Introduction
Glioblastomas, the most common primary malignant
brain tumors, infiltrate the surrounding normal brain tissues, and
therefore are almost always non-curable even with surgical
resection. Despite recent refined therapeutic strategies, the
regrowth of tumor cells residing in the adjacent brain inevitably
occurs, resulting in a dismal prognosis for patients with
glioblastoma (1). Recently,
mesenchymal stem cells (MSCs) that have inherent tumor-tropic
properties have been tested as a vehicle for delivery of
therapeutic genes such as suicide genes and cytokine genes in
experimental gliomas (2–13). MSCs used as a vehicle for suicide
gene therapy have obtained sufficiently effective results both in
intracranial glioma (14) and in
the leptomeningeal glioma models (15). However, little is known about the
mechanisms involved in the migratory activity of MSCs toward
tumors.
Cyclin-dependent kinase (CDK) inhibitor
p27Kip1 is a well-characterized tumor suppressor
and is frequently down-regulated by enhanced degradation of p27 in
malignancies. The decreased expression of p27 is usually correlated
with increased tumor aggressiveness and poor clinical outcome.
Notably, high p27 levels correlate with high tumor grade, poor
prognosis and increased metastasis. This has been observed, for
instance, in various types of tumors (breast, cervix, esophagus and
uterus) and in certain types of lymphomas and leukemias (16–18).
These observations suggest that deregulation of p27 in tumors may
serve to uncouple it from its cell cycle-inhibitory function,
possibly by being excluded from the nucleus. Once in the cytoplasm,
p27 may exert other functions, such as the regulation of cell
migration, thereby promoting tumor progression and invasiveness
(19,20).
When MSCs are used as a vehicle for glioma gene
therapy, highly migratory MSCs would be more efficient. However,
little is known about whether p27 is involved in the tumor-tropic
properties of MSCs. In the present study, we investigated the
influence of p27 on MSC migration by using MSCs derived from
p27-null and wild-type mice and found that the motility of
p27−/− MSCs was impaired and the numbers of actin stress
fibers of these cells were increased. The in vivo migratory
activity of the p27−/− MSCs toward the tumor in the
mouse brain was lower than that of the p27+/+ MSCs,
suggesting that p27 acted as a stimulator during the migration
process of MSCs.
Materials and methods
Isolation and culture of MSCs
All following experiments were performed according
to the Rules of Animal Experimentation and the Guide for the Care
and Use of Laboratory Animals of the Hamamatsu University School of
Medicine. p27−/− and p27+/+ C57BL/6 mice (8
weeks old) were sacrificed with ether, and the marrow tissue was
obtained from the femurs and tibias as previously described
(21). A single-cell suspension
was obtained by gently aspirating the tissue several times using
the same needle and syringe in 5 ml Murine MSC Growth Medium
(MMSCGM; StemCell Technologies Inc., British Columbia, Canada),
washed one time with 10 ml fresh MMSCGM and passed through a 70-μm
nylon strainer (Falcon, Becton Dickinson Labware, Franklin lakes,
NJ, USA). The cells were then plated into a 25-cm2
tissue culture flask in 5 ml MMSCGM and incubated at 37°C under 5%
CO2. The non-adherent cells were removed by replacing
the medium 24 h after the initial culture. The residual attached
cells were maintained at 37°C in 5% CO2 by exchanging
the medium with fresh medium at 5-day intervals. These cells are
designated as MSCs in the present study.
Wound healing assay
Cells were seeded at 80% confluence in 60-mm dishes
and grown for an additional 24 h. A linear scratch, ∼1 cm wide, was
performed using a rubber policeman across the diameter of the
plate. This was then rinsed with phosphate-buffered saline (PBS).
Cells were fed with growth medium supplement. Cells were incubated
for 24 h, rinsed with PBS, and fixed for 5 min in 95% ethanol/5%
acetic acid at room temperature. For each plate, images were
captured using a dissection microscope (Zeiss) at a magnification
of x20. Then the distance the cells had migrated from the scratch
line at each time point was measured in mm. Cells were pretreated
with 10 μg/ml mitomycin C for 3 h to block cell division in order
to rule out the possibility that the differences in motility were
due to the differences in cell proliferation.
BrdU labeling and immunohistological
analysis
For the in vivo transplantation experiments,
MSC cultures grown for 5 days were pulsed for 48 h with 5 μM
5-bromo-2-deoxyuridine (BrdU) (Sigma) in Eagle's minimal essential
medium supplemented with 10% FBS or for 24 h with 10 μg/ml
bisbenzimide (Sigma) before harvest (22). Cells were harvested by incubation
with 0.25% trypsin for 5 min at room temperature followed by gentle
scraping. For BrdU immunostaining, the DNA was first denatured by
incubating the brain sections (6 μm) in 50% formamide 2X SSC at
65°C for 2 h and then in 2 N HCl at 37°C for 30 min. The sections
were then rinsed with PBS and treated with 1%
H2O2 to block endogenous peroxidase. The
sections were incubated with a mouse monoclonal antibody agaist
BrdU (1:500, Sigma) overnight and incubated with biotinylated
secondary antibody (Dako) for 1 h. Control experiments consisted of
staining brain coronal tissue sections as described above, but the
primary antibodies were omitted.
In vivo migratory capacity of MSCs toward
brain tumors in nude mice
To compare the in vivo migratory capacity and
tropism of p27−/− and p27+/+ MSCs toward
glioma, we injected 2×104 C6 rat glioma cells into one
side of the mouse brain hemisphere and 1×105
BrdU-labeled MSCs into the opposite hemisphere. The method of cell
implantation was the same as described previously (21). Briefly, 10 BALB/c nude mice (6
weeks old, Nippon SLC, Hamamatsu, Japan) were anesthetized with 0.4
ml/100 g equithesin and placed in a stereotaxic apparatus
(Narishige Scientific Instrument Lab., Tokyo, Japan). A 25-gauge
needle was inserted into the target point (0.2 mm posterior to the
bregma, 2 mm left of the midline, 3 mm ventral from the dura), and
2×104 C6 cells were injected with a 10-μl microsyringe
(Hamilton Company, Reno, NV, USA) and a microinjector (Harvard
Apparatus Inc., South Natick, MA, USA) for 5 min. After one week,
2×104 BrdU-labeled MSCs (p27−/− or
p27+/+, n=5 for each group) were injected at the mirror
point in the contralateral hemisphere (0.2 mm posterior, 2 mm
right). The animals were sacrificed on day 10. Serial coronal
sections (5 μm) were obtained and stained with hematoxylin and
eosin, and the tumor area of each section was measured using NIH
Image software (rsbweb.nih.gov). The adjacent sections
were stained with the BrdU antibody as described above, and the MSC
infiltrating area was detected. The migration potential of MSCs was
defined as the MSC infiltration area/C6 tumor region.
Results
p27−/− MSCs exhibit no
different morphological characteristics but have elevated
growth
The murine p27Kip1 genomic locus
comprises three exons spanning ∼4 kb. The targeting construct was
designed to delete the exon 1 and 2 of the
p27Kip1 gene, since the protein-coding region
resides only in exons 1 and 2 (23). MSCs were isolated from
p27−/− and p27+/+ mice as described in
Materials and methods. There was no difference in the morphological
characteristics between p27−/− and p27+/+
MSCs, but the p27−/− MSCs had an elevated proliferation
rate compared to p27+/+ MSCs due to the function of p27
as an inhibitor of CDK. The doubling growth time of
p27+/+ MSCs (122 h) was more than twice that of
p27−/− MSCs (60 h).
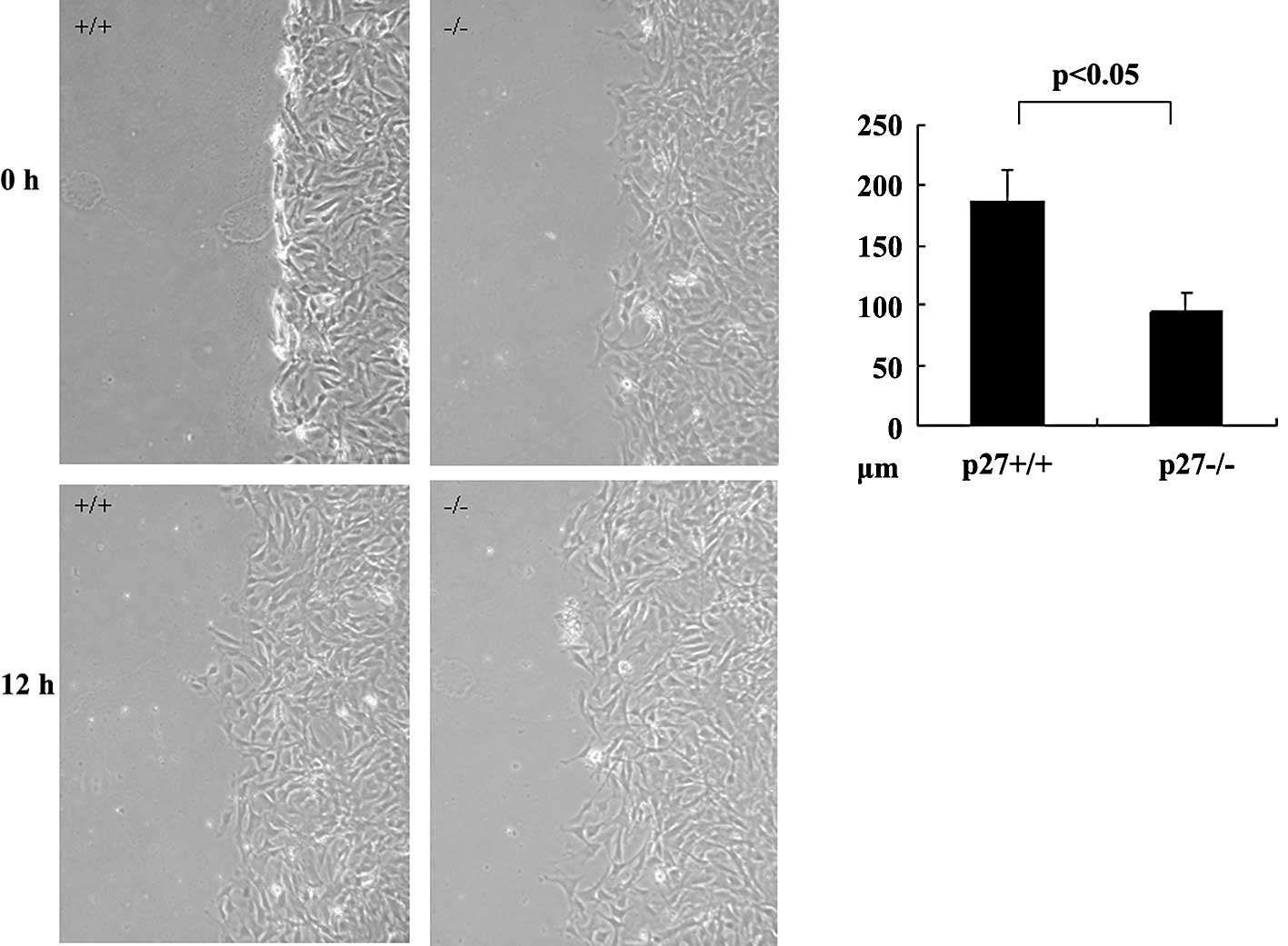
Impaired motility in p27−/−
MSCs
To further characterize the function of p27 during
MSC migration, the motility of MSCs derived from p27−/−
and p27+/+ mice was measured using the wound healing
assay. The wound healing assay was designed as a method of
simulating the ability of a cell to reconstruct a tissue. Cell
motility in this assay system is dependent upon reorganization of
the actin cytoskeleton and the assembly and disassembly of focal
adhesion complexes, processes which are governed by the Rho family
GTPases Rho, Rac and Cdc42 (24).
Following wounding of a confluent cell monolayer, p27−/−
MSCs exhibited a significant reduction in cell motility compared
with cells derived from wild-type MSCs 24 h later (p<0.05,
Fig. 1).
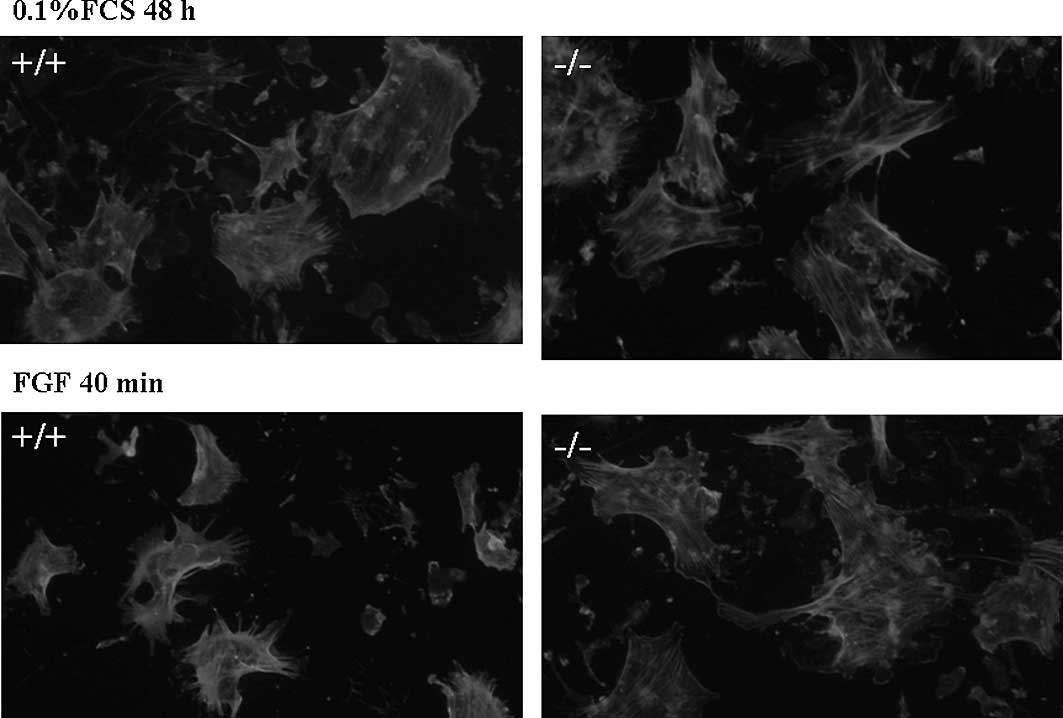
Increased numbers of actin stress fibers
in p27−/− MSCs
It was reported that p27−/− fibroblasts
have elevated amounts of endogenous Rho-GTP due to inhibition of
RhoA by p27. p27−/− fibroblasts had Rho-dependent
cellular phenotypes, including increased numbers of focal adhesions
and actin stress fibers, increased phosphorylation of cofilin (a
target molecule of the Rho pathway), and a marked decrease in
motility (25). We therefore
surveyed the actin cytoskeleton of p27−/− and
p27+/+ MSCs by immunocytochemistry using phalloidin.
Since various cytokines (including EPO, IL-6, SDF1-β, FGF and VEGF)
showed the ability to increase the migratory activity of MSCs, we
analyzed the effects of FGF on the actin cytoskeleton. Wild-type
MSCs had few actin stress fibers in serum-starved conditions, and
FGF stimulation evoked a dramatic rearrangement of the actin
cytoskeleton (Fig. 2, left). In
contrast, p27−/− cells had an extensive network of
stress fibers in the absence of serum, and FGF stimulation
substantially failed to induce the actin rearrangement (Fig. 2, right).
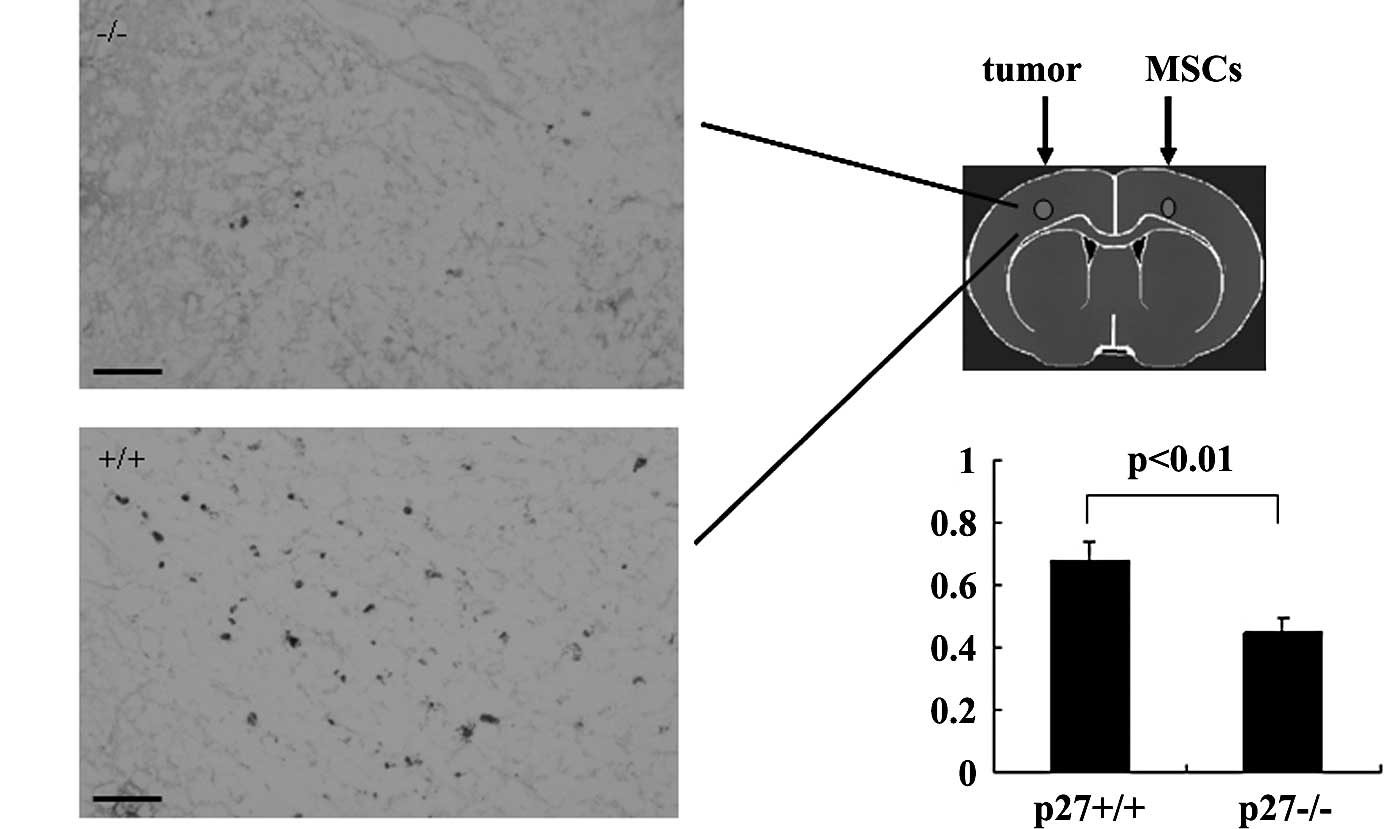
Decreased in vivo migratory capacity of
p27−/− MSCs toward tumors
MSCs have the capacity to migrate specifically
toward tumors. To further compare the tropism of p27−/−
and p27+/+ MSCs, we detected their migratory capacity
toward tumors. We injected BrdU-labeled MSCs on the opposite
hemisphere of the tumor inoculation. MSCs migrated across the
corpus callosum toward the tumor and ultimately entered the tumor
on the opposite side of the brain (Fig. 3A). We compared the tropism and
infiltrative potential of p27+/+ and p27−/−
MSCs using the ratio of the area of the BrdU-labeled MSC
infiltration region divided by the area of the tumor region. We
found significantly fewer labeled p27−/− MSCs in the
tumor compared with p27+/+ MSCs (p<0.01, Fig. 3B). Very few p27+/+ MSCs
were found in the normal brain tissue beyond the injection
site.
Discussion
Since Aboody and colleagues (26) first demonstrated the potent
migratory ability of neural stem cells to brain tumors, this
ability of neural stem cells has been confirmed by numerous
studies, including ours (27). It
has also been demonstrated that an established rat glioma can be
successfully treated by genetically engineered neural stem cells
(21). However, there is a
limitation to using neural stem cells obtained from the patients
with glioblastomas due to the invasiveness and the low
proliferative activity of neural stem cells obtained from adult
brains. As an alternative, we have been testing the use of MSCs
obtained from the bone marrow of patients instead of neural stem
cells. It has previously been demonstrated that rat brain tumors
can be effectively treated by rat MSCs transduced with the herpes
simplex virus-thymidine kinase gene, the same gene we used with
neural stem cells (14). If MSCs
can be used for the treatment of malignant glioma that deeply
infiltrates the surrounding normal brain tissues, the potency of
the tumor-homing activity of MSCs is particularly important for the
success of this treatment strategy. However, the precise mechanisms
of tumor tropism of neural and other types of stem cells are still
unknown. Some recent reports suggest several molecular mechanisms
of MSC migration toward gliomas (28–30).
p27, a CDK inhibitor, has also been known to regulate cell
migration as well as cell proliferation. Therefore, in the present
study we investigated the role of p27 on the migratory activity of
MSCs.
We measured the motility of MSCs derived from
p27-null mouse using a wound healing assay and found that
p27−/− MSCs had decreased motility and increased numbers
of actin stress fibers. Consistent with the results in mouse
embryonic fibroblasts, p27 works as a stimulator during the
migratory process of MSCs via modulation of the Rho pathway
(25). MSCs are known to have
strong tropism toward tumors, and when MSCs are implanted in one
hemisphere and tumor cells in the other hemisphere, most MSCs
travel across the corpus callosum and gather around the tumor
(5,8,9,12,13,31).
Our in vivo study, using a contralateral hemisphere
injection model, demonstrated that the migratory activity of MSCs
toward tumors was also significantly decreased in p27−/−
MSCs.
p27 generally suppresses CDK activity in
proliferating cells. Another role of p27 in cell migration has been
recently suggested in vitro. However, the physiological
importance of p27 in cell migration remains elusive, since
p27-deficient mice have no obvious migration defect-related
phenotypic abnormality.p27 has been alternatively reported as an
inhibitor or stimulator of cell migration in primary or stabilized
cells of different origins. Controversial results for the role of
p27 in cell motility are mainly attributable to both types of cells
and the methods of motility assay used. Cell migration is a dynamic
process governed by intracellular and extracellular stimuli that
promote the formation of focal adhesions between the cell membrane
and the extracellular matrix. In some cell types p27 increases cell
motility, whereas in others it decreases it. These differences
possibly originate from cell type-specific variation in the
relative balance between Rho and Rac activity.
Motility assays mainly consist of the
fibronectin-coated transwell assay and the wound healing assay.
Cell migration through the pores of fibronectin-coated transwells
is promoted by amoeboid movement that responds to attractant
stimuli lacking an obvious polarization, which depends largely on
propulsive forces and on cytoplasmic streaming for their motility
(32). In this case, p27 may act
as an inhibitor of cell migration by altering microtubule
stability, which then impairs the cytoskeletal modifications
necessary for the cell to move. The wound healing assay is an in
vitro directional motility assay designed to simulate the
ability of cells to reconstruct tissues. It is possible that p27,
contributing to stabilize the perinuclear network of microtubules,
enforces cell polarity and favors the movements of highly polarized
cells. p27−/− fibroblasts failed to reorient
glutamylated microtubules to the scratched area during the wound
healing assay, since they migrated toward the wounded area with
altered cell trajectories (33).
Accordingly, p27 expression stimulates wound healing cell motility
by decreasing the RhoA-ROCK1 activity and inhibits the Rho pathway
by blocking the guanine-nucleotide exchange-mediated activation of
Rho (25).
Our data suggested that p27 increases cell migration
under both in vitro and in vivo conditions as shown
previously (25). It seems that
p27 regulates the migration process of MSCs via modulation of the
Rho pathway, since p27−/− MSCs showed increased numbers
of stress fibers and were largely refractory to FGF stimuli. p27
may be acting as a tumor suppressor and as an oncogene, depending
on its subcellular localization, which may explain the different
regulatory functions of p27 in different cell lines. The results of
the present study would open a window on the mechanisms
contributing to the regulation of MSCs migration, though further
studies are obviously required to understand the behaviors of stem
cells in the brain. We believe that knowledge of the mechanisms
involved in the migratory process and enhancement of the potency of
MSC migration are directly related to the efficacy of stem
cell-based strategies for glioma therapy.
References
|
1.
|
DeAngelis LM: Brain tumors. N Engl J Med.
344:114–123. 2001. View Article : Google Scholar
|
|
2.
|
Kim SM, Lim JY, Park SI, et al: Gene
therapy using TRAIL-secreting human umbilical cord blood-derived
mesenchymal stem cells against intracranial glioma. Cancer Res.
68:9614–9623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kinoshita Y, Kamitani H, Mamun MH, et al:
A gene delivery system with a human artificial chromosome vector
based on migration of mesenchymal stem cells towards human
glioblastoma HTB14 cells. Neurol Res. 2009, (E-pub ahead of
print).
|
|
4.
|
Kucerova L, Altanerova V, Matuskova M,
Tyciakova S and Altaner C: Adipose tissue-derived human mesenchymal
stem cells mediated prodrug cancer gene therapy. Cancer Res.
67:6304–6313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lee J, Elkahloun AG, Messina SA, et al:
Cellular and genetic characterization of human adult bone
marrow-derived neural stem-like cells: a potential antiglioma
cellular vector. Cancer Res. 63:8877–8889. 2003.PubMed/NCBI
|
|
6.
|
Menon LG, Kelly K, Yang HW, Kim SK, Black
PM and Carroll RS: Human bone marrow-derived mesenchymal stromal
cells expressing S-TRAIL as a cellular delivery vehicle for human
glioma therapy. Stem Cells. 27:2320–2330. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Miletic H, Fischer YH, Litwak S, et al:
Bystander killing of malignant glioma by bone marrow-derived
tumor-infiltrating progenitor cells expressing a suicide gene. Mol
Ther. 15:1373–1381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Nakamizo A, Marini F, Amano T, et al:
Human bone marrow-derived mesenchymal stem cells in the treatment
of gliomas. Cancer Res. 65:3307–3318. 2005.PubMed/NCBI
|
|
9.
|
Nakamura K, Ito Y, Kawano Y, et al:
Antitumor effect of genetically engineered mesenchymal stem cells
in a rat glioma model. Gene Ther. 11:1155–1164. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Sasportas LS, Kasmieh R, Wakimoto H, et
al: Assessment of therapeutic efficacy and fate of engineered human
mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci USA.
106:4822–4827. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Uchibori R, Okada T, Ito T, et al:
Retroviral vector-producing mesenchymal stem cells for targeted
suicide cancer gene therapy. J Gene Med. 11:373–381. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Wu X, Hu J, Zhou L, et al: In vivo
tracking of superparamagnetic iron oxide nanoparticle-labeled
mesenchymal stem cell tropism to malignant gliomas using magnetic
resonance imaging. Laboratory investigation. J Neurosurg.
108:320–329. 2008. View Article : Google Scholar
|
|
13.
|
Yuan X, Hu J, Belladonna ML, Black KL and
Yu JS: Interleukin-23-expressing bone marrow-derived neural
stem-like cells exhibit antitumor activity against intracranial
glioma. Cancer Res. 66:2630–2638. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Amano S, Li S, Gu C, et al: Use of
genetically engineered bone marrow-derived mesenchymal stem cells
for glioma gene therapy. Int J Oncol. 35:1265–1270. 2009.PubMed/NCBI
|
|
15.
|
Gu C, Li S, Tokuyama T, Yokota N and Namba
H: Therapeutic effect of genetically engineered mesenchymal stem
cells in rat experimental leptomeningeal glioma model. Cancer Lett.
298:256–262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Bloom J and Pagano M: Deregulated
degradation of the cdk inhibitor p27 and malignant transformation.
Semin Cancer Biol. 13:41–47. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Philipp-Staheli J, Payne SR and Kemp CJ:
p27(Kip1): regulation and function of a haploinsufficient tumor
suppressor and its misregulation in cancer. Exp Cell Res.
264:148–168. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Gao Y, Kitagawa K, Hiramatsu Y, et al:
Up-regulation of GPR48 induced by down-regulation of p27Kip1
enhances carcinoma cell invasiveness and metastasis. Cancer Res.
66:11623–11631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Rodier G, Montagnoli A, DiMarcotullio L,
et al: p27 cytoplasmic localization is regulated by phosphorylation
on Ser10 and is not a prerequisite for its proteolysis. EMBO J.
20:6672–6682. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Ishida N, Hara T, Kamura T, Yoshida M,
Nakayama K and Nakayama KI: Phosphorylation of p27Kip1 on serine 10
is required for its binding to CRM1 and nuclear export. J Biol
Chem. 277:14355–14358. 2002. View Article : Google Scholar
|
|
21.
|
Li S, Tokuyama T, Yamamoto J, Koide M,
Yokota N and Namba H: Bystander effect-mediated gene therapy of
gliomas using genetically engineered neural stem cells. Cancer Gene
Ther. 12:600–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Chen J, Zhang ZG, Li Y, et al: Intravenous
administration of human bone marrow stromal cells induces
angiogenesis in the ischemic boundary zone after stroke in rats.
Circ Res. 92:692–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Nakayama K, Ishida N, Shirane M, et al:
Mice lacking p27(Kip1) display increased body size, multiple organ
hyperplasia, retinal dysplasia, and pituitary tumors. Cell.
85:707–720. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Nobes CD and Hall A: Rho GTPases control
polarity, protrusion, and adhesion during cell movement. J Cell
Biol. 144:1235–1244. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Besson A, Gurian-West M, Schmidt A, Hall A
and Roberts JM: p27Kip1 modulates cell migration through the
regulation of RhoA activation. Genes Dev. 18:862–876. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Aboody KS, Brown A, Rainov NG, et al:
Neural stem cells display extensive tropism for pathology in adult
brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA.
97:12846–12851. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Li S, Gao Y, Tokuyama T, et al:
Genetically engineered neural stem cells migrate and suppress
glioma cell growth at distant intracranial sites. Cancer Lett.
251:220–227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Xu G, Jiang XD, Xu Y, et al:
Adenoviral-mediated interleukin-18 expression in mesenchymal stem
cells effectively suppresses the growth of glioma in rats. Cell
Biol Int. 33:466–474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Ho IA, Chan KY, Ng WH, et al: Matrix
metalloproteinase 1 is necessary for the migration of human bone
marrow-derived mesenchymal stem cells toward human glioma. Stem
Cells. 27:1366–1375. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Zhao D, Najbauer J, Garcia E, et al:
Neural stem cell tropism to glioma: critical role of tumor hypoxia.
Mol Cancer Res. 6:1819–1829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Bexell D, Gunnarsson S, Tormin A, et al:
Bone marrow multi-potent mesenchymal stroma cells act as
pericyte-like migratory vehicles in experimental gliomas. Mol Ther.
17:183–190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Webb DJ and Horwitz AF: New dimensions in
cell migration. Nat Cell Biol. 5:690–692. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Baldassarre G, Belletti B, Nicoloso MS, et
al: p27(Kip1)-stathmin interaction influences sarcoma cell
migration and invasion. Cancer Cell. 7:51–63. 2005. View Article : Google Scholar : PubMed/NCBI
|