Introduction
There is increasing evidence that the stroma is
involved in the growth and metastasis of several types of cancer,
including gastric (1), breast
(2,3) and prostate (4) cancer. The stroma consists of a
variety of components, including fibroblasts, macrophages, and the
extracellular matrix (5,6). Among these cells, fibroblasts
constitute a major stromal compartment and play a critical role in
the regulation of tumor growth. Among the various types of
fibroblasts, myofibroblasts, which are distinct from normal
fibroblasts in their expression of both vimentin and α-smooth
muscle actin (α-SMA), have recently been implicated to have
important functions in epithelial solid tumor biology, such as
neoplastic progression, tumor growth and metastasis in vitro
(7–9). De Wever et al emphasized the
myofibroblast as a driver of invasive cancer growth; the role of
myofibroblasts in tissue development was also proposed (10).
Scirrhous gastric cancer cells proliferate and
extensively invade the submucosa in the gastric submucosa
accompanied by abundant fibrosis (11). Interactions have been reported to
exist between scirrhous gastric cancer cells and orthotopic
fibroblasts, thus suggesting that the proliferation of scirrhous
gastric carcinoma is related to growth factor production by gastric
fibroblasts (12). Myofibroblasts
have also been shown to be present in gastric cancer, but their
histogenesis remains unclear. Despite increasing reports
illustrating the role of tumor-stroma in cancer progression, no
consensus has been reached regarding whether myofibroblasts
regulate tumor development positively or negatively. Few reports of
clinical studies of scirrhous gastric cancer discuss the
significance of myofibroblasts. Therefore, the present study was
performed to investigate the significance of myofibroblast
expression in gastric carcinomas.
Materials and methods
Clinical materials
A total of 265 patients who had undergone resection
of a primary gastric tumor at our institute were enrolled in this
study. Tumor specimens were fixed in 10% formaldehyde solution and
embedded in paraffin. Sections (4-μm) were cut and mounted on glass
slides. The pathologic diagnoses and classifications were made
according to the Japanese Classification of Gastric Carcinoma
(13). The median follow-up time
for all 265 patients was 58 months (range, 1–177 months). The
median follow-up time for the patients that succumbed to the
disease was 25 months (n=88) compared with 75 months for surviving
patients (n=177). Thirty-one patients were lost during more than 60
months of follow-up. Kaplan-Meier overall survival curves were
calculated from the date of surgery.
Antibodies and reagents
A mouse monoclonal antibody which recognizes α-SMA
(clone 1A4) and a mouse monoclonal antibody which recognizes
vimentin (clone Vim 3B4) were purchased from DakoCytomation
(Cambridge, UK). Normal rabbit serum, normal mouse immunoglobulin
G, biotinylated rabbit anti-mouse immunoglobulin G,
streptavidin-peroxidase reagent and diaminobenzidine were purchased
from Nichirei Corp. (Tokyo, Japan).
Immunohistochemical techniques
Since there is no myofibroblast-specific
immunocytochemical marker, characterization of human
tumor-associated myofibroblasts is based on a combination of
positive markers such as vimentin and α-SMA. The methods for the
immunohistochemical determination of α-SMA and vimentin are
described in detail in the manufacturer's instructions. Briefly,
the slides were deparaffinized in xylene and hydrated in decreasing
concentrations of ethyl alcohol. The tissues were heated for 20 min
at 105°C and at 0.4 kg/cm2 by autoclave in Target
Retrieval Solution (Dako Co., Carpinteria, CA). The sections were
then dewaxed and incubated with 3% hydrogen peroxide v/v in
methanol for 15 min to block endogenous peroxidase activity. Next,
the sections were washed in phosphate-buffered saline (PBS) and
incubated in 10% normal rabbit serum v/v for 10 min to reduce
non-specific antibody binding. The specimens were incubated with
α-SMA antibodies (1:200) or vimentin antibodies (1:200) for 1 h at
room temperature followed by three washes with PBS. Sections were
incubated with biotinylated rabbit anti-mouse immunoglobulin G for
30 min, followed by three washes with PBS. Slides were treated with
streptavidin-peroxidase reagent for 15 min and washed with PBS
three times. Finally, the slides were incubated in PBS
diaminobenzidine and 1% hydrogen peroxide v/v for 20 sec,
counterstained with Mayer’s hematoxylin and mounted.
Immunohistochemical determination of
α-smooth muscle actin and vimentin
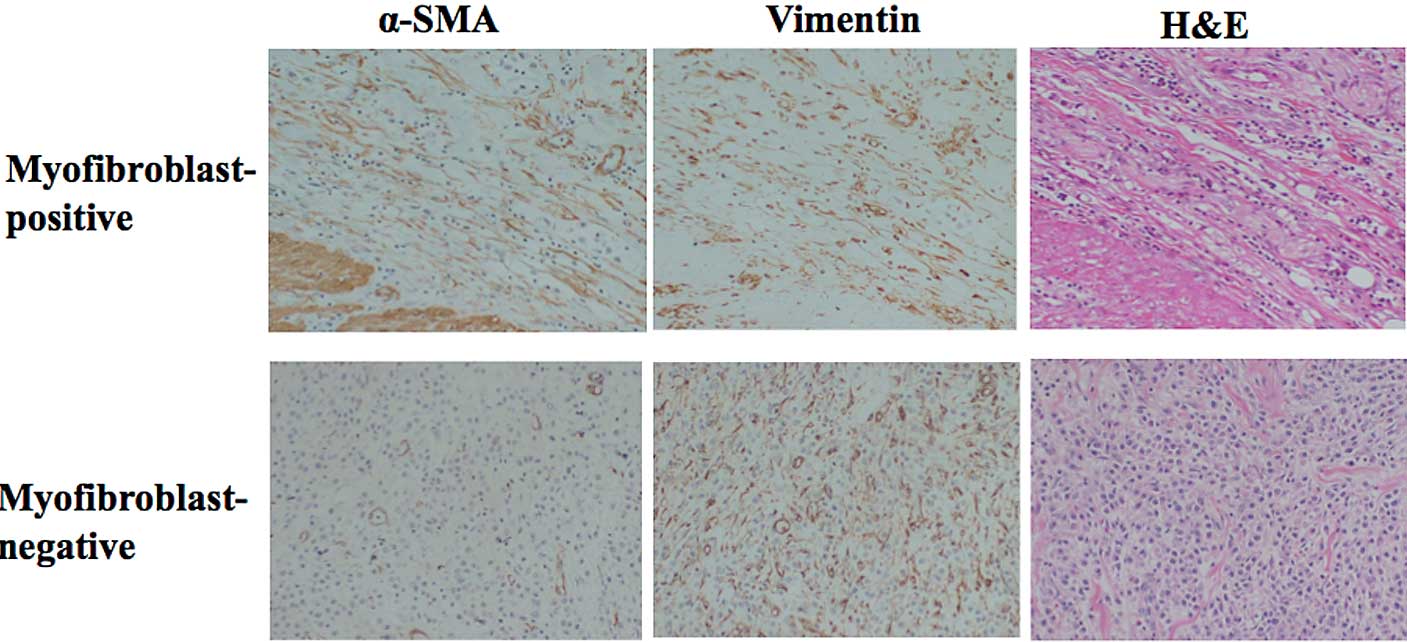
The tumor specimens showed various staining patterns
against the anti-α-SMA and anti-vimentin antibodies.
Vimentin-positive stromal cells were considered to be fibroblasts.
Myofibroblasts were defined as fibroblasts which were positive for
α-SMA staining. Smooth muscle was defined as being α-SMA-positive
and vimentin-negative. The myofibroblast expression level was
semi-quantitatively analyzed according to the percentage of
fibroblasts showing α-SMA positivity: 0, 0%; 1+, 1–24%; 2+, 25–49%;
3+, ≥50%. Myofibroblast expression was considered positive when
scores were ≥2+, and negative when scores were ≤1+ (Fig. 1). The slides were interpreted by
two investigators without knowledge of the corresponding
clinicopathological data.
Statistical analysis
The χ2 test was used to determine the
significance of the differences between the covariates. Survival
durations were calculated using the Kaplan-Meier method and were
analyzed by the log-rank test to compare the cumulative survival
durations in the patient groups. The Cox proportional hazards model
was used to compute univariate and multivariate hazards ratios for
the study parameters. For all tests, a p-value <0.05 was defined
as statistically significant. The SPSS software program (SPSS
Japan, Tokyo, Japan) was used for the analyses.
Results
Correlation between the
clinicopathological factors and myofibroblast expession
Myofibroblast expression was positive in 92 (35%) of
the 265 gastric carcinoma specimens, in 16 (13%) of the 119 early
stage gastric carcinoma specimens, and in 76 (52%) of the 146
advanced gastric carcinoma specimens. The relationships between
myofibroblast positivity and clinicopathological features of the
tumors are shown in Table I. A
significantly (p<0.001) high frequency of myofibroblast
positivity was observed in the advanced gastric cancers (76 of 146)
in comparison to the early stage cancers (16 of 119). Therefore, a
statistically significant correlation was found between
myofibroblast expression and scirrhous type gastric cancer
(p<0.001). Myofibroblast positivity was also significantly
present in patients with lymph node metastasis (p<0.001),
positive cytology (p=0.005) and lymphatic invasion (p<0.001).
‘Cytology’ is defined as peritoneal lavage cytology at laparotomy
as a standard method for the detection of free tumor cells. There
was no statistically significant association between myofibroblast
positivity and histological type, peritoneal dissemination and
venous invasion.
 | Table I.Correlation between
clinicopathological factors and myofibroblast expression. |
Table I.
Correlation between
clinicopathological factors and myofibroblast expression.
| Myofibroblast
expressiona
| |
|---|
| Clinicopathological
factors | Positive n=92
(35%) | Negative n=173
(65%) | p-value |
|---|
| Invasion depth | | | |
| Early stage
cancer | 16 (13%) | 103 (87%) | |
| Advanced
cancer | 76 (52%) | 70 (48%) | <0.001 |
| Macroscopic
typeb | | | |
| Type 0, 1, 2,
3 | 65 (29%) | 157 (71%) | |
| Type 4 (scirrhous
type) | 27 (63%) | 16 (37%) | <0.001 |
| Histological
type | | | |
| Differentiated | 34 (30%) | 79 (70%) | |
|
Undifferentiated | 58 (38%) | 94 (62%) | 0.172c |
| Peritoneal
metastasis | | | |
| Positive | 12 (57%) | 9 (43%) | |
| Negative | 80 (33%) | 164 (67%) | 0.024 |
| Venous invasion | | | |
| Positive | 25 (45%) | 31 (55%) | |
| Negative | 67 (32%) | 142 (68%) | 0.079 |
| Lymph node
metastasis | | | |
| Positive | 57 (51%) | 54 (49%) | |
| Negative | 35 (23%) | 119 (77%) | <0.001 |
| Lymphatic
invasion | | | |
| Positive | 56 (48%) | 60 (52%) | |
| Negative | 36 (24%) | 113 (76%) | <0.001 |
| Cytologyc | | | |
| Positive | 19 (56%) | 15 (44%) | |
| Negative | 73 (32%) | 158 (68%) | 0.005 |
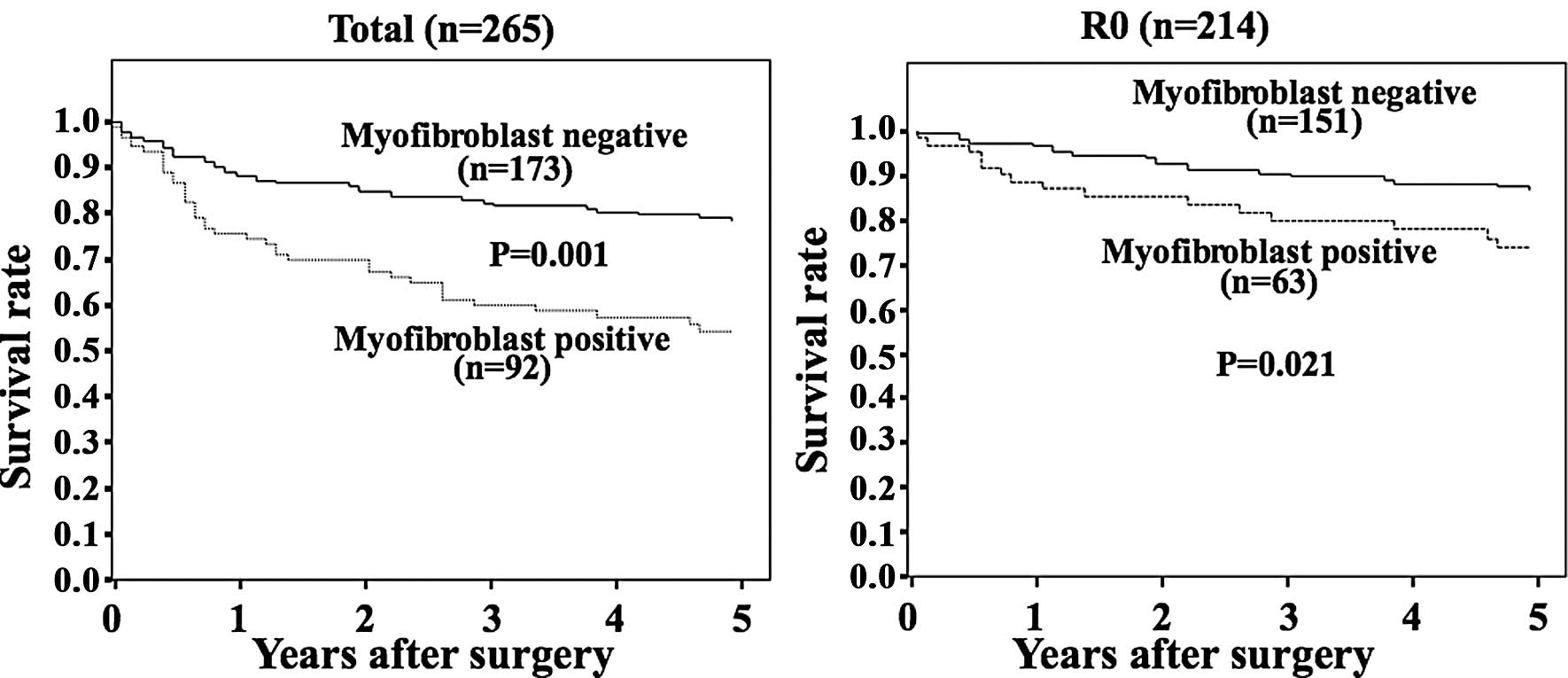
Survival
The prognosis of patients with tumors positive for
myofibroblast expression was significantly (p<0.001) worse than
that of patients with tumors negative for myofibroblast expression
(Fig. 2A). The 5-year survival of
the patients with myofibroblast-positive tumors was 57% in
comparison to 79% for those patients with negative tumors. The
prognosis of patients who underwent a curative resection (R0) with
myofibroblast-positive tumors was significantly (p<0.0210) worse
than the prognosis of patients with tumors negative for
myofibloblast expression (Fig.
2B). Univariate analysis revealed that myofibroblast expression
(p=0.003), advanced cancer (p<0.001), peritoneal dissemination
(p<0.001), venous invasion (p=0.042) and lymph node metastasis
(p<0.001) were significantly correlated with patient survival
(Table II). Multivariate analysis
indicated that the macroscopic type and peritoneal dissemination
were independent prognostic factors (Table III), while myofibroblast expression
(p=0.290) was not an independent prognostic factor.
 | Table II.Univariate analysis with respect to
overall survival in gastric cancer. |
Table II.
Univariate analysis with respect to
overall survival in gastric cancer.
| Parameter | Risk ratio | 95% CI | p-value |
|---|
| Myofibroblast
expression | | | |
| Positive vs.
negative | 1.482 | 1.146–1.917 | 0.003 |
| Invasion depth | | | |
| Early stage vs.
advanced cancer | 1.692 | 1.323–2.163 | <0.001 |
| Macroscopic type | | | |
| Type 1, 2, 3
vs. | 3.366 | 2.389–4.742 | <0.001 |
| Type 4 (scirrhous
type) | | | |
| Histological
type | | | |
| Diffuse vs.
intestinal | 1.084 | 0.848–1.386 | 0.520 |
| Peritoneal
metastasis | | | |
| Positive vs.
negative | 6.462 | 3.926–10.638 | <0.001 |
| Cytology | | | |
| Positive vs.
negative | 4.417 | 3.002–6.501 | <0.001 |
| Venous invasion | | | |
| Positive vs.
negative | 1.363 | 1.011–1.838 | 0.042 |
| Lymph node
metastasis | | | |
| Positive vs.
negative | 1.911 | 1.490–2.450 | <0.001 |
| Lymphatic
invasion | | | |
| Positive vs.
negative | 1.663 | 1.299–2.130 | <0.001 |
 | Table III.Multivariate analysis with respect to
overall survival in gastric cancer. |
Table III.
Multivariate analysis with respect to
overall survival in gastric cancer.
| Parameter | Risk ratio | 95% CI | p-value |
|---|
| Myofibroblast
expression | | | |
| Positive vs.
negative | 1.076 | 0.664–1.744 | 0.766 |
| Invasion depth | | | |
| Early stage vs.
advanced cancer | 3.366 | 1.401–8.088 | 0.007 |
| Macroscopic
type | | | |
| Type 1, 2, 3
vs. | 4.344 | 2.136–8.838 | <0.001 |
| Type 4 (scirrhous
type) | | | |
| Histological
type | | | |
| Diffuse vs.
intestinal | 1.118 | 0.586–2.123 | 0.735 |
| Peritoneal
metastasis | | | |
| Positive vs.
negative | 2.184 | 1.162–4.105 | 0.015 |
| Venous
invasion | | | |
| Positive vs.
negative | 1.543 | 0.869–2.741 | 0.139 |
| Lymph node
metastasis | | | |
| Positive vs.
negative | 1.308 | 0.675–2.533 | 0.426 |
Discussion
The present study demonstrated that myofibroblast
expression was significantly associated with advanced stage
diffuse-type gastric cancer, particularly scirrhous type and
distant metastasis. In the present study, 43 cases of Type 4 showed
diffusely infiltrating carcinoma accompanied by extensive stromal
fibroblasts, which is distinguished as scirrhous gastric carcinoma
(11). This type of carcinoma
accounts for approximately 10% of all gastric carcinomas, and
patients presenting with this type are associated with a poorer
prognosis in comparison to other types of gastric carcinomas, thus
reflecting a rapid proliferation of cancer cells (14). Interactions between scirrhous
gastric cancer cells and orthotopic fibro-blasts have been
previously reported (12).
Myofibroblasts, among orthotopic stromal cells, might thus play an
important role in cancer progression in the development of
scirrhous type gastric cancer. Orimo et al found that
myofibroblasts exhibited significant positive signals of various
growth factors in breast cancer (9,15).
Our previous study indicated that keratinocyte growth factor,
transforming growth factor-β and hepatocyte growth factor secreted
by human gastric fibroblasts might stimulate proliferation and
invasion of human scirrhous gastric carcinoma cells (16,17).
Myofibroblasts are thought to accelerate the aggressive phenotype
of scirrhous gastric carcinoma via these growth factors.
The prognosis of patients with
myofibroblast-positive tumors such as colorectal cancer (18) was reported to be significantly
worse than the prognosis of patients with myofibroblast-negative
tumors, thus suggesting that myofibroblast expression is indicative
of high malignancy and might also be associated with diffusely
invasive growth and poor prognosis. Overexpression of
myofibroblasts might be a useful prognostic indicator, while
multivariate analysis revealed that myofibroblast expression was
not an independent prognostic factor.
Differentiation from resident stromal fibroblasts
into myofibroblasts is induced by paracrine signals generated by
repairing or inflamed tissues. Among these signals, TGF-β is a
well-known inducer of myofibroblasts which stimulates fibroblasts
to differentiate into myofibroblasts in cancer tissues (2,19,20).
Overexpression of TGF-ß is reported to accelerate metastasis and is
thus correlated with the poor prognosis of gastric tumors,
particularly for scirrhous gastric carcinoma (21,22).
Moreover, TGF-β is produced to a greater extent by most scirrhous
gastric cancer cells than by other types of gastric cancer cells
(23). These findings suggest that
myofibroblasts induced by TGF-β from scirrhous gastric cancer cells
might thus be responsible for the poor prognosis observed for
scirrhous gastric cancer.
In conclusion, myofibroblasts in the
microenvironment of gastric cancer were found to be significantly
associated with an advanced stage, particularly for the
macroscopically scirrhous type gastric carcinoma. Overexpression of
myofibroblasts may therefore be a useful prognostic indicator for
such cases.
Acknowledgements
We thank Masako Shinkawa (Osaka City
University Graduate School of Medicine) for the technical advice on
the immunohistochemical staining. This study was supported, in
part, by Grants-in-Aid for Scientific Research (nos. 18591475,
20591073 and 18390369) from the Ministry of Education, Science,
Sports, Culture and Technology of Japan.
References
|
1.
|
Nakayama H, Enzan H, Miyazaki E and Toi M:
Alpha smooth muscle actin-positive stromal cells in gastric
carcinoma. J Clin Pathol. 55:741–744. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ronnov-Jessen L, Petersen OW, Koteliansky
VE and Bissell MJ: The origin of the myofibroblasts in breast
cancer. Recapitulation of tumor environment in culture unravels
diversity and implicates converted fibroblasts and recruited smooth
muscle cells. J Clin Invest. 95:859–873. 1995. View Article : Google Scholar
|
|
3.
|
Chauhan H, Abraham A, Phillips JR, Pringle
JH, Walker RA and Jones JL: There is more than one kind of
myofibroblast: analysis of CD34 expression in benign, in situ, and
invasive breast lesions. J Clin Pathol. 56:271–276. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Tuxhorn JA, McAlhany SJ, Dang TD, Ayala GE
and Rowley DR: Stromal cells promote angiogenesis and growth of
human prostate tumors in a differential reactive stroma (DRS)
xenograft model. Cancer Res. 62:3298–3307. 2002.PubMed/NCBI
|
|
5.
|
Albini A and Sporn MB: The tumour
microenvironment as a target for chemoprevention. Nat Rev Cancer.
7:139–147. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Barth PJ and Westhoff CC: CD34+
fibrocytes: morphology, histogenesis and function. Curr Stem Cell
Res Ther. 2:221–227. 2007.
|
|
7.
|
Eyden B: The myofibroblast: a study of
normal, reactive and neoplastic tissues, with an emphasis on
ultrastructure. J Submicrosc Cytol Pathol. 231–296. 2007.
|
|
8.
|
Eyden B: The myofibroblast: phenotypic
characterization as a prerequisite to understanding its functions
in translational medicine. J Cell Mol Med. 12:22–37. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Orimo A, Gupta PB, Sgroi DC, et al:
Stromal fibroblasts present in invasive human breast carcinomas
promote tumor growth and angiogenesis through elevated SDF-1/CXCL12
secretion. Cell. 121:335–348. 2005. View Article : Google Scholar
|
|
10.
|
De Wever O, Demetter P, Mareel M and
Bracke M: Stromal myofibroblasts are drivers of invasive cancer
growth. Int J Cancer. 123:2229–2238. 2008.PubMed/NCBI
|
|
11.
|
Tahara E: Growth factors and oncogenes in
human gastrointestinal carcinomas. J Cancer Res Clin Oncol.
116:121–131. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Yashiro M, Chung YS and Sowa M: Role of
orthotopic fibroblasts in the development of scirrhous gastric
carcinoma. Jpn J Cancer Res. 85:883–886. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Japanese Gastric Cancer A. Japanese
Classification of Gastric Carcinoma – 2nd English edition. Gastric
Cancer. 1:10–24. 1998.
|
|
14.
|
Maruyama M, Sasaki T and Kubota H:
[Endoscopic treatment of early cancer of the large bowel]. Gan No
Rinsho. 32:1309–1316. 1986.
|
|
15.
|
Orimo A, Tomioka Y, Shimizu Y, et al:
Cancer-associated myofibroblasts possess various factors to promote
endometrial tumor progression. Clin Cancer Res. 7:3097–3105.
2001.PubMed/NCBI
|
|
16.
|
Nakazawa K, Yashiro M and Hirakawa K:
Keratinocyte growth factor produced by gastric fibroblasts
specifically stimulates proliferation of cancer cells from
scirrhous gastric carcinoma. Cancer Res. 63:8848–8852. 2003.
|
|
17.
|
Inoue T, Chung YS, Yashiro M, et al:
Transforming growth factor-beta and hepatocyte growth factor
produced by gastric fibroblasts stimulate the invasiveness of
scirrhous gastric cancer cells. Jpn J Cancer Res. 88:152–159. 1997.
View Article : Google Scholar
|
|
18.
|
Tsujino T, Seshimo I, Yamamoto H, et al:
Stromal myofibroblasts predict disease recurrence for colorectal
cancer. Clin Cancer Res. 13:2082–2090. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Wipff PJ, Rifkin DB, Meister JJ and Hinz
B: Myofibroblast contraction activates latent TGF-beta1 from the
extracellular matrix. J Cell Biol. 179:1311–1323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Ronnov-Jessen L and Petersen OW: Induction
of alpha-smooth muscle actin by transforming growth factor-beta 1
in quiescent human breast gland fibroblasts. Implications for
myofibroblast generation in breast neoplasia. Lab Invest.
68:696–707. 1993.
|
|
21.
|
Saito H, Tsujitani S, Oka S, et al: The
expression of transforming growth factor-beta1 is significantly
correlated with the expression of vascular endothelial growth
factor and poor prognosis of patients with advanced gastric
carcinoma. Cancer. 86:1455–1462. 1999. View Article : Google Scholar
|
|
22.
|
Kinugasa S, Abe S, Tachibana M, et al:
Overexpression of transforming growth factor-beta1 in scirrhous
carcinoma of the stomach correlates with decreased survival.
Oncology. 55:582–587. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Yoshida K, Yokozaki H, Niimoto M, Ito H,
Ito M and Tahara E: Expression of TGF-beta and procollagen type I
and type III in human gastric carcinomas. Int J Cancer. 44:394–398.
1989. View Article : Google Scholar : PubMed/NCBI
|