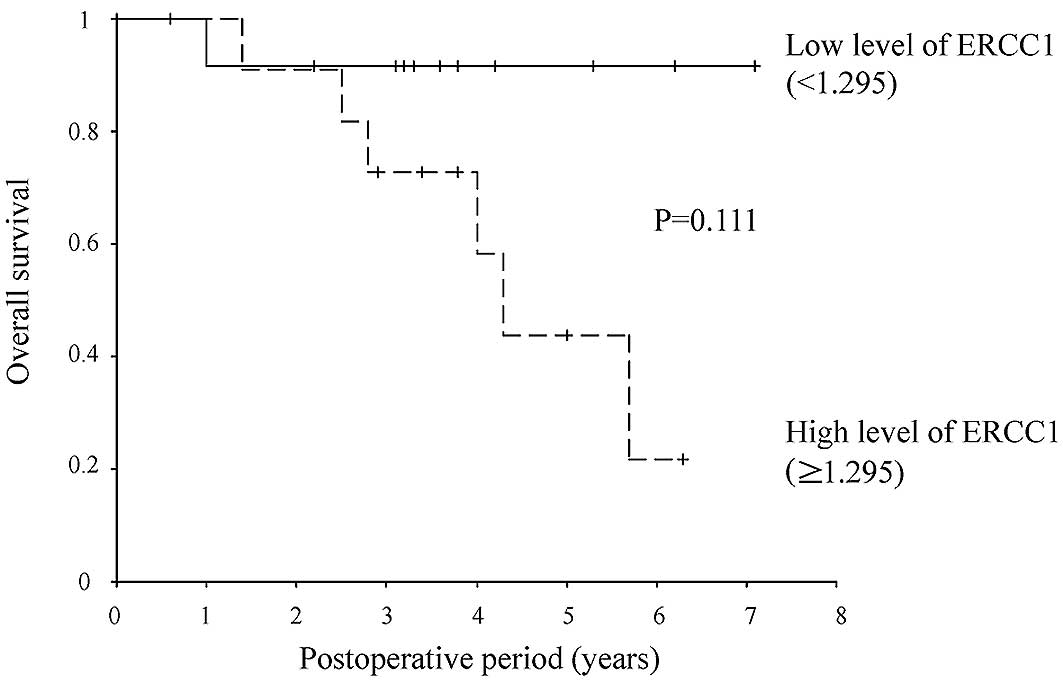
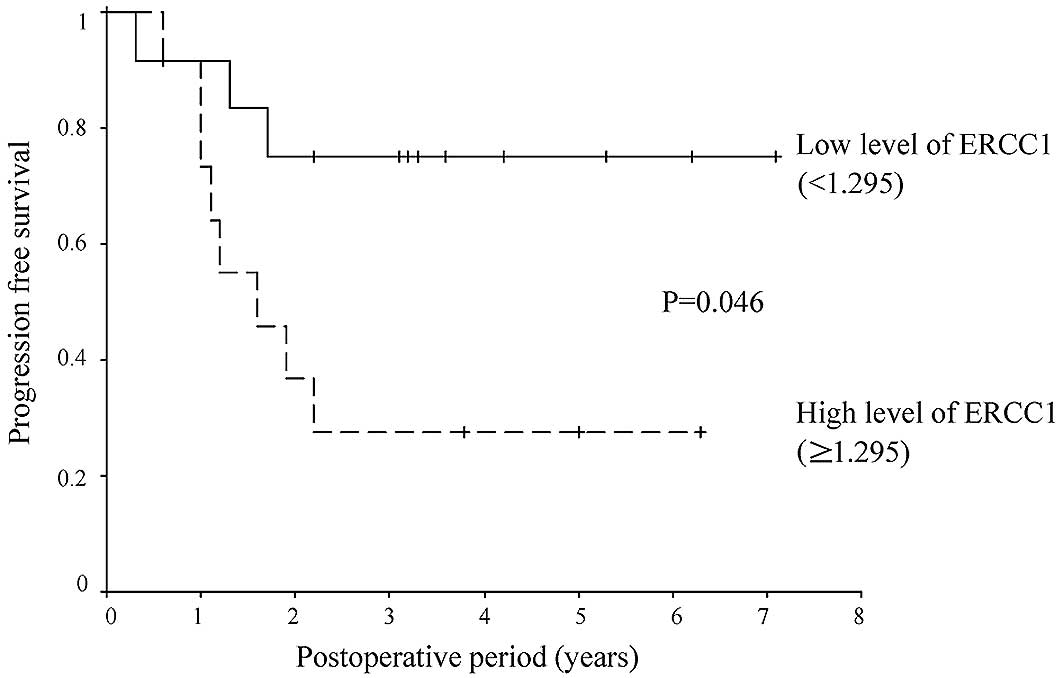
low-expression group (25%) relapsed. The relapse-free survival rate
was lower in the ERCC1 high-than in the ERCC1 low-expression group
(HR, 3.891; 95% CI, 1.023–14.808; P=0.046) (Figs. 2 and 3).
 | Table II.Univariate analysis of recurrent
factors of gastric and colon cancer (n=45). |
Table II.
Univariate analysis of recurrent
factors of gastric and colon cancer (n=45).
| Variable | Mean ± SD | Hazard ratio | 95% CI | P-value |
|---|
| TS | 2.53±1.25 | 1.047 | 0.746–1.471 | 0.790 |
| DPD | 0.68±0.85 | 0.957 | 0.523–1.749 | 0.885 |
| TP | 4.96±3.17 | 0.996 | 0.869–1.143 | 0.959 |
| FPGS | 1.44±1.03 | 1.047 | 0.720–1.522 | 0.810 |
| GGH | 2.95±1.55 | 1.174 | 0.816–1.689 | 0.387 |
| DHFR | 2.54±0.96 | 0.691 | 0.370–1.291 | 0.247 |
| ERCC1 | 1.79±0.80 | 1.649 | 0.992–2.741 | 0.054 |
| Topo1 | 1.66±0.77 | 1.168 | 0.579–2.357 | 0.665 |
| VEGF | 6.75±4.73 | 0.986 | 0.903–1.077 | 0.755 |
| EGFR | 3.35±11.58 | 0.922 | 0.709–1.199 | 0.544 |
 | Table VII.Univariate analysis of recurrent
factors of colon cancer (n=24). |
Table VII.
Univariate analysis of recurrent
factors of colon cancer (n=24).
| Variable | Mean ± SD | Hazard ratio | 95% CI | P-value |
|---|
| Age | 66.4±7.9 | 1.009 | 0.938–1.086 | 0.807 |
| Gender
(male/female) | 14/10 | 0.999 | 0.304–3.279 | 0.999 |
| Organ
(colon/rectum) | 15/9 | 0.954 | 0.279–3.262 | 0.940 |
| Depth of tumor
invasion (sm, mp/ss, se) | 3/21 | 0.037 | 0.000–37.694 | 0.352 |
| Lymph node
metastasis (no/yes) | 5/19 | 1.389 | 0.300–6.439 | 0.675 |
| Pathological stage
(II/III) | 5/19 | 1.389 | 0.300–6.439 | 0.675 |
Discussion
In this study, we analyzed the genes related to
chemotherapeutic sensitivity in gastric and colorectal cancer
patients who received adjuvant chemotherapy with orally active
derivatives of fluoropyrimidine after undergoing curative surgery.
We demonstrated that ERCC1 overexpression was related to relapse in
colorectal cancer patients.
ERCC1 is a nucleotide excision repair (NER)-related
gene which is involved in the excision and repair of damaged DNA.
ERCC1 forms a complex with a protein named XRF and repairs DNA
interstrand cross-links (23).
Since DNA damage induces cell death and gene mutations, the
possible causes of aging and carcinogenesis, respectively, DNA
repair mechanisms are very important. In platinum-based drugs such
as cisplatin, platinum molecules bind to the DNA in tumor cells
forming adducts that inhibit DNA replication. This process is
considered to be the main molecular mechanism of the anti-tumor
action of these drugs (23,24).
In 1992, Dabholkar et al measured ERCC1
expression in ovarian tumor tissues and reported for the first time
in a clinical study that the expression of ERCC1 is related to
cisplatin-resistance (25). In
vitro studies have also shown that the expression of ERCC1 mRNA
is related to resistance to platinum-based drugs in cell lines
derived from ovarian, head and neck, bladder, testicular and
non-small cell lung cancers (24).
Additionally, retrospective clinical studies have indicated that
the expression of ERCC1 mRNA is related to resistance to
platinum-based drugs in gastric, ovarian, colorectal, esophageal
and non-small cell lung cancers (24–35).
5-FU + oxaliplatin therapy was administered to patients with
advanced gastric cancer, and the expression of ERCC1, TS and
glutathione S-transferase P1 (GSTP1) were measured
immunohistochemically. Patients with positive ERCC1 expression had
a lower overall survival rate (30). In patients with metastatic
colorectal cancer who received oxaliplatin + 5-FU combination
therapy, ERCC1 overexpression was related to chemotherapeutic
resistance and a short survival time, and ERCC1 was suggested to be
a potentially useful biomarker (24). In all of these studies, the
therapies were performed in combination with platinum-based drugs,
while no reports analyzing the relationships between 5-FU
monotherapy or orally active derivatives of fluoropyrimidine and
ERCC1 were found in a PubMed search.
A relation between ERCC1 expression and CPT-11
sensitivity has also been reported. First-line CPT-11 therapy is
effective in colorectal cancers overexpressing ERCC1, EGFR and
GSTP1, and the overexpression of both ERCC1 and EGFR has been
associated with relapse-free survival (36). ERCC1 and EGFR are expected to be
useful for evaluating the response of FOLFIRI and CPT-11 +
cetuximab therapies, which are currently administered to patients
with unresectable advanced/recurrent colorectal cancer.
5-FU therapy is also effective against metastatic
advanced colorectal cancer with low expression levels of
intratumoral TS mRNA or protein (10–14).
In addition, 5-FU adjuvant chemotherapy is effective against
colorectal cancer with a high expression level of intratumoral TS
protein (15–19). 5-FU is also effective against
advanced head and neck cancer when the expression level of DPD is
low (20). Furthermore, the effect
of 5-FU therapy can be reportedly predicted by measuring the
expression levels of intratumoral TS, TP and DPD in metastatic
colorectal cancer (21).
To summarize these numerous reports, since cancer
cells overexpressing ERCC1 are adept at repairing DNA damaged by
antitumor drugs, patients with such tumors have a high risk of
cancer relapse, even after the administration of anticancer drugs.
Therefore, ERCC1 might be a useful marker gene for predicting
anticancer drug sensitivity. In the cases examined in this study,
1.295 was set as the cut-off value for ERCC1 overexpression based
on the results of the ROC curve; values of 1.295 or higher were
defined as high-expression, while values less than 1.295 were
defined as low-expression. Using this classification, the
high-expression patient group was found to have a high risk of
relapse. Thus, patients with high intratumoral levels of ERCC1
expression have a higher risk of cancer relapse and should receive
frequent follow-up examinations after surgery; additional
chemotherapies might also need to be considered.
In conclusion, the overexpression of the ERCC1 gene
is useful for predicting relapse in colorectal cancer patients who
received adjuvant chemotherapy with orally active derivatives of
fluoropyrimidine after undergoing curative surgery.
References
|
1.
|
Matsuda T, Marugame T, Kamo K, Katanoda K,
Ajiki W and Sobue T; The Japan Cancer Surveillance Research Group:
Cancer incidence and incidence rates in Japan in 2002: based on
data from 11 population-based cancer registries. Jpn J Clin Oncol.
38:641–648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Parkin DM, Whelan SL, Ferlay J, Teppo L
and Thomas DB: Cancer incidence in five continents. IARC Scientific
Publications no. 155. Vol. 8, Lyon,. 2002
|
|
3.
|
WHO databank: http://www.depdb.iarc.fr/who/menu.htmluri.
|
|
4.
|
Duschinsky R, Pleven E and Heidelberger C:
The synthesis of 5-fluoropyrimidines. J Am Chem Soc. 79:45591957.
View Article : Google Scholar
|
|
5.
|
Heidelberger C, Chaudhuri NK, Danneberg P,
Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E and
Scheiner J: Fluorinated pyrimidines, a new class of
tumour-inhibitory compounds. Nature. 179:663–666. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
MacDonald JS, Schin P, Woolley PV, Smythe
T, Ueno W, Hoth D, Smith F, Boiron M, Gisselbrecht C, Brunet R and
Lagarde C: 5-Fluorouracil, doxorubicin and mitomycin (FAM)
combination chemotherapy for advanced gastric cancer. Ann Intern
Med. 93:533–536. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Scheithauer W, Rosen H, Kornek GV, Sebesta
C and Depisch D: Randomised comparison of combination chemotherapy
plus supportive care with supportive care alone in patients with
metastatic colorectal cancer. BMJ. 306:752–755. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Colorectal Cancer Collaborative Group:
Palliative chemotherapy for advanced colorectal cancer: systematic
review and metaanalysis. BMJ. 321:531–535. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Meyerhardt JA and Mayer RJ: Systemic
therapy for colorectal cancer. N Engl J Med. 352:476–487. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Leichman CG, Lenz H-J, Leichman L,
Danenberg K, Baranda J, Groshen S, Boswell W, Metzger R, Tan M and
Danenberg PV: Quantitation of intratumoral thymidylate synthase
expression predicts for disseminated colorectal cancer response and
resistance to protracted-infusion fluorouracil and weekly
leucovorin. J Clin Oncol. 15:3223–3229. 1997.
|
|
11.
|
Lenz H-J, Hayashi K, Salonga D, Danenberg
KD, Danenerg PV, Metzger R, Banerjee D, Bertino JR, Groshen S,
Leichman LP and Leichman CG: p53 point mutations and thymidylate
synthase messenger RNA levels in disseminated colorectal cancer: an
analysis of response and survival. Clin Cancer Res. 4:1243–1250.
1998.PubMed/NCBI
|
|
12.
|
Cascinu S, Ashele C, Barni S, Debernardis
D, Baldo C, Tunesi G, Catalano V, Staccioli MP, Brenna A, Muretto P
and Catalano G: Thymidylate synthase protein expression in advanced
colon cancer: correlation with the site of metastasis and the
clinical response to leucovorin-modulated bolus 5-fluorouracil.
Clin Cancer Res. 5:1996–1999. 1999.
|
|
13.
|
Bathe OF, Franceschi D, Livingstone AS,
Moffat FL, Tian E and Ardalan B: Increased thymidylate synthase
gene expression in liver metastases from colorectal carcinoma:
implications for chemotherapeutic options and survival. Cancer J
Sci Am. 5:34–40. 1999.PubMed/NCBI
|
|
14.
|
Paradiso A, Simone G, Petroni S, Leone B,
Vallejo C, Lacava J, Romero A, Machiavelli M, Lena MD, Allegra CJ
and Johnson PG: Thymidylate synthase and p53 primary tumor
expression as predictive factors for advanced colorectal cancers.
Br J Cancer. 82:560–567. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Johnson PG, Fisher ER, Rockette HE, Fisher
B, Wolmark N, Drake JC, Chabner BA and Allegra CJ: The role of
thymidilate synthase expression in prognosis and outcome of
adjuvant chemotherapy in patients with rectal cancer. J Clin Oncol.
12:2640–2647. 1994.PubMed/NCBI
|
|
16.
|
Takenoue T, Nagawa H, Matsuda K, Fujii S,
Nita ME, Hatano K, Kitayama J, Tsuruo T and Muto T: Relation
between thymidilate synthase expression and survival in colon
carcinoma, and determination of appropriate application of
5-fluorouracil by immunohistochemical method. Ann Surg Oncol.
7:193–198. 2000. View Article : Google Scholar
|
|
17.
|
Elder D, Glimelius B, Hallstrom M,
Jakobsen A, Johnson PG, Magnusson I, Ragnhammar P and Blomgren H:
Thymidilate synthase expression in colorectal cancer: a prognostic
and predictive marker of benefit from adjuvant fluorouracil-based
chemotherapy. J Clin Oncol. 20:1721–1728. 2002. View Article : Google Scholar
|
|
18.
|
Findlay MPN, Cunningham D, Morgan G,
Clinton S, Hardcastle A and Aherne GW: Lack of correlation between
thymidylate synthase levels in primary colorectal tumors and
subsequent response to chemotherapy. Br J Cancer. 75:903–909. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ashele C, Debernardis D, Tunesi G, Maley F
and Sobrero A: Thymidilate synthase protein expression in primary
colorectal cancer compared with the corresponding distant
metastases and relationship with the clinical response to
5-fluorouracil. Clin Cancer Res. 6:4797–4802. 2000.
|
|
20.
|
Etienne MC, Cheradama S, Fischel JL,
Formento P, Dassonville O, Renee N, Schneider M, Thyss A, Demard F
and Milano G: Response to flurouracil therapy in cancer patients:
the role of tumoral dihydropyrimidine dehydrogenase activity. J
Clin Oncol. 13:1663–1670. 1995.PubMed/NCBI
|
|
21.
|
Salonga D, Danenberg KD, Johnson M,
Metzger R, Groshen S, Tsao-Wei DD, Lenz HJ, Leichman CG, Leichman
L, Diasio RB and Danenberg PV: Colorectal tumors responding to
5-fluorouracil have low gene expression levels of dihydropyrimidine
dehydrogenase, thymidilate synthase and thymidine phosphorylase.
Clin Cancer Res. 6:1322–1327. 2000.
|
|
22.
|
Fukui Y, Oka T, Nagayama S, Danenberg PV,
Danenberg KD and Fukushima M: Thymidilate synthase,
dihydropyrimidine dehydrogenase, orotate phosphoribosyltransferase
mRNA and protein expression levels in solid tumors in a large scale
population analysis. Int J Mol Med. 22:709–716. 2008.
|
|
23.
|
Houtsmuller AB, Rademakers S, Nigg AL,
Hoogstraten D, Hoeijmakers JHJ and Vermeulen W: Action of DNA
repair endonuclease ERCC1/XRF in living cells. Science.
284:958–961. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Altaha R, Liang X, Yu JJ and Reed E:
Excision repair cross complementing-group1: gene expression and
platinum resistance. Int J Mol Med. 14:959–970. 2004.PubMed/NCBI
|
|
25.
|
Dabholkar M, Bostick-Bruton F, Weber C,
Bohr VA, Egwuagu C and Reed E: ERCC1 and ERCC2 expression in
malignant tissues from ovarian cancer patients. J Natl Cancer Inst.
84:1512–1517. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Dabholkar M, Vionnet J, Bostick-Bruton F,
Yu JJ and Reed E: Messenger RNA levels of XPAC and ERCC1 in ovarian
cancer tissue correlate with response to platinum-based
chemotherapy. J Clin Invest. 94:703–708. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Joshi MB, Shirota Y, Danenberg KD, Conlon
DH, Salonga DS, Herndon JE II, Danenberg PV and Harpole DH Jr: High
gene expression of TS1, GSTP1 and ERCC1 are risk factors for
survival in patients treated with trimodality therapy for
esophageal cancer. Clin Cancer Res. 11:2215–2221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Langer R, Specht K, Becker K, Ewald P,
Bekesch M, Sarbia M, Busch R, Feith M, Stein HJ, Siewert JR and
Hofler H: Association of pretherapeutic expression of
chemotherapy-related genes with response to neoadjuvant
chemotherapy in Barrett carcinoma. Clin Cancer Res. 11:7462–7469.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Lord RVN, Brabender D, Gandara D, Alberola
V, Camps C, Domine M, Cardenal F, Sanchez JM, Gumerlock PH, Taron
M, Sanchez JJ, Danenberg KD, Danenberg PV and Rosell R: Low ERCC1
expression correlates with prolonged survival after ciplatin plus
gemcitabine chemotherapy in non-small cell lung cancer. Clin Cancer
Res. 8:2286–2291. 2002.PubMed/NCBI
|
|
30.
|
Kwon HC, Roh MS, Oh SY, Kim SH, Kim JS and
Kim HJ: Prognostic value of expression of ERCC1, thymidylate
synthase and glutathione S-transferase P1 for
5-fluorouracil/oxalip-latin chemotherapy in advanced gastric
cancer. Ann Oncol. 18:504–509. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Reed E, Dabholkar M, Thornton K, Thompson
C, Yu JJ and Bostick-Bruton F: Evidence for in the appearance of
mRNAs of nucleotide excision repair genes, in human ovarian cancer
tissue. Oncol Rep. 7:1123–1128. 2000.PubMed/NCBI
|
|
32.
|
Rosell R, Lord RV, Taron M and Reguart N:
DNA repair and cisplatin resistance in non-small cell lung cancer.
Lung Cancer. 38:217–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Olaussen KA, Dunant A, Fouret P, Brambilla
E, Andre F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH,
Stahel R, Sabatier L, Pignon JP, Tursz T, Chevalier TL and Soria
JC: DNA repair by ERCC1 in non-small cell lung cancer and
cisplatin-based adjuvant chemotherapy. N Engl J Med. 355:983–991.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Shirota Y, Stoehlmacher J, Brabender J,
Xiong YP, Uetake H, Danenberg KD, Groshen S, Tsao-Wei DD, Danenberg
PV and Lenz HJ: ERCC1 and thymidylate synthase mRNA levels predict
survival for colorectal cancer patients receiving combination
oxaliplatin and fluorouracil chemotherapy. J Clin Oncol.
19:4298–4304. 2001.
|
|
35.
|
Warnecke-Eberz U, Metzger R, Miyazono F,
Baldus SE, Neiss S, Brabender J, Schaefer H, Doerfler W,
Bollschweiler E, Dienes HP, Mueller RP, Danenberg PV, Hoelscher AH
and Schneider PM: High specificity of quantitative excision repair
cross-complementing 1 messenger RNA expression for prediction of
minor histopathological response to neoadjuvant radiochemotherapy
in esophageal cancer. Clin Cancer Res. 10:3794–3799. 2004.
View Article : Google Scholar
|
|
36.
|
Vallbohmer D, Iqbal S, Yang DY, Rhodes KE,
Zhang W, Gordon M, Fazzone W, Schultheis AM, Sherrod AE, Danenberg
KD and Lenz HJ: Molecular determinants of irinotecan efficacy. Int
J Cancer. 119:2435–2442. 2006. View Article : Google Scholar : PubMed/NCBI
|