Introduction
Growth factors constitute a family of
multifunctional proteins that play important roles in the growth,
development and maintenance of tissues (1). However, they also promote malignant
phenotypes by enhancing cellular proliferation, survival,
migration, invasiveness, acquisition of new vasculature and the
ability of tumor cells to escape detection by immune cells
(2). Growth factors such as
transforming growth factor-α (TGF-α) and macrophage colony
stimulating factor (m-CSF) have been shown to regulate meningioma
growth. Several angiogenic growth factors including vascular
endothelial growth factor, (VEGF), placental growth factor,
hepatocyte growth factor/scatter factor and basic fibroblast growth
factor (bFGF), have also been shown to be potential factors in
intracranial meningiomas (3–5).
The granulin family of growth factors are from
leukocytes and are known to mediate cell cycle progression and the
cell motility of epithelial and mesenchymal cells (6,7).
High levels of granulin expression have been found in several types
of cancers including those of the kidney, brain and stomach
(8–10). Overexpression of granulin has also
been linked to the growth and tumorigenicity of human breast
carcinomas (11,12). Furthermore, granulin mRNA levels
are elevated in high-grade primary brain tumors (9). Meningiomas are common brain tumors
and most are benign in their pathology. However, meningiomas
frequently accompany peritumoral brain edema (PTBE) associated with
malignant brain tumors. Meningioma develops in the meninges and
originates from a multiple layer of mesenchymal cells (13). Granulin, which is mostly expressed
in epithelial cell tumors, has not previously been reported in
intracranial meningiomas, and its clinical implication in
meningiomas has not been verified.
In this study, we investigated granulin expression
in intracranial meningiomas and analyzed the association of this
growth factor with clinical parameters including demographic data,
tumor size and PTBE volume.
Materials and methods
Patients
Samples were obtained from 79 consecutive patients
who underwent surgical removal of brain tumors that were confirmed
pathologically as meningiomas between October 2002 and August 2005.
The patient group included 15 (19%) men and 64 (81%) women ranging
in age from 16 to 84 years (median 59 years). Tumor size and PTBE
volume were measured on pre-operative magnetic resonance (MR)
images. The greatest anteroposterior (a) and lateral (b) diameters
were obtained on axial contrast enhanced T1-weighted
(T1W) images, and the greatest height (c) of the tumor
on the coronal contrast enhanced T1W MR images was
measured to calculate the tumor size. The PTBE volume was estimated
in the same manner on T2-weighted (T2W) MR
images. The volume was calculated by the formula V (cm3)
= 4/3 × πabc.
Tumor specimens
Freshly excised meningioma tissues were collected
during craniotomies for their resection or during biopsy. The
tissues were stored quickly in a deep freezer at −70°C prior to
processing.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Anonymized samples of frozen meningiomas were
retrieved from the deep freezer, and RNA extraction was carried out
using the SV Total Isolation system (Promega, Madison, WI, USA)
according to the manufacturer’s protocol. For each reverse
transcription reaction, we combined 25 μl Access Quick Master Mix
2X (Promega), 1 μl upstream primer (5′-TCC ACG TGC TGT GTT ATG
GT-3′), 1 μl downstream primer (5′-CTG CCC TGT TAG TCC TCT GG-3′),
5 μg RNA template and nuclease-free water to a final volume of 50
μl. AMV reverse transcriptase (1 μl) was added to the above mixture
as the final component and mixed by gentle vortexing. Reaction
tubes were incubated at 45°C for 45 min. The PCR reaction was
carried out using 3 μl of the reverse transcription reaction
product. Thirty-five cycles of touchdown PCR were performed
according to the manufacturer’s protocol and consisted of 95°C for
3 min, a one-degree decline in annealing temperature 55°C for 1
min, extension reaction at 72°C for 6 min and then maintaining the
reaction at 4°C overnight. After that, PCR products were stored at
−20°C. A single reaction void of template was performed with each
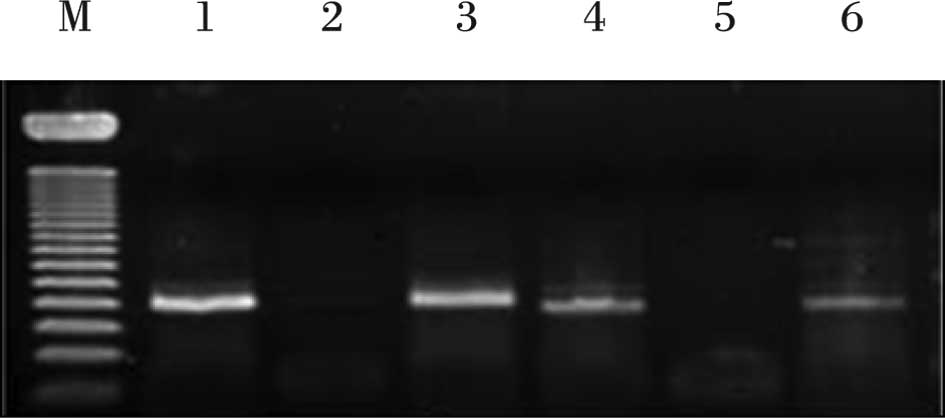
experiment as a negative control. After mixing 8 μl of PCR products
and 2 μl of loading buffer, mixtures were electrophoresed on a 2%
agarose gel, stained with ethidium bromide and visualized by
ultraviolet illumination (Fig. 1).
The optical density (OD) for granulin mRNA concentration was
measured by spectrometry, and its ratio to β-actin concentration
was obtained.
Statistical analysis
Data were analyzed using SPSS version 11 (SPSS Inc.,
Chicago, IL, USA). The Student’s t-test was employed to compare the
differences in tumor size and PTBE volume between the
granulin-positive and -negative tumors. Correlations of mRNA
concentrations to tumor and PTBE volumes were analyzed by Pearson’s
correlation test. All data are expressed as the mean ± standard
deviation. Differences with a P-value of <0.05 were regarded as
statistically significant.
Results
Patient characteristics
Granulin was expressed in tumors of 36.7% (n=29) of
the patients with meningioma. The mean age of patients who were
positive for expression of granulin was 53.7±1.6 years and
58.4±16.4 years for patients with granulin-negative tumors. The
difference in ages between the two groups was not statistically
significant (P>0.05). Granulin was expressed in 24 of 64 female
patients (37.5%) and in 5 of 15 male patients (33.3%). The
frequency of granulin expression according to gender was also not
significantly different (P>0.05) (Table I).
 | Table I.Patient characteristics and granulin
expression in 79 patients with meningioma. |
Table I.
Patient characteristics and granulin
expression in 79 patients with meningioma.
| Factors | Granulin expression
| P-value |
|---|
| (+) | (−) | |
|---|
| Mean age (years) | 53.7±1.6 | 58.4±16.4 | 0.149 |
| Gender |
| Male (n=15) | 5 (33%) | 10 (67%) | >0.050a |
| Female (n=64) | 24 (38%) | 40 (62%) | |
| Mean tumor volume
(cm3) | 51.5±5.9 | 24.9±2.8 | <0.050 |
| Mean PTBE volume
(cm3) | 104.2±15.9 | 52.9±10.6 | 0.010 |
Correlation of tumor volume and granulin
expression
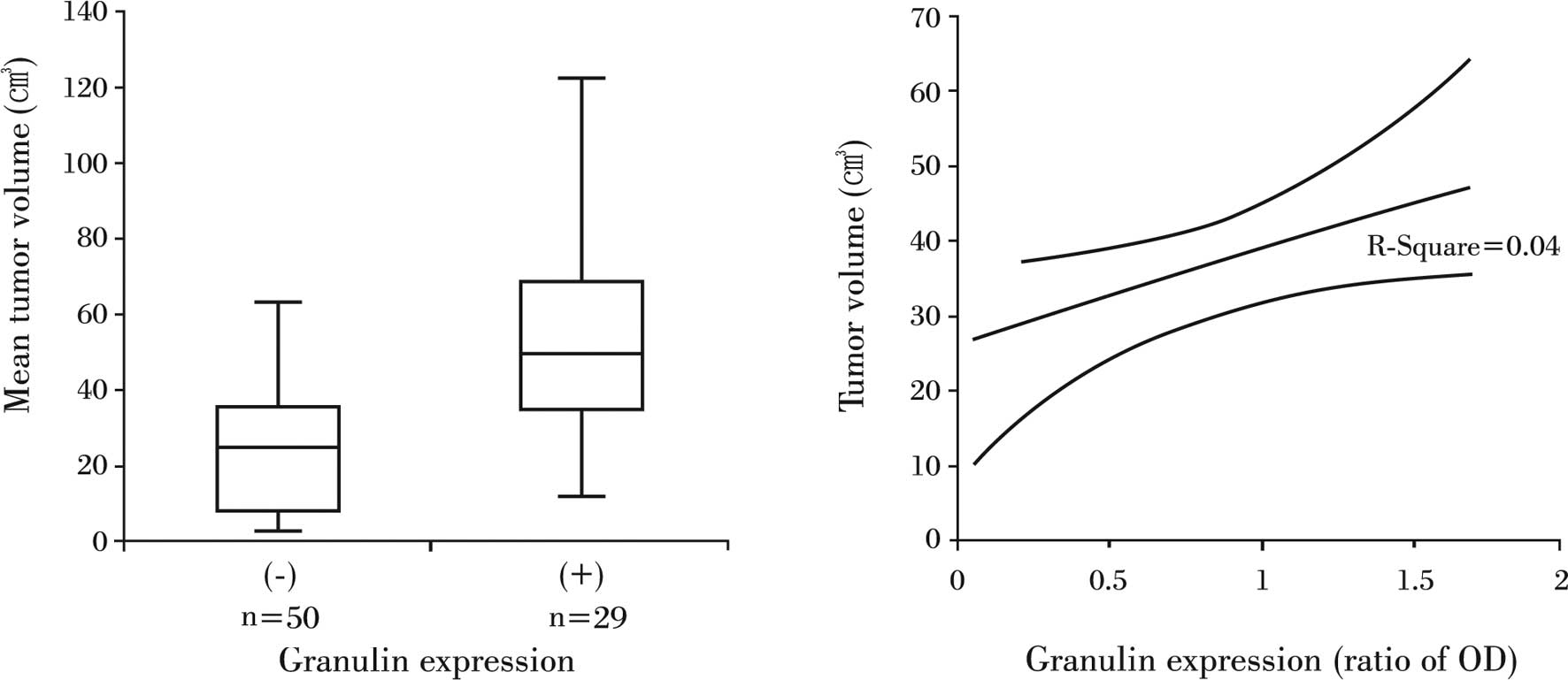
The mean tumor volume for all of the patients was
34.7±3.1 cm3. The mean tumor volume for the 50 patients
with granulin-negative tumors was 24.9±2.8 cm3, whereas
the average volume for patients with granulin-expressing tumors was
51.5±5.9 cm3, which was a statistically significant
difference (P<0.05) (Fig. 2A).
The relative ratio of OD for granulin detected in
granulin-expressing tumors was 0.57±0.47 (range 0–1.58), but it did
not correlate to tumor volume (R2=0.04, P>0.05)
(Fig. 2B).
Relationship between PTBE volume and
granulin expression
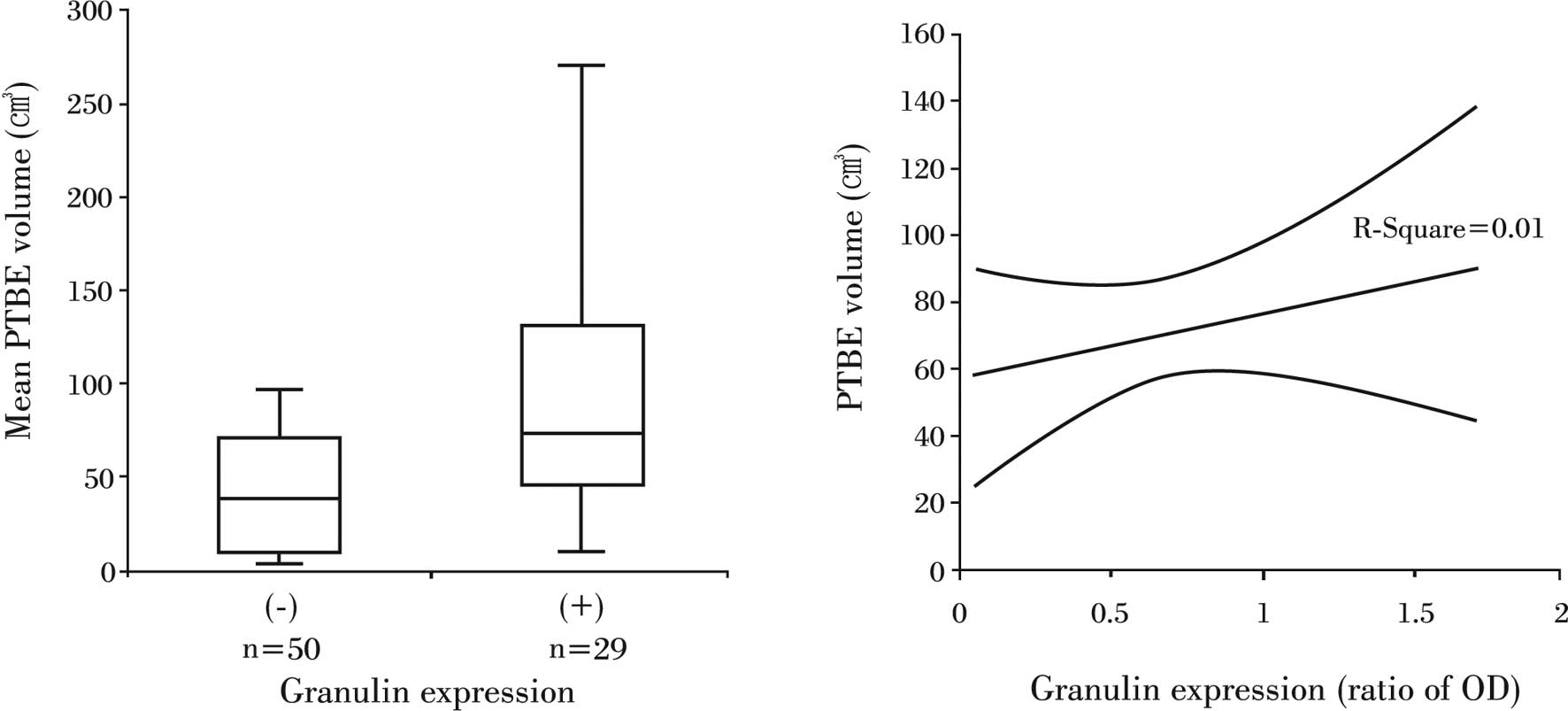
The mean PTBE volume for all of the patients was
71.7±9.3 cm3. The mean PTBE volume was 52.9±10.6
cm3 in patients whose tumors did not express granulin
expression, while it was 104.2±15.9 cm3 in patients with
granulin-positive tumors. This marked difference in PTBE volume
according to granulin expression was also significant (P<0.05)
(Fig. 3A). However, as with tumor
volume, the relative amount of granulin did not correlate to PTBE
volume (R2=0.01, P>0.05) (Fig. 3B).
Discussion
We observed granulin expression in the intracranial
meningiomas and found that it correlated to tumor size and PTBE
volume. However, the differences we observed in granulin expression
according to the demographic factors of age and gender were not
significant.
Growth factors identified to date, such as TGF-β,
m-CSF and lysophosphatic acid have been shown to replicate cancer
cell growth and survival in vitro and in vivo
(15–19). The role of growth factors in
intracranial meningioma development and progression appears to be
complex and multifactorial (13).
Compared to other well-established growth factors, such as
insulin-like growth factor, VEGF and fibroblast growth factor,
information about granulin is much more limited, although it has
been shown that the granulin gene is readily induced when quiescent
cells are aroused into a state of proliferation and motility, such
as in neoplastic transformation, or in the case of tissue injury of
dermal connective tissue (20).
Four isoforms of granulin, A, B, C and D, have been
isolated from human inflammatory cells. Granulin A was found to be
the most abundant and has been characterized in full using
microsequencing techniques. Partial amino-terminal sequences were
obtained for granulins B, C and D, and these sequences indicate
that all four human granulins are closely related. A fifth human
granulin, granulin F, has recently been isolated from urine
(21). In humans, the granulin
precursor is 593 amino acids long, and each of the five human
granulins that have been isolated as individual peptides is
represented in the common precursor. The human granulin gene is
located on chromosome 17, and the protein-coding region of the
granulin gene is constructed of 12 exons. The intronic splice sites
are positioned approximately in the middle of each granulin motif
such that, at the genetic level, the 12-cysteine motif is split
into two hemigranulin subdomains (22,23).
Also of interest are the surprising parallels between the granulin
and epidermal growth factor systems. There is no direct correlation
between epidermal growth factor receptor overexpression and
granulin-induced growth regulation. In a study of brain tumors it
was found that granulin mRNA was expressed predominantly in glial
cell tumors, while expression was not detected in non-tumor brain
tissues. This finding suggests that granulin may play a role in the
pathogenesis and/or malignant progression of primary brain tumors
(9).
In a previous study, the highest levels of granulin
were found in the placenta and the spleen, although levels were
also high in several reproductive tissues, most notably the ovary,
and also in the epidermis (24).
Granulin modulates the growth of epithelial and mesenchymal cells
in vitro, and high levels of expression have been found in
several types of cancers (8–10).
Overexpression of granulin has also been linked to the growth and
tumorigenicity of human breast carcinoma (11,12).
Previous studies have shown that granulin expression occurs
predominantly in epithelial cells, with little expression in
mesenchymal cells, muscle or endothelium (25). In this study, we also observed
granulin expression in meningiomas originating from mesenchymal
cells.
Granulin E promotes neuronal survival and enhances
neurite outgrowth in cultured neurons (26). The differential expression of
granulin in human gliomas was confirmed by Northern blot analysis,
which showed a transcript of 2.1-kb expressed in 86% (18 of 21) of
human gliomas. It is possible that granulin expression is mitigated
by radiation and/or is related to higher malignancy and tumor
progression (9). The results of
our study showed that granulin expression was correlated to tumor
size and PTBE development of intracranial meningiomas. This finding
suggests that granulin may affect the progression of meningioma as
it does in gliomas. However, one limitation of our study is that
granulin was investigated at the mRNA level, and additional studies
may be required to verify granulin expression at the protein
level.
In conclusion, we confirmed the expression of
granulin in intracranial meningiomas and found that its expression
is correlated to tumor size and PTBE volume. This information
provides a novel insight into the molecular biology of intracranial
meningiomas and suggests a potential target for management of
unresectable or malignant meningiomas. Further study is required to
verify the role of granulin in the molecular biology of
intracranial meningiomas.
Acknowledgements
This study was supported by the
research fund of Hanyang University (HY-2006-C).
References
|
1.
|
Cross M and Dexter TM: Growth factors in
development, transformation and tumorigenesis. Cell. 64:271–284.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Morrison RS, Jarell AD and Schuster JM:
Growth factors and brain tumors. Youmans Neurological Surgery. Winn
HR: Saunders; Philadelphia: pp. 725–738. 2004
|
|
3.
|
Lingood RM, Hsu DW, Efird JT and Pardo FS:
TGF alpha expression in meningioma-tumor progression and
therapeutic response. J Neurooncol. 26:45–51. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Braun B, Lange M, Oeckler R and Mueller
MM: Expression of G-CSF and GM-CSF in human meningiomas correlates
with increased tumor proliferation and vascularization. J
Neurooncol. 68:131–140. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lamszus K, Lengler U, Schmidt NO, Stavrou
D, Ergün S and Westphal M: Vascular endothelial growth factor,
basic fibroblast growth factor and placenta growth factor in human
meningiomas and their relation to angiogenesis and malignancy.
Neurosurgery. 46:938–948. 2000.PubMed/NCBI
|
|
6.
|
Bateman A and Bennett HP: The granulin
gene family from cancer to dementia. Bioassay. 31:1245–1254. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Bateman A, Belcourt D, Benett HPJ, Hazure
C and Solomon S: Granulins, a novel class of peptides from
leukocytes. Biochem Biophys Res Commun. 173:1161–1168. 1993.
View Article : Google Scholar
|
|
8.
|
Donald CD, Laddu A, Chandham P, et al:
Expression of progranulin and the epithelin/granulin precursor
acrogranin correlates with neoplastic state in renal epithelium.
Anticancer Res. 21:3739–3742. 2001.PubMed/NCBI
|
|
9.
|
Liau LM, Lallone RL, Seitz RS, et al:
Identification of a human glioma-associated growth factor gene,
granulin, using differential immuno-absorption. Cancer Res.
60:1353–1360. 2000.PubMed/NCBI
|
|
10.
|
Line AA, Stengrevics Z, Slucka GL,
Jankevics E and Rees RC: Serological identification and expression
analysis of gastric cancer-associated genes. Br J Cancer.
86:1824–1830. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Lu R and Serrero G: Inhibition of PC
cell-derived growth factor (PCDGF, epithelin/granulin precursor)
expression by antisense PCDGF cDNA transfection inhibits
tumorigenicity of the human breast carcinoma cell line MDA-MB-468.
Proc Natl Acad Sci USA. 97:3993–3998. 2000. View Article : Google Scholar
|
|
12.
|
Lu R and Serrero G: Stimulation of PC
cell-derived growth factor (epithelin/granulin precursor)
expression by estradiol in human breast cancer cell. Biochem
Biophys Res Commun. 256:204–207. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Otsuka S, Tamiya T, Ono Y, et al: The
relationship between peritumoral edema and the exression of
vascular endothelial growth factor and its receptors in
intracranial meningiomas. J Neurooncol. 70:349–357. 2004.
View Article : Google Scholar
|
|
14.
|
Haddad GF, Al-Mefty O and Abdulrauf SI:
Meningiomas. Youmans Neurological Surgery. Winn HR: Saunders;
Philadelphia: pp. 1099–1131. 2004
|
|
15.
|
Davidson B, Alejandro E, Florenes VA,
Goderstad JM, Kristensen GB, Trope CG and Kohn EC:
Granulin-epithelin precursor is a novel prognostic marker in
epithelial ovarian carcinoma. Cancer. 100:2139–2147. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Davidson B, Risberg R, Reich R and Berner
A: Effusion cytology in ovarian cancer – new molecular methods as
aids to diagnosis and prognosis. Clin Lab Med. 23:729–754.
2003.
|
|
17.
|
He Z and Bateman A: Progranulin
(granulin-epithelin precursor, PC-cell derived growth factor,
acrogranin) mediates tissue repair and tumorigenesis. J Mol Med.
81:600–612. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Jemal H, Murray T, Samuels A, Ghafoor A,
Ward E and Thun MJ: Cancer Statistics 2003. CA Cancer J Clin.
53:5–26. 2003. View Article : Google Scholar
|
|
19.
|
Ong CHP and Bateman A: Progranulin
(granulin-epithelin precursor, PC-cell derived growth factor,
acrogranin) in proliferation and tumorigenesis. Histol Histopathol.
18:1275–1288. 2003.PubMed/NCBI
|
|
20.
|
Hoque M, Young TM, Lee CG, Serrero G,
Mathews MB and Pe’ery T: The growth factor granulin interacts with
cyclin T1, and modulates p-TEFb-dependent transcription. Mol Cell
Biol. 23:1688–1702. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Sparro G, Galdenzi G, Eleuteri AM,
Angeletti M, Schroeder W and Fioretti E: Isolation and N-terminal
sequence of multiple forms of granulins in human urine. Protein
Expr Purif. 10:169–174. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Bhandari V and Bateman A: Structure and
chromosomal location of the human granulin gene. Biochem Biophys
Res Commun. 188:57–63. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Baba T, Hoff HB III, Nemoto H, Lee H, Orth
J, Arai Y and Gertan GL: Acrogranin, an autosomal cysteine-rich
glycoprotein, is the precursors of the growth-modulating peptides,
granulin and epithelins and is expressed in somatic as well as male
germ cells. Mol Reprod Dev. 34:233–243. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Bhandari V, Giad A and Bateman A: The cDNA
structure, tissue distribution and cellular localization of the rat
granulin precursors: a novel growth factor-like protein.
Endocrinology. 133:2682–2689. 1993.PubMed/NCBI
|
|
25.
|
Bhandari V, Palfree RG and Bateman A:
Isolation and sequence of the granulin precursor cDNA from human
bone marrow reveals tandem cysteine-rich granulin domains. Proc
Natl Acad Sci USA. 89:1715–1719. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Van Damme P, van Hoecke A, Lambrechts D,
Vanacker P, Bogaert E, van Swieten J, Carmeliet P, van Den Bosch L
and Robberecht W: Progranulin functions as a neurotrophic factor to
regulate neurite outgrowth and enhance neuronal survival. J Cell
Biol. 181:37–41. 2008.PubMed/NCBI
|