Introduction
Black chokeberry (Aronia melanocarpa) is a
shrub of the Rosaceae family (1).
It originates from North America (1) and bears deep-purple fruits
approximately 5-10 mm in diameter. It has been cultivated in
Russia, other European countries and more recently, in Japan. The
fruits of the black chokeberry (hereafter designated as aronia
berries) are excellent sources of anthocyanins and reportedly have
the highest anthocyanin content among a variety of commonly
consumed foods (2). They are
particularly rich in the anthocyanin, cyanidin-3-galactoside
(3), and contain various
functional components, including carotenoids (i.e.,
β-cryptoxanthin, β-carotene and lutein) and omega-3 fatty acids
(i.e., α-linolenic acid).
Anthocyanins are associated with a bitter and
astringent taste; therefore, aronia berries are generally processed
into food products, including juice, jam and wine. Previous reports
have demonstrated that aronia juice and its extract have
health-promoting properties, such as antioxidant (4-7),
anti-hypertensive (8,9) and anti-coagulant (10) properties; they also have the
ability to improve glucose levels and lipid metabolism (11,12)
and exert hepatoprotective effects (13). During the processing of aronia
juice, the residual fruit and peel (hereafter designated as juice
residue) remain underutilized, even though they are rich in
anthocyanins (14), fiber and
other functional components. Therefore, research into the
utilization of these by-products is warranted. The authors have
previously reported that ethanol extract from aronia juice residue
activates lipolysis in adipocytes (15).
Factors, such as a high-fat diet and a sedentary
lifestyle induce energy imbalance and fat accumulation in the body,
giving rise to obesity. Obesity is a central factor in the
development of insulin resistance. Insulin resistance impairs
glucose tolerance, leading to the elevation of the plasma
concentrations of triglycerides and free fatty acids via the
acceleration of the lipogenic pathway (16). Consequently, obesity is closely
related to a number of chronic diseases, including diabetes,
dyslipidemia, non-alcoholic fatty liver disease and cardiovascular
disorders, among others (17).
Regular exercise and the control of energy intake are the main
recommendations for the prevention and treatment of obesity.
However, diet supplementation with functional foods may further
promote healthy metabolic function in a convenient and accessible
manner.
The present study aimed to investigate whether
aronia juice residue and its ethanol extract can improve metabolic
dysfunction. Thus, the effectiveness of aronia, including its
various functional components, on glucose and lipid metabolism in
high-fat diet-fed mice were investigated.
Materials and methods
Materials
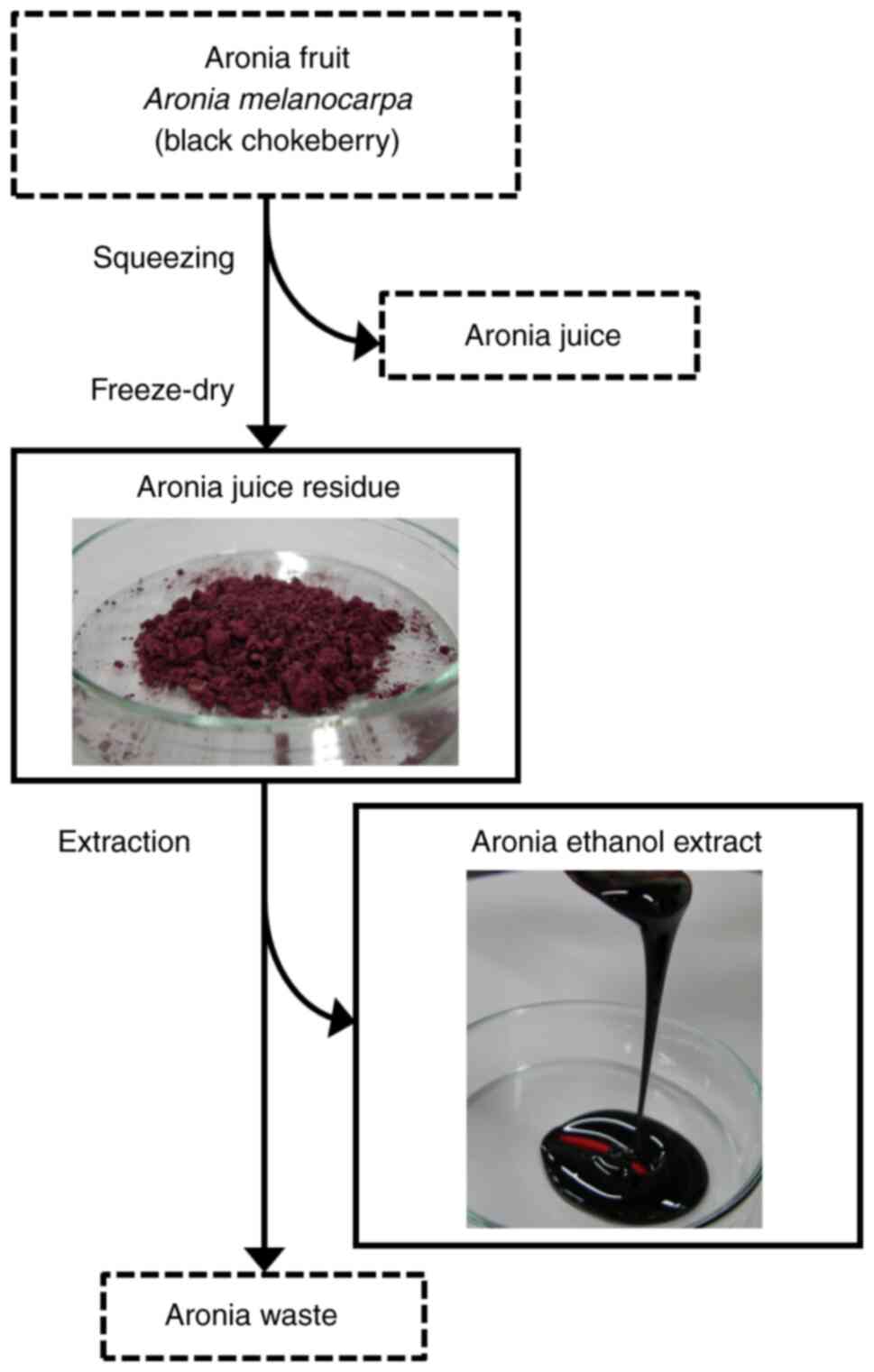
Aronia juice residue and ethanol extract (Fig. 1) were obtained from the Hokkaido
Research Organization, Food Processing Research Center (Hokkaido,
Japan). The nutritional composition of 100 g of aronia juice
residue included: Water, 9.8 g; ash, 1.8 g; protein, 7.4 g; lipid,
7.8 g; and carbohydrate, 73.2 g. Sucrose and L-cystine were
purchased from Kanto Chemical Co., Ltd. Choline bitartrate and
tert-butylhydroquinone were obtained from Sigma-Aldrich;
Merck KGaA. Soybean oil and lard were purchased from Wako Pure
Chemical Industries, Ltd. and Hayashi Chemical Industry Co., Ltd.,
respectively. Other ingredients used for the mouse diets were
obtained from CLEA Japan Inc. For the lipase inhibition assay,
triolein, pancreatin from porcine pancreas, and Trizma base were
purchased from Sigma-Aldrich Japan K.K., whereas sodium
taurocholate was obtained from Wako Pure Chemical Industries,
Ltd.
Preparation of ethanol extract from
aronia juice residue
Freeze-dried aronia juice residue was homogenized
using a Polytron homogenizer (Central Scientific Commerce, Inc.)
with 20 volumes of 99% ethanol and extracted overnight. The extract
was separated from the residue via filtration utilizing a filter
paper (no. 4A) under reduced pressure. This extraction procedure
was repeated twice, and the extract was mixed. Excess ethanol in
the extract was removed via evaporation under reduced pressure. The
yield of the ethanol extraction was 35.7%. The remaining of the
extract was used in the animal diets.
Determination of total polyphenol and
anthocyanin contents in aronia materials
Total polyphenol and anthocyanin contents in aronia
fruit, juice and juice residue were measured using the Folin-Denis
method as previously described (18). The anthocyanin content in the
aronia ethanol extract was analyzed by high-performance liquid
chromatography (HPLC) using the method described in the study by
Cassinese et al (19). The
SHIMADZU CLASS-VP HPLC system and photodiode-array detector
(SPD-M1010Avp) equipped with an Inertsil ODS-3 column (250x4.6 mm
i.d., 5 µm, GL Sciences Inc.) were used. HPLC mobile phases were
solvent A (10% formic acid) and solvent B (10% formic acid-50%
acetonitrile). Elution was performed as follows: 0-30 min, 0-80% B;
30-40 min 80% B. The flow rate was maintained at 1.0 ml/min at
40˚C. The injection volume of the sample was 20 µl and anthocyanin
was detected at 520 nm. The total polyphenol and anthocyanin
contents were expressed as mg of chlorogenic acid and
cyanidin-3-glucoside equivalent, respectively, in each material.
Four anthocyanin content included in the aronia ethanol extract was
mg of each anthocyanin using calibration curve by each anthocyanin
as external standard method (the contents of cyanidin-3-arabinoside
and cyanidin-3-xyloside were quantified by the curve of
cyaniding-3-galactoside).
Animals and diets
Male C57BL/6J mice (4 weeks old, 28 mice, 15.5 g
average body weight) were purchased from Charles River Laboratories
Japan, Inc. The mice were housed individually in plastic cages
(13.6x20.8x11.5 cm) under controlled temperature (23±1˚C), humidity
(45-60%), and a 12/12-h light and dark cycle, with lights on at
8:00 a.m.; the experimental animals were allowed free access to
water and the respective diet throughout the experimental period.
The basal diet contained 7% soybean oil based on the American
Institute of Nutrition (AIN-93G) composition (20). Following a 1-week acclimation on
the basal diet, the mice were assigned into 4 experimental groups
(7 mice per group) depending on the different diets given (Table I) as follows: i) The high-fat diet
group; ii) high-fat diet with 2% aronia juice residue; iii)
high-fat diet with 1% aronia ethanol extract; and iv) high-fat diet
with 2% aronia ethanol extract group. The mice were fed their
respective diets for 26 days, and were then sacrificed under
anesthesia (diethyl ether; 1.9% diethyl ether for inhalant
anesthesia in a glass container) following 12 h of fasting (9:00
a.m., light period). After a terminal blood sample was collected
into a blood collection tube (BD Vacutainer, BD Biosciences), the
liver, epididymal, mesenteric, perirenal, retroperitoneal and
inguinal white adipose tissues (WATs) were immediately excised and
weighed. A specimen of liver tissue was conserved in
RNAlater® (Sigma-Aldrich; Merck KGaA) for use in reverse
transcription-quantitative polymerase chain reaction (PCR)
analysis. The animal experiments were approved by the Ethical
Committee of Experimental Animal Care at Hokkaido University
(Permission no. 09-0094).
 | Table IComposition of the 4 experimental
diets used in the present study. |
Table I
Composition of the 4 experimental
diets used in the present study.
| | Aronia |
|---|
| | | Juice residue | Ethanol
extract |
|---|
| Ingredients
(g/kg) | High-fat diet | 2% | 1% | 2% |
|---|
| Cornstarch | 181.28 | 166.27 | 181.28 | 181.28 |
| Casein | 258.00 | 258.00 | 258.00 | 258.00 |
| Dextrized
cornstarch | 60.20 | 55.21 | 60.20 | 60.20 |
| Sucrose | 100.00 | 100.00 | 100.00 | 100.00 |
| Soybean oil | 70.00 | 70.00 | 60.00 | 50.00 |
| Lard | 230.00 | 230.00 | 230.00 | 230.00 |
| Cellulose | 50.00 | 50.00 | 50.00 | 50.00 |
| Mineral mix
(AIN-93G-MX) | 35.00 | 35.00 | 35.00 | 35.00 |
| Vitamin mix
(AIN-93G-VX) | 10.00 | 10.00 | 10.00 | 10.00 |
| L-cystine | 3.00 | 3.00 | 3.00 | 3.00 |
| Choline
bitartrate | 2.50 | 2.50 | 2.50 | 2.50 |
|
tert-Butylhydroquinone | 0.02 | 0.02 | 0.02 | 0.02 |
| Aronia juice
residue | - | 20.00 | - | - |
| Aronia ethanol
extract | - | - | 10.00 | 20.00 |
Serum glucose and lipid levels
To obtain serum, the collected blood samples were
incubated at 25˚C for 30 min and then centrifuged at 1,300 x g for
10 min at 25˚C. The levels of serum lipids [triglycerides, total
cholesterol, high-density lipoprotein (HDL) cholesterol,
low-density lipoprotein (LDL) cholesterol and free fatty acids]
were analyzed at the Hakodate City Medical Association inspection
center.
Extraction of total lipids from liver
tissue
Total lipids were extracted as previously described
by Folch et al (21).
Briefly, 100 mg of liver tissue were homogenized with 5 volumes of
chloroform-methanol (2/1, v/v) for 2 min, and total lipids were
extracted. The extracts were filtered and dried with an evaporator
and a vacuum pump.
Measurement of triglyceride and total
cholesterol levels in liver tissue
Total lipids extracted from the liver were
solubilized by the addition of 200 µl Triton X-100-methanol (1/1,
v/v) and then dried with nitrogen gas and a vacuum pump. The
triglyceride and total cholesterol levels were measured using
commercial kits, L type TG Wako and Cholesterol C-test Wako (Wako
Pure Chemical Industries, Inc.), respectively, following the
manufacturer's instructions.
RT-qPCR for the quantification of mRNA
expression in liver tissue
Total RNA was extracted from the liver tissue using
the RNeasy Mini kit (Qiagen, Inc.) following the manufacturer's
instructions. Briefly, 30 mg of liver tissue in RNAlater was
homogenized at 4,000 rpm for 30 sec utilizing Micro Smash (Tomy
Seiko Co., Ltd.) with 2 zirconia beads in 300 µl RLT buffer.
cDNA was synthesized using total RNA by a reverse
transcription reaction with the High-Capacity cDNA Archive kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.). qPCR was
performed with an ABI Prism 7500 sequence detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The PCR cycling
conditions were as follows: 50˚C for 2 min and 95˚C for 10 min,
followed by 40 cycles at 95˚C for 15 sec and 60˚C for 1 min.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), sterol regulatory
element-binding protein-1c (SREBP-1c), fatty acid synthase (FAS),
stearoyl-CoA desaturase-1 (SCD1), peroxisome proliferator activated
receptor α (PPARα), long-chain fatty acyl-CoA synthase (ACSL),
carnitine palmitoyltransferase 1a (CPT1a), carnitine
palmitoyltransferase 2 (CPT2), uncoupling protein 2 (UCP2),
hydroxymethylglutaryl-CoA (HMG-CoA) reductase, sterol 14
α-demethylase (CYP51), and cholesterol 7 alpha-hydroxylase (CYP7A1)
mRNA expression levels were measured using Taq Man Gene Expression
Assays (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
following PCR primers were purchased from Applied Biosystems;
Thermo Fisher Scientific, Inc. for use in these assays:
Mm99999915_g1 (Gapdh), Mm00550338_m1 (Srebp-1c), Mm00662319_m1
(Fas), Mm00772290_m1 (Scd1), Mm00440939_m1 (Ppara), Mm00495907_g1
(Ucp2), Mm00550438_m1 (Cpt1a), Mm00487202_m1 (Cpt2), Mm00484217_m1
(Acsl1), Mm01282499_m1 (Hmg-coa reductase), Mm00490968_m1 (Cyp51)
and Mm00484152_m1 (Cyp7a1).
Lipase inhibition assay
Lipase activity was determined by the amount of free
fatty acids released from triolein. The inhibition of lipase
activity was calculated based on the reduction in activity
following the addition of aronia ethanol extract. The substrate
(200 µl of 10 mM triolein dissolved in n-hexane) was placed
into a glass vial, and the solvent was removed using nitrogen gas.
The substrate emulsion was prepared by sonication of triolein with
1 ml of 20 mM sodium taurocholate and 0, 2.5, 5.0, 7.5, or 10.0
mg/ml of aronia ethanol extract in 100 mM Tris-HCl buffer (pH 8.0)
for 5 min. The mixture was incubated with 1 ml lipase solution [10
mg/ml pancreatin from porcine pancreas (Wako Pure Chemical
Industries) in 100 mM Tris-HCl buffer (pH 8.0)] for 6 h at 37˚C
with a magnetic stirrer. The enzyme reaction was terminated by the
addition of 2 volumes n-hexane and centrifugation at 550 x g
for 5 min at 25˚C. The amount of free fatty acids was quantified by
colorimetry, with the absorbance of the n-hexane fraction at
550 nm using the NEFA C-test Wako kit (Wako Pure Chemical
Industries).
Statistical analysis
Data are expressed as the means ± standard deviation
(SD) or as box-and-whisker plots (n=7 mice per group for in
vivo experiments and n=3 per group for in vitro
experiments). Statistical significance was determined by one-way
analysis of variance (ANOVA). When statistically significant
differences (P<0.05) were observed, Dunnett's test was used for
multiple comparisons. P<0.05 or P<0.01 were considered to
indicate statistically significant differences. Data were analyzed
utilizing Excel Tokei software 6.0 (Esumi Co., Ltd.).
Results
Aronia berries are known as excellent sources of
polyphenols and particularly, anthocyanins. First, the present
study determined the total polyphenol and anthocyanin contents in
the aronia materials used. The total polyphenol contents of aronia
fruit, juice and juice residue were 491.4±52.2, 57.5±0.78 and
433.9±51.4 mg/100 g dry matter, respectively (Table II). The distribution of the
anthocyanin content in each material demonstrated an almost equal
ratio to that of the total polyphenol content. These results
indicate that the majority of polyphenols and anthocyanins of the
aronia fruit remain in its juice residue instead of in the juice
when the fruit is squeezed. Anthocyanins in aronia fruit, juice and
juice residue accounted for almost all of the total polyphenol
content. Furthermore, the anthocyanin content in the aronia ethanol
extract was 1446.7±63.4 mg/100 g dry matter and was 3.4-fold times
higher than that in the juice residue. The aronia ethanol extract
included several glycosides of cyanidin, predominantly
cyanidin-3-galactoside and cyanidin-3-arabinoside (886.7±4.7 mg and
456.7±41.1 mg/100 g dry matter, respectively) (Table II).
 | Table IITotal polyphenol and anthocyanin
contents in aronia materials. |
Table II
Total polyphenol and anthocyanin
contents in aronia materials.
|
Polyphenols/anthocyanins | Aronia |
|---|
| Polyphenols (mg/100
g dry matter) | Fruits | Juice | Juice residue | Ethanol
extract |
|---|
| Total polyphenol
content (chlorogenic acid equivalent) | 491.4±52.2 | 57.5±0.78 | 433.9±51.4 | |
| Anthocyanin content
(cyanidin 3-glucoside equivalent) | 486.2±45.4 | 57.1±0.76 | 429.1±4.6 | 1446.7±63.4 |
| Anthocyanins
(mg/100 g dry matter) | Ethanol
extract | | | |
|
Cyanidin-3-galactoside | 886.7±4.7 | | | |
|
Cyanidin-3-arabinoside | 456.7±41.1 | | | |
|
Cyanidin-3-xyloside | 60.0±8.2 | | | |
| Cyanidin
3-glucoside | 43.3±9.4 | | | |
To utilize these potential aronia by-products, the
effects of aronia juice residue and its ethanol extract on glucose
and lipid metabolism were evaluated through the administration of
high-fat diets, including aronia by-products, to C57BL/6J mice.
Following the experimental feeding period, the final
body weights of the mice did not differ significantly among the
mice from the 4 experimental groups (Table III), although the food intake was
higher in the aronia juice residue group than in the high-fat diet
group. Furthermore, the administration of aronia juice residue and
ethanol extract did not significantly change the weight of the
liver and white adipose tissues (Table III). The blood glucose levels
were decreased in mice fed the aronia juice residue (107.0±16.8
mg/dl) or ethanol extracts (102.3±17.7 and 101.6±32.4 mg/dl for 1
and 2% extract, respectively) compared to those in mice fed the
high-fat diet (130.7±44.8 mg/dl), although differences among the
groups did not reach statistical significance (Table III).
 | Table IIIEffects of aronia juice residue and
ethanol extract on body parameters, food intake and blood glucose
levels in C57/BL6J mice fed the fed high-fat diet. |
Table III
Effects of aronia juice residue and
ethanol extract on body parameters, food intake and blood glucose
levels in C57/BL6J mice fed the fed high-fat diet.
| | Aronia |
|---|
| | | Juice residue | Ethanol
extract |
|---|
| Parameter | High-fat diet | 2% | 1% | 2% |
|---|
| Final body weight
(g) | 27.2±1.0 | 28.0±2.2 | 27.9±1.8 | 27.0±1.5 |
| Food intake (g/26
days) | 77.4±9.3 |
90.9±6.5a | 81.5±4.1 | 86.5±11.6 |
| Liver (g/100 g body
weight) | 3.9±0.4 | 3.7±0.2 | 3.6±0.1 | 3.9±0.1 |
| White adipose
tissues (g/100 g body weight) | 5.7±0.7 | 6.2±1.2 | 5.9±0.8 | 5.9±0.7 |
| Blood glucose level
(mg/dl) | 130.7±44.8 | 107.0±16.8 | 102.3±17.7 | 101.6±32.4 |
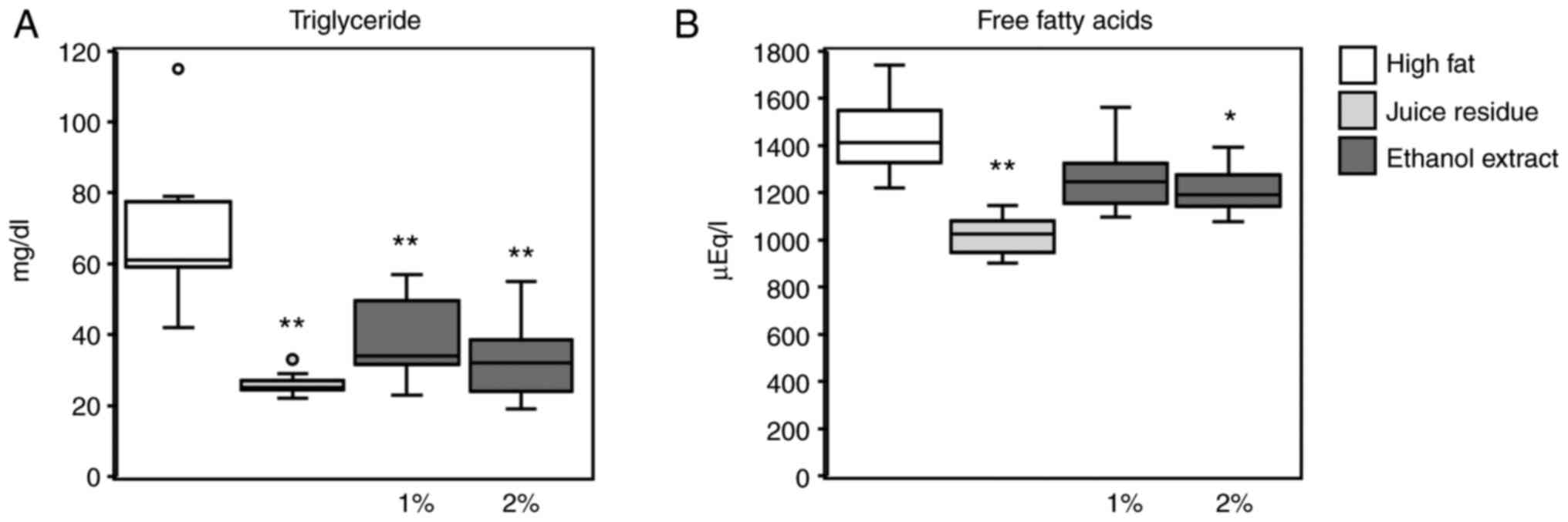
The serum triglyceride levels were significantly
lower in mice fed the 2% aronia juice residue (26.1±3.7 mg/dl), and
1 and 2% aronia ethanol extract diets (39.4±12.5 and 33.0±12.4
mg/dl, respectively) than those in the high-fat diet-fed mice
(70.1±23.3 mg/dl) (Fig. 2A).
Furthermore, the serum free fatty acid levels were decreased in the
2% juice residue and 2% aronia ethanol extract groups compared to
the high-fat diet group (Fig. 2B).
Although the serum free fatty acid levels in the 1% ethanol extract
group were reduced compared to those in the high-fat diet group,
the difference was not significant.
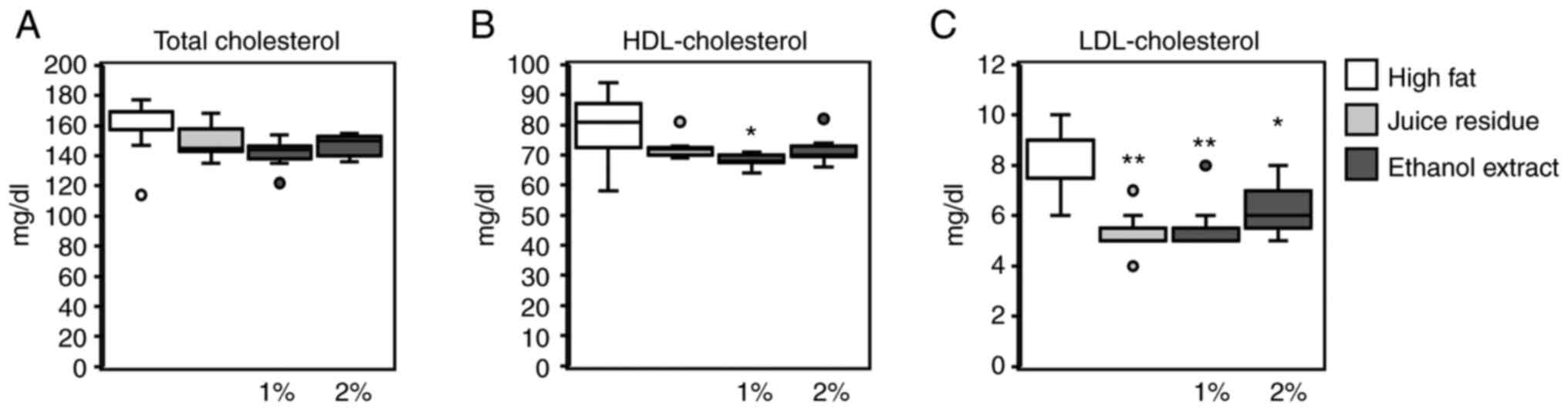
The total cholesterol levels in serum did not differ
significantly among the 4 groups (Fig.
3A). However, the HDL cholesterol levels were significantly
lower in mice the fed 1% aronia ethanol extract diet than in the
mice fed the high-fat diet (Fig.
3B). The levels of LDL cholesterol were significantly lower in
the mice fed the aronia juice residue, and the 1 and 2% aronia
ethanol extract diets than in the mice fed the high-fat diet
(Fig. 3C).
Due to the observed reductions in triglycerides,
free fatty acids and the LDL cholesterol content, the levels of
total hepatic lipids, triglycerides and cholesterol were measured
(Table IV). The total liver lipid
and triglyceride content did not differ significantly among the 4
experimental groups. Significant increases (P<0.05) in total
cholesterol levels were observed in the livers of the mice fed the
aronia juice residue and 1% ethanol extract compared to the mice
fed the high-fat diet (Table IV).
The mice fed the 2% ethanol extract also exhibited an increase in
total cholesterol levels compared to the mice fed the high-fat
diet, although the difference was not significant.
 | Table IVLevels of total lipids, triglycerides
and total cholesterols in the livers of C57BL/6J mice fed the
high-fat diet with aronia juice residue and ethanol extract. |
Table IV
Levels of total lipids, triglycerides
and total cholesterols in the livers of C57BL/6J mice fed the
high-fat diet with aronia juice residue and ethanol extract.
| | Aronia |
|---|
| | | Juice residue | Ethanol
extract |
|---|
| Parameter | High-fat | 2% | 1% | 2% |
|---|
| Total lipids (mg/g
liver) | 73.2±14.7 | 115.3±43.4 | 72.2±34.7 | 104.1±36.3 |
| Triglycerides (mg/g
liver) | 22.5±10.4 | 20.0±7.1 | 20.1±9.7 | 19.1±6.9 |
| Total cholesterols
(mg/g liver) | 4.05±1.00 |
6.15±1.47a |
6.26±2.03a | 5.63±1.49 |
To further investigate the effects of aronia juice
residue and its ethanol extract on lipid metabolism, the hepatic
expression levels of genes involved in lipid synthesis and
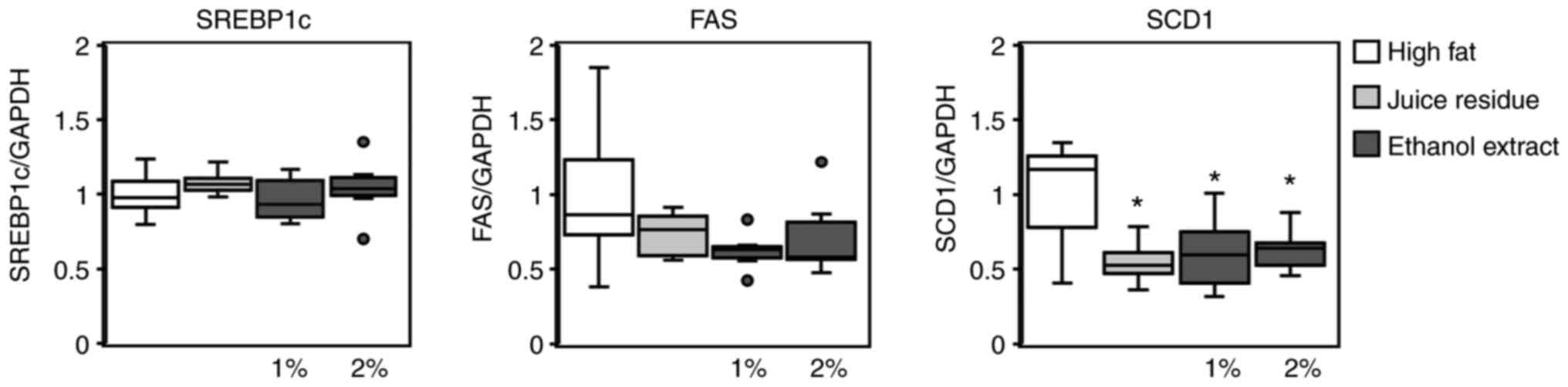
degradation were measured. As shown in Fig. 4, the hepatic mRNA levels of genes
implicated in lipogenesis (i.e., SREBP-1c, FAS and SCD1) were
determined. SREBP-1c expression was not affected by the
administration of aronia juice residue or ethanol extract (Fig. 4). By contrast, the FAS and SCD1
mRNA levels were decreased in mice fed the aronia juice residue or
its ethanol extract compared to the mice fed the high-fat diet,
although the differences in FAS expression were not significant
(Fig. 4). In addition, the hepatic
expression levels of genes associated with lipolysis were measured
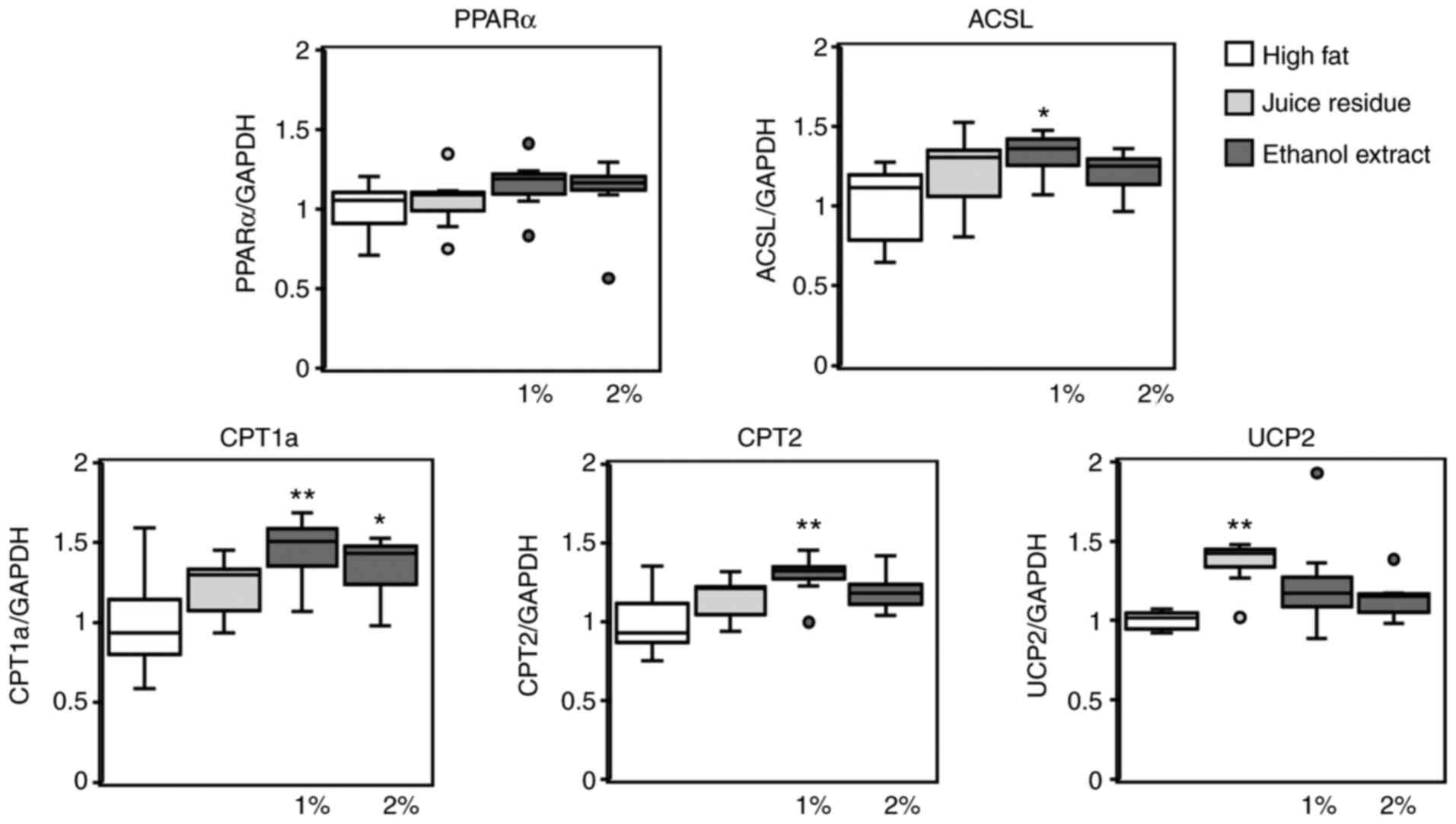
(Fig. 5). The PPARα mRNA levels
did not differ significantly among the 4 experimental groups.
However, the expression of ACSL, CPT1a and CPT2 was significantly
upregulated in the mice fed the 1% aronia ethanol extract compared
to the mice fed the high-fat diet (Fig. 5). Of note, CPT1a was the only gene
whose expression was significantly increased in the mice fed the 2%
ethanol extract. Moreover, UCP2 expression was significantly
upregulated in the mice fed the aronia juice residue compared to
the mice fed the high-fat diet (Fig.
5).
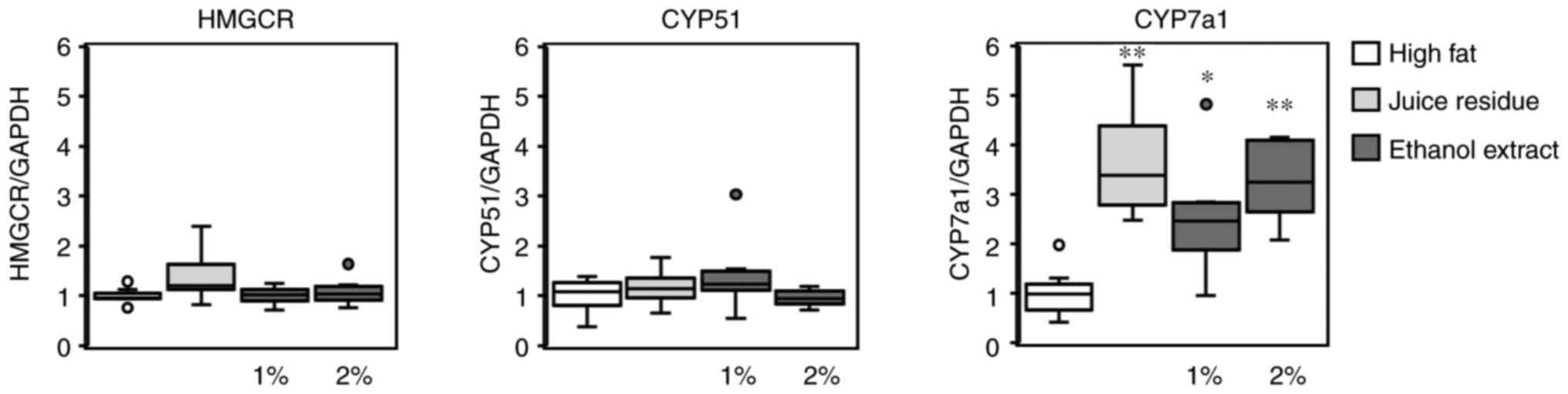
To determine the cause of the observed decrease in
serum LDL cholesterol levels in mice fed the aronia juice residue
or its ethanol extract, the hepatic mRNA levels of genes involved
in cholesterol metabolism were measured. As shown in Fig. 6, the expression levels of HMG-CoA
reductase and CYP51, which are involved in cholesterol synthesis,
were not significantly affected by the inclusion of aronia in the
diet (Fig. 6). On the contrary,
the mRNA levels of CYP7A1, which is a rate-limiting enzyme in the
hepatic catabolism of cholesterol into bile acid, were markedly and
significantly increased with the inclusion of aronia juice residue
(3.7-fold) and 1% or 2% ethanol extract (2.5- or 3.3-fold,
respectively) in the diet compared to the high-fat diet.
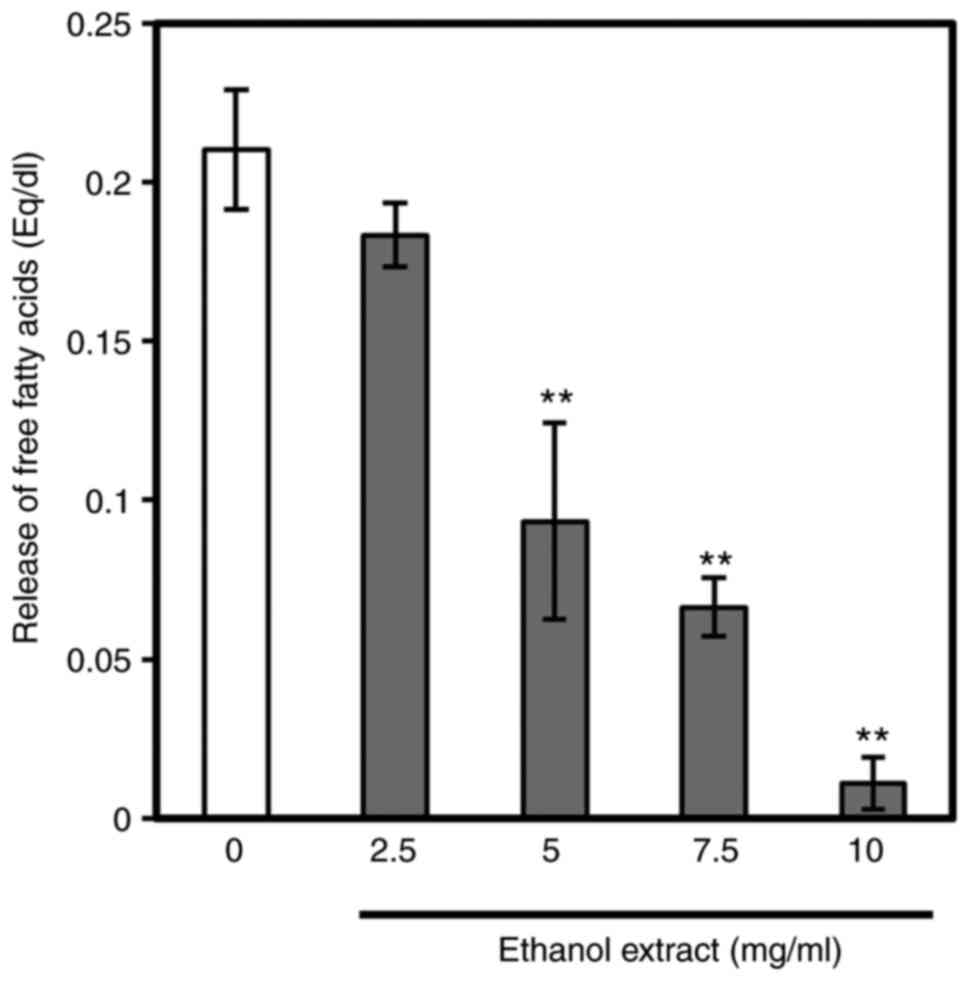
Subsequently, to investigate whether aronia ethanol
extract affects triglyceride absorption, the inhibition of lipase
activity in vitro was evaluated (Fig. 7). Ethanol extract of aronia juice
residue lowered the release of free fatty acids from triolein in a
dose-dependent manner with significant decreases observed at the 5,
7.5 and 10 mg/ml concentrations. The IC50 value for
pancreatic lipase was 4.6 mg/ml ethanol extract.
Discussion
Black chokeberry has numerous potential health
benefits due to its high levels of anthocyanins and other
functional compounds. The processed juice residue is also rich in
these compounds, but is currently underutilized due to a lack of
research into its health-promoting effects and functionality.
The objective of the present study was to determine
the bio-functional effects of aronia juice residue and its ethanol
extract on glucose and lipid metabolism in high-fat diet-fed mice.
Following a 26-day feeding trial involving mice on a high-fat diet
or a high-fat diet supplemented with aronia juice residue (2%) or
ethanol extract (1% or 2%), no significant differences were
detected in body weight, liver weight WAT weight, or blood glucose
levels among the 4 groups. However, serum triglyceride, free fatty
acid and LDL-cholesterol levels were significantly lower in mice
fed aronia juice residue and ethanol extract than in mice fed a
high-fat control diet. These data suggest that the administration
of aronia juice residue or its ethanol extract prevents the
development of elevated serum lipid profiles in high-fat diet-fed
mice.
Dyslipidemia, such as hyper-LDL cholesterolemia and
hypertriglyceridemia, is one of the health issues associated with
obesity and leads to arteriosclerosis. Takahashi et al
(12) previously reported that a
4-week feeding period with 1.7% aronia phytochemicals (i.e., 0.4%
anthocyanins) suppressed visceral fat accumulation in mice fed a
high-fat diet. Furthermore, this treatment decreased serum LDL
cholesterol and glucose levels (12). In the present study,
supplementation with aronia juice residue or its ethanol extract
did not affect visceral fat accumulation or blood glucose levels;
however, the results of the present are consistent with those of
the study by Takahashi et al (12) in terms of the LDL cholesterol
reduction as a result of aronia supplementation. Moreover,
Valcheva-Kuzmanova et al (22) reported that the administration of
aronia fruit juice suppressed the plasma total cholesterol, LDL
cholesterol and triglyceride levels. As indicated by serum
triglyceride and LDL cholesterol levels, these parameters may be
improved by common components of aronia juice and its residue
(i.e., anthocyanins and carotenoids). Furthermore, aronia juice
residue contains a considerable amount of mainly insoluble dietary
fibers (23). A previous study
found that the insoluble, fiber-rich fraction of carrot pomace
lowered serum triglyceride and total cholesterol levels in hamsters
(24). The aronia juice residue
used in the present study contained anthocyanin and carbohydrate
(429.1 mg and 73.2 g in 100 g dry matter, respectively). The
present study did not determine the dietary fiber content in aronia
juice residue; however, a previous study demonstrated that dietary
fiber accounted for approximately 40% of carbohydrate in aronia
fruit (25). Therefore, the
observed improvement in lipid metabolism in the present study may
have occurred due to the combined effects of the functional
components of aronia juice residue, such as anthocyanin and dietary
fibers.
Lipid metabolism is primarily controlled by a
combination of hepatic enzymes and transcription factors. SREBP-1c
is a master regulator of de novo lipogenesis and regulates
hepatic transcription of lipogenic enzymes, such as FAS and
SCD1(26). A high-fat diet induces
the expression of these lipogenic genes in the liver (27). In a previous study, Park et
al (28) reported that an
8-week administration of 1% aronia powder with a high-fat diet
decreased the hepatic mRNA expression of SREBP1 and FAS in mice
with non-alcoholic fatty liver disease. In the present study,
aronia juice residue and its ethanol extract did not affect hepatic
SREBP1 expression. However, the SCD1 mRNA levels decreased
significantly in mice fed high-fat diets supplemented with aronia
compared to the high-fat diet-fed control mice. These results
suggest that aronia juice residue and its ethanol extract
suppressed de novo lipogenesis through a mechanism
independent of SREBP-1c, contrary to the findings of the study by
Park et al (28). In terms
of hepatic lipolysis-related mRNA expression, PPARα levels, a
master regulator of fatty acid oxidation, were not affected by
aronia juice residue or its ethanol extract. However, the mRNA
levels of ACSL, CPT1a and CPT2, which are enzymes targeted by PPARα
in the promotion of acyl CoA formation or mitochondrial β-oxidation
(29), were significantly
increased in mice fed a high-fat diet supplemented with aronia
ethanol extract in the present study. Furthermore, supplementation
with aronia juice residue increased the UCP2 mRNA levels, without
affecting the PPARα levels, even though UCP2 gene expression in
hepatocytes is upregulated by PPARα activators (30). The present findings indicate that
aronia juice residue and its ethanol extract regulate these genes
without affecting the PPARα pathway. These findings also suggest
that aronia juice residue and its ethanol extract reduce serum
triglyceride and free fatty acid levels through distinct mechanisms
by altering the expression of different hepatic genes. In the
present study, a dose-dependent decrease in serum triglyceride and
free fatty acid levels was observed with the administration of 1
and 2% ethanol extracts with the diet. However, the effect on
hepatic gene expression, and particularly, on lipolysis-related
enzymes, was not dose-dependent. Further studies are required to
elucidate the mechanism through which hepatic lipogenic and
lipolytic gene expression is involved in the observed ethanol
extract-induced decrease in serum triglyceride and free fatty acid
levels.
Another mechanism that may explain the changes
detected in the serum lipid profile may be the inhibition of
dietary lipid absorption. Pancreatic lipase hydrolyzes
triglycerides into fatty acids and glycerols and is responsible for
the hydrolysis of 50-70% of total dietary fats (31). Dietary fat is absorbed by the small
intestine, only when it has been hydrolyzed by a pancreatic lipase
(32). Therefore, the inhibition
of lipase activity is a potential mechanism for the regulation of
serum triglyceride and free fatty acid levels. In the present
study, the ethanol extract of aronia juice residue inhibited lipase
activity in a dose-dependent manner. Previous studies have
demonstrated that anthocyanin-rich phytochemicals from aronia
berries and polyphenol-rich extracts from various berries also
inhibit lipase activity (12,33).
The present data revealed that aronia ethanol extract was rich in
anthocyanins (1.45 g/100 g dry matter, Table II), including
cyanidin-3-galactoside and cyanidin-3-arabinoside. Based on these
results, the improved serum lipid profile observed in the present
study may have been partially caused by the inhibition of
pancreatic lipase due to anthocyanins in the ethanol extract of
aronia juice residue.
The results of the present study also demonstrate a
decrease in serum LDL cholesterol in high-fat diet-fed mice as a
result of supplementation with aronia juice residue or its ethanol
extract. Furthermore, the total hepatic cholesterol levels
increased in mice fed aronia juice residue or 1% ethanol extract.
Circulating cholesterol levels are regulated by a balance between
cholesterol biosynthesis, catabolism and transfer into peripheral
tissues. Focusing on cholesterol biosynthesis in the liver, the
mRNA levels of HMG-CoA reductase and CYP51, which are involved in
cholesterol synthesis, were not affected by supplementation with
aronia juice residue and its ethanol extract. However, the
expression of CYP7A1, which is a rate-limiting enzyme that converts
cholesterol to bile acid, markedly increased with the
administration of aronia juice residue or ethanol extract. Previous
studies have also revealed the upregulation of hepatic CYP7A1
expression by anthocyanin (34)
and procyanidin administration (35). These data suggest that the aronia
juice residue and ethanol extract containing anthocyanins that were
used in our study may regulate serum LDL-cholesterol through
hepatic cholesterol excretion, not cholesterol synthesis.
Furthermore, the decrease in plasma LDL cholesterol levels is
caused by an increased LDL receptor activity (36). A previous study revealed that red
grape juice with anthocyanins upregulated both the activity and
expression of the LDL receptor and total cholesterol content in
HepG2 cells in the presence of LDL (37). Although the present study did not
evaluate the activity of the hepatic LDL receptor, these findings
raise the possibility that aronia juice residue and its ethanol
extract may decrease serum LDL-cholesterol levels by incorporating
cholesterol into the liver via LDL receptor activation.
In conclusion, aronia juice residue and its ethanol
extract exhibited anti-hypolipidemic activities in high-fat
diet-fed mice by the regulation of hepatic gene expression related
to fatty acid synthesis, fatty acid oxidation, cholesterol
degradation and lipase inhibition. The results suggest that the
underutilized aronia juice residue and its ethanol extract may be
potentially used as ingredients in the functional food industry.
However, there are some limitations, including the lack of
measuring protein expression and enzyme activities in lipid
metabolic pathway. Further research is required to determine their
effectiveness in more detail and the optimal applications for their
utilization in foods.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data used during the present study are available
from the corresponding author on reasonable request.
Authors' contributions
YH, TO and MH performed the experiments in this
study. NM, YH, TS, TO, KM and MH contributed to the design and
interpretation of the study, as well as in the writing and revision
of the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participant
All animal procedures were carried out according to
the protocol approved by the Ethical Committee of Experimental
Animal Care at Hokkaido University (Permission no. 09-0094) in
Japan.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kokotkiewicz A, Jaremicz Z and Luczkiewicz
M: Aronia plants: A review of traditional use, biological
activities, and perspectives for modern medicine. J Med Food.
13:255–269. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wu X, Beecher GR, Holden JM, Haytowitz DB,
Gebhardt SE and Prior RL: Concentrations of anthocyanins in common
foods in the United States and estimation of normal consumption. J
Agric Food Chem. 54:4069–4075. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wu X, Gu L, Prior RL and McKay S:
Characterization of anthocyanins and proanthocyanidins in some
cultivars of ribes, aronia, and sambucus and their antioxidant
capacity. J Agric Food Chem. 52:7846–7856. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pilaczynska-Szczesniak L,
Skarpanska-Steinborn A, Deskur E, Basta P and Horoszkiewicz-Hassan
M: The influence of chokeberry juice supplementation on the
reduction of oxidative stress resulting from an incremental rowing
ergometer exercise. Int J Sport Nutr Exerc Metab. 15:48–58.
2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kähkönen MP, Hopia AI, Vuorela HJ, Rauha
JP, Pihlaja K, Kujala TS and Heinonen M: Antioxidant activity of
plant extracts containing phenolic compounds. J Agric Food Chem.
47:3954–3962. 1999.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zheng W and Wang SY: Oxygen radical
absorbing capacity of phenolics in blueberries, cranberries,
chokeberries, and lingonberries. J Agric Food Chem. 51:502–509.
2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rugină D, Sconţa Z, Leopold L, Pintea A,
Bunea A and Socaciu C: Antioxidant activities of chokeberry
extracts and the cytotoxic action of their anthocyanin fraction on
HeLa human cervical tumor cells. J Med Food. 15:700–706.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tjelle TE, Holtung L, Bøhn SK, Aaby K,
Thoresen M, Wiik SÅ, Paur I, Karlsen AS, Retterstøl K, Iversen PO
and Blomhoff R: Polyphenol-rich juices reduce blood pressure
measures in a randomised controlled trial in high normal and
hypertensive volunteers. Br J Nutr. 114:1054–1063. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ciocoiu M, Badescu L, Miron A and Badescu
M: The involvement of a polyphenol-rich extract of black chokeberry
in oxidative stress on experimental arterial hypertension. Evid
Based Complement Alternat Med. 2013(912769)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sikora J, Broncel M, Markowicz M,
Chałubiński M, Wojdan K and Mikiciuk-Olasik E: Short-term
supplementation with Aronia melanocarpa extract improves
platelet aggregation, clotting, and fibrinolysis in patients with
metabolic syndrome. Eur J Nutr. 51:549–556. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mu J, Xin G, Zhang B, Wang Y, Ning C and
Meng X: Beneficial effects of Aronia melanocarpa berry
extract on hepatic insulin resistance in type 2 diabetes mellitus
rats. J Food Sci. 85:1307–1318. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Takahashi A, Shimizu H, Okazaki Y,
Sakaguchi H, Taira T, Suzuki T and Chiji H: Anthocyanin-rich
phytochemicals from aronia fruits inhibit visceral fat Accumulation
and hyperglycemia in high-fat diet-induced dietary obese rats. J
Oleo Sci. 64:1243–1250. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kujawska M, Ignatowicz E, Ewertowska M,
Oszmiański J and Jodynis-Liebert J: Protective effect of chokeberry
on chemical-induced oxidative stress in rat. Hum Exp Toxicol.
30:199–208. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vagiri M and Jensen M: Influence of juice
processing factors on quality of black chokeberry pomace as a
future resource for colour extraction. Food Chem. 217:409–417.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ohta T, Nashida-Hosotani Y, Hosokawa M and
Miyashita K: The effects of ethanol extract from aronia
juice-residue on lipid metabolism. Presented at the International
Society for Nutraceuticals and Functional Foods (ISNFF), Sapporo,
pp137, 2011.
|
|
16
|
Rask-Madsen C and Kahn CR: Tissue-specific
insulin signaling, metabolic syndrome, and cardiovascular disease.
Arterioscler Thromb Vasc Biol. 32:2052–2059. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jonathan Q and Purnell JQ: Definitions,
classification, and epidemiology of obesity. In: Endotext
[Internet]. Feingold KR, Anawalt B, Boyce A, et al (eds).
urihttp://MDText.comsimpleMDText.com,
Inc., South Dartmouth, MA, 2000.
|
|
18
|
Kuwabara H, Takanami S, Oguri I, Yoshida T
and Nakajima T: Influence of ascorbic acid on polyphenol
determination in fruit juice. Res Rep Food Technol Res Inst Nagano
Prefect. 13:49–53. 1985.(In Japanese).
|
|
19
|
Cassinese C, de Combarieu E, Falzoni M,
Fuzzati N, Pace R and Sardone N: New liquid chromatography method
with ultraviolet detection for analysis of anthocyanins and
anthocyanidins in vaccinium myrtillus fruit dry extracts and
commercial preparations. J AOAC Int. 90:911–919. 2007.PubMed/NCBI
|
|
20
|
Reeves PG, Nielsen FH and Fahey GC Jr:
AIN-93 purified diets for laboratory rodents: Final report of the
American institute of nutrition ad hoc writing committee on the
reformulation of the AIN-76A rodent diet. J Nutr. 123:1939–1951.
1993.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Folch J, Lees M and Sloane Stanley GH: A
simple method for the isolation and purification of total lipides
from animal tissues. J Biol Chem. 226:497–509. 1957.PubMed/NCBI
|
|
22
|
Valcheva-Kuzmanova S, Kuzmanov K, Mihova
V, Krasnaliev I, Borisova P and Belcheva A: Antihyperlipidemic
effect of Aronia melanocarpa fruit juice in rats fed a
high-cholesterol diet. Plant Foods Hum Nutr. 62:19–24.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wawer I, Wolniak M and Paradowska K: Solid
state NMR study of dietary fiber powders from aronia, bilberry,
black currant and apple. Solid State Nucl Magn Reson. 30:106–113.
2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hsu PK, Chien PJ, Chen CH and Chau CF:
Carrot insoluble fiber-rich fraction lowers lipid and cholesterol
absorption in hamsters. LWT-Food Sci Technol. 39:338–343. 2006.
|
|
25
|
Tanaka T and Tanaka A: Chemical components
and characteristics of black chokeberry. J Jpn Soc Food Sci
Technol. 48:606–610. 2001.
|
|
26
|
Shimano H, Yahagi N, Amemiya-Kudo M, Hasty
AH, Osuga J, Tamura Y, Shionoiri F, Iizuka Y, Ohashi K, Harada K,
et al: Sterol regulatory element-binding protein-1 as a key
transcription factor for nutritional induction of lipogenic enzyme
genes. J Biol Chem. 274:35832–35839. 1999.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang Z, Kim JH, Jang YS, Kim CH, Lee JY
and Lim SS: Anti-obesity effect of Solidago virgaurea var. gigantea
extract through regulation of adipogenesis and lipogenesis pathways
in high-fat diet-induced obese mice (C57BL/6N). Food Nutr Res.
61(1273479)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Park H, Liu Y, Kim HS and Shin JH:
Chokeberry attenuates the expression of genes related to de novo
lipogenesis in the hepatocytes of mice with nonalcoholic fatty
liver disease. Nutr Res. 36:57–64. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rakhshandehroo M, Knoch B, Müller M and
Kersten S: Peroxisome proliferator-activated receptor alpha target
genes. PPAR Res. 2010(612089)2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nakatani T, Tsuboyama-Kasaoka N, Takahashi
M, Miura S and Ezaki O: Mechanism for peroxisome
proliferator-activated receptor-alpha activator-induced
up-regulation of UCP2 mRNA in rodent hepatocytes. J Biol Chem.
277:9562–9569. 2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Birari RB and Bhutani KK: Pancreatic
lipase inhibitors from natural sources: Unexplored potential. Drug
Discov Today. 12:879–889. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Guo L, Gao Z, Zhang L, Guo F, Chen Y, Li Y
and Huang C: Saponin-enriched sea cucumber extracts exhibit an
antiobesity effect through inhibition of pancreatic lipase activity
and upregulation of LXR-β signaling. Pharm Biol. 54:1312–1325.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
McDougall GJ, Kulkarni NN and Stewart D:
Berry polyphenols inhibit pancreatic lipase activity in vitro. Food
Chem. 115:193–199. 2009.
|
|
34
|
Wang D, Xia M, Gao S, Li D, Zhang Y, Jin T
and Ling W: Cyanidin-3-O-β-glucoside upregulates hepatic
cholesterol 7α-hydroxylase expression and reduces
hypercholesterolemia in mice. Mol Nutr Food Res. 56:610–621.
2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Del Bas JM, Fernández-Larrea J, Blay M,
Ardèvol A, Salvadó MJ, Arola L and Bladé C: Grape seed procyanidins
improve atherosclerotic risk index and induce liver CYP7A1 and SHP
expression in healthy rats. FASEB J. 19:479–481. 2005.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Momtazi AA, Banach M, Pirro M, Katsiki N
and Sahebkar A: Regulation of PCSK9 by nutraceuticals. Pharmacol
Res. 120:157–169. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Dávalos A, Fernández-Hernando C, Cerrato
F, Martínez-Botas J, Gómez-Coronado D, Gómez-Cordovés C and
Lasunción MA: Red grape juice polyphenols alter cholesterol
homeostasis and increase LDL-receptor activity in human cells in
vitro. J Nutr. 136:1766–1773. 2006.PubMed/NCBI View Article : Google Scholar
|