Introduction
Malignant melanoma (MM) is a highly invasive fatal
skin cancer arising from the abnormal proliferation of epidermal
melanocytes (1). Its resistance to
conventional cancer therapies including irradiation and
chemotherapy (2) renders its
treatment difficult.
MK615, an extract mixture of the Japanese apricot
Prunus mume Sieb. Et Zucc, Ume, at a neutral pH, contains
several triterpenoids (3),
compounds derived from 30-carbon precursors (4). These compounds have been demonstrated
to exert anti-neoplastic effects (5,6). The
extract of Ume has been reported to exert anticancer effects
against gastric (3), breast
(7), hepatocellular (8), colon (9) and pancreatic cancers (10). The mechanisms underlying these
effects include the induction of apoptosis (3,7) and
autophagy (9) and the suppression
of Aurora A kinase (8,10) in cancer cells.
In the present study, after obtaining marked results
by treating a patient with advanced MM with MK615, we investigated
the mechanisms underlying the effect of MK615 using the human MM
cell line SK-MEL28.
Patients and methods
Patients
The patient, a 67-year-old Japanese woman diagnosed
with MM, provided prior written informed consent for use of her
data in this report.
Chemicals
MK615 and Misatol® GL, containing an
additive to render it drinkable, were provided by AdaBio Co. Ltd.
(Takasaki, Japan).
TUNEL staining and evaluation of
apoptosis in vivo
Apoptosis in skin cells from the patient was
evaluated by the TdT-mediated dUTP-biotin nick end-labeling (TUNEL)
method using the ApoTag® Plus Peroxidase in situ
Apoptosis Detection kit (Chemicon, Temecula, CA) according to the
manufacturer’s instructions. Cell nuclei were stripped of proteins
by incubation with 20 μg/ml Proteinase K (Invitrogen, Carlsbad,
CA). The immune complexes were visualized by incubating skin
sections with DAB-nickel (Vector, Burlingame, CA). They were then
counterstained with methyl-green. The apoptotic index was estimated
by determining the percentage of apoptotic cells under a light
microscope at x400 magnification. A minimum of 2,000 cells was
counted in tumor sections. Positively stained tumor cells
exhibiting the morphological characteristics of apoptosis were
identified using standard criteria (11).
Cell lines
Human SK-MEL28 melanoma cells were maintained in
DMEM medium containing 10% fetal calf serum. The cells were
confirmed to be mycoplasma-free.
Cell growth analysis
The growth of SK-MEL28 cells in the presence or
absence of MK615 was determined by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assay.
Cell cycle analysis
The cell cycle was analyzed by the propidium iodide
(PI) method of Biswas et al (12). SK-MEL28 cells (1×105/ml)
were grown for 24 h in 60-mm culture dishes containing 0, 10 or 20
μl/ml MK615, scraped off, and centrifuged at 1,500 rpm for 10 min.
The cell pellets were fixed in 70% ethanol at −20°C for 1 h,
incubated in 0.25 mg/ml ribonuclease A in PBS at room temperature,
stained with PI (0.125 mg/ml in PBS) for 30 min, and subjected to
flow cytometric analysis. Cells in the sub-G1 phase were considered
to be apoptotic.
Determination of apoptosis in vitro by
fluorescence microscopy
We determined apoptosis in vitro by
immunofluorescence microscopy (13). Briefly, SK-MEL28 cells
(5×104/well) in 4-well culture dishes were treated with
0, 10 or 20 μl/ml MK615 for 2, 4 and 8 h and incubated with Annexin
V-fluorescein isothiocyanate (FITC) (Immunotech, France) according
to the manufacturer’s instructions. Cell nuclei were stained with
4′,6-diamidino-2-phenylindole (DAPI; Dojindo, Kumamoto, Japan) and
examined under an Axioskop microscope (Carl Zeiss, Oberkochen,
Germany).
Western blotting
Cell lysate samples were subjected to 12%
SDS-polyacrylamide gel electrophoresis (PAGE), and the separated
proteins were then transferred to a nitrocellulose membrane (GE
Healthcare Bio-sciences KK, Piscataway, NJ) as described previously
(14). The membrane was blocked
with 5% non-fat dry milk in Tris-buffered saline (TBS; pH 7.4)
containing 0.02% Tween-20 (TBST) for 1 h at room temperature and
then incubated with the first antibody in TBST containing 1%
non-fat dry milk (overnight at 4°C). After washing, the membrane
was incubated with horseradish peroxidase (HRP)-conjugated
anti-rabbit IgG polyclonal antibody (Invitrogen) diluted 1:3000 in
TBST containing 2.5% non-fat dry milk (1 h at room temperature).
The membrane was washed again, and the immunoreactive bands were
visualized using the ECL detection system (GE Healthcare
Bio-sciences KK).
Immunofluorescence microscopy
SK-MEL28 cells on culture slides were fixed in 4%
paraformaldehyde and blocked with 1% bovine serum albumin (Sigma,
St. Louis, MO) in PBS. After washing, the cells were incubated with
RAGE antibody (Santa Cruz, CA) and washed. The slides were then
incubated with Alexa-fluor 488 goat anti-rabbit IgG (Invitrogen)
diluted 1:200, washed, and stained with DAPI. Cells were visualized
under an Axioskop microscope.
Statistical analysis
Statistical analysis was carried out using the
Student’s t-test (15); p-values
<0.05 were regarded as significant.
Results
Improvement of clinical manifestations in
the MK615-treated MM patient
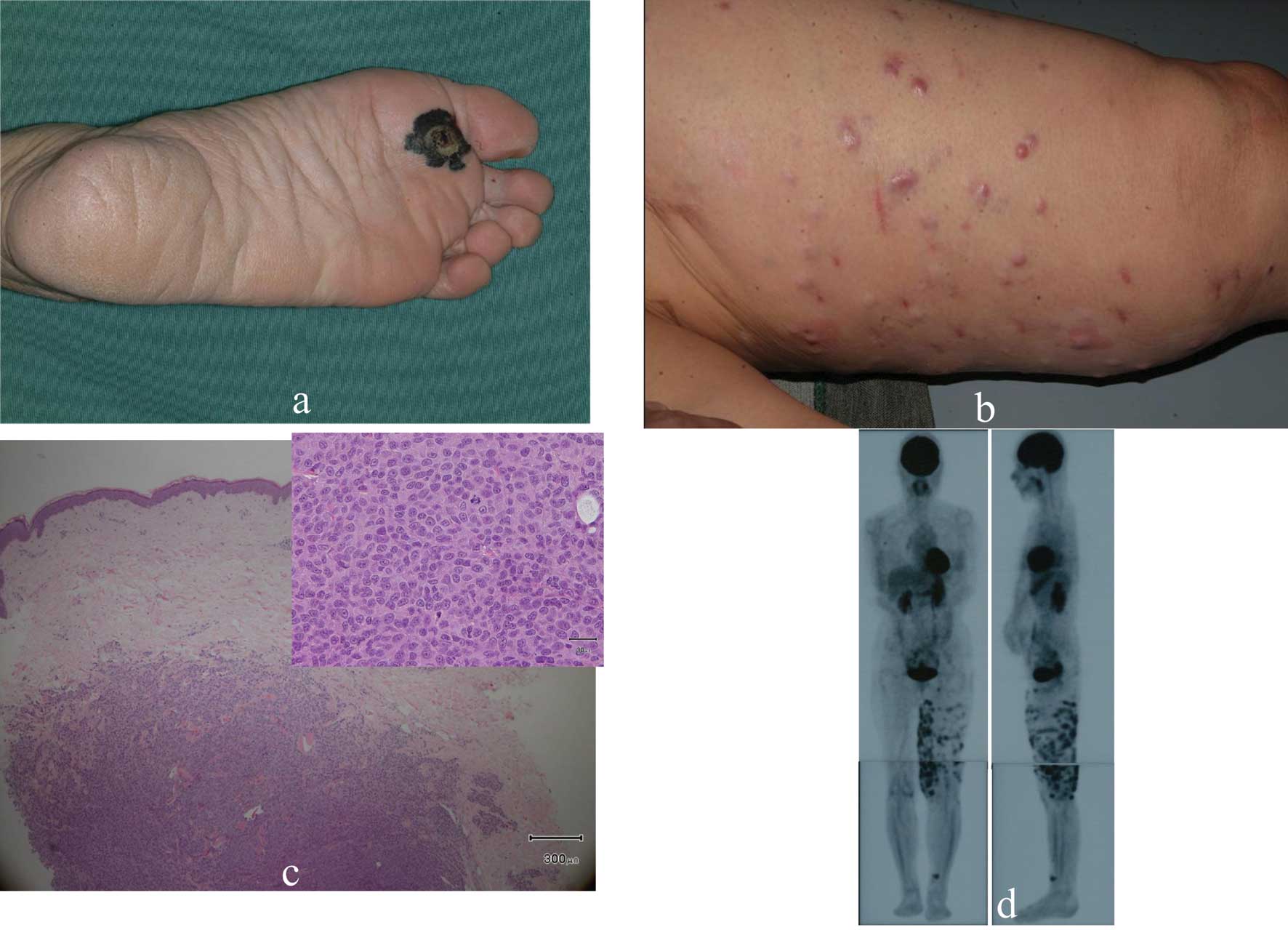
This 67-year-old Japanese woman presented with a
black nodule and a freckle on her left sole. Based on clinical
examinations a diagnosis of MM was made (Fig. 1a). We resected the primary
cutaneous lesions and dissected the popliteal, inguinal and iliac
lymph nodes. Multiple nodules appeared on her left thigh 15 months
later (Fig. 1b). Histological
examination of biopsy specimens disclosed intradermal tumor nests
consisting of highly atypical cells positive for S100 protein,
HMB45 and Melan-A (Fig. 1c).
Positron emission tomography (PET) showed highly increased glucose
uptake by multiple lesions on the left thigh (Fig. 1d). The lesions were diagnosed as
cutaneous in-transit metastasis of MM, and although we delivered
3×106 units of IFN-β intravenously daily, the number of
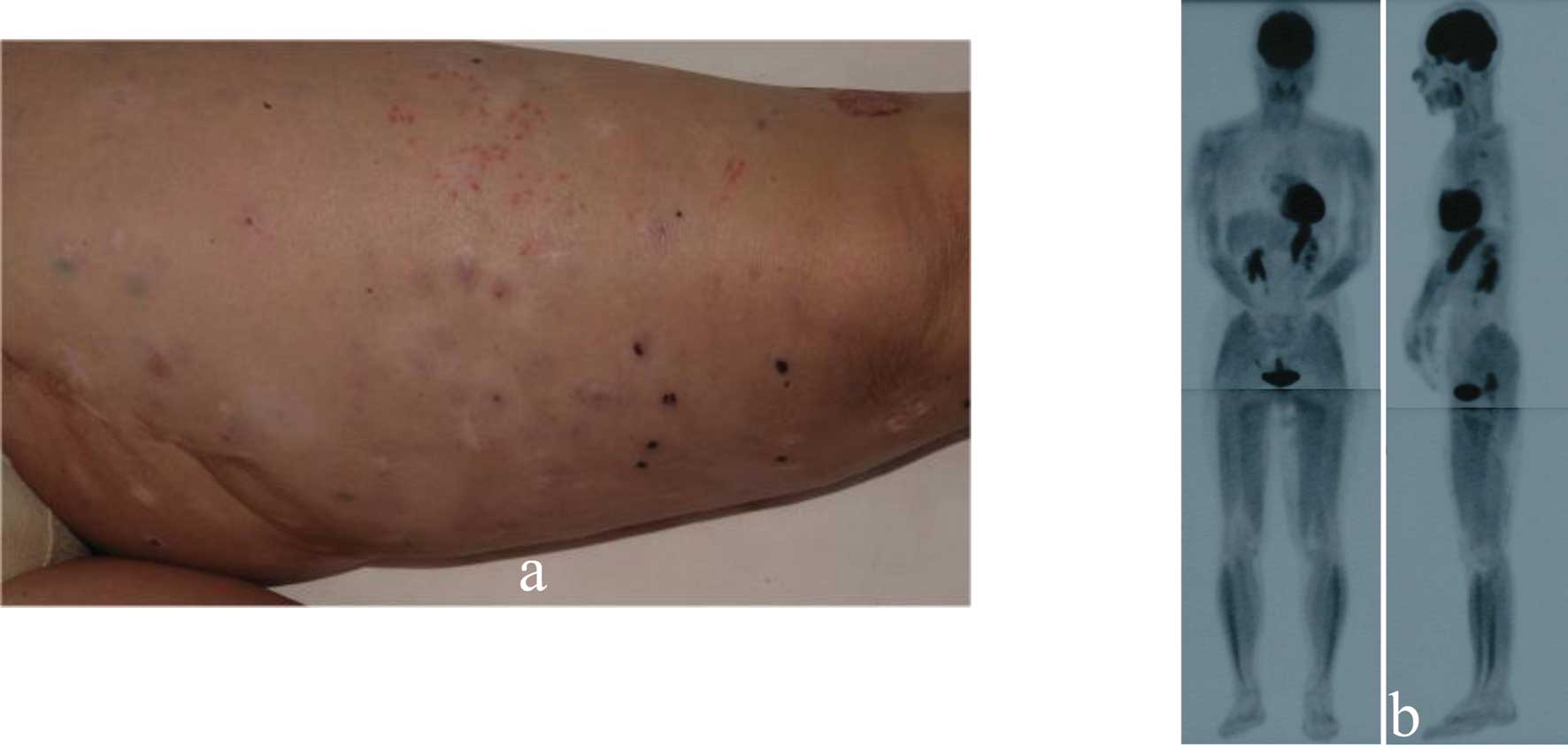
in-transit metastatic lesions gradually increased. After 4 months,
we administered 13 g Misatol® GL perorally daily. After
5 months, the number of in-transit metastatic lesions was
significantly decreased (Fig. 2a),
and de-pigmentation was observed on some of the regressed tumors.
On PET scans, the lesions on her left thigh were strikingly
improved after MK615 administration (Fig. 2b). The apoptotic index determined
by the TUNEL technique was clearly higher after MK615 treatment
(0.462±0.203 vs. 0.165±0.039%; p=0.00568).
Inhibition of SK-MEL28 cell growth by
MK615
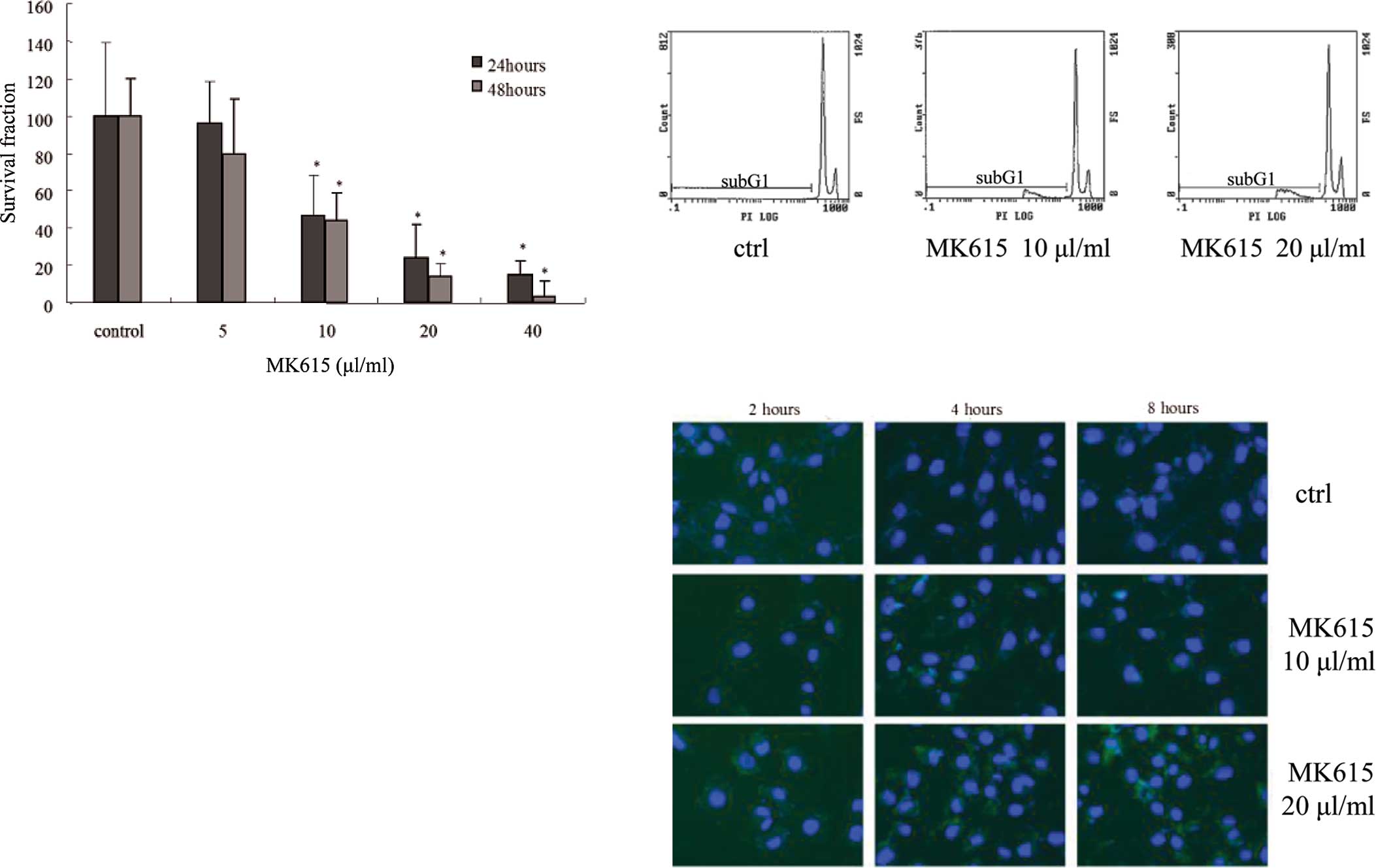
The growth of SK-MEL28 cells in the presence or
absence of MK615 was determined by MTT assay. The viability of
MK615-treated SK-MEL28 cells was significantly decreased in a
dose-dependent manner after a 24- and 48-h incubation (Fig. 3a).
Cell cycle retardation of SK-MEL28 cells
by MK615
To examine the effects of MK615 on apoptosis we
assessed the cell cycle of SK-MEL28 cells by flow cytometry using
PI staining. In the presence of 10 and 20 μl/ml MK615, the
proportion of sub-G1 cells was increased in a dose-dependent manner
(Fig. 3b); 19.97±3.90 and
23.07±2.73% at 10 and 20 μl/ml MK615, respectively, and
significantly higher than in cells cultured in the absence of MK615
(p=0.00284 and 0.00041, Table
I).
 | Table I.The proportion of sub-G1 SK-MEL28
cells after exposure to the indicated doses of MK615. |
Table I.
The proportion of sub-G1 SK-MEL28
cells after exposure to the indicated doses of MK615.
| MK615 (μl/ml) | sub-G1 (%) |
|---|
| 0 |
1.80±0.50a |
| 10 |
19.97±3.90b |
| 20 |
23.07±2.73c |
MK615-induced SK-MEL28 apoptosis
Fluorescence staining with Annexin V showed that at
10 and 20 μl/ml, MK615 induced apoptosis as reflected by
phosphatidylserine externalization. The number of apoptotic cells
increased in a time-dependent manner (Fig. 3c).
Attenuation of RAGE expression in
SK-MEL28 cells by MK615
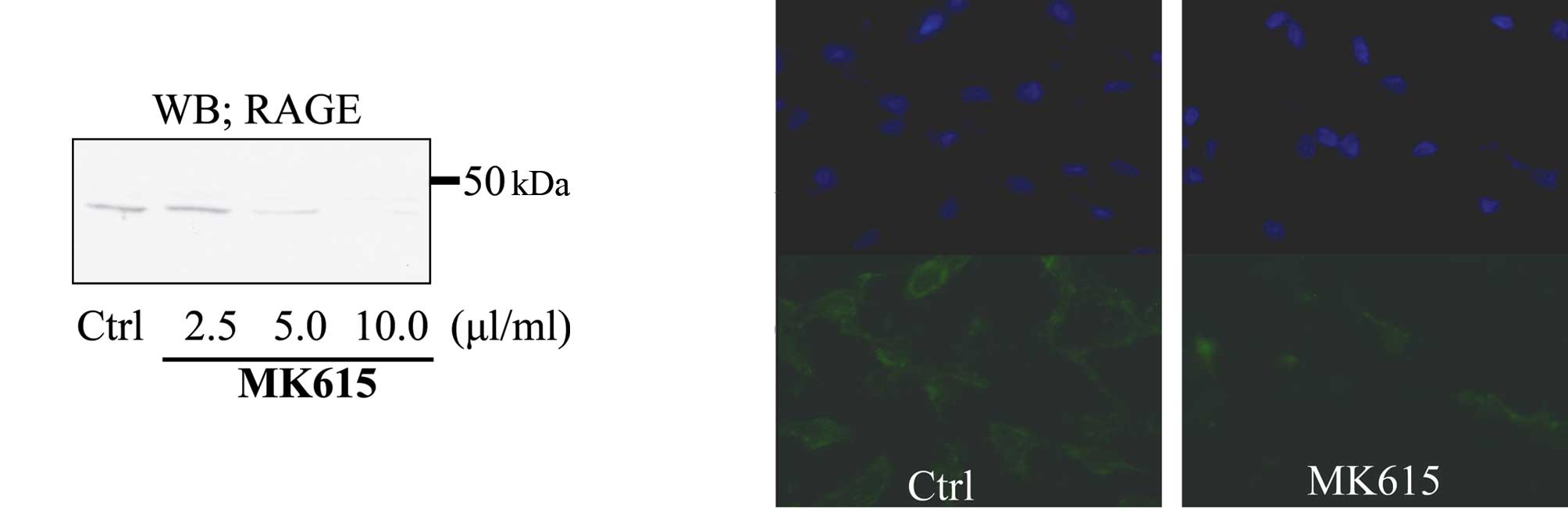
Western blotting showed that MK615 inhibited RAGE
expression by SK-MEL28 cells in a dose-dependent manner (Fig. 4a). Immunofluorescence analysis
revealed that at 10 μl/ml, MK615 abolished the expression of RAGE
on the cell membrane (Fig.
4b).
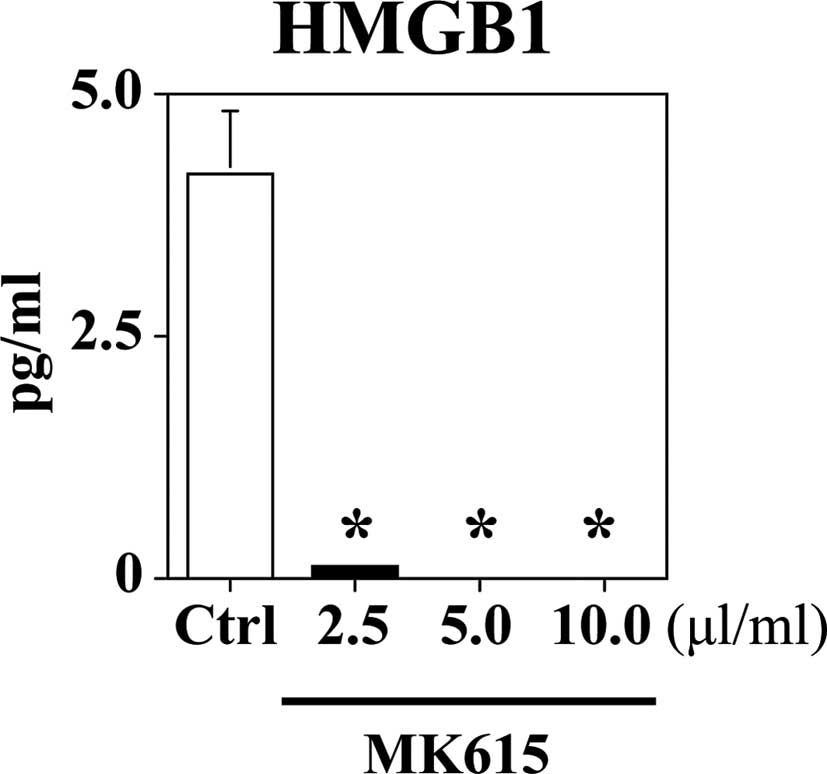
MK615 inhibits HMGB1 release by SK-MEL28
cells
The concentration of HMGB1 in SK-MEL28 cell
supernatants is shown in Fig. 5.
The SK-MEL28 cells spontaneously released 4.2±0.6 pg/ml HMGB1.
MK615 significantly suppressed HMGB1 release in a dose-dependent
manner.
Discussion
The Japanese apricot Ume has been accepted for
centuries as a traditional medicine in Japan. It contains several
chemical substances, e.g. citric and malic acid, cyanogenic
glycosides and triterpenoids (3).
Triterpenoids are natural or synthetic compounds derived from
30-carbon precursors (4). They
have been shown to exert anti-neoplastic effects (5,7).
MK615 is a neutral pH extract mixture of the Ume fruit that
contains several triterpenoids. It has been reported to exert
anti-cancer effects against gastric (3), breast (7), hepatocellular (8), colon (9) and pancreatic cancer (10).
Here, we demonstrated that the Ume extract MK615 at
a neutral pH suppressed cutaneous and in-transit meta-static
lesions in a patient with MM. Pre- and post-treatment comparison
showed that the apoptotic index of the tumor cells was
significantly increased by MK615. We attributed the de-pigmentation
of the regressed tumor portions to the activation of T-cell
immunity against MM and melanocytes (16). Triterpenoids have been reported to
regulate the cell cycle. Sun et al (17) showed that, in HeLa cells,
triterpenoids induced apoptosis by cell cycle deviation resulting
in S-phase accumulation and a decrease in G0-G1 phase cells. Other
researchers (6) have documented
triterpenoid-induced G2-M phase arrest, suggesting that cell cycle
deviation plays a key role in the induction of apoptosis. Gutterman
et al (5) demonstrated
that, in pancreatic cancer cell lines, Ume extract increased the
proportion of cells in the G2-M phase and inhibited Aurora A and B
kinases. Aurora kinases are key mediators of cell deviation by
controlling chromatoid segregation (18–20).
In our study, the exposure of SK-MEL28 melanoma cells to MK615
resulted in an increase in cells in the sub-G1 phase. Moreover, we
detected Annexin V-positive apoptotic cells 2 h after the start of
MK615 exposure, and their number continued to increase up to 8 h.
These data suggest that MK615 induces apoptosis in MM cells by
another pathway.
RAGE, a multi-ligand receptor, is highly expressed
and is associated with cell invasion and migration (21). In a primary tumor model, anti-RAGE
and soluble RAGE partially inhibited local tumor growth (21). Therefore, RAGE blockade may
contribute to reducing tumor growth and invasion.
HMGB1, the ligand of RAGE, has two distinct
functions in cellular systems. In the nucleus, HMGB1 acts as an
intracellular regulator of the transcription process and plays a
crucial role in the maintenance of DNA functions (22). In the extracellular space, HMGB1 is
released by all eukaryotic cells upon necrosis or by macrophages in
response to inflammatory stimuli such as endotoxin. Extracellular
HMGB1 plays a major role in several diseases such as sepsis
(22), rheumatoid arthritis,
disseminated intravascular coagulation, periodontitis,
xenotransplantation and atherosclerosis (13,22–25).
Extracellular matrix associated HMGB1 promotes
neurite outgrowth and provides a surface for the assembly of
protease complexes in the fibrinolytic system, which can contribute
to cell mobility. In view of the increased expression of HMGB1 and
RAGE in tumors, the tumor bed may be an ideal locus for the effects
of RAGE/HMGB1 interactions on cell migration and invasion.
Our study demonstrated that MK615 decreased the
expression of RAGE in SK-MEL28 cells. Moreover, it significantly
suppressed the release of HMGB1 by SK-MEL28 cells. Our results
suggest that MK615 inhibits cell migration and invasion.
In summary, MK615 was effective in a patient with
advanced MM. Our in vitro studies showed that it inhibited
the growth of MM cells and increased the proportion of apoptotic
cancer cells. MK615 may be a valuable tool for treating MM and
other malignant tumors and further studies to evaluate its clinical
effectiveness and to elucidate the precise mechanisms of its
actions are warranted.
Abbreviations:
|
MM,
|
malignant melanoma;
|
|
RAGE,
|
receptor for advanced glycation end
products;
|
|
HMGB1,
|
high mobility group box protein 1;
|
|
TdT,
|
terminal deoxynucleotidyl
transferase;
|
|
TUNEL,
|
TdT-mediated dUTP-biotin nick
end-labeling;
|
|
PBS,
|
phosphate-buffered saline;
|
|
DAB,
|
diaminobenzidine;
|
|
MTT,
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide;
|
|
PI,
|
propidium iodide;
|
|
FITC,
|
fluorescein isothiocyanate;
|
|
DAPI,
|
4′,6-diamidino-2-phenylindole;
|
|
PET,
|
positron emission tomography;
|
|
PAGE,
|
polyacrylamide gel
electrophoresis;
|
|
TBS,
|
tris-buffered saline;
|
|
HRP,
|
horseradish peroxidase
|
Acknowledgements
We thank Nobue Uto, Tomoka Nagasato
and Tomomi Morizono for their excellent technical assistance. This
study was supported by research grants from the Ministry of
Education, Culture, Sports, Science, and Technology of Japan, by
Grants-in-Aid 21390483 (to K.K.).
References
|
1.
|
Houghton AN and Polsky D: Focus on
melanoma. Cancer Cell. 2:275–278. 2002. View Article : Google Scholar
|
|
2.
|
Soengas MS and Lowe SW: Apoptosis and
melanoma chemo-resistance. Oncogene. 22:3138–3151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Adachi M, Suzuki Y, Mizuta T, Osawa T,
Suzuki K, Shiojima K, Arai Y, Masuda K, Uchiyama M and Oyamada T:
The Japanese apricot ‘Prunus mume Sieb. Et Zucc’ (Ume) is a
rich natural source of novel anti-cancer substance. Int J Food
Prop. 10:375–384. 2007.
|
|
4.
|
Xu R, Frazio GC and Matsuda PT: On the
origins of triterpenoid skeletal diversity. Phytochemistry.
65:261–291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Gutterman JU, La HT, Yang P, Haridas V,
Gaikwad A and Marcus S: Effects of the tumor inhibitory
triterpenoid avicin G on cell integrity, cytokinesis, and protein
ubiquitination in fission yeast. Proc Natl Acad Sci USA.
102:12771–12776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ramachandran C, Rabi T, Fonseca HB,
Melnick SJ and Escalon EA: Novel plant triterpenoid drug amooranin
overcomes multidrug resistance in human leukemia and colon
carcinoma cell lines. Int J Cancer. 105:784–789. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Nakagawa A, Sawada T, Okada T, Ohsawa T,
Adachi M and Kubota K: New antineoplastic agent, MK615, from Ume (a
variety of) Japanese apricot inhibits growth of breast cancer cells
in vitro. Breast J. 13:44–49. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Okada T, Sawada T, Osawa T, Adachi M and
Kubota K: A novel anti-cancer substance, MK615, from ume, a variety
of Japanese apricot, inhibits growth of hepatocellular carcinoma
cells by suppressing Aurora A kinase activity.
Hepatogastroenterology. 54:1770–1774. 2007.
|
|
9.
|
Mori S, Sawada T, Okada T, Ohsawa T,
Adachi M and Keiichi K: New anti-proliferative agent, MK615, from
Japanese apricot ‘Prunus mume’ induces striking autophagy in
colon cancer cells in vitro. World J Gastroenterol. 13:6512–6517.
2007.PubMed/NCBI
|
|
10.
|
Okada T, Sawada T, Osawa T, Adachi M and
Kubota K: MK615 inhibits pancreatic cancer cell growth by dual
inhibition of Aurora A and B kinases. World J Gastroenterol.
14:1378–1382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ansari B, Coates PJ, Greenstein BD and
Hall PA: In situ end-labeling detects DNA strand breaks in
apoptosis and other physiological and pathological states. J
Pathol. 170:1–8. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Biswas KK, Sarker KP, Abeyama K, Kawahara
K, Iino S, Otsubo Y, Saigo K, Izumi H, Hashiguchi T, Yamakuchi M,
Yamaji K, Endo R, Suzuki K, Imaizumi H and Haruyama I: Membrane
cholesterol but not putative receptors mediates anandamide-induced
hepatocyte apoptosis. Hepatology. 38:1167–1177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kawahara K, Setoyama K, Kikuchi K, Biswas
KK, Kamimura R, Iwata M, Ito T, Morimoto Y, Hashiguchi T, Takao S
and Maruyama I: HMGB1 release in co-cultures of porcine endothelial
and human T cells. Xenotransplantation. 14:636–641. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Morimoto Y, Kawahara KI, Tancharoen S,
Kikuchi K, Matuyama T, Hashiguchi T, Izumi Y and Maruyama I: Tumor
necrosis factor-alpha stimulates gingival epithelial cells to
release high mobility-group box 1. J Periodontal Res. 43:76–83.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zar JH: Biostatistical Analysis. 3rd
edition. Prentice Hall; New Jersey: 1996
|
|
16.
|
Lengagne R, Le Gal FA, Garcette M, Fiette
L, Ave P, Kato M, Briand JP, Massot C, Nakashima I, Rĕnia L,
Guillet JG and Prĕvost-Blondel A: Spontaneous vitiligo in an
animal model for human melanoma: Role of tumor-specific
CD8+ T cells. Cancer Res. 64:1496–1501. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Sun HX, Zheng QF and Tu J: Induction of
apoptosis in HeLa cells by 3beta-hydroxy-12-oleanen-27-oic acid
from the rhizomes of Astilbe chinensis. Bioorg Med Chem.
14:1189–1198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Fu J, Bian M, Jiang Q and Zhang C: Roles
of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res.
5:1–10. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Naruganahalli KS, Lakshmanan M, Dastidar
SG and Ray A: Therapeutic potential of Aurora kinase inhibitors in
cancer. Curr Opin Investig Drugs. 7:1044–1051. 2006.PubMed/NCBI
|
|
20.
|
Brittle AL and Ohkura H: Centrosome
maturation: Aurora lights the way to the poles. Curr Biol.
15:880–882. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Taguchi A, Blood DC, del Toro G, Canet A,
Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kisilinger
T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM and Schmidt
AM: Blockade of RAGE-amphoterin signaling suppresses tumor growth
and metastases. Nature. 18:354–360. 2000.
|
|
22.
|
Wang H, Bloom O, Zhang M, Vishnubhakat JM,
Ombrellio M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L,
Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina
PE, Abumrad NN, Sama A and Tracey KJ: HMG-1 as a late mediator of
endotoxin lethality in mice. Science. 285:m248–251. 1999.
View Article : Google Scholar
|
|
23.
|
Taniguchi N, Kawahara K, Yone K,
Hashiguchi T, Yamakuchi M, Goto M, Inoue K, Yamada S, Ijiri K,
Matunaga S, Nakajima T, Komiya S and Maruyama I: High mobility
group box chromosomal protein 1, a DNA binding cytokine, induces
arthritis. Arthritis Rheum. 48:971–981. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Ito T, Kawahara K, Okamoto K, Yamada S,
Yasuda M, Imaizumi H, Nawa Y, Meng X, Shrestha B, Hashiguchi T and
Maruyama I: Proteolytic cleavage of high mobility group box 1
protein by thrombomodulin complexes. Arterioscler Thromb Vasc Biol.
28:1825–1830. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Inoue K, Kawahara K, Biswas KK, Ando K,
Mitsudo K, Nobuyoshi M and Maruyama I: HMGB1 expression by
activated vascular smooth muscle cells in advanced human
atherosclerosis. Cardiovasc Pathol. 16:136–143. 2007. View Article : Google Scholar : PubMed/NCBI
|