Introduction
In the US, an estimated 57,760 patients were
diagnosed with renal cell carcinoma (RCC) in 2009, of which 12,980
patients succumbed to the disease (1). The prognosis for RCC patients with
progressive or recurring disease is particularly poor. RCC is
relatively resistant to radiotherapy and chemotherapy, and only a
minority of patients respond to specific immunotherapy with
interleukin-2 and interferon-α (2). Recently, anti-angiogenic therapy
targeting the angiogenesis pathway was approved for the treatment
of advanced stage RCC patients (3–5).
Although this treatment appears to be promising, there remains a
lack of curative therapy for advanced stage RCC, and currently
almost all patients with this condition are incurable. Recent
advances in genetic and molecular biology have led to the use of
novel approaches, such as gene and virus therapy, for the treatment
of RCC. Adenovirus serotype 5 (Ad5) vectors have been traditionally
used in pre-clinical experiments and clinical trials (6). Ad5 vectors mediate their attachment
and entry into cells using the Coxsackie and adenovirus receptor
(CAR) (7). Renal carcinoma cell
lines generally express low levels of CAR; as a result Ad5 viruses
have a low transduction efficacy in these cells (8).
In order to overcome the low transduction efficacy
of Ad5 in cells lacking CAR, several researchers have developed
fiber-modified Ad vectors with a broader tropism (9). Adenovirus serotype 35 (Ad35) vectors
do not interact with CAR, but instead bind to CD46 receptors (also
known as MCP-membrane co-factor protein), which are ubiquitously
expressed in almost all human cells (10–12).
Ad vector containing chimeric type 5 and type 35 fiber proteins
(Ad5/F35) is capable of CAR-independent tropism in target cells
expressing CD46 on the membrane. Several groups have investigated
the utility of Ad5/F35 for the development of transduction efficacy
in cells lacking CAR (13–17). In this study, we evaluated the
ability of the Ad5/F35 vector to transfer genes to several human
renal cell lines, in comparison to the Ad5 vector.
Materials and methods
Cell lines and cell culture
Established cell lines derived from human renal
carcinoma cell lines, namely ACHN and Caki-1, were obtained from
the Cell Resource Center for Biomedical Research Institute of
Development, Aging and Cancer, Tohoku University (Sendai, Japan).
The RCC4-VHL human renal carcinoma cell line was purchased from the
European Collection of Animal Cell Cultures (ECACC; Salisbury, UK).
The 786-O human renal carcinoma cell line was purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA). The
HEK293 cells were purchased from the RIKEN Bioresource Center
(Tsukuba, Japan).
Caki-1 and 786-O cells were maintained in RPMI-1640
medium (Life Technologies, Inc., Gaithersburg, MD, USA) containing
10% fetal bovine serum (FBS) and antibiotics (50 μg/ml streptomycin
sulfate and 50 IU/ml penicillin). RCC4-VHL and HEK293 cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM; Nacalai
Tesque, Inc., Kyoto, Japan) containing 10% FBS, antibiotics (50
μg/ml streptomycin sulfate and 50 IU/ml penicillin), 2 mM glutamine
and 0.5 g/l geneticin. ACHN were maintained in modified Eagle’s
medium (MEM; Nacalai Tesque, Inc.) containing 10% FBS, 2 mM
glutamine, 10 mM non-essential amino acids and antibiotics (50
μg/ml streptomycin sulfate and 50 IU/ml penicillin). All cell lines
were maintained at 37°C in a humidified incubator with a 5%
CO2 atmosphere and 97% relative humidity, and were
sub-cultured on reaching 80% confluence using 1 mmol/l EDTA-0.025%
trypsin for 3–5 min. The cells were transferred 2–3 times/week into
fresh growth medium.
In vitro real-time quantitative reverse
transcription-PCR assay
Total cellular RNA was isolated from all cell lines
using a TaKaRa RNA extraction kit (Parex), and was reverse
transcribed using a reverse transcription kit (TaKaRa RNA PCR kit
Ver. 3.0) following the manufacturer’s protocol. The resulting cDNA
was amplified with CAR, CD46 and GAPDH sequence-specific primers
(40 cycles, 95°C for 15 sec, 60°C for 1 min) using TaqMan chemistry
in the StepOnePlus Real-Time PCR System v2.0 (Applied Biosystems).
Table I shows the sequences of the
TaqMan probes and primers for CAR, CD46 and GAPDH. All
primers/probes were purchased from Biosearch Technologies
Japan.
 | Table I.Primers and probes. |
Table I.
Primers and probes.
| CAR | |
| Forward primer |
5′-CAGAAGCTACATCGGCAGTAATCA-3′ |
| Reverse primer |
5′-CTCTGAGGAGTGCGTTCAAAGTC-3′ |
| Probe | 5′-d
FAM-TCCATGTCTCCTTCCAACATGGAAGGA-TAMRA-3′ |
| CD46 | |
| Forward primer |
5′-GGTGTTGCTGCTGTACTCCTTCT-3′ |
| Reverse primer |
5′-CCAATGAGCTCCATAGCTTCAA-3′ |
| Probe | 5′-d
FAM-CGATGCCTGTGAGGAGCCACCAAC-BHQ-1-3′ |
| GAPDH | |
| Forward primer |
5′-GAAGGTGAAGGTCGGAGTC-3′ |
| Reverse primer |
5′-GAAGATGGTGATGGGATTTC-3′ |
| Probe | 5′-d
FAM-CAAGCTTCCCGTTCTCAGCC-BHQ-1-3′ |
Adenovirus vector preparation
The transduction efficiency of Ad5-LacZ and
Ad5/F35-LacZ, chimeric type 5 and type 35 fiber proteins expressing
LacZ driven by the cytomegalovirus promoter, were tested (15). Vectors were purified through two
rounds of CsCl gradient ultracentrifugation using standard methods.
Serial dilutions of the viruses were used to infect HEK293 cells
for a plaque assay. The infectious titer of the purified Ad vectors
was determined by triplicate 50% tissue culture infective dose
(TCID50) assays using HEK293 cells, and expressed as plaque-forming
units (pfU)/ml.
Transduction efficacy of adenovirus
vectors
In order to determine transduction efficacy in each
cell line, 1×105 cells were prepared in a 24-well plate
and infected with Ad5-LacZ or Ad5/F35-LacZ. After 48 h, the
transduction efficacy was assessed by β-galactosidase (β-gal)
staining and expressed as blue titer units (btU)/ml.
Statistical analysis
Statistical significance was determined using ANOVA
and Bonferroni correction. A p-value of <0.05 was considered
statistically significant.
Results
Relative quantification of CAR and CD46
mRNA expression
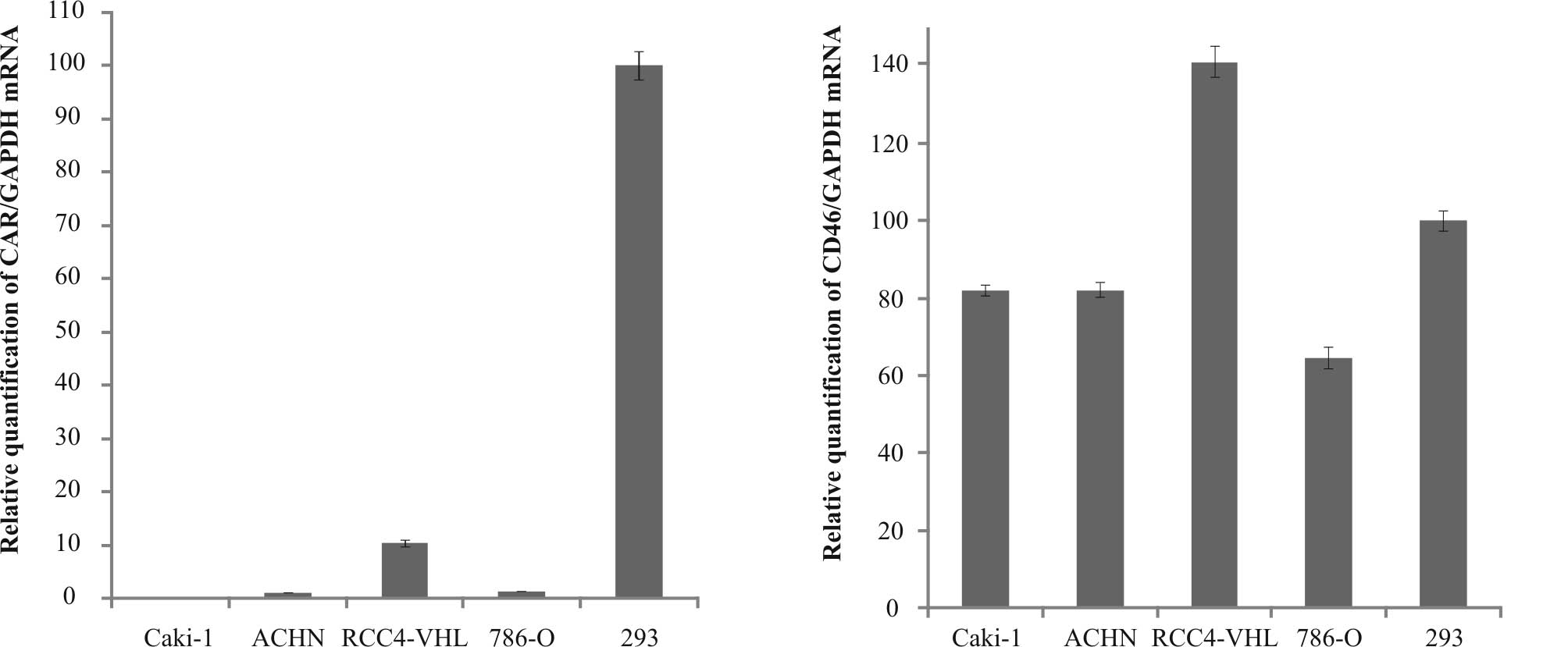
Fig. 1 shows the
mean relative quantifications of CAR and CD46 mRNA expression as
detected in the cell lines. In order to normalize for differences
in the quantity of total RNA, GAPDH was selected as the endogenous
RNA control. HEK293 cells were selected as the positive control.
Relative quantification was calculated with the value obtained from
HEK293 cells set to 100%. Levels of CAR mRNA expression were
considerably higher in the RCC4-VHL cell line compared to the
Caki-1 (>100-fold), ACHN (>10-fold) and 786-O (8-fold) cell
lines. However, the level of CAR mRNA expression in the RCC4-VHL
cells was still much lower (∼0.1-fold) than that in the HEK293 cell
line. The levels of CD46 mRNA in all the RCC cell lines were
relatively high compared to the expression of CAR mRNA. The
relative quantification of CD46 mRNA expression in the Caki-1, ACHN
and 786-O cell lines was 81.8, 82.1 and 64.6%, respectively.
Expression was higher (141.1%) in the RCC4-VHL cells than in the
HEK293 cells.
Transduction efficacy of adenovirus
vectors
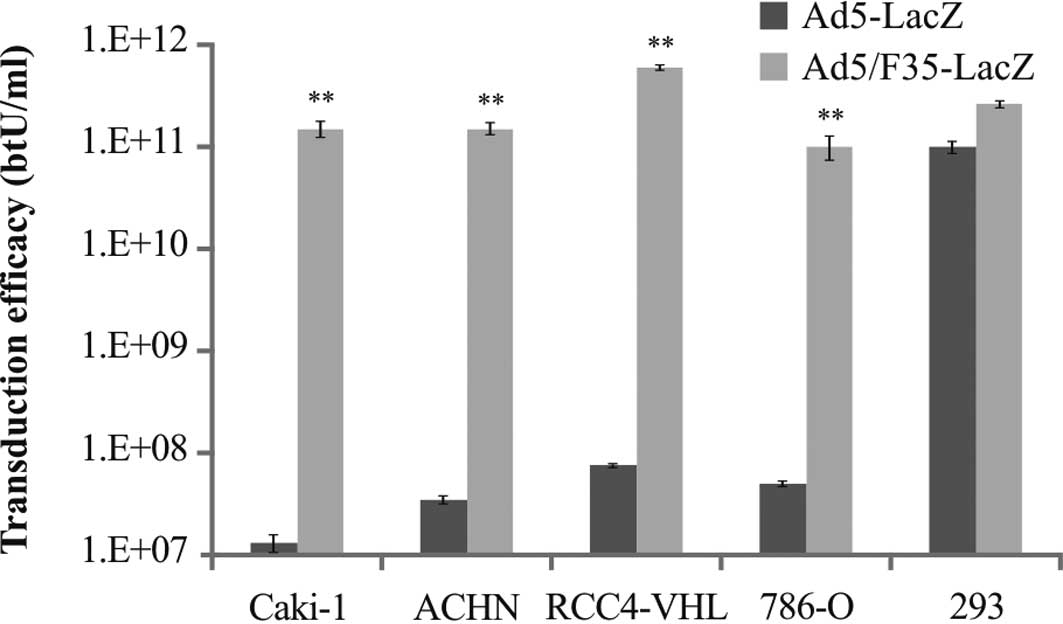
In order to assess transduction efficacy in all the
cell lines, both Ad5-LacZ and Ad5/F35-LacZ were utilized. The
transduction efficacy of Ad5-LacZ in Caki-1, ACHN, RCC4-VHL and
786-O cells was 1.3×107, 3.5×107,
7.5×107 and 5×107 btU/ml, respectively. By
contrast, the transduction efficacy of Ad5/F35-LacZ in Caki-1,
ACHN, RCC4-VHL and 786-O cells was 1.9×1011,
1.8×1011, 6.6×1011 and 6.0×1010
btU/ml, respectively (Fig. 2). The
transduction efficacy of Ad5/F35-LacZ was significantly higher in
all the RCC cell lines compared to that of Ad5-LacZ
(p<0.01).
Discussion
Gene therapy using Ad5 vector is a promising
therapeutic strategy for the treatment of various types of cancer.
However, this approach has yet to be developed for use in cases of
RCC, possibly due to the low transduction efficiency of the Ad5
vector in these cells. To date, only 12 protocols for the gene
therapy of RCC have been applied in a clinical setting, and only
one of these used the Ad5 vector. By contrast, more than 990
protocols have been described for other cancers (Gene Therapy
Clinical Trials Worldwide, 2009; http://www.wiley.co.uk/genetherapy/clinical/).
In the present study, we attempted to increase the
transduction efficiency of the Ad vector in renal carcinoma cells,
and thus evaluated a newly developed adenovirus vector,
Ad5/F35-LacZ. This vector is capable of CAR-independent tropism in
target cells expressing CD46 on the membrane. The results revealed
that the transduction efficiency of Ad5/F35-LacZ in each of the
tested renal carcinoma cell lines was significantly higher than
that of the Ad5 vector (Fig. 2),
and almost paralleled the relative quantifications of CD46 mRNA
expression (Fig. 1B).
Previously, we examined the transduction efficiency
of Ad5 vector containing the RGD motif in the HI loop of the Ad
fiber knob (Ad-RGD vector) (18).
This vector is capable of CAR-independent tropism in target cells
expressing αvβ3 and/or αvβ5 integrin on the membrane (19). Also, this vector was expected to
have a high efficacy in renal carcinoma cells due to the
overexpression of αvβ3 integrin in these cells (20). We demonstrated a significant
increase in transduction efficacy of 125- to 1,800-fold in human
RCC cell lines compared to the transduction efficacy associated
with the Ad5 vector (18). The
results also showed that the transduction efficacy of Ad5/F35-LacZ
was significantly increased by 8.3-, 10-, 37.5- and 20-fold in the
ACHN, Caki-1, RCC4-VHL and 786-O RCC cells, respectively, compared
to the Ad-RGD vector. Previous investigators have demonstrated an
antitumor effect in various cancer cell lines using an oncolytic
Ad5/F35 vector (21,22). The transduction efficacy of the
Ad5/F35 vector in the cell lines used in these studies was higher
than that of the Ad5 vector. In agreement with these studies, our
findings suggest that, in RCC, an oncolytic Ad5/F35 vector
containing the E1a gene controlled by a RCC-specific
promoter may achieve a greater antitumor effect than gene therapy
using a conventional Ad5 vector.
In this study, we demonstrated a marked increase in
the transduction efficacy of renal carcinoma cells using an Ad
vector containing chimeric type 5 and type 35 fiber proteins. It
may therefore be preferable to use the Ad vector described in this
study to target renal carcinoma cells. The application of our
findings may lead to more effective novel gene therapy approaches
to renal cell carcinoma.
Acknowledgements
The authors wish to thank Satoko
Kodama for help in the completion of the manuscript.
References
|
1.
|
American Cancer Society: Cancer Facts and
Figures 2009. American Cancer Society; Atlanta: 2009
|
|
2.
|
Coppin C, Porzsolt F, Awa A, Kumpf J,
Coldman A and Wilt T: Immunotherapy for advanced renal cell cancer.
Cochrane Database Syst Rev. 1:CD0014252005.
|
|
3.
|
Castellano D, Del Muro XG, Pérez-Gracia
JL, González-Larriba JL, Abrio MV, Ruiz MA, Pardo A, Guzmán C,
Cerezo SD and Grande E: Patient-reported outcomes in a phase III,
randomized study of sunitinib versus interferon-α as first-line
systemic therapy for patients with metastatic renal cell carcinoma
in a European population. Ann Oncol. 20:1803–1812. 2009.PubMed/NCBI
|
|
4.
|
Rini BI, Halabi S, Taylor J, Small EJ and
Schilsky RL; Cancer and Leukemia Group B: Cancer and Leukemia Group
B 90206: a randomized phase III trial of interferon-alpha or
interferon-alpha plus anti-vascular endothelial growth factor
antibody (bevacizumab) in metastatic renal cell carcinoma. Clin
Cancer Res. 10:2584–2586. 2004. View Article : Google Scholar
|
|
5.
|
Yang JC, Haworth L, Sherry RM, Hwu P,
Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX and
Rosenberg SA: A randomized trial of bevacizumab, an anti-vascular
endothelial growth factor antibody, for metastatic renal cancer. N
Engl J Med. 349:427–434. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hamilton MM, Byrnes GA, Gall JG, Brough
DE, King CR and Wei LL: Alternate serotype adenovector provides
long-term therapeutic gene expression in the eye. Molecular Vision.
14:2535–2546. 2008.PubMed/NCBI
|
|
7.
|
Bergelson JM, Cunningham JA, Droguett G,
Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowel RL and
Finberg RW: Isolation of a common receptor for Coxsackie B viruses
and adenoviruses 2 and 5. Science. 275:1320–1323. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Okegawa T, Sayne JR, Nutahara K, Pong RC,
Saboorian H, Kabbani W, Higashihara E and Hsieh JT: A histone
deacetylase inhibitor enhances adenoviral infection of renal cancer
cells. J Urol. 177:1148–1156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Mizuguchi H and Hayakawa T: Targeted
adenovirus vectors. Hum Gene Ther. 15:1034–1044. 2004. View Article : Google Scholar
|
|
10.
|
Gaggar A, Shayakhmetov DM and Lieber A:
CD46 is a cellular receptor for group B adenoviruses. Nat Med.
9:1408–1412. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Segerman A, Atkinson JP, Marttila M,
Dennerquist V, Wadell G and Arnberg N: Adenovirus type 11 uses CD46
as a cellular receptor. J Virol. 77:9183–9191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Marttila M, Persson D, Gustafsson D,
Liszewski MK, Atkinson JP, Wadell G and Arnberg N: CD46 is a
cellular receptor for all species B adenoviruses except types 3 and
7. J Virol. 79:14429–14436. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Toyoda E, Doi R, Kami K, Mori T, Ito D,
Koizumi M, Kida A, Nagai K, Ito T, Masui T, Wada M, Tagawa M and
Uemoto S: Adenovirus vectors with chimeric type 5 and 35 fiber
proteins exhibit enhanced transfection of human pancreatic cancer
cells. Int J Oncol. 33:1141–1147. 2008.PubMed/NCBI
|
|
14.
|
Yu L, Shimozato O, Li Q, Kawamura K, Ma G,
Namba M, Ogawa T, Kaiho I and Tagawa M: Adenovirus type 5
substituted with type 11 or 35 fiber structure increases its
infectivity to human cells enabling dual gene transfer in
CD46-dependent and independent manners. Anticancer Res.
27:2311–2316. 2007.PubMed/NCBI
|
|
15.
|
Mizuguchi H and Hayakawa T: Adenovirus
vectors containing chimeric type 5 and type 35 fiber proteins
exhibit altered and expanded tropism and increase the size limit of
foreign genes. Gene. 20:69–77. 2002. View Article : Google Scholar
|
|
16.
|
Ni S, Gaggar A, Di Paolo N, Li ZY, Liu Y,
Strauss R, Sova P, Morihara J, Feng Q, Kiviat N, Touré P, Sow PS
and Lieber A: Evaluation of adenovirus vectors containing serotype
35 fibers for tumor targeting. Cancer Gene Ther. 13:1072–1081.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yu L, Takenobu H, Shimozato O, Kawamura K,
Nimura Y, Seki N, Uzawa K, Tanzawa H, Shimada H, Ochiai T and
Tagawa M: Increased infectivity of adenovirus type 5 bearing type
11 or type 35 fibers to human esophageal and oral carcinoma cells.
Oncol Rep. 14:831–835. 2005.PubMed/NCBI
|
|
18.
|
Terao S, Acharya B, Suzuki T, Aoi T, Naoe
M, Hamada K, Mizuguchi H and Gotoh A: Improved gene transfer into
renal carcinoma cells using adenovirus vector containing RGD motif.
Anticancer Res. 29:2997–3002. 2009.PubMed/NCBI
|
|
19.
|
Koizumi N, Mizuguchi H, Kondoh M, Fujii M,
Nakanishi T, Utoguchi N and Watanabe Y: Efficient gene transfer
into differentiated human trophoblast cells with adenovirus vector
containing RGD motif in the fiber protein. Biol Pharm Bull.
29:1297–1299. 2006. View Article : Google Scholar
|
|
20.
|
Wechsel HW, Petri E, Feil G, Nelde HJ,
Bichler KH and Loesr W: Renal cell carcinoma: immunohistological
investigation of expression of the integrin alpha v beta 3.
Anticancer Res. 19:1529–1532. 1999.PubMed/NCBI
|
|
21.
|
Chen L, Chen D, Gong M, Na M, Li L, Wu H,
Jiang L, Qian Y, Fang G and Xue X: Concomitant use of Ad5/35
chimeric oncolytic adenovirus with TRAIL gene and taxol produces
synergistic cytotoxicity in gastric cancer cells. Cancer Lett.
284:141–148. 2009. View Article : Google Scholar
|
|
22.
|
Toivonen R, Suominen E, Grenman R and
Savontaus M: Retargeting improves the efficacy of a
telomerase-dependent oncolytic adenovirus for head and neck cancer.
Oncol Rep. 21:165–171. 2009.PubMed/NCBI
|