Global health is threatened by an ongoing outbreak
of COVID-19, a respiratory disease caused by the novel coronavirus,
SARS-CoV-2, which was first identified in December, 2019 in Wuhan,
China (1). As of August 28, 2020,
>24.7 million individuals had been infected with the virus
worldwide, and approximately 838,000 have succumbed to the disease
(2). In total, seven types of
coronaviruses, namely severe acute respiratory syndrome coronavirus
(SARS-CoV), Middle East respiratory syndrome coronavirus
(MERS-CoV), severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2), OC43, NL63, 229E and HKU1, are known to infect
humans. Of these, the first three can cause fatal infections,
whereas the latter four typically cause mild common cold-associated
symptoms, particularly in immunocompromised individuals (3). COVID-19 is highly contagious and
infected patients exhibit symptoms of fever, pneumonia,
thrombocytopenia, cough, dyspnea, myalgia and asthenia (4).
As of August 5, 2020, no vaccine or successful
treatment for COVID-19 has been reported and only supportive care,
personal protection, early diagnosis and isolation are available to
reduce the spread and severity of the infection (5). Huang et al (6) reported that patients with COVID-19
develop acute respiratory distress syndrome, followed by anemia,
acute heart injuries and secondary infections. Empirical therapy
with antibiotics (including cephalosporins, azithromycin,
vancomycin, quinolones, tigecycline and carbapenems), antivirals
(including lopinavir, ritonavir, remdesivir and oseltamivir) and
corticosteroids (including dexamethasone and methylprednisolone)
has thus been used for the treatment of patients with
COVID-19(7). The clinical efficacy
of all of these treatments, however, warrants further
confirmation.
Research proposals and clinical trials have
suggested that some treatments, including supplements and
phytochemicals, have the potential to help fight coronavirus
infection. A recent study suggested that the risk of becoming
infected could be reduced by vitamin D3 supplementation. Serum
concentrations >40-60 ng/ml (100-150 nmol/l) were suggested to
be required in order to prevent infection, with even higher doses
required to treat patients already infected (8). In addition, due to its antiviral and
immunomodulatory properties, zinc supplementation may also be
considered for use in the prevention or treatment of
COVID-19(9). Zhang et al
(10) proposed that melatonin,
which has antioxidant and anti-inflammatory properties, together
with a good safety profile, and also potentially modulates the
immune system, improves sleep quality, and reduces vessel
permeability, anxiety and the use of sedatives, may lead to better
clinical outcomes for patients with COVID-19. Ang et al
(11) analyzed the potential of
traditional herbal medicines, which contained a total of 56 herbs,
for the treatment of patients with COVID-19. The detailed review by
Islam et al (12) also
reported that a wide range of herbal compounds, including
tylophorine, lycorine, ouabain, silvestrol, homoharringtonine and
7-methoxycryptopleurine, broadly suppressed different
coronaviruses, with IC50 values ranging from 12 to 143
nM. Yu et al (13) also
demonstrated that the plant-derived flavonoids, myricetin and
scutellarein, are inhibitors of SARS-CoV helicase. Collectively,
the above-mentioned studies suggest that natural products and
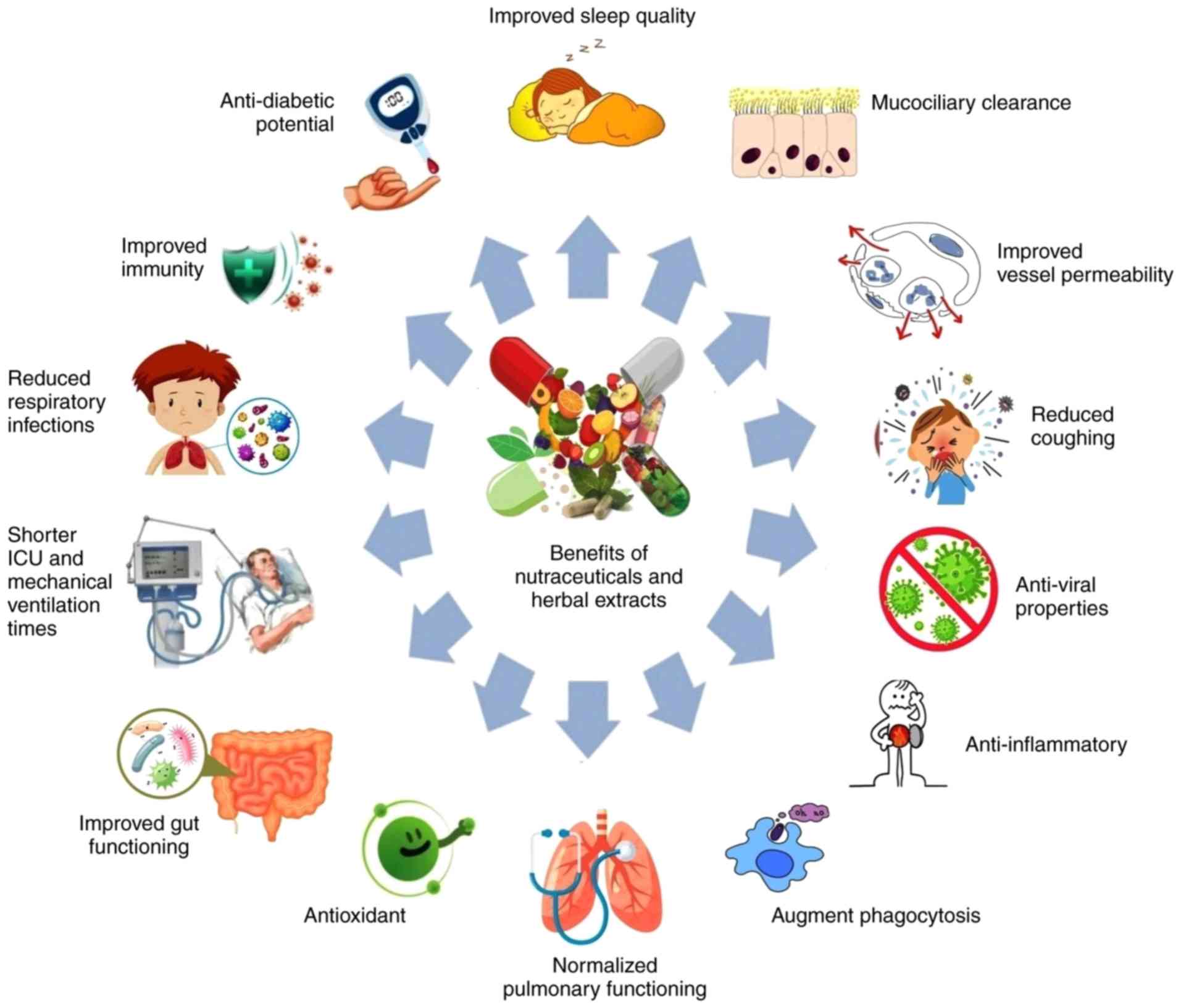
dietary supplements may help in the fight against COVID-19. A
pictorial representation of the potential beneficial effects of
nutraceuticals and herbal extracts against COVID-19 is depicted in
Fig. 1.
With regards to the recent increase in the number of
published articles on COVID-19, including reports of infected
cases, fatalities, disease severity and vulnerability (21), the purpose of the present review
article is to draw the attention of medical and pharmacy
professionals towards nutraceuticals and herbal extracts for the
treatment of COVID-19. The present review focuses on reported facts
and figures to highlight the potential of natural products to
strengthen the immunity of the general population, and to pave the
way for the identification of drugs which may be used in the
treatment of patients with COVID-19. Mathematical models predict
that reported numbers of cases of COVID-19 will continue to
increase until a vaccine is made available to the global market
(22). The present review article
highlights the therapeutic significance of some promising natural
products with activity against viruses in general, and COVID-19 in
particular.
A daily intake of 20-50 µg of vitamin D was recently
recommended for obese individuals, healthcare workers and smokers
in order to enhance their resistance to COVID-19 infection
(23). Yalaki et al
(24) reported that
supplementation with vitamin D in patients suffering from acute
bronchiolitis increased immunity and normalized pulmonary function.
Other studies have also associated the administration of vitamin D
with the reduced likelihood of developing respiratory infections
(25,26). The administration of high-dose
vitamin C (1,000-6,000 mg) has also been reported to decrease the
time spent by critically ill patients on mechanical ventilation by
25%, and also reduced their length of stay in an intensive care
unit (27). Of note, vitamin C has
also been proven to be effective against asthma induced by the
common cold (28,29).
The importance of selenium for optimal immune
function has been emphasized in the literature (30). Selenium provides resistance against
viral infections through its redox homeostasis and antioxidant
properties (31). Selenium
deficiency has been associated with impaired immune function,
likely due to increased oxidative stress in the host organism
(32). This can lead to
alterations in the viral genome, which may increase virulence and
boost pathogenicity (33). Dietary
selenium has been shown to improve immunity against the lethal H1N1
influenza virus infection (34-36)
and can also be potentially used in the current battle against
COVID-19(37).
NAC, which is derived from the naturally occurring
amino acid, cysteine, is most commonly prescribed to patients
suffering from various respiratory complications, including
respiratory tract infections, idiopathic pulmonary fibrosis and
chronic bronchitis (54-56).
The antioxidant and mucolytic effects of NAC have been reported to
significantly improve the function of airways and to reduce COPD
exacerbations (57). NAC treatment
has also been reported to inhibit the RSV infection of human
alveolar epithelial (A549) cells and to reduce mucin release
(58). In a previous review
article, Sadowska (59) concluded
that NAC may be beneficial in the management of COPD, since it
would promote clearance of mucus and alleviate oxidative stress and
inflammation. Taken together, these results demonstrate the clear
potential of NAC as an adjuvant supplement for COVID-19 patients
(60,61).
Arginine has been reported to act synergistically
with virucidal conditions, such as high temperatures and acidic pH
levels, and can thus potentially inactivate enveloped viruses
(62,63). Tsujimoto et al (64) successfully inactivated herpes
simplex virus type 2 (HSV-2) using a solution of arginine.
Similarly, influenza A has been shown to be inactivated under
similar conditions (65).
Recently, Meingast and Heldt (63)
suggested that arginine inactivates viruses through a variety of
mechanisms, including pore formation and destabilization of the
viral membrane, the inhibition of the function of non-structural
proteins, the suppression of protein-protein interactions and
aggregation. Ikeda et al (66) suggested that arginine associates
with multiple sites on viral particles, thereby affecting
glycoprotein-lipid interactions on the viral envelope. Due to the
low cytotoxicity of arginine, a previous study demonstrated the
possible use of an intranasal spray containing an aqueous solution
of arginine to inhibit influenza A infection in vivo
(67). In a NC/Nga mouse model of
asthma, arginine was found to contribute to improved asthmatic
symptoms by reducing airway inflammation in lung tissue and
altering L-arginine metabolism (68).
Nutritional supplementation with probiotics has been
reported to be beneficial for patients suffering from respiratory
tract infections (74-76).
Strasser et al (77)
reported that various strains of probiotics, including
Lactococcus lactis W58, Lactobacillus brevis W63,
Enterococcus faecium W54, Lactobacillus acidophilus
W22, Bifidobacterium lactis W51 and Bifidobacterium
bifidum W23, helped to reduce the incidence of upper
respiratory tract infections (URTIs) in trained athletes, without
altering performance. Another probiotic strain, Lactobacillus
casei Zhang, which exhibits immunomodulatory, anti-inflammatory
and anti-oxidative effects, has been shown to alleviate the
symptoms of URTI and restore gastrointestinal health in adults and
elderly subjects (78). RSV
infection has also been reported to be suppressed by various
probiotic strains, including Lactobacillus gasseri SBT2055
and Lactobacillus rhamnosus CRL1505 (79,80).
The results of the meta-analysis by Kang et al (81) revealed the efficacy of probiotics
in the treatment of common cold infections. During the COVID-19
pandemic, the National Administration of Traditional Chinese
Medicine and the Chinese National Health Commission recommended the
use of probiotic therapy to control coronavirus infection (82); however, the effectiveness of
probiotics in reducing the mortality rate of patients in intensive
care units remains uncertain. Jayawardena et al (83) suggested the use of probiotics as a
dietary supplement to prevent infection of susceptible populations
with SARS-CoV-2. Since probiotics are readily available as dietary
supplements and have negligible side-effects if administered at the
correct doses, they may thus provide a useful intervention strategy
against COVID-19 (84-86).
A study to evaluate whether dietary supplementation with
Lactobacillus coryniformis K8 can help to prevent healthcare
workers from contracting COVID-19 was registered at ClinicalTrials.gov (NCT04366180) on April 28,
2020.
Fats, which can be classified as saturated or
unsaturated, form an essential part of the human diet and play a
vital role in nutrition and health (87,88).
Fats serve as a main source of energy, participate in cell
signaling and responses, and play a structural role as part of the
cell membrane. Omega-3 polyunsaturated fatty acids have been
reported to confer health benefits in patients suffering from
respiratory complications, such as ARDS, COPD, impaired oxygenation
and pulmonary fibrosis (89-92)
and are attracting considerable attention due to their
anticoagulant properties and ability to reduce inflammation
(93). The consumption of omega-3
polyunsaturated fatty acids has been associated with a number of
physiological alterations, including the production of lung
surfactants, host-microbial interactions, alterations in blood
rheology and the production of endogenous eicosanoids (94).
β-glucans, which are potent activators of immune
cells, including neutrophils, natural killer cells and macrophages,
exert a favorable effect on the host defense system (107). In addition to the
immunomodulatory effects, the administration of β-glucans has been
shown to reduce the susceptibility of healthy subjects to URTIs and
to decrease the severity of URTIs in infected subjects (108-111).
β-glucans have also been shown to exhibit antiviral activity
against HSV-1 (112,113) and influenza virus (114,115). More recently, it was suggested
that β-glucans can help to reduce morbidity and mortality
associated with COVID-19 (116,117).
The use of phytochemicals and natural products for
the treatment of various diseases is gaining worldwide attention
(118,119). Prior to the discovery of
antibiotics, herbal extracts played an important role in the
treatment of diseases (120), and
purified natural products and herbal extracts now provide a rich
pool of compounds for the development of novel antiviral drugs
(121). Lin et al
(122) summarized the antiviral
activity of various natural products and herbal medicines against
some notable viral pathogens, including RSV, measles virus, dengue
virus, influenza virus, human immunodeficiency virus (HIV), HSV,
HCV, hepatitis B virus, enterovirus 71, coxsackievirus and
coronavirus.
The present review attempted to highlight the
potential of various nutraceuticals and herbal extracts as possible
treatments for COVID-19. Although strong evidence for the potential
of these compounds to combat the ongoing COVID-19 pandemic has
already appeared in the literature, new evidence is gradually
emerging (140). The reported
clinical data are, however, still inconclusive and there are also
inconsistencies within the data, since some clinical studies did
not achieve the desired effects. These inconsistencies seem to be
related to a number of factors, including the dose used, the
heterogeneity of the target population, the plasma concentration,
the beginning and duration of the treatment and the route of
administration (141). Taking
these factors into consideration, randomized and controlled trials
are required to resolve these controversies and to clarify issues
around the use of these compounds. In addition to an increase in
the reported number of cases, some patients who have recovered from
coronavirus are testing positive again (142). Clinical validation of compounds
that could possibly help to combat the COVID-19 pandemic is thus
urgently required.
No funding was received.
Not applicable.
AHT and MMJ conceived this review. AHT wrote and
revised the manuscript. All authors read and approved the final
version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Zhu N, Zhang D, Wang W, Li X, Yang B, Song
J, Zhao X, Huang B, Shi W, Lu R, et al: A novel coronavirus from
patients with pneumonia in China, 2019. N Engl J Med. 382:727–733.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Worldometer. COVID-19 Coronavirus
Pandemic. Dover, Delaware: uriWorldometers.infosimpleWorldometers.info., 2020
Available online at: uriwww.worldometers.info/coronavirussimplewww.worldometers.info/coronavirus
(accessed August 28, 2020).
|
|
3
|
Andersen KG, Rambaut A, Lipkin WI, Holmes
EC and Garry RF: The proximal origin of SARS-CoV-2. Nat Med.
26:450–452. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Contini C, Di Nuzzo M, Barp N, Bonazza A,
De Giorgio R, Tognon M and Rubino S: The novel zoonotic COVID-19
pandemic: An expected global health concern. J Infect Dev Ctries.
14:254–264. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Prada V, Benedetti L, Cocito D, Briani C,
Orazio EN, Gallia F, Antonini G, Manganelli F, Fabrizi GM, Germano
F, et al: High-dose immunoglobulin pulse therapy and risk of
Covid19 infection. J Neurol. 1–3. 2020.doi:
10.1007/s00415-020-10146-5 (Epub ahead of print). PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ahmed SS: The coronavirus disease 2019
(COVID-19): A review. J Adv Med Med Res 32: 1-9, 2020.
|
|
8
|
Grant WB, Lahore H, McDonnell SL, Baggerly
CA, French CB, Aliano JL and Bhattoa HP: Evidence that vitamin D
supplementation could reduce risk of influenza and COVID-19
infections and deaths. Nutrients. 12(988)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang L and Liu Y: Potential interventions
for novel coronavirus in China: A systematic review. J Med Virol.
92:479–490. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang R, Wang X, Ni L, Di X, Ma B, Niu S,
Liu C and Reiter RJ: COVID-19: Melatonin as a potential adjuvant
treatment. Life Sci. 250(117583)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ang L, Lee HW, Kim A, Lee JA, Zhang J and
Lee MS: Herbal medicine for treatment of children diagnosed with
COVID-19: A review of guidelines. Complement Ther Clin Pract.
39(101174)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Islam MT, Sarkar C, El-Kersh DM, Jamaddar
S, Uddin SJ, Shilpi JA and Mubarak MS: Natural products and their
derivatives against coronavirus: A review of the non-clinical and
pre-clinical data. Phytother Res: Apr 4, 2020 doi: 10.1002/ptr.6700
(Epub ahead of print).
|
|
13
|
Yu MS, Lee J, Lee JM, Kim Y, Chin YW, Jee
JG, Keum YS and Jeong YJ: Identification of myricetin and
scutellarein as novel chemical inhibitors of the SARS coronavirus
helicase, nsP13. Bioorg Med Chem Lett. 22:4049–4054.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ashour HM, Elkhatib WF, Rahman MM and
Elshabrawy HA: Insights into the recent 2019 novel Coronavirus
(SARS-CoV-2) in light of past human coronavirus outbreaks.
Pathogens. 9(186)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chandrasekharan JA, Marginean A and
Sharma-Walia N: An insight into the role of arachidonic acid
derived lipid mediators in virus associated pathogenesis and
malignancies. Prostaglandins Other Lipid Mediat. 126:46–54.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Innes JK and Calder PC: Omega-6 fatty
acids and inflammation. Prostaglandins Leukot Essent Fatty Acids.
132:41–48. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Duvall MG and Levy BD: DHA- and
EPA-derived resolvins, protectins, and maresins in airway
inflammation. Eur J Pharmacol. 785:144–155. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chiurchiù V, Leuti A, Dalli J, Jacobsson
A, Battistini L, Maccarrone M and Serhan CN: Proresolving lipid
mediators resolvin D1, resolvin D2, and maresin 1 are critical in
modulating T cell responses. Sci Transl Med.
8(353ra111)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ramsden CE: Breathing easier with fish
oil-a new approach to preventing asthma? N Engl J Med.
375:2596–2598. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Das UN: Can bioactive lipids inactivate
coronavirus (COVID-19)? Arch Med Res. 51:282–286. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Velavan TP and Meyer CG: The COVID-19
epidemic. Trop Med Int Health. 25:278–280. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Victor A: Mathematical predictions for
COVID-19 as a global pandemic. Available at SSRN 3555879, 2020.
|
|
23
|
McCartney DM and Byrne DG: Optimisation of
Vitamin D status for enhanced immuno-protection against Covid-19.
Ir Med J. 113(58)2020.PubMed/NCBI
|
|
24
|
Yalaki Z, Taşar MA, Oney H and Gokceoğlu
AU: Comparison of viral agents and Vitamin D levels in children
with acute bronchiolitis infection. J Pediatr Inf. 13:e14–e20.
2019.
|
|
25
|
Larkin A and Lassetter J: Vitamin D
deficiency and acute lower respiratory infections in children
younger than 5 years: Identification and treatment. J Pediatr
Health Care. 28:572–582. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Loeb M, Dang AD, Thiem VD, Thanabalan V,
Wang B, Nguyen NB, Tran HTM, Luong TM, Singh P, Smieja M, et al:
Effect of Vitamin D supplementation to reduce respiratory
infections in children and adolescents in Vietnam: A randomized
controlled trial. Influenza Other Respir Viruses. 13:176–183.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hemilä H and Chalker E: Vitamin C may
reduce the duration of mechanical ventilation in critically ill
patients: A meta-regression analysis. J Intensive Care.
8(15)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hemilä H: Vitamin C and common
cold-induced asthma: A systematic review and statistical analysis.
Allergy Asthma Clin Immunol. 9(46)2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jeong YJ, Kim JH, Kang JS, Lee WJ and
Hwang YI: Mega-dose vitamin C attenuated lung inflammation in mouse
asthma model. Anat Cell Biol. 43:294–302. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wiesner-Reinhold M, Schreiner M,
Baldermann S, Schwarz D, Hanschen FS, Kipp AP, Rowan DD,
Bentley-Hewitt KL and McKenzie MJ: Mechanisms of selenium
enrichment and measurement in brassicaceous vegetables, and their
application to human health. Front Plant Sci.
8(1365)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Guillin OM, Vindry C, Ohlmann T and
Chavatte L: Selenium, selenoproteins and viral infection.
Nutrients. 11(2101)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xu J, Gong Y, Sun Y, Cai J, Liu Q, Bao J,
Yang J and Zhang Z: Impact of selenium deficiency on inflammation,
oxidative stress, and phagocytosis in mouse macrophages. Biol Trace
Elem Res. 194:237–243. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Harthill M: Micronutrient selenium
deficiency influences evolution of some viral infectious diseases.
Biol Trace Elem Res. 143:1325–1336. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yu L, Sun L, Nan Y and Zhu LY: Protection
from H1N1 influenza virus infections in mice by supplementation
with selenium: A comparison with selenium-deficient mice. Biol
Trace Elem Res. 141:254–261. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li Y, Lin Z, Guo M, Zhao M, Xia Y, Wang C,
Xu T and Zhu B: Inhibition of H1N1 influenza virus-induced
apoptosis by functionalized selenium nanoparticles with amantadine
through ROS-mediated AKT signaling pathways. Int J Nanomedicine.
13:2005–2016. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li Y, Lin Z, Gong G, Guo M, Xu T, Wang C,
Zhao M, Xia Y, Tang Y, Zhong J, et al: Inhibition of H1N1 influenza
virus-induced apoptosis by selenium nanoparticles functionalized
with arbidol through ROS-mediated signaling pathways. J Mater Chem
B Mater Biol Med. 7:4252–4262. 2019.
|
|
37
|
Kieliszek M and Lipinski B: Selenium
supplementation in the prevention of coronavirus infections
(COVID-19). Med Hypotheses. 143(109878)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wessels I, Maywald M and Rink L: Zinc as a
gatekeeper of immune function. Nutrients. 9(1286)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pae M and Wu D: Nutritional modulation of
age-related changes in the immune system and risk of infection.
Nutr Res. 41:14–35. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Barnett JB, Hamer DH and Meydani SN: Low
zinc status: A new risk factor for pneumonia in the elderly? Nutr
Rev. 68:30–37. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Walker CLF, Rudan I, Liu L, Nair H,
Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H and Black RE:
Global burden of childhood pneumonia and diarrhoea. Lancet.
381:1405–1416. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kumar NS, Jayaprakash S and Kavitha D: Low
serum Zinc level-a possible Marker of severe pneumonia. J Med Sci
Clin Res. 5:21554–21570. 2017.
|
|
43
|
Shah UH, Abu-Shaheen AK, Malik MA, Alam S,
Riaz M and Al-Tannir MA: The efficacy of zinc supplementation in
young children with acute lower respiratory infections: A
randomized double-blind controlled trial. Clin Nutr. 32:193–199.
2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Suara RO and Crowe JE: Effect of zinc
salts on respiratory syncytial virus replication. Antimicrob Agents
Chemother. 48:783–790. 2004.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Truong-Tran AQ, Carter J, Ruffin R and
Zalewski PD: New insights into the role of zinc in the respiratory
epithelium. Immunol Cell Biol. 79:170–177. 2001.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Roscioli E, Jersmann HP, Lester S, Badiei
A, Fon A, Zalewski P and Hodge S: Zinc deficiency as a
codeterminant for airway epithelial barrier dysfunction in an ex
vivo model of COPD. Int J Chron Obstruct Pulmon Dis. 12:3503–3510.
2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Darma A, Athiyyah AF, Ranuh RG, Merbawani
W, Setyoningrum RA, Hidajat B, Hidayati SN, Endaryanto A and
Sudarmo SM: Zinc Supplementation effect on the bronchial cilia
length, the number of cilia, and the number of intact bronchial
cell in Zinc deficiency rats. Indones Biomed J. 12:78–84. 2020.
|
|
48
|
Woodworth BA, Zhang S, Tamashiro E,
Bhargave G, Palmer JN and Cohen NA: Zinc increases ciliary beat
frequency in a calcium-dependent manner. Am J Rhinol Allergy.
24:6–10. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Te Velthuis AJ, van den Worm SH, Sims AC,
Baric RS, Snijder EJ and van Hemert MJ: Zn(2+) inhibits coronavirus
and arterivirus RNA polymerase activity in vitro and zinc
ionophores block the replication of these viruses in cell culture.
PLoS Pathog. 6(e1001176)2010.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kumar A, Kubota Y, Chernov M and Kasuya H:
Potential role of Zinc supplementation in prophylaxis and treatment
of COVID-19. Med Hypotheses. 144(109848)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wessling-Resnick M: Crossing the Iron
gate: Why and how transferrin receptors mediate viral entry. Annu
Rev Nutr. 38:431–458. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ali MK, Kim RY, Karim R, Mayall JR, Martin
KL, Shahandeh A, Abbasian F, Starkey MR, Loustaud-Ratti V,
Johnstone D, et al: Role of iron in the pathogenesis of respiratory
disease. Int J Biochem Cell Biol. 88:181–195. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Jayaweera JAAS, Reyes M and Joseph A:
Childhood iron deficiency anemia leads to recurrent respiratory
tract infections and gastroenteritis. Sci Rep.
9(12637)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Homma S, Azuma A, Taniguchi H, Ogura T,
Mochiduki Y, Sugiyama Y, Nakata K, Yoshimura K, Takeuchi M and
Kudoh S: Japan NAC Clinical Study Group: Efficacy of inhaled
N-acetylcysteine monotherapy in patients with early stage
idiopathic pulmonary fibrosis. Respirology. 17:467–477.
2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Blasi F, Page C, Rossolini GM, Pallecchi
L, Matera MG, Rogliani P and Cazzola M: The effect of
N-acetylcysteine on biofilms: Implications for the treatment of
respiratory tract infections. Respir Med. 117:190–197.
2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Cazzola M, Calzetta L, Page C, Jardim J,
Chuchalin AG, Rogliani P and Matera MG: Influence of
N-acetylcysteine on chronic bronchitis or COPD exacerbations: A
meta-analysis. Eur Respir Rev. 24:451–461. 2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Santus P, Corsico A, Solidoro P, Braido F,
Di Marco F and Scichilone N: Oxidative stress and respiratory
system: Pharmacological and clinical reappraisal of
N-acetylcysteine. COPD. 11:705–717. 2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Mata M, Sarrion I, Armengot M, Carda C,
Martinez I, Melero JA and Cortijo J: Respiratory syncytial virus
inhibits ciliagenesis in differentiated normal human bronchial
epithelial cells: Effectiveness of N-acetylcysteine. PLoS One.
7(e48037)2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Sadowska AM: N-Acetylcysteine mucolysis in
the management of chronic obstructive pulmonary disease. Ther Adv
Respir Dis. 6:127–135. 2012.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Andreou A, Trantza S, Filippou D, Sipsas N
and Tsiodras S: COVID-19: The potential role of copper and
N-acetylcysteine (NAC) in a combination of candidate antiviral
treatments against SARS-CoV-2. In Vivo. 34:1567–1588.
2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Jaiswal N, Bhatnagar M and Shah H:
N-acetycysteine: A potential therapeutic agent in COVID-19
infection. Med Hypotheses. 144(110133)2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ohtake S, Arakawa T and Koyama AH:
Arginine as a synergistic virucidal agent. Molecules. 15:1408–1424.
2010.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Meingast C and Heldt CL:
Arginine-enveloped virus inactivation and potential mechanisms.
Biotechnol Prog. 36(e2931)2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Tsujimoto K, Uozaki M, Ikeda K, Yamazaki
H, Utsunomiya H, Ichinose M, Koyama AH and Arakawa T:
Solvent-induced virus inactivation by acidic arginine solution. Int
J Mol Med. 25:433–437. 2010.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Yamasaki H, Tsujimoto K, Koyama AH, Ejima
D and Arakawa T: Arginine facilitates inactivation of enveloped
viruses. J Pharm Sci. 97:3067–3073. 2008.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Ikeda K, Yamasaki H, Minami S, Suzuki Y,
Tsujimoto K, Sekino Y, Irie H, Arakawa T and Koyama AH: Arginine
inactivates human herpesvirus 2 and inhibits genital herpesvirus
infection. Int J Mol Med. 30:1307–1312. 2012.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ikeda K, Yamasaki H, Suzuki Y, Koyama AH
and Arakawa T: Novel strategy with acidic arginine solution for the
treatment of influenza A virus infection. Exp Ther Med. 1:251–256.
2010.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Zhang R, Kubo M, Murakami I, Setiawan H,
Takemoto K, Inoue K, Fujikura Y and Ogino K: l-Arginine
administration attenuates airway inflammation by altering
l-arginine metabolism in an NC/Nga mouse model of asthma. J Clin
Biochem Nutr. 56:201–207. 2015.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Pierre JF, Heneghan AF, Lawson CM,
Wischmeyer PE, Kozar RA and Kudsk KA: Pharmaconutrition review:
Physiological mechanisms. JPEN J Parenter Enteral Nutr. 37 (5
Suppl):51S–65S. 2013.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Lee CH, Kim HK, Kim JM, Ayush O, Im SY, Oh
DK and Lee HK: Glutamine suppresses airway neutrophilia by blocking
cytosolic phospholipase A(2) via an induction of MAPK
phosphatase-1. J Immunol. 189:5139–5146. 2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Lai CC, Liu WL and Chen CM: Glutamine
attenuates acute lung injury caused by acid aspiration. Nutrients.
6:3101–3116. 2014.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Oliveira GP, De Abreu MG, Pelosi P and
Rocco PR: Exogenous glutamine in respiratory diseases: Myth or
reality? Nutrients. 8(76)2016.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Romano L, Bilotta F, Dauri M, Macheda S,
Pujia A, De Santis GL, Tarsitano MG, Merra G, Di Renzo L, Esposito
E and De Lorenzo A: Short report-medical nutrition therapy for
critically ill patients with COVID-19. Eur Rev Med Pharmacol Sci.
24:4035–4039. 2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
West NP, Horn PL, Pyne DB, Gebski VJ,
Lahtinen SJ, Fricker PA and Cripps AW: Probiotic supplementation
for respiratory and gastrointestinal illness symptoms in healthy
physically active individuals. Clin Nutr. 33:581–587.
2014.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Maldonado J, Cañabate F, Sempere L, Vela
F, Sánchez AR, Narbona E, López-Huertas E, Geerlings A, Valero AD,
Olivares M and Lara-Villoslada F: Human milk probiotic
Lactobacillus fermentum CECT5716 reduces the incidence of
gastrointestinal and upper respiratory tract infections in infants.
J Pediatr Gastroenterol Nutr. 54:55–61. 2012.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Ozen M, Kocabas Sandal G and Dinleyici EC:
Probiotics for the prevention of pediatric upper respiratory tract
infections: A systematic review. Expert Opin Biol Ther. 15:9–20.
2015.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Strasser B, Geiger D, Schauer M, Gostner
JM, Gatterer H, Burtscher M and Fuchs D: Probiotic supplements
beneficially affect tryptophan-kynurenine metabolism and reduce the
incidence of upper respiratory tract infections in trained
athletes: A randomized, double-blinded, placebo-controlled trial.
Nutrients. 8(752)2016.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Hor YY, Lew LC, Lau ASY, Ong JS, Chuah LO,
Lee YY, Choi SB, Rashid F, Wahid N, Sun Z, et al: Probiotic
Lactobacillus casei Zhang (LCZ) alleviates respiratory,
gastrointestinal & RBC abnormality via immuno-modulatory,
anti-inflammatory & anti-oxidative actions. J Funct Foods.
44:235–245. 2018.
|
|
79
|
Kitazawa H and Villena J: Modulation of
respiratory TLR3-anti-viral response by probiotic microorganisms:
Lessons learned from Lactobacillus rhamnosus CRL1505. Front
Immunol. 5(201)2014.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Eguchi K, Fujitani N, Nakagawa H and
Miyazaki T: Prevention of respiratory syncytial virus infection
with probiotic lactic acid bacterium Lactobacillus gasseri
SBT2055. Sci Rep. 9(4812)2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Kang EJ, Kim SY, Hwang IH and Ji YJ: The
effect of probiotics on prevention of common cold: A meta-analysis
of randomized controlled trial studies. Korean J Fam Med. 34:2–10.
2013.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Mak JWY, Chan FKL and Ng SC: Probiotics
and COVID-19: One size does not fit all. Lancet Gastroenterol
Hepatol. 5:644–645. 2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Jayawardena R, Sooriyaarachchi P,
Chourdakis M, Jeewandara C and Ranasinghe P: Enhancing immunity in
viral infections, with special emphasis on COVID-19: A review.
Diabetes Metab Syndr. 14:367–382. 2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Morais AHA, Passos TS, Maciel BLL and da
Silva-Maia JK: Can probiotics and diet promote beneficial immune
modulation and purine control in coronavirus infection? Nutrients.
12(1737)2020.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Infusino F, Marazzato M, Mancone M, Fedele
F, Mastroianni CM, Severino P, Ceccarelli G, Santinelli L,
Cavarretta E, Marullo AGM, et al: Diet supplementation, probiotics,
and Nutraceuticals in SARS-CoV-2 infection: A scoping review.
Nutrients. 12(1718)2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Baud D, Agri VD, Gibson GR, Reid G and
Giannoni E: Using probiotics to flatten the curve of coronavirus
disease COVID-2019 pandemic. Front Public Health.
8(186)2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Ibarguren M, López DJ and Escribá PV: The
effect of natural and synthetic fatty acids on membrane structure,
microdomain organization, cellular functions and human health.
Biochim Biophys Acta. 1838:1518–1528. 2014.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Thom G and Lean M: Is there an optimal
diet for weight management and metabolic health? Gastroenterology.
152:1739–1751. 2017.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Lemoine S CM, Brigham EP, Woo H, Hanson
CK, McCormack MC, Koch A, Putcha N and Hansel NN: Omega-3 fatty
acid intake and prevalent respiratory symptoms among U.S. adults
with COPD. BMC Pulm Med. 19(97)2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Escamilla-Nuñez MC, Barraza-Villarreal A,
Hernández-Cadena L, Navarro-Olivos E, Sly PD and Romieu I: Omega-3
fatty acid supplementation during pregnancy and respiratory
symptoms in children. Chest. 146:373–382. 2014.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Singer P and Shapiro H: Enteral omega-3 in
acute respiratory distress syndrome. Curr Opin Clin Nutr Metab
Care. 12:123–128. 2009.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Abidi A, Kourda N, Feki M and Ben Khamsa
S: Protective effect of Tunisian flaxseed oil against
bleomycin-induced pulmonary fibrosis in rats. Nutr Cancer.
72:226–238. 2020.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Whyand T, Hurst JR, Beckles M and Caplin
ME: Pollution and respiratory disease: Can diet or supplements
help? A review. Respir Res. 19(79)2018.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Knapp HR: Omega-3 fatty acids in
respiratory diseases: A review. J Am Coll Nutr. 14:18–23.
1995.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Miyata J and Arita M: Role of omega-3
fatty acids and their metabolites in asthma and allergic diseases.
Allergol Int. 64:27–34. 2015.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Yates CM, Calder PC and Rainger GE:
Pharmacology and therapeutics of omega-3 polyunsaturated fatty
acids in chronic inflammatory disease. Pharmacol Ther. 141:272–282.
2014.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Morita M, Kuba K, Ichikawa A, Nakayama M,
Katahira J, Iwamoto R, Watanebe T, Sakabe S, Daidoji T, Nakamura S,
et al: The lipid mediator protectin D1 inhibits influenza virus
replication and improves severe influenza. Cell. 153:112–125.
2013.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Linday LA, Shindledecker RD, Tapia-Mendoza
J and Dolitsky JN: Effect of daily cod liver oil and a
multivitamin-mineral supplement with selenium on upper respiratory
tract pediatric visits by young, inner-city, Latino children:
Randomized pediatric sites. Ann Otol Rhinol Laryngol. 113:891–901.
2004.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Da Boit M, Gabriel BM, Gray P and Gray SR:
The effect of fish oil, vitamin D and protein on URTI incidence in
young active people. Int J Sports Med. 36:426–430. 2015.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Philpott JD, Witard OC and Galloway SD:
Applications of omega-3 polyunsaturated fatty acid supplementation
for sport performance. Res Sports Med. 27:219–237. 2019.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Langlois PL, D'Aragon F, Hardy G and
Manzanares W: Omega-3 polyunsaturated fatty acids in critically ill
patients with acute respiratory distress syndrome: A systematic
review and meta-analysis. Nutrition. 61:84–92. 2019.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Calder PC: Omega-3 fatty acids and
inflammatory processes: From molecules to man. Biochem Soc Trans.
45:1105–1115. 2017.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Imai Y: Role of omega-3 PUFA-derived
mediators, the protectins, in influenza virus infection. Biochim
Biophys Acta. 1851:496–502. 2015.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Messina G, Polito R, Monda V, Cipolloni L,
Di Nunno N, Di Mizio G, Murabito P, Carotenuto M, Messina A,
Pisanelli D, et al: Functional role of dietary intervention to
improve the outcome of COVID-19: A hypothesis of work. Int J Mol
Sci. 21(3104)2020.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Hammock BD, Wang W, Gilligan MM and
Panigrahy D: Eicosanoids: The overlooked storm in COVID-19? Am J
Pathol. 190:1782–1788. 2020.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Torrinhas RS, Calder PC, Lemos GO and
Waitzberg DL: Parenteral fish oil, an adjuvant pharmacotherapy for
COVID-19? Nutrition. 81(110900)2020.PubMed/NCBI View Article : Google Scholar
|
|
107
|
De Marco Castro E, Calder PC and Roche HM:
β-1,3/1,6-glucans and immunity: State of the art and future
directions. Mol Nutr Food Res. (e1901071)2020.doi:
10.1002/mnfr.201901071 (Epub ahead of print).
|
|
108
|
Murphy EA, Davis JM, Brown AS, Carmichael
MD, Carson JA, Van Rooijen N, Ghaffar A and Mayer EP: Benefits of
oat β-glucan on respiratory infection following exercise stress:
Role of lung macrophages. Am J Physiol Regul Integr Comp Physiol.
294:R1593–R1599. 2008.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Dharsono T, Rudnicka K, Wilhelm M and
Schoen C: Effects of yeast (1,3)-(1,6)-beta-glucan on severity of
upper respiratory tract infections: a double-blind, randomized,
placebo-controlled study in healthy subjects. J Am Coll Nutr.
38:40–50. 2019.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Jesenak M, Urbancikova I and Banovcin P:
Respiratory tract infections and the role of biologically active
polysaccharides in their management and prevention. Nutrients.
9(779)2017.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Talbott S and Talbott J: Effect of BETA 1,
3/1, 6 GLUCAN on upper respiratory tract infection symptoms and
mood state in marathon athletes. J Sports Sci Med. 8:509–515.
2009.PubMed/NCBI
|
|
112
|
Urbancikova I, Hudackova D, Majtan J,
Rennerova Z, Banovcin P and Jesenak M: Efficacy of Pleuran
(β-Glucan from Pleurotus ostreatus) in the management of
herpes simplex virus type 1 infection. Evid Based Complement
Alternat Med. 2020(8562309)2020.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Murphy EA, Davis JM, Brown AS, Carmichael
MD, Ghaffar A and Mayer EP: Effects of oat β-glucan on the
macrophage cytokine response to herpes simplex virus 1 infection in
vitro. J Interferon Cytokine Res. 32:362–367. 2012.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Muramatsu D, Iwai A, Aoki S, Uchiyama H,
Kawata K, Nakayama Y, Nikawa Y, Kusano K, Okabe M and Miyazaki T:
β-glucan derived from Aureobasidium pullulans is effective
for the prevention of influenza in mice. PLoS One.
7(e41399)2012.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Vetvicka V and Vetvickova J: Glucan
supplementation enhances the immune response against an influenza
challenge in mice. Ann Transl Med. 3(22)2015.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Geller A and Yan J: Could the induction of
trained immunity by β-Glucan serve as a defense against COVID-19?
Front Immunol. 11(1782)2020.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Murphy EJ, Masterson C, Rezoagli E,
O'Toole D, Major I, Stack GD, Lynch M, Laffey JG and Rowan NJ:
β-Glucan extracts from the same edible shiitake mushroom Lentinus
edodes produce differential in-vitro immunomodulatory and pulmonary
cytoprotective effects-Implications for coronavirus disease
(COVID-19) immunotherapies. Sci Total Environ.
732(139330)2020.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Singh S, Sharma B, Kanwar SS and Kumar A:
Lead phytochemicals for anticancer drug development. Front Plant
Sci. 7(1667)2016.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Altemimi A, Lakhssassi N, Baharlouei A,
Watson DG and Lightfoot DA: Phytochemicals: Extraction, isolation,
and identification of bioactive compounds from plant extracts.
Plants. 6(42)2017.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Prasanna G, Ujwal A, Diliprajudominic S,
Marimuthu T and Saraswathi NT: A new pipeline to discover
antimycotics by inhibiting ergosterol and riboflavin synthesis: The
inspirations of Siddha medicine. Med Chem Res. 23:2651–2658.
2014.
|
|
121
|
Oliveira AF, Teixeira RR, Oliveira ASD,
Souza AP, Silva MLD and Paula SO: Potential Antivirals: Natural
products targeting replication enzymes of dengue and chikungunya
viruses. Molecules. 22(505)2017.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Lin LT, Hsu WC and Lin CC: Antiviral
natural products and herbal medicines. J Tradit Complement Med.
4:24–35. 2014.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Cheng PW, Ng LT, Chiang LC and Lin CC:
Antiviral effects of saikosaponins on human coronavirus 229E in
vitro. Clin Exp Pharmacol Physiol. 33:612–616. 2006.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Lau KM, Lee KM, Koon CM, Cheung CS, Lau
CP, Ho HM, Lee MY, Au SW, Cheng CH, Lau CB, et al: Immunomodulatory
and anti-SARS activities of Houttuynia cordata. J
Ethnopharmacol. 118:79–85. 2008.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Maree JE and Viljoen AM: Phytochemical
distinction between Pelargonium sidoides and Pelargonium
reniforme-A quality control perspective. S Afr J Bot. 82:83–91.
2012.
|
|
126
|
Careddu D and Pettenazzo A: Pelargonium
sidoides extract EPs 7630: A review of its clinical efficacy
and safety for treating acute respiratory tract infections in
children. Int J Gen Med. 11:91–98. 2018.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Baars EW, Zoen EBV, Breitkreuz T, Martin
D, Matthes H, von Schoen-Angerer T, Soldner G, Vagedes J, van
Wietmarschen H, Patijn O, et al: The contribution of complementary
and alternative medicine to reduce antibiotic use: A narrative
review of health concepts, prevention, and treatment strategies.
Evid Based Complement Alternat Med. 2019(5365608)2019.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Michaelis M, Doerr HW and Cinatl J Jr:
Investigation of the influence of EPs® 7630, a herbal
drug preparation from Pelargonium sidoides, on replication
of a broad panel of respiratory viruses. Phytomedicine. 18:384–386.
2011.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Fiore C, Eisenhut M, Krausse R, Ragazzi E,
Pellati D, Armanini D and Bielenberg J: Antiviral effects of
Glycyrrhiza species. Phytother Res. 22:141–148.
2008.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Feng Yeh C, Wang KC, Chiang LC, Shieh DE,
Yen MH and San Chang J: Water extract of licorice had anti-viral
activity against human respiratory syncytial virus in human
respiratory tract cell lines. J Ethnopharmacol. 148:466–473.
2013.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Ahmad A, Husain A, Mujeeb M, Khan SA,
Najmi AK, Siddique NA, Damanhouri ZA and Anwar F: A review on
therapeutic potential of Nigella sativa: A miracle herb.
Asian Pac J Trop Biomed. 3:337–352. 2013.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Dajani EZ, Shahwan TG and Dajani NE:
Overview of the preclinical pharmacological properties of
Nigella sativa (black seeds): A complementary drug with
historical and clinical significance. J Physiol Pharmacol.
67:801–817. 2016.PubMed/NCBI
|
|
133
|
Li SY, Chen C, Zhang HQ, Guo HY, Wang H,
Wang L, Zhang X, Hua SN, Yu J and Xiao PG: Identification of
natural compounds with antiviral activities against SARS-associated
coronavirus. Antiviral Res. 67:18–23. 2005.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Cui JD: Biotechnological production and
applications of Cordyceps militaris, a valued traditional
Chinese medicine. Crit Rev Biotechnol. 35:475–484. 2015.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Ashraf SA, Elkhalifa AE, Siddiqui AJ,
Patel M, Awadelkareem AM, Snoussi M, Ashraf MS, Adnan M and Hadi S:
Cordycepin for health and wellbeing: A Potent bioactive metabolite
of an entomopathogenic cordyceps medicinal fungus and its
nutraceutical and therapeutic potential. Molecules.
25(2735)2020.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Daba G: The endless nutritional and
pharmaceutical benefits of the Himalayan gold, Cordyceps;
Current knowledge and prospective potentials. Biofarmasi J Nat Prod
Biochem. 18:70–77. 2020.
|
|
137
|
Yang M, Shang YX, Tian ZY, Xiong M, Lu CL,
Jiang Y, Zhang Y, Zhang YY, Jin XY, Jin QB, et al: Characteristics
of registered studies for Coronavirus disease 2019 (COVID-19): A
systematic review. Integr Med Res. 9(100426)2020.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Boone HA, Medunjanin D and Sijerčić A:
Review on potential of phytotherapeutics in fight against COVID-19.
Int J Innov Sci Res Technol. 5:481–491. 2020.
|
|
139
|
Suwannarach N, Kumla J, Sujarit K,
Pattananandecha T, Saenjum C and Lumyong S: Natural bioactive
compounds from fungi as potential candidates for protease
inhibitors and immunomodulators to apply for coronaviruses.
Molecules. 25(1800)2020.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Umesh Kundu D, Selvaraj C, Singh SK and
Dubey VK: Identification of new anti-nCoV drug chemical compounds
from Indian spices exploiting SARS-CoV-2 main protease as target. J
Biomol Struct Dyn. 1–9. 2020.doi: 10.1080/07391102.2020.1763202
(Epub ahead of print). PubMed/NCBI View Article : Google Scholar
|
|
141
|
Paintsil E and Cheng YC: Antiviral agents.
Encyclopedia Microbiology. 176–225. 2019.doi:
10.1016/B978-0-12-801238-3.02387-4.
|
|
142
|
Xing Y, Mo P, Xiao Y, Zhao O, Zhang Y and
Wang F: Post-discharge surveillance and positive virus detection in
two medical staff recovered from coronavirus disease 2019
(COVID-19), China, January to February 2020. Euro Surveill.
25(2000191)2020.PubMed/NCBI View Article : Google Scholar
|