Introduction
Methotrexate (MTX) or N-4-(2,4-diamino-6-pteridinyl)-methylamino benzoyl-L-glutamic acid is an anti-metabolite drug widely used against a range of diseases, including cancer, and several immunological disorders, such as rheumatoid arthritis, juvenile idiopathic arthritis, psoriasis, etc. (1-3). When MTX enters a cell, it is immediately polyglutamated, competitively binds with dihydrofolate reductase with an affinity 1,000-fold greater than its substrate folate, thus inhibits the conversion of dihydrofolate to tetrahydrofolate, hence interfering with DNA synthesis, repair and replication (4). In spite of its efficacy in treating these diseases, it can cause significant toxicity, including nephrotoxicity, pulmonary toxicity, oral mucositis, intestinal toxicity, neurotoxicity, reproductive toxicity (it impairs oocyte quality and testicular toxicity) and hepatotoxicity, as well as immunosuppression (5-14). However, in spite of its effectiveness in treating these diseases, the therapeutic application of MTX is usually limited due to its severe toxicity. The prevalence rates of MTX-induced acute kidney injury, pulmonary disorder and liver damage range between 2-12, 7.6-11.6 and 5-10%, respectively, thus increasing its chances of discontinuation (15-18). MTX is known to induce oxidative stress by increasing reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation, which may be involved in its associated toxicities (19). Several mechanisms underlying MTX-induced nephrotoxicity have been proposed; among these most commonly described mechanisms are the crystallization of MTX on the lumen of renal tubules and the constriction of afferent capillaries. This crystallization often induces cell membrane alterations or injury on the lumen of renal tubules, resulting in renal tubular necrosis, the generation of excessive ROS and causing progressive oxidative damage (20,21). An increase in serum renal toxicity marker enzymes shortly following MTX administration is a distinctive key feature of acute kidney injury. Several mechanisms account for MTX-induced potential pulmonary injury. The most commonly described mechanism of MTX-induced pulmonary toxicity is ROS-induced parenchymal lung injury, as well as interstitial and alveolar fibrosis. Another possible mechanism is via pro-inflammatory cytokine-mediated lung injury. MTX-induced liver disease is one of the most common, and potentially fatal adverse effects. The mechanisms underlying hepatotoxicity are considered to be direct toxicity possibly due to local folate deficiency, thus inhibiting the synthesis of purines and pyrimidines, and decreasing DNA and RNA synthesis (4). To prevent these extreme toxicities, a rigorously standardized combinational therapeutic approach with natural phytochemicals is widely accepted.
Pharmaceuticals and nutritional sciences have recently witnessed the emergence of a new research field studying the impact of nutrition on human and animal health. At the present time, considerable attention is being paid to functional foods, which, in principle, apart from their classical notion of ‘adequate nutrition’, that is, a diet that provides basic nutritional functions, is being gradually replaced by ‘functional nutrition’; i.e., the food components have the potential to improve general physiological functions and reduce the risk of developing chronic diseases. There has been a virtual explosion of interest in identifying foods, food extracts and phytochemical formulations from plant sources that are able to mitigate oxidative and inflammatory stress (22). The frequent consumption of fruits can boost human health and help to prevent diseases.
Pithecellobium dulce (P. dulce) (Roxb) Benth (Fabaceae) is widely distributed in the tropics, particularly in America and Asia. Members of this genus greatly resemble tamarind, and hence widely known as Manila tamarind. This is a small to medium-sized, spiny, evergreen tree that grows throughout the plains of India and Andamans. The fruits of P. dulce are edible and have marked nutritional benefits. It contains 2.3-3 g proteins, 0.4-0.5 g fats, 8.2-19.6 g carbohydrates, 79 calories of food energy, 13 mg calcium, 42 mg phosphorous, 0.5 mg iron, 19 mg sodium, 20.2 mg potassium, 25 IU vitamin A, 0.24 mg thiamine, 0.1 mg riboflavin, 0.6 mg niacin, and 133 mg ascorbic acid (23-25). A variety of potential antioxidants have been isolated and characterized from the fruit of P. dulce that promises its therapeutic efficacy. The high-performance liquid chromatography (HPLC) analysis of the hydroalcoholic extract of the fruit arils of P. dulce has revealed the presence of a high content of flavonoid and phenolic compounds, such as ellagic acid, kaempferol, mandelic acid, rutin, naringenin and quercetin (26). Saponins, such as pithedulosides A-G, dulcin and pithedulosides H-K isolated from the seed extract of P. dulce have been reported to exhibit potent antioxidant, anti-inflammatory and anti-fungal properties (27-30). Anthocyanin, water-soluble polysaccharides (PDP-1, PDP-2 and PDP-3), N-malonyl tryptophan, triterpene and saponins from the fruit arils of P. dulce are known to possess anti-hyperglycemic, antioxidant, antiparasitic and glucosidase inhibitory activity.
The anti-inflammatory, antitumor and chemoprotective efficacy of the fruit extract of P. dulce (FPD) has been explored by the authors (24,25). The present study examined the protective effects of FPD on hematology, relative organ weight, hepatic and renal toxicity marker levels, tissue antioxidant and stress marker levels, as well as on histopathological changes in the liver, kidney and lung tissue during MTX induced experimental toxicity.
Materials and methods
Drugs and chemicals
MTX, silymarin (SLM), 2,2-diphenyl 1 picrylhydrazyl (DPPH), ascorbic acid, deoxy-ribose, ethylene-diamine, tetra-acetic acid (EDTA), sodium cyanide (NaCN), p-nitroblue tetrazolium chloride (NBT), riboflavin, eosin and α-tocopherol were purchased from Sigma-Aldrich; Merck KGaA. Riboflavin, sodium nitroprusside (SNP), sulphanilamide, trichloroacetic acid (TCA) and thiobarbituric acid (TBA) were purchased from HiMedia Laboratories Pvt. Ltd. Sodium dodecyl sulfate (SDS) and Harris hematoxylin were purchased from Spectrum Reagents and Chemicals Pvt. Ltd. Glutathione and 5-dithiobis-2-nitrobenzoic acid (DTNB; Ellmans reagent) were obtained from Molecular Probes Life Technologies (USA). Drabkin's reagent was purchased from Agappe Diagnostics Ltd. The creatinine, urea, serum glutamate-pyruvate transaminase (SGPT), alkaline phosphatase (ALP) and serum glutamic oxaloacetic transaminase (SGOT) analysis kits were purchased from Coral Clinical Systems. The highly specific sandwich ELISA kits for quantifying mouse IL-1β, TNF- α and IL-6 levels were purchased from PeproTech, Inc. All other chemicals used for experimental purposes were of analytical grade.
Preparation of P. dulce extract
The fresh fruits of P. dulce were collected from the herbal garden of the Centre for Indian Medicinal Heritage (CIMH), Kanjikode, India. The plant specimen was authenticated by a taxonomist at the Botanical Survey of India, Coimbatore (no. FAB/02/2018). A voucher specimen was deposited at the herbarium at the Division of Cancer Research, Regional Cancer Centre (RCC), Thiruvananthapuram, India.
The collected fresh fruits of P. dulce were dried (45˚C) for 2 weeks and pulverized. The finely powdered samples (20 g) were defatted with petroleum ether for 4 h followed by subsequent extraction with methanol, and water (1:1) using a Soxhlet apparatus. The crude extract was filtered through a filter (45 µm), and concentrated under reduced pressure (40˚C) for 45 min using a rotary evaporator. The yield of the extract was 11% [w/w].
Phytochemical screening
The FPD thus obtained was evaluated for determining the occurrence of various phytochemical constituents, such as flavonoids, terpenoids, glycosides, alkaloids, phenols, tannins, saponins, xanthoproteins and quinones using the following simple qualitative test (31): Terpenoid: To 0.5 ml of extract (10 mg/ml), 2 ml of chloroform were added, followed by the careful addition of concentrated sulphuric acid (H2SO4). The appearance of reddish-brown coloration at the interface was considered as a confirmation of the presence of terpenoids. Flavonoids: 50 mg of extract were mixed with 10 ml of distilled water and filtered. To the aqueous filtrate, an aliquot of 5 ml of dilute ammonia solution, and concentrated H2SO4 were added. The formation of yellow coloration corroborates the presence of flavonoids. Glycosides: To 5 ml of extract (10 mg/ml), 2 ml of glacial acetic acid, and one drop of 2% ferric chloride (FeCl3) solution were added, followed by the careful addition of 1 ml of concentrated H2SO4. The development of a brown ring at the interface confirms the presence of glycosides. Tannins: 5 mg of the extract were mixed with 2 ml of water. The mixture was then thoroughly mixed and filtered. The formation of blue, black, or brownish-green color following the addition of 0.1% FeCl3 confirms the presence of tannins. Phenols: 5 ml of extract solution (10 mg/ml) were mixed with 1 ml of 5% FeCl3 solution and boiled in a water bath. The appearance of greenish-black coloration corroborates the presence of phenols. Alkaloids: 0.5 g of FPD was heated with 8 ml of 1% hydrochloric acid (HCl). Following filtration, the filtrate was divided into two portions. Mayer's reagent (0.136 g of mercuric chloride (HgCl2 ) and 0.5 g of potassium iodide (KI) were mixed in 10 ml distilled water) was added to one portion of the filtrate, and Wagner's reagent (0.6 g of KI and 0.2 g of iodine was weighed, and dissolved in 10 ml of distilled water) to the other. The presence of alkaloids was indicated by the formation of cream (for Mayer's reaction), and brown or reddish precipitate (for Wagner's reaction). Saponins: 0.5 gm of FPD was suspended in 20 ml of distilled water and heated for 5 min. Following filtration, 5 ml of distilled water were suspended with 10 ml of the aforementioned filtrate and mixed vigorously. The presence of saponins was confirmed by the development of emulsion after vigorously mixing the froth with 3 drops of olive oil. Xanthoproteins: To 1 ml of extract (10 mg/ml), a few drops of concentrated nitric acid (HNO3) and ammonia (NH3) solution were added. The presence of xanthoproteins was indicated by the development of a yellow coloration. Quinones: To 1 ml of extract (10 mg/ml), 10% of aqueous sodium hydroxide (NaOH) solution was added. The appearance of bluish-green or red coloration corroborates the presence of quinones.
High performance thin-layer chromatography (HPTLC), polyphenolic profiling and quantification using liquid chromatography-mass spectrometry (LC-MS/MS)
HPTLC was performed on HPTLC plate's silica gel 60F 254, 7.0X10.0 cm plates (Merck KGaA), with toluene:ethyl acetate:methanol:formic acid (7:3:1:0.5) as a mobile phase. A CAMAG automatic TLC sampler 4 (ATS4) 170206 was equipped with a 25 µl syringe. A CAMAG TLC scanner 160623 was used for detection purposes. WIN CATS planar chromatography software was used to densitometrically quantify the bands. After HPTLC plate development, the TLC plates were dried and the chromatogram was visualized under 254 and 366 nm, and visible region using CAMAG TLC scanner 3.
A total of 28 polyphenols (catechin, quinine, catechol, epigallocatechin, tocopherol, naringenin, chlorogenic acid, gallic acid, syringic acid, epicatechin, caffeic acid, vanillic acid, ferulic acid, quercetin, myricetin, p-coumaric acid, apigenin, luteolin, kaempferol, rutin, shikimic acid, morin, diadzein, hesperetin, ellagic acid, cinnamic acid, genistein and chrysin) were quantitatively analyzed using the LC-MS/MS system (Nexera with LCMS-8045 (Shimadzu Corporation)-HPLC (Nexera LC-30 AD). It was coupled with an autosampler (SIL-30AC), prominence diode array detector (SPD-M20), temperature-controlled column oven (CTO-20AC) and a triple quadrupole mass spectrometer (Nexera with LCMS-8045 (Shimadzu Corporation). Working standards (0.01-1 µg/ml) were prepared by diluting the stock solution with water. The quantification of all polyphenols was performed on a Shimadzu Shim-pack GISS C18 column (150x2.1 mm), with solvent A [water/formic acid (100/0.1%)] and solvent B (100% methanol) as a mobile phase. A linear gradient elution system was used to elute the polyphenols. The detailed analytical procedure was as follows: 5% of solvent B (0.5 to 1.9 min), 98% of solvent B (2.0 to 10.0 min), 98% of solvent B (10.1 to 15 min) and 5% of solvent B (15.1 to 17 min). The flow rate was 0.3 ml/min with an injection volume of 10 µl, and an oven temperature of 40˚C. The detection of polyphenols was performed in multiple reaction monitoring (MRM) mode using LC-MS/MS with positive, and negative modes of ion switching electrospray ionization (ESI) method. LC-MS/MS data acquisition and processing were performed using Lab Solutions Software 5.6 (Shimadzu Corporation). Each calibration solution was performed in triplicate, and the results obtained are expressed as µg/g with SD (n=3).
In vitro antioxidant experiments to evaluate the free radical scavenging and lipid peroxidation inhibiting efficacy of FPD
The DPPH free radical scavenging activity of the FPD was estimated by the method described in the study by Magalhaes et al (32). Various concentrations of FPD (20, 40, 60, 80, 100, 200, 300 and 400 µg/ml) and the standard (3 ml) were added to 1 ml of 0.1 mM of DPPH solution and shaken vigorously. Following incubation for 30 min at room temperature (25±3˚C), the scavenging ability of FPD was quantified spectrophotometrically at 517 nm against 95% methanol as a blank. Ascorbic acid was used as the standard.
The superoxide scavenging activity was determined by the method previously described by McCord and Fridovich, with minor modifications (33). The reaction mixture (3 ml) was prepared by mixing 200 µl of 6 µM EDTAwith 3 µg NaCN, 100 µl of 50 µM NBT, 50 µl of 2 µM riboflavin and 2.65 ml of 67 mM phosphate buffer (pH 7.8), prior to the addition of 100 µl of various concentrations of FPD (20, 40, 60, 80, 100, 200, 300 and 400 µg/ml). Ascorbic acid was used as standard. Following 15 min of illumination, the absorbance was read at 560 nm using PowerWave XS plate reader, BioTek Instruments Inc.
The iron (Fe3+)-ascorbate-EDTA-hydrogen peroxide (H2O2) system (Fenton reaction) was employed to assay the hydroxyl radical scavenging activity by the methods described in the studies by Kunchandy and Rao (34) and Awah et al (35) with minor modifications. Briefly, the reaction mixture (1 ml) was prepared by the addition of 100 µl of 2-deoxy-D-ribose (28 mM in 20 mM phosphate buffer, pH 7.4), 100 µl of 0.1 mM FeCl3, 100 µl of 1 mM H2O2, 100 µl of 0.1 mM ascorbic acid, and 500 µl of various concentrations (20, 40, 60, 80, 100, 200, 300 and 400 µg/ml) of extract/control. The mixture was incubated for 1 h at 37˚C. Furthermore, 1 ml of 1% TBA (in 500 µl of 50 mM NaOH), and 1,000 µl of 2.8% TCA were added to the reaction mixture. The mixture was again incubated for 20 min at 100˚C. After cooling, the absorbance was measured spectrophotometrically at 532 nm using PowerWave XS plate reader, BioTek Instruments Inc. In this experiment, α-tocopherol was used as the standard.
The nitric oxide (NO) radical scavenging activity of FPD was determined by the procedure described by Green et al (36). Briefly, the reaction mixture (2 ml) was prepared by the addition of 10 mM SNP in phosphate-buffered saline (PBS), and 1 ml of various concentrations (20, 40, 60, 80, 100, 200, 300 and 400 µg/ml) of extract/control. The aforementioned mixture was incubated for 150 min at 25˚C. Following incubation, 1 ml of the mixture was removed, and an equal amount of Griess reagent [0.1% N-(1-Naphthyl)ethylenediamine dihydrochloride (NED) in 2% phosphoric acid (H3PO4), 1% sulfanilamide] was added. α-tocopherol was used as the standard. The absorbance was measured at 546 nm nm using PowerWave XS plate reader, BioTek Instruments Inc. Thiobarbituric acid reactive substances (TBARS) assay was used to estimate the degree of lipid peroxidation with slight modifications (37,38). The reaction mixture (500 µl) was prepared by the addition of 100 µl of mouse liver homogenate (25%), 50 µl of 150 mM potassium chloride (KCl) and 100 µl of 0.8 mM of ferrous sulfate (FeSO4). Following incubation for 60 min at 37˚C, 400 µl from the aforementioned mixture were treated with 1.5 ml of 20% acetic acid (CH3COOH; pH 3.5), 1.5 ml of 0.8% TBA, 0.2 ml 8.1% SDS and 500 µl of 20% TCA. The mixture was then mixed thoroughly, and again incubated for 60 min on a boiling water bath (100˚C). After cooling, 5 ml of n-butanol were added to the reaction mixture and mixed vigorously. Following centrifugation at 1,792 x g for 10 min at 37˚C, the absorbance of the upper butanol layer was measured at 532 nm using PowerWave XS plate reader, BioTek Instruments Inc.
The capability of FPD to scavenge radical was calculated using the following formula: Percentage inhibition of radical=[A0-A1/A0] x100, where A0 was the OD of the control, and A1 was the OD of the sample/standard. IC50 values were calculated using software program Easy Plot (Epw.32).
In vivo antioxidant experiments to evaluate the mitigating effect of FPD against MTX-induced oxidative stress and associated tissue injury Animals
BALB/c mice (6-8 weeks old, weighing 22-25 g) were purchased from the Sree Chitra Thirunal Institute for Medical Sciences and Technology (SCTIMST), Thiruvananthapuram, India. The animals were acclimatized for 1 month prior to the study. The experimental animals were housed under standard animal housing conditions (25±2˚C, 50% relative humidity and 12-h light/dark cycle) and were provided with rodent chow (VRK Nutritional Solutions, Maharashtra, India), and tap water ad libitum. The study was approved by the Institutional Animal Ethics Committee (IAEC), Regional Cancer Centre (RCC), Thiruvananthapuram, India (IAEC/RCC no. 6/18).
Experimental design
Following acclimatization under laboratory conditions, the BALB/c mice (n=24) were randomly divided into 4 groups (n=6). Group 1 was served as the sham-operated (sham) control. Group 2 served as the MTX control and was intraperitoneally administered with a single dose of MTX [20 mg/kg body weight (BW), intraperitoneally (i.p.)] on day 5 (39,40). The mice in groups 3 and 4 were treated with the standard drug, SLM and FPD at a dose of 50 and 40 mg/kg BW, respectively via oral gavage for 10 consecutive days (25,41). On day 5, a single dose of MTX was administered to the animals in groups 3 and 4 at a concentration of 20 mg/kg BW, i.p. All animals were euthanized on day 11 of the experiment by cervical dislocation. Blood was collected by cardiac puncture immediately following euthanasia for determining hematological parameters [total white blood cell (WBC) count and the hemoglobin (Hb) level]. Serum was separated by centrifugation at 2,000 x g for 10 min at 4˚C, and was used for quantifying the liver and kidney toxicity markers, as well as pro-inflammatory cytokines. All vital organs such as the spleen, kidney, liver, thymus and lungs were excised, and washed thoroughly in PBS (pH 7.4) for determining relative organ weight. Liver, kidney and lung homogenates were prepared using Tris HCl buffer (pH 8.7) for estimating enzymatic [superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase] and non-enzymatic antioxidant levels [total reduced glutathione (GSH)], as well as oxidative stress markers [malondialdehyde (MDA; marker of lipid peroxidation) and nitric oxide (NO)] levels. A portion of the liver, kidney, and lungs were fixed in 10% formalin and subjected for histopathological analysis.
Investigation of the effect of FPD on hematological parameters, BW and relative organ weight during MTX-induced oxidative stress
Changes in average BW of all the animals were measured at the beginning (day 0) prior to FPD administration, and then every 3 days thereafter, continuing throughout the entire experimental period (i.e., up to 10 days). Following euthanization, relative organ weight, and blood parameters were determined. The vital organs of experimental animals were excised immediately, washed thoroughly using PBS (pH 7.4). The data for organ weight are expressed as the relative organ weight (absolute organ weight/BW of the mice x100). Blood was collected via cardiac puncture immediately following euthanasia, and hematological parameters, such as Hb content, and total leukocyte count (WBC count) were also determined.
Investigation of the effects of FPD on hepatic and renal marker enzymes during MTX-induced oxidative stress
All animals were euthanized on the 11th day of the experiment by cervical dislocation. Separated serum was used for estimating hepatic and renal toxicity marker enzymes, such as SGOT, SGPT, bilirubin, ALP, urea and creatinine, measured using standard diagnostic kits from Coral Clinical Systems.
Investigation of the effects of FPD on tissue antioxidant and oxidative stress marker levels during MTX-induced oxidative stress
All animals were euthanized on the 11th day of the experiment by cervical dislocation. Liver, lung and kidney tissue samples were dissected out, washed with physiological saline, minced and homogenized in 25% (w/v) ice-cold 0.1 M Tris buffer (pH 7.4) using a homogenizer, and the resulting homogenate was then used for estimating the level of antioxidant enzymes and oxidative stress markers, such as catalase, GPx, SOD, GSH, MDA and NO (33,36-38).
Investigation of the effects of FPD on liver, kidney and lung histopathology during MTX-induced oxidative stress
A portion of the liver, kidney and lungs from each group was excised and fixed in 10% formalin. The preserved samples were then processed for routine paraffin wax block preparation. Following several steps of dehydration with alcohol i.e., 70% alcohol for 1 h at 37˚C, 80% alcohol for 1 h at 37˚C, 90% alcohol for 1 h at 37˚C and 100% alcohol for 2 h at 37˚C. The tissue specimens were then incubated with xylene for 2 h at 37˚C. The specimens were then embedded in paraffin blocks and 4-µm-thick sections of samples were prepared using a Weswox optic rotary microtome, MT-1090. A and then transferred the tissue section into a clean glass slide. The slide was then heat-fixed at 60˚C for 1 h and then incubated in xylene for 10 min at 37˚C. This was followed by rehydration using 100% alcohol for 1 h at 37˚C, 90% alcohol for 1 h at 37˚C, 80% alcohol for 1 h at 37˚C and 70% alcohol for 2 h at 37˚C. The tissue specimen was then rinsed with water. The tissue specimen was then stained with hematoxylin for 2 min at 37˚C, then rinsed with water for 1 min at 37˚C, then dipped the slide in acid alcohol solution for removing excess stain, and rinsed again with water for 1 min at 37˚C. This was followed by immersion of the slide in 0.3% ammonium hydroxide solution for 1 min, and rinsing again with water for 1 min at 37˚C, and further immersion in eosin for 45 sec, washing with water for 1 min, and dehydration using 70% alcohol for 1 h at 37˚C, 80% alcohol for 1 h at 37˚C, 90% alcohol for 1 h at 37˚C and 100% alcohol for 2 h at 37˚C. The mounted specimens were analyzed and examined under Olympus CKX53 microscope (Olympus Corporation) by a certified pathologist. A total of three sections/mouse was analyzed, and random sections on each slide were used for analysis.
Investigation of the effect of FPD on the levels of pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6 during MTX-induced oxidative stress
On day 11, blood was collected by cardiac puncture, and serum was separated by centrifugation (2,000 x g) for 10 min at 4˚C. The serum was then used for quantifying the level of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) using highly specific sandwich ELISA kits as per the manufacturer's instructions (PeproTech, Inc.).
Statistical analysis
Values were expressed as the mean ± standard deviation (SD). The significant result difference between the groups was determined using one-way ANOVA followed by Dunnett's test using Instat version 3.0 software (GraphPad Software Inc.). A P-value <0.05 was considered to indicate a statistically significant difference.
Results
Phytochemical screening of FPD
The phytochemical screening of FPD revealed the presence of bioactive components, such as flavonoids, terpenoids, glycosides, alkaloids, phenols, tannins, xanthoprotiens and saponins. Quinones were found to be absent in FPD. The results are presented in Table I. The pharmacological activity of FPD is mainly attributed to the existence of these bioactive compounds.
 |
Table I
Phytochemical components in FPD.
|
Table I
Phytochemical components in FPD.
| Sl. no. |
Component |
In FPD |
| 1 |
Flavonoids |
+ |
| 2 |
Terpenoids |
+ |
| 3 |
Glycosides |
+ |
| 4 |
Alkaloids |
+ |
| 5 |
Phenols |
+ |
| 6 |
Tannins |
+ |
| 7 |
Saponins |
+ |
| 8 |
Xanthoproteins |
+ |
| 9 |
Quinones |
- |
Findings of HPTLC and LC-MS/MS analyses
HPTLC analysis of FPD revealed several peaks of phytocompounds. The obtained retardation factor (Rf) values of the different peaks are summarized in Fig. 1A-c, B-c and C-c. The HPTLC chromatogram is depicted in Fig. 1A-a and -b, B-a and -b, and C-a and -b, which revealed a few bands at 254 and 366 nm, and visible range, respectively. At 254 nm, five distinct peak profiles were obtained, and the % area of respective peaks was as follows: 2.30, 7.76, 22.62, 6.66 and 60.67. At 366 nm, five distinct peak profiles were obtained, and the % area of respective peaks was as follows: 13.77, 13.13, 9.61 and 63.49. At visible range, eight distinct peak profiles were obtained, and the area % of respective peaks are as follows: 3.27, 0.39, 0.91, 5.64, 0.70, 7.21, 38.10 and 43.79.
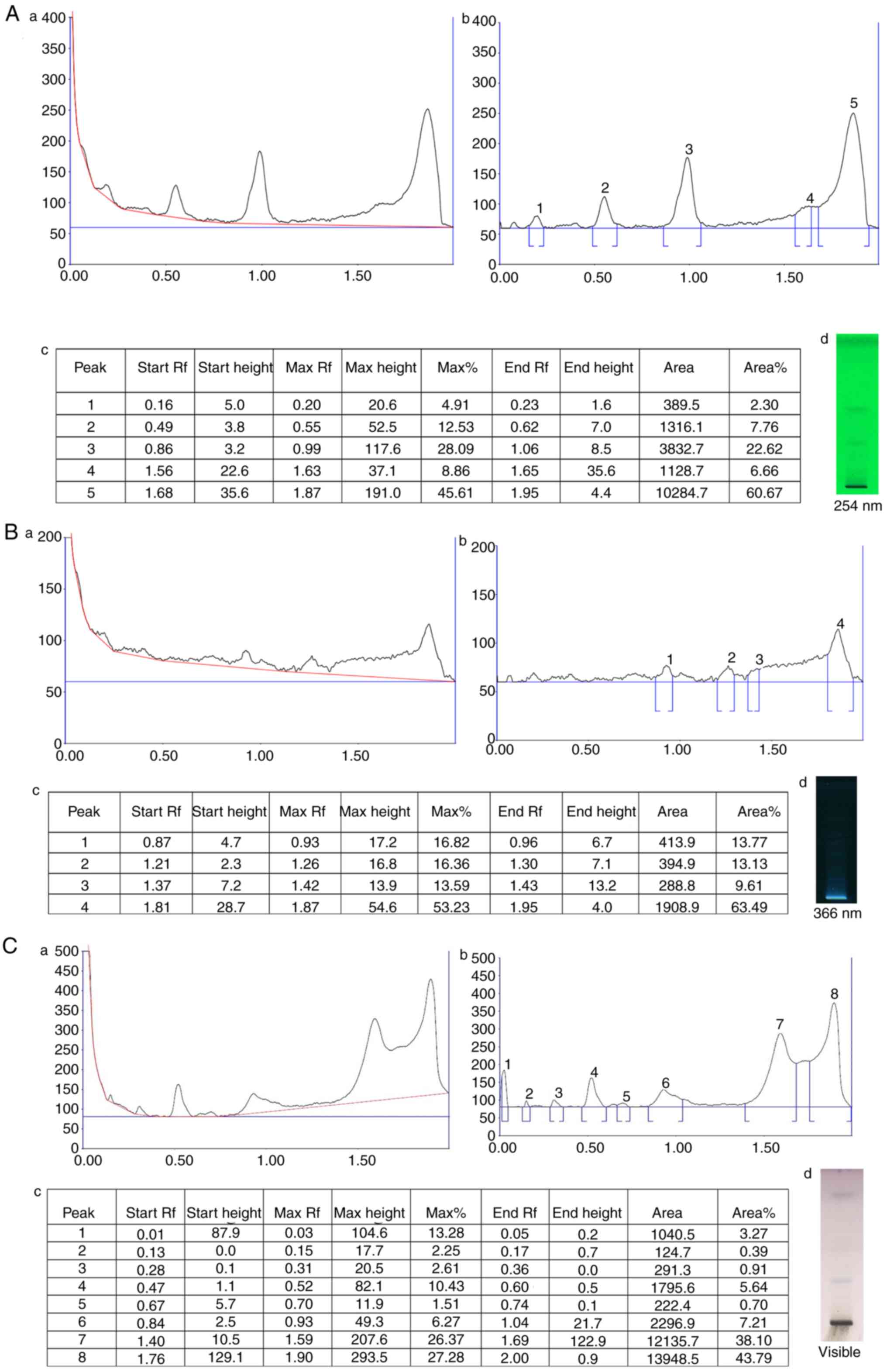 |
Figure 1
HPTLC chromatogram of FPD. (A-a) HPTLC chromatogram of FPD at 254 nm. (A-b) Peak analysis of HPTLC chromatogram at 254 nm. (A-c) Peak area with Rf values of obtained chromatogram at 254 nm. (A-d) Image documentation of developed HPTLC plate at 254 nm. (B-a) HPTLC chromatogram of FPD at 366 nm. (B-b) Peak analysis of HPTLC chromatogram at 366 nm (B-c) Peak area with Rf values of obtained chromatogram at 366 nm. (B-d) Image documentation of developed HPTLC plate at 366 nm. (C-a) HPTLC chromatogram of FPD at visible range. (C-b) Peak analysis of HPTLC chromatogram at visible range. (C-c) Peak area with Rf values of obtained chromatogram at visible range. (C-d) Image documentation of developed HPTLC plate at visible range. HPTLC, High performance thin layer chromatography; FPD, fruit extract of Pihecellobium dulce.
|
LC-MS/MS chromatograms of the standards and extract, and polyphenols identified in FPD are depicted in Fig. 2A and B. The presence of 28 different polyphenols in FPD was analyzed (Fig. 2C), which included naringenin [8.37576 ppm; Rt, 7.28 min; parent ion (m/z), 273.20; molecular ion (m/z), 153.05/147.15; ion mode, positive], tocopherol [0.96868 ppm; Rt, 12.87 min; parent ion (m/z), 429.50; molecular ion (m/z), 163.15/205.05; ion mode, positive], gallic acid [0.14716 ppm; Rt, 1.91 min; parent ion (m/z), 169.20; molecular ion (m/z), 125.05/81.00; ion mode, negative), catechin [0.01646 ppm; Rt, 6.75 min; parent ion (m/z), 291.20; molecular ion (m/z), 139.10/165.05; ion mode, positive], quinine [0.00196 ppm; Rt, 6.88 min; parent ion (m/z), 325.20; molecular ion (m/z), 307.10/184.05; ion mode, positive], epicatechin [0.0607 ppm; Rt, 6.77 min; parent ion (m/z), 289.00; molecular ion (m/z), 245.20/205.20; ion mode, positive], caffeic acid [0.00615 ppm; Rt, 6.91 min; parent ion (m/z), 179.20; molecular ion (m/z), 135.15/134.10; ion mode, negative], epigallocatechin [0.0229 ppm; Rt, 2.01 min; parent ion (m/z), 456.90; molecular ion (m/z), 169.15/125.05; ion mode, negative], myricetin [0.00901 ppm; Rt, 7.65 min; parent ion (m/z), 317.00; molecular ion (m/z), 151.20/179.20; ion mode, negative], quercetin [0.00801 ppm; Rt, 7.92 min; parent ion (m/z), 301.20; molecular ion (m/z), 151.05/179.00; ion mode, negative], luteolin [0.00079 ppm; Rt, 7.92 min; parent ion (m/z), 285.20; molecular ion (m/z), 151.10/175.05; ion mode, negative], apigenin [0.01047 ppm; Rt, 8.18 min; parent ion (m/z), 216.20; molecular ion (m/z), 149.05/151.00; ion mode, negative], kaempferol [0.01142 ppm; Rt, 7.83 min; parent ion (m/z), 285.20; molecular ion (m/z), 159.15/187.05; ion mode, negative], rutin [0.01ppm; Rt, 7.34 min; parent ion (m/z), 609.20; molecular ion (m/z), 300.00/301.20; ion mode, negative], hesperetin [0.00812 ppm; Rt, 7.86 min; parent ion (m/z), 301.20; molecular ion (m/z), 164.10/286.05; ion mode, negative], shikimic acid [0.01559 ppm; Rt, 1.76 min; parent ion (m/z), 172.90; molecular ion (m/z), 111.20; ion mode, negative], genistein [0.00514 ppm; Rt, 7.81 min; parent ion (m/z), 269.20; molecular ion (m/z), 133.20/132.05; ion mode, negative], cinnamic acid [0.01075 ppm; Rt, 7.93 min; parent ion (m/z), 147.00; molecular ion (m/z), 103.5; ion mode, negative]. The results revealed that FPD included several potent antioxidants.
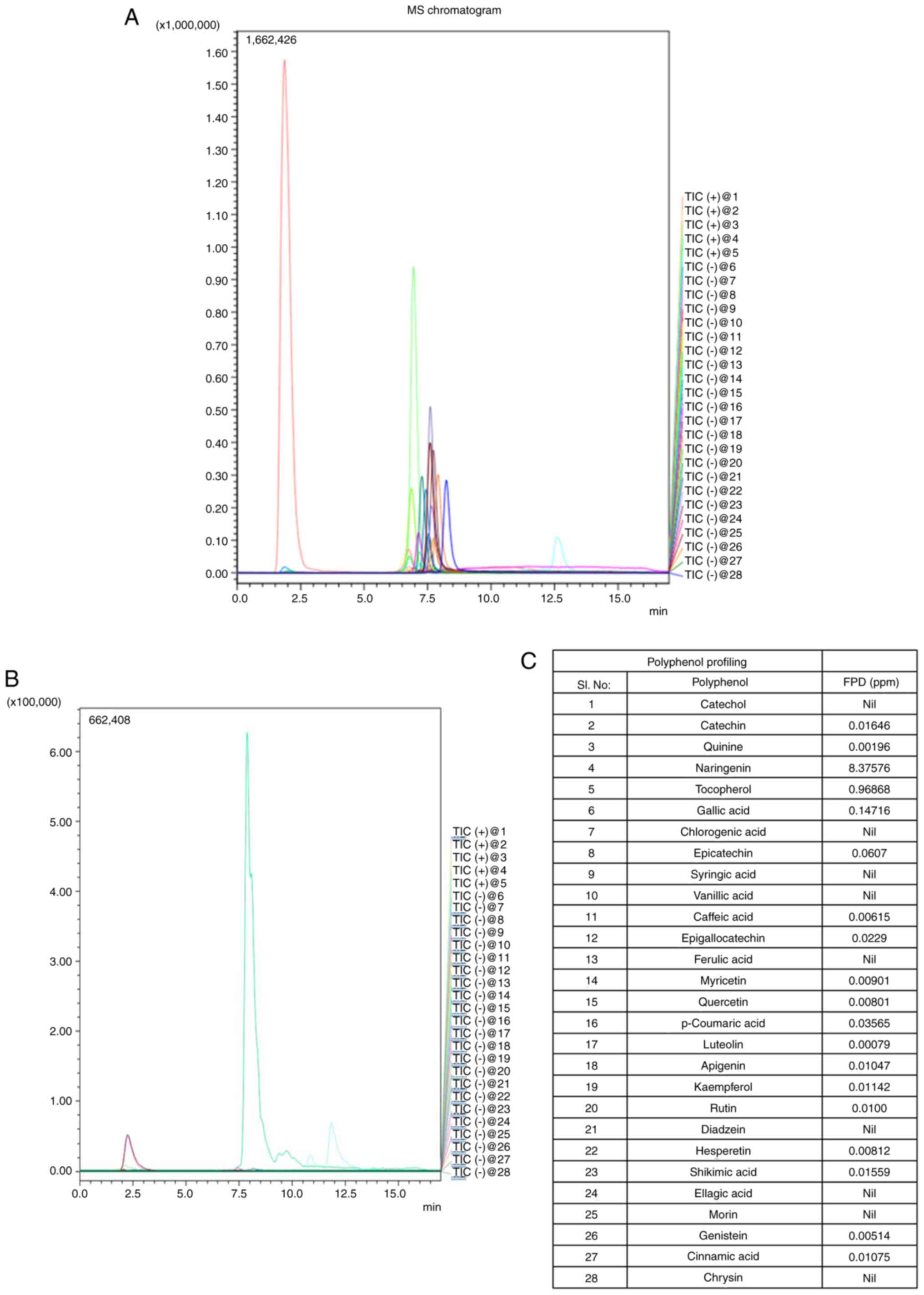 |
Figure 2
Polyphenolic profiling of FPD and its quantification using LC-MS/MS: Chromatogram of FPD. (A) LC-MS/MS chromatogram showing standard polyphenols. (B) LC-MS/MS chromatogram showing polyphenol concentration in FPD. (C) LC-MS/MS analysis of polyphenols in FPD. FPD, fruit extract of Pihecellobium dulce; LC-MS/MS, liquid chromatography-mass spectrometry.
|
In vitro free radical scavenging and lipid peroxidation inhibitory effect of FPD
The results of the DPPH scavenging activity of FPD are shown in Fig. 3A. The DPPH scavenging activity of various concentrations of FPD was compared with the same concentrations of the standard (ascorbic acid) ranging from 20 to 400 µg/ml. FPD exhibit notable concentration-dependent scavenging activity. FPD exhibited an IC50 value of 97.2 µg/ml, whereas for ascorbic acid, it was 60.878 µg/ml. However, the scavenging activity of ascorbic acid was found to be markedly high as compared to that of the extract.
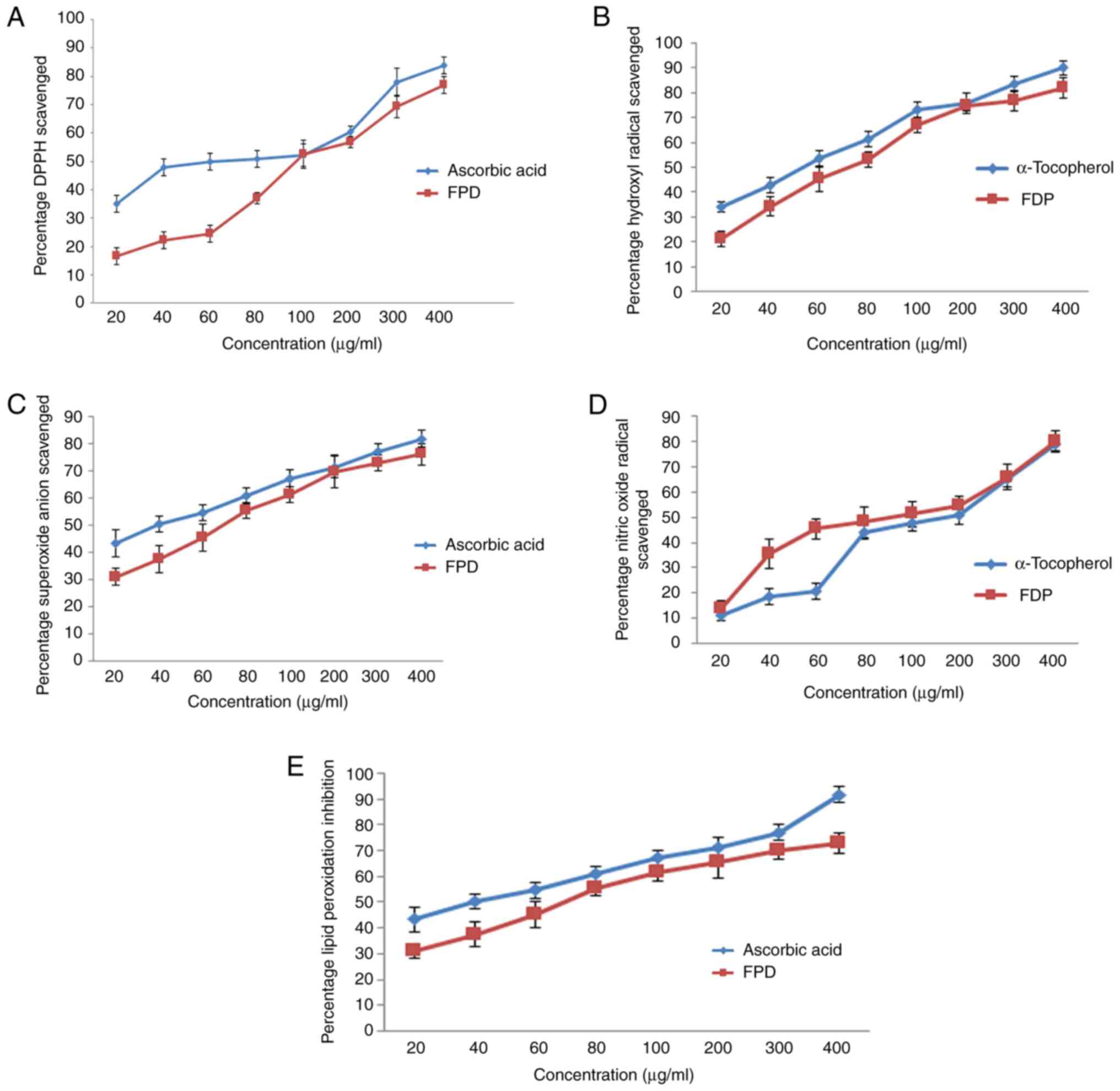 |
Figure 3
Evaluation of in vitro free radical scavenging efficacy of FPD. (A) DPPH scavenging activity of FPD and standard (ascorbic acid) at various concentrations. (B) Hydroxyl radical scavenging activity of FPD and standard (α-tocopherol) at various concentrations. (C) Super oxide anion scavenging activity of FPD and standard (ascorbic acid) at various concentrations. (D) Nitric oxide radical scavenging activity of FPD and standard (α-tocopherol) at various concentrations. (E) Lipid peroxidation inhibition activity of FPD and standard (ascorbic acid) at various concentrations. All values are expressed as the mean ± SD. FPD, fruit extract of Pihecellobium dulce; DPPH, 2,2-diphenyl 1 picrylhydrazyl.
|
The hydroxyl radical scavenging activity of various concentrations of FPD was compared with the same concentrations of the standard (α-tocopherol) ranging from 20 to 400 µg/ml, and is depicted in Fig. 3B. The hydroxyl scavenging activity of FPD was elevated markedly with escalating concentrations of FPD. The IC50 values were 71.7 and 52.04 µg/ml for FPD extract, and ascorbic acid, respectively. However, the scavenging activity of standard α-tocopherol was found to be higher compared with that of FPD.
The results of the superoxide scavenging activity of FPD are depicted in Fig. 3C. FPD exhibited prominent scavenging activity in a concentration-dependent manner up to 200 µg/ml; a further increase in the concentration did not lead to any marked increase in superoxide scavenging capacity. The IC50 values were 50.4 and 40.1 µg/ml for FPD and ascorbic acid, respectively. Ascorbic acid exhibited a higher superoxide scavenging activity compared with that of FPD.
Effect of FPD on NO radical scavenging activity and lipid peroxidation inhibition in vitro
The results of the NO scavenging activity of FPD are shown in Fig. 3D. The free radical scavenging activity of various concentrations of FPD was compared with the same concentrations of the standard (α-tocopherol) ranging from 20 to 400 µg/ml. FPD exhibited higher scavenging efficacy compared to that of control. IC50 value of FPD and α-tocopherol were found to be 93.2 and 113.9 µg/ml respectively. Since FPD was capable of scavenging the NO radical even at lower concentrations, it hence may be a promising agent for scavenging NO radicals in biological systems, and may be used for regulating certain pathophysiological conditions which occur due to the excessive generation of NO.
The anti-lipid peroxidation efficacy of different concentrations of FPD was compared with the same concentrations of standard (ascorbic acid) ranging from 20 to 400 µg/ml and is shown in Fig. 3E. The results revealed that various concentrations of FPD exhibited a concentration-dependent anti-lipid peroxidation activity. The IC50 values of FPD and ascorbic acid were found to be 70.1, and 39.6 µg/ml, respectively. The results demonstrated that FPD exhibited promising antioxidant scavenging activity, and may thus be used to reduce or terminate the progression of various types of oxidative stress and inflammatory-related diseases.
Effect of FPD on hematological parameters, BW and relative organ weight during MTX-induced oxidative stress
The effects of different treatment regimens on the total WBC count are depicted in Fig. 4A. The administration of MTX resulted in a reduction in the total WBC count compared with the SLM plus MTX-treated group (MTX + SLM) and the FPD plus MTX-treated group (MTX + FPD); the corresponding values were 4,583.26±881.63/mm3, 19,066.56±1,527.32/mm3 and 19,275.33±1,471.24/mm3, respectively. On the contrary, co-treatment with SLM and FPD increased the WBC count. Thus, co-treatment with FPD significantly (P<0.01) attenuated MTX-induced immunosuppression.
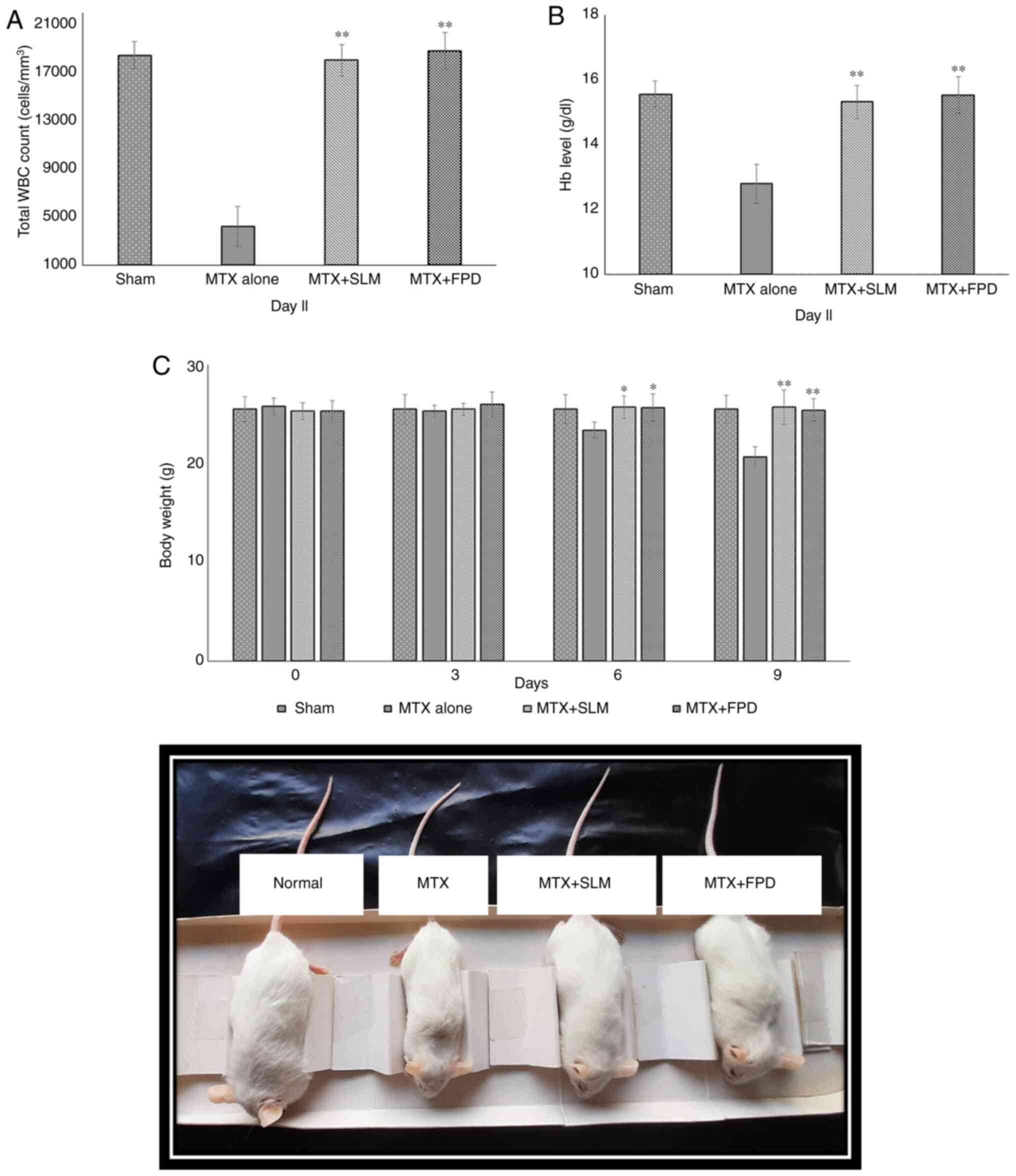 |
Figure 4
(A) Effect of FPD on the total leukocyte count (WBCs). (B) Effect of FPD on the Hb level. (C) Assessment of body weight of animals following the respective treatments. The body weight of each animal was monitored every 3rd day (starting from day 0, prior to the first treatment) and statistical analysis was performed. All values shown are the mean ± SD. *P<0.05 and **P<0.01, MTX alone vs. MTX + FPD or MTX + SLM. Hb, hemoglobin; MTX, methotrexate; SLM, silymarin; FPD, fruit extract of Pihecellobium dulce; WBC, white blood cell.
|
The effects of the different treatment regimens on the Hb content are presented in Fig. 4B. In the present study, at the end of the experiment, the MTX-treated group exhibited a significant decrease in Hb content (12.89±0.58 gm/dl), whereas the MTX + SLM and MTX + FPD groups exhibited a significant (P<0.01) increase in the Hb content, with the corresponding values being 14.88±0.69 and 15.24±0.78 gm/dl, respectively.
A schematic of the effects of the different treatment regimens on BW is shown in Fig. 4C. The MTX-treated group exhibited a decrease in BW (20.67±1.03 g; P<0.01) compared with the MTX + SLM (25.7±1.73 g) and MTX + FPD groups (25.4±1.40 g).
Changes in the relative organ weights of the spleen, thymus, liver, kidney and lungs are depicted in Table II. The relative organ weights of the organs mentioned above was significantly decreased (P<0.01) in MTX treated group compared with the MTX + SLM and MTX + FPD groups. However, these changes were reversed following the co-administration of SLM and FPD with MTX.
 |
Table II
Effect of treatments on relative organ weights.
|
Table II
Effect of treatments on relative organ weights.
| |
Relative organ weight (g/100 g body weight) |
| Group |
Spleen |
Thymus |
Liver |
Kidney |
Lungs |
| Sham |
0.46±0.04 |
0.26±0.02 |
5.58±0.52 |
1.36±0.05 |
1.15±0.20 |
| MTX |
0.30±0.02 |
0.10±0.02 |
4.40±0.34 |
1.29±0.10 |
0.80±0.05 |
| MTX + SLM |
0.47±0.03a |
0.18±0.07a |
5.70±0.16a |
1.38±0.04 |
0.98±0.13a |
| MTX + FPD |
0.41±0.05a |
0.21±0.02a |
5.31±0.26a |
1.33±0.07b |
1.08±0.10a |
Effect of FPD on hepatic and renal marker enzymes during MTX-induced oxidative stress
The effects of FPD on serum hepatic (SGPT, SGOT, bilirubin and ALP) and renal (urea and creatinine) toxicity marker levels are presented in Tables III and IV, respectively. The administration of MTX significantly (P<0.01) exacerbated the serum SGOT (23.84±8.09 U/l), SGPT (82.04±9.30 U/l), bilirubin (2.02±0.28 g/l), ALP (7.21±0.58 U/l), urea (2.17±0.27 g/l) and creatinine (3.74±0.50 g/l) levels in serum compared with the MTX + SLM (SGOT, 14.19±3.24 U/l; SGPT, 41.33±5.13 U/l; bilirubin, 1.19±0.24 g/l; ALP, 4.05±0.65 U/l; urea, 1.55±0.27 g/l; and creatinine, 3.03±0.09 g/l) and MTX + FPD (SGOT, 12.64±2.80 U/l; SGPT, 45.33±6.43 U/l; bilirubin, 1.16±0.16 g/l; ALP, 3.94±0.22 U/l; urea, 1.17±0.06 g/l; and creatinine, 3.10±0.14 g/l) groups.
 |
Table III
Effect of FPD on serum SGOT, SGPT, bilirubin and ALP levels in experimental animals treated with MTX.
|
Table III
Effect of FPD on serum SGOT, SGPT, bilirubin and ALP levels in experimental animals treated with MTX.
| Groups |
SGOT (U/l) |
SGPT (U/l) |
Bilirubin (g/l) |
ALP (U/l) |
| Sham |
11.99±3.80 |
39.88±6.23 |
1.06±0.26 |
3.88±0.54 |
| MTX alone |
23.84±8.09 |
82.04±9.30 |
2.02±0.28 |
7.21±0.58 |
| MTX + SLM |
14.19±3.24a |
41.33±5.13a |
1.19±0.24a |
4.05±0.65a |
| MTX + FPD |
12.64±2.80a |
45.33±6.43a |
1.16±0.16a |
3.924±0.22a |
 |
Table IV
Effect of FPD on serum urea and creatinine levels in experimental animals treated with MTX.
|
Table IV
Effect of FPD on serum urea and creatinine levels in experimental animals treated with MTX.
| |
Urea (g/l) |
Creatinine (g/l) |
| Sham |
1.39±0.09 |
2.91±0.28 |
| MTX alone |
2.17±0.27 |
3.74±0.50 |
| MTX + SLM |
1.55±0.27a |
3.03±0.09a |
| MTX + FPD |
1.17±0.06a |
3.1±0.14a |
Effects of FPD on enzymic and non-enzymic antioxidant levels during MTX-induced oxidative stress
The effects of treatment regimens on liver, kidney, and lungs oxidative parameters are illustrated in Fig. 5. Compared with the MTX + FPD group, the administration of MTX significantly reduced the levels of antioxidant enzymes, such as GSH (Fig. 5C), SOD (Fig. 5D), GPx (Fig. 5E) and catalase (Fig. 5F), while on the contrary, the levels of antioxidant stress markers, such as MDA (Fig. 5B) and NO (Fig. 5A) were elevated, indicating an impaired antioxidant defense capacity and consequently, increased oxidative stress. However, combination therapy with MTX with FPD (MTX + FPD) led to a significant reversal of these outcomes (P<0.01).
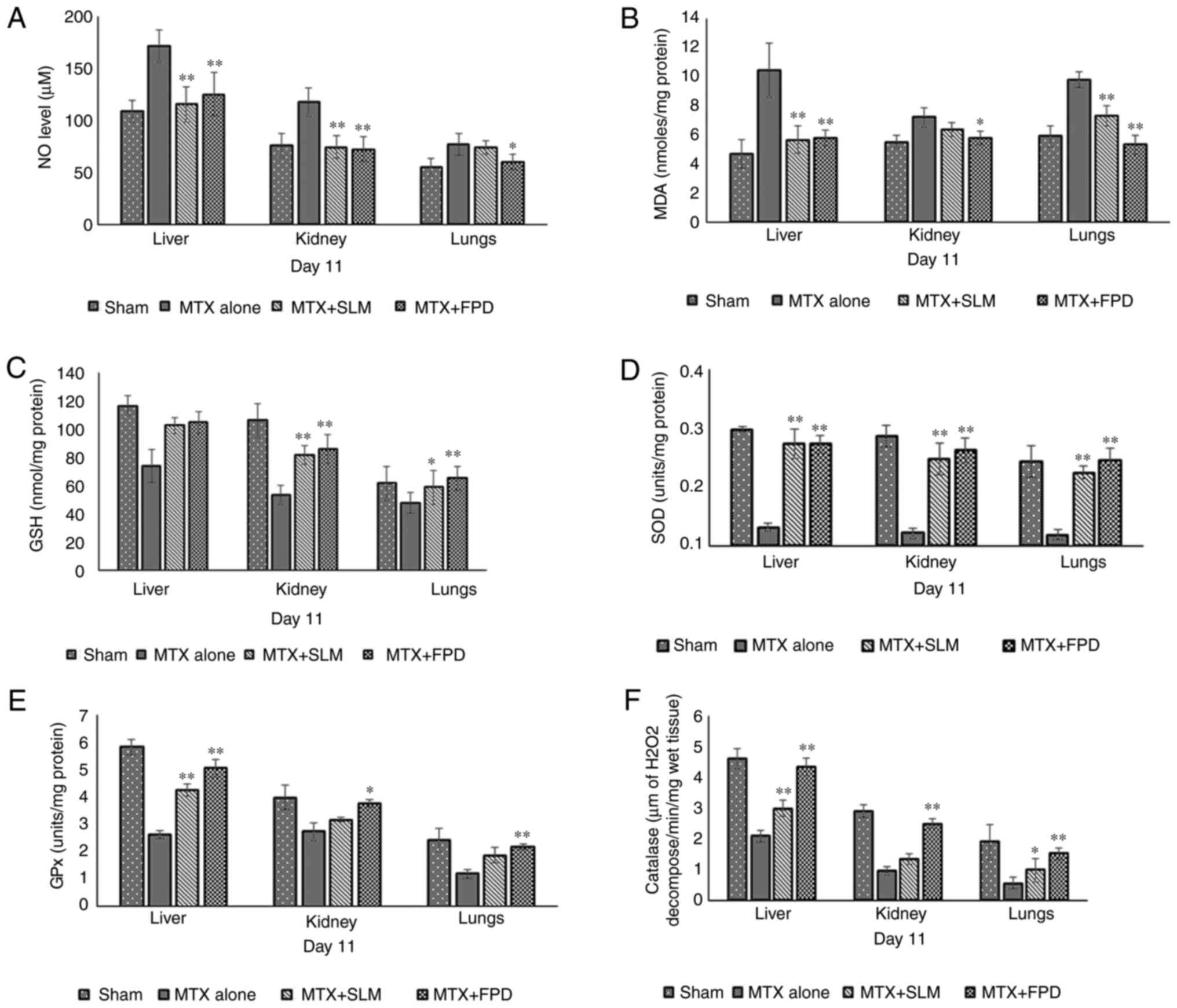 |
Figure 5
Effects of FPD on liver, kidney and lung markers (A) NO; (B) MDA; (C) GSH; (D) SOD; (E) GPx and (F) catalase levels following the respective treatments. All values shown are the mean ± SD. *P<0.05 and **P<0.01, MTX alone vs. MTX + FPD or MTX + SLM. MTX, methotrexate; SLM, silymarin; FPD, fruit extract of Pihecellobium dulce; NO, nitric oxide; MDA, malondialdehyde; GSH, glutathione; GPx, glutathione peroxidase; SOD, superoxide dismutase.
|
Effect of FPD on liver, kidney and lung histopathology during MTX-induced oxidative stress
The effects of FPD on MTX-induced histopathological changes in the liver, kidney and lung tissue samples are illustrated in Fig. 6A-C, respectively. The histological evaluation of the liver samples of mice treated with MTX exhibited an altered lobular architecture with interface hepatitis and periportal inflammation. By contrast, the administration of FPD reduced MTX-induced hepatic toxicity. The kidney sections from the MTX-treated group exhibited tubular epithelial loss and inflammatory cell infiltration. However, treatment with FPD mitigated MTX-induced nephrotoxicity. The administration of MTX led to severe lung damage, including the scattered infiltration of lymphocytes and blood vessel congestion. However, treatment with FPD significantly reduced MTX-induced pulmonary toxicity, as evidenced by the normal morphology of the septal wall, alveoli and blood vessels.
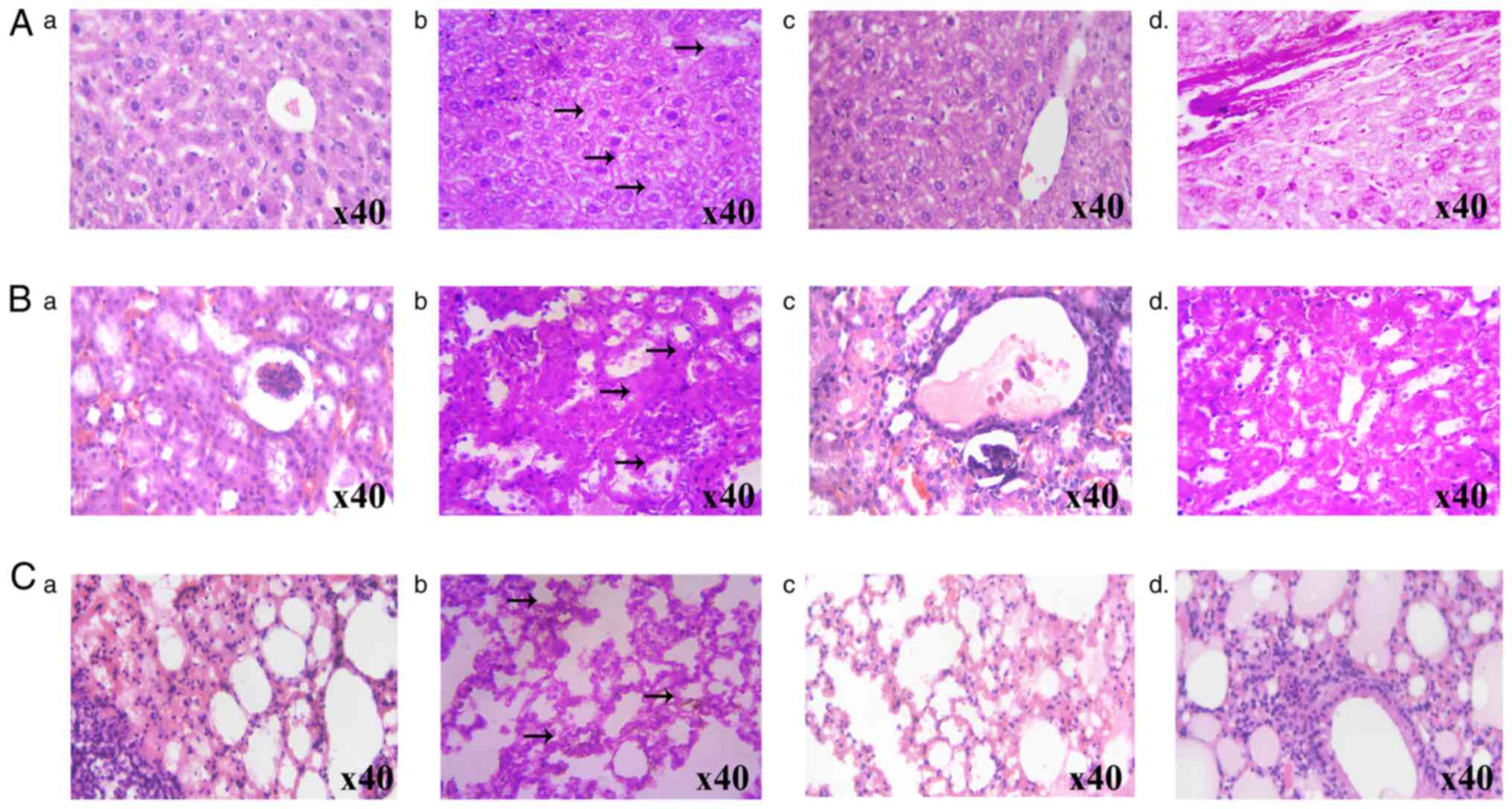 |
Figure 6
Protective effect of FPD on liver, kidney and lungs histology during MTX-induced toxicity. (A) Protective effect of FPD on liver histology during MTX-induced toxicity. (a) Section from normal mouse. (b) MTX alone group (arrows represent altered lobular architecture with inflammatory cell infiltration). (c) MTX + SLM group. (d) MTX + FPD group. (B) Photomicrographs of mouse kidney following the respective treatments. (a) Section from normal mouse. (b) MTX alone group (arrows represent focal epithelial loss and inflammatory cell infiltration). (c) MTX + SLM group. (dd) MTX + FPD group (C) Protective effect of FPD on lung histology during MTX-induced toxicity. (a) Section from normal mouse. (b) MTX alone group (arrows represent periportal inflammation, cytoplasmic vacuolation and scattered infiltration of lymphocytes) (c) MTX + SLM group. (d) MTX + FPD group. MTX, methotrexate; SLM, silymarin; FPD, fruit extract of Pihecellobium dulce.
|
Effect of FPD on the level pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6 during MTX-induced oxidative stress
The administration of a high dose of MTX markedly elevated the serum cytokine levels in the experimental mice. However, treatment with FPD along with MTX reduced the levels of serum pro-inflammatory cytokines (Fig. 7). The administration of MTX resulted in an elevation in the levels of pro-inflammatory cytokines, such as TNF-α (140±23.34 pg/ml), IL-1β (179.23±29.49 pg/ml) and IL-6 (187.39±20.49 pg/ml) in serum. However, the administration of FPD significantly (P<0.01) decreased the levels of TNF-α (106.93±29.69 pg/ml), IL-1β (120.90±22.00 pg/ml) and IL-6 (130.40±19.09 pg/ml) compared with MTX alone group.
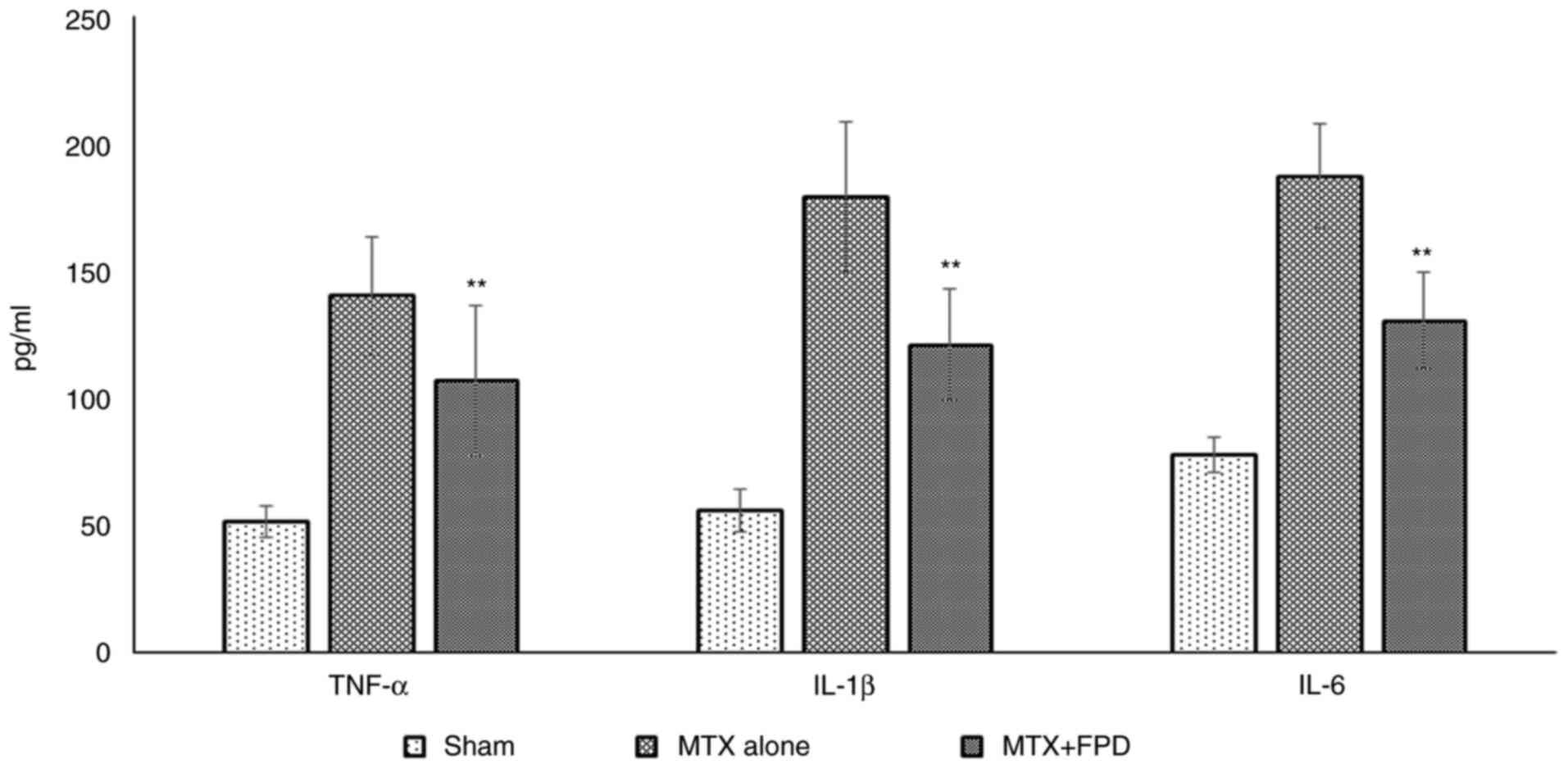 |
Figure 7
Pro-inflammatory cytokine (TNF-α, IL-1β and IL-6 and) profiling. Statistical analysis was performed using one-way ANOVA. All values were expressed as the mean ± SD. **P<0.01 compared with MTX alone. MTX, methotrexate; FPD, fruit extract of Pihecellobium dulce.
|
Discussion
Preliminary qualitative phytochemical analysis of FPD revealed the presence of diverse phytochemical components, which includes flavonoids, terpenoids, glycosides, alkaloids, phenols, tannins, saponins and xanthoproteins, and has been reported to possess anti-inflammatory, antioxidant, antiparasitic, antimicrobial and anticancer activity. Over the past few years, flavonoids have elicited increasing attention due to their distinct potential health benefits (42-46). Research on flavonoids has been rapidly gaining momentum due to their broad spectrum of biological and pharmacological activities (47). Flavonoids can reduce the risk of developing coronary atherosclerosis, diabetes mellitus, ischemic damage, the aging process and cancer. However, the edifice of evidence suggests that flavonoids have several other biological properties, which include anti-inflammatory, antioxidant, antimicrobial and antitumor activities (48). Oxidative stress is a known contributor to a variety of disorders and hence over the past few decades, there has been a resurgence of interest towards antioxidant screening studies driven by the hope of identifying an effective alternative medicine from natural resources. FPD contains several other bioactive components, including terpenoids, glycosides, alkaloids, phenols, tannins, saponins and xanthoproteins, which possess a distinct range of functions in biological systems. Terpenoids are naturally occurring extensive and diverse structural phytochemical compounds, which display a wide array of biological and pharmacological functions. Studies on alkaloids and saponins have demonstrated that they have a wide range of anti-inflammatory, antidiabetic, hypocholesterolemic, anti-fungal, fungistatic, molluscicidal, anticancer and anti-bacterial activities. Moreover, tannins have been shown to possess excellent antioxidant and immunomodulatory properties (49-52).
In the present study, LC-MS/MS analysis of FPD revealed the presence of several flavonoids and phenolic compounds, including naringenin, quercetin, catechin, tocopherol, quinine, gallic acid, epicatechin, caffeic acid, epigallocatechin, myricetin, p-coumaric acid, luteolin, apigenin, kaempferol, rutin, cinnamic acid, hesperetin, shikimic acid and genistein, which have been reported to possess potent antioxidant properties (53-71). By LC-MS/MS analysis, the major compound identified was naringenin. Naringenin is one of the key bioactive flavonoids known to have several biological properties, which include antioxidant, antitumor, antiviral, anti-inflammatory, cardioprotective and antiadipogenic effects (72).
The free radical scavenging activity of antioxidants on DPPH is considered to be due to their hydrogen-donating property (73). DPPH assay has been used as an index that ascertains the antioxidant activity of FPD extract. In the present study, the increased reduction of DPPH radicals with escalating concentrations of FPD, and the reference compound, ascorbic acid, was observed. Therefore, it can be hypothesized that FPD contains some active constituents that have potent DPPH radical quenching properties, by reducing DPPH purple-colored methanolic solution to its corresponding yellow-colored diphenyl picryl hydrazine. Lower absorbance is an indicator of higher antioxidant efficacy in terms of hydrogen ion donating ability.
Superoxide anion radicals are highly unstable free radicals mainly produced by activated phagocytes, such as eosinophils, neutrophils, monocytes, and macrophages. Cells have developed an elaborate complex system of enzymatic and non-enzymatic antioxidant defense mechanisms, which can counteract these indigenously developed ROS. SOD is one such key powerful detoxification enzyme found in almost all living organisms and is responsible for protecting cells from oxidative-stress induced cell death. The excessive generation of free radicals disrupts homeostasis between oxidants and antioxidants, invariably accompanies cellular damage (74). In the present study, the superoxide radical scavenging activity of FPD was measured based on NBT reduction. Superoxide radical scavenging assay measures the reducing potential of FPD reacting with superoxide anions were generated from the in vitro system reduces the yellow-colored NBT to form the blue formazan. FPD and the reference compound, ascorbic acid, exhibited concentration-depended superoxide anion scavenging activity. Hence the results presented herein strongly established the antioxidant efficacy of FPD.
Among ROS, hydroxyl radicals are the most potent, extremely reactive, highly lethal and damaging oxidants that can interact with chromatin; exposure to these radicals causes extensive damage, which include a wide range of sugar- and base-derived products, protein fragmentation, DNA-protein cross-links and DNA strand breaks (75-77). Hydroxyl radical scavenging assay measures the reducing potential of FPD reacting with the hydroxyl radicals generating from the in vitro system by fenton reaction. In the present study, the hydroxyl scavenging activity of FPD was markedly elevated with escalating concentrations of FPD. Hence the results strongly suggested these as promising free radical scavengers.
It has been long been recognized that generations of free radicals can inflict the oxidative deterioration of polyunsaturated fatty acids (PUFAs) (78-81). In the present study, the amelioration of hydroxyl-radical induced lipid peroxidation in mouse livers by FPD was evaluated. FPD exerted an inhibitory effect on lipid peroxidation in a concentration-depended manner. These results suggested that FPD can prevent the deleterious effects induced by ROS by disrupting the chain reactions responsible for lipid peroxidation, thereby maintaining cellular integrity.
Reactive nitrogen species (RNS) and NO are highly unstable freely diffusible free radicals that readily attack chromatin leading to deamination of cytosine, adenine, and guanine. The toxicity induced by NO markedly increases by reacting with superoxide anion radical, forming an extremely reactive peroxynitrite radical (ONOO-). NO is a potent pleiotropic intracellular and intercellular signaling molecule having an impressive spectrum of diverse physiological and pathophysiological functions. NO can react rapidly with molecular oxygen to form nitrogen oxides (NO2, N3O4, and N2O4). The higher nitrogen oxides either interact with certain biomolecules such as amines, and thiols or otherwise hydrolyze to produce nitrite (NO2-), and nitrate (NO3-). Furthermore, NO can also interact rapidly with superoxide radical forming ONOO-. The degree of tissue damage is mainly persuaded by microenvironmental conditions under which NO is released. All these intermediators and progenitor products induce deamination in cytosine, adenine and guanine. Chronic exposure to NO radicals is associated with various types of disorders, including AIDS, juvenile diabetes, cancer, multiple sclerosis, arthritis, Alzheimer's and ulcerative colitis (82-84). In the present study, in NO radical scavenging assay, the absorbance at 546 nm was decreased markedly with escalating concentrations of FPD extract. Fostering results suggested that FPD could be used as a potential candidate for relieving antioxidant stress, and thus preventing cancer.
Furthermore, the in vivo antioxidant efficacy of FPD was analyzed in the present study. MTX is a folate antimetabolite agent used as the first choice for the treatment of different types of malignancies and autoimmune diseases. However, treatment with MTX is associated with severe adverse effects, including hepatotoxicity, myelotoxicity, pancytopenia, acute renal failure, liver fibrosis, uremia and hematuria, thus limiting its clinical use (85-87). Findings of Mukherjee et al (88) suggested that MTX induced hepatorenal toxicity via oxidative stress, inflammation and apoptosis. Thus, a combined therapy with agents having antioxidant, and anti-inflammatory activity is highly recommended, which may thus reduce toxicity and increase the treatment efficacy. Myelosuppression and pancytopenia are the most common hematological toxicities occurring during MTX treatment. In the present study, the co-administration of FPD with MTX attenuated MTX-induced leukopenia and myelotoxicity, as evidenced by the total WBC count measurement and Hb level analysis. Moreover, combined treatment with FPD with MTX exerted a prominent suppressive effect against the MTX-induced decrease in splenic and thymic weight, suggesting the potential role of FPD over MTX-induced immunotoxicity.
Hepatotoxicity is another most common complication associated with MTX treatment. An increment in the levels of serum SGOT, SGPT and ALP may be attributed to the damaged structural integrity of the hepatic membrane (possibly via lipid peroxidation and oxidative stress) (87). In the present study, a marked elevation in serum hepatotoxicity markers, such as SGPT, SGOT and ALP was observed following the administration of MTX. Co-treatment with FPD and MTX significantly reduced the elevated levels of serum toxicity markers, indicating its supreme role in restoring or ameliorating MTX-induced hepatocellular damage. The reason for the coordinated effects may be attributed to their anti-inflammatory and antioxidant properties.
The administration of MTX leads to the upregulation of nephrotoxicity marker enzymes (urea and creatinine), which ultimately culminate the disruption of glomerular apparatus (40). In the present study, co-treatment with FPD and MTX significantly attenuated serum nephrotoxicity markers, suggesting the nephroprotective role of FPD. Combined treatment with MTX and FPD may thus be beneficial in attenuating the detrimental effects induced by MTX.
It has been demonstrated that MTX administration significantly increases hepatocellular ROS and NO levels (88). The excessive generation of ROS can contribute to tissue damage, as evaluated by LPO, SOD and GSH levels. GSH and SOD play a crucial role in ameliorating MTX-induced oxidative damage in tissues. As was expected, in accordance with the reports of other findings, in the present study, the administration of MTX led to a significant reduction in tissue GSH and SOD levels, and increased the LPO level (89,90). The administration of a high dose of MTX significantly increased the levels of serum inflammatory mediators, such as TNF-α, IL-1β and IL-6. However, FPD significantly attenuated the inflammatory responses by regulating the level of pro-inflammatory cytokines. Combined treatment with MTX and FPD significantly reversed the multiple pathophysiological conditions induced by MTX. The effects of FPD were presumably attributed to its free radical scavenging antioxidant properties. A conceptual diagram of the antioxidant mechanisms of FPD is presented in Fig. 8.
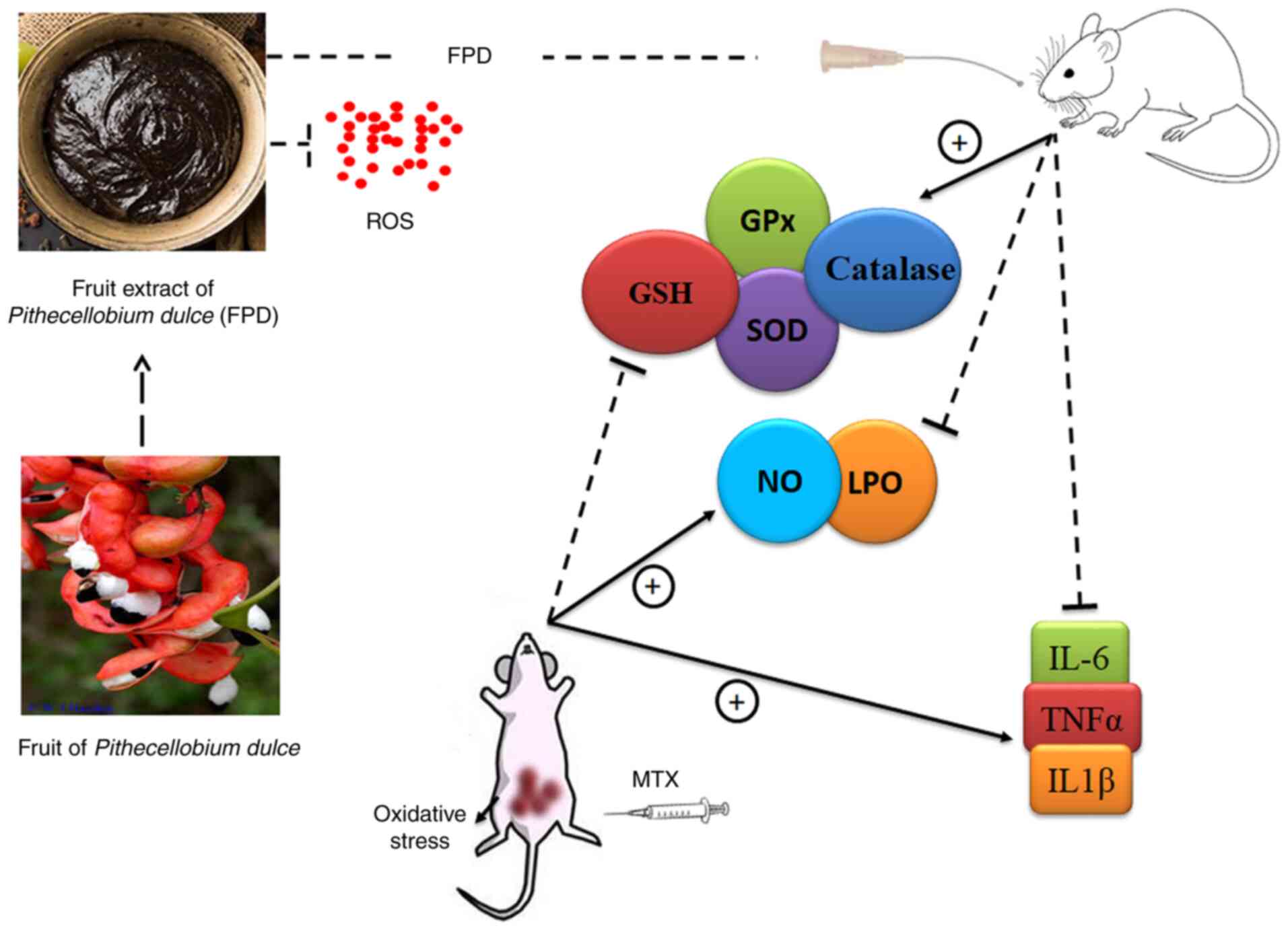 |
Figure 8
Conceptual diagram of the antioxidant mechanisms of FPD. FPD, fruit extract of Pihecellobium dulce. ROS, reactive oxygen species; NO, nitric oxide; MDA, malondialdehyde; GSH, glutathione; GPx, glutathione peroxidase; SOD, superoxide dismutase.
|
In conclusion, the present study demonstrates that FPD contains higher concentrations of tannins, flavonoids, phenolics, saponins and several other bioactive components. Furthermore, the results revealed that supplementation with FPD significantly prevented oxidative stress-associated tissue damage induced by MTX; FPD may have great potential for application in future disease therapeutic strategies. However, further studies are required to better ascertain and disseminate its pharmacological properties, which may provide aid in the discovery of novel drugs and the developmental arena.
Acknowledgements
The authors are thankful to Dr Rekha A. Nair, Director, Regional Cancer Centre (RCC) and Dr S. Kannan, Head, Division of Cancer Research, RCC for providing valuable support required for the study. The authors also acknowledge Dr Nisha Prakasan of CSIR-NIIST for assisting with the LC-MS/MS analysis.
Funding
The present study was supported by the Council of Scientific and Industrial Research University Grants Commission [20/12/2015 (ii) EU‑V] and [09/553(0022)/2016‑EMR‑1] in the form of a senior research fellowship for the study.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SSD was involved in the study methodology and investigation, as well as in the writing of the original draft, visualization, and data collection and interpretation. SD was involved in formal analysis and investigation. RPM was involved in data analysis and interpretation. CG was involved in the study conceptualization, in the writing, reviewing and editing of the manuscript, in study supervision, project administration, funding acquisition, and data analysis and interpretation. CG and SSD confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Institutional Animal Ethics Committee (IAEC), Regional Cancer Centre (RCC), Thiruvananthapuram. (IAEC/RCC NO. 6/18).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Pincus T, Yazici Y, Sokka T, Aletaha D and Smolen JS: Methotrexate as the anchor drug for the treatment of early rheumatoid arthritis. Clin Exp Rheumatol. 21 (5 Suppl 31):S179–S185. 2003.PubMed/NCBI
|
|
2
|
Foell D, Frosch M, Schulze zur Wiesch A, Vogl T, Sorg C and Roth J: Methotrexate treatment in juvenile idiopathic arthritis: When is the right time to stop? Ann Rheum Dis. 63:206–208. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Czarnecka-Operacz M and Sadowska-Przytocka A: The possibilities and principles of methotrexate treatment of psoriasis-the updated knowledge. Postepy Dermatol Alergol. 31:392–400. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Howard SC, McCormick J, Pui CH, Buddington RK and Harvey RD: Preventing and managing toxicities of high-dose methotrexate. Oncologist. 21:1471–1482. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wiczer T, Dotson E, Tuten A, Phillips G and Maddocks K: Evaluation of incidence and risk factors for high-dose methotrexate-induced nephrotoxicity. J Oncol Pharm Pract. 22:430–436. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lateef O, Shakoor N and Balk RA: Methotrexate pulmonary toxicity. Expert Opin Drug Saf. 4:723–730. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim J, Kim Y, Choi J, Jung H, Lee K, Kang J, Park N, Rim YA, Nam Y and Ju JH: Recapitulation of methotrexate hepatotoxicity with induced pluripotent stem cell-derived hepatocytes from patients with rheumatoid arthritis. Stem Cell Res Ther. 9(357)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ajmani S, Preet Singh Y, Prasad S, Chowdhury A, Aggarwal A, Lawrence A, Misra R, Mishra R and Agarwal V: Methotrexate-induced pancytopenia: A case series of 46 patients. Int J Rheum Dis. 20:846–851. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Driehuis E, Oosterom N, Heil SG, Muller IB, Lin M, Kolders S, Jansen G, de Jonge R, Pieters R, Clevers H and van den Heuvel-Eibrink MM: Patient-derived oral mucosa organoids as an in vitro model for methotrexate induced toxicity in pediatric acute lymphoblastic leukemia. PLoS One. 15(e0231588)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tang D, Zeng T, Wang Y, Cui H, Wu J, Zou B, Tao Z, Zhang L, Garside GB and Tao S: Dietary restriction increases protective gut bacteria to rescue lethal methotrexate-induced intestinal toxicity. Gut Microbes. 12(1714401)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Burmester GR, Kaeley GS, Kavanaugh AF, Gabay C, MacCarter DK, Nash P, Takeuchi T, Goss SL, Rodila R, Chen K, et al: Treatment efficacy and methotrexate-related toxicity in patients with rheumatoid arthritis receiving methotrexate in combination with adalimumab. RMD Open. 3(e000465)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kroll M, Kaupat-Bleckmann K, Mörickel A, Altenl J, Schewel DM, Stanullal M, Zimmermann M, Schrappe M and Cario G: Methotrexate-associated toxicity in children with Down syndrome and acute lymphoblastic leukemia during consolidation therapy with high dose methotrexate according to ALL-BFM treatment regimen. Haematologica. 105:1013–1020. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Naewla S, Sirichoat A, Pannangrong W, Chaisawang P, Wigmore P and Welbat JU: Hesperidin alleviates methotrexate-induced memory deficits via hippocampal neurogenesis in adult rats. Nutrients. 11(936)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ozcicek F, Kara AV, Akbas EM, Kurt N, Yazici GN, Cankaya M, Mammadov R, Ozcicek A and Suleyman H: Effects of anakinra on the small intestine mucositis induced by methotrexate in rats. Exp Anim. 69:144–152. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Widemann BC, Balis FM, Kim A, Boron M, Jayaprakash N, Shalabi A, O Brien M, Eby M, Cole DE, Murphy RF, et al: Glucarpidase, leucovorin, and thymidine for high-dose methotrexate-induced renal dysfunction: Clinical and pharmacologic factors affecting outcome. J Clin Oncol. 28:3979–3986. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Valade S, Mariotte E, Azoulay E and Darmon M: High-dose methotrexate in ICU patients: A retrospective study. Ann Intensive Care. 10(81)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Skeoch S, Weatherley N, Swift AJ, Oldroyd A, Johns C, Hayton C, Giollo A, Wild JM, Waterton JC, Buch M, et al: Drug-induced interstitial lung disease: A systematic review. J Clin Med. 10(356)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Conway R and Carey JJ: Risk of liver disease in methotrexate treated patients. World J Hepatol. 9:92–1100. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Elango T, Dayalan H, Gnanaraj P, Malligarjunan H and Subramanian S: Impact of methotrexate on oxidative stress and apoptosis markers in psoriatic patients. Clin Exp Med. 14:431–437. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Albrecht K and Muller-Ladner U: Side effects and management of side effects of methotrexate in rheumatoid arthritis. Clin Exp Rheumatol. 28 (5 Suppl 61):S95–S101. 2010.PubMed/NCBI
|
|
21
|
Pazhayattil GS and Shirali AC: Drug-induced impairment of renal function. Int J Nephrol Renovasc Dis. 7:457–468. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mozaffarian D, Rosenberg I and Uauy R: History of modern nutrition science-implications for current research, dietary guidelines, and food policy. BMJ. 361(k2392)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ponmozhi P, Geetha M, Saravana Kumar M and Suganya Devi P: Extraction of anthocyanin and analysing its antioxidant properties from Pithecellobium dulce fruit pericarp. Asian J Pharm Clin Res. 4:41–45. 2011.
|
|
24
|
Dhanisha SS, Drishya S and Guruvayoorappan C: Fruit extract of Pithecellobium dulce (FPD) ameliorates carrageenan-induced acute inflammatory responses via regulating pro-inflammatory mediators. J Food Biochem. 44(e13329)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dhanisha SS, Drishya S and Guruvayoorappan C: Pithecellobium dulce fruit extract mitigates cyclophosphamide-mediated toxicity by regulating proinflammatory cytokines. J Food Biochem. 44(e13083)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Megala J and Geetha A: Free radical scavenging and H+, K+- ATPase inhibition activities of Pithecellobium dulce. Food Chem. 121:1120–1128. 2010.
|
|
27
|
Nigam SK, Gopal M, Uddin R, Yoshikawa K, Kawamoto M and Arihara S: Pithedulosides A-G, oleanane glycosides from Pithecellobium dulce. Phytochem. 44:1329–1334. 1997.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sahu NP and Mahato SB: Anti-inflammatory triterpene saponins of Pithecellobium dulce: Characterization of an echinocystic acid bisdesmoside. Phytochem. 37:1425–1427. 1994.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bautista-Banios S, Garcı́a-Domı́nguez E, Barrera-Necha LL, Reyes Chilpa R and Wilson CL: Seasonal evaluation of the postharvest fungicidal activity of powders and extracts of huamuchil (Pithecellobium dulce): Action against Botrytriscinerea, Penicillium digitatum and Rhizopus stolonifer of strawberry fruit. Postharvest Biol Technol. 29:81–92. 2003.
|
|
30
|
Dhanisha SS, Drishya S and Guruvayoorappan C: Traditional knowledge to clinical trials: A review on nutritional and therapeutic potential of Pithecellobium dulce. J Basic Clin Physiol Pharmacol: Feb 9, 2021 (Epub ahead of print).
|
|
31
|
Parekh J and Chanda S: Phytochemical screening of some plants from Western regions of India. Plant Arch. 8:657–662. 2008.
|
|
32
|
Magalhaes LM, Segundo MA, Reis S and Lima JLFC: Automatic method for determination of total antioxidant capacity using 2,2-diphenyl-1-picrylhydrazyl assay. Analy Chem Acta. 558:310–318. 2006.
|
|
33
|
McCord MJ and Fridowich I: Superoxide dismutase, an enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 9244:6049–6055. 1969.PubMed/NCBI
|
|
34
|
Kunchandy E and Rao MNA: Effect of curcumin on hydroxyl radical generation through Fenton reaction. Int J Pharm. 57:173–176. 1989.
|
|
35
|
Awah FM, Uzoegwu PN, Oyugi JO, Rutherford J, Ifeonu P, Yao X, Fowke KR and Eze MO: Free radical scavenging activity and immunomodulatory effect of Stachytarpheta angustifolia leaf extract. Food Chem. 119:1409–1416. 2010.
|
|
36
|
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS and Tannenbaum SR: Analysis of nitrate, nitrite and 15N nitrite in biological fluids. Anal Biochem. 126:131–138. 1982.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bishayee S and Balasubramaniyam AS: Lipid peroxide formation in rat brain. J Neurochem. 18:909–920. 1971.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ohkawa H, Ohishi N and Yagi K: Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95:351–358. 1979.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Cetin A, Kaynar L, Kocyigit I, Hacioglu SK, Saraymen R, Ozturk A, Sari I and Sagdic O: Role of Grape seed extract on methotrexate induced oxidative stress in rat liver. Am J Chin Med. 36:861–872. 2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Abdel-Daim MM, Khalifa HA, Abushouk AI, Dkhil MA and Al-Quraishy SA: Diosmin attenuates methotrexate-induced hepatic, renal, and cardiac injury: A biochemical and histopathological study in mice. Oxid Med Cell Longev. 2017(3281670)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Karimi G, Vahabzadeh M, Lari P, Rashedinia M and Moshiri M: Silymarin a promising pharmacological agent for treatment of diseases. Iran J Basic Med sci. 14:308–317. 2011.PubMed/NCBI
|
|
42
|
Charalampos P, Konstantina L, Olga KM, Panagiotis Z and Vassileia JS: Antioxidant capacity of selected plant extracts and their essential oils. Antioxidants. 2:11–22. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Hayash T, Sawa K, Kawasaki M, Arisawa M, Shimizu M and Morita N: Inhibition of cow's milk xanthine oxidase by flavonoids. J Nat Prod. 51:345–348. 1988.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Metodiewa D, Kochman A and Karolczak S: Evidence for antiradical and antioxidant properties of four biologically active N,N, diethylaminoethyl ethers of flavanone oximes: A comparison with natural polyphenolic flavonoid (rutin) action. Biochem Mol Biol Int. 41:1067–1075. 1997.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Havsteen B: The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 96:67–202. 2002.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Narender PD, Ganga R, Sambasiva E, Mallikarjuna T and Praneeth VS: Quantification of phytochemical constituents and in vitro antioxidant activity of Mesua ferrea leaves. Asian Pac J Trop. 2 (Suppl):S539–S542. 2012.
|
|
47
|
Hertog MG, Sweetnam PM, Fehily AM, Elwood PC and Kromhout D: Antioxidant flavanols and ischemic heart disease in a welsh population of men: The Caerphilly study. Am J Clin. 65:1489–1494. 1997.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kattan MB and Hollman PC: Dietary flavonoids and cardiovascular disease. Nutr Metab Cardiovasc Dis. 81(4)1998.
|
|
49
|
Badri S, Reddy BV, Basha SK, Anasuya D and Bhavani K: A review on pharmacological activities of alkaloids. World J Curr Med Pharm Res. 1:230–234. 2019.
|
|
50
|
Arpita R: A review on the alkaloids an important therapeutic compound from plants. Int J Plant Biotechnol. 3:2456–0162. 2017.
|
|
51
|
Lacaille-Dubois MA and Wagner H: A review of the biological and pharmacological activities of saponins. Phytomed. 2:363–86. 1996.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Barbosa ADP: An overview on the biological and pharmacological activities of saponins. Int J Pharm Pharm Sci. 6:47–50. 2014.
|
|
53
|
Rashmi R, Bojan Magesh S, Mohanram Ramkumar K, Suryanarayanan S and Venkata Subba Rao M: Antioxidant potential of naringenin helps to protect liver tissue from streptozotocin-induced damage. Rep Biochem Mol Biol. 7:76–84. 2018.PubMed/NCBI
|
|
54
|
Xu D, Hu MJ, Wang YQ and Cui YL: Antioxidant activities of quercetin and its complexes for medicinal application. Molecules. 24(1123)2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Bernatoniene J and Kopustinskiene DM: The role of catechins in cellular responses to oxidative stress. Molecules. 23(965)2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Frankel EN: The antioxidant and nutritional effects of tocopherols, ascorbic acid and beta-carotene in relation to processing of edible oils. Bibl Nutr Dieta. 43:297–312. 1989.PubMed/NCBI View Article : Google Scholar
|
|
57
|
He K, Nukada H, Urakami T and Murphy MP: Antioxidant and pro-oxidant properties of pyrroloquinoline quinone (PQQ): Implications for its function in biological systems. Biochem Pharmacol. 65:67–74. 2003.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Velderrain-Rodríguez GR, Torres-Moreno H, Villegas-Ochoa MA, Ayala-Zavala JF, Robles-Zepeda RE, Wall-Medrano A and González-Aguilar GA: Gallic acid content and an antioxidant mechanism are responsible for the antiproliferative activity of ‘Ataulfo' mango peel on LS180 cells. Molecules. 23(695)2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Pushp P, Sharma N, Joseph GS and Singh RP: Antioxidant activity and detection of (-)epicatechin in the methanolic extract of stem of Tinospora cordifolia. J Food Sci Technol. 50:567–572. 2013.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Gülçin I: Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology. 217:213–220. 2006.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Henning SM, Niu Y, Liu Y, Lee NH, Hara Y, Thames GD, Minutti RR, Carpenter CL, Wang H and Heber D: Bioavailability and antioxidant effect of epigallocatechin gallate administered in purified form versus as green tea extract in healthy individuals. J Nutr Biochem. 16:610–616. 2005.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Barzegar A: Antioxidant activity of polyphenolic myricetin in vitro cell-free and cell-based systems. Mol Biol Res Commun. 5:87–95. 2016.PubMed/NCBI
|
|
63
|
Kilic I and Yesilogu Y: Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim Acta A Mol Biomol Spectrosc. 115:719–724. 2013.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Kang KA, Piao MJ, Ryu YS, Hyun YJ, Park JE, Shilnikova K, Zhen AX, Kang HK, Koh YS, Jeong YJ and Hyun JW: Luteolin induces apoptotic cell death via antioxidant activity in human colon cancer cells. Int J Oncol. 51:1169–1178. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Salehi B, Venditti A, Sharifi-Rad M, Kręgiel D, Sharifi-Rad J, Durazzo A, Lucarini M, Santini A, Souto EB, Novellino E, et al: The therapeutic potential of apigenin. Int J Mol Sci. 20(1305)2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Chen AY and Chen YC: A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 138:2099–2107. 2013.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Enogieru AB, Haylett W, Hiss DC, Bardien S and Ekpo OE: Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid Med Cell Longev. 2018(6241017)2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Sova M: Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev Med Chem. 12:749–767. 2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Yang HL, Chen SC, Senthil Kumar KJ, Yu KN, Lee Chao PD, Tsai SY, Hou YC and Hseu YC: Antioxidant and anti-inflammatory potential of hesperetin metabolites obtained from hesperetin-administered rat serum: An ex vivo approach. J Agric Food Chem. 60:522–532. 2012.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Sun JY, You CY, Dong K, You HS and Xing JF: Anti-inflammatory, analgesic and antioxidant activities of 3,4-oxo-isopropylidene-shikimic acid. Pharm Biol. 54:2282–2287. 2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Park CE, Yun H, Lee EB, Min BI, Bae H, Choe W, Kang I, Kim SS and Ha J: The antioxidant effects of genistein are associated with AMP-activated protein kinase activation and PTEN induction in prostate cancer cells. J Med Food. 13:815–820. 2010.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Salehi B, Fokou PVT, Sharifi-Rad M, Zucca P, Pezzani R, Martins N and Sharifi-Rad J: The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals (Basel). 12(11)2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Baumann J, Wurn G and Bruchlausen FV: Prostaglandin synthetase inhibiting O2 radical scavenging properties of some flavonoids and related phenolic compounds. Arch Pharm Res. 307:1–77. 1979.
|
|
74
|
Wettasinghe M and Shahidi F: Evening primrose meal: A source of natural antioxidants and scavenger of hydrogen peroxide and oxygen-derived free radicals. J Agric Food Chem. 47:1801–1818. 1999.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Mohammed AA and Ibrahim AA: Pathological roles of reactive oxygen species and their defence mechanism. Saudi Pharm J. 12:1–18. 2004.
|
|
76
|
Stohs SJ and Bagchi D: Oxidative mechanisms in the toxicity of metals ions. Free Radic Biol Med. 18:321–336. 1995.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Lloyd RV, Hanna PM and Mason RP: The origin of the hydroxyl radical oxygen in the fenton reaction. Free Radic Biol Med. 22:885–888. 1997.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Yin H, Xu L and Porter NA: Free radical lipid peroxidation: Mechanisms and analysis. Chem Rev. 10:5944–5972. 2011.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Kinnunen PKJ, Kaarniranta K and Mahalka AK: Protein-oxidized phospholipid interactions in cellular signaling for cell death: From biophysics to clinical correlations. Biochim Biophys Acta. 10:2446–2455. 2012.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Reis A and Spickett CM: Chemistry of phospholipid oxidation. Biochim Biophys Acta. 1818:2374–2387. 2012.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Volinsky R and Kinnunen PK: Oxidized phosphatidylcholines in membrane-level cellular signaling: From biophysics to physiology and molecular pathology. FEBS J. 280:2806–2816. 2013.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Chiueh CC: Neuroprotective properties of nitric oxide. Ann NY Acad Sci. 890:301–311. 1999.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Beckman JS and Koppenol WH: Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am J Physiol. 271:1424–1437. 1996.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Welch G and Loscalto J: Nitric oxide and the cardiovascular system. J Cardiothorac Surg. 9:361–371. 1994.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Hersh EM, Wong VG, Henderson ES and Freirich EJ: Hepatotoxic effects of methotrexate. Cancer. 19:600–606. 1966.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Hall PD, Jenner MA and Ahern MJ: Hepatotoxicity in a rat model caused by orally administered methotrexate. Hepatol. 14:906–910. 1991.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Tousson E, Atteya E, El-Atrash E and Jeweely OI: Abrogation by Ginkgo Byloba leaf extract on hepatic and renal toxicity induced by methotrexate in rats. Cancer Res Treat. 2:44–51. 2014.
|
|
88
|
Mukherjee S, Ghosh S, Choudhury S, Adhikary A, Manna K, Dey S and Chattopadhyay S: Pomegranate reverses methotrexate-induced oxidative stress and apoptosis in hepatocytes by modulating Nrf2-NF-κB pathways. J Nutr Biochem. 24:2040–2050. 2013.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Olayinka ET, Ore A, Adeyemo OA and Ola OS: Ameliorative effect of gallic acid on methotrexate-induced hepatotoxicity and nephrotoxicity in rat. J Xenobiot. 6(6092)2016.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Saka S and Aouacheri O: The investigation of the oxidative stress-related parameters in high the investigation of the oxidative stress-related parameters in high doses methotrexate-induced albino wistar rats. J Bioequiv Availab. 9(2)2017.
|






















