Introduction
Heat shock proteins (HSPs) are induced when cells
are exposed to biological stress such as heat stress and chemical
stress (1). It is generally
recognized that high-molecular-weight HSPs such as HSP70, HSP90 and
HSP110 act as molecular chaperones in protein folding,
oligomerization and translocation in cells (2). On the other hand, functions of the
low-molecular-weight HSPs with molecular masses from 10 to 30 kDa,
such as HSP27, αB-crystallin and HSP20 are less understood than
those of the high-molecular-weight HSPs (2). The low-molecular-weight HSPs share
high homology in amino acid sequences ‘α-crystallin domain’
(2). It has been shown that HSP27
activity is regulated by post-translational modification such as
phosphorylation (3). Under
unstimulated conditions, HSP27 exists as a high-molecular-weight
aggregated form and is rapidly dissociated as a result of
phosphorylation (4,5). Although the phosphorylation-elicited
dissociation from the aggregated form reportedly correlates with
the loss of molecular chaperone activity (4,5), the
physiological significance of phosphorylated HSP27 remains
controversial.
Bone metabolism is regulated by two functional
cells, osteoblasts and osteoclasts, responsible for bone formation
and bone resorption, respectively (6). The maintenance of bone structures and
bone remodeling results from the coupling process; bone resorption
by activated osteoclasts with subsequent deposition of new matrix
by osteoblasts. In osteoblasts, it has been shown that the
down-regulation of proliferation is accompanied by a transient
increase in HSP27 mRNA expression (7). In addition, heat-stimulated induction
of HSP27 is reportedly facilitated by estrogen (8). These findings lead us to speculate
that HSP27 plays an important role in osteoblast cell functions.
However, the exact role of HSP27 in osteoblasts has not yet been
clarified.
Prostaglandins (PGs) act as autacoids in osteoblasts
(9,10). Among PGs, PGD2 has been implicated
in the control of osteoblast cell function and bone anabolism
(10). It has been shown that
PGD2 stimulates collagen synthesis during calcification
of osteoblasts (11). In addition,
PGD2 produced by osteoblasts reportedly modulates
expression of osteoprotegerin and RANKL in osteoblasts (12). In our previous study (13), we reported that PGD2
stimulates interleukin (IL)-6 synthesis via Ca2+
mobilization in osteoblast-like MC3T3-E1 cells. In addition, we
showed that PGD2 stimulates the induction of HSP27 via
p44/p42 mitogen-activated protein (MAP) kinase, p38 MAP kinase and
SAPK/JNK among the MAP kinase superfamily (14) in MC3T3-E1 cells (15,16).
However, the exact mechanism behind PGD2-stimulated
HSP27 induction in osteoblasts remains to be elucidated.
It is well known that Rho and the downstream
effector, Rho-associated kinase (Rho-kinase), play important roles
in a variety of cellular functions such as smooth muscle
contraction and cell motility (17–19).
With regard to osteoblasts, it has been reported that Rho and p38
MAP kinase are involved in the endothelin-1-induced expression of
prostaglandin endoperoxide G/H synthase mRNA in osteoblasts
(20). In addition, it has been
shown that the Rho/Rho-kinase pathway stimulates osteoblast
proliferation whereas it has an inhibitory role in osteoblast
differentiation (21). In a recent
study (22), we demonstrated that
Rho-kinase functions at the point of p38 MAP kinase but not p44/p42
MAP kinase activation in the PGD2-stimulated synthesis
of IL-6 in osteoblast-like MC3T3-E1 cells. In the present study, we
investigated the involvement of Rho-kinase in the
PGD2-stimulated HSP27 induction in these cells.
Materials and methods
Materials
PGD2 and HSP70 antibodies were obtained
from R&D Systems, Inc. (Minneapolis, MN). Y27632 was obtained
from Calbiochem-Novabiochem Co. (La Jolla, CA). Hydroxyfasudil
(fasudil) was purchased from Sigma (St. Louis, MO).
Phospho-specific SAPK/JNK antibodies and SAPK/JNK antibodies were
purchased from Cell Signaling, Inc. (Beverly, MA). Glyceraldehyde 3
phosphate dehydrogenase (GAPDH) antibodies and HSP27 antibodies for
Western blot analysis, and HSP27 antibodies for immunofluorescence
microscopy studies were obtained from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA). Alexa Fluor 488®-conjugated calf
anti-goat antibodies and Alexa Fluor 555® phalloidin
were obtained from Invitrogen Corporation, Inc. (Carlsbad, CA).
DAPI was obtained from Wako Pure Chemical Industries, Ltd. (Tokyo,
Japan). The ECL Western blot detection system was purchased from GE
Healthcare UK Ltd. (Buckinghamshire, UK). Other materials and
chemicals were obtained from commercial sources. Y27632 was
dissolved in dimethyl sulfoxide. The maximum concentration of
dimethyl sulfoxide was 0.1%, which did not affect the assay for
Western blot analysis or the immunofluorescence microscopy
study.
Cell culture
Cloned osteoblast-like MC3T3-E1 cells derived from
newborn mouse calvaria (23) were
maintained as previously described (24). Briefly, the cells were cultured in
α-minimum essential medium (α-MEM) containing 10% fetal calf serum
(FCS) at 37°C in a humidified atmosphere of 5% CO2/95%
air. The cells were seeded into 90-mm diameter dishes
(20×104/dish) for Western blot analysis or 35-mm
diameter glass-bottom dishes (3×104/dish) for
immunofluorescence microscopy study. After 5 days, the medium was
exchanged with α-MEM containing 0.3% FCS. The cells were then used
for experiments after 48 h.
Western blot analysis
Western blot analysis was performed as described
previously (25). In brief, the
cultured cells were pretreated with various doses of Y27632 or
fasudil for 60 min and then stimulated by PGD2 in the
presence of the inhibitors in α-MEM containing 0.3% FCS for the
indicated periods. The cells were washed twice with
phosphate-buffered saline and then lysed, homogenized and sonicated
in a lysis buffer containing 62.5 mM Tris/HCl (pH 6.8), 3% sodium
dodecyl sulfate (SDS), 50 mM dithiothreitol and 10% glycerol.
SDS-polyacrylamide gel electrophoresis (PAGE) was performed
according to Laemmli in 10% polyacrylamide gel (26). The protein was fractionated and
transferred onto an Immun-Blot PVDF membrane (Bio-Rad, Hercules,
CA). Membranes were blocked with 5% fat-free dry milk in
Tris-buffered saline-Tween-20 (TBS-T; 20 mM Tris/HCl, pH 7.6, 137
mM NaCl, 0.1% Tween-20) for 2 h before incubation with the primary
antibodies. Western blot analysis was performed using HSP27, HSP70
and GAPDH antibodies with peroxidase-labeled antibodies raised in
goat anti-rabbit IgG which were used as second antibodies.
Peroxidase activity on PVDF membranes was visualized on X-ray film
by means of the ECL Western blot detection system. All of the
Western blot analyses were repeated at least three times in
independent experiments.
Immunofluorescence microscopy study
The cultured cells were pretreated with various
doses of Y27632 or fasudil at the indicated concentrations for 1 h
and then exposed to PGD2 (10 μM) or vehicle for 12 h.
They were then fixed with 3% paraformaldehyde for 10 min on ice and
exposed to 0.1% Triton X-100 for 10 min to permeabilize the cell
membrane. They were then exposed to anti-HSP27 antibodies (1:100
dilution) in the presence of 1% BSA for 1 h, followed by exposure
to Alexa Fluor 488-conjugated calf anti-goat IgG antibodies (1:500)
for 1 h. Finally, they were exposed to Alexa Fluor 555 phalloidin
and DAPI for 20 min. The cells were examined by fluorescence
microscopy (Biorevo, BZ-9000; Keyence, Tokyo, Japan) according to
the manufacturer’s protocol.
Statistical analysis
All data are presented as the mean ± SEM of
triplicate determinations. The data were analyzed by ANOVA followed
by Bonferroni method for multiple comparisons between pairs, and
p<0.05 was considered significant.
Results
Effects of Y27632 or fasudil on HSP27
induction stimulated by PGD2 in MC3T3-E1 cells
We previously reported that PGD2
stimulated HSP27 induction in osteoblast-like MC3T3-E1 cells
(15). In addition, we showed that
Rho kinase was activated by PGD2 in these cells
(22). In the present study, we
examined the effect of Y27632, a specific inhibitor of Rho-kinase
(19), on HSP27 induction
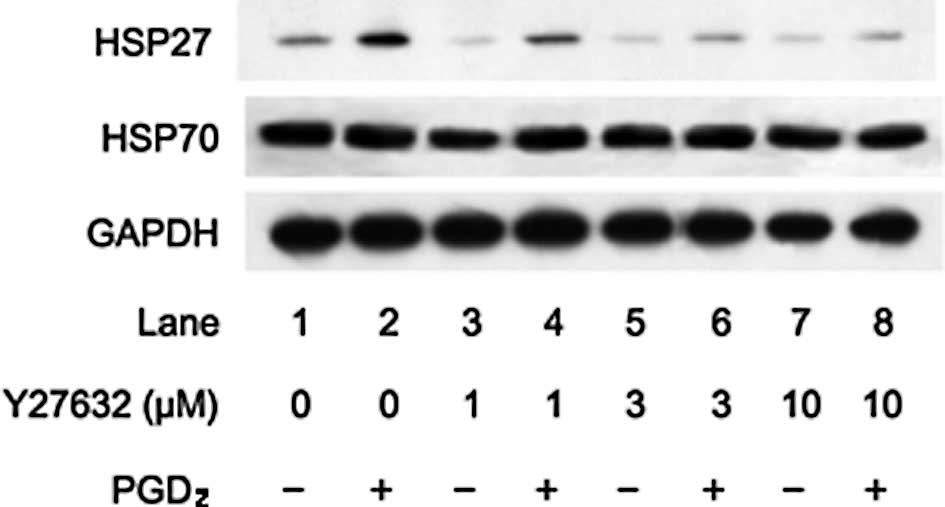
stimulated by PGD2. Y27632 significantly suppressed the
PGD2-stimulated HSP27 induction in a dose-dependent
manner in the range between 1 and 10 μM (Fig. 1). As well, we also found that
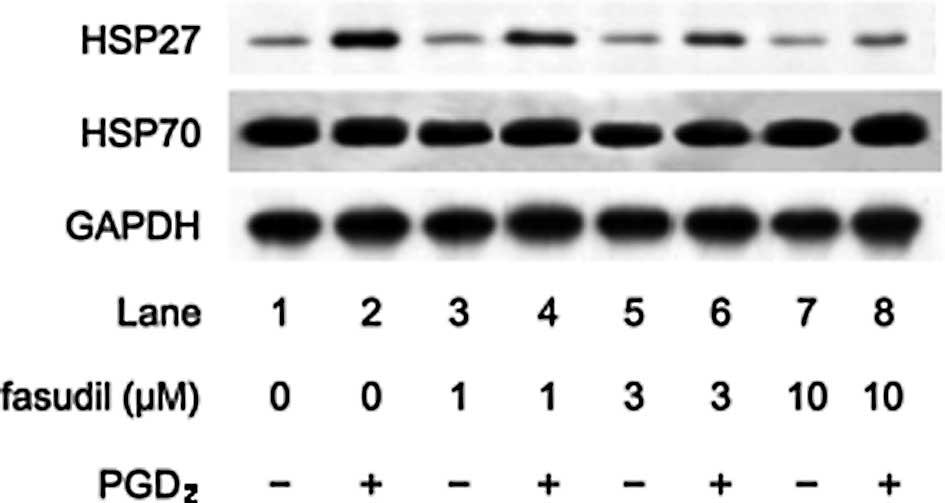
fasudil dose dependently reduced the PGD2-stimulated
HSP27 induction (Fig. 2). We
previously demonstrated that PGD2 did not affect the
levels of HSP70, a high-molecular-weight HSP, in MC3T3-E1 cells
(15,16). In the present study, we found that
Y27632 or fasudil had little effect on the levels of HSP70 in the
presence of PGD2 (Figs.
1 and 2).
Effects of Y27632 and fasudil on HSP27
induction stimulated by PGD2 in MC3T3-E1 cells by
immunofluorescence microscopy study
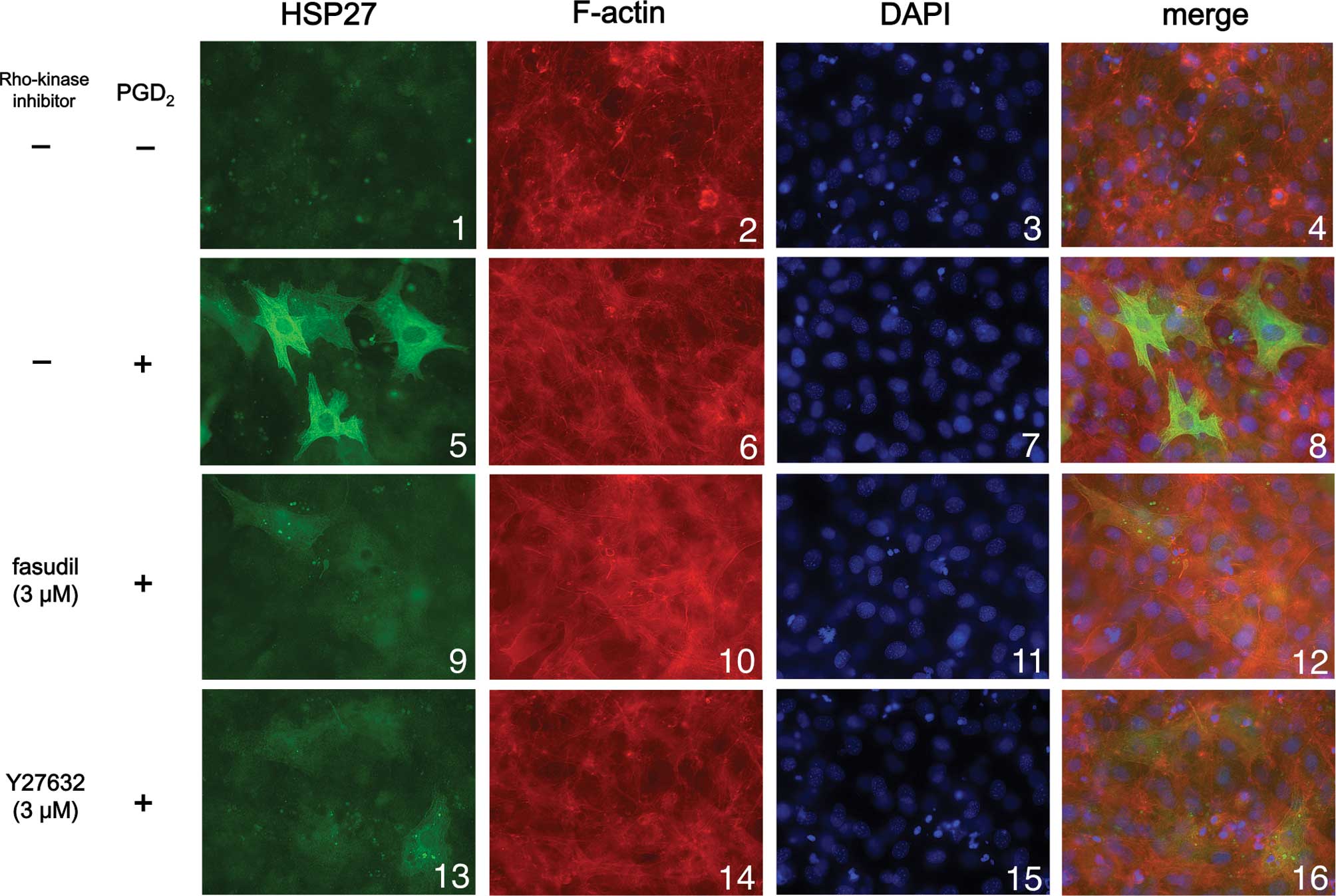
We next examined the effect of Y27632 or fasudil on
HSP27 induction stimulated by PGD2 using
immunofluorescence microscopy. We found that PGD2
markedly stimulated HSP27 induction (green signal) in the cytosol
of the osteoblast-like MC3T3-E1 cells (Fig. 3; panel 5 in comparison with panel
1), consistent with the results shown in Figs. 1 and 2. Either Y27632 (3 μM) or fasudil (3 μM)
markedly suppressed HSP27 induction stimulated by PGD2
(Fig. 3; panels 9 and 13 in
comparison with panel 5), also consistent with the results shown in
Figs. 1 and 2.
Effects of Y27632 or fasudil on the
FGF-2-induced phosphorylation of SAPK/JNK in MC3T3-E1 cells
We previously reported that PGD2
stimulates HSP27 induction via p44/p42 MAP kinase, p38 MAP kinase
and SAPK/JNK in osteoblast-like MC3T3-E1 cells (15,16).
We investigated whether the effect of Rho-kinase on the
PGD2-stimulated HSP27 induction is dependent on the
activation of these three MAP kinases in these cells. In our
previous study (22), we showed
that Rho-kinase activated by PGD2 inhibits p38 MAP
kinase phosphorylation but not p44/p42 MAP kinase in MC3T3-E1
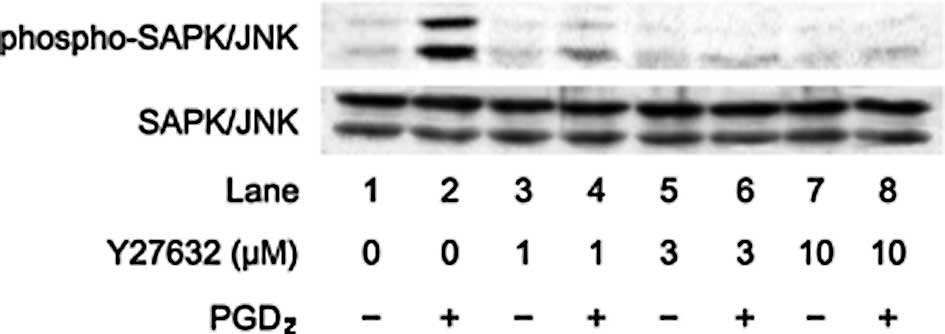
cells. Therefore, we next examined the effects of Rho-kinase
inhibitors on the PGD2-induced phosphorylation of
SAPK/JNK. Y27632 markedly attenuated the PGD2-induced
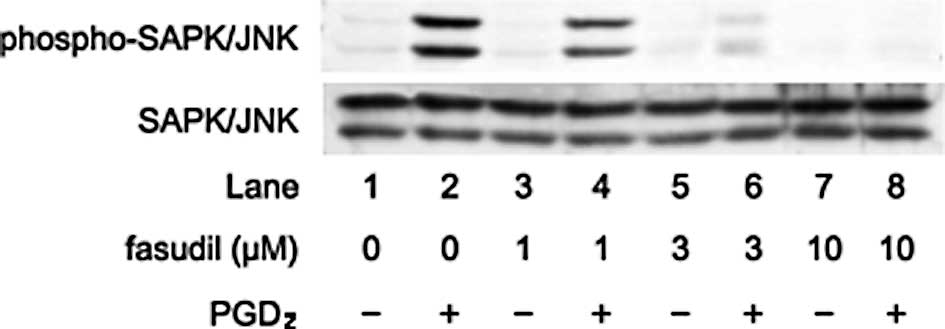
phosphorylation levels of SAPK/JNK (Fig. 4). In addition, fasudil also reduced
the phosphorylation levels of SAPK/JNK by PGD2 (Fig. 5).
Discussion
We previously demonstrated that PGD2
activates Rho-kinase in osteoblast-like MC3T3-E1 cells (22). In the present study, we showed that
Y27632 and fasudil markedly reduced the PGD2-stimulated
HSP27 induction, a low-molecular-weight HSP, in these cells.
Additionally, we also found that Y27632 and fasudil markedly
reduced PGD2-stimulated HSP27 induction in
immunofluorescence microscopy studies. Based on our findings, it is
probable that Rho-kinase is involved in PGD2-stimulated
HSP27 induction in osteoblast-like MC3T3-E1 cells.
In our previous studies (15,16),
PGD2 stimulated the induction of HSP27 via p44/p42 MAP
kinase, p38 MAP kinase and SAPK/JNK among the MAP kinase
superfamily (14) in
osteoblast-like MC3T3-E1 cells. It is well known that three MAP
kinases are the central elements used by mammalian cells to
transduce diverse messages (14).
Furthermore, we showed that Rho-kinase functions at a point
upstream from p38 MAP kinase in PGD2-induced IL-6
synthesis in MC3T3-E1 cells and that Rho-kinase inhibitors failed
to affect the PGD2-stimulated phosphorylation of p44/p42
MAP kinase (22). Therefore, it
seems unlikely that Rho-kinase affects the
PGD2-stimulated HSP27 induction through the modulation
of p44/p42 MAP kinase in MC3T3-E1 cells. In the present study, we
showed that Y27632 and fasudil markedly attenuated the
PGD2-induced phosphorylated levels of SAPK/JNK. Taking
our findings into account as a whole, it is most likely that
Rho-kinase functions at a point upstream from SAPK/JNK and p38 MAP
kinase among the MAP kinase superfamily in the
PGD2-stimulated HSP27 induction in osteoblast-like
MC3T3-E1 cells.
Rho-kinase is well known to play an important role
in a variety of cellular functions, particularly vascular smooth
muscle contraction (17–19). In bone metabolism, it has been
reported that the activation of Rho-kinase suppresses the
differentiation of osteoblasts and induces their proliferation
(21). Our present results reveal
that Rho-kinase stimulated by PGD2 acts as a positive
regulator in HSP27 induction in osteoblasts. It is generally
recognized that most HSPs including HSP27 function mainly as
molecular chaperones in protein folding, oligomerization and
translocation (2). Although the
physiological significance of HSP27 in osteoblasts has not yet been
clarified, our finding that SAPK/JNK and p38 MAP kinase, but not
p44/p42 MAP kinase, are regulated by Rho-kinase suggests the
importance of the fine tuning of MAP kinase-mediated HSP27
induction stimulated by PGD2, which is significantly
correlated with IL-6 synthesis (22), in bone remodeling. Our present
findings regarding the involvement of Rho-kinase in the induction
of HSP27 in osteoblasts present novel aspects of Rho-kinase and/or
HSP27 as therapeutic targets for metabolic or inflammatory bone
diseases such as osteoporosis and rheumatic arthritis. However, the
exact role of Rho-kinase in osteoblasts remains to be clarified.
Further investigations are necessary to elucidate the exact roles
of Rho-kinase in bone metabolism.
In conclusion, our results strongly suggest that
Rho-kinase inhibitors suppress PGD2-stimulated HSP27
induction via the activation of both SAPK/JNK and p38 MAP kinase in
osteoblasts.
Acknowledgements
We are grateful to Yoko Kawamura for
her skillful technical assistance. This investigation was supported
in part by a Grant-in-Aid for Scientific Research (19591042) from
the Ministry of Education, Science, Sports and Culture of Japan,
the Foundation for Growth Science, the Research Grants for
Longevity Sciences (21A-4), Research on Proteomics and Research on
Longevity Sciences from the Ministry of Health, Labour and Welfare
of Japan.
References
|
1.
|
Hendrick JP and Hartl FU: Molecular
chaperone functions of heat-shock proteins. Annu Rev Biochem.
62:349–384. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Benjamin IJ and McMillan DR: Stress (heat
shock) proteins: molecular chaperones in cardiovascular biology and
disease. Circ Res. 83:117–132. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Landry J, Lambert H, Zhou M, Lavoie JN,
Hickey E, Weber LA and Anderson CW: Human HSP27 is phosphorylated
at serines 78 and 82 by heat shock and mitogen-activated kinases
that recognize the same amino acid motif as S6 kinase II. J Biol
Chem. 267:794–803. 1992.PubMed/NCBI
|
|
4.
|
Kato K, Hasegawa K, Goto S and Inaguma Y:
Dissociation as a result of phosphorylation of an aggregated form
of the small stress protein, hsp27. J Biol Chem. 269:11274–11278.
1994.PubMed/NCBI
|
|
5.
|
Rogalla T, Ehrnsperger M, Preville X,
Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo AP,
Buchner J and Gaestel M: Regulation of Hsp27 oligomerization,
chaperone function, and protective activity against oxidative
stress/tumor necrosis factor α by phosphorylation. J Biol Chem.
274:18947–18956. 1999.PubMed/NCBI
|
|
6.
|
Nijweide PJ, Burger EH and Feyen JH: Cells
of bone: proliferation, differentiation, and hormonal regulation.
Physiol Rev. 66:855–886. 1986.PubMed/NCBI
|
|
7.
|
Shakoori AR, Oberdorf AM, Owen TA, Weber
LA, Hickey E, Stein JL, Lian JB and Stein GS: Expression of heat
shock genes during differentiation of mammalian osteoblasts and
promyelocytic leukemia cells. J Cell Biochem. 48:277–287. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Cooper LF and Uoshima K: Differential
estrogenic regulation of small M(r) heat shock protein expression
in osteoblasts. J Biol Chem. 269:7869–7873. 1994.PubMed/NCBI
|
|
9.
|
Morgan EF, Barnes GL and Einhorn TA: The
bone organ system: Form and function. Osteoporosis. 3rd edition.
Marcus R, Feldman D, Nelson D and Rosen CJ: Elsevier Press; Boston:
pp. 3–25. 2008
|
|
10.
|
Hikiji H, Takato T, Shimizu T and Ishii S:
The roles of prostanoids, leukotrienes, and platelet-activating
factor in bone metabolism and disease. Prog Lipid Res. 47:107–126.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Tasaki Y, Takamori R and Koshihara Y:
Prostaglandin D2 metabolite stimulates collagen
synthesis by human osteoblasts during calcification.
Prostaglandins. 41:303–313. 1991.PubMed/NCBI
|
|
12.
|
Gallant MA, Samadfam R, Hackett JA,
Antoniou J, Parent JL and de Brum-Fernandes AJ: Production of
prostaglandin D(2) by human osteoblasts and modulation of
osteoprotegerin, RANKL, and cellular migration by DP and CRTH2
receptors. J Bone Miner Res. 20:672–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Tokuda H, Kozawa O, Harada A and Uematsu
T: Prostaglandin D2 induces interleukin-6 synthesis via
Ca2+ mobilization in osteoblasts: regulation of protein
kinase C. Prostaglandins Leukot Essent Fatty Acids. 61:189–194.
1999.
|
|
14.
|
Widmann C, Gibson S, Jarpe MB and Johnson
GJ: Mitogen-activated protein kinase: conservation of a
three-kinase module from yeast to human. Physiol Rev. 79:143–180.
1999.PubMed/NCBI
|
|
15.
|
Kozawa O, Otsuka T, Hatakeyama D, Niwa M,
Matsuno H, Ito H, Kato K, Matsui N and Uematsu T: Mechanism of
prostaglandin D2-stimulated heat shock protein 27
induction in osteoblasts. Cell Signal. 13:535–541. 2001.PubMed/NCBI
|
|
16.
|
Yoshida M, Niwa M, Ishisaki A, Hirade K,
Ito H, Shimizu K, Kato K and Kozawa O: Methotrexate enhances
prostaglandin D2-stimulated heat shock protein 27
induction in osteoblasts. Prostaglandins Leukot Essent Fatty Acids.
71:351–362. 2004.PubMed/NCBI
|
|
17.
|
Fukata Y, Amano M and Kaibuchi K:
Rho-Rho-kinase pathway in smooth muscle contraction and
cytoskeletal reorganization of non-muscle cells. Trends Pharmacol
Sci. 22:32–39. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Riento K and Ridley AJ: Rocks:
multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol.
4:446–456. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Shimokawa H and Rashid M: Development of
Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol
Sci. 28:296–302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Windischhofer W, Zach D, Fauler G,
Raspotnig G, Kofeler H and Leis HJ: Involvement of Rho and p38 MAPK
in endothelin-1-induced expression of PGHS-2 mRNA in
osteoblast-like cells. J Bone Miner Res. 17:1774–1784. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Harmey D, Stenbeck G, Nobes CD, Lax AJ and
Grigoriadis AE: Regulation of osteoblast differentiation by
Pasteurella multocida toxin (PMT): a role for Rho GTPase in
bone formation. J Bone Miner Res. 19:661–670. 2004.
|
|
22.
|
Tokuda H, Takai S, Matsushima-Nishiwaki R,
Hanai Y, Adachi S, Minamitani C, Mizutani J, Otsuka T and Kozawa O:
Function of Rho-kinase in prostaglandin D2-induced
interleukin-6 synthesis in osteoblasts. Prostaglandins Leukot
Essent Fatty Acids. 79:41–46. 2008. View Article : Google Scholar
|
|
23.
|
Sudo H, Kodama H, Amagai Y, Yamamoto S and
Kasai S: In vitro differentiation and calcification in a new clonal
osteogenic cell line derived from newborn mouse calvaria. J Cell
Biol. 96:191–198. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Kozawa O, Suzuki A, Tokuda H and Uematsu
T: Prostaglandin F2α stimulates interleukin-6 synthesis
via activation of PKC in osteoblast-like cells. Am J Physiol.
272:E208–E211. 1997.PubMed/NCBI
|
|
25.
|
Kato K, Ito H, Hasegawa K, Inaguma Y,
Kozawa O and Asano T: Modulation of the stress-induced synthesis of
hsp27 and αB-crystallin by cyclic AMP in C6 rat glioma cells. J
Neurochem. 66:946–950. 1996.
|
|
26.
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|